Introduction
As the most common renal parenchymal tumor, renal
cell carcinoma (RCC) accounts for 2% of all adult malignant tumors
(1). It is ranked third as the most
common urinary tumor, following prostatic cancer and carcinoma of
the urinary bladder. Its annual incidence rate and mortality rate
are 271,000 and 116,000, respectively (2). In addition, the incidence rate shows a
persistent increasing tendency. Due to differences in diagnosis,
the reported incidence rates of RCC in Europe and America are
higher compared with those in Asia and Africa at present (3,4). In
addition, the RCC morbidity of males is higher than that of
females. Specifically, the incidence and mortality rates of males
are 167,000 and 72,000, respectively, while those of female are
103,000 and 44,000, respectively (5).
Research in recent years has shown that the general
biological characteristic of tumors is rampant growth. The main
molecular mechanisms present extreme cell proliferation with too
little apoptosis. Faithful cell replication is to be ensured and
controlled by restriction points (6). The overexpression of cyclin D1 and E
can promote the G/S limiting point of cells and cause excessive
cell proliferation; moreover, cell cycle confusion is a key link of
the genome (7). It may further
increase the probability of carcinogenesis of labile cells, and
thereby participate in tumorigenesis and development. At present,
the relationship between tumors and the cell cycle is one of the
main topics concerning tumorigenesis and development as well as
gene therapy (8).
A large number of studies have demonstrated that p53
can transcribe the levels of numerous targeted genes and microRNAs
(miRNAs) under induction of stress. Being able to damage DNA by
stress, p53 mainly blocks the cell cycle and/or introduces cell
apoptosis as a tumor-suppressor gene. It causes not only
accumulation of DNA damage, but also cancer. For more than 50% of
human types of cancers, p53 is mutated. Therefore, p53 in cells is
recognized as a classic Knudson-type tumor inhibitor.
Actually, p21 can induce cells to convert to S and
G2 phases (9). However, transient
cell cycle arrest cannot eradicate tumors. In fact, low-dose mutant
cell stress, or a low amount of cell mutation, may lead to
monitoring point of cell cycle arrest and cytothesis (9). After the checking point of cytothesis,
cells undergo cell cycle. That is to say, the cells maintain
continuous growth and proliferation (9,10).
First detected in 1993, miRNAs are uncoded single
small molecules containing 17–25 nucleotides (nts), which
horizontally adjust genetic expression after transcription. Studies
have shown that ~30% of human genes are regulated by miRNAs. They
play a vital role in cellular differentiation, growth,
proliferation, programmed cell death (apoptosis) and metabolism.
Given this, they are also closely related to tumors. They can
regulate the development and metastasis of tumors as oncogenes or
cancer-suppressor genes. Among them, miRNA-21 (miR-21) has been
given much attention, and has been reported in much research
concerning tumors. Nevertheless, to the best of our knowledge, the
underlying mechanisms of miR-21 in relation to advanced
clinicopathological features and poor prognosis in patients with
RCC have not been previously studied. On this basis, we explored
whether upregulation of miR-21 expression predicates advanced
clinicopathological features and poor prognosis in patients with
RCC and the possible mechanism.
Materials and methods
Samples and cases
Sixty-eight patients (RCC and normal adjacent
tissues) were collected from July 2014 to December 2014 at West
China Hospital, Sichuan University. Blood and tissue samples from
patients were collected and saved at −80°C.
Quantitative real-time PCR
Total RNA was extracted from blood, tissue samples
or cells using an RNeasy Mini kit from Qiagen (Valencia, CA, USA).
cDNA was prepared from total RNA (1 µg) using a reverse
transcription system (Promega, Madison, WI, USA). 7500 Fast
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was
used to execute and analyze the gene expression of miR-21. Thermal
cycling conditions consisted of 95°C for 40 sec, 40 cycles of 95°C
for 30 sec and 60°C for 30 sec according to the TaqMan Fast
Universal PCR protocol.
Cell lines and cell culture
Human kidney cell line HRPTEpiC and RCC cell line
A-498 were cultured with RPMI-1640 medium (Invitrogen, Carlsbad,
CA, USA) containing 10% heat-inactivated fetal bovine serum (FBS;
Biological Industries, Kibbutz Beit Haemek, Israel), 50 mg/ml
penicillin and 50 mg/ml streptomycin (Invitrogen) in an incubator
with a humidified atmosphere of 95% air and 5% CO2 at
37°C using a microplate reader (ELX800; GE, USA).
Cell transfection
A-498 cells were transiently transfected with
miR-21, anti-miR-21 or anti-miR negative control using
Lipofectamine 2000 (Invitrogen). The cells were pelleted after 24 h
of transfection for cell proliferation, apoptosis, RNA and protein
extraction.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
A-498 cells transfected with miRNAs were seeded on
96-well plates, and collected and centrifuged at 700 × g for 1 min.
Every well was administered 20 µl MTT (5 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) and incubated for 4 h in an incubator with a
humidified atmosphere of 95% air and 5% CO2 at 37°C.
Dimethyl sulfoxide (DMSO) (150 µl) was added to incubate for 10 min
at room temperature while being shaken. For assessment of cell
proliferation the optical density was read at 570 nm using a
microplate reader (ELX800).
Apoptosis assay
A-498 cells transfected with miRNAs were seeded on
6-well plates, and collected and centrifuged at 700 × g for 1 min.
A-498 cells were stained with 5 µl Annexin V-Alexa and propidium
iodide (PI) for 15 min at room temperature in the dark. The
apoptosis rate of the A-498 cells was analyzed by FACScan flow
cytometry using CellQuest 3.3 analysis software (Becton-Dickinson,
San Jose, CA, USA).
Caspase-3 activity
A-498 cells transfected with miRNAs were seeded on
6-well plates, and collected and centrifuged at 700 × g for 1 min.
A-498 cells were washed once in phosphate-buffered saline (PBS) and
lysed in RIPA lysis and extraction buffer (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). The protein concentration was
determined using a BCA protein assay kit (Beyotime, Jiangsu,
China). Equal sample volumes were incubated with 1 M DTT and the
labeled caspase-3 substrate DEVD-p-nitroanilide (DEVD-pNA) for 4 h
at 37°C. Caspase-3 activity was quantified by measuring the
absorbance at 405 nm using a microplate reader (ELX800).
Western blot analysis
A-498 cells transfected with miRNAs were seeded on
6-well plates, and collected and centrifuged at 700 × g for 1 min.
A-498 cells were washed once in PBS and lysed in RIPA lysis and
extraction buffer. The protein concentration was determined using a
BCA protein assay kit. Equal sample volumes were loaded onto 8–10%
polyacrylamide-SDS gel and were then transferred to an Immobilon-P
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA).
The membrane was incubated with blocking buffer and then incubated
overnight at 4°C with anti-p53, anti-CDKN1A p21, anti-cyclin E2,
anti-Bax and anti-β-actin (1:4,000; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) followed by incubation with the corresponding
secondary antibodies.
