Introduction
Gastric cancer (GC) poses a significant health
burden worldwide despite its declining incidence. GC is often
diagnosed in advanced stages and has a poor prognosis. Globally,
the incidence of GC ranks fourth for cancers in men and fifth in
women, but its death rate is similar to that of lung cancer.
Seventy percent of GC-related deaths occur in developing regions,
with ~40% in China (1,2). The endemic regions are Asia, Eastern
Europe and South America. The incidence of GC has declined over
time due to improving living standards (3–5).
Based on our clinical experience, surgery continues
to be the primary modality for managing early-stage GC; however,
the survival rate of advanced GC is still poor since 80% of
patients who underwent a curative resection develop locoregional or
distant recurrence (6).
The first human cell line was established in a
Baltimore laboratory over 50 years ago by Gey et al
(7). Since then, numerous cancer
cell lines have been established from human tumours; these cell
lines have served as important experimental tools in oncology
research. Currently, >30 GC cell lines are available (8–13)
(Table I).
 | Table IDocumented human gastric cell
lines. |
Table I
Documented human gastric cell
lines.
| Cell line | Age (years) | Gender | Source of
culture | Race | Differentiation | Primary culture | Refs. |
|---|
| AGS | – | – | Primary tumour | American |
Moderately-poorly | 1979 | (8) |
| KATO-III | 55 | Male | Pleural effusion | Japanese | Signet ring cell | 1974-4-10 | (9) |
| MKN-28 | 70 | Female | Lymph node meta | Japanese | Moderately
differentiated | 1975-8-19 | (9) |
| MKN-45 | 62 | Female | Liver meta | Japanese | Poorly
differentiated | 1976-9-5 | (9) |
| MKN-74 | 37 | Male | Liver meta | Japanese | Moderately
differentiated | 1976-7-2 | (9) |
| MKN-7 | 39 | Male | Lymph node
meta | Japanese | Well
differentiated | 1975-7-5 | (9) |
| KWS-1 | 42 | Male | Ascites | Japanese | Poorly
differentiated | 1982-10-13 | (9) |
| OKAJIMA | 38 | Male | Pleural
effusion | Japanese | Poorly
differentiated | 1976-11-19 | (9) |
| SNU-1 | 44 | Male | Primary tumour | Korean | Poorly
differentiated | 1984-4 | (10) |
| SNU-5 | 33 | Female | Ascites | Korean | Poorly
differentiated | 1987-6 | (10) |
| SNU-16 | 33 | Female | Ascites | Korean | Poorly
differentiated | 1987-7 | (10) |
| NCI-N87 | – | Male | Liver meta | American | Well
differentiated | 1976-8 | (10) |
| SNU-719 | 53 | Male | Primary tumour | Korean | Moderately
differentiated | 1991-7 | (11) |
| SNU-216 | 46 | Female | Lymph node
meta | Korean | Moderately
differentiated | 1989-7 | (11) |
| SNU-484 | 53 | Male | Primary tumour | Korean | Poorly
differentiated | 1990-8 | (11) |
| SNU-520 | 60 | Female | Primary tumour | Korean | Poorly
differentiated | 1990-10 | (11) |
| SNU-601 | 34 | Male | Ascites | Korean | Signet ring
cell | 1991-2 | (11) |
| SNU-620 | 59 | Female | Ascites | Korean | Poorly
differentiated | 1991-3 | (11) |
| SNU-638 | 48 | Male | Ascites | Korean | Poorly
differentiated | 1991-3 | (11) |
| SNU-668 | 63 | Male | Ascites | Korean | Signet ring
cell | 1991-5 | (11) |
| NCC-19 | 56 | Male | Primary tumour | Korean | Moderately
differentiated | 2002-3 | (12) |
| NCC-20 | 50 | Female | Ascites | Korean | – | 2002-2 | (12) |
| NCC-24 | 49 | Male | Primary tumour | Korean | Signet ring
cell | 2002-2 | (12) |
| NCC-59 | 62 | Male | Ascites | Korean | Moderately
differentiated | 2002-11 | (12) |
| SNU-1750 | 65 | Male | Primary tumour | Korean | Poorly
differentiated | 2001-4 | (12) |
| SNU-1967 | 41 | Female | Ascites | Korean | Poorly
differentiated | 2002-3 | (12) |
| NU-GC-2 | – | – | Lymph node
meta | Japanese | Poorly
differentiated | – | (13) |
| NU-GC-2 | – | – | Brachial muscle
meta | Japanese | Poorly
differentiated | – | (13) |
| NU-GC-2 | – | – | Lymph node
meta | Japanese | Partial signet ring
cell | – | (13) |
These cell lines have many advantages: they are very
easy to handle, they are an infinite source of self-replicating
cells, they have a relatively high degree of homogeneity, and they
are easy to replace with frozen stocks if they become contaminated.
However, these cell lines also have some shortcomings: cell lines
easily have genotypic and phenotypic drift in culture; this drift
is particularly frequent in the more commonly used cell lines,
particularly those that have been deposited in Cell Banks for
numerous years. Through specific mutations, various subpopulations
that are fast growing or more malignant may arise as time goes on
(14–16). Another important consideration for
cell lines is cross-contamination or misidentification (17,18).
