Liver cancer is a highly prevalent disease worldwide
and one of the leading causes of cancer death in the world
(1). An estimated 782,500 new liver
cancer cases and 745,500 deaths occurred worldwide during 2012,
with China alone accounting for approximately 50% of the total
number of cases and deaths. Despite the declining rate for liver
cancer in China, population growth and ageing still led to a large
and rising number of new cases in 2015 (2). Most (70–90%) primary liver cancers
occurring worldwide are hepatocellular carcinoma (HCC). Because of
the presence of advanced disease or poor liver function, many HCC
patients are not candidates for curative surgical treatments
(resection or transplantation). These patients may be eligible for
treatment with locoregional therapies (LRTs) including ablative and
endovascular therapies, and/or with cytostatic targeted molecular
systemic therapies such as sorafenib, which achieve some survival
benefits for unresectable HCC (3).
Accurately determining tumor response after therapy has become
essential in the management of HCC for determining efficacy of
therapy (4), subsequent therapeutic
planning and as a surrogate marker for improved survival (5). Lack of objective response after one or
more LRTs is associated with poor survival, although it may be
influenced by the type of LRTs used (6–8) and
pattern of tumor progression (intrahepatic or extrahepatic)
(9).
Here, we review various proposed clinical response
criteria of HCC to LRTs from morphological to functional imaging
biomarkers, assess their accuracy for determining tumor response
after LRTs and discuss their challenges in clinical practice.
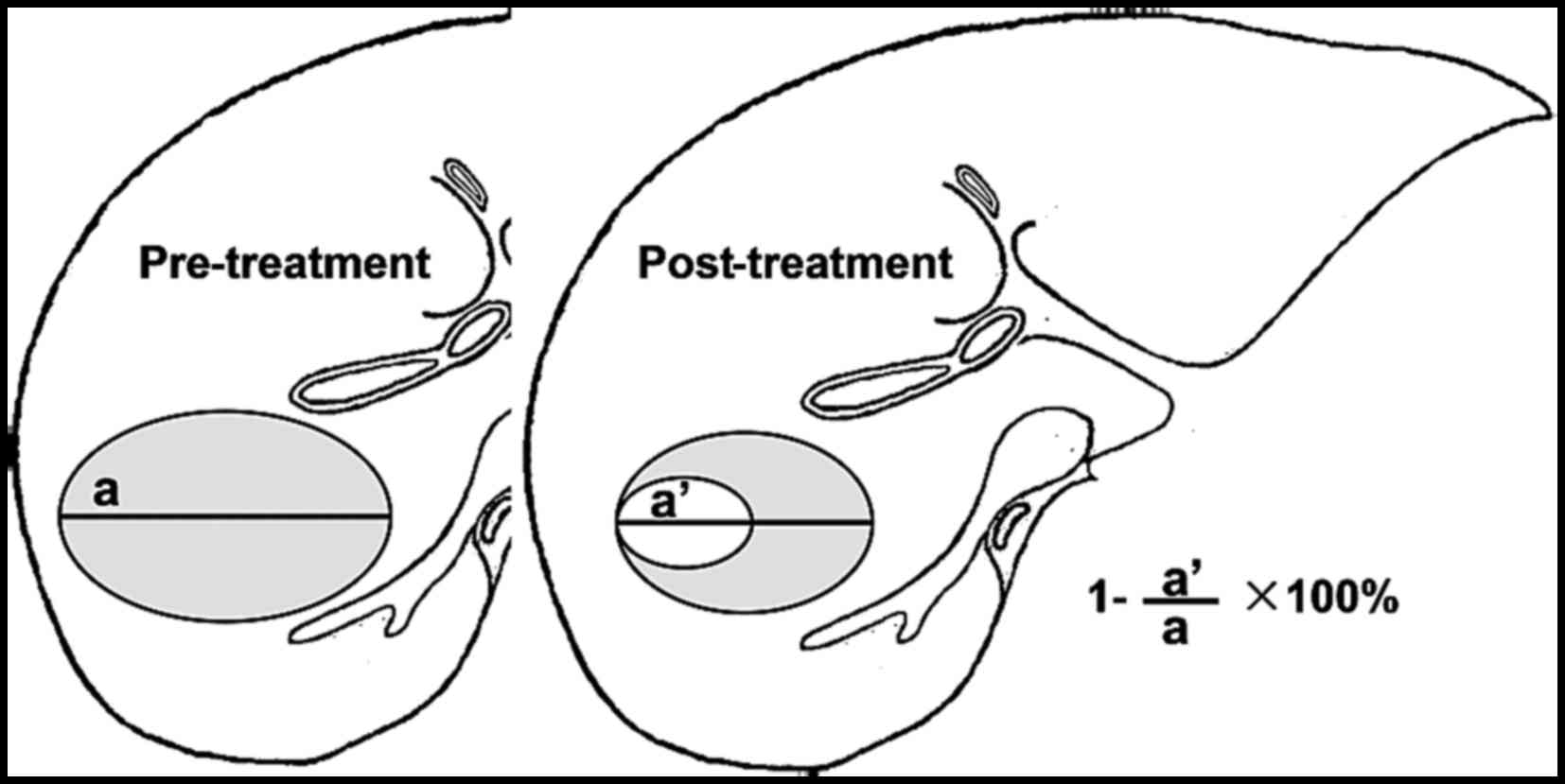
In 1981, the World Health Organization (WHO)
proposed the first tumor response criterion after therapy (WHO
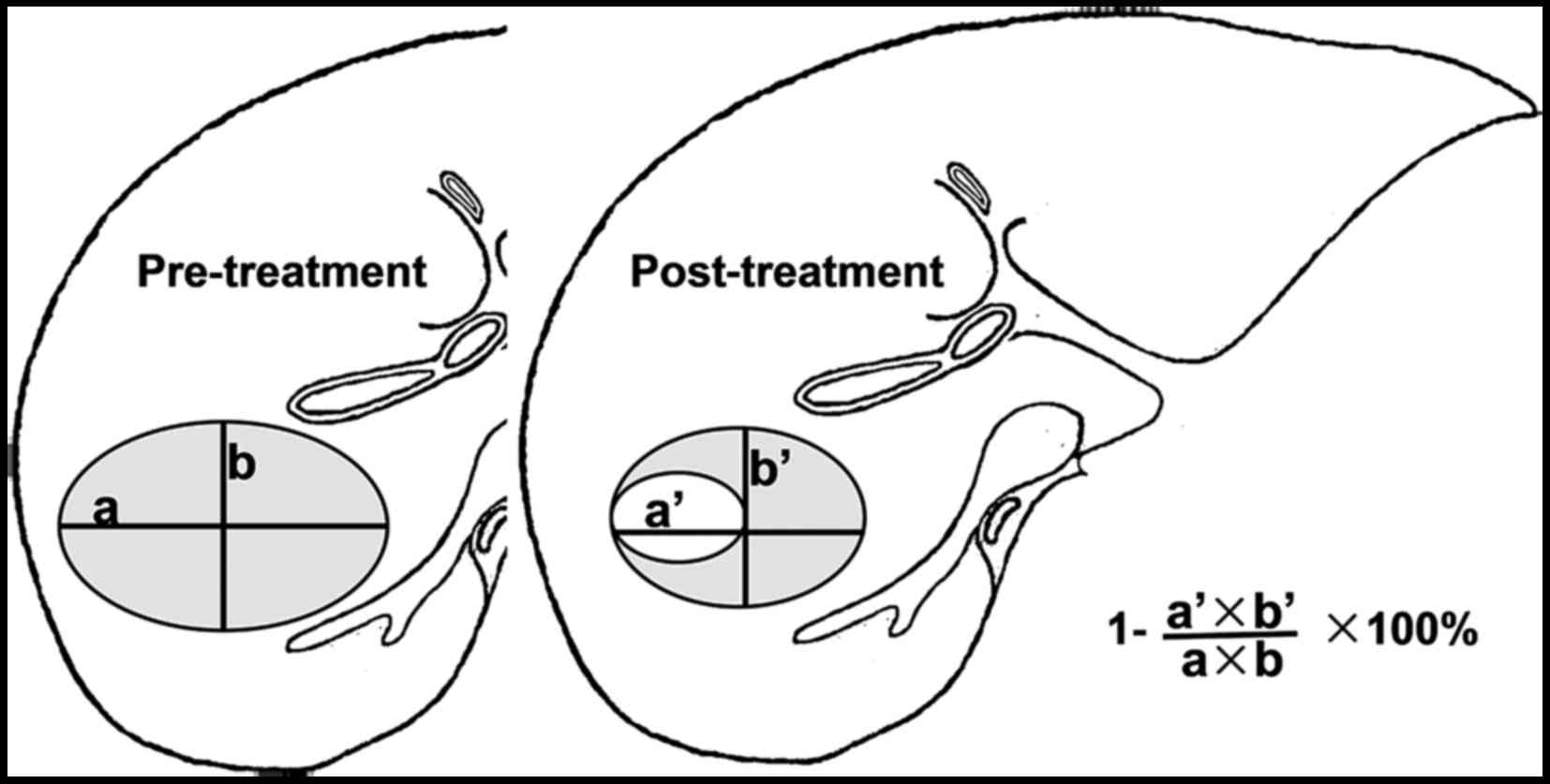
criterion) (10) (Fig. 1). In 2000, the Response Evaluation
Criteria in Solid Tumors 1.0 (RECIST version 1.0) was proposed
(11) and updated (RECIST version
1.1) in 2009 (12) (Table I and Fig. 2), which addressed the shortcoming of
the WHO criterion. The objective the WHO and RECIST response
criteria assess overall tumor burden using morphological tumor-size
measurements on imaging and require assessment at baseline and on
follow-up imaging. Objective response assessment is based on
morphological tumor-size change after therapy and classified into
complete response (CR), partial response (PR), no change (NC) and
progressive disease (PD) (Table
I).