Statistical analysis
To analyze baseline characteristics, the continuous
variables are presented as mean ± SD. Student's t-test was used to
compare the different groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-12 expression and adjacent
non-neoplastic and RCC tissues
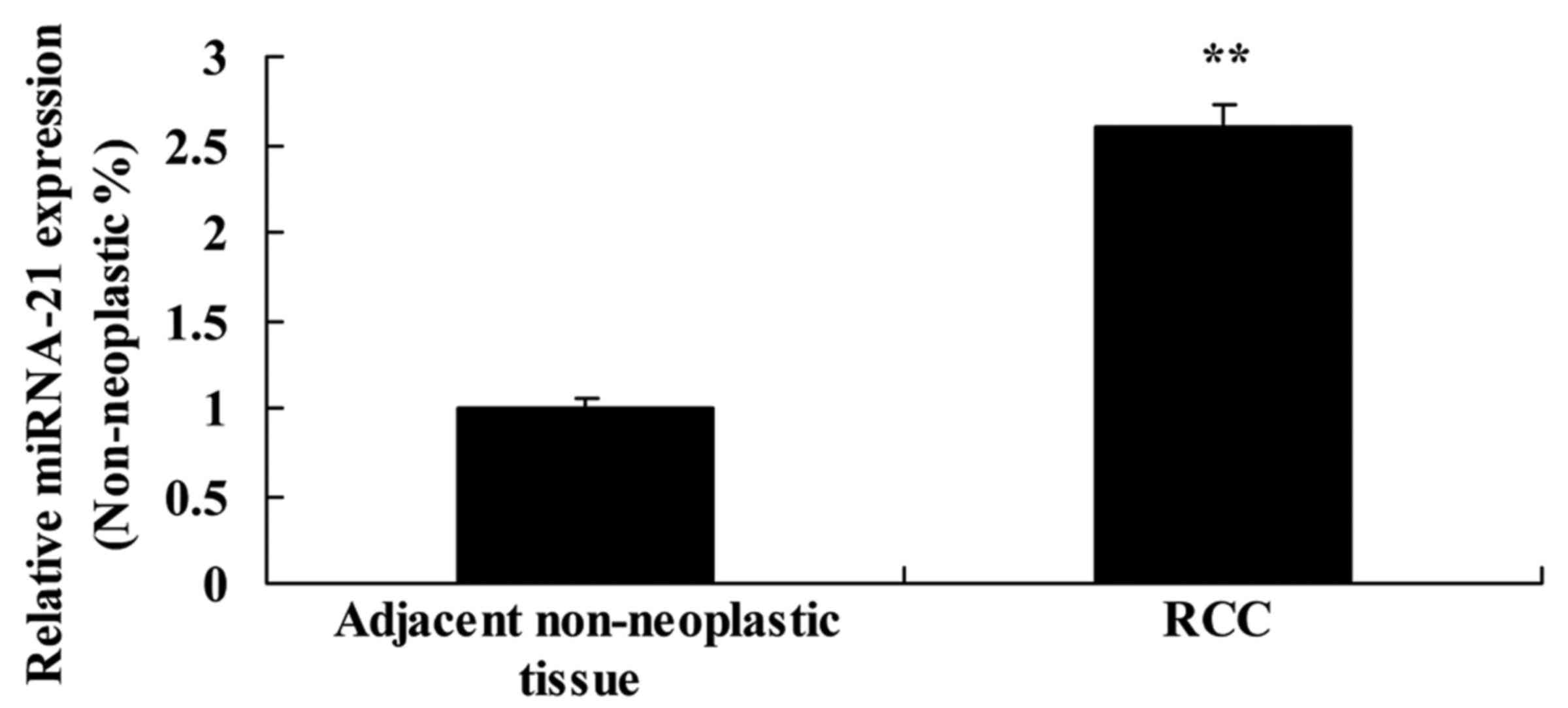
To evaluate the expression of miR-21 expression in
adjacent non-neoplastic and RCC tissues, miR-21 expression was
detected by real-time PCR. As shown in Fig. 1, the expression of miR-21 in RCC
tissues was much higher than that noted in the adjacent
non-neoplastic tissues.
Expression of miR-21 and
clinicopathological features
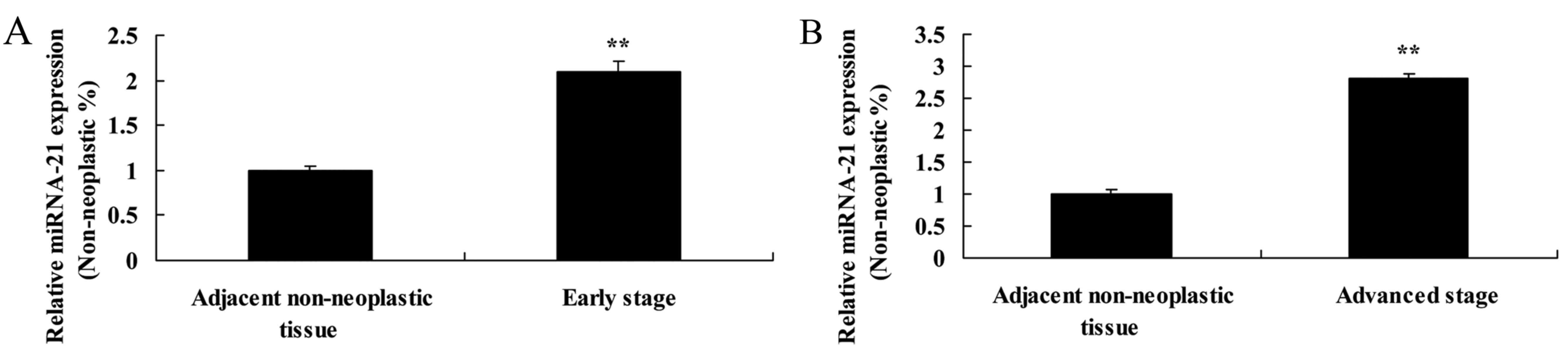
We investigated the correlation between the
expression of miR-21 and the clinicopathological features of the
patients with RCC (Table I). The
expression of miR-21 in patients with early stage RCC or advantaged
stage RCC was higher than that detected in the adjacent
non-neoplastic tissues, respectively (Fig. 2A and B).
 | Table I.Correlation of miR-21 expression and
the clinicopathological features of the RCC cases. |
Table I.