The cell lines in current use are primarily derived from
late-stage, poorly differentiated and metastatic tumours,
particularly ascites or pleural effusions, which have accumulated
the mutations required for indefinite growth in vitro
(19); thus, the majority of these
cell lines are aggressive and unrepresentative of the diverse
tumour types, grades or stages as well as of the tumour progression
indications that are observed in primary cancer. Thus, studies
using these cell lines are biased towards more progressive and
malignant cell lines or those established from late-stage disease.
For these reasons, using cell lines that are derived directly from
early-stage and well-differentiated primary tumours would be more
meaningful, particularly since most drug therapies are directed
against these types of tumours (15).
However, despite our growing understanding of this
disease, the precise molecular and genetic steps for the
oncogenesis of GC remain largely unknown; thus, there is a need to
better understand the molecular pathogenesis of this disease. For
this purpose, a well-characterized cell line may be an
indispensable tool.
We report a new human expanding-type GC cell line,
XGC-2, from a Chinese patient. This newly established cell line may
be a useful model for the study of GC pathogenesis.
Materials and methods
Specimen collection
The specimens used in the present study were
obtained with written informed consent from a 72-year-old Chinese
male patient who underwent surgical resection at the First
Affiliated Hospital of Xiamen University (Xiamen, China) for GC in
the cardio-fundal junction of the stomach. The size of the original
tumour was 6.5×4.5 cm. No distant metastasis was detected at the
time of surgical resection. The tumour was histopathologically
classified as a moderately differentiated gastric tubular
adenocarcinoma.
The methods are parallel or identical to those
employed by Wang et al as follows in the next six paragraphs
(20).
Primary culture and establishment of the
XGC-2 cell line
Tumour specimens were rinsed twice with sterile
phosphate-buffered saline (PBS) containing antibiotics. The mucosa
of tumour specimens was removed using a scalpel, and samples were
then enzymatically disaggregated after incubation with collagenase
type II and neutral protease solution at 37°C in a humidified
atmosphere containing 5% CO2. After samples were
incubated for ~0.5 h, small clusters of tumour cells were isolated,
and 5 ml foetal calf serum (FBS) (Gibco, Grand Island, NY, USA) was
added to terminate the digestion. Then, the digested tumour
fragments and fluid were filtered through a 200 mesh sieve, and the
filtrate was centrifuged at 1,000 rpm for 5 min. The supernatant
was removed, and the remaining cells were resuspended in Dulbecco's
modified Eagle's medium (DMEM) and Ham's F-12 medium (1:1) (Gibco)
supplemented with penicillin (100 U/ml), streptomycin (100
µg/ml), heat-inactivated 2% FBS, hEGF (0.1 ng/ml), bFGF (0.1
ng/ml), hydrocortisone (25 µg/ml), fluconazole (40
µg/ml) (Gibco) and M-plasmocin (25 µg/ml)
(Invitrogen); seeded into 12-well culture plates; and cultivated at
37°C in a humidified atmosphere of 5% CO2 in air. The
growth medium was replaced every 2–3 days, and the plate was
regularly checked for epithelial cells and fibroblast outgrowth.
Whether fibroblast growth was observed during primary culture, a
scraping method was used to obtain a pure tumour cell population.
Twenty days after primary culture, the cells completely covered the
bottom of the plate and were passaged, and the culture medium was
changed to complete DMEM supplemented with 10% FBS, penicillin (100
U/ml) and streptomycin (100 µg/ml). Currently, the cell line
has been cultured for >60 passages. The cells were tested for
mycoplasma contamination, and the result was negative. The cell
line was designated XGC-2 (Xiamen-gastric cancer).
Morphology of the XGC-2 cell line
Cells were directly imaged without staining under a
phase contrast microscope.
Cell growth properties
Cells were plated in 96-well plates at 1,000
cells/well and cultured in DMEM containing 10% FBS for various
durations. Cell numbers were measured by MTT assay, which was
performed according to the manufacturer's protocol. The doubling
times were determined from the growth curve.
Plating and colony-forming
efficiency
Clone formation of different cell clones in soft
agar was used to assess their growth capabilities. Cell suspensions
were prepared in complete culture medium at low densities
(1×104 cells/ml). These suspensions (0.1 ml) were seeded
in a 12-well plate and incubated in a humidified 5% Co2
atmosphere at 37°C. Cultures were incubated for 14 days and
regularly observed; colonies (≥10 cells) were counted by
microscopic examination.
A single-cell suspension (1×104 cells/ml)
was prepared. Agar (0.6%) was mixed with DMEM and added to 6-well
plates as the lower layer. Then, 0.3% agar was mixed with the cell
suspension, and the mixture was added to the same 6-well plate as
the upper layer. Every well contained 1,000 cells. The plates were
incubated at 37°C in a humidified incubator containing 5%
Co2. Fourteen days later, cell colonies were counted
under a microscope, and the clone formation rates were calculated
using the following formula: Clone formation rate (%) = (number of
colonies/number of cells inoculated) × 100%.
Chromosome analysis
Cells were karyotyped using a standard air-dried
method after treatment with a final concentration of 0.05 mg/ml
colcemid for 2 h when the cells were in the exponential growth
phase. The cells were analysed using trypsin G banding. A total of
200 metaphase spreads were examined to determine the modal number.
Karyotyping was performed according to the International System for
Human Cytogenetic Nomenclature (2005). Chromosome analysis was
carried out on the cell line at passage 45.
