Introduction
Glioblastoma multiforme (GBM) is the most common
primary malignant brain tumor in adults. Patients with GBM have a
poor prognosis, and have a median survival of 1 year despite
aggressive therapy; fewer than 5% survive for 5 years (1). One reason for this poor prognosis is
that GBM has the ability to infiltrate and invade the surrounding
normal brain tissue and thereby avoid surgical and radiological
interventions. These residual metastatic cancer cells contribute to
recurrence, leading to the fatal outcome of this disease (2). While invading through these spaces,
glioma cells typically undergo several biological changes,
including an increase in their ability to degrade the extracellular
matrix (ECM) (3). Many studies have
implicated matrix-metalloproteinases (MMPs) in the degradation of
the ECM, and increased expression of several MMPs occurs in cancer
cells compared with their normal cell counterparts, including
glioma cells (4).
Forkhead box O (FOXO) transcription factors have
critical roles in a number of physiological and pathological
processes. In mammals, four subfamily members have been identified,
FoxO1, FoxO3, FoxO4, and FoxO6 (5).
Of the Forkhead transcription factors, FoxO3a is the member that
has emerged as a versatile target for multiple disorders, including
various tumors. Hu and colleagues have shown that FoxO3a is linked
to the poor survival of patients with breast tumors (6). In addition to breast cancer (7–9),
FoxO3a has been shown to be involved in several other tumor types,
including prostate cancer, glioblastoma and leukemia (10–15).
The role of FoxO3a in cancer progression is primarily attributed to
the two most significant cellular processes regulated by FoxO3a: i)
FoxO3a activation increases the levels of cell cycle inhibitor
proteins p21 and p27, both of which subsequently suppress the G1 to
S cell cycle transition (16–18),
and ii) the expression of FoxO3a leads to pro-apoptosis (19,20)
through the modulation of the expression of its target genes. For
example, FoxO3A induces tumor necrosis factor-related
apoptosis-induced ligand (TRAIL) and the BH3-only proteins Noxa and
Bim (21).
Prior studies advocated that FoxO3a functions as a
tumor suppressor and that it is likely to be an important target
for the inhibition of cancer cell progression. However, recent
studies highlight the opposing functions of the protein. In
invasive ductal breast carcinoma, FoxO3a is linked to lymph node
metastasis and poor survival, and its activation does not induce
cell death or cell cycle arrest (8). The overexpression of FoxO3a in the
MDA-MB-231 cell line results in greatly increased cancer cell
invasion by elevating the expression of matrix metalloproteinase 9
(MMP9) and MMP13, both of which have been causally linked to the
invasion and progression of numerous human solid tumors (22). In colon cancer, FoxO3a synergizes
with beta-catenin to promote cell metastasis while conferring
resistance to FoxO3a-induced apoptosis (23). These lines of evidence have
challenged the previously defined role of FoxO3a in tumor
progression. The role of FoxO3a is disputable in glioma, as the
protein was revealed to mediate slow proliferation in GSCs
(24), implying however its
involvement in tumor suppression (25).
Materials and methods
Cell lines and cell culture
The glioma cell line U251 was obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). U87,
A172 and T98g cell lines were gifts from the Neuroscience Institute
of Soochow University. The normal human astrocyte 1800 cell line
(HA1800) was obtained from the American Type Culture Collection
(Manassas, VA, USA). All cells were cultured at 37°C in a
humidified atmosphere containing 5% CO2 and maintained
in Dulbecco's modified Eagle's medium (DMEM; Gibco, Paisley, UK)
supplemented with 10% heat inactivated fetal bovine serum (FBS;
Gibco, Carlsbad, CA, USA), 100 µg/ml streptomycin and 1 U/ml
penicillin.
Establishment of stable cell lines
DNA oligos designed by the BLOCK-iT RNAi designer
(Invitrogen) encoding human FoxO3a-short hairpin RNAs
[5′-GCTCTTGGTGGA TCATCAA-3′ (FoxO3a-knockdown1) and 5′-GCATGTTCA
ATGGGAGCTTGGA-3′ (FoxO3a-knockdown2)] were synthesized and cloned
into the pHY-LV-KD1.1 (Hanyin Biotech, Shanghai, China) vector to
generate pHY-FoxO3a-KD1 and pHY-FoxO3a-KD2. A vector expressing
shRNA targeted against an irrelevant sequence (shRNA-NC) was used
as a negative control. The full-length human FoxO3a cDNA was
purchased from Open Biosystems and sub-cloned into the PHY-LV-OE1.6
vector (Hanyin Biotech). The recombined vector expressing FoxO3a
was designated pHY-FoxO3a-OE. The Trans-Lentiviral Packaging System
and Vira Power Lentiviral Expression System (Invitrogen) were used
to produce shRNA and overexpress lentiviruses, respectively. shRNA
lentiviruses were used to silence FoxO3a expression in U251 cells,
while lentiviruses for overexpression were transduced into U87
cells. Stable cells, including FoxO3a-KD1 and FoxO3a-KD2 (both of
which are U251 parental cell line knockdown derivatives), and
FoxO3a-OE (U87 cells stably expressing FoxO3a), were generated
using one week of puromycin selection.