LRTs for HCC induce tumor necrosis and reduced
vascularity. Some LRTs initially lead to even an increase in
apparent tumor size due to extensive necrosis. This is a major
limitation of morphological (size-based) imaging response criteria
for assessing response of HCC to LRTs. Therefore, morphological
(size-based) imaging response criteria for determination of
therapeutic success that rely solely on change in tumor size after
therapy may be inappropriate to HCC treated with LRTs (13). In addition, more objective imaging
response criteria that are specific to the therapy type have been
developed (14–20).
In summary, for the determination of therapeutic
success of HCC to LRTs, morphological (size-based) imaging
measurements using WHO, RECIST 1.0 and RECIST 1.1 criteria may not
be applicable because these therapies result in tumor necrosis
regardless of change in size.
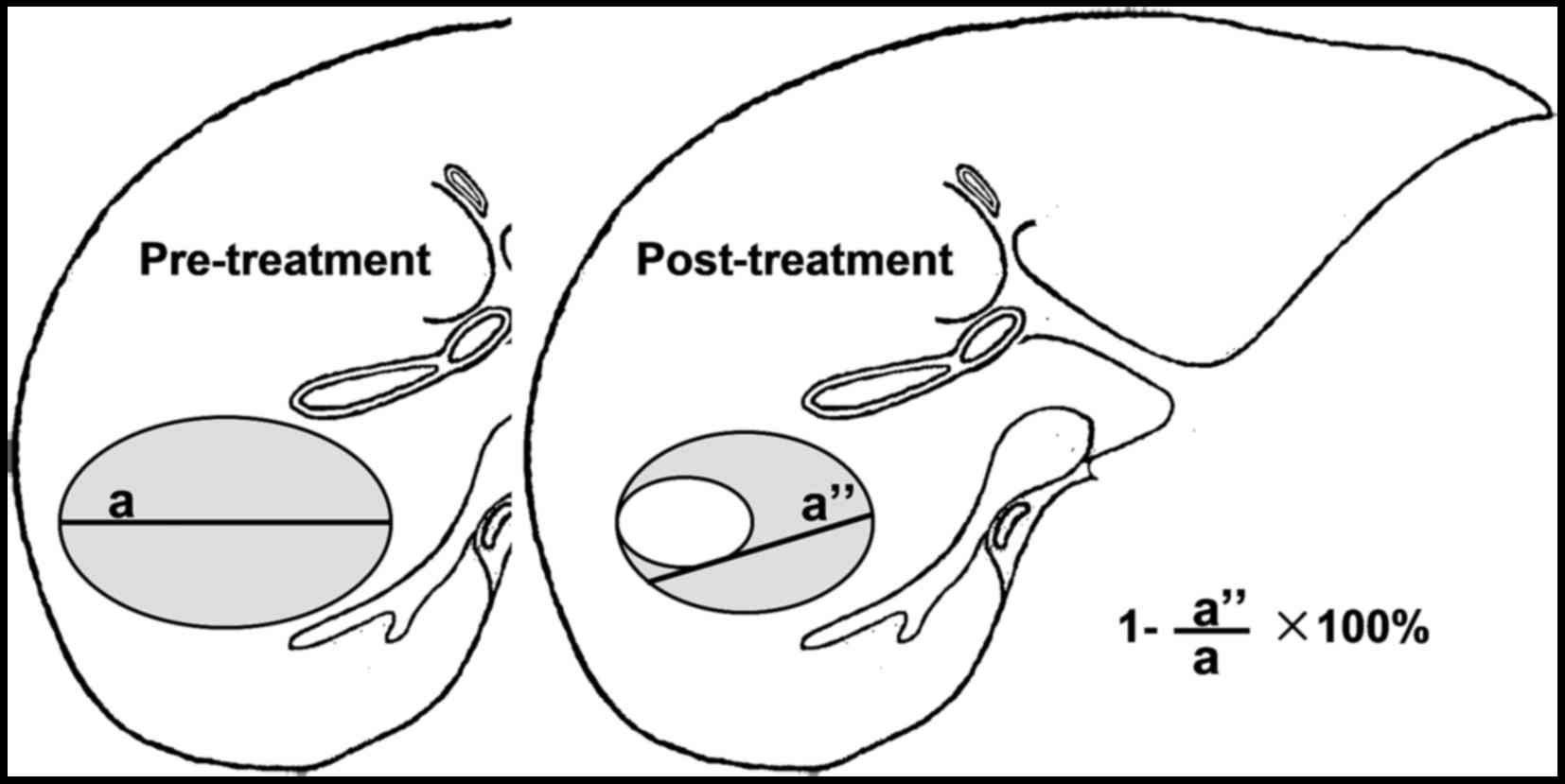
In 2000, The European Association for the Study of
the Liver (EASL) proposed determination of therapeutic success of
HCC to LRTs should measure the size of residual viable tumor rather
than the overall size of the tumor (14) (Table
I and Fig. 3). In 2005, the
American Association for the Study of Liver Diseases (AASLD)
guideline cited this concept (15).