Tumourigenicity in nude mice
The study protocol for mice was approved by the
Xiamen University Experimental Animal Care Commission. Briefly,
cells at passage 30 were prepared to determine their
tumourigenicity in nude mice. Cultured cells (2×107
cells/ml) were harvested, washed, resuspended in 0.1 ml complete
DMEM, and subcutaneously injected into the right flanks of three
4-week-old female nude mice. The animals were examined every week
for the development of tumours. Tumour-bearing mice were
sacrificed. Tumour tissue was excised, fixed in 10% formalin and
processed for routine histopathological examination.
Immunohistochemistry
Cell monolayers at passage 35 were subcultured and
grown on sterile microscope slides. After confluent growth was
obtained, the slides were washed with PBS, fixed in 4%
paraformaldehyde for 15 min, air-dried and treated with 0.5% Triton
X-100 for 20 min. PBS was used as the negative control, and the
slides were then overlaid with the following antibodies: mouse
monoclonal antibody directed against human cytokeratin, rabbit
monoclonal antibody directed against Ki-67, mouse monoclonal
antibody directed against human carcinoembryonic antigen, mouse
monoclonal antibody directed against human E-cadherin, and mouse
monoclonal antibody directed against human vimentin. Slides were
incubated with antibodies for 60 min and thoroughly washed with
PBS. A biotinylated rabbit anti-mouse IgG was subsequently applied
for 15–20 min and samples were washed. Then, a solution of DAB was
added to the slides, and the slides were incubated for 1–5 min at
room temperature. Finally, the slides were rinsed with distilled
water, counterstained with haematoxylin and eosin (H&E) and
examined by light microscopy.
Statistical analysis
The statistical significance of differences between
experimental groups and controls was determined by Student's
t-test. Values of P≤0.05 were considered to indicate a
statistically significant result.
Results
A new GC cell line, designated XGC-2, was
successfully established in vitro with a fresh aseptic
specimen derived from the primary tumour of a patient with GC. In
this culture, we succeeded in freezing, thawing and subculturing
cells for >60 generations in DMEM supplemented with 10% FBS.
In vitro characteristics of XGC-2
cells
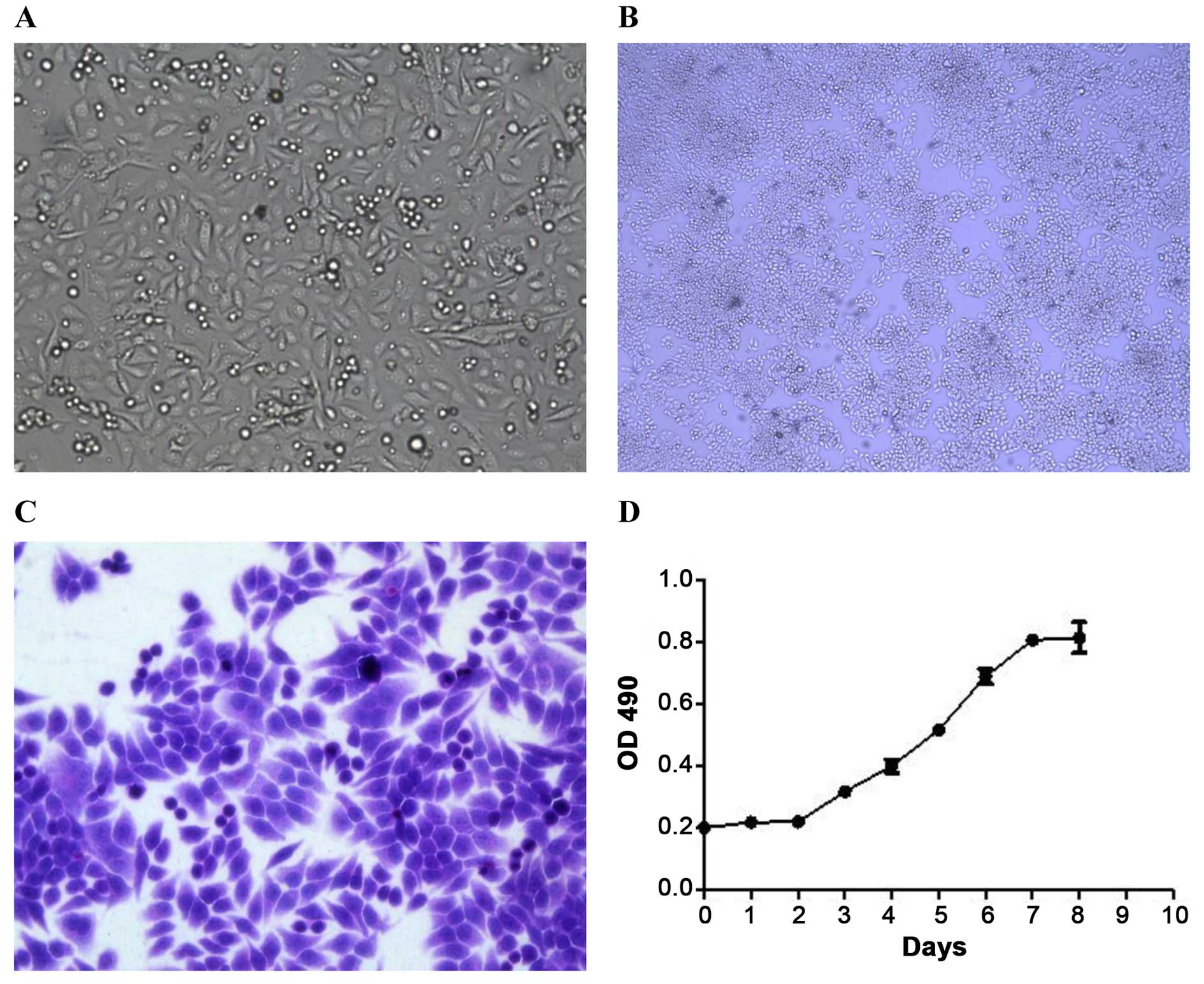
The XGC-2 cell line grew as an adherent monolayer
with characteristic epithelial morphology and showed no significant
differences in successive culturing (Fig. 1A–C). The growth of the XGC-2 cell
line was assayed by the MTT method. The XGC-2 cell line showed
vigorous growth tendency and a doubling time of ~60 h (Fig. 1D).