Cell proliferation
Cell counts were determined using Cell Counting
Kit-8 (Dojindo, Japan). A total of 1×103 GBM cells were
plated onto 96-well culture plates in triplicate, and cell growth
was determined daily for 5 days using a tetrazolium salt-based
colorimetric assay (Dojindo Molecular Technologies) according to
the manufacturer's protocol. Absorbance was measured at 450 nm.
Three independent experiments were performed.
Transwell invasion assay
For the Transwell assay, 2×104 stable
cells were plated into 24-well Boyden chambers (Corning Costar,
Cambridge, MA, USA) with an 8-µm pore polycarbonate membrane
coated with 30 µg of Matrigel (BD Biosciences, San Jose, CA,
USA). Cells were plated in the upper chamber with 200 µl of
serum-free medium, and medium containing 20% FBS was added to the
lower chamber to serve as a chemoattractant. After 36 h, the cells
were washed 3 times with PBS. Non-invasive cells were removed from
the upper well using cotton swabs, and the invasive cells were then
fixed with paraformaldehyde for 15 min, air-dried, and stained with
0.1% crystal violet for 15 min. The cells were imaged with a
digital camera.
Real-time PCR
Total RNA was extracted from stable cell lines and
GBM parental cell lines. cDNA was prepared using 1 µg of
total RNA from each sample and using specific primers (Applied
Biosystems). Six nanograms of cDNA were then used for real-time PCR
analysis in a final reaction volume of 20 µl. Samples were
analyzed in triplicate and statistical analysis was performed using
the t-test.
Western blots
Total protein was extracted and separated by gel
electrophoresis. Protein was then transferred to nitrocellulose
membranes and probed overnight using the appropriate primary
antibodies. The antibodies used were MMP1, MMP2, MMP3, MMP9 and