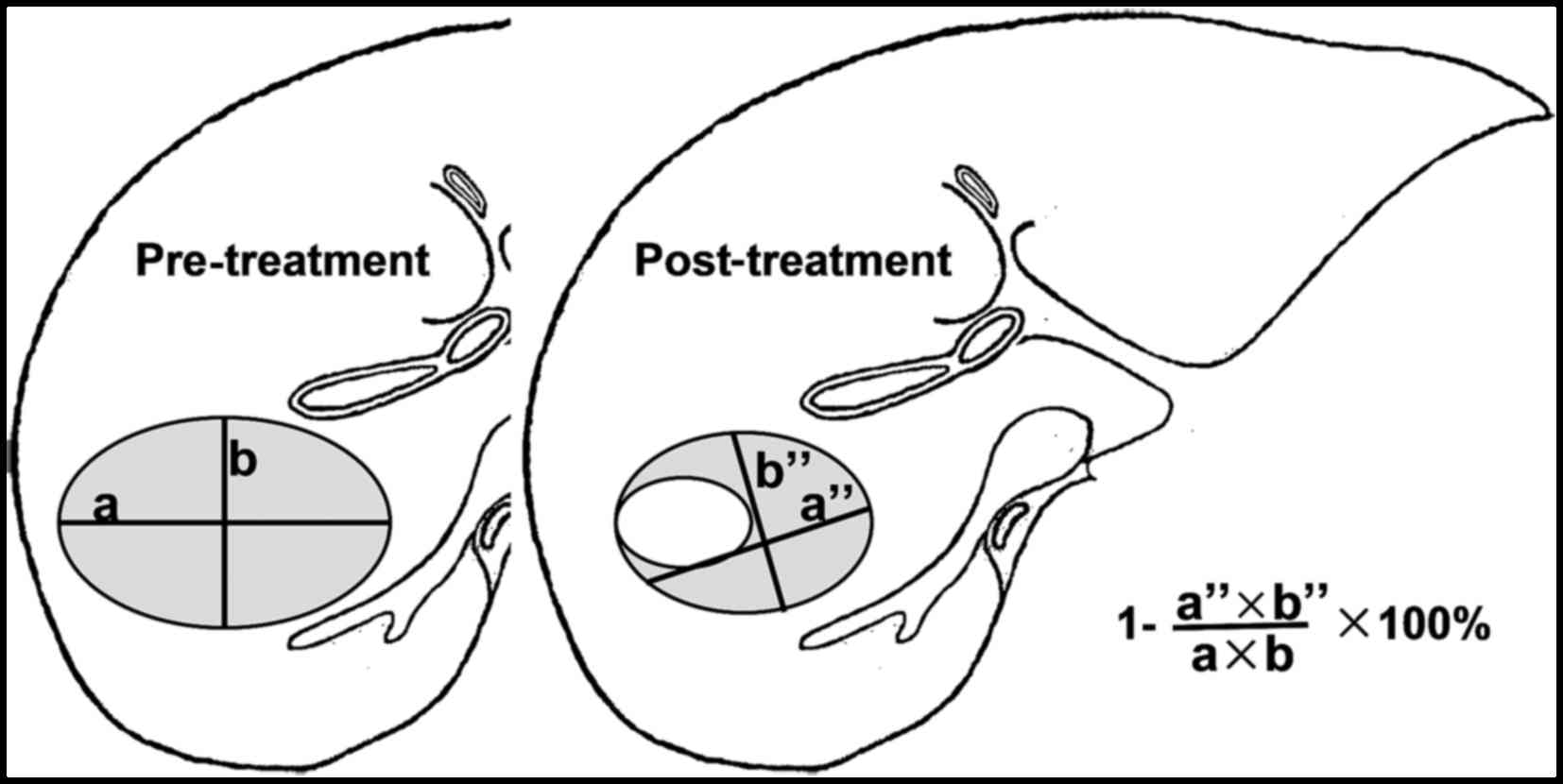
In 2010, the EASL published the modified response evaluation
criteria in solid tumors (mRECIST) criteria for HCC response to
LRTs based on measuring only the viable enhancing areas of the HCC
after LRTs, excluding portion of necrosis. It is as an amendment
and update to the AASLD-JNCI Expert Panel Criteria and are based on
the most recent 2009 RECIST 1.1 and EASL 2000 criteria (16) (Table
I and Fig. 4). The mRECIST
criteria assesses HCC at baseline, after LRTs and overall response.
In the process of clinical application, high quality arterial-phase
enhanced CT/MR imaging is required. There are also other response
criteria for HCC response to LRTs based on enhancement CT/MRI exam.
In 1994, the Liver Cancer Study Group of Japan used a similar
system (RECICL) (17) to assess
direct treatment effect and overall disease status after therapy,
which was updated in 2004, and again in 2009 (18,19).
In 2007, Choi et al (20)
proposed the Choi Response Criteria for HCC response to
antivascular therapy based on tumor density and volume.
Therefore, the EASL and the AASLD-JNCI and mRESIST
criteria suggested assessing response criteria for HCC response to
LRTs based on the size of viable enhancing tumor rather than
overall tumor size including area of necrosis. These criteria have
been shown to correlate well with histopathologic response than WHO
and RECIST criteria. Previous studies of patients with HCC treated
with ablation (21,22), TACE (6–8,21,23–26),
and transarterial radioembolization (TARE) with Y90 (24,25)
have shown that mRECIST and EASL criteria have excellent
intercriterion concordance and are more accurate at predicting
complete histopathologic response (23) and survival after therapy than WHO
and RECIST criteria (25).
First of all, a major shortcoming of
enhancement-based models of imaging response systems for HCC
response to LRTs is that they assess all target and non-target
lesions in the entire liver without taking into consideration that
different tumors in the same patient are not treated at the same
time (27). Thus, the overall
prognosis in such patients is unclearly determined by the behavior
of the treated or untreated tumors. Recent studies have shown one
or two primary target lesion responses to LRTs correlate well with
disease progression and survival in patients with solitary and
multifocal HCC (25,28,29).
Additionally, the EASL and mRECIST criteria do not take into
consideration the change of the overall tumor size after LRTs as an
index of response, because several studies have shown the reduction
in overall tumor size correlates well with long-term response
(25,30). Secondly, for enhancement-based
models of imaging response systems for HCC response to LRTs, any
single enhancement-based imaging technique may not be adequate for
assessment of response to all types of LRTs (30). For example, all enhancement-based
models of imaging response systems (except for the Choi Response
Criteria) do not accurately reflect the result of therapies that
result in decreased enhancement without frank necrosis, such as
transarterial chemoembolization (TACE) (30).
In summary, enhancement-based systems of response to
LRTs assessing change in size of viable enhancing tumor rather than
overall tumor size have been shown to correlate well with
histopathologic response and are more accurate at predicting
survival after therapy than WHO and RECIST size-based models. Any
single enhancement-based system may not be adequate for assessment
of response to all types of LRTs. Of course, the three-dimensional
volumetric tumor measurements could be more accurately identify the
result of HCC response to LRTs, and it is a priority in future
clinical trial research (16).
Almost all enhancement-based models of imaging
response systems (except for the Choi Response Criteria) do not
accurately reflect the result of therapies that result in decreased
enhancement without frank necrosis, such as conventional TACE or
radioembolization (30).
Furthermore, HCC exhibits local alterations in microvascular
anatomy, demonstrating neoangiogenesis, promoted by vascular
endothelial growth factor (VEGF). Antiangiogenic therapies have
demonstrated promising results in HCC. The result of HCC treated
with molecular targeted agents showed that they initially suppress
tumor growth by downregulating angiogenesis and it is not
sufficient to assess treatment response by measurement of tumor
size or the residual viable tumor size (31). Since the therapeutic effects on the
tumor microvascular environment alter tissue perfusion, physiologic
imaging techniques such as dynamic contrast-enhanced ultrasound
(D-CEUS), CT or MR perfusion imaging begins to play a critical role
in the evaluation of therapies that result in decreased enhancement
without necrosis (32,33).
The fundamental principle of perfusion imaging is
based on DCE imaging techniques that compute the temporal changes
in tissue enhancement after intravenous administration of contrast
media. A variety of imaging protocols have been proposed for
perfusion imaging and the protocol selection should be made on the
availability of the scanner technology and the pertinent
physiologic parameter of interest. The computed perfusion
parameters are dependent on the scan protocol and the mathematical
model/software for image processing (34,35).
The commonly described perfusion CT parameters include blood flow
(BF), blood volume (BV), permeability surface area (PS), time to
peak enhancement (TTP) and transfer constant (Ktrans).
Similarly for perfusion MR, transfer constant (Ktrans)
is the most accepted quantitative surrogate end point from
compartment models (36,37).