Correlation of miR-21 expression and
the clinicopathological features of the RCC cases.
| Patient features | Low miR-21 | High miR-21 | P-value |
|---|
| Age (years) |
|
| 0.761 |
|
<50 | 8 | 12 |
| ≥50 | 25 | 23 |
| Tumor stage |
|
| 0.021 |
| Early
(I–II) | 11 | 7 |
| Advanced
(III–IV) | 19 | 31 |
| Tumor size
(cm) |
|
| 0.393 |
|
<5 | 14 | 16 |
| ≥5 | 21 | 17 |
| AFP level
(ng/ml) |
|
| 0.112 |
|
≤20 | 14 | 11 |
|
>20 | 20 | 21 |
| Venous
infiltration |
|
| 0.001 |
|
Absent | 28 | 10 |
|
|
Present | 9 | 21 |
|
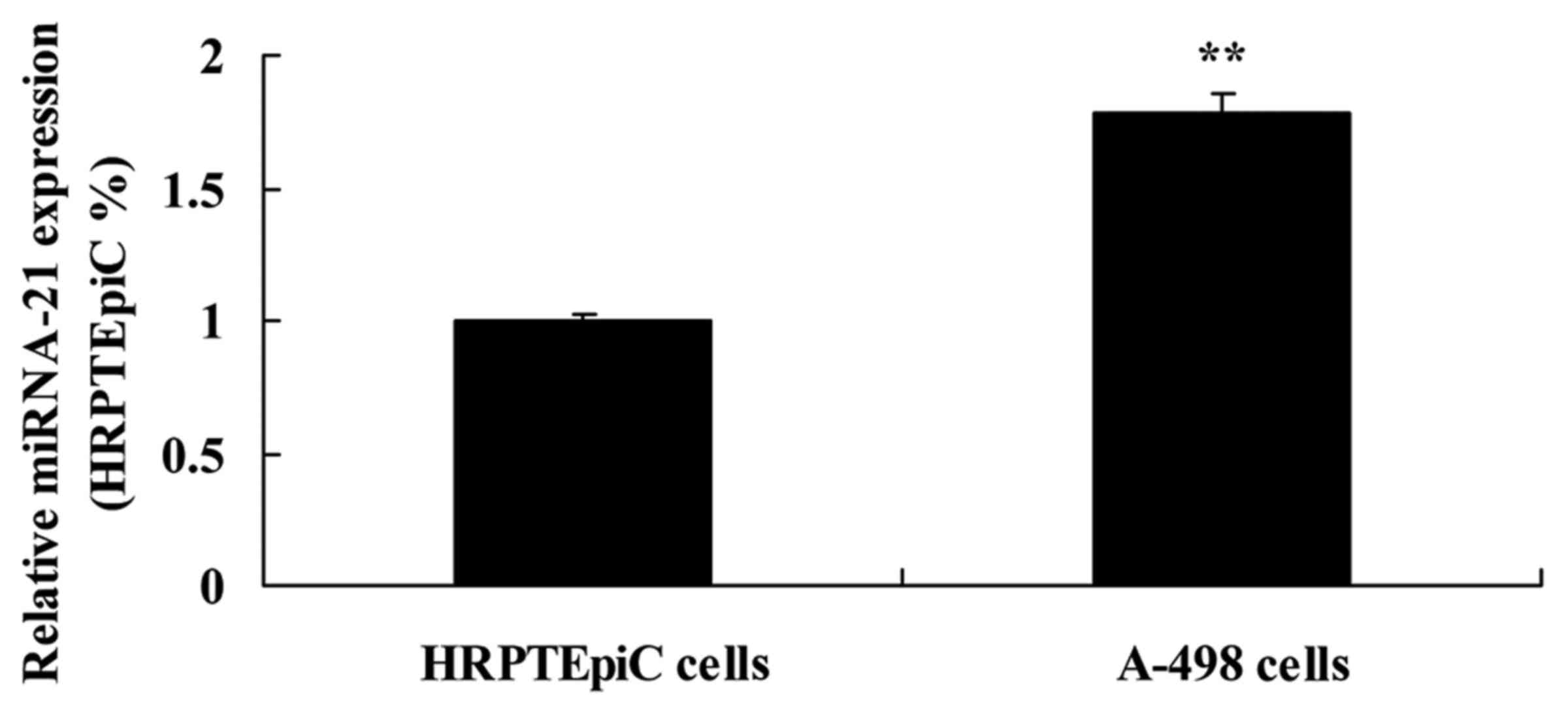
Expression of miR-21 in HRPTEpiC and
A-498 cells
We used HRPTEpiC and A-498 cells to detect the
expression of miR-21. Similarly, the expression of miR-21 in A-498
cells was markedly higher than that in the HRPTEpiC cells (Fig. 3).
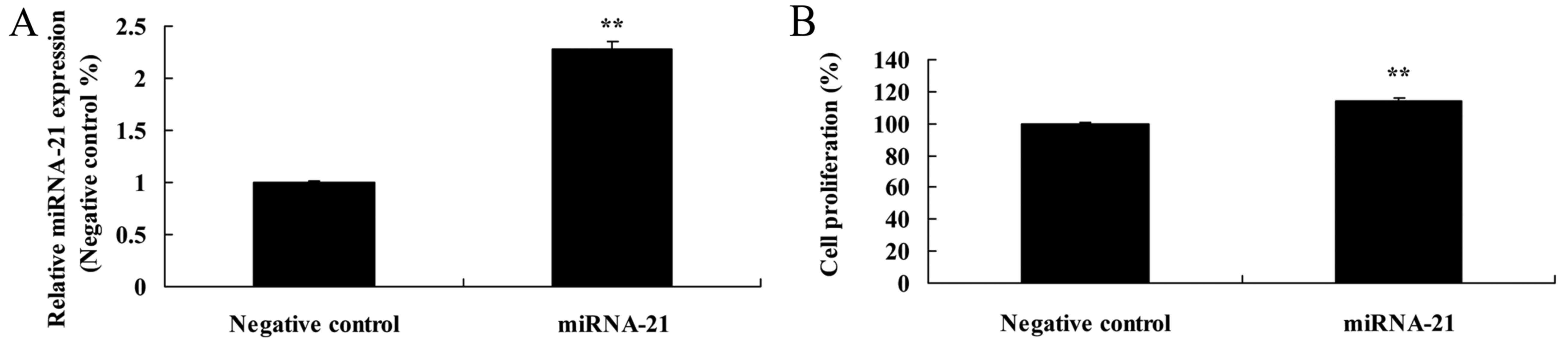
Effect of the overexpression of miR-21
on cell proliferation of A-498 cells
As shown in Fig. 4A,
the miR-21 plasmid increased the expression of miR-21 in the A-498
cells, as compared to the negative control. Meanwhile, the miR-21
plasmid facilitated the cell proliferation of the A-498 cells, as
compared to the proliferation noted in the cells transfected with
the negative control (Fig. 4B).
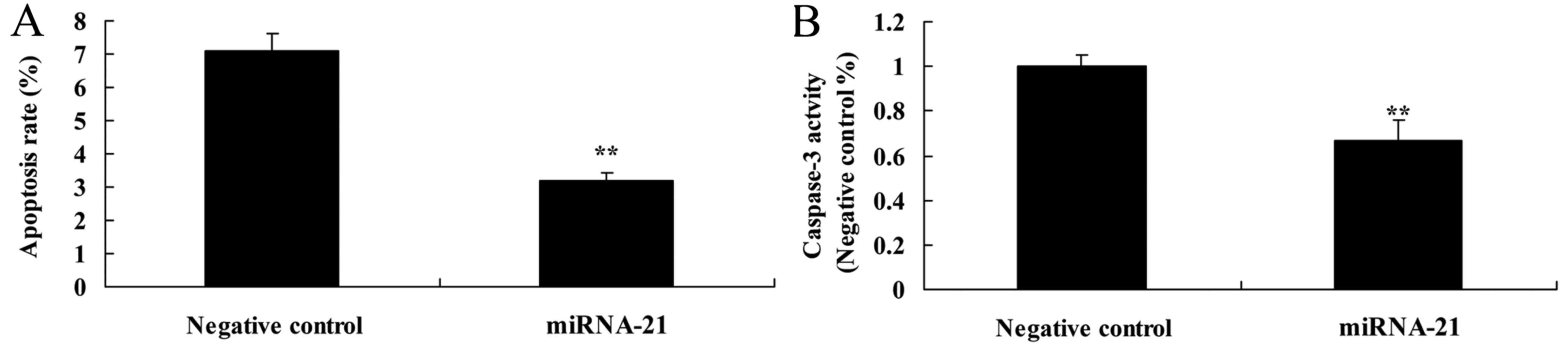
Effect of the overexpression of miR-21
on apoptosis and caspase-3 activity in the A-498 cells
To determine whether overexpression of miR-21
affects apoptosis and caspase-3 activity of A-498 cells, flow
cytometry and ELISA kit were used to analyze apoptosis and
caspase-3 activity of the A-498 cells. Compared with the
anti-miR-negative control, the apoptosis rate and caspase-3
activity were suppressed in the cells in the miR-21-overexpressing
group (Fig. 5A and B).