Plating and colony-forming
efficiency
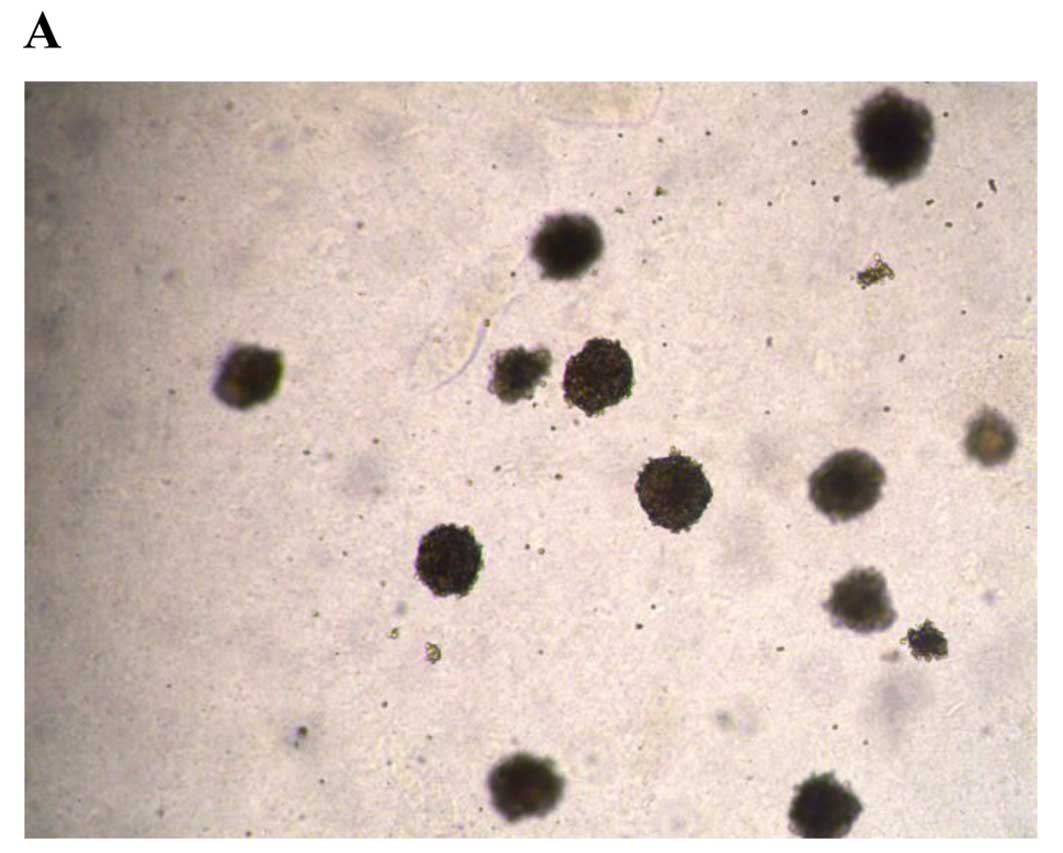
To assess the anchorage-independent growth abilities
of XGC-2 cells, plating and colony-forming assays were performed.
XGC-2 cells showed strong anchorage-independent growth. A
colony-forming efficiency of 45.8% was obtained when cells were
plated at a concentration of 500 cells/well (Fig. 2A). Colonies were visible and densely
packed 14 days after inoculation. The plating efficiency of XGC-2
cells after seeding at low cell densities was 8.13% (Fig. 2B).