CEUS and D-CEUS were acknowledged to be a feasible
examination for evaluating dynamic changes in tumor vascularity in
patients with HCC undergoing antiangiogenic target therapy
(38) and chemoembolization
(39). Previously, HCC shows
hyper-vascularised tumor enhancement type. After treatment, it
shows lack of contrast enhancement, whereas still viable tumor
shows arterial-enhancing and subsequent washout (40). CEUS and D-CEUS could be used to
identify the result of HCC response to antiangiogenic therapies,
predict tumor responses and patient survival (38). The times to peak intensity, mean
transit time (MTT) values and area under the curve (AUC) levels,
correlated well with tumor responses and survival rates (32). In addition, AUC, time to peak
intensity and slope of wash-in were positively associated with
progression free survival (PFS) (32). In fact, CEUS and D-CEUS are low cost
and good safety examinations can provide both morphological and
functional data. In addition, its important role in clinical
application has been recently highlighted by EFSUMB guidelines
(41). For example this panel of
experts recognised the important role of CEUS in the very early
evaluation of ablative treatment as a guidance for immediate
retreatment of residual unablated tumor (41). More recently, three-dimensional CEUS
technique (3D CEUS) has been reported to improve the study of tumor
vascularity, thus, allowing the response evaluation of HCC
treatments in the three orthogonal planes. Nevertheless, 3D CEUS
may be limited by the spatial resolution of the current 3D probes
in the assessment of therapeutic response of HCC treated with
ablative treatments compared to conventional CEUS (42). Additionally, the best timing and the
best quantitative dynamic parameters for the assessment HCC
response to LRTs are still unclear.
On perfusion CT, HCC has been reported to show
substantially higher perfusion (high BF, BV and PS with low MTT)
compared to normal liver tissue (43). After antiangiogenic drugs or HCC
directed therapies, decrease in tumor perfusion parameters has been
shown within days of initiation of treatment (43,44).
Similarly, Zhu et al (43)
have shown that HCC nodules showing more substantial reduction in
tumor permeability (Ktrans) on perfusion MR soon after
sunitinib, had better long-term outcome. Liang et al
(45) reported Signal parameters of
DCE-MRI over tumor and liver parenchyma correlated with tumor
response and survival, respectively, in HCC patients receiving
radiotherapy combination with an anti-angiogenic agent.
With CT, relatively high radiation dose and limited
coverage of the anatomy are two major drawbacks of perfusion
technique. Several efforts are being made with low dose scanning
approaches (46). Likewise, there
is no consensus on a scanning protocol or a mathematical model
specific for HCC. Since the liver has a dual arterial and portal
venous perfusion, the scan protocols should ideally include dual
inputs to estimate quantitative perfusion parameters for hepatic
tumors. However, due to larger tumor burden in advanced HCC and
frequent occurrence of angioinvasion into the portal venous system,
single arterial input is often applied as a simplifying assumption
(47). More recently, volume
perfusion CT (VPCT) enables quantification of perfusion in tumor
tissue in absolute values by measuring flow and concentration of
iodinated contrast medium during a time period within blood vessels
and tissue generating time density curves (TDC) (48). This technique is also designed to
calculate separately hepatic arterial and portal venous blood flow
to the liver and liver tumors based on input functions obtained by
regions of interest (ROIs) set in the spleen and the portal vein,
the former representing a substitute for direct hepatic arterial
measurements. VPCT have been used to characterize HCC (49) and monitor the HCC response to TACE
and analysis of TACE-impact on tumor and uninvolved liver
parenchymal perfusion at day one post-TACE (50).
MR imaging has several advantages over CT, including
the lack of ionizing radiation. Therefore, it has the ability to
image whole organs repeatedly and dynamically with high temporal
resolution, and the possibility of repeating the study multiple
times after treatment. A variety of imaging protocols, other than
DCE imaging technique after intravenous gadolinium contrast, have
been proposed for perfusion MR imaging. Transcatheter intraarterial
perfusion (TRIP) MR imaging involves direct catheter-based
intraarterial injection of contrast material (51), which offers a functional alternative
to conventional digital subtraction angiography in the assessment
of tumor perfusion changes during TACE (52). Recently, unenhanced MR perfusion
imaging using the arterial spin labeling (ASL) technique was also
introduced to quantify perfusion in the liver (53). This perfusion method, which does not
require the use of contrast media, is a non-invasive MR perfusion
technique that may offer great potential as an alternative imaging
method for pure liver portal perfusion (53). There are several drawbacks in liver
perfusion MRI technique. General challenges confronting perfusion
MR include lack of accepted standards of image acquisition and
analysis, variable reproducibility and no established response
evaluation criteria. Furthermore, unlike the linear relationship
between iodine concentration and Hounsfield units on CT, the
relationship between gadolinium concentration and signal intensity
(SI) is non-linear with MR imaging, complicating quantitative
perfusion measurements.
In summary, the possibility of evaluating tissue
vascularization through perfusion imaging has led to the
exploration of these imaging techniques as new assessment tools in
order to measure the effectiveness of intraarterial therapy with or
without antiangiogenic therapies for HCC. Different imaging
biomarkers for assessing response to therapy in HCC derive from
different imaging technique and the protocol selection should be
made on the availability of the scanner technology and the
pertinent physiologic parameter of interest. Further studies are
warranted to determine the still unclear aspects such as the best
timing and the best quantitative dynamic parameter for the
assessment of response to HCC treatment.
Diffusion-weighted MR imaging (DWI) has the unique
ability of being able to provide information that reflects tissue
cellularity and cellular membrane integrity (54). Moreover, apparent diffusion
coefficient (ADC) measurement on an ADC map can be quantified by
acquiring images with a different gradient duration and amplitude
(i.e., b-value). DWI and ADC maps reflect the water molecule
diffusion in tissue and can discriminate viable tumor from necrotic
tissue. Viable tumor cells have intact membranes that restrict
water molecules, whereas necrotic tissue shows increased water
molecule diffusion as a result of cell membrane disruption
(55). This makes it an attractive
and useful technique for the assessment of tumor response after
LRTs in patients with HCC.
The visual assessment of DWI, which includes images
at higher b-values (≥500 sec/mm2), may aid to
distinguish the different components of HCC (viable and necrotic
components) following LRTs. As a general observation, necrotic HCC
tissues (liquefaction or coagulation necrosis) secondary to LRTs
typically show lower signal intensity on higher b-value images than
viable tissues. ADC has also been used for early evaluation and
prediction response to LRTs (56–64).
An increase in ADC values has been reported following
radioembolization (56,57) and chemoembolization (58–62) in
the early post-treatment period (a few days up to 2 weeks) with
measurable differences before and after treatment (58–62),
but the treatment effect was noted 1–3 months after treatment.
The role of the pretreatment ADC value in predicting
the response to LRTs have also been investigated with discordant
results (58,65), which may be related to the nature of
tumors with or without necrotic tissue before treatment (66). Recent studies have also shown that
pre-treatment ADC values as well as changes in ADC values after
treatment may provide useful information for predicting survival
for patients with unresectable hepatocellular carcinoma (63,67–69).
Vandecaveye et al (63)
reported that 1-month response determined with apparent diffusion
coefficient is an independent predictor of outcome for HCC treated
with chemoembolization. Several studies have also shown that the
pretreatment ADC values of liver malignancy can be a predictive
factor of tumor response to RFA therapy (70); Mori et al (70) reported that the signal intensity of
HCC on the ADC map was strongly associated with outcome after RFA.
Hypointensity on the ADC map was the strongest independent factor
related to recurrence and survival after RFA, even for small HCC
(70).
In summary, previous studies of DWI in monitoring
HCC response to LRTs, have uniformly reported increasing ADC during
therapy onset proceeding anatomic size changes (71). DWI should be recommended as a
routine method for evaluation of HCC response to LRTs, however, not
in substitution but rather in combination with enhancement (EASL,
mRECIST) criteria (72), because at
this point, this technology is evolving with no accepted protocols
and quantified standards, although the National Cancer Institute
(NCI) has recognized the potential of this technique and has
proposed consensus guidelines for DWI to meet minimum standards for
its use as an effective image biomarker (73). In patients with contraindication to
contrast agents or with slight-enhancement lesions, DWI can be
considered a reasonable alternative to enhancement (EASL, mRECIST)
criteria. However, further technological improvements (i.e.,
intravoxel incoherent motion diffusion-weighted MRI with
bi-exponential diffusion model) and technique standardization are
still required to use DWI at its full potential (74–76).