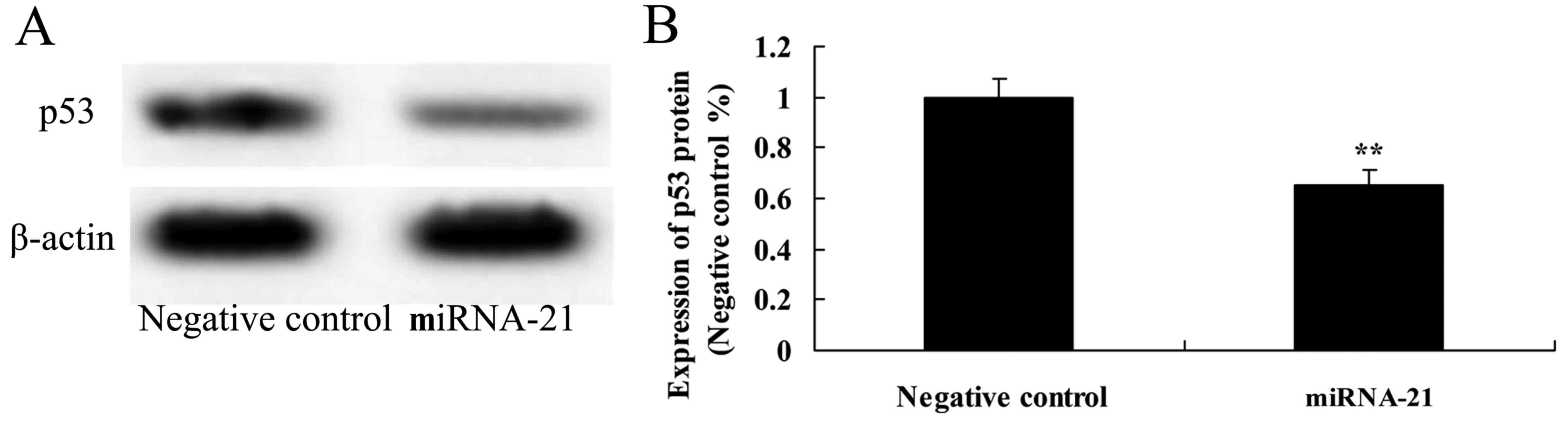
Effect of the overexpression of miR-21
on the expression of p53
To determine the mechanism of miR-21 that affects
A-498 cells, p53 protein expression was investigated in the present
study. Overexpression of miR-21 observably suppressed the protein
expression of p53, as compared to the negative control (Fig. 6).
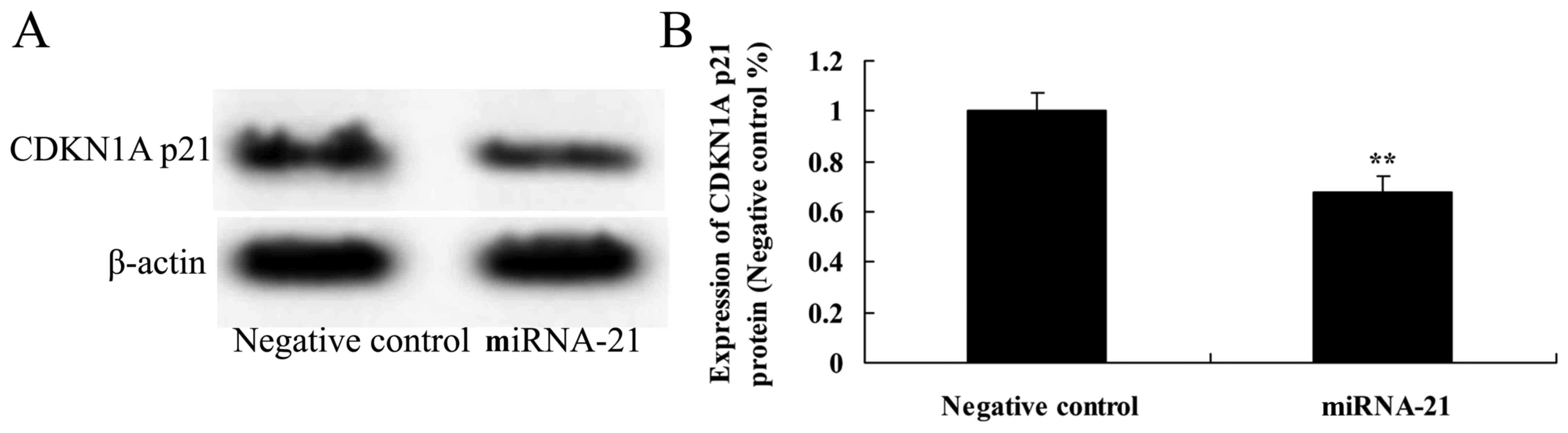
Effect of the overexpression of miR-21
on the expression of CDKN1A p21
To ascertain whether overexpression of miR-21 is
involved in the expression of CDKN1A p21 in A-498 cells, CDKN1A p21
protein expression was detected using western blot analysis. The
protein expression of CDKN1A p21 was observably inhibited following
the overexpression of miR-21, as compared to cells transfected with
the negative control (Fig. 7).