MMP13 (Santa Cruz), cyclin D1 (CST, 2926), p21 (abcam, ab7960) and
p27 (CST, 2552). Nuclear and cytoplasmic fractions of total protein
were separated using NE-PER Nuclear and Cytoplasmic Extraction
Reagent (Thermo Scientific, Rockford, IL, USA) and were then
subjected to western blot analysis.
Statistical analysis
The data shown in the graphs represent the mean
values ± SD of three independent experiments. The difference among
groups was determined by ANOVA, and comparison between two groups
was analyzed by Student's t-test. A value of P<0.05 was
considered statistically significant.
Results
Expression and subcellular localization
of FoxO3a in U87 and U251 cells
To investigate the expression pattern of FoxO3a in
glioma cell lines, we employed western blotting to detect the
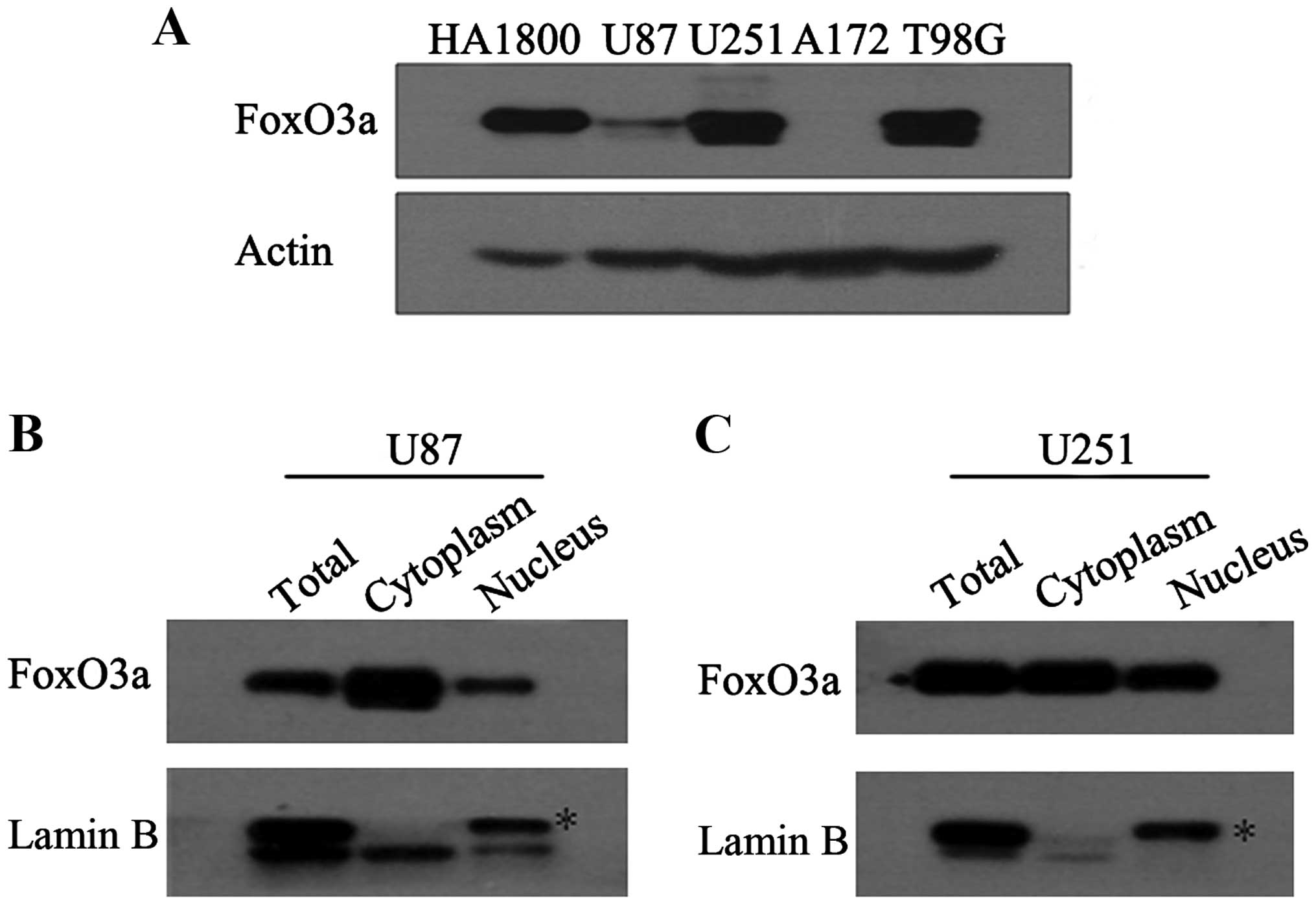
protein level of FoxO3a. It was observed that among the four
carcinoma cell lines (U87, U251, A172, and T98g), the FoxO3a levels
in A172 and U87 were lower than in U251 and T98g (Fig. 1A). This finding is partially similar
to that observed in a previous study (25). To explore the role of FoxO3a in
glioma cell lines, U87 and U251 cells were selected for further
investigation because not only did the two cell lines display a
relatively inverse FoxO3a expression pattern, but they are also
reliable glioma cell models that are known to mimic the salient
features of human GBM in vitro. As FoxO3a transcriptional
activity requires its nuclear accumulation, we detected its protein
distribution in the nucleus and cytoplasm. Western blot analysis
showed that a relatively high proportion of overall protein
(nucleus: cytoplasm) accumulated in the nuclear fraction (Fig. 1B and C).
Effect of FoxO3a on proliferation
Because of the differential expression pattern of
FoxO3a in U251 and U87 cells, we established stable cell lines
