Introduction
Cervical cancer is the seventh most common cancer
overall in human populations and the second most common type of
cancer in women in many developing countries (1–3). In
Taiwan, cervical cancer is the tenth most common cause of cancer
associated death with rates of 5.5/100,000 persons/year based on
the 2012 report from the Department of Health, Executive Yuan,
Taiwan. The treatments for cervical cancer include surgery,
radiotherapy, chemotherapy or combination radiotherapy with
chemotherapy. However, the reduction in quality of patients' life
caused by the high toxic effects of chemotherapeutic drugs makes it
unsatisfactory (4,5). Numerous studies have been undertaken
to find new therapies, mechanisms or compounds for cervical cancer
treatment. Natural products have been used to treat cancer patients
and those compounds can be used as complementary and/or alternative
therapies in psychiatric medicine (6).
Curcumin is one of the plant pigments and was
obtained from turmeric (Curcuma longa L.), which has been
demonstrated to have wound healing function in diabetic animals
(7), and anti-inflammatory
(8), antibacterial (9) and antioxidant (10) properties. Furthermore, substantial
evidence has shown that curcumin could be used as anti-carcinogenic
substance through the inhibition of cell proliferation, inductions
of cancer cell apoptosis, inhibition of angiogenesis and tumor
metastasis (11–13). Curcumin has been shown to involve
downregulation of nuclear factor-κB and the serine/threonine kinase
Akt and is independent of tubulin polymerization (14). Curcumin is safe to human in phase I
clinical trials for up to 12 g/day orally and causes histologic
improvement of precancerous lesions in some patients; thus it
suggested that these doses of curcumin is biologically active
(15,16).
Several studies have shown that curcumin-mediated
cell cycle arrest involved DNA damage signaling in colorectal
carcinoma HCT116 cells (17), human
bladder cancer T24 cells (18) and
primary adult T-cell leukemia cells (19). It was reported that curcumin induced
reactive oxygen species (ROS) production in human colon cancer
HCT116 cells (20), nuclear DNA
damage in human gastric mucosa cells, lymphocytes (21) and in human hepatoma HepG2 cells
(22). Furthermore, there is no
available information to show curcumin induced DNA damage in human
cervical cancer cells and the exact nature of the trigger and the
mechanisms involved are unclear.
Curcumin has been demonstrated to present antitumor
activity against many types of human cancers (23–26).
However, the inhibitory effects and possible mechanisms of curcumin
induced DNA damage in human cervical cancer cells remain to be
determined. Therefore, in this study, we used human cervical cancer
HeLa cells to further characterize curcumin-induced DNA damage
in vitro. Curcumin treatment generally resulted in decreased
viable cells and DNA damage in vitro. We further
investigated DNA damage associated protein expression and we found
that curcumin inhibited DNA damage and repair associated proteins
in HeLa cells.
Materials and methods
Chemicals and reagents
Curcumin, dimethyl sulfoxide (DMSO), propidium
iodide (PI), trypsin-EDTA, penicillin-streptomycin,
anti-O6-methylguanine-DNA methyltransferase (MGMT) (cat.
no. M3068), anti-PARP (cat. no. P248), anti-p-ATMSer1981 (cat. no.
SBA4300100) and anti-β-actin (cat. no. A5316) were purchased from
Sigma Chemical Co. (St. Louis, MO, USA). Anti-DNA-PK (cat. no.
PC127) was purchased from Calbiochem (San Diego, CA, USA).
Anti-p-H2A.X (cat. no. GTX80694), anti-breast cancer 1 (BRCA1)
(cat. no. GTX70111) and anti-MDM2 (cat. no. GTX110608) were
purchased from GeneTex, Inc. (Irvine, CA, USA). Anti-mediator of
DNA damage checkpoint 1 (MDC1) (cat. no. 05-1572) and anti-p53
(cat. no. 04-241) were purchased from Millipore Corp. (Billerica,
MA, USA). Anti-p-ATRSer428 (cat. no. 2853) was purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Dulbecco's
modified Eagle's medium (DMEM) medium and fetal bovine serum (FBS)
were purchased from Gibco®, Invitrogen Life Technologies
(Carlsbad, CA, USA). Curcumin was first dissolved in DMSO at 1
mM.
Cell culture
The human cervical cancer HeLa cell line was
purchased from the Food Industry Research and Development Institute
(Hsinchu, Taiwan). Cells were placed into 75 cm2 tissue
culture flasks in DMEM medium supplemented with 10% FBS and 1%
penicillin-streptomycin (100 U/ml penicillin and 100 µg/ml
streptomycin). Then cells were grown at 37°C in a humidified
atmosphere of 95% air and 5% CO2 as previously described
(27,28).
Cell morphology examination and cellular
viability assay
HeLa cells (1×105 cells/well) were kept
on the 12-well cell culture cluster overnight and then were treated
with 0, 12, 13 and 14 µM curcumin for 48 h. After
incubation, cells were examined by contrast-phase microscopy for
cell morphological changes. Trypsin was added to the cells,
harvested and rinsed three times with in phosphate-buffered saline
(PBS). All cells were stained with PI (5 µg/ml) in PBS and
the total percentage of cell viability was measured by flow
cytometry (Becton-Dickinson, San Jose, CA, USA) as previously
described (27).
Comet assay (single cell gel
electrophoresis)
HeLa cells (1×105 cells/well) were kept
on 12-well cell culture plate for 24 h and then treated with 13
µM of curcumin or 0.5% H2O2 (positive
control) for 0, 6, 24 and 48 h. At the end of incubation, aliquots
of 105 cells from each treatment were collected and cast
into miniature LMA gels on microscope slides as previously
described (27), followed by lysing
in situ to relax the compacted DNA in nuclei of cells. Cells
were electrophoresed and DNA was visualized and photographed by EB
staining under fluorescence microscopy. Comets (DNA damage) of
cells on slides were quantitated for comet tail lengths by the
CometScore™ Freeware analysis (TriTek Corp., Sumerduck, VA, USA)
(27).
4′,6-Diamidino-2-phenylindole
dihydrochloride (DAPI) staining
HeLa cells (1.5×105 cells/well) were
maintained on 6-well cell culture plate for 24 h and then were
incubated with 13 µM curcumin for 0, 6, 24 and 48 h. At the
end of incubation, cells were fixed with 4% formaldehyde in PBS for
10 min, followed by washing with PBS and then were stained by DAPI
for 1 h at 37°C. Cells from each treatment were examined and
photographed by using a fluorescence microscope at x200 as
previously described (27).
Apo-BrdU (bromolated deoxyuridine
triphosphate nucleotides) TUNEL assays
To evaluate HeLa cell DNA fragmentation, the HeLa
cells (1.5×105 cells/well) were maintained on 6-well
plate and treated with 0 and 13 µM of curcumin for 0, 12, 24
and 48 h. Cells were fixed in 4% formaldehyde in PBS for 15 min,
and then washed twice with ice-cold PBS, and incubated in the dark
for 60 min at 37°C in 50 µl of DNA Labeling Solution each
assay. TUNEL assays were performed by fluorescence microscope in
triplicate on three independent experiments. TUNEL staining was
performed according to the manufacturer's instructions (Apo-BrdU
in situ DNA fragmentation assay kit; BioVision, Inc.,
Milpitas, CA, USA) (27).