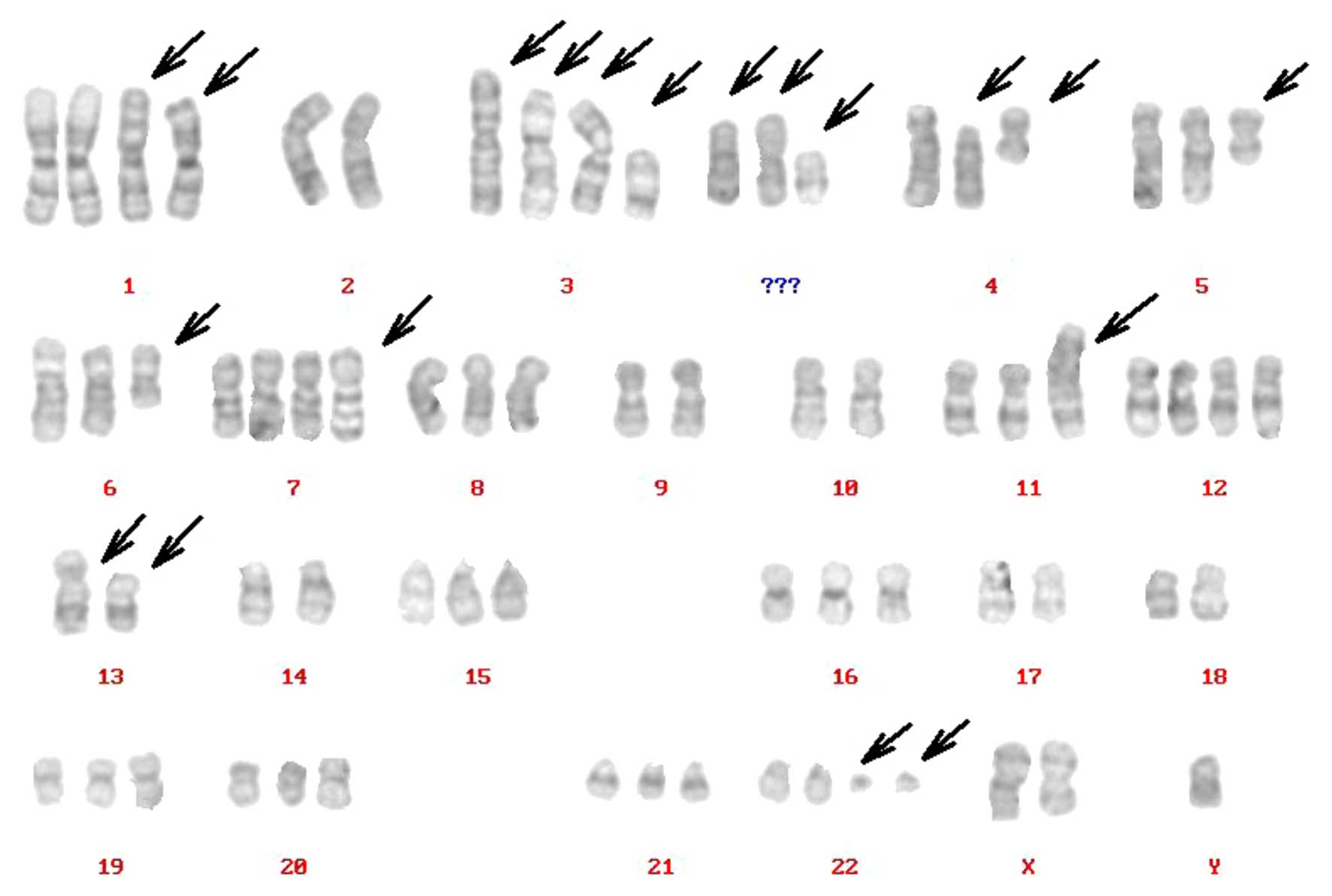
Karyotype of the XGC-2 cell line
Complicated karyotypes were revealed in the XGC-2
cell line, which included gains, losses, translocations and other
abnormalities, as determined by G-band analysis. The number of
chromosomes ranged between 23 and 137; however, in 86% of the cells
studied in the XGC-2 cell line, the chromosome counts were tightly
clustered around the modal numbers and ranged only from 46 to 69
chromosomes/cell. The arrowheads indicate rearranged chromosomes.
The representative karyotype was: 70,XY,del(X) (q22),der(1),+der(1),−2,der(3)t(3;4),der(3)t(3;5),der(3)t(3;13), del(3),del(4)(p10),i(4)(p10),i(5)(p10),del(6)(q21),+der(7)add(7)
(q36),−9,−10,der(11),+12,der(13)add(13p),der(13),−14,−17,−18, 22ph, +mar1,+mar2,+mar3
(Fig. 3).
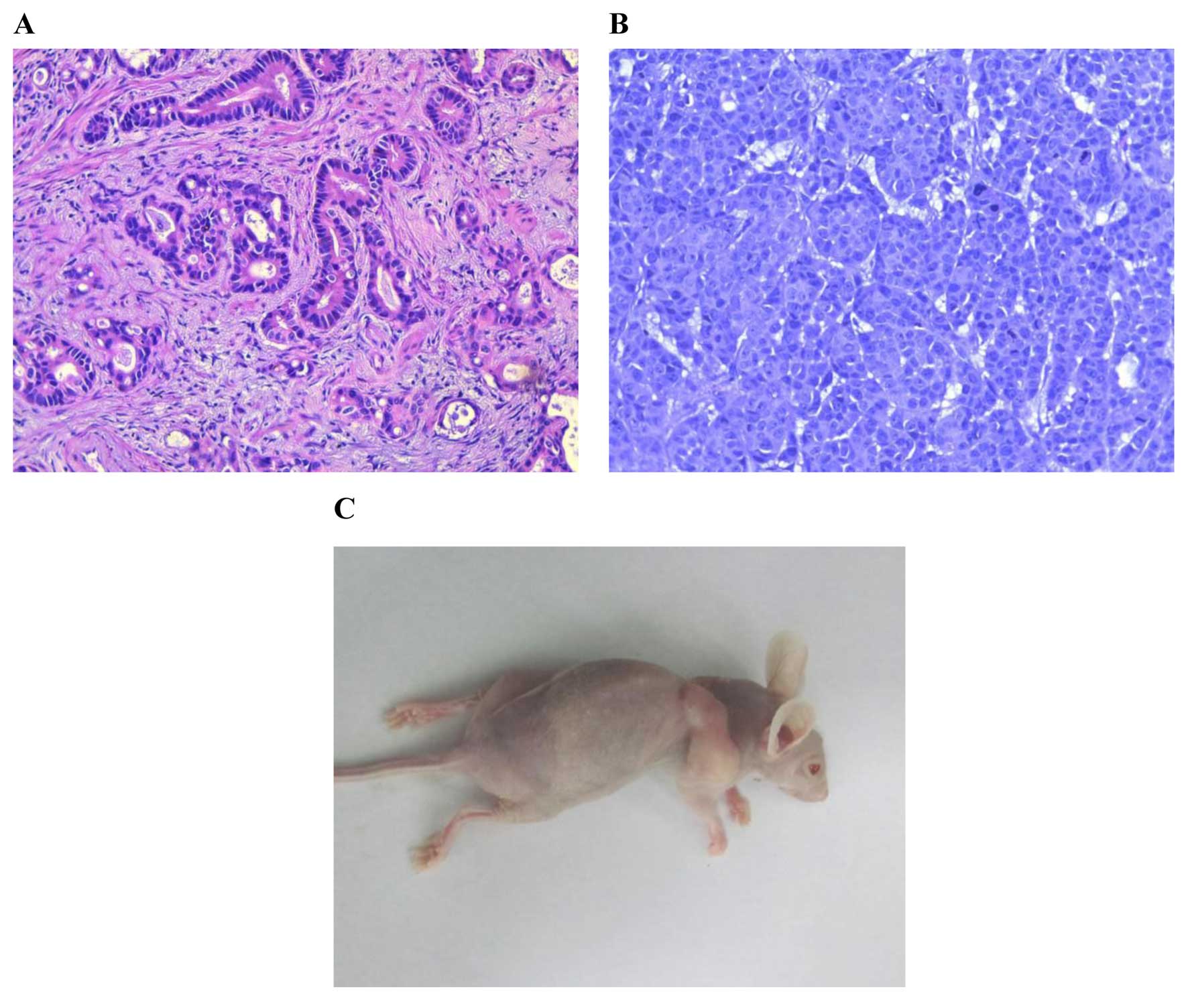
Histological examination
At 2 weeks after XGC-2 cells were subcutaneously
injected into mice, tumours were found at the site of inoculation
(Fig. 4C). The subcutaneous tumours
were removed for histological examination and were compared with
the original primary tumour. Both primary tumour (Fig. 4A) and subcutaneous tumours (Fig. 4B) were characterized by typical
gastric tubular adenocarcinoma (H&E) features, and all were
moderately differentiated.
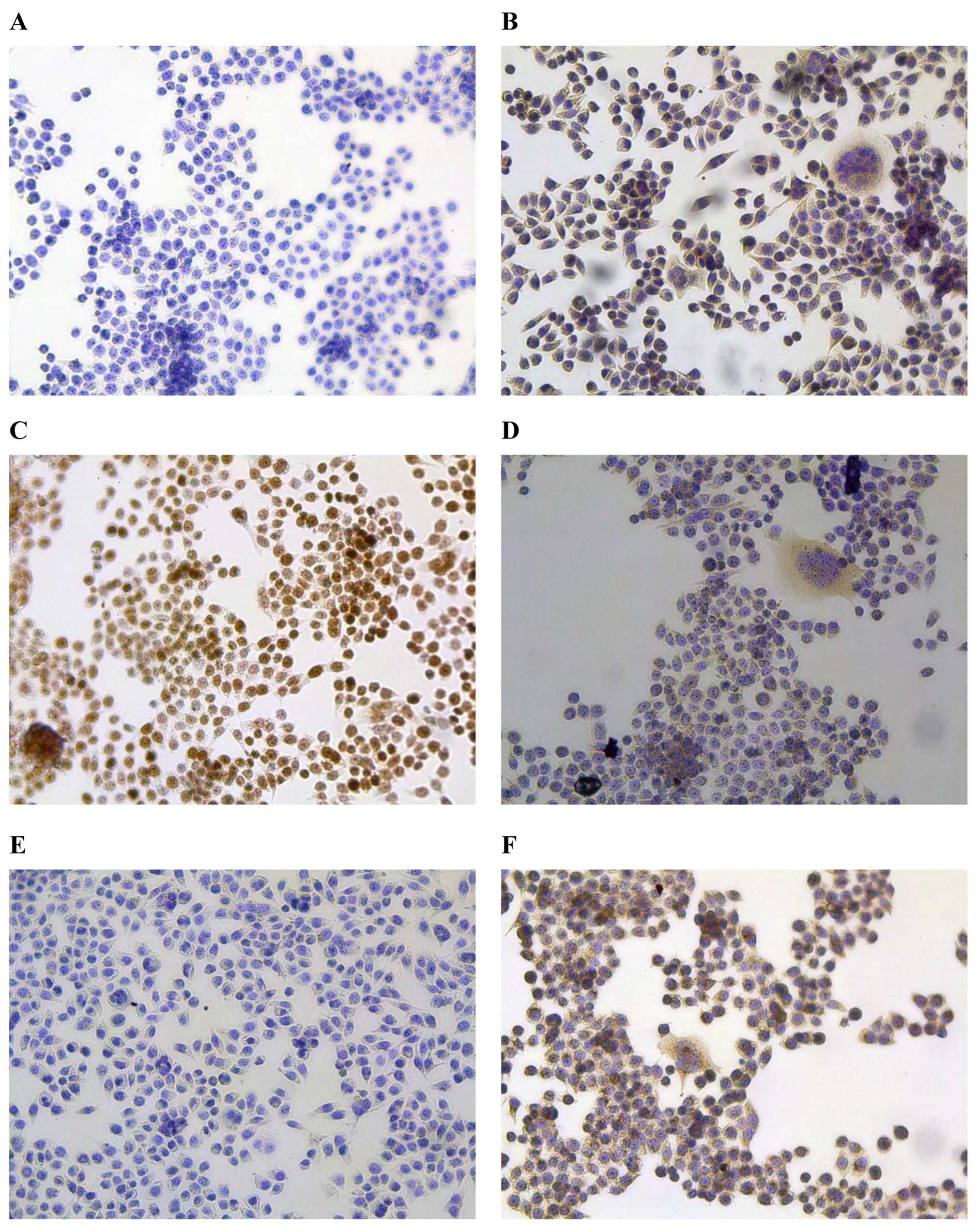
Immunohistochemistry
As one of the main objectives in establishing human
tumour lines is to use them to study tumour-specific marker(s), we
evaluated this cell line for its capacity to express commonly used
biomarkers. PBS was used as the negative control (Fig. 5A). The results of
immunohistochemical staining revealed that CEA, a well-known
biomarker for digestive system tumours, was positive in XGC-2 cells
(Fig. 5B). Ki-67, a protein
associated with active cell proliferation, was expressed at very
high levels in the nucleus (≥80.6%), indicating the poor prognosis
of the donor patient (Fig. 5C).
Cytokeratin staining showed that the XGC-2 cells had an epithelial
phenotype (Fig. 5D); however,
E-cadherin, an epithelial marker, was downregulated in XGC-2 cells
