Introduction
Renal cell carcinoma (RCC) is the most common
malignant cancer in the adult kidney, and accounts for
approximately 85% of all primary malignant kidney tumors and 3% of
all cancers in adults (1,2). Despite increased early detection of
RCC and more frequent surgery, 30% of RCC patients develop
metastases after surgery, which are associated with poor prognosis
(3–5). Therefore, there is an urgent need to
elucidate the mechanisms of RCC which regulate the initiation and
progression of RCC to provide useful information for the clinical
management of RCC.
MicroRNAs (miRNAs) are small endogenous non-coding
RNAs composed of ~19–25 nucleotides that negatively regulate gene
expression by degrading target mRNAs or repressing protein
translation by binding to the 3′ untranslated region (3′UTR) of
their target mRNAs (6).
Accumulating studies suggest that deregulation of miRNAs is
associated with carcinogenesis (7).
miRNAs have been shown to function as either oncogenes or tumor
suppressors in different types of cancer, which manifest as the
regulation of cellular proliferation, cell death and angiogenesis
in tumor progression (8,9). Numerous related studies have been
carried out in RCC and have shown that dysregulation in miRNAs are
involved in the occurrence and progression of RCC by regulating the
expression of their target oncogenes and tumor suppressors
(10,11), suggesting that miRNAs could serve as
diagnostic markers or therapeutic agents for RCC.
Deregulation of miR-148a has been reported in
several types of cancer, including breast cancer (12), hepatocellular carcinoma (13), osteosarcoma (14), gastric cancer (15), colorectal cancer (16), medulloblastoma (17), bladder cancer (18) and non-small cell lung cancer
(19). miR-148a has been previously
characterized as a tumor suppressor or oncogene with functions in
regulating cell proliferation, apoptosis, and migration and
invasion in several types of cancer (12–20).
However, the detailed biological function and underlying molecular
mechanisms of miR-148a in human RCC remain unclear. Therefore, the
aims of this study were to investigate the clinical significance,
biological function and underlying molecular mechanisms of miR-148a
in RCC.
Materials and methods
Human RCC clinical specimens
A total of 52 paired clear cell RCC and
corresponding adjacent noncancerous tissues (ANT) were obtained
sequentially from patients who underwent radical nephrectomy in the
People's Hospital of Qinghai (Xining, China) between July 2013 and
July 2015. Noncancerous renal tissues were obtained at least 5 cm
away from the tumor site. All tissue specimens were snap-frozen in
liquid nitrogen until use. The basic clinical characteristics of
these patients were collected, and are listed in Table I. The study protocol was approved by
the Ethics Committee of the People's Hospital of Qinghai and a
written informed consent was obtained from all patients involved in
this study.
 | Table I.Correlation between
clinicopathological features and miR-148a expression in tissues of
the RCC cases. |
Table I.
Correlation between
clinicopathological features and miR-148a expression in tissues of
the RCC cases.
|
|
| miR-148a
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of cases | Low, n (%) | High, n (%) | P-value |
|---|
| Age (years) |
|
|
| >0.05 |
|
<55 | 22 | 12 (54.5) | 10 (45.5) |
|
|
≥55 | 30 | 16 (53.3) | 14 (46.7) |
|
| Gender |
|
|
| >0.05 |
|
Male | 29 | 15 (51.7) | 14 (48.2) |
|
|
Female | 23 | 13 (56.5) | 10 (43.5) |
|
| TNM stage |
|
|
| <0.01 |
|
T1-T2 | 36 | 13 (36.1) | 23 (63.9) |
|
|
T3-T4 | 16 | 15 (93.7) | 1 (6.3) |
|
| Tumor size
(cm) |
|
|
| <0.01 |
|
<5 | 32 | 11 (34.5) | 21 (63.5) |
|
| ≥5 | 20 | 17 (85.0) | 3 (15.0) |
|
| Lymph node
metastasis |
|
|
| <0.01 |
| No | 35 | 13 (37.1) | 22 (62.9) |
|
|
Yes | 17 | 15 (88.2) | 2 (10.8) |
|
Cell culture
Four human RCC cell lines (786-O, ACHN, Caki-1, and
Caki-2) and human renal proximal tubule epithelial cell line (HK-2)
were all obtained from American Type Culture Collection (ATCC;
Rockville, MD, USA). The cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) (both from Gibco,
Grand Island, NY, USA), 50 U/ml penicillin or 50 µg/ml streptomycin
at 37°C in a 5% CO2 humidified incubator.
RNA extraction and real-time PCR
assays for miR-148a and AKT2 detection
Total RNA was extracted from the cultured cell and
tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The RNA was
quantified using NanoDrop ND-100 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA) at 260 nm. For detection of the
miR-148a expression level, quantitative PCR was performed using
TaqMan miRNA assays with specific primers for miR-148a according to
the protocol with endogenous U6 snRNA as the control under an ABI
PRISM 7900 sequence detection system (both from Applied Biosystems
Life Technologies, Foster City, CA, USA). For detection of AKT2
mRNA expression, cDNA was synthesized from 500 ng total RNA using
the Primer Script RT reagent kit (Takara Bio, Inc., Otsu, Japan).
Quantitative PCR analysis of the AKT2 expression at the mRNA level
was performed using Fast SYBR-Green Master Mix (Applied Biosystems
Life Technologies) under ABI PRISM 7900 sequence detection system,
with GAPDH used as an internal control. The primers of AKT2 and
GAPDH used in this study were previously described (21). The relative miRNA or mRNA expression
was quantified by measuring cycle threshold (Ct) values and
normalized using the 2−∆∆Ct method.
Cell transfection
The miR-148a mimic (miR-148a) and corresponding
negative control (miR-NC) were designed and purchased from RiboBio
(Guangzhou, China). siRNAs against AKT2 (si-AKT2) and the
corresponding scramble control (si-NC) were purchased from
GenePharma (Shanghai, China). The AKT2 expression vector (pcDNA 3.0
containing AKT2 coding region) was obtained from Dr Ju Peng (Jilin
University, Changchun, China), which was used for the ‘rescue’
experiments. Transfection was performed using Lipofectamine 2000
(Invitrogen) according to the manufacturer's instructions.
Cell proliferation and colony
formation assays
Cell proliferation was assessed using a tetrazolium
salt (WST-8)-based colormetric assay provided by the Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Briefly,
transfected cells were seeded into 96-well plates (5×103
cells/well) with 100 µl culture medium. At the indicated
time-points (24, 48 and 72 h), 10 µl of CCK-8 solution was added to
the wells and cultured additionally for 4 h at 37°C. Cell viability
was determined from absorbance readings at 450 nm using a
microplate reader (Bio-Rad Laboratories, Gaithersburg, MD,
USA).