Western blotting
HeLa cells (1.5×106 cells/dish) were
maintained on a 10-cm dish with DMEM medium containing 10% FBS for
24 h and were incubated with 13 µM of curcumin for 0, 6, 24
and 48 h. After treatment, cells were collected and suspended in
sodium dodecyl sulphate (SDS) buffer followed by sonication and
boiling for 10 min as described previously (27). The total protein in each sample was
quantitated. A total 30 µg from each sample was
electrophoresed by 10% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE), transferred to PVDF membrane, and were immunoblotted as
previously described (27).
Membranes were transferred, and then were followed by staining with
primary antibodies such as anti-p-ATM, anti-p-ATR, anti-p53,
anti-MDM2, and anti-p-p53, anti-BRCA1, anti-DNA-PK, anti-MDC1,
anti-p-H2A.X, anti-PARP and anti-MGMT at 4°C (overnight), each
membrane was then washed. After washing, all membranes were stained
by secondary antibody, washed and were visualized with a
chemiluminescent detection system and the protein expressions were
quantitated as described by the manufacturer (27,29).
Confocal laser microscopy
HeLa cells (1.5×105 cells/well) were
maintained on 6-well plate and treated with 0 and 13 µM of
curcumin for 48 h. Cells were rinsed and fixed in 4% formaldehyde
in PBS for 15 min as previously described (30). Cells were washed and permeablized
with 0.1% Triton X-100 in PBS followed by washing and blocking with
5% BSA in PBS for 60 min and then were washed with PBS. The primary
anti-p-H2A.X, anti-p53 and anti-p-p53 (green fluorescence) were
used to stain cells and then followed by secondary antibody
(FITC-conjugated goat anti-mouse IgG) staining. The PI (red
fluorescence) used for nuclei staining and were photomicrographed
under a Leica TCS SP2 confocal spectral microscope as previously
described (30).
Statistical analysis
All quantitative data were presented as the mean ±
standard deviation (SD) from three independent experiments.
Student's t-test was used to compare means between the control and
curcumin treated groups. The P<0.05 was considered as the
significant level.
Results
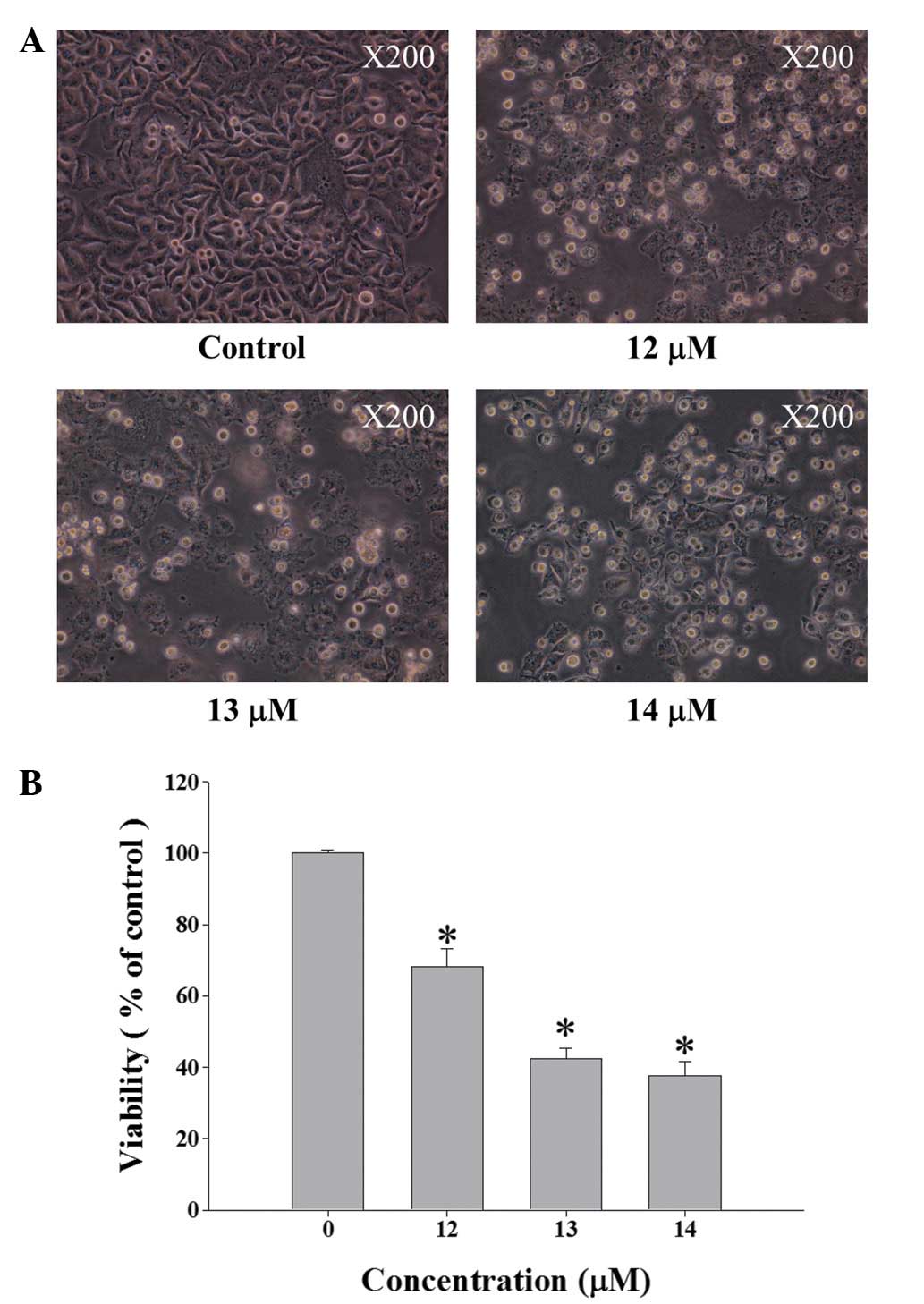
Curcumin induces cell morphological
changes and decreases the total percentage of HeLa viable
cells
HeLa cells were incubated with 0, 12, 13 and 14
µM curcumin for 48 h and then were examined for cell
morphology and were collected for percentage of viable cell
determinations and results are shown in Fig. 1A and B. The results from Fig. 1A indicated that curcumin induced
cell morphological changes and the total viable cells were
decreased when compared to control (without curcumin
treatment).