containing FoxO3a overexpression and knockdown in the corresponding
parental cell lines using a lentivirus-mediated approach. Stable
transfectants were obtained through puromycin selection. In
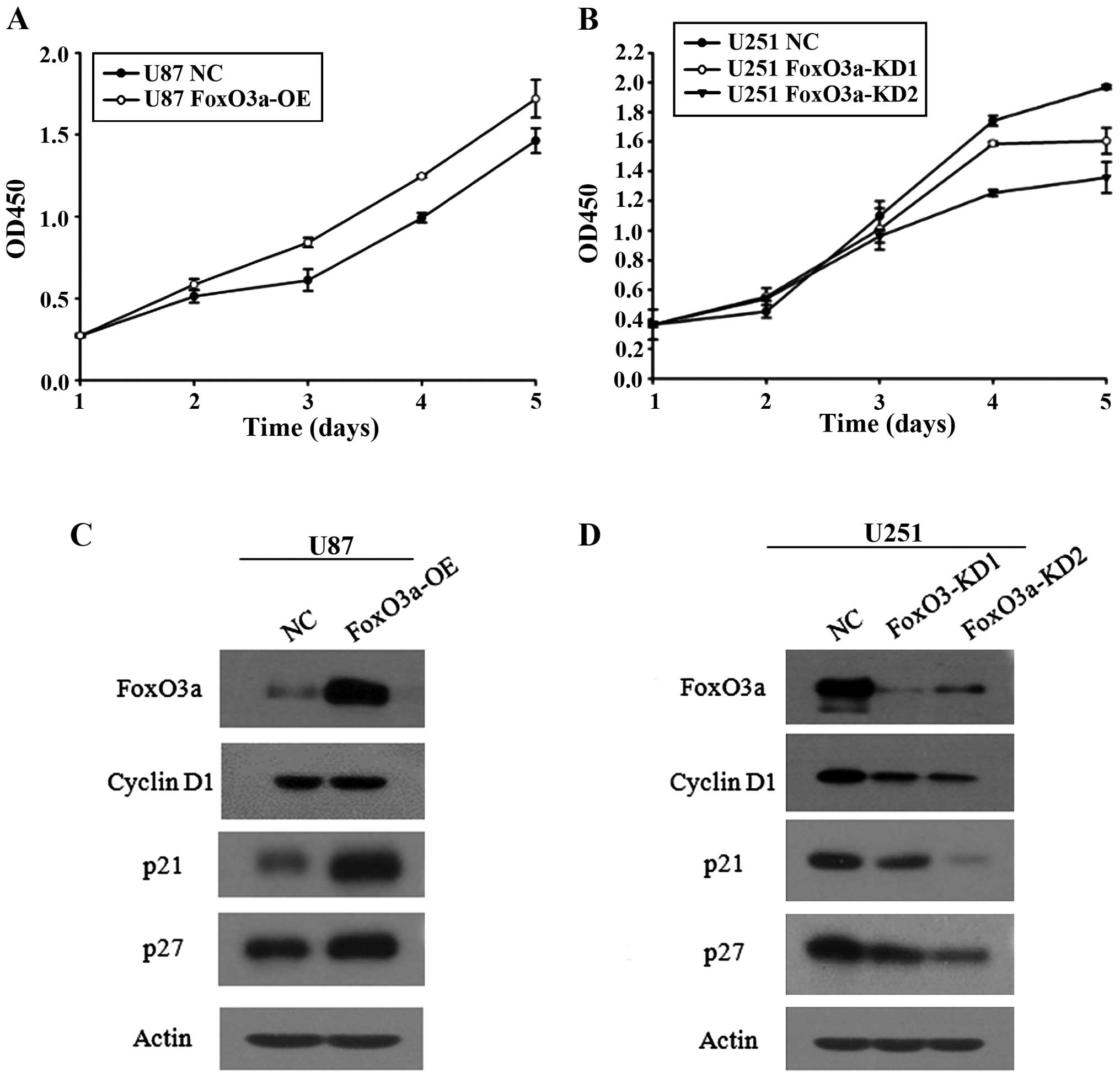
parallel cohorts of two clones, designated FoxO3a-KD1 and
FoxO3a-KD2, we observed that knockdown of FoxO3a reduced U251
proliferation and that FoxO3a-KD1 had effects on cell proliferation
despite better silencing (Fig. 2B).
By contrast, increasing FoxO3a expression in U87 cells, which have
relatively low levels of endogenous FoxO3a, promoted cell
proliferation (Fig. 2A). To further
confirm that FoxO3a promoted cell proliferation in these two cell
models, we detected the expression of FoxO3a target genes
associated with cell arrest and cell growth, including p27, p21 and
cyclin D1. Silencing FoxO3a led to p27, p21 and cyclin D1
downregulation (Fig. 2D), whereas
elevating FoxO3a expression upregulated these target genes
(Fig. 2C). In effect, FoxO3a was
able to promote cell proliferation, as determined by an in
vitro proliferation assay, suggesting that FoxO3a has a
dominant effect on cell growth over cell arrest. We then determined
whether FoxO3a expression could trigger apoptosis. The findings
from these stable glioma cells indicated that FoxO3a exerted little
effect on apoptosis (data not shown).
Effect of FoxO3a on invasion of glioma
cells
To study the role of FoxO3a in glioma cell invasion
in vitro, we analyzed the invasion potential of the stably
transfected cells expressing FoxO3a using a matrigel invasion
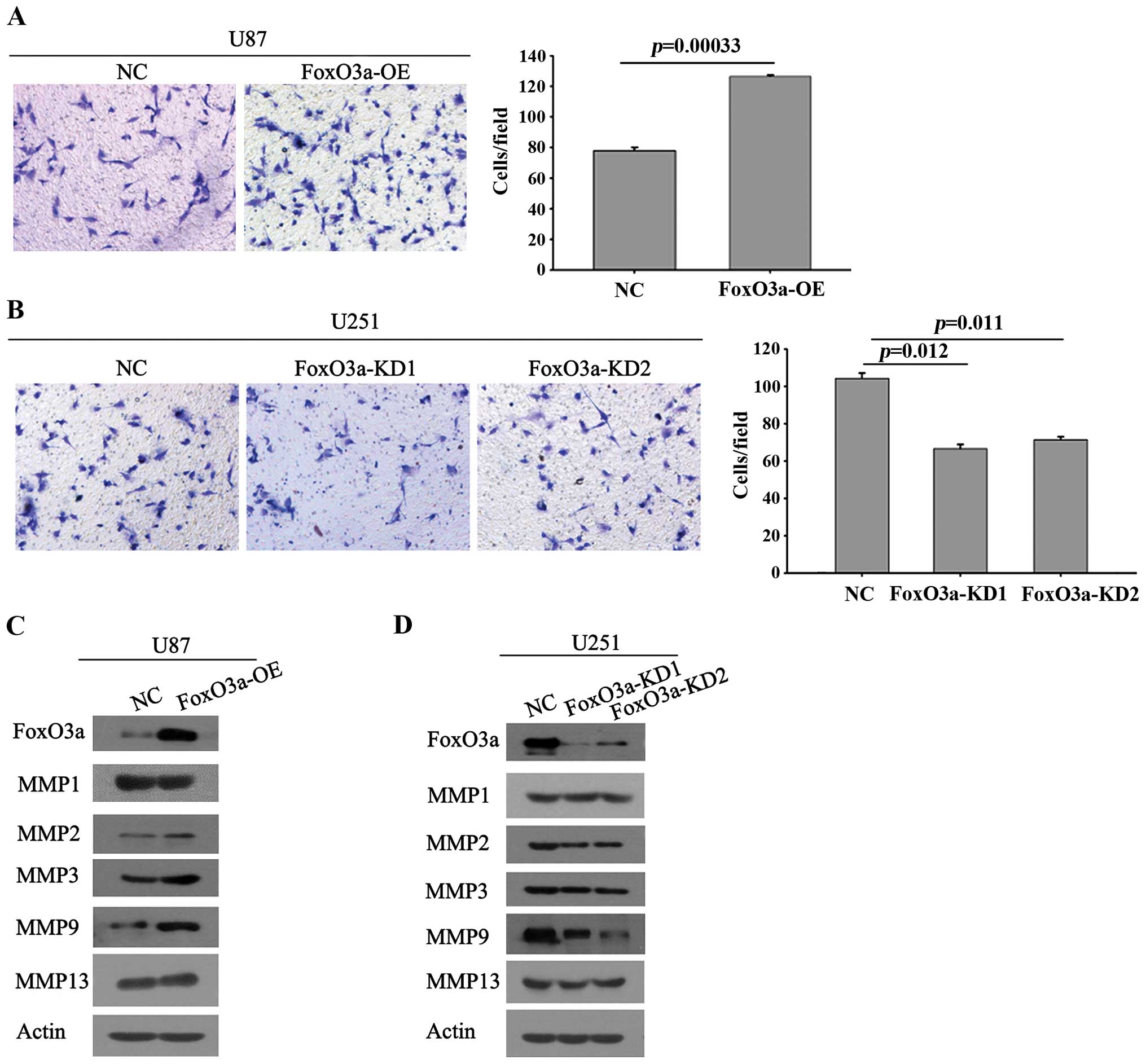
chamber assay. As shown in Fig. 3A,
ectopic expression of FoxO3a in U87 cells led to a significant
increase in invasion compared with the control of glioma cells. By
contrast, knockdown of FoxO3a in U251 cells led to a marked