(Fig. 5E). Vimentin, the
characteristic protein of mesenchymal cells, was expressed
(Fig. 5F), suggesting that the
XGC-2 cell had metastatic potential.
Discussion
In 1977, based on patterns of growth and
invasiveness, Ming divided gastric carcinomas into two types:
expanding and infiltrative. The 5-year survival rate of patients
with expanding-type gastric cancer (GC) was 27.4%, while patients
with infiltrative-type GC had a 5-year survival rate of only 9.9%.
The two types have different biological behaviours and clinical
prognosis. However, only a few studies on these types of GC have
been performed due to the lack of appropriate cell lines. There
have been no studies on the establishment of expanding- and
infiltrative-type GC cell lines (21).
In the present study, a GC cell line (XGC-2) derived
from a primary tumour that was shown to have characteristics of
moderately differentiated adenocarcinoma by histological
examination was established from a Chinese male patient. The XGC-2
cells grew as an adherent monolayer with characteristic epithelial
morphology and had a population doubling time of ~60 h. Cultured
cells showed comparable morphology to the primary culture following
subculture passages. The XGC-2 cells were continuously grown for ~7
months, undergoing >60 passages, and growth continued even after
recovery from cryopreservation. The XGC-2 cells also formed
colonies in soft agar and plating assays. Furthermore, injection of
the XGC-2 cells into nude mice resulted in tumour growth, and the
histological features of the tumour resembled those of the original
primary tumour. Immunohistochemistry revealed that XGC-2 cells were
of epithelial origin, but the cells also showed vrious
characteristics of mesenchymal cells, suggesting that the XGC-2
cells have metastatic potential.
Chromosomal aberrations including gains, losses,
translocations and other abnormalities, are observed in almost all
malignant tumours. To date, only a limited number of studies have
investigated the cytogenetic changes in GC, and the molecular
carcinogenesis of GC remains unclear (22–24).