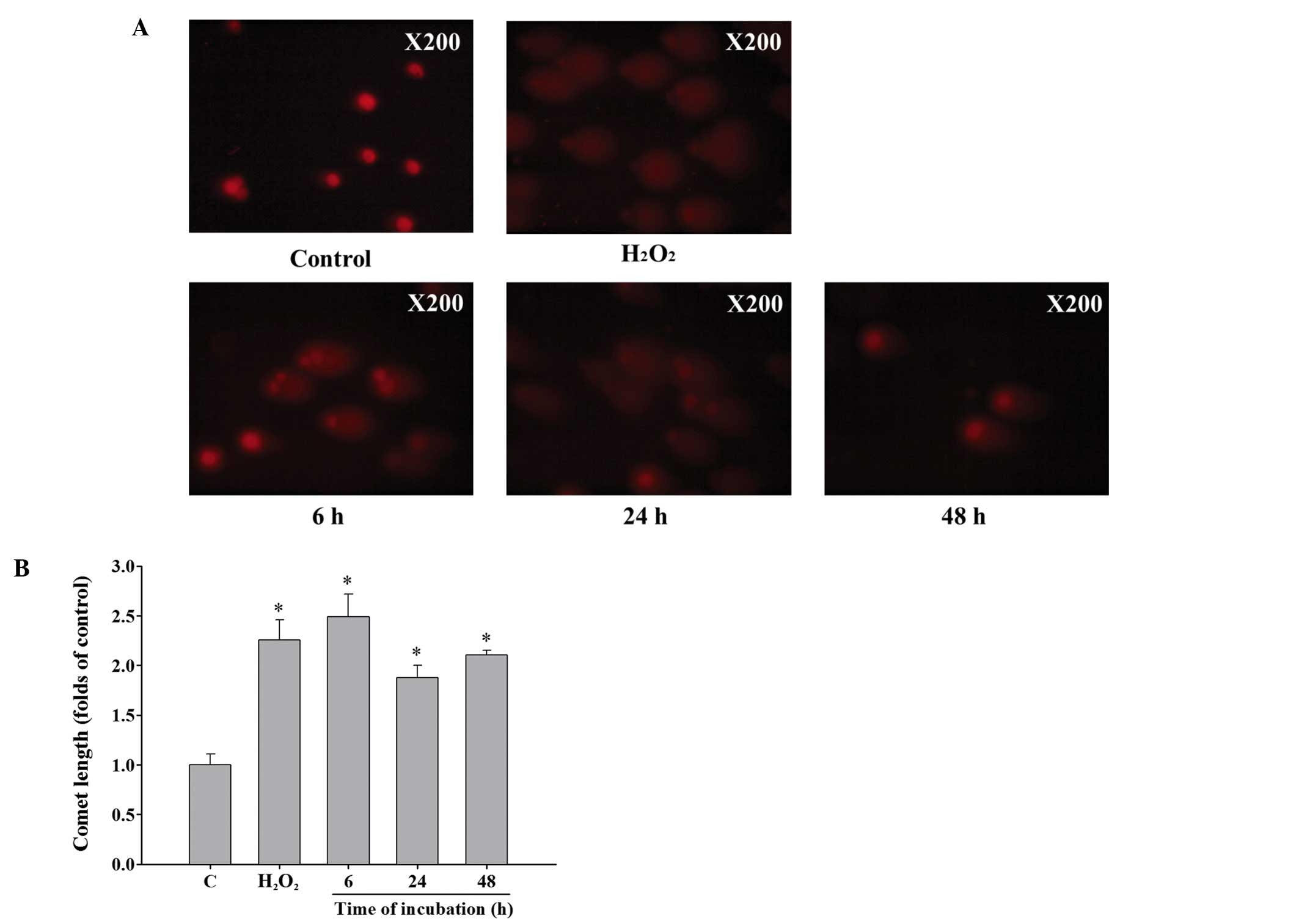
Curcumin induces DNA damage of HeLa
cells
Cells were incubated with 13 µM curcumin for
0, 6, 24 and 48 h and then collected for comet assay. Results from
Fig. 2A and B demonstrated that 13
µM curcumin induced comet tail (DNA damage) production from
single cell electrophoresis when compared with control in HeLa
cells (Fig. 2A). The longer the
incubation time, the longer the comet tail length which indicated
that DNA damage is time-dependent caused by curcumin in HeLa cells
(Fig. 2B).
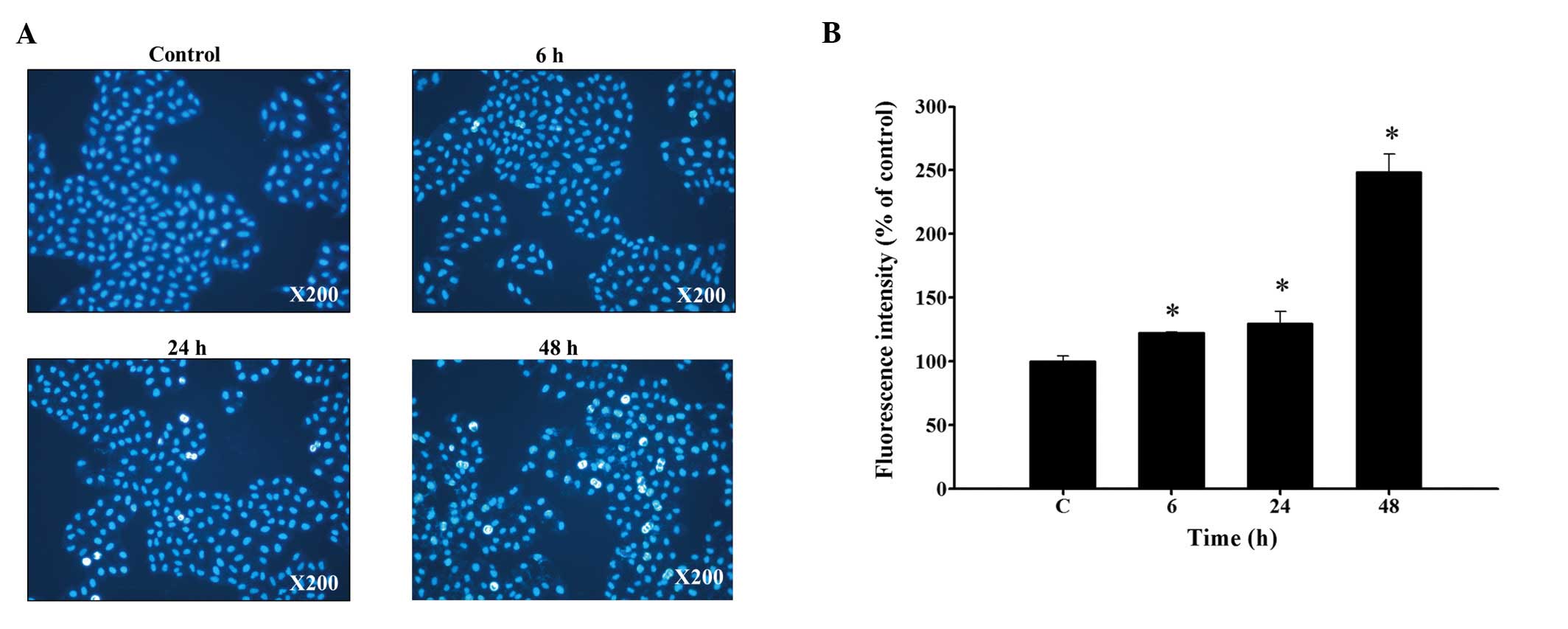
Curcumin induces chromatin condensation
of HeLa cells
For further confirming whether or not curcumin
decreased the total viable cells through apoptosis of HeLa cells,
cells were incubated with 13 µM of curcumin for 0, 6, 24 and
48 h, and were stained by DAPI to investigate the formation of
chromatin condensation which were characterized by nuclear
fluorescence (white color). Results from Fig. 3A and B indicated that curcumin
induced chromatin condensation in a time-dependent manner. The
chromatin condensation was based on the higher fluoresence (DAPI
staining) compared to control under fluorescence microscope
examination in HeLa cells.