decrease in invasion, as observed by the smaller number of cells
penetrating through the chambers (Fig.
3B). The findings from the two cell models demonstrate that
FoxO3a affects the invasion of glioma cells. Furthermore, we
detected the expression of key MMP members associated with tumor
metastasis. The results showed that FoxO3a overexpression induced
significantly elevated expression of MMP9, while other well-defined
candidates associated with metastasis, such as MMP2 and MMP13, did
not show any marked increase (Fig.
3C). Furthermore, the U251 stable cell line FoxO3a-KD1 showed
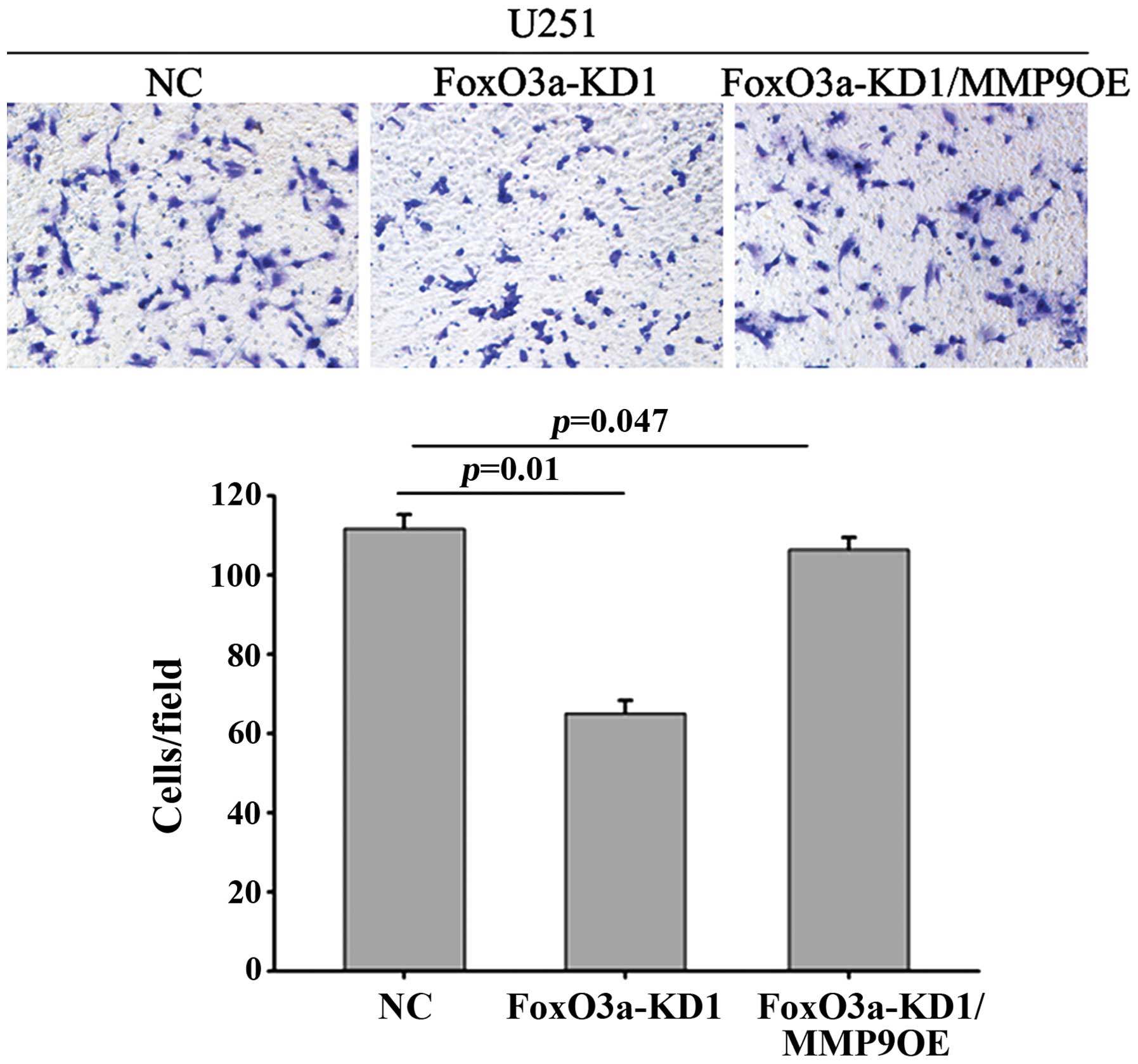
attenuated MMP9 protein levels and decreased invasion (Fig. 3D). Following MMP9 overexpression,
the impaired invasive ability mediated by FoxO3a depletion appeared
to recover (Fig. 4).
Discussion
In the present study, we investigated the FoxO3a
expression spectrum in several glioma cell lines and used the U251
and U87 cell lines to further investigate the role of FoxO3a in
glioma cells. FoxO3a overexpression in U87 cells did not attenuate
the cell proliferative capacity but instead increased cell
proliferation. This finding is consistent with observations in U251
cells, where the silencing of FoxO3a caused a decrease in
proliferation. Unlike most researchers, who claim that FoxO3a can
trigger apoptosis, we did not observe that FoxO3a expression
induced apoptosis in glioma cells (data not shown). Furthermore, we
determined whether FoxO3a had an impact on cell invasion. It was
observed that the downregulation of FoxO3a significantly inhibited
the invasion of U251 cells, whereas the overexpression of FoxO3a
enhanced the invasion of U87 cells. Following stable FoxO3a
overexpression and knockdown in cells, we detected changes in the