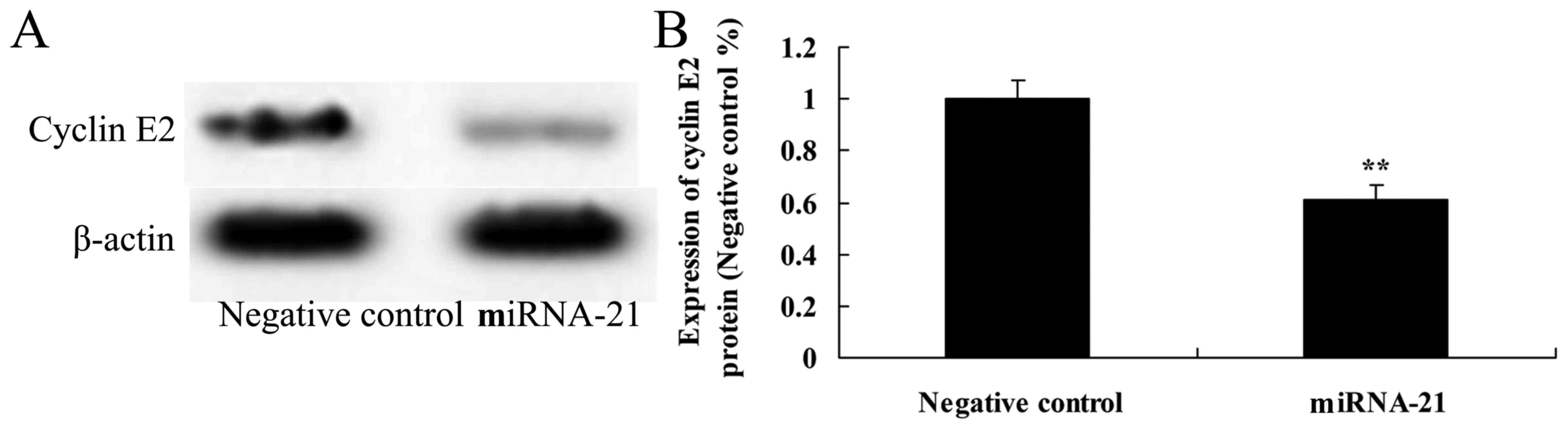
Effect of the overexpression of miR-21
on the expression of cyclin E2
To ascertain how the overexpression of miR-21
affects the expression of cyclin E2 in A-498 cells, western blot
analysis was used to reveal the protein expression of cyclin E2. We
observed a significant inhibition of cyclin E2 protein expression
following overexpression of miR-21 in the A-498 cells, as compared
to the negative control. Thus, miR-21 affects the cell cycle of
A-498 cells (Fig. 8).
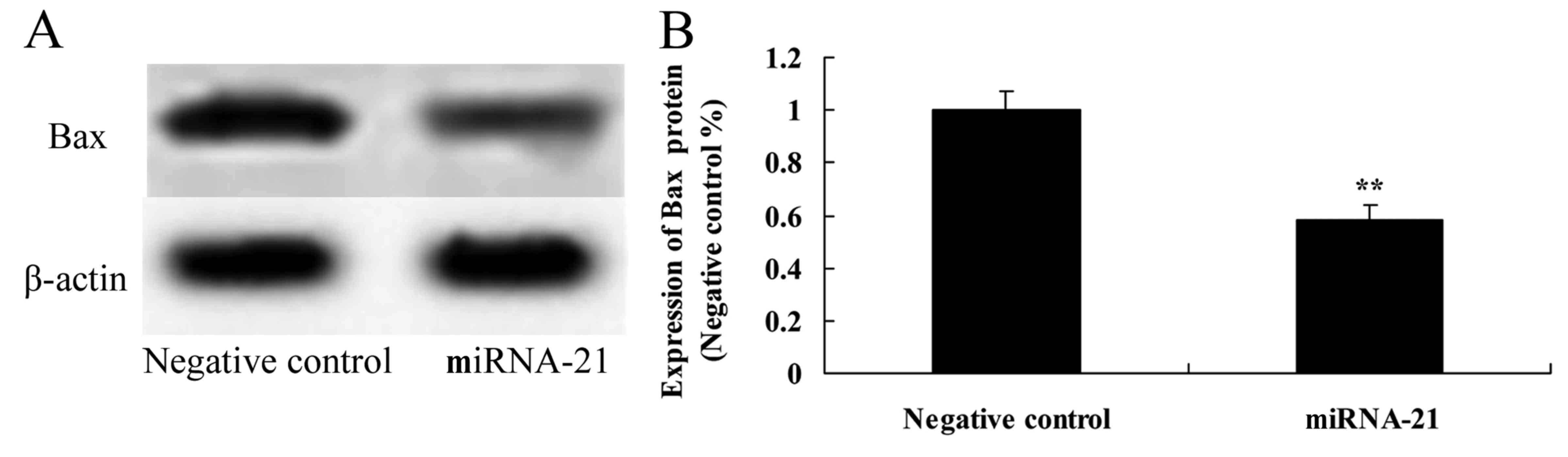
Effect of the overexpression of miR-21
on the expression of Bax
To further explore how the overexpression of miR-21
affects the Bax protein expression in A-498 cells, western blot
analysis was used to reveal Bax protein expression. As shown in
Fig. 9, overexpression of miR-21
significantly suppressed the protein expression of Bax in the A-498
cells, as compared to the negative control.
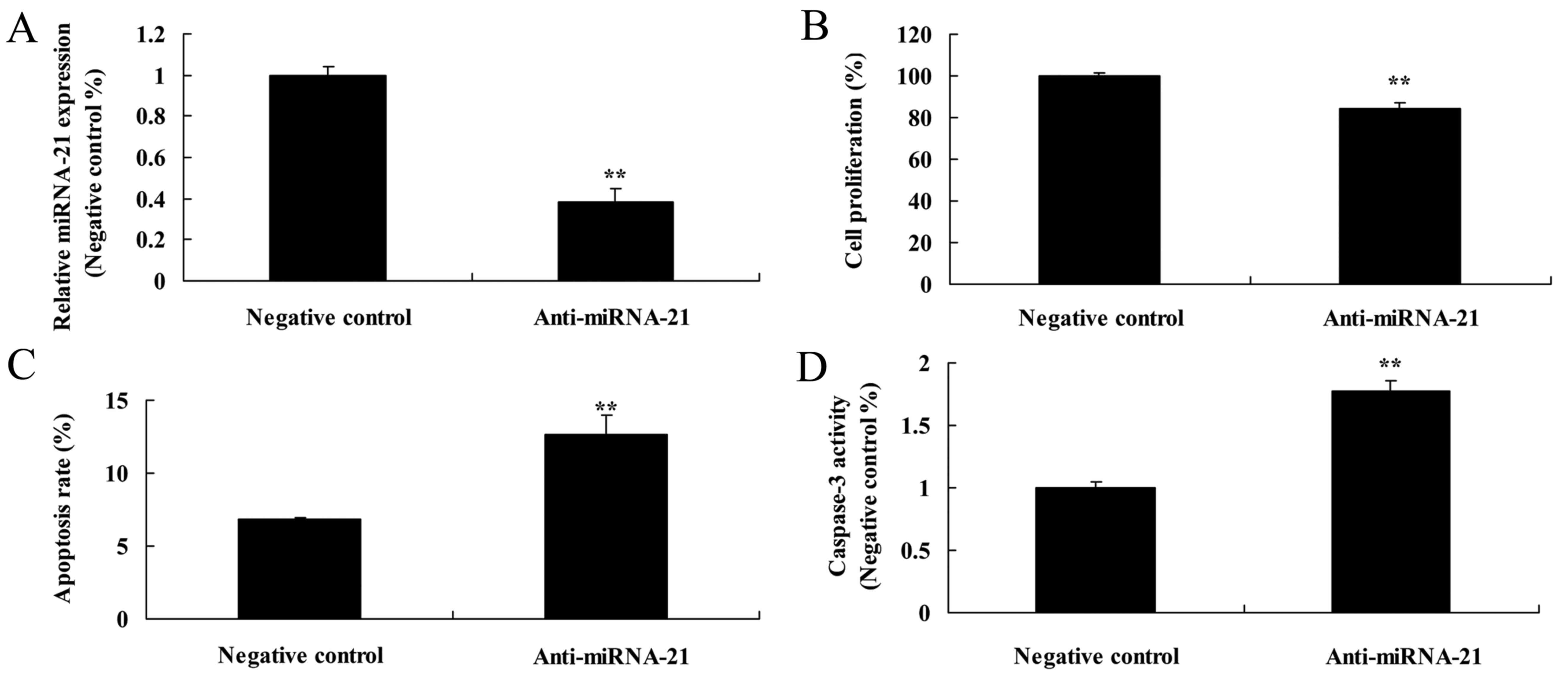
Effect of the downregulation of miR-21
on cell proliferation and apoptosis in the A-498 cells
We further ascertained how downregulation of miR-21
affects the cell proliferation and apoptosis of A-498 cells. miR-21
expression in the anti-miR-21 expression group was lower than that
of the negative control group (Fig.