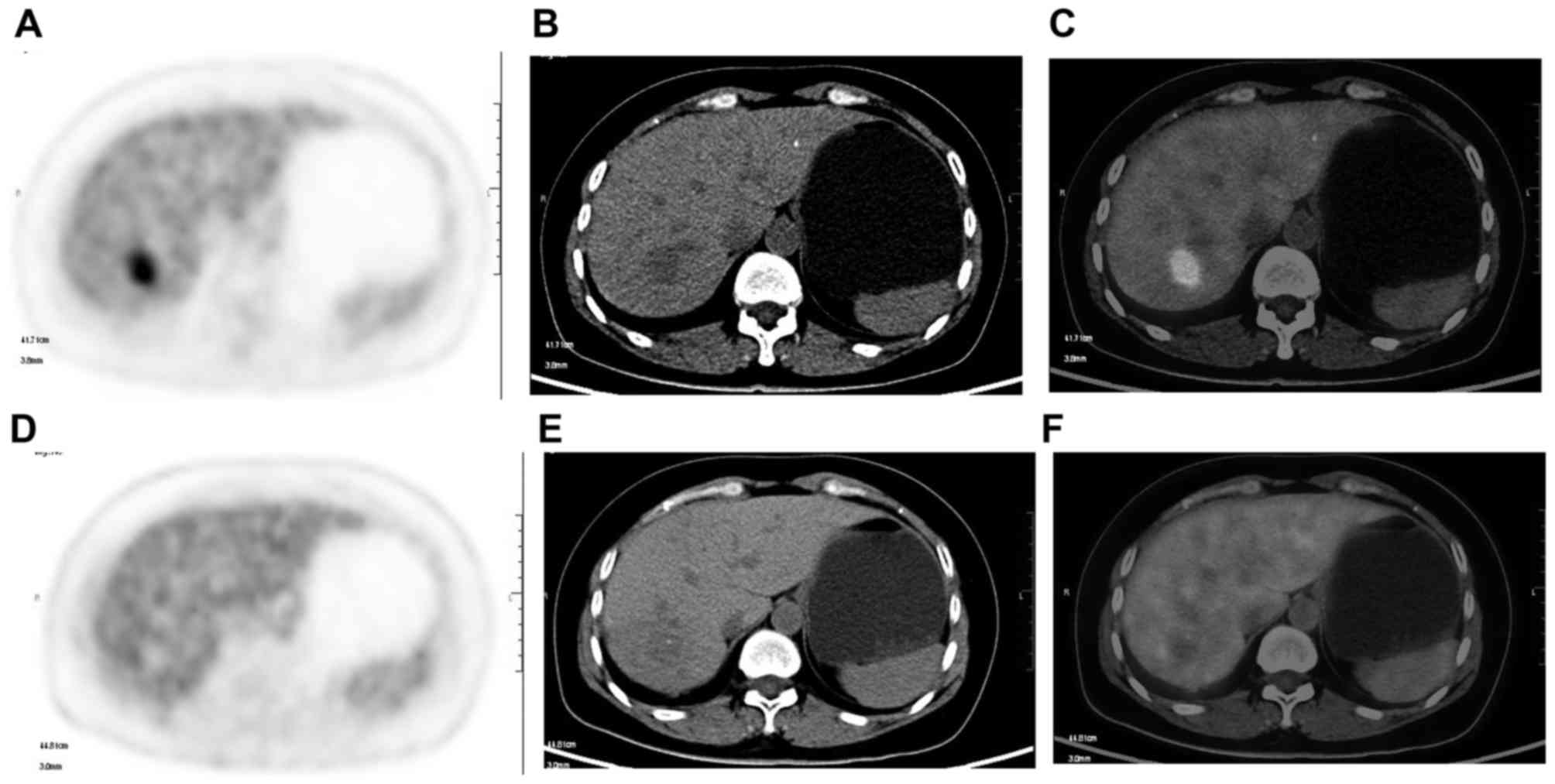
In summary, PET and PET-CT have proven value in the
imaging-based diagnosis of recurrent disease following LRTs of
liver malignancies, and repeat treatment is often initiated solely
based on this imaging modality. 18F-FDG is not a
tumor-specific tracer and the reproducibility of SUV is influenced
by the time of image acquisition from tracer injection. Given the
limitations of FDG in HCC, other tracers with different molecules
including choline-based tracers are being investigated (87).
Integrated PET-MRI is a new imaging modality,
combining the advantages of FDG-PET with the ability of MRI to
detect small liver tumors without CT radiation exposure. Recent
results show that detection sensitivity for hepatic metastases,
through post hoc fusion of FDG-PET images and 1.5 Tesla
contrast-enhanced (Gadolinium) MRI obtained from two different
scanners, is significantly higher than for PET-CT (96). PET-MRI appears to offer higher
lesion conspicuity and diagnostic confidence compared to PET-CT
(97), and this additional
information can influence clinical management of cancer patients.
The combined advantages of detection of smaller hepatic tumors with
a higher sensitivity and detection of focal FDG uptake suggestive
for local tumor progression indicates that PET-MRI could provide
complementary information and facilitate improved clinical decision
making (98). FDG PET-MRI could
potentially improve the accuracy of (early) detection of
progressive disease, and thus, allow swifter and more effective
decision-making regarding appropriate treatment (99).
It is important that early and accurate assessment
of the efficacy of HCC following intra-arterial (i.e.,
chemoembolization and radioembolization) and ablative (i.e.,
radiofrequency ablation and cryoablation) therapies in making
therapeutic decisions, such as whether to repeat, interrupt or
completely terminate therapy. Functional imaging has an important
position in assessing tumor response in locoregional therapy for
HCCs, which induce biologic changes that may be detected by
functional imaging much earlier than morphological imaging. An
ideal imaging biomarker should be able to detect an immediate
response to any therapeutic regimen in one examination. Although
promising, none of these functional imaging biomarkers have gone
through all the required steps of standardization and validation
and established accepted criteria for clinical practice. At present
in clinical practice, different imaging biomarkers for assessing
response to therapy in HCC derive from different imaging technique
and the protocol selection should be made on the availability of
the scanner technology and the pertinent physiologic parameter of
interest. Therefore, it is unlikely to be the sole functional
imaging biomarker of HCC response after LRTs. A combination of
enhancement (EASL, mRECIST) criteria and functional imaging has
been stated to be better in assessing therapeutic response of HCC
after LRTs and in providing more information to guide future
therapy.
This study was supported by the National Natural
Science Foundation of China (No. 81301262, 81571629 and
81301218).
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaghmai V, Besa C, Kim E, Gatlin JL,
Siddiqui NA and Taouli B: Imaging assessment of hepatocellular
carcinoma response to locoregional and systemic therapy. AJR Am J
Roentgenol. 201:80–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz
A, Ryu RK, Baker TB, Ibrahim SM, Abecassis MI, Miller FH, Sato KT,
et al: Chemoembolization for hepatocellular carcinoma:
Comprehensive imaging and survival analysis in a 172-patient
cohort. Radiology. 255:955–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeo DM, Choi JI, Lee YJ, Park MY, Chun HJ
and Lee HG: Comparison of RECIST, mRECIST, and choi criteria for
early response evaluation of hepatocellular carcinoma after
transarterial chemoembolization using drug-eluting beads. J Comput
Assist Tomogr. 38:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gillmore R, Stuart S, Kirkwood A,
Hameeduddin A, Woodward N, Burroughs AK and Meyer T: EASL and
mRECIST responses are independent prognostic factors for survival
in hepatocellular cancer patients treated with transarterial
embolization. J Hepatol. 55:1309–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prajapati HJ, Spivey JR, Hanish SI,
El-Rayes BF, Kauh JS, Chen Z and Kim HS: mRECIST and EASL responses
at early time point by contrast-enhanced dynamic MRI predict
survival in patients with unresectable hepatocellular carcinoma
(HCC) treated by doxorubicin drug-eluting beads transarterial
chemoembolization (DEB TACE). Ann Oncol. 24:965–973. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reig M, Rimola J, Torres F, Darnell A,
Rodriguez-Lope C, Forner A, Llarch N, Ríos J, Ayuso C and Bruix J:
Postprogression survival of patients with advanced hepatocellular
carcinoma: Rationale for second-line trial design. Hepatology.