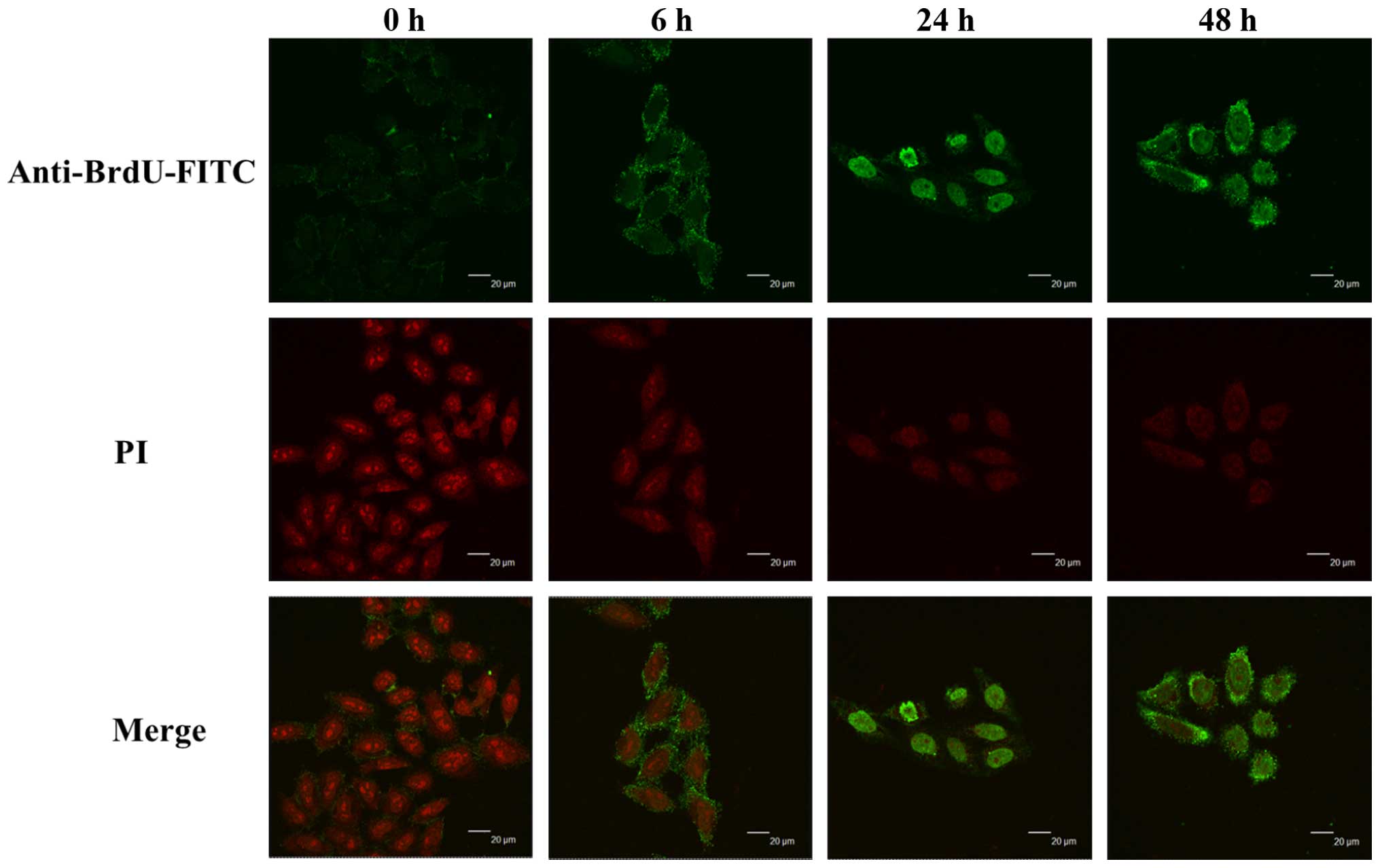
Curcumin induces DNA fragmentation of
HeLa cells
HeLa cells were incubated with 13 µM curcumin
for 0, 6, 24 and 48 h, and then collected for Apo-BrdU TUNEL
assays. In order to investigate the expression of the BrdU in HeLa
cells. Cells were exposed to 13 µM of curcumin for 0, 6, 24
and 48 h, and then were examined by confocal microscopy and results
are shown in Fig. 4. Comparing with
control group, the BrdU-FITC in cells treated with curcumin was
found to increase the incorporation with DNA strand breaks and were
visualized in the nucleus as shown in the Fig. 4 (merge image). The result
demonstrated that curcumin induced DNA fragmentation that may be
through the overexpression of BrdU in nuclei in HeLa cells.
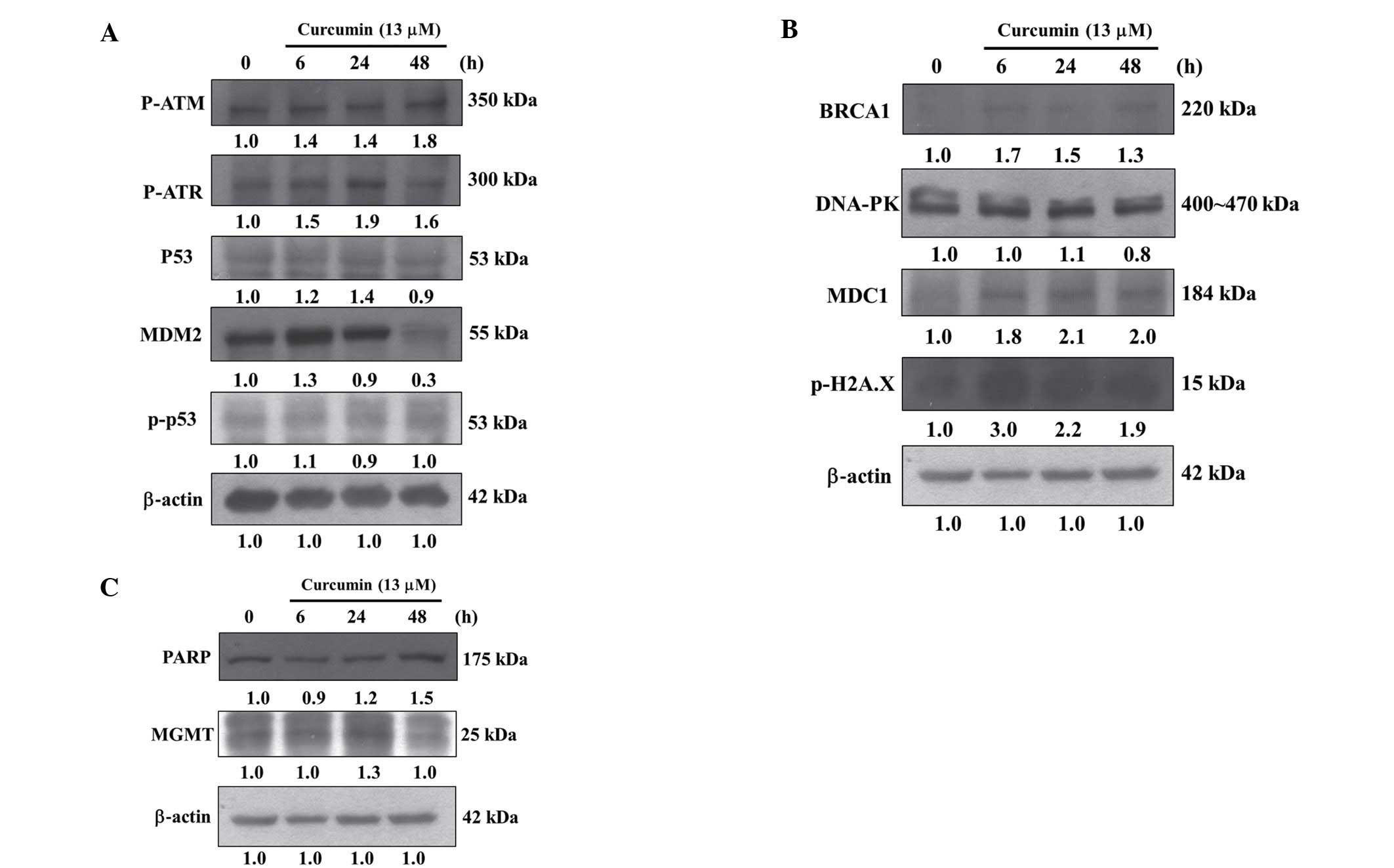
Curcumin affects DNA damage associated
proteins of HeLa cells
In order to further investigate curcumin induced DNA
damage via affect DNA damage and repair associated protein
expression in HeLa cells, cells were treated with 13 µM of
curcumin for 0, 6, 24 and 48 h and then total protein from cells
were quantitated and DNA damage associated proteins were examined
by western blotting and results are shown in Fig. 5A–C. These results demonstrated that
curcumin significantly increased the amounts of proteins such as
p-ATM, p-ATR, p53 and MDM2 (Fig.