10A). Meanwhile, downregulation of miR-21 markedly suppressed
the cell proliferation of the A-498 cells, as compared to the
negative control (Fig. 10B).
However, downregulation of miR-21 also markedly induced the
apoptosis and caspase-3 activity in the A-498 cells, as compared to
the negative control (Fig. 10C and
D).
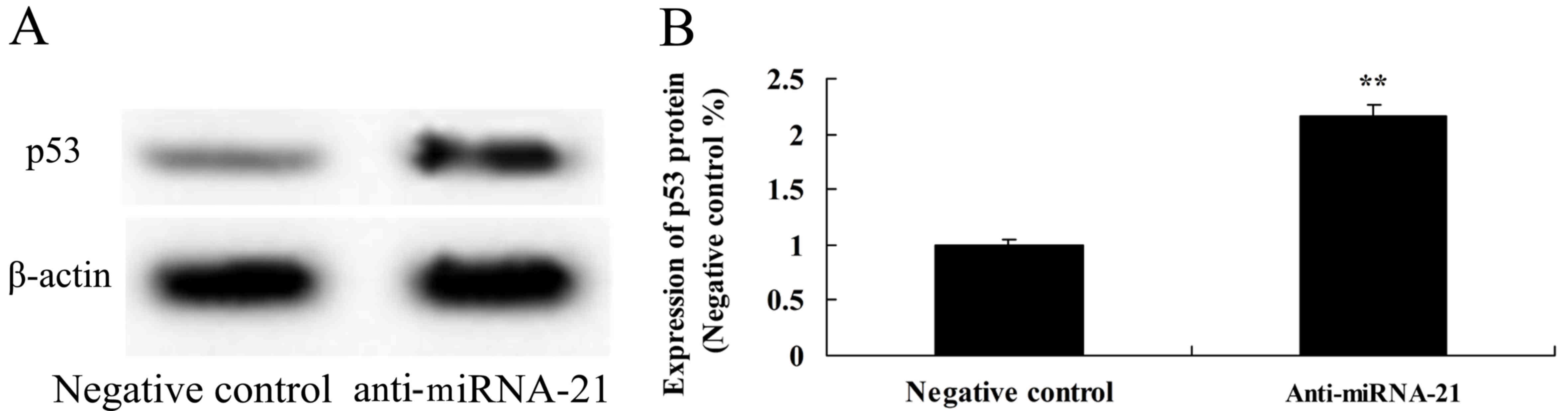
Effect of the downregulation of miR-21
on p53 protein expression in the A-498 cells
To further observe whether downregulation of miR-21
is involved in the expression of p53 in A-498 cells, p53 protein
expression was detected using western blot analysis. On the
contrary, downregulation of miR-21 significantly activated the
protein expression of p53, as compared to the negative control
(Fig. 11).
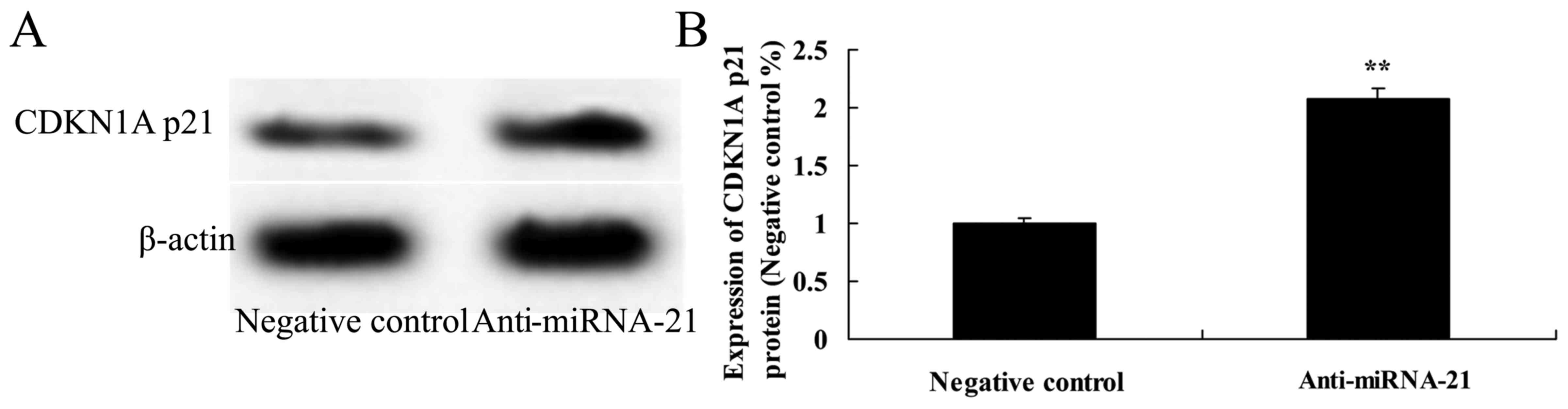
Effect of the downregulation of miR-21
on CDKN1A p21 expression in the A-498 cells
To next ascertain whether downregulation of miR-21
affects CDKN1A p21 protein expression in the A-498 cells, CDKN1A
p21 protein expression was also analyzed using western analysis. As
shown in Fig. 12, downregulation
of miR-21 significantly promoted CDKN1A p21 protein expression in
the A-498 cells, as compared to the negative control.
Effect of the downregulation of miR-21
on cyclin E2 expressions in the A-498 cells
To further ascertain whether the downregulation of
miR-21 affects cyclin E2 expression in the A-498 cells, we assessed
cyclin E2 expressions using western blot experiments.
Downregulation of miR-21 significantly increased cyclin E2
expressions in the A-498 cells, as compared to the negative control
(Fig. 13).