protein levels of MMPs. The results showed that FoxO3a manipulated
MMP9 expression while having no effect on other MMPs.
Overexpression of MMP9 in FoxO3a-KD1 cells restored the compromised
invasive ability mediated by FoxO3a silencing, indicating that
FoxO3a increases glioma cell invasion through the induction of MMP9
alone.
Despite technological advances in surgical and
radiological techniques, malignant gliomas often recur within 1–2
cm of the original tumor site (2).
Key characteristic features, such as the extensive infiltration of
invasive cells into surrounding normal tissue, allow gliomas to
evade surgical removal and radiation therapy. Despite the need for
improved therapies, the molecular mechanisms responsible for
invasion remain largely undefined. This encourages the
investigation of many cancer-related genes, including FoxO3a. We
observed that FoxO3a promoted the invasion of glioma cells, which
is at odds with previous studies defining FoxO3a as a tumor
suppressor. Nonetheless, several recent studies favor our findings
demonstrating that FoxO3a participates in cell invasion: i) FoxO3a
activation in breast cancer tissue was significantly associated
with lymph node metastasis (8), ii)
depletion of FoxO3a resulted in a significant decrease in the
invasive migration of MDA-MB-435 cells (22) and iii) high levels of activated
FoxO3a were present in cells with a high metastatic potential.
In addition, we observed that FoxO3a promoted cell
invasion through the induction of MMP9 (Fig. 3C and D). MMP9, a key member of the
MMP family, is linked to metastasis in a variety of cancer types
(26). Overexpression of MMP9 is
also associated with a high metastatic potential in rat
osteosarcoma cell lines (27).
Chemically-induced skin tumors exhibited high MMP9 expression only
in invasive skin carcinomas (28).