For the colony formation assay, 1×103
transfected cells were plated on 6-well plates and incubated for 10
days. Then, the cells were rinsed with PBS and fixed with 1%
formaldehyde for 30 min and stained with 1% crystal violet for 10
min. Finally images were captured of the colonies and the number of
colonies was counted under a light microscope (Olympus, Tokyo,
Japan).
Wound healing assays
For the wound healing assay, transfected cells were
seeded on 6-well plates with fresh medium containing 10% FBS at a
density of 2×105 cells/well. After formation of a
confluent monolayer of cells, the membrane was scratched using a
sterile 100-µl pipette. The cell culture medium was replaced and
images of the wound were taken at different time-points (0 and 24 h
after scratching) under a light microscope (Olympus). To assess the
migration rate, we measured the fraction of cell coverage across
the line.
Invasion assays
A 24-well Transwell plate with 8-µm pore
polycarbonate membrane inserts (Corning Costar Corp., Cambridge,
MA, USA) was employed to assess invasive capacity of the cells
according to the manufacturer's instructions. Briefly,
3×104 transfected cells suspended in serum-free medium
were added to the upper chamber pre-coated with Matrigel (BD
Biosciences, Bedford, MA, USA). Medium containing 10% FBS was added
to the bottom chamber as a chemoattractant. After 48 h of
incubation, the cells invading into the lower surface of the
membrane were fixed and stained with methanol mixed with crystal
violet and then counted under a light microscope (Olympus).
Vector construction and luciferase
reporter assay
A luciferase reporter assay was performed using the
firefly luciferase-expressing vector psiCHECK2 (Promega Corp.,
Madison, WI, USA). A wild-type 3′UTR segment of AKT2 mRNA
containing the putative miR-148a binding sites was amplified and
cloned into the XhoI and NotI sites downstream of the
luciferase reporter gene in psiCHECK-2, named as Wt-AKT2-3′UTR.
Mutations of their 3′UTR sequence were created using QuickChange
Site-Directed Mutagenesis kit (Agilent Technologies, Inc., Palo
Alto, CA, USA) following the manufacturer's instructions, referred
to Mut-AKT2-3′UTR. For the luciferase activity assay, cells were
seeded into 12-well plates overnight before transfection, and then
co-transfected with 100 ng of psiCHECK-2 vectors, which harbored
the AKT2 3′UTR wild-type or mutant constructs, 100 ng pRL-TK
Renilla luciferase report vector as the internal control,
100 nM of miR-148a or miR-NC. After 48 h, the luciferase activity
was measured with a dual-luciferase assay kit (Promega Corp.)
according to the manufacturer's instructions. Renilla
luciferase activity was normalized to firefly luciferase
activity.
Western blot analysis
The cells or tissues were harvested and incubated on
ice with lysis buffer (Boster Inc., Shanghai, China). Total protein
concentration was detected using a bicinchoninic acid (BCA) protein
assay kit (Boster Inc.); 50 µg of protein was electrophoresed
through 10% SDS polyacrylamide gels and were then transferred to a
PVDF membrane (Millipore Corp., Billerica, MA, USA). Membranes were
blocked using 5% non-fat milk for 1 h and blotted in antibodies
against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), AKT2,
AKT, p-Akt (Ser473), mTOR and p-mTOR (Ser2448) at 4°C overnight.
All antibody were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). After washing with TBST, the membranes were
incubated in HRP-conjugated goat anti-mouse or anti-rabbit
secondary antibodies (Santa Cruz Biotechnology, Inc.) for 2 h at
room temperature. Proteins band were visualized with an ECL
chemiluminescent kit (ECL-Plus; Thermo Fisher Scientific, Waltham,
MA, USA) and exposure to chemiluminescent film.
In vivo tumor growth assay
All experimental procedures involving animals were
performed in accordance with the Guidelines for the Care and Use of
Laboratory Animals and Institutional Ethical Guidelines of People's
Hospital of Qinghai. The animal studies and experimental protocol
were approved by the Institutional Animal Care and Use Committee of
the People's Hospital of Qinghai. To establish RCC xenografts,
2×106 786-O cells stably expressing miR-148a or miR-NC
were subcutaneously implanted into the flanks of male BALB/c-nude
mice (n=10 per group, 3–4 weeks of age). Tumor sizes were measured
with callipers to estimate the volume (V) from day 5 to day 30
after injection using the formula: V (mm3) =
[width2 (mm2) × length (mm)]/2. Thirty days
after implantation, the animals were sacrificed, and tumors were
dissected and weighed. A part of the tumor tissues was frozen in
liquid nitrogen and stored at −80°C for qRT-PCR and western blot
analysis.
Statistical analysis
All data are expressed as mean ± SD (standard
deviation) from at least three independent experiments. Differences
were determined by two-tailed Student's t-test by one-way ANOVA.
Associations of miR-148a expression and AKT2 expression were
estimated using Spearman's correlation analysis. Statistical
analysis was performed using the SPSS® Statistical
Package, version 19.0 (SPSS Inc., Chicago, IL, USA) for
Windows®. In all cases, P<0.05 was considered
statistically significant.