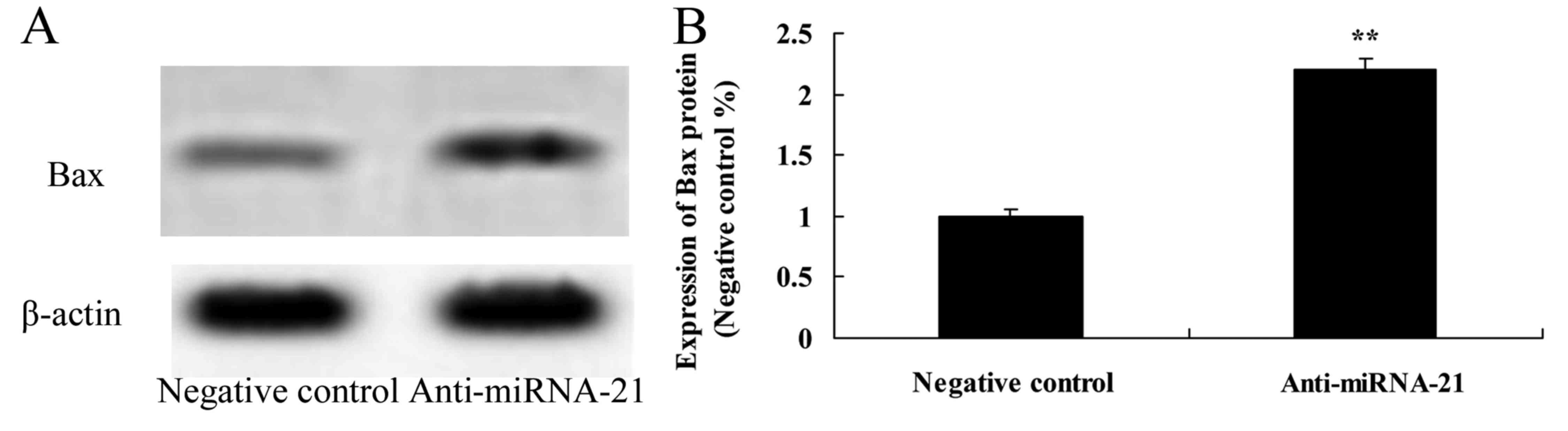
Effect of the downregulation of miR-21
on Bax expression in the A-498 cells
We further ascertained how the downregulation of
miR-21 affects Bax expression in the A-498 cells. Using western
blot experiments we assessed Bax expressions. Downregulation of
miR-21 significantly increased Bax expression in the A-498 cells,
as compared to the negative control (Fig. 14).
Discussion
Originating from renal tubular epithelial cells
(RTECs), renal cell carcinoma (RCC) is a common malignant tumor of
the urinary system (11). As a
common malignant renal tumor, it accounts for approximately 2–3% of
all adult solid malignant tumors, and 85–90% of primary malignant
neoplasms (12). RCC tissue typing
mainly includes clear cell carcinoma, chromophobe cell tumor,
collecting duct carcinoma and unclassified RCC. The morbidity of
RCC in different countries or regions is diversified (13). Specifically, it is higher in
developed countries than in developing countries. The morbidity and
mortality rate of RCC in China have been presenting an escalating
trend in recent years (14). We
found that the expression of miR-21 in RCC tissues was much higher
than that in adjacent non-neoplastic tissues. Meanwhile, the
expression of miR-21 in RCC patient tissues at the early or
advanced stage was higher than that in the adjacent non-neoplastic
tissues.
Research has also shown that miR-21 plays an
important role in a variety of malignant tumors (including tumors
in solid organs and the blood system) (15,16).
As the only miRNA overexpressed in almost all malignant solid
tumors, it has been regarded as a proto-oncogene. Generally, it is
upregulated in a variety of tumor tissues and cells, such as
glioma, cervical, ovarian, bladder, prostatic, lung, breast,
thyroid, esophageal and liver cancer, and bile duct carcinoma,
pancreatic, colorectal and gastric cancer, B cell lymphoma,
leukemia and multiple myelomas. Owing to its participation in cell
differentiation, proliferation and apoptosis (15–18),
it is closely related to genesis and development, as well as
infiltration and metastasis (19).
Moreover, the overexpression of miR-21 was found to increase cell
proliferation, and inhibit apoptosis and caspase-3 activity in RCC
A-498 cells.
Briefly, p53 can induce cell cycle progression and
regulate G2/M transformation. It not only prevents cells from
entering into the mitotic phase by inhibiting cdc2 (20), but also inhibits cyclin B1 and
blocks the cells at the G2 phase (21). In addition, p53 enables the
transcriptional activation of cell cycle protein relying on kinase
inhibitor p21 protein expression, thus, as to block the cell cycle
at the G1 phase (21,22). Ma et al suggested that miR-21
may be beneficial in apoptosis-inducing cancer therapies for
p53-deficient tumors (23).
Observably, the overexpression of miR-21 affects the protein
expression of p53 in A-498 cells.
It is noteworthy that p21 can play the role of an
oncogene. The root cause is that p21 can be phosphorylated by Akt
and is located in the cytoplasm (24). Nonetheless, the cytoplasmic
localized p21 inhibits caspase-3, and thus restrains apoptosis. In
addition, it can form a composite with procaspase-3 to resist
Fas-mediated apoptosis (10).
Consequently, the functional loss of p53 of transcription factor
p21 may verify tumor inhibition and promote cancer activity of
cytoplasmic localization in various situations. With two different
functions of p21, p53 can generally activate the downstream of cell
cycle inhibitors (25). In the
present study, it was found that miR-21 regulated CDKN1A p21
protein expression in the A-498 cells. Haghpanah et al
suggested that miR-21 enhances differentiation/apoptosis by
increasing the expression of p21 in anaplastic thyroid cancer
(26).
The increase in p21 protein expression may result in
the blocking of the cell cycle at G1/S. However, there are reports
concerning DNA damage induced by exogenous factors such as UV
irradiation (27) in recent years.
Generally, p21 is activated to upregulate and p53 is utilized to
inhibit cyclin/CDK composite, so as to induce blocking of the cell
cycle at the S phase (28). Based
on a large number of studies, p53 can induce apoptosis. As a
transcription factor, it is of vital importance to clarify the
target genes related to the regulation of apoptosis (22). Additionally, wild-type p53 can
combine with the bax promoter, and increase bax expression at the
transcription level. Bax is a family member of Bcl-2 (28). It can form heterology of two
polymers to inhibit the activity of the Bcl-2 family, which plays
an important role in apoptosis and cancer (27). For instance, Bcl-2 is able to
control cytochrome c to release from mitochondria, thus
activating caspase-9, −9 and −3, eventually leading to apoptosis
(29). The present study results
showed that miR-21 affects cyclin E2 protein expression in the
A-498 cells. Zaman et al reported that miR-21 can not only
serve as a tumor marker in kidney cancer survival but also affects
cyclin E2 protein expression (30).