Increased expression of MMP9 has been found in anaplastic
astrocytomas and GBM (29), and
this increased expression is also correlated with the progression
of highly malignant human gliomas in vivo. In MDA-MB-231
cells, it appears that MMP9 expression, combined with the
expression of other MMP members, such as MMP2 and MMP13, could lead
to cell invasion (22). However, we
did not observe that FoxO3a induced the expression of MMP2 and
MMP13, instead we only observed the induction of MMP9 (Fig. 3C and D).
Similar findings have been reported in studies of
spontaneous murine breast carcinomas, in which researchers
discovered that MMP9 was the only metalloproteinase whose
expression correlated with the metastatic properties of a parental
cell line derivative (30). Using
AG1478, researchers observed significantly reduced MMP9 activity,
but not MMP2 activity, and the reduced MMP9 activity correlated
with a decrease in cell invasion (31). In addition, GBM patient specimens
were shown to express high levels of MMP9 protein, but low levels
of other MMPs, and MMP9 was present in human GBM tumor masses as
well as in tumor cells diffusely invading adjacent brain tissue
(31), suggesting that manipulation
of the MMP9 protein level alone might render glioma cells capable
or incapable of invasion. Our findings have validated the
relationship between MMP9 and glioma cell invasion. Ectopic
expression of MMP9 restored the invasive potential that would have
otherwise been attenuated by the silencing of FoxO3a (Fig. 4), indicating that FoxO3a-induced
MMP9 alone may play a critical role in cell invasion. However, the
MMP9 promoter does not contain a FoxO3a DNA binding motif. This
implies that FoxO3a may cooperate with a specific transcription
factor of MMP9 (SMAD3/4 or NF-κB), thereby activating expression
through the promoter, which lacks FOXO binding sites.
The effect of FoxO3a on cell proliferation and
apoptosis was investigated, and our findings indicated that FoxO3a
did not induce either cell death or cell cycle arrest. On the
contrary, FoxO3a increased cell proliferation. FoxO3a target genes
associated with cell proliferation and apoptosis were concurrently
activated. Of note, we observed the upregulation of FASLG and HIPK3
(data not shown), two genes that are co-regulated by FoxO3a and
beta-catenin in colon cancer (23).
This finding provides a clue for further investigations exploring
the unknown mechanisms that FoxO3a uses to override its own
anti-proliferative function in glioma cells. For instance, we might
test whether the coordinated mechanism of FoxO3a and beta-catenin
that has been unraveled in colon cancer (23) has a role in gliomas.
Few studies have correlated FoxO3a with the
promotion of cancer progression. On the contrary, most studies have
defined FoxO3a as a tumor suppressor. Herein, by providing
compelling evidence with respect to cell proliferation and
invasion, we propose that in glioma cells, FoxO3a plays a part in
cancer progression.
Acknowledgments
We are grateful to the National Natural Science
Foundation of China (Grant # 31260225) for funding this
research.
References
|
1
|
Ohgaki H: Genetic pathways to
glioblastomas. Neuropathology. 25:1–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou LC, Veeravagu A, Hsu AR and Tse VC:
Recurrent glioblastoma multiforme: A review of natural history and
management options. Neurosurg Focus. 20:E52006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tonn JC and Goldbrunner R: Mechanisms of
glioma cell invasion. Local Therapies for Glioma Present Status and
Future Developments. Springer; pp. 163–167. 2003, View Article : Google Scholar
|
|
5
|
Fu Z and Tindall DJ: FOXOs, cancer and
regulation of apoptosis. Oncogene. 27:2312–2319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu M, Lee D, Xia W, Xia W, Golfman LS,
Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, et al: IkappaB kinase
promotes tumourigenesis through inhibition of Forkhead
transcription factor FOXO3a. Cell. 117:225–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J-Y, Chang C-J, Xia W, Wang Y, Wong
KK, Engelman JA, Du Y, Andreeff M, Hortobagyi GN and Hung MC:
Activation of FOXO3a is sufficient to reverse mitogen-activated
protein/extracellular signal-regulated kinase kinase inhibitor
chemoresistance in human cancer. Cancer Res. 70:4709–4718. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu
LH, Ng TT, Karadedou CT, Millour J, Ip YC, Cheung YN, et al:
Constitutively nuclear FOXO3a localization predicts poor survival
and promotes Akt phosphorylation in breast cancer. PLoS One.
5:e122932010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lam M, Carmichael AR and Griffiths HR: An
aqueous extract of Fagonia cretica induces DNA damage, cell cycle
arrest and apoptosis in breast cancer cells via FOXO3a and p53
expression. PLoS One. 7:e401522012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla S, Bhaskaran N, Maclennan GT and
Gupta S: Deregulation of FoxO3a accelerates prostate cancer
progression in TRAMP mice. Prostate. 73:1507–1517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukla S, Bhaskaran N, Babcook MA, Fu P,
Maclennan GT and Gupta S: Apigenin inhibits prostate cancer
progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway.