58:2023–2031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruix J, Sherman M, Llovet JM, Beaugrand
M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M
and Rodés J: EASL Panel of Experts on HCC; European Association for
the Study of the Liver: Clinical management of hepatocellular
carcinoma. Conclusions of the Barcelona-2000 EASL conference. J
Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khalili K, Kim TK, Jang HJ, Yazdi LK,
Guindi M and Sherman M: Indeterminate 1–2-cm nodules found on
hepatocellular carcinoma surveillance: Biopsy for all, some, or
none? Hepatology. 54:2048–2054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kudo M, Kubo S, Takayasu K, Sakamoto M,
Tanaka M, Ikai I, Furuse J, Nakamura K and Makuuchi M: Liver Cancer
Study Group of Japan (Committee for Response Evaluation Criteria in
Cancer of the Liver, Liver Cancer Study Group of Japan): Response
Evaluation Criteria in Cancer of the Liver (RECICL) proposed by the
Liver Cancer Study Group of Japan (2009 Revised Version). Hepatol
Res. 40:686–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo M, Ueshima K, Kubo S, Sakamoto M,
Tanaka M, Ikai I, Furuse J, Murakami T, Kadoya M and Kokudo N:
Liver Cancer Study Group of Japan: Response Evaluation Criteria in
Cancer of the Liver (RECICL) (2015 Revised version). Hepatol Res.
46:3–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi H, Charnsangavej C, Faria SC,
Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA and
Benjamin RS: Correlation of computed tomography and positron
emission tomography in patients with metastatic gastrointestinal
stromal tumor treated at a single institution with imatinib
mesylate: Proposal of new computed tomography response criteria. J
Clin Oncol. 25:1753–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forner A, Ayuso C, Varela M, Rimola J,
Hessheimer AJ, De Lope CR, Reig M, Bianchi L, Llovet JM and Bruix
J: Evaluation of tumor response after locoregional therapies in
hepatocellular carcinoma: Are response evaluation criteria in solid
tumors reliable? Cancer. 115:616–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Guo Z, Si T and Wang H: EASL and
mRECIST responses are independent predictors of survival in
hepatocellular carcinoma patients treated with cryoablation. Eur J
Gastroenterol Hepatol. 25:620–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bargellini I, Vignali C, Cioni R, Petruzzi
P, Cicorelli A, Campani D, De Simone P, Filipponi F and Bartolozzi
C: Hepatocellular carcinoma: CT for tumor response after
transarterial chemoembolization in patients exceeding Milan
criteria - selection parameter for liver transplantation.
Radiology. 255:289–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Memon K, Kulik L, Lewandowski RJ, Wang E,
Riaz A, Ryu RK, Sato KT, Marshall K, Gupta R, Nikolaidis P, et al:
Radiographic response to locoregional therapy in hepatocellular
carcinoma predicts patient survival times. Gastroenterology.
141:526–535.e2.. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riaz A, Miller FH, Kulik LM, Nikolaidis P,
Yaghmai V, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Gupta R, et
al: Imaging response in the primary index lesion and clinical
outcomes following transarterial locoregional therapy for
hepatocellular carcinoma. JAMA. 303:1062–1069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shim JH, Lee HC, Kim SO, Shin YM, Kim KM,
Lim YS and Suh DJ: Which response criteria best help predict
survival of patients with hepatocellular carcinoma following
chemoembolization? A validation study of old and new models.
Radiology. 262:708–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salem R, Miller FH, Yaghmai V and
Lewandowski RJ: Response assessment methodologies in hepatocellular
carcinoma: Complexities in the era of local and systemic
treatments. J Hepatol. 58:1260–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim BK, Kim SU, Kim MJ, Kim KA, Kim DY,
Park JY, Ahn SH, Han KH and Chon CY: Number of target lesions for
EASL and modified RECIST to predict survivals in hepatocellular
carcinoma treated with chemoembolization. Clin Cancer Res.
19:1503–1511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shim JH, Lee HC, Won HJ, Shin YM, Kim KM,
Lim YS and Suh DJ: Maximum number of target lesions required to
measure responses to transarterial chemoembolization using the
enhancement criteria in patients with intrahepatic hepatocellular
carcinoma. J Hepatol. 56:406–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riaz A, Memon K, Miller FH, Nikolaidis P,
Kulik LM, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Mulcahy MF, et
al: Role of the EASL, RECIST, and WHO response guidelines alone or
in combination for hepatocellular carcinoma: Radiologic-pathologic
correlation. J Hepatol. 54:695–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwazawa J, Ohue S, Hashimoto N, Yasumasa
K, Abe H and Mitani T: Bevacizumab-induced hypovascular
hepatocellular carcinoma treated by transarterial chemoembolization
in a patient with metastatic colon cancer. J Vasc Interv Radiol.
21:412–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zocco MA, Garcovich M, Lupascu A, Di
Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME,
Ponziani F, Caracciolo G, et al: Early prediction of response to
sorafenib in patients with advanced hepatocellular carcinoma: The
role of dynamic contrast enhanced ultrasound. J Hepatol.
59:1014–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roccarina D, Garcovich M, Ainora ME,
Riccardi L, Pompili M, Gasbarrini A and Zocco MA: Usefulness of
contrast enhanced ultrasound in monitoring therapeutic response
after hepatocellular carcinoma treatment. World J Hepatol.
7:1866–1874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miles KA, Hayball MP and Dixon AK:
Functional images of hepatic perfusion obtained with dynamic CT.
Radiology. 188:405–411. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kambadakone AR and Sahani DV: Body
perfusion CT: Technique, clinical applications, and advances.
Radiol Clin North Am. 47:161–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morgan B, Thomas AL, Drevs J, Hennig J,
Buchert M, Jivan A, Horsfield MA, Mross K, Ball HA, Lee L, et al:
Dynamic contrast-enhanced magnetic resonance imaging as a biomarker
for the pharmacological response of PTK787/ZK 222584, an inhibitor
of the vascular endothelial growth factor receptor tyrosine
kinases, in patients with advanced colorectal cancer and liver
metastases: Results from two phase I studies. J Clin Oncol.
21:3955–3964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Laarhoven HW, Rijpkema M, Punt CJ,
Ruers TJ, Hendriks JC, Barentsz JO and Heerschap A: Method for
quantitation of dynamic MRI contrast agent uptake in colorectal
liver metastases. J Magn Reson Imaging. 18:315–320. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lassau N, Koscielny S, Chami L, Chebil M,
Benatsou B, Roche A, Ducreux M, Malka D and Boige V: Advanced
hepatocellular carcinoma: Early evaluation of response to
bevacizumab therapy at dynamic contrast-enhanced US with
quantification-preliminary results. Radiology. 258:291–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shaw CM, Eisenbrey JR, Lyshchik A, OKane
PL, Merton DA, Machado P, Pino L, Brown DB and Forsberg F:
Contrast-enhanced ultrasound evaluation of residual blood flow to
hepatocellular carcinoma after treatment with transarterial
chemoembolization using drug-eluting beads: A prospective study. J
Ultrasound Med. 34:859–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marcus CD, Ladam-Marcus V, Cucu C, Bouché
O, Lucas L and Hoeffel C: Imaging techniques to evaluate the
response to treatment in oncology: Current standards and
perspectives. Crit Rev Oncol Hematol. 72:217–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Claudon M, Dietrich CF, Choi BI, Cosgrove
DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC,
et al: World Federation for Ultrasound in Medicine; European
Federation of Societies for Ultrasound: Guidelines and good
clinical practice recommendations for Contrast Enhanced Ultrasound
(CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in
cooperation with representatives of AFSUMB AIUM, ASUM, FLAUS and
ICUS. Ultrasound Med Biol. 39:187–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bartolotta TV, Taibbi A, Matranga D,
Midiri M and Lagalla R: 3D versus 2D contrast-enhanced sonography
in the evaluation of therapeutic response of hepatocellular
carcinoma after locoregional therapies: Preliminary findings.