5A), BRCA1, DNA-PK, MDC1 and p-H2A.X (Fig. 5B), PARP and MGMT (Fig. 5C) in HeLa cells.
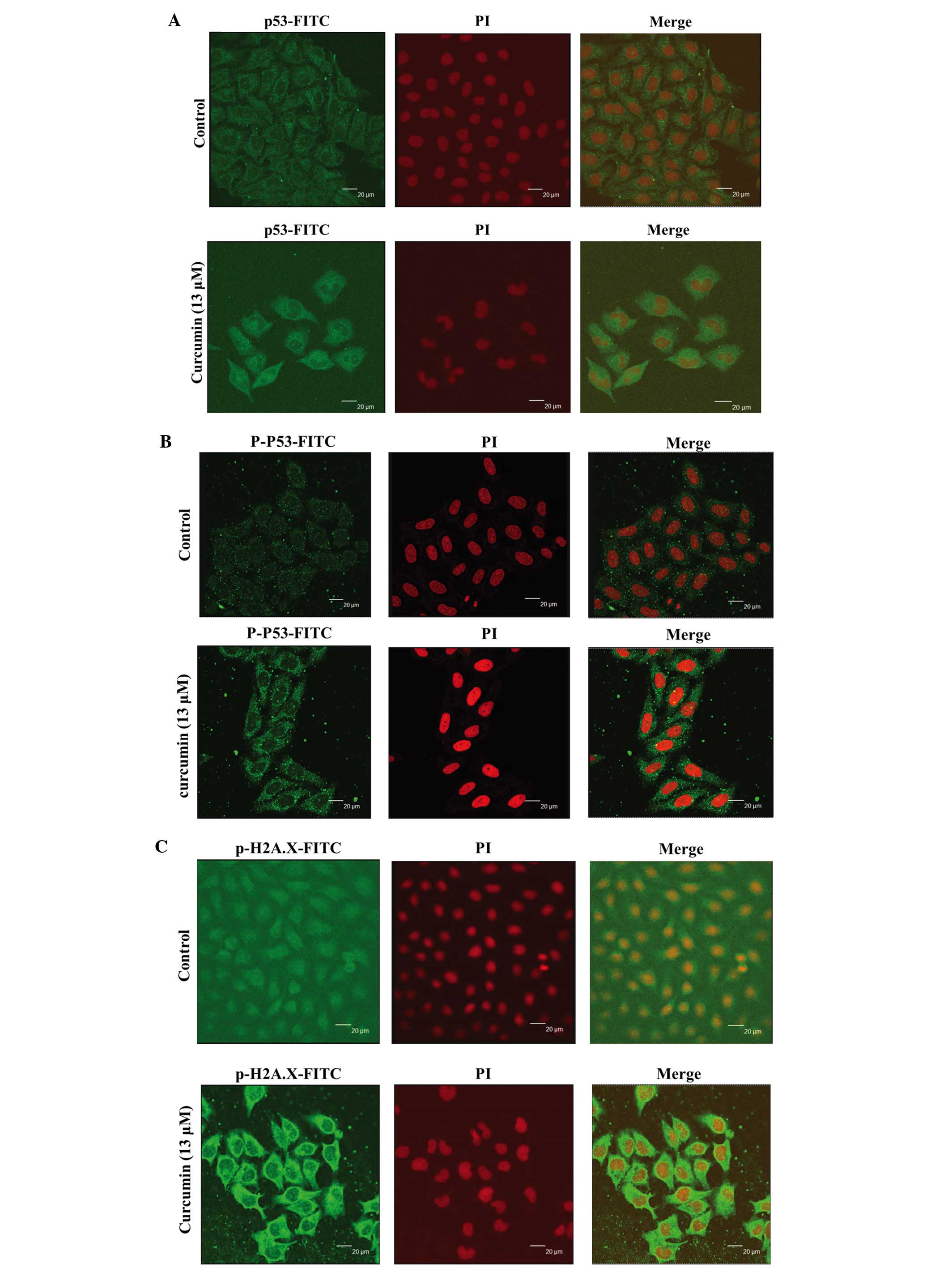
Curcumin affects the translocation of
p53, p-p53 and p-H2A.X in HeLa cells
Based on the results from western blotting (Fig. 5A and B) indicated that curcumin
increased the amounts of p53, p-p53 and p-H2A.X in HeLa cells, we
investigated the translocation and increase of the proteins in HeLa
cells. Cells were exposed to 13 µM of curcumin for 48 h and
then examined by confocal microscopy and results are shown in
Fig. 6. Contrasting to the control,
the p53 (Fig. 6A), p-p53 (Fig. 6B) and p-H2A.X (Fig. 6C) in cells treated with curcumin was
found to increase the cytosol, and more labelled p53, p-p53 and
p-H2A.X were visualized in the nucleus as shown in the Fig. 6 (Merge image). These observations
indicated that curcumin induced DNA damage and repair that may be
via the translocation of p53, p-p53 and p-H2A.X from cytoplasm into
nuclei in HeLa cells.
Discussion
It is well documented that curcumin induced cell
death may be via cell cycle arrest and induction of apoptosis in
many human cancer cell lines, furthermore, animal studies revealed
that oral administration of curcumin inhibited the incidence of
cancers and it is under clinical trials to various cancers and
related diseases (9). It was also
reported that curcumin induced DNA damage and repair in several
human cancer cells including human peripheral blood mononuclear
cells (PBMCs) which was measured by the comet assay (21,31).