Results
miR-148a is downregulated in RCC cell
lines and tissues
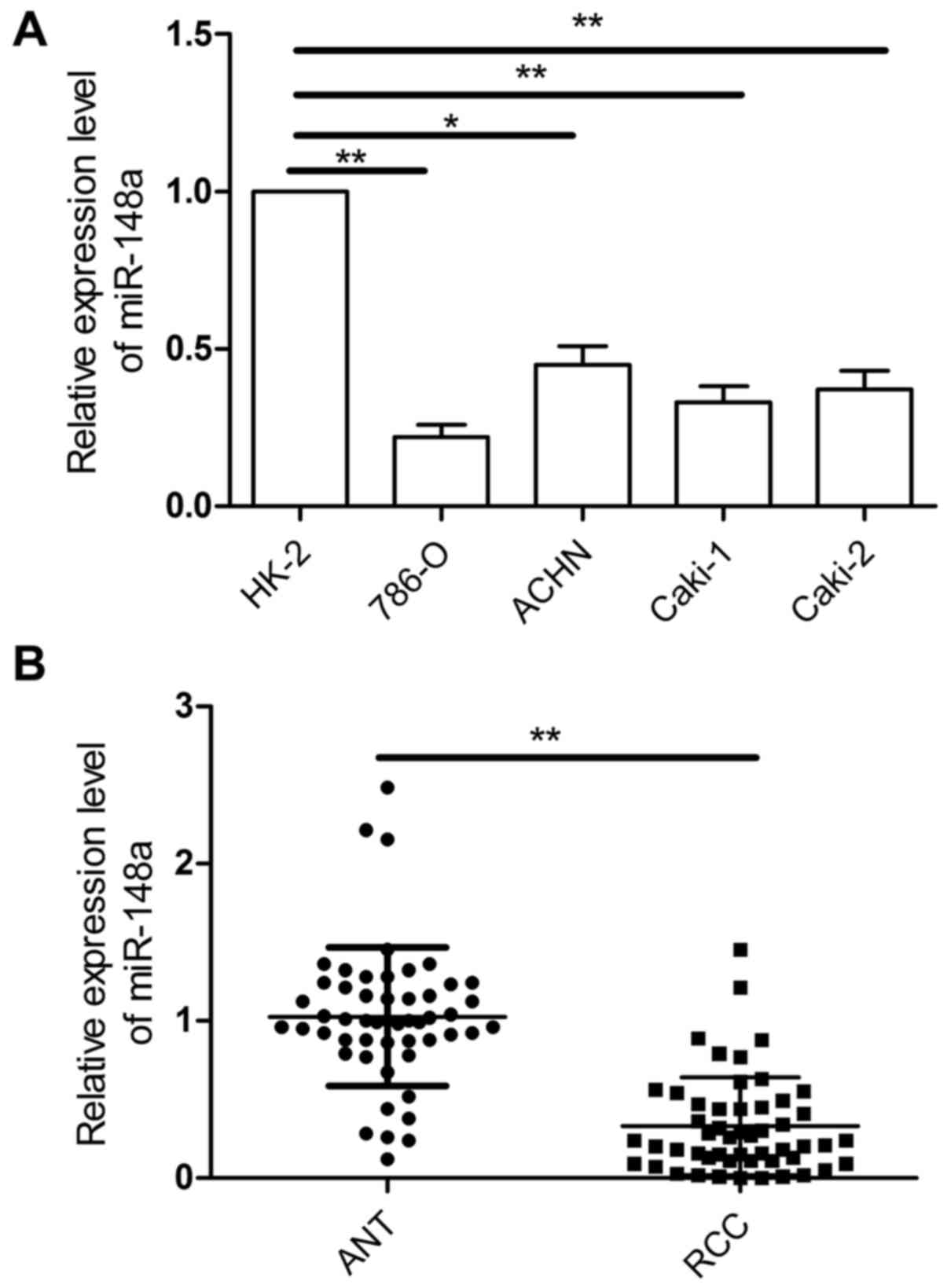
To investigate the role of miR-148a in RCC
development and progression, the expression of miR-148a was
examined in four human RCC cell lines (786-O, ACHN, Caki-1, and
Caki-2) and a human renal proximal tubule epithelial cell line
(HK-2). Results of qRT-PCR showed that expression of miR-148a was
decreased in all of the RCC cell lines compared with the HK-2 cells
(Fig. 1A). In addition, the levels
of miR-148a in 52 RCC tissues and ANT were detected by qRT-PCR.
Compared with the noncancerous tissues, the miR-148a expression
level was downregulated (P<0.001; Fig. 1B). Clinical patients were divided
into two groups according to the median of miR-148a expression in
the RCC samples. The downregulation of miR-148a was significantly
associated with large tumor size, advanced TNM stage, and lymph
node metastasis (Table I). Taken
together, these results suggest miR-148a as a potential biomarker
for prediction of prognosis in RCC.
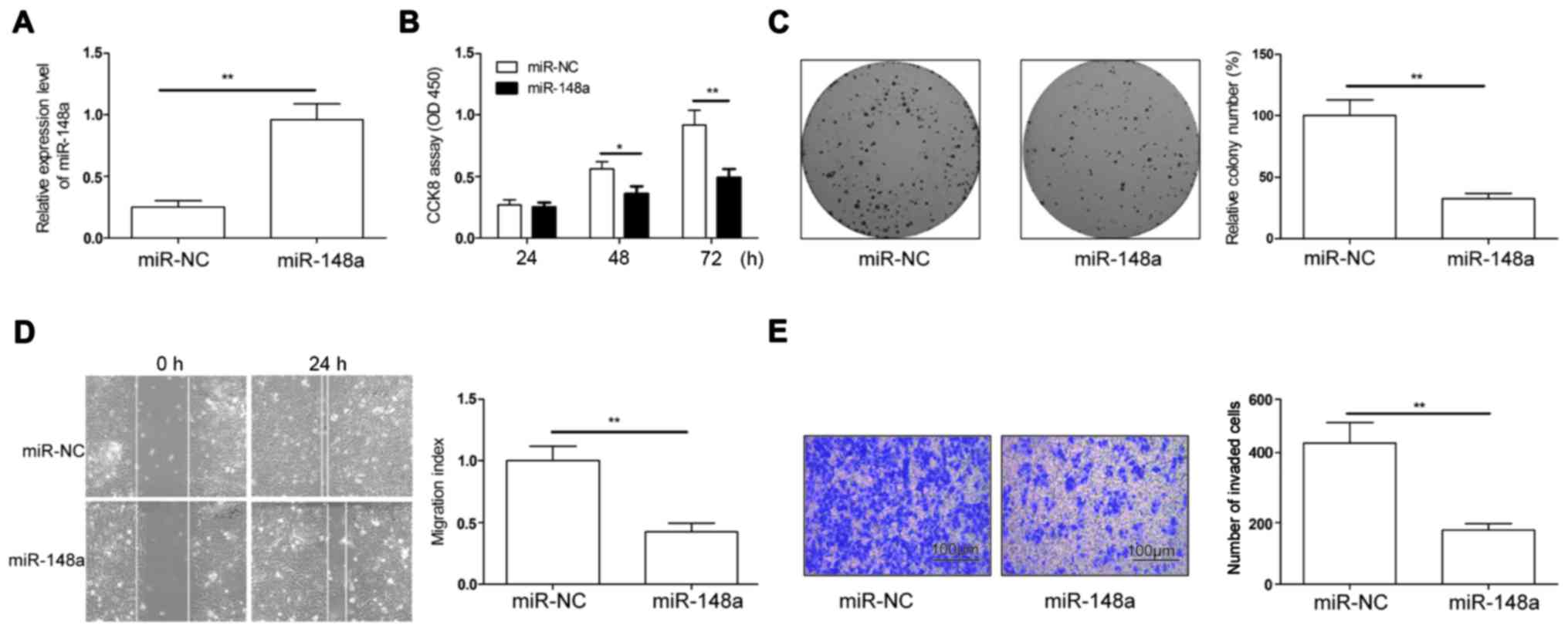
miR-148a inhibits proliferation,
migration and invasion of RCC cells in vitro
To determine the biological function of miR-148a in
RCC progression, we re-introduced miR-148a into 786-O cells, which
exploited the lowest expression among the four RCC cell lines
(Fig. 1A). miR-148a expression was
significantly increased in the 786-O cells transfected with
miR-148a mimics compared to those transfected with negative control
mimic (miR-NC), as shown by qRT-PCR (Fig. 2A). CCK-8 assay showed that miR-148a
overexpression significantly inhibited cell proliferation (Fig. 2B). Consistently, ectopic miR-148a
expression dramatically suppressed colony formation (Fig. 2C). Given that miR-148a expression
was associated with lymph node metastasis in RCC patients, we next
examined whether miR-148a could affect RCC cell migration and
invasion. Results showed that miR-148a overexpression distinctly
abrogated the migration and invasion of 786-O cells (Fig. 2D and E). Our results suggest that
miR-148a suppresses proliferation, migration and invasion of RCC
cells.