Carcinogenesis. 35:452–460. 2014. View Article : Google Scholar :
|
|
12
|
Chen Q, Ganapathy S, Singh KP, Shankar S
and Srivastava RK: Resveratrol induces growth arrest and apoptosis
through activation of FOXO transcription factors in prostate cancer
cells. PLoS One. 5:e152882010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunayama J, Sato A, Matsuda K, Tachibana
K, Watanabe E, Seino S, Suzuki K, Narita Y, Shibui S, Sakurada K,
et al: FoxO3a functions as a key integrator of cellular signals
that control glioblastoma stem-like cell differentiation and
tumorigenicity. Stem Cells. 29:1327–1337. 2011.PubMed/NCBI
|
|
14
|
Wang W, Li N-N, Du Y, Lv F-F and Lin G-Q:
FoxO3a and nilotinib-induced erythroid differentiation of CML-BC
cells. Leuk Res. 37:1309–1314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruvolo PP: The Herculean task of killing
cancer cells: Suppression of FOXO3A in acute leukemia involves a
hydra of multiple survival kinases. Cell Cycle. 11:2589. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kops GJ, Medema RH, Glassford J, Essers
MA, Dijkers PF, Coffer PJ, Lam EW and Burgering BM: Control of cell
cycle exit and entry by protein kinase B-regulated forkhead
transcription factors. Mol Cell Biol. 22:2025–2036. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hauck L, Harms C, Grothe D, An J, Gertz K,
Kronenberg G, Dietz R, Endres M and von Harsdorf R: Critical role
for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to
statin signaling in cardiac myocytes. Circ Res. 100:50–60. 2007.
View Article : Google Scholar
|
|
18
|
Miyauchi H, Minamino T, Tateno K, Kunieda
T, Toko H and Komuro I: Akt negatively regulates the in vitro
lifespan of human endothelial cells via a p53/p21-dependent
pathway. EMBO J. 23:212–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rathbone CR, Booth FW and Lees SJ: FoxO3a
preferentially induces p27Kip1 expression while impairing muscle
precursor cell-cycle progression. Muscle Nerve. 37:84–89. 2008.
View Article : Google Scholar
|
|
20
|
Roy SK, Chen Q, Fu J, Shankar S and
Srivastava RK: Resveratrol inhibits growth of orthotopic pancreatic
tumors through activation of FOXO transcription factors. PLoS One.
6:e251662011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obexer P, Geiger K, Ambros PF, Meister B
and Ausserlechner MJ: FKHRL1-mediated expression of Noxa and Bim
induces apoptosis via the mitochondria in neuroblastoma cells. Cell
Death Differ. 14:534–547. 2007. View Article : Google Scholar
|
|
22
|
Storz P, Döppler H, Copland JA, Simpson KJ
and Toker A: FOXO3a promotes tumor cell invasion through the
induction of matrix metalloproteinases. Mol Cell Biol.
29:4906–4917. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tenbaum SP, Ordóñez-Morán P, Puig I,
Chicote I, Arqués O, Landolfi S, Fernández Y, Herance JR, Gispert
JD, Mendizabal L, et al: β-catenin confers resistance to PI3K and
AKT inhibitors and subverts FOXO3a to promote metastasis in colon
cancer. Nat Med. 18:892–901. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osuka S, Sampetrean O, Shimizu T, Saga I,
Onishi N, Sugihara E, Okubo J, Fujita S, Takano S, Matsumura A, et
al: IGF1 receptor signaling regulates adaptive radioprotection in
glioma stem cells. Stem Cells. 31:627–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang
HT and Li X: microRNA-155 regulates cell proliferation and invasion
by targeting FOXO3a in glioma. Oncol Rep. 30:2111–2118.
2013.PubMed/NCBI
|
|
26
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Troussard AA, Costello P, Yoganathan TN,
Kumagai S, Roskelley CD and Dedhar S: The integrin linked kinase
(ILK) induces an invasive phenotype via AP-1 transcription
factor-dependent upregulation of matrix metalloproteinase 9
(MMP-9). Oncogene. 19:5444–5452. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kupferman ME, Fini ME, Muller WJ, Weber R,
Cheng Y and Muschel RJ: Matrix metalloproteinase 9 promoter
activity is induced coincident with invasion during tumor
progression. Am J Pathol. 157:1777–1783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rao JS, Yamamoto M, Mohaman S, Gokaslan
ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL
and Sawaya RE: Expression and localization of 92 kDa type IV
collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp
Metastasis. 14:12–18. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Xiao A, diPierro CG, Carpenter JE,
Abdel-Fattah R, Redpath GT, Lopes MB and Hussaini IM: An extensive
invasive intracranial human glioblastoma xenograft model: Role of
high level matrix metalloproteinase 9. Am J Pathol. 176:3032–3049.
2010. View Article : Google Scholar : PubMed/NCBI
|