Recently, it was reported that curcumin can induce apoptosis of
normal resting human T cells that is not connected with DNA damage
(17). However, there is no
available information to show curcumin induced DNA damage and
affect DNA damage and repair associated protein expression in human
cervical cancer cells. Therefore, we investigated whether or not
curcumin induced DNA damage in HeLa cells and we found that: i)
curcumin decreased the percentage of viable cells (Fig. 1); ii) a time-dependent increase in
DNA damage was measured by comet assay (Fig. 2); iii) curcumin induced chromatin
condensation time-dependently which was examined by DAPI staining
(Fig. 3); iv) curcumin induced DNA
fragmentation which was examined by TUNEL assay (Fig. 4); v) curcumin significantly
increased p-ATM, p-ATR, p53 and MDM2 (Fig. 5A), BRCA1, MDC1 and p-H2A.X (Fig. 5B), PARP and MGMT (Fig. 5C) in HeLa cells; and vi) curcumin
induced DNA damage and repair that may also involve p53, p-p53 and
p-H2A.X (Fig. 6) translocation from
cytoplasm into nuclei that were measured by confocal laser
microscopy in HeLa cells.
Several studies have shown that curcumin induced DNA
damage in cancer cells (32).
Herein, we also showed that curcumin induced DNA damage (Fig. 3) and chromatin condensation
(Fig. 2) in HeLa cells. Both were
assayed by single cell electrophoresis (comet assay) and DAPI
staining, respectively. Both assays are well known for measuring
DNA damage (32,33) and chromatin condensation (34,35),
respectively. There are studies that cells can repair DNA damage
from agent induction and also can use DNA repair systems to
eliminate these damaged DNA even to repair DNA base for cell
maintaining survival (36,37). In addition, previous studies showed
that DNA fragmentation was closely related with apoptosis (27,38).
Our result showed that curcumin induced DNA fragmentation in
Fig. 4 as well. The above
descriptions indicated that curcumin could inhibit HeLa cell
proliferation by induced DNA damage.
Results from western blotting indicated that
curcumin increased DNA damage and repair associated protein
expression such as p-ATM, p-ATR, p53 and MDM2 (Fig. 5A), BRCA1, MDC1 and p-H2A.X (Fig. 5B), PARP and MGMT (Fig. 5C), however, the protein levels of
p-p53 (Fig. 5A) and DNA-PK
(Fig. 5B) were no significantly
affected. It was reported that MGMT in human cervical cancer
(39,40) and polymorphism in MGMT increases the
susceptibility of women to cervical carcinoma (40), herein; we found that curcumin
inhibited the expression of MGMT in HeLa cells (Fig. 5). It was reported that inhibiting
MGMT could increase tumor susceptibility (41). MGMT can repair the pre-mutagenic,
pre-carcinogenic and pre-toxic DNA damage
O6-methylguanine (42)
and MGMT has been recognized as an important change that takes
place in cervical cancer (43).
BRCA1, is a tumor-suppressor gene, whose mutation
has been correlated with the appearance of breast and/or ovarian
cancer. Herein, we found that curcumin increased the expression of
BRCA1 in HeLa cells (Fig. 5B). The
BRCA1 gene products have been demonstrated to be associated with
DNA damage repair, transcriptional control, cell growth, and
apoptosis (44,45). NSCLC patients with reduced
BRCA1 mRNA expression levels have greater overall survival
benefit (46). Numerous studies
have reported that MDC1 plays important roles in DNA damage
response pathway (47) and have
been shown also in human cervical cancer (48). Our findings showed that curcumin
increased MDC1 expression in HeLa cells (Fig. 5B). Recently, it was reported that
MDC1 seems to be related to the oncogenic potential of cervical
cancer, furthermore, the suppression of its expression can inhibit
cancer cell growth (48) and MDC1
could be a potential therapeutic target in human cervical
cancer.
Fig. 5 shows that
curcumin increased p53, p-p53 and p-H2A.X, respectively, in HeLa
cells. We also used confocal laser microscopy to measure the
translocation of p53 (Fig. 6A),
p-p53 (Fig. 6B) and p-H2A.X
(Fig. 6C) from cytoplasm to nuclei.
It is well known that after DNA damage is present in cells, the
protein level of p53 (a transcription factor) will increase. The
p53 have been shown to mediate G1 arrest for cells in
response to genotoxic stress and allowing time for DNA repair
(49). Herein, we found p53 was
increased but p-p53 was not significantly increased. The
phosphorylation of p53 has been shown to be the one type of
upstream signal for triggering the p53 regulatory functions
(50). The phospho-H2A.X, is a
reliable marker of DNA double-strand breaks (DSBs) (51) and the H2A.X deficient mice will have
higher radiosensitivity (52). In
the present studies, we found that curcumin increased the
expression of p-H2A.X (Fig. 6C)
that was also confirmed by confocal laser microscopic examination
(Fig. 6).
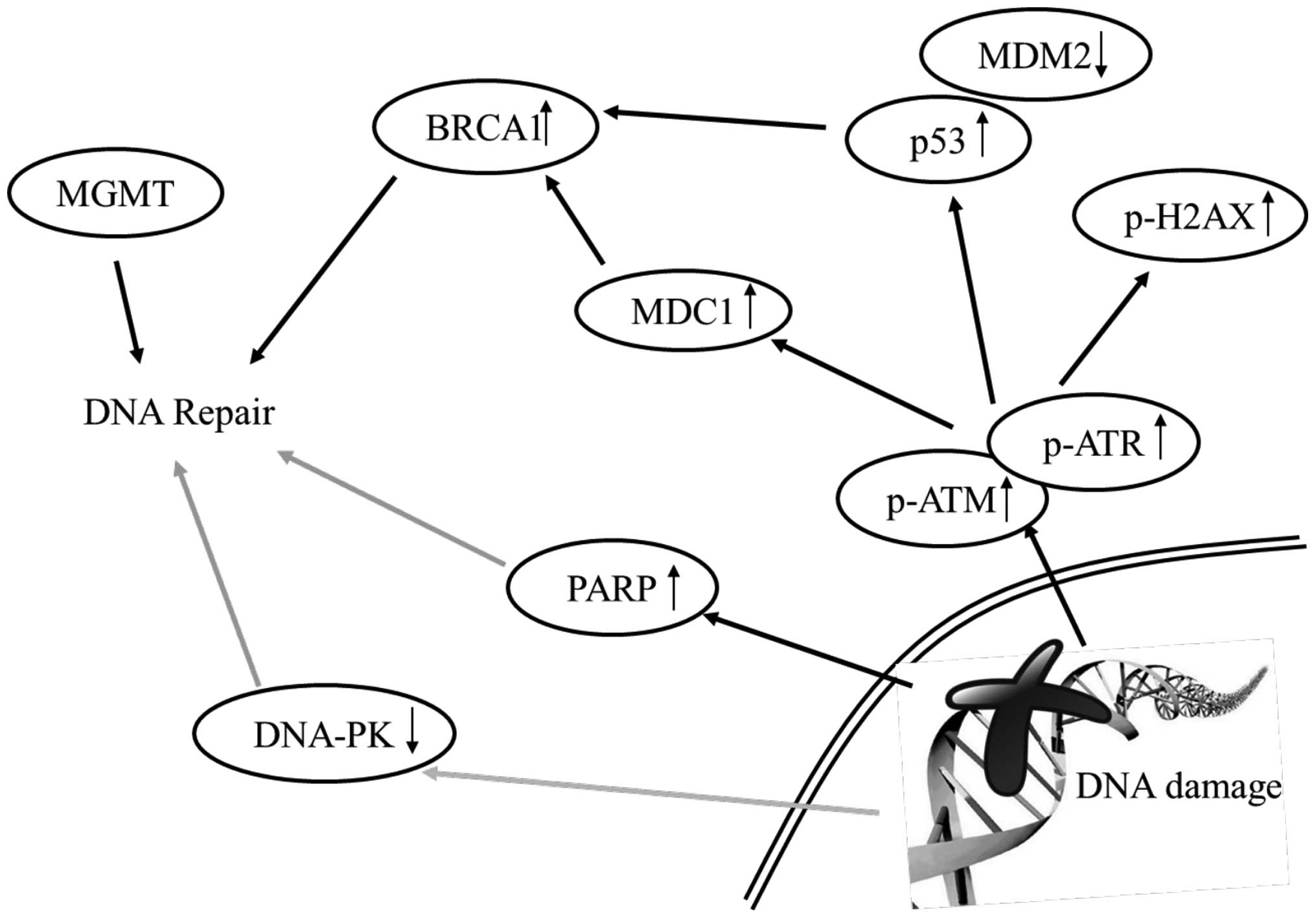
In conclusion, the results of the present study
indicate that: i) curcumin induced cytotoxic effects (cell death)
in HeLa cells; ii) curcumin induced DNA damage and cell death which
were measured by comet assay and DAPI staining, respectively, in
HeLa cells; and iii) curcumin increased the proteins levels of
p-ATM, p-ATR, MGMT, BRCA1, MDC1, p53, p-p53 and p-H2A.X that may
lead to cell death in HeLa cells as summarized in Fig. 7.