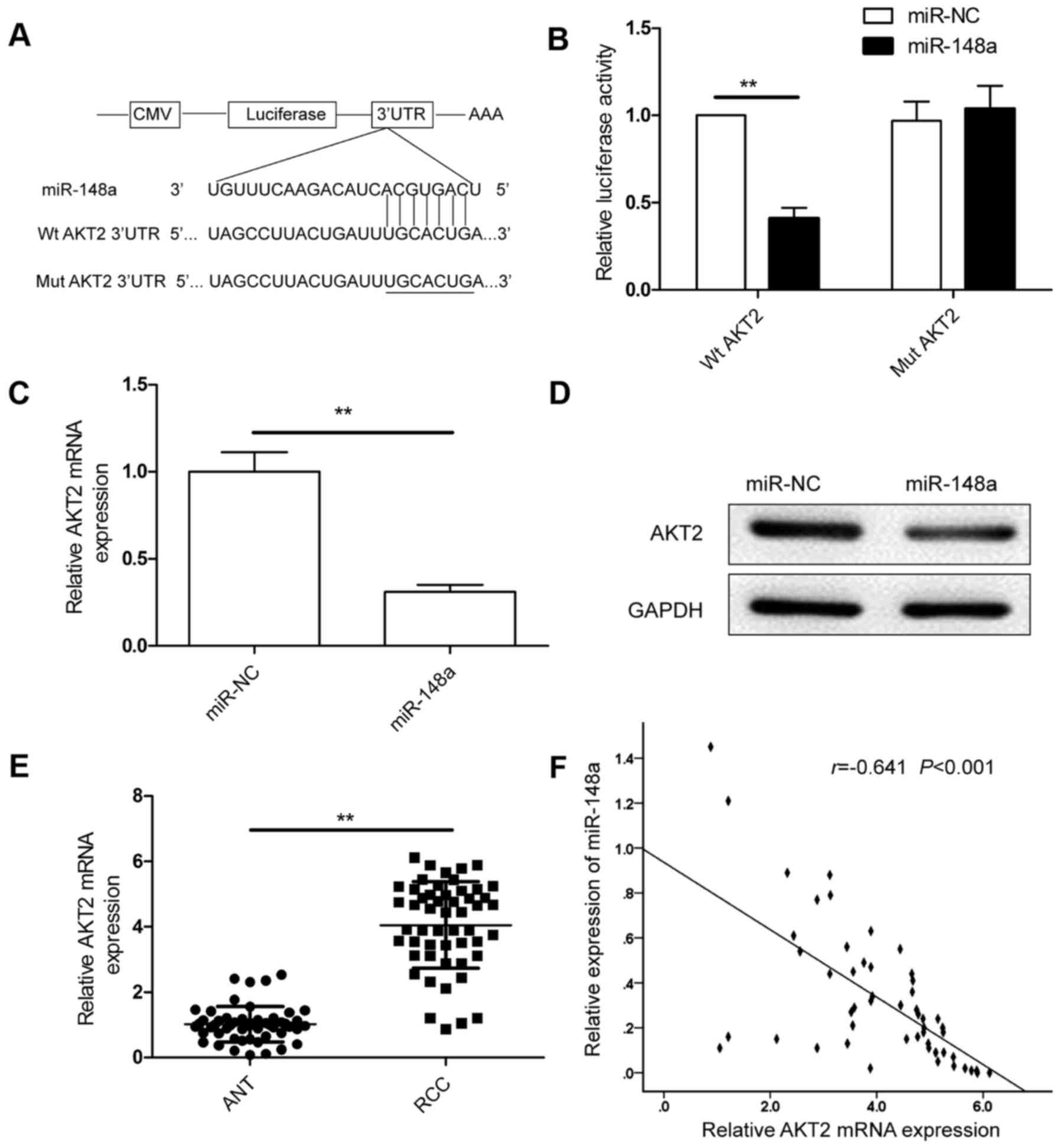
AKT2 is the direct target of miR-148a
in RCC cells
According to the bioinformatic analysis, the 3′UTR
of AKT2 was complementarily matched to the miR-148a sequence
(Fig. 3A). To test whether miR-148a
was able to regulate AKT2 directly, we performed dual luciferase
reporter experiments, and found that miR-148a significantly reduced
luciferase activity of wild-type AKT2 3′UTR, whereas the luciferase
activity of mutant AKT2 3′UTR remained unchanged (Fig. 3B). Furthermore, miR-148a
overexpression blocked AKT2 expression at both the mRNA and protein
levels in the RCC cells (Fig. 3C and
D). The expression of AKT2 was increased in the RCC clinical
samples (Fig. 3E), and was
inversely correlated with miR-148a in the RCC tissues (r=−0.641,
P<0.001; Fig. 3F). These data
supported our hypothesis that miR-148a directly targets the AKT2
gene in RCC.