Radiol Med (Torino). 120:695–704. 2015. View Article : Google Scholar
|
|
43
|
Zhu AX, Holalkere NS, Muzikansky A, Horgan
K and Sahani DV: Early antiangiogenic activity of bevacizumab
evaluated by computed tomography perfusion scan in patients with
advanced hepatocellular carcinoma. Oncologist. 13:120–125. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang PC, Chang HJ, Hsu C, Chen LT, Shih
TT and Liu TW: Perfusion parameters of dynamic contrast-enhanced
magnetic resonance imaging predict outcomes of hepatocellular
carcinoma receiving radiotherapy with or without thalidomide.
Hepatol Int. 9:258–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Griffiths JR, Tate AR, Howe FA and Stubbs
M: Group on MRS Application to Cancer: Magnetic Resonance
Spectroscopy of cancer-practicalities of multi-centre trials and
early results in non-Hodgkins lymphoma. Eur J Cancer. 38:2085–2093.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schwarz AJ, Maisey NR, Collins DJ,
Cunningham D, Huddart R and Leach MO: Early in vivo detection of
metabolic response: A pilot study of 1H MR spectroscopy in
extracranial lymphoma and germ cell tumours. Br J Radiol.
75:959–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bota S, Piscaglia F, Marinelli S,
Pecorelli A, Terzi E and Bolondi L: Comparison of international
guidelines for noninvasive diagnosis of hepatocellular carcinoma.
Liver Cancer. 1:190–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaufmann S, Horger T, Oelker A, Kloth C,
Nikolaou K, Schulze M and Horger M: Characterization of
hepatocellular carcinoma (HCC) lesions using a novel CT-based
volume perfusion (VPCT) technique. Eur J Radiol. 84:1029–1035.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kaufmann S, Horger T, Oelker A, Beck S,
Schulze M, Nikolaou K, Ketelsen D and Horger M: Volume perfusion
computed tomography (VPCT)-based evaluation of response to TACE
using two different sized drug eluting beads in patients with
nonresectable hepatocellular carcinoma: Impact on tumor and liver
parenchymal vascularisation. Eur J Radiol. 84:2548–2554. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang D, Bangash AK, Rhee TK, Woloschak GE,
Paunesku T, Salem R, Omary RA and Larson AC: Liver tumors:
Monitoring embolization in rabbits with VX2 tumors - transcatheter
intraarterial first-pass perfusion MR imaging. Radiology.
245:130–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Larson AC, Wang D, Atassi B, Sato KT, Ryu
RK, Lewandowski RJ, Nemcek AA Jr, Mulcahy MF, Kulik LM, Miller FH,
et al: Transcatheter intraarterial perfusion: MR monitoring of
chemoembolization for hepatocellular carcinoma - feasibility of
initial clinical translation. Radiology. 246:964–971. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Katada Y, Shukuya T, Kawashima M, Nozaki
M, Imai H, Natori T and Tamano M: A comparative study between
arterial spin labeling and CT perfusion methods on hepatic portal
venous flow. Jpn J Radiol. 30:863–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koh DM and Collins DJ: Diffusion-weighted
MRI in the body: Applications and challenges in oncology. AJR Am J
Roentgenol. 188:1622–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Youn BJ, Chung JW, Son KR, Kim HC, Jae HJ,
Lee JM, Song IC, Kim IO and Park JH: Diffusion-weighted MR:
Therapeutic evaluation after chemoembolization of VX-2 carcinoma
implanted in rabbit liver. Acad Radiol. 15:593–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng J, Miller FH, Rhee TK, Sato KT,
Mulcahy MF, Kulik LM, Salem R, Omary RA and Larson AC:
Diffusion-weighted MR imaging for determination of hepatocellular
carcinoma response to yttrium-90 radioembolization. J Vasc Interv
Radiol. 17:1195–1200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kamel IR, Reyes DK, Liapi E, Bluemke DA
and Geschwind JF: Functional MR imaging assessment of tumor
response after 90Y microsphere treatment in patients with
unresectable hepatocellular carcinoma. J Vasc Interv Radiol.
18:49–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yuan Z, Ye XD, Dong S, Xu LC, Xu XY, Liu
SY and Xiao XS: Role of magnetic resonance diffusion-weighted
imaging in evaluating response after chemoembolization of
hepatocellular carcinoma. Eur J Radiol. 75:e9–e14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chung JC, Naik NK, Lewandowski RJ, Deng J,
Mulcahy MF, Kulik LM, Sato KT, Ryu RK, Salem R, Larson AC, et al:
Diffusion-weighted magnetic resonance imaging to predict response
of hepatocellular carcinoma to chemoembolization. World J
Gastroenterol. 16:3161–3167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kubota K, Yamanishi T, Itoh S, Murata Y,
Miyatake K, Yasunami H, Morio K, Hamada N, Nishioka A and Ogawa Y:
Role of diffusion-weighted imaging in evaluating therapeutic
efficacy after transcatheter arterial chemoembolization for
hepatocellular carcinoma. Oncol Rep. 24:727–732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bonekamp S, Jolepalem P, Lazo M, Gulsun
MA, Kiraly AP and Kamel IR: Hepatocellular carcinoma: Response to
TACE assessed with semiautomated volumetric and functional analysis
of diffusion-weighted and contrast-enhanced MR imaging data.
Radiology. 260:752–761. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sahin H, Harman M, Cinar C, Bozkaya H,
Parildar M and Elmas N: Evaluation of treatment response of
chemoembolization in hepatocellular carcinoma with
diffusion-weighted imaging on 3.0-T MR imaging. J Vasc Interv
Radiol. 23:241–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vandecaveye V, Michielsen K, De Keyzer F,
Laleman W, Komuta M, Op De beeck K, Roskams T, Nevens F, Verslype C
and Maleux G: Chemoembolization for hepatocellular carcinoma:
1-month response determined with apparent diffusion coefficient is
an independent predictor of outcome. Radiology. 270:747–757. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vouche M, Salem R, Lewandowski RJ and
Miller FH: Can volumetric ADC measurement help predict response to
Y90 radioembolization in HCC? Abdom Imaging. 40:1471–1480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mannelli L, Kim S, Hajdu CH, Babb JS and
Taouli B: Serial diffusion-weighted MRI in patients with
hepatocellular carcinoma: Prediction and assessment of response to
transarterial chemoembolization. Preliminary experience. Eur J
Radiol. 82:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yuan Z, Li WT and Peng WJ: Pre-treatment
apparent diffusion coefficient is imaging biomarker for prediction
of response to chemoembolization in hepatocellular carcinoma. Eur J
Radiol. 82:e901–e902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dong S, Ye XD, Yuan Z, Xu LC and Xiao XS:
Relationship of apparent diffusion coefficient to survival for
patients with unresectable primary hepatocellular carcinoma after
chemoembolization. Eur J Radiol. 81:472–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Corona-Villalobos CP, Halappa VG, Bonekamp
S, Eng J, Reyes D, Cosgrove D, Rastegar N, Pan L, Pawlik TM and
Kamel IR: Functional magnetic resonance imaging response of
targeted tumor burden and its impact on survival in patients with
hepatocellular carcinoma. Invest Radiol. 50:283–289. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ye XD, Li WT and Yuan Z: Apparent
diffusion coefficients at diffusion-weighted MR imaging: Potential
predictors of survival in patients with hepatocellular carcinoma
treated with chemoembolization. Radiology. 272:920–921. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mori Y, Tamai H, Shingaki N, Moribata K,
Deguchi H, Ueda K, Inoue I, Maekita T, Iguchi M, Kato J, et al:
Signal intensity of small hepatocellular carcinoma on apparent
diffusion coefficient mapping and outcome after radiofrequency
ablation. Hepatol Res. 45:75–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ye XD, Li WT and Yuan Z: Is volumetric
functional MR imaging superior to current anatomic imaging response
criteria for hepatocellular carcinoma after intraarterial therapy?