Acknowledgments
This study was supported by the grant no. 103-47
from the Cheng Hsin General Hospital (Taipei, Taiwan). Experiments
and data analysis were performed in part through the use of the
Medical Research Core Facilities Center, Office of Research and
Development at China Medical University, Taichung, Taiwan.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding Y, Yang M, She S, Min H, Xv X, Ran X,
Wu Y, Wang W, Wang L, Yi L, et al: iTRAQ-based quantitative
proteomic analysis of cervical cancer. Int J Oncol. 46:1748–1758.
2015.PubMed/NCBI
|
|
3
|
Smith HO, Tiffany MF, Qualls CR and Key
CR: The rising incidence of adenocarcinoma relative to squamous
cell carcinoma of the uterine cervix in the United States - a
24-year population-based study. Gynecol Oncol. 78:97–105. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gascoigne KE and Taylor SS: Cancer cells
display profound intra- and interline variation following prolonged
exposure to antimitotic drugs. Cancer Cell. 14:111–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rein DT and Kurbacher CM: The role of
chemotherapy in invasive cancer of the cervix uteri: Current
standards and future prospects. Anticancer Drugs. 12:787–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiappedi M and Bejor M: Herbals and
natural dietary supplements in psychiatric practice. Recent Patents
CNS Drug Discov. 5:164–171. 2010. View Article : Google Scholar
|
|
7
|
Sidhu GS, Mani H, Gaddipati JP, Singh AK,
Seth P, Banaudha KK, Patnaik GK and Maheshwari RK: Curcumin
enhances wound healing in streptozotocin induced diabetic rats and
genetically diabetic mice. Wound Repair Regen. 7:362–374. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Niederau C and Göpfert E: The effect of
chelidonium- and turmeric root extract on upper abdominal pain due
to functional disorders of the biliary system. Results from a
placebo-controlled double-blind study. Med Klin (Munich).
94:425–430. 1999.In German. View Article : Google Scholar
|
|
9
|
Duvoix A, Blasius R, Delhalle S,
Schnekenburger M, Morceau F, Henry E, Dicato M and Diederich M:
Chemopreventive and therapeutic effects of curcumin. Cancer Lett.
223:181–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiagarajan R and Manikandan R:
Antioxidants and cataract. Free Radic Res. 47:337–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basnet P and Skalko-Basnet N: Curcumin: An
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: Molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar
|
|
14
|
Bava SV, Puliappadamba VT, Deepti A, Nair
A, Karunagaran D and Anto RJ: Sensitization of taxol-induced
apoptosis by curcumin involves down-regulation of nuclear
factor-kappaB and the serine/threonine kinase Akt and is
independent of tubulin polymerization. J Biol Chem. 280:6301–6308.
2005. View Article : Google Scholar
|
|
15
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as 'Curecumin': From kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008. View Article : Google Scholar
|
|
16
|
Strimpakos AS and Sharma RA: Curcumin:
Preventive and therapeutic properties in laboratory studies and
clinical trials. Antioxid Redox Signal. 10:511–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu JJ, Cai YJ and Ding J: Curcumin induces
DNA damage and caffeine-insensitive cell cycle arrest in colorectal
carcinoma HCT116 cells. Mol Cell Biochem. 354:247–252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park C, Kim GY, Kim GD, Choi BT, Park YM
and Choi YH: Induction of G2/M arrest and inhibition of
cyclooxygenase-2 activity by curcumin in human bladder cancer T24
cells. Oncol Rep. 15:1225–1231. 2006.PubMed/NCBI
|
|
19
|
Tomita M, Kawakami H, Uchihara JN,
Okudaira T, Masuda M, Takasu N, Matsuda T, Ohta T, Tanaka Y,
Ohshiro K, et al: Curcumin (diferuloylmethane) inhibits
constitutive active NF-kappaB, leading to suppression of cell
growth of human T-cell leukemia virus type I-infected T-cell lines
and primary adult T-cell leukemia cells. Int J Cancer. 118:765–772.
2006. View Article : Google Scholar
|
|
20
|
Giri AK, Das SK, Talukder G and Sharma A:
Sister chromatid exchange and chromosome aberrations induced by
curcumin and tartrazine on mammalian cells in vivo. Cytobios.
62:111–117. 1990.PubMed/NCBI
|
|
21
|
Blasiak J, Trzeciak A and Kowalik J:
Curcumin damages DNA in human gastric mucosa cells and lymphocytes.
J Environ Pathol Toxicol Oncol. 18:271–276. 1999.
|
|
22
|
Cao J, Jia L, Zhou HM, Liu Y and Zhong LF:
Mitochondrial and nuclear DNA damage induced by curcumin in human
hepatoma G2 cells. Toxicol Sci. 91:476–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim B, Kim HS, Jung EJ, Lee JY, K Tsang B,
Lim JM and Song YS: Curcumin induces ER stress-mediated apoptosis
through selective generation of reactive oxygen species in cervical
cancer cells. Mol Carcinog. 55:918–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewinska A, Adamczyk J, Pajak J, Stoklosa
S, Kubis B, Pastuszek P, Slota E and Wnuk M: Curcumin-mediated
decrease in the expression of nucleolar organizer regions in
cervical cancer (HeLa) cells. Mutat Res Genet Toxicol Environ
Mutagen. 771:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh M and Singh N: Molecular mechanism
of curcumin induced cytotoxicity in human cervical carcinoma cells.