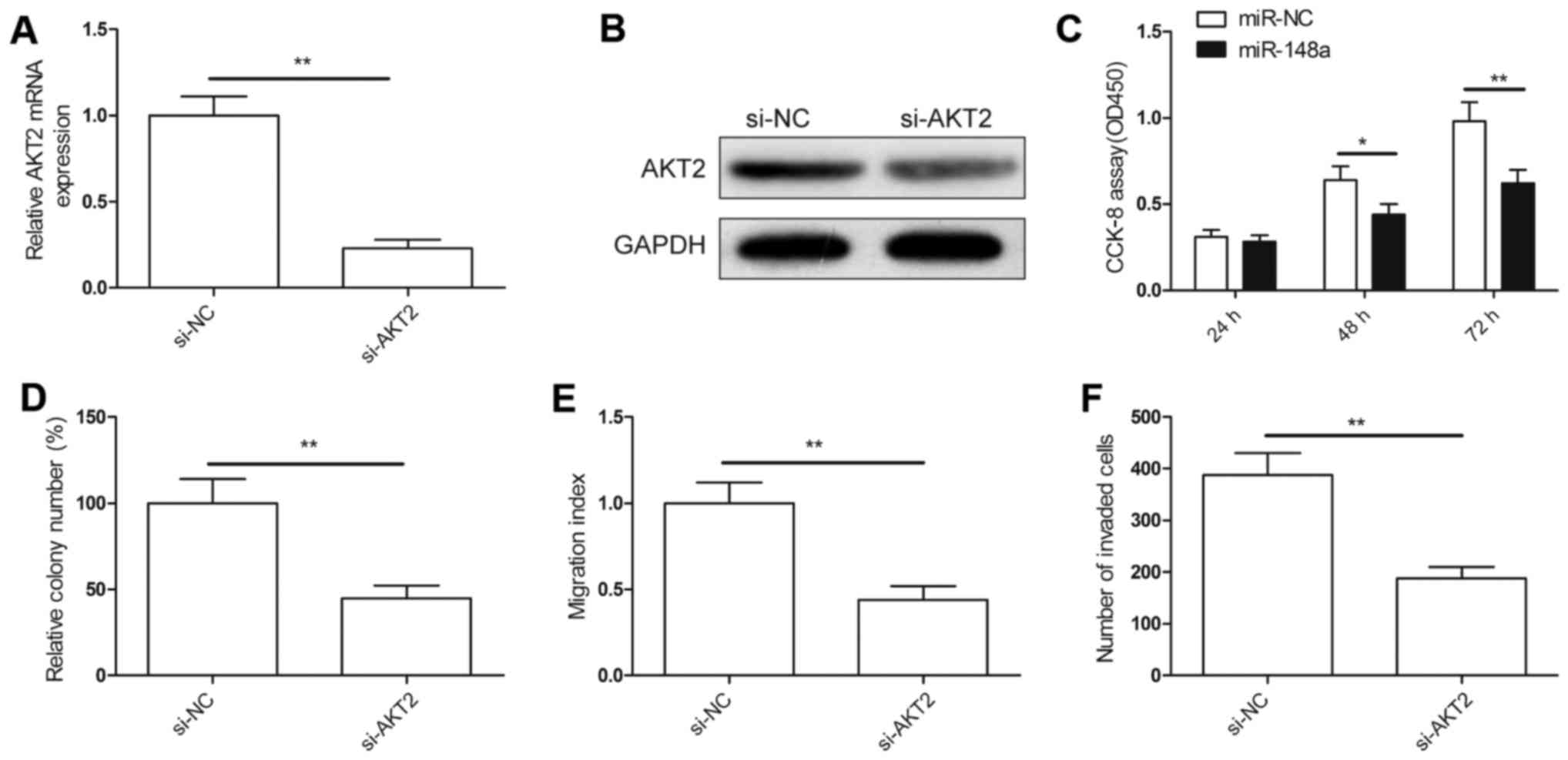
miR-148a suppresses cell proliferation
and migration via the suppression of AKT2
We assessed whether targeting of AKT2 would mimic
miR-148a-induced phenotypes on cell proliferation migration, and
invasion. Firstly, we knocked down AKT2 in the 786-O cells by
si-AKT2, and found that AKT2 expression was inhibited both at the
mRNA and protein levels (Fig. 4A and
B). In addition, we demonstrated that downregulation of AKT2
efficiently inhibited cell proliferation (Fig. 4C), decreased colony formation
ability (Fig. 4D), and suppressed
migration (Fig. 4E) and invasion
(Fig. 4F) in the 786-O cells, and
had effects similar to those of the overexpression of miR-148a.
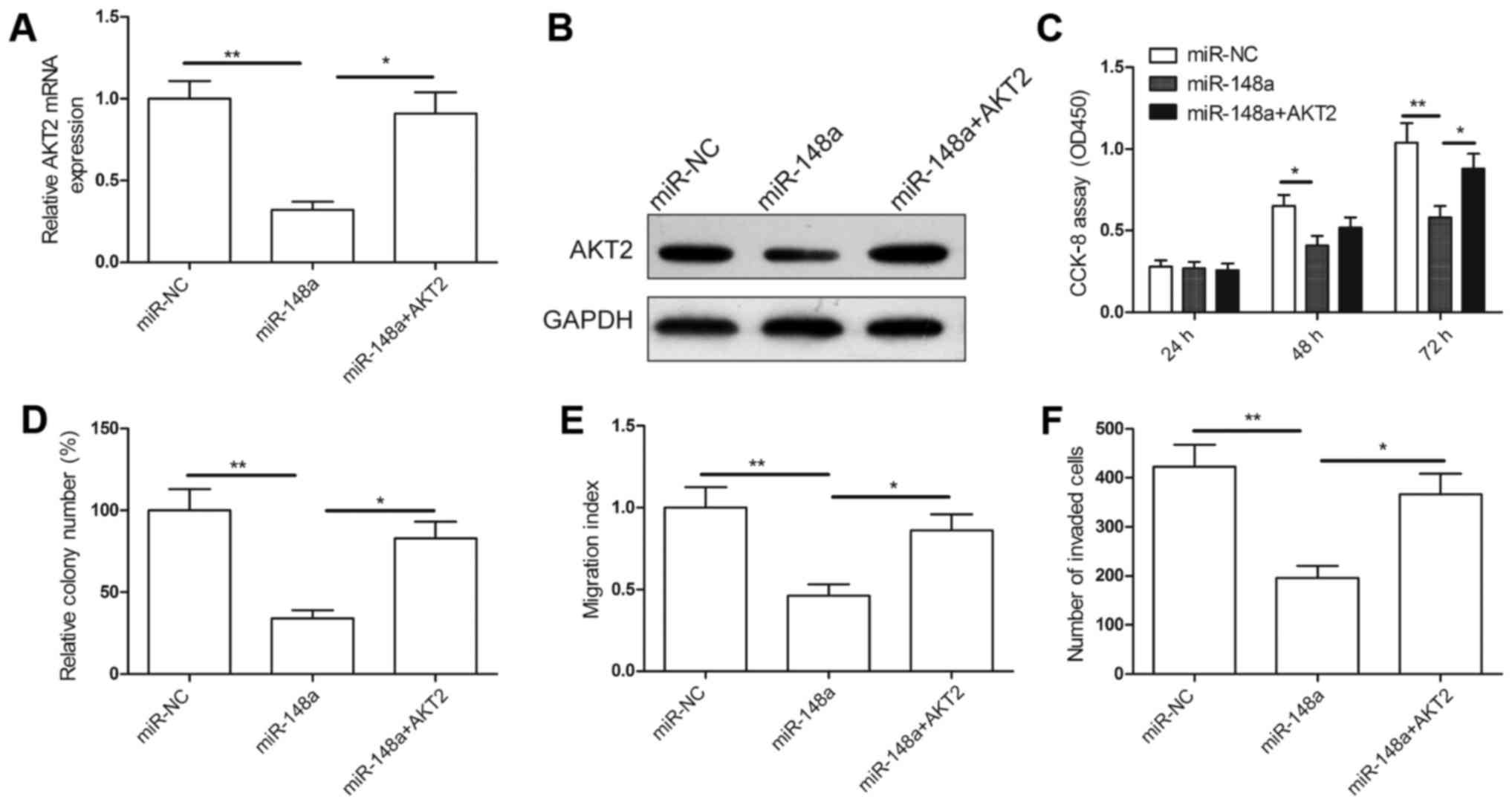
Next, we examined whether AKT2 overexpression could
rescue the inhibitory effects of miR-148a on RCC cell
proliferation, migration and invasion. The overexpressing AKT2
plasmid was introduced in the 786-O cells that had been transfected
with the miR-148a mimic and mimic NC. RT-PCR and western blot
analysis confirmed that the miR-338-3p mimics markedly and
specifically decreased AKT2 expression, whereas transfection of the
overexpressing AKT2 plasmid restored AKT2 expression in the 786-O
cells (Fig. 5A and B). Our results
also demonstrated that reintroduction of AKT2 significantly
abrogated the suppression of cell proliferation, colony formation,
migration and invasion in the 786-O cells induced by miR-148a
(Fig. 5C-F). Collectively, these
results suggest that miR-148a suppresses cell proliferation and
migration in RCC cells via the suppression of AKT2.