Radiology. 271:619–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang Y, Zhao J, Guo D, Zhong W and Ran L:
Evaluation of short-term response of high intensity focused
ultrasound ablation for primary hepatic carcinoma: Utility of
contrast-enhanced MRI and diffusion-weighted imaging. Eur J Radiol.
79:347–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Padhani AR, Liu G, Koh DM, Chenevert TL,
Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M,
Collins D, et al: Diffusion-weighted magnetic resonance imaging as
a cancer biomarker: Consensus and recommendations. Neoplasia.
11:102–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Park YS, Lee CH, Kim JH, Kim IS, Kiefer B,
Seo TS, Kim KA and Park CM: Using intravoxel incoherent motion
(IVIM) MR imaging to predict lipiodol uptake in patients with
hepatocellular carcinoma following transcatheter arterial
chemoembolization: A preliminary result. Magn Reson Imaging.
32:638–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Koh DM: Science to practice: Can
intravoxel incoherent motion diffusion-weighted MR imaging be used
to assess tumor response to antivascular drugs? Radiology.
272:307–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yuan Z, Zhang J, Yang H, Ye XD, Xu LC and
Li WT: Diffusion-weighted MR imaging of hepatocellular carcinoma:
Current value in clinical evaluation of tumor response to
locoregional treatment. J Vasc Interv Radiol. 27:20–30. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hiraoka A, Hirooka M, Ochi H, Koizumi Y,
Shimizu Y, Shiraishi A, Yamago H, Tanihira T, Miyata H, Ninomiya T,
et al: Importance of screening for synchronous malignant neoplasms
in patients with hepatocellular carcinoma: Impact of FDG PET/CT.
Liver Int. 33:1085–1091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shiomi S and Kawabe J: Clinical
applications of positron emission tomography in hepatic tumors.
Hepatol Res. 41:611–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lee JW, Yun M, Cho A, Han KH, Kim DY, Lee
SM and Lee JD: The predictive value of metabolic tumor volume on
FDG PET/CT for transarterial chemoembolization and transarterial
chemotherapy infusion in hepatocellular carcinoma patients without
extrahepatic metastasis. Ann Nucl Med. 29:400–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lee JW, Oh JK, Chung YA, Na SJ, Hyun SH,
Hong IK, Eo JS, Song BI, Kim TS, Kim do Y, et al: Prognostic
significance of 18F-FDG uptake in hepatocellular carcinoma treated
with transarterial chemoembolization or concurrent
chemoradiotherapy: A multicenter retrospective cohort study. J Nucl
Med. 57:509–516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hartenbach M, Weber S, Albert NL,
Hartenbach S, Hirtl A, Zacherl MJ, Paprottka PM, Tiling R,
Bartenstein P, Hacker M, et al: Evaluating treatment response of
radioembolization in intermediate-stage hepatocellular carcinoma
patients using 18F-Fluoroethylcholine PET/CT. J Nucl Med.
56:1661–1666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jo IY, Son SH, Kim M, Sung SY, Won YK,
Kang HJ, Lee SJ, Chung YA, Oh JK and Kay CS: Prognostic value of
pretreatment 18F-FDG PET-CT in radiotherapy for patients with
hepatocellular carcinoma. Radiat Oncol J. 33:179–187. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cho E, Jun CH, Kim BS, Son DJ, Choi WS and
Choi SK: 18F-FDG PET CT as a prognostic factor in hepatocellular
carcinoma. Turk J Gastroenterol. 26:344–350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ma W, Jia J, Wang S, Bai W, Yi J, Bai M,
Quan Z, Yin Z, Fan D, Wang J, et al: The prognostic value of
18F-FDG PET/CT for hepatocellular carcinoma treated with
transarterial chemoembolization (TACE). Theranostics. 4:736–744.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Young H, Baum R, Cremerius U, Herholz K,
Hoekstra O, Lammertsma AA, Pruim J and Price P: European
Organization for Research and Treatment of Cancer (EORTC) PET Study
Group: Measurement of clinical and subclinical tumour response
using [18F]-fluorodeoxyglucose and positron emission tomography:
Review and 1999 EORTC recommendations. Eur J Cancer. 35:1773–1782.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving Considerations for PET response
criteria in solid tumors. J Nucl Med. 50 Suppl 1:122S–150S. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kuang Y, Salem N, Tian H, Kolthammer JA,
Corn DJ, Wu C, Wang F, Wang Y and Lee Z: Imaging lipid synthesis in
hepatocellular carcinoma with [methyl-11c]choline: Correlation with
in vivo metabolic studies. J Nucl Med. 52:98–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao
JC, Hsu JS and Liu GC: Early response of hepatocellular carcinoma
to transcatheter arterial chemoembolization: Choline levels and MR
diffusion constants - initial experience. Radiology. 239:448–456.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kuo YT, Li CW, Chen CY, Jao J, Wu DK and
Liu GC: In vivo proton magnetic resonance spectroscopy of large
focal hepatic lesions and metabolite change of hepatocellular
carcinoma before and after transcatheter arterial chemoembolization
using 3.0-T MR scanner. J Magn Reson Imaging. 19:598–604. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu B, Peng WJ, Wang PJ, Gu YJ, Li WT, Zhou
LP, Tang F and Zhong GM: In vivo 1H magnetic resonance spectroscopy
in evaluation of hepatocellular carcinoma and its early response to
transcatheter arterial chemoembolization. Chin Med Sci J.
21:258–264. 2006.PubMed/NCBI
|
|
91
|
Bian DJ, Xiao EH, Hu DX, Chen XY, Situ WJ,
Yuan SW, Sun JL and Yang LP: Magnetic resonance spectroscopy on
hepatocellular carcinoma after transcatheter arterial
chemoembolization. Chin J Cancer. 29:198–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Schilling A, Gewiese B, Berger G,
Boese-Landgraf J, Fobbe F, Stiller D, Gallkowski U and Wolf KJ:
Liver tumors: Follow-up with P-31 MR spectroscopy after local
chemotherapy and chemoembolization. Radiology. 182:887–890. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Meyerhoff DJ, Karczmar GS, Valone F,
Venook A, Matson GB and Weiner MW: Hepatic cancers and their
response to chemoembolization therapy. Quantitative image-guided
31P magnetic resonance spectroscopy. Invest Radiol. 27:456–464.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yuan Z, Ye XD, Dong S, Xu LC and Xiao XS:
Evaluation of early imaging response after chemoembolization of
hepatocellular carcinoma by phosphorus-31 magnetic resonance
spectroscopy-initial experience. J Vasc Interv Radiol.
22:1166–1173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yuan Z, Li WT, Ye XD, Zhu HY and Peng WJ:
Novel functional magnetic resonance imaging biomarkers for
assessing response to therapy in hepatocellular carcinoma. Clin
Transl Oncol. 16:599–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Donati OF, Hany TF, Reiner CS, von
Schulthess GK, Marincek B, Seifert B and Weishaupt D: Value of
retrospective fusion of PET and MR images in detection of hepatic
metastases: Comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced
MRI. J Nucl Med. 51:692–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beiderwellen K, Gomez B, Buchbender C,
Hartung V, Poeppel TD, Nensa F, Kuehl H, Bockisch A and Lauenstein
TC: Depiction and characterization of liver lesions in whole body
[18F]-FDG PET/MRI. Eur J Radiol. 82:e669–e675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Catalano OA, Rosen BR, Sahani DV, Hahn PF,
Guimaraes AR, Vangel MG, Nicolai E, Soricelli A and Salvatore M:
Clinical impact of PET/MR imaging in patients with cancer
undergoing same-day PET/CT: Initial experience in 134 patients: a
hypothesis-generating exploratory study. Radiology. 269:857–869.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Nielsen K, Scheffer HJ, Pieters IC, van
Tilborg AA, van Waesberghe JH, Oprea-Lager DE, Meijerink MR,
Kazemier G, Hoekstra OS, Schreurs HW, et al: The use of PET-MRI in
the follow-up after radiofrequency- and microwave ablation of
colorectal liver metastases. BMC Med Imaging. 14:272014. View Article : Google Scholar : PubMed/NCBI
|