Mol Cell Biochem. 325:107–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sreekanth CN, Bava SV, Sreekumar E and
Anto RJ: Molecular evidences for the chemosensitizing efficacy of
liposomal curcumin in paclitaxel chemotherapy in mouse models of
cervical cancer. Oncogene. 30:3139–3152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang AC, Lin TP, Weng YS, Ho YT, Lin HJ,
Huang LJ, Kuo SC and Chung JG: Ethyl
2-[N-m-chlorobenzyl-(2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01006) induces
apoptosis in human cervical cancer HeLa cells. Anticancer Res.
27:2505–2514. 2007.PubMed/NCBI
|
|
28
|
Leung YM, Wong KL, Chen SW, Lu DY, Kuo CS,
Chen YR, Chen YW and Cheng TH: Down-regulation of voltage-gated
Ca2+ channels in Ca2+ store-depleted rat
insulinoma RINm5F cells. Biomedicine. 3:130–139. 2013. View Article : Google Scholar
|
|
29
|
Lin MC, Tsai SY, Wang FY, Liu FH, Syu JN
and Tang FY: Leptin induces cell invasion and the upregulation of
matrilysin in human colon cancer cells. Biomedicine. 3:174–180.
2013. View Article : Google Scholar
|
|
30
|
Wu LY, Lu HF, Chou YC, Shih YL, Bau DT,
Chen JC, Hsu SC and Chung JG: Kaempferol induces DNA damage and
inhibits DNA repair associated protein expressions in human
promyelocytic leukemia HL-60 cells. Am J Chin Med. 43:365–382.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Błasiak J, Trzeciak A, Małecka-Panas E,
Drzewoski J, Iwanienko T, Szumiel I and Wojewódzka M: DNA damage
and repair in human lymphocytes and gastric mucosa cells exposed to
chromium and curcumin. Teratog Carcinog Mutagen. 19:19–31. 1999.
View Article : Google Scholar
|
|
32
|
Ashby J, Tinwell H, Lefevre PA and Browne
MA: The single cell gel electrophoresis assay for induced DNA
damage (comet assay): Measurement of tail length and moment.
Mutagenesis. 10:85–90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pool-Zobel BL, Lotzmann N, Knoll M,
Kuchenmeister F, Lambertz R, Leucht U, Schröder HG and Schmezer P:
Detection of genotoxic effects in human gastric and nasal mucosa
cells isolated from biopsy samples. Environ Mol Mutagen. 24:23–45.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marchissio MJ, Francés DE, Carnovale CE
and Marinelli RA: Evidence for necrosis, but not apoptosis, in
human hepatoma cells with knockdown of mitochondrial aquaporin-8.
Apoptosis. 19:851–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng B, Wu L, Ma L, Liu S, Li L, Xie W
and Li X: Telekin induces apoptosis associated with the
mitochondria-mediated pathway in human hepatocellular carcinoma
cells. Biol Pharm Bull. 36:1118–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olive PL, Banáth JP and Durand RE:
Detection of etoposide resistance by measuring DNA damage in
individual Chinese hamster cells. J Natl Cancer Inst. 82:779–783.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tice RR, Andrews PW and Singh NP: The
single cell gel assay: A sensitive technique for evaluating
intercellular differences in DNA damage and repair. Basic Life Sci.
53:291–301. 1990.PubMed/NCBI
|
|
38
|
Malhotra P, Adhikari M, Singh SK and Kumar
R: N-acetyl tryptophan glucopyranoside (NATG) provides
radioprotection to murine macrophage J774A.1 cells. Free Radic Res.
49:1488–1498. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Banzai C, Nishino K, Quan J, Yoshihara K,
Sekine M, Yahata T and Tanaka K; Gynecological Cancer Registry of
Niigata: Promoter methylation of DAPK1, FHIT, MGMT, and CDKN2A
genes in cervical carcinoma. Int J Clin Oncol. 19:127–132. 2014.
View Article : Google Scholar
|
|
40
|
Huang J, Ye F, Chen H, Lu W and Xie X:
Amino acid substitution polymorphisms of the DNA repair gene MGMT
and the susceptibility to cervical carcinoma. Carcinogenesis.
28:1314–1322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verbeek B, Southgate TD, Gilham DE and
Margison GP: O6-Methylguanine-DNA methyltransferase
inactivation and chemotherapy. Br Med Bull. 85:17–33. 2008.
View Article : Google Scholar
|
|
42
|
Christmann M, Verbeek B, Roos WP and Kaina
B: O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal
tissues and tumors: Enzyme activity, promoter methylation and
immunohistochemistry. Biochim Biophys Acta. 1816:179–190.
2011.PubMed/NCBI
|
|
43
|
Dueñas-González A, Lizano M, Candelaria M,
Cetina L, Arce C and Cervera E: Epigenetics of cervical cancer. An
overview and therapeutic perspectives. Mol Cancer. 4:382005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abramovitch S, Glaser T, Ouchi T and
Werner H: BRCA1-Sp1 interactions in transcriptional regulation of
the IGF-IR gene. FEBS Lett. 541:149–154. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maor SB, Abramovitch S, Erdos MR, Brody LC
and Werner H: BRCA1 suppresses insulin-like growth factor-I
receptor promoter activity: Potential interaction between BRCA1 and
Sp1. Mol Genet Metab. 69:130–136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taron M, Rosell R, Felip E, Mendez P,
Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ,
et al: BRCA1 mRNA expression levels as an indicator of
chemoresistance in lung cancer. Hum Mol Genet. 13:2443–2449. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lou Z, Minter-Dykhouse K, Franco S,
Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J,
Nussenzweig A, Paull TT, et al: MDC1 maintains genomic stability by
participating in the amplification of ATM-dependent DNA damage
signals. Mol Cell. 21:187–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amichay K, Kidron D, Attias-Geva Z,
Schayek H, Sarfstein R, Fishman A, Werner H and Bruchim I: BRCA1 is
expressed in uterine serous carcinoma (USC) and controls
insulin-like growth factor I receptor (IGF-IR) gene expression in
USC cell lines. Int J Gynecol Cancer. 22:748–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prives C and Hall PA: The p53 pathway. J
Pathol. 187:112–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gorgoulis VG, Zacharatos P, Mariatos G,
Liloglou T, Kokotas S, Kastrinakis N, Kotsinas A, Athanasiou A,
Foukas P, Zoumpourlis V, et al: Deregulated expression of c-mos in
non-small cell lung carcinomas: Relationship with p53 status,
genomic instability, and tumor kinetics. Cancer Res. 61:538–549.
2001.PubMed/NCBI
|
|
51
|
Bonner WM, Redon CE, Dickey JS, Nakamura
AJ, Sedelnikova OA, Solier S and Pommier Y: GammaH2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Celeste A, Petersen S, Romanienko PJ,
Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B,
Coppola V, Meffre E, Difilippantonio MJ, et al: Genomic instability
in mice lacking histone H2AX. Science. 296:922–927. 2002.
View Article : Google Scholar : PubMed/NCBI
|