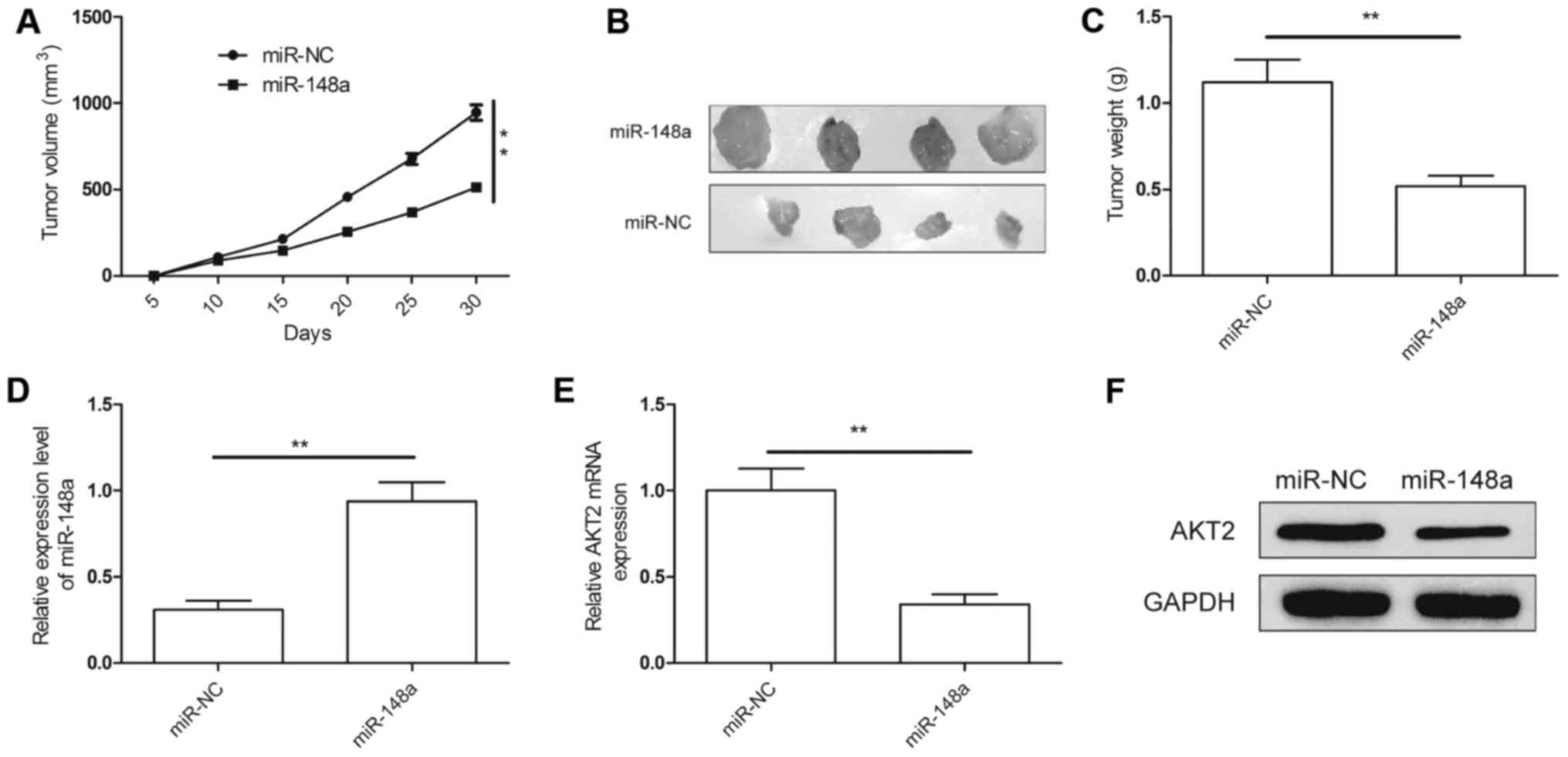
miR-148a inhibits tumor growth in
vivo
An animal experiment was employed to verify the
function of miR-148a in RCC growth. 786-O/miR-148a and 786-O/miR-NC
cells were subcutaneously injected into nude mice. Tumor sizes were
measured every 5 days until the mice were sacrificed at 30 days
post-implantation. The results demonstrated that tumor growth was
slower in the 786-O/miR-148 group compared with that in the miR-NC
group (Fig. 6A). Thirty days after
injection, the nude mice were sacrificed, and the tumor tissues
were stripped and weighed. The tumor size (Fig. 6B) and weight (Fig. 6C) were significantly decreased in
the 786-O/miR-148a group compared with the size and weight in the
786-O/miR-NC group. In addition, we also found that the miR-148a
expression level was upregulated (Fig.
6D), while the AKT2 expression at the mRNA level (Fig. 6E) and protein level (Fig. 6F) was downregulated in the
786-O/miR-148a group. These results suggest that miR-148a
suppresses RCC tumorigenicity in vivo by repressing AKT2
expression.
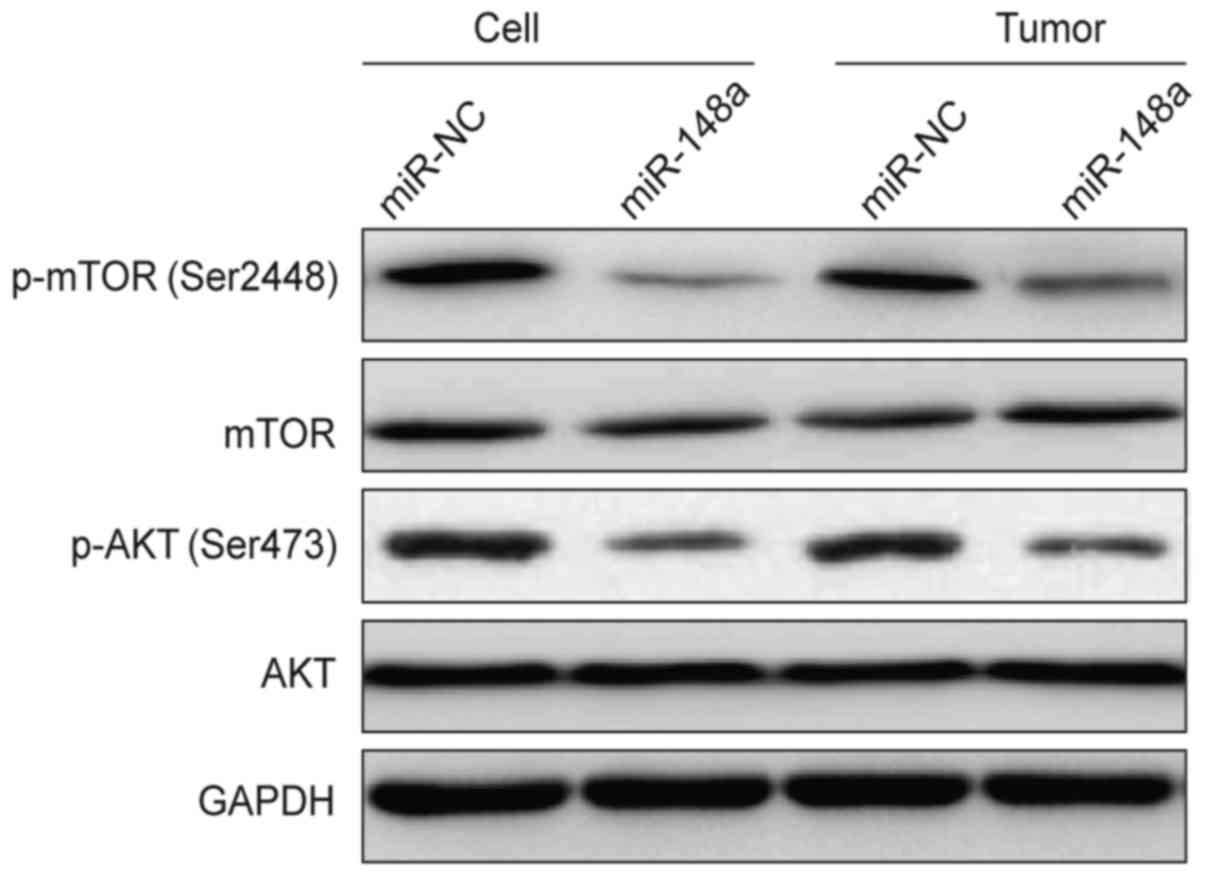
miR-148a inhibits the Akt pathway in
vitro and in vivo
AKT2 belongs to the Akt family that functions as the
hub in the PI3K/Akt signaling pathway. We therefore assessed the
levels of AKT2 downstream proteins including p-Akt (Ser473) and
p-mTOR (Ser2448) in the 786-O cells 48 h after transfection with
miR-148a or miR-NC, and found that overexpression of miR-148a
decreased the phosphorylation levels of Akt and mTOR, whereas the
total AKT and mTOR level were not changed (Fig. 7). Moreover, we also determined
expression of these proteins in tumor tissues from the RCC
xenograft mice. Our results further showed that p-Akt and p-mTOR
expression was decreased in the 786-O/miR-148a group compared to
that in the 786-O/miR-NC group (Fig.
7). These results suggest that miR-148a overexpression inhibits
the AKT signaling pathway in vitro and in vivo.
Discussion
Recent advances have revealed that dysregulation of
miRNAs is involved in the progression and development of cancer
(8,9). Modulation of miRNA expression has been
proposed to be a feature of various types of cancer, including RCC,
which may benefit the development of diagnostic markers and
therapeutic agents for RCC (10,11).
Here, we found that miR-148a was significantly downregulated in RCC
cell lines and tissue specimens relative to normal renal cells and
adjacent noncancerous tissues. Meanwhile, we also discovered that
miR-148a inhibited RCC growth and metastasis by regulating AKT2
expression. These findings provided a mechanism by which miR-148a
suppresses tumorigenesis in RCC.
miR-148a, a member of the miR-148 family, has been
reported to correlate with diverse biological functions, including
proliferation, migration, invasion, and cell cycle progression
(12–19). In multiple types of tumors, miR-148a
functions as a tumor suppressor to block the growth and metastasis
of cancer cells (12–14,16–20).
On the contrary, in glioblastoma and gastric cancer, miR-148a acts
as an oncomiR to promote cancer cell proliferation and survival
(15,22). These studies indicated that miR-148a
can acts as an oncomiR or tumor suppressor, depending on tumor
type. In the present study, we provide the first demonstration that
miR-148a expression was downregulated in human RCC tissues and cell
lines. We also found that restoration of miR-148a inhibited RCC
cell proliferation, colony formation, migration and invasion in
vitro, as well as suppressed RCC tumor growth in vivo.
Thus, our in vitro and in vivo findings together
suggest that miR-148a functions as a tumor suppressor in RCC.
It is well known that miRNAs exert their roles in
tumor proliferation, apoptosis, migration, invasion and metastasis
through regulation of target gene expression (7). Thus, to explore the mechanism
underlying the function of miR-148a in RCC, the miR-148a target was
predicted using biological software. We identified AKT2 as a
potential candidate. AKT2, an isoform of the AKT family, has been
reported to function as an oncogene by primarily enhancing the
survival, migration and invasion of cancer cells (23–25).
Recently studies have shown that AKT2 expression was elevated in
RCC tissues, and it has an important role in the pathogenesis and
progression of RCC (26). In
addition, AKT2 has been shown to be regulated by multiple miRNAs,
such as miR-29b (21), miR-124
(27), miR-612 (28), miR-137 (29) and miR-615 (30). In this study, we indentified AKT2 as
a target of miR-148a in RCC. We also found that downregulation of
AKT2 phenotypically copied miR-148a-induced phenotypes, whereas
re-expression of AKT2 reversed the suppressive effects of miR-148a
in RCC cells. These results suggest that miR-148a exerts tumor
suppressor roles in RCC by repressing AKT2.
AKT2 is a significant member of the PI3K/AKT
pathway, which is recognized as one of the most frequently
activated signaling pathways in human cancers (31,32).
This pathway is involved in regulating various cancer processes,
such as cell proliferation, apoptosis, migration, and metastasis
(33,34). It has been reported that AKT exerts
its biological function by phosphorylating its downstream
substrates including mTOR (35,36).
Therefore, we investigated whether miR-148a affects the AKT pathway
and its downstream protein. We found that overexpression of
miR-148a significantly suppressed the phosphorylation of AKT, as
well as inhibited its downstream effectors, p-mTOR expression, but
not total AKT and mTOR in vitro and in vivo. These
findings suggest that miR-148 inhibits RCC growth via regulating
the AKT signaling pathway.