In the present study, we found that XGC-2 cells revealed common
chromosomal alterations and a marked tendency to triploidy.
However, the Y chromosome was detected in XGC-2 cells, in contrast
to previous studies (25,26). The biological significance of the Y
chromosome in tumour development is unknown, and further
investigation is needed to elucidate the detailed mechanism.
Metastasis, the spread of tumour cells from the
primary site through lymphatic and blood vessels or another route
to distant sites followed by continued growth and formation of the
same type of primary tumour, is a characteristic of malignant
tumours and is the main cause of patients death. Many potential
pathways and molecules are involved in the metastatic process
(27). Epithelial-to-mesenchymal
transition (EMT) is a key step towards cancer metastasis, which is
characterized by the loss of epithelial cell markers of cell-cell
adhesion molecules, such as E-cadherin (28,29).
The expression of this protein is downregulated during the
acquisition of metastatic potential at late stages of epithelial
tumour progression (30). In the
present study, E-cadherin expression was downregulated and vimentin
expression was upregulated; however, the biological behaviours and
clinical prognosis of expanding-type GCs are relatively better than
those of infiltrative-type GCs, suggesting that the metastatic
potential of the cells was due to not only the loss of E-cadherin,
but also other factors that are crucial for metastatic processes,
such as those that degrade and remodel the extracellular matrix and
promote angiogenesis. The detailed mechanisms require further
study.
In conclusion, we report the establishment and
characterization of a new human expanding-type GC cell line derived
from a primary tumour and termed XGC-2. Future studies of tumour
biology, cellular and molecular carcinogenesis, and biomarkers for
early diagnosis and drug responses to new therapeutic agents are
needed for a better understanding of GC. This cell line should
provide us with a new experimental model for future research of
this disease.