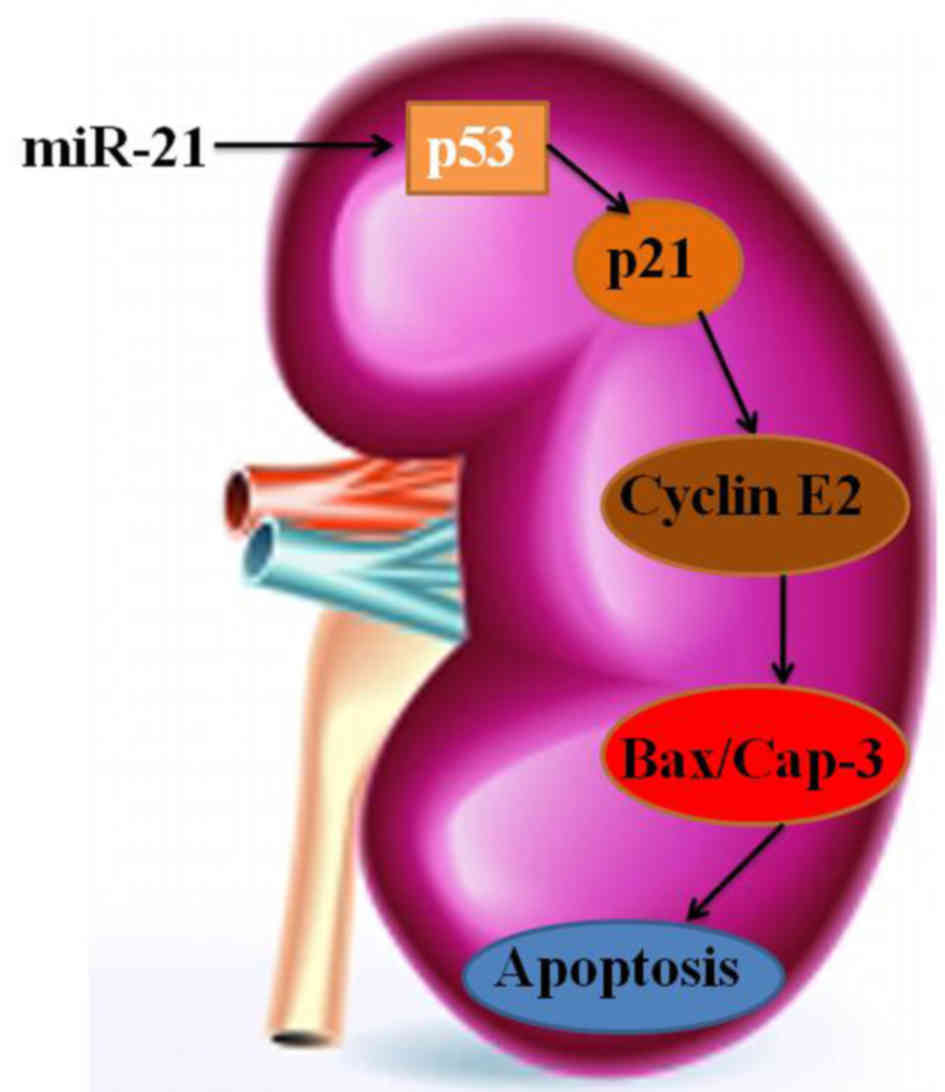
In conclusion, our results demonstrated that miR-21
expression predicates advanced clinicopathological features and
poor prognosis in patients with RCC through p53/p21-cyclin
E2/caspase-3 (Fig. 15). Moreover,
examining new targets and biological factors may facilitate to
clarify the miR-21/p53/p21-cyclin E2/caspase-3 pathway in RCC.
References
|
1
|
Ghosh A, Jana M, Modi K, Gonzalez FJ, Sims
KB, Berry-Kravis E and Pahan K: Activation of peroxisome
proliferator-activated receptor α induces lysosomal biogenesis in
brain cells: Implications for lysosomal storage disorders. J Biol
Chem. 290:10309–10324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patterson CM, Wong JM, Leinninger GM,
Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones
JC, Kennedy RT, et al: Ventral tegmental area neurotensin signaling
links the lateral hypothalamus to locomotor activity and striatal
dopamine efflux in male mice. Endocrinology. 156:1692–1700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levy O, Mortensen LJ, Boquet G, Tong Z,
Perrault C, Benhamou B, Zhang J, Stratton T, Han E, Safaee H, et
al: A small-molecule screen for enhanced homing of systemically
infused cells. Cell Reports. 10:1261–1268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marcu R, Kotha S, Zhi Z, Qin W, Neeley CK,
Wang RK, Zheng Y and Hawkins BJ: The mitochondrial permeability
transition pore regulates endothelial bioenergetics and
angiogenesis. Circ Res. 116:1336–1345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kannan K, Coarfa C, Chao PW, Luo L, Wang
Y, Brinegar AE, Hawkins SM, Milosavljevic A, Matzuk MM and Yen L:
Recurrent BCAM-AKT2 fusion gene leads to a constitutively activated
AKT2 fusion kinase in high-grade serous ovarian carcinoma. Proc
Natl Acad Sci USA. 112:E1272–E1277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan MH, Chang YH, Tsai ML, Lai CS, Ho SY,
Badmaev V and Ho CT: Pterostilbene suppressed
lipopolysaccharide-induced up-expression of iNOS and COX-2 in
murine macrophages. J Agric Food Chem. 56:7502–7509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sayer RD, Wright AJ, Chen N and Campbell
WW: Dietary approaches to stop hypertension diet retains
effectiveness to reduce blood pressure when lean pork is
substituted for chicken and fish as the predominant source of
protein. Am J Clin Nutr. 102:302–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT
and Pan MH: Pterostilbene, a natural analogue of resveratrol,
potently inhibits 7,12-dimethylbenz[a]anthracene
(DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse
skin carcinogenesis. Food Funct. 3:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Liu S, Xu Y, Zhang A, Jiang J, Tan
W, Xing J, Feng G, Liu H, Huo F, et al: Human umbilical
cord-derived mesenchymal stem cells downregulate inflammatory
responses by shifting the Treg/Th17 profile in experimental
colitis. Pharmacology. 92:257–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu W, Konermann A, Guo T, Jäger A, Zhang
L and Jin Y: Canonical Wnt signaling differently modulates
osteogenic differentiation of mesenchymal stem cells derived from
bone marrow and from periodontal ligament under inflammatory
conditions. Biochim Biophys Acta. 1840:1125–1134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aggarwal BB, Gupta SC and Sung B:
Curcumin: An orally bioavailable blocker of TNF and other
pro-inflammatory biomarkers. Br J Pharmacol. 169:1672–1692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore JN, Cook JA, Morris DD, Halushka PV
and Wise WC: Endotoxin-induced procoagulant activity, eicosanoid
synthesis, and tumor necrosis factor production by rat peritoneal
macrophages: Effect of endotoxin tolerance and glucan. Circ Shock.
31:281–295. 1990.PubMed/NCBI
|
|
13
|
Park K, Ju WC, Yeo JH, Kim JY, Seo HS,
Uchida Y and Cho Y: Increased OPG/RANKL ratio in the conditioned
medium of soybean-treated osteoblasts suppresses RANKL-induced
osteoclast differentiation. Int J Mol Med. 33:178–184.
2014.PubMed/NCBI
|
|
14
|
Hodge G, Hodge S, Holmes-Liew CL, Reynolds
PN and Holmes M: Loss of glucocorticoid receptor from
pro-inflammatory T cells after lung transplant. J Heart Lung
Transplant. 33:957–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinez-Schlurmann NI, Rampa S, Speicher
DG and Allareddy V, Rotta AT and Allareddy V: Prevalence,
predictors and outcomes of cardiopulmonary resuscitation in
hospitalized adult stem cell transplant recipients in the United
States: Not just opening the black box but exploring an opportunity
to optimize! Bone Marrow Transplant. 50:1578–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarmento CA, Rodrigues MN, Bocabello RZ,