In conclusion, the results presented here
demonstrated that the miR-148a expression level was downregulated
in RCC cell lines and tissues, and its expression level was
significantly associated with large tumor size, advanced TNM stage
and lymph node metastasis, and that miR-148a acts as a tumor
suppressor inhibiting the proliferation, migration and invasion of
RCC cells, as well as suppressing tumor growth in vivo by
negative regulation of its target AKT2 and regulation of the AKT
signaling pathway. Thus, miR-1 may be a potential therapeutic
target for RCC treatment.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pantuck AJ, Zisman A and Belldegrun AS:
The changing natural history of renal cell carcinoma. J Urol.
166:1611–1623. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ying SY, Chang DC, Miller JD and Lin SL:
The microRNA: Overview of the RNA gene that modulates gene
functions. Methods Mol Biol. 342:1–18. 2006.PubMed/NCBI
|
|
7
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu L, Li H, Chen L, Ma X, Gao Y, Li X,
Zhang Y, Fan Y and Zhang X: MicroRNAs as prognostic molecular
signatures in renal cell carcinoma: A systematic review and
meta-analysis. Oncotarget. 6:32545–32560. 2015.PubMed/NCBI
|
|
11
|
Li JY, Yong TY, Michael MZ and Gleadle JM:
Review: The role of microRNAs in kidney disease. Nephrology
(Carlton). 15:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Zhang Y, Jasper J, Lykken E,
Alexander PB, Markowitz GJ, McDonnell DP, Li QJ and Wang XF:
MiR-148a functions to suppress metastasis and serves as a
prognostic indicator in triple-negative breast cancer. Oncotarget.
7:20381–20394. 2016.PubMed/NCBI
|
|
13
|
Pan L, Huang S, He R, Rong M, Dang Y and
Chen G: Decreased expression and clinical significance of miR-148a
in hepatocellular carcinoma tissues. Eur J Med Res. 19:682014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma W, Zhang X, Chai J, Chen P, Ren P and
Gong M: Circulating miR-148a is a significant diagnostic and
prognostic biomarker for patients with osteosarcoma. Tumour Biol.
35:12467–12472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia J, Guo X, Yan J and Deng K: The role
of miR-148a in gastric cancer. J Cancer Res Clin Oncol.
140:1451–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hibino Y, Sakamoto N, Naito Y, Goto K, Oo
HZ, Sentani K, Hinoi T, Ohdan H, Oue N and Yasui W: Significance of
miR-148a in colorectal neoplasia: Downregulation of miR-148a
contributes to the carcinogenesis and cell invasion of colorectal
cancer. Pathobiology. 82:233–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yogi K, Sridhar E, Goel N, Jalali R, Goel
A, Moiyadi A, Thorat R, Panwalkar P, Khire A, Dasgupta A, et al:
MiR-148a, a microRNA upregulated in the WNT subgroup tumors,
inhibits invasion and tumorigenic potential of medulloblastoma
cells by targeting neuropilin 1. Oncoscience. 2:334–348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lombard AP, Mooso BA, Libertini SJ, Lim
RM, Nakagawa RM, Vidallo KD, Costanzo NC, Ghosh PM and Mudryj M:
miR-148a dependent apoptosis of bladder cancer cells is mediated in
part by the epigenetic modifier DNMT1. Mol Carcinog. 55:757–767.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joshi P, Jeon YJ, Laganà A, Middleton J,
Secchiero P, Garofalo M and Croce CM: MicroRNA-148a reduces
tumorigenesis and increases TRAIL-induced apoptosis in NSCLC. Proc
Natl Acad Sci USA. 112:8650–8655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Q, Jiang Y, Yin Y, Li Q, He J, Jing Y,
Qi YT, Xu Q, Li W, Lu B, et al: A regulatory circuit of
miR-148a/152 and DNMT1 in modulating cell transformation and tumor
angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 5:3–13.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Li H, Liu X, Xu D and Wang F:
MicroRNA-29b regulates TGF-β1-mediated epithelial-mesenchymal
transition of retinal pigment epithelial cells by targeting AKT2.
Exp Cell Res. 345:115–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim J, Zhang Y, Skalski M, Hayes J, Kefas
B, Schiff D, Purow B, Parsons S, Lawler S and Abounader R:
microRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to
regulate EGFR and apoptosis in glioblastoma. Cancer Res.
74:1541–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pereira L, Horta S, Mateus R and Videira
MA: Implications of Akt2/Twist crosstalk on breast cancer
metastatic outcome. Drug Discov Today. 20:1152–1158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal E, Brattain MG and Chowdhury S:
Cell survival and metastasis regulation by Akt signaling in
colorectal cancer. Cell Signal. 25:1711–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng GZ, Zhang W and Wang LH: Regulation
of cancer cell survival, migration, and invasion by Twist: AKT2
comes to interplay. Cancer Res. 68:957–960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toschi A, Lee E, Gadir N, Ohh M and Foster
DA: Differential dependence of hypoxia-inducible factors 1 alpha
and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 283:34495–34499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang CF, Li DM, Shi ZM, Wang L, Liu MM,
Ge X, Liu X, Qian YC, Wen YY, Zhen LL, et al: Estrogen regulates
miRNA expression: Implication of estrogen receptor and miR-124/AKT2
in tumor growth and angiogenesis. Oncotarget. 7:36940–36955.
2016.PubMed/NCBI
|
|
28
|
Sheng L, He P, Yang X, Zhou M and Feng Q:
miR-612 negatively regulates colorectal cancer growth and
metastasis by targeting AKT2. Cell Death Dis. 6:e18082015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu L, Chen J, Ding C, Wei S, Zhu Y, Yang
W, Zhang X, Wei X and Han D: MicroRNA-137 contributes to dampened
tumorigenesis in human gastric cancer by targeting AKT2. PLoS One.
10:e01301242015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai Y, Li J, Li J, Liu Y and Zhang B:
MiR-615 inhibited cell proliferation and cell cycle of human breast
cancer cells by suppressing of AKT2 expression. Int J Clin Exp Med.
8:3801–3808. 2015.PubMed/NCBI
|
|
31
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robbins HL and Hague A: The PI3K/Akt
pathway in tumors of endocrine tissues. Front Endocrinol
(Lausanne). 6:1882016.PubMed/NCBI
|
|
34
|
Guo H, German P, Bai S, Barnes S, Guo W,
Qi X, Lou H, Liang J, Jonasch E, Mills GB, et al: The PI3K/AKT
pathway and renal cell carcinoma. J Genet Genomics. 42:343–353.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JJ, Loh K and Yap YS: PI3K/Akt/mTOR
inhibitors in breast cancer. Cancer Biol Med. 12:342–354.
2015.PubMed/NCBI
|
|
36
|
Hudes GR: Targeting mTOR in renal cell
carcinoma. Cancer 115 (Suppl 10). 2313–2320. 2009. View Article : Google Scholar
|