Acknowledgments
The present study was supported by the national
natural Science Foundation (nos. 81172283 and 81372616).
References
|
1
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Bu XL, Wang QY, Hu PJ and Chen MH:
Decreasing seroprevalence of Helicobacter pylori infection during
1993–2003 in Guangzhou, southern China. Helicobacter. 12:164–169.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawakami E, Machado RS, Ogata SK and
Langner M: Decrease in prevalence of Helicobacter pylori infection
during a 10-year period in Brazilian children. Arq Gastroenterol.
45:147–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyerhardt JA and Fuchs CS: Adjuvant
therapy in gastric cancer: Can we prevent recurrences? Oncology.
17:714–733. 2003.PubMed/NCBI
|
|
7
|
Gey GO, Coffman WD and Kubicek MT: Tissue
culture studies of the proliferative capacity of cervical carcinoma
and normal epithelium. Cancer Res. 12:264–265. 1952.
|
|
8
|
Barranco SC, Townsend CM Jr, Casartelli C,
Macik BG, Burger NL, Boerwinkle WR and Gourley WK: Establishment
and characterization of an in vitro model system for human
adenocarcinoma of the stomach. Cancer Res. 43:1703–1709.
1983.PubMed/NCBI
|
|
9
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
10
|
Park JG, Frucht H, LaRocca RV, Bliss DP
Jr, Kurita Y, Chen TR, Henslee JG, Trepel JB, Jensen RT, Johnson
BE, et al: Characteristics of cell lines established from human
gastric carcinoma. Cancer Res. 50:2773–2780. 1990.PubMed/NCBI
|
|
11
|
Park JG, Yang HK, Kim WH, Chung JK, Kang
MS, Lee JH, Oh JH, Park HS, Yeo KS, Kang SH, et al: Establishment
and characterization of human gastric carcinoma cell lines. Int J
Cancer. 70:443–449. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ku JL, Kim KH, Choi JS, Kim SH, Shin YK,
Chang HJ, Bae JM, Kim YW, Lee JH, Yang HK, et al: Establishment and
characterization of six human gastric carcinoma cell lines,
including one naturally infected with Epstein-Barr virus. Cell
Oncol. 35:127–136. 2012. View Article : Google Scholar
|
|
13
|
Akiyama S, Amo H, Watanabe T, Matsuyama M,
Sakamoto J, Imaizumi M, Ichihashi H, Kondo T and Takagi H:
Characteristics of three human gastric cancer cell lines, NU-GC-2,
NU-GC-3 and NU-GC-4. Jpn J Surg. 18:438–446. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burdall SE, Hanby AM, Lansdown MR and
Speirs V: Breast cancer cell lines: Friend or foe? Breast Cancer
Res. 5:89–95. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osborne CK, Hobbs K and Trent JM:
Biological differences among MCF-7 human breast cancer cell lines
from different laboratories. Breast Cancer Res Treat. 9:111–121.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR, et al: Check your cultures! A list of cross-contaminated
or misidentified cell lines. Int J Cancer. 127:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christgen M and Lehmann U: MDA-MB-435: The
questionable use of a melanoma cell line as a model for human
breast cancer is ongoing. Cancer Biol Ther. 6:1355–1357. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheung PF, Yip CW, Ng LW, Lo KW, Wong N,
Choy KW, Chow C, Chan KF, Cheung TT, Poon RT, et al: Establishment
and characterization of a novel primary hepatocellular carcinoma
cell line with metastatic ability in vivo. Cancer Cell Int.
14:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JH, Li LF, Yu Y, Li B, Jin HJ, Shen
DH, Li J, Jiang XQ and Qian QJ: Establishment and characterization
of a cell line, EH-GB2, derived from hepatic metastasis of
gallbladder cancer. Oncol Rep. 27:775–782. 2012.
|
|
21
|
Ming SC: Gastric carcinoma. A
pathobiological classification. Cancer. 39:2475–2485. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S, Hu L, Huang XH, Cao LQ, Chan KW,
Wang Q and Guan XY: Establishment and characterization of a human
cholangiocarcinoma cell line. Oncol Rep. 18:1195–1200.
2007.PubMed/NCBI
|
|
23
|
Limpaiboon T, Tapdara S, Jearanaikoon P,
Sripa B and Bhudhisawasdi V: Prognostic significance of
microsatellite alterations at 1p36 in cholangiocarcinoma. World J
Gastroenterol. 12:4377–4382. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uhm KO, Park YN, Lee JY, Yoon DS and Park
SH: Chromosomal imbalances in Korean intrahepatic
cholangiocarcinoma by comparative genomic hybridization. Cancer
Genet Cytogenet. 157:37–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wada M, Yokota J, Mizoguchi H, Terada M
and Sugimura T: Y chromosome abnormality in human stomach and lung
cancerJpn. J Cancer Res. 78:780–783. 1987.
|
|
26
|
Yanagihara K, Seyama T, Tsumuraya M,
Kamada N and Yokoro K: Establishment and characterization of human
signet ring cell gastric carcinoma cell lines with amplification of
the c-myc oncogene. Cancer Res. 51:381–386. 1991.PubMed/NCBI
|
|
27
|
Geiger TR and Peeper DS: Metastasis
mechanisms. Biochim Biophys Acta. 1796:293–308. 2009.PubMed/NCBI
|
|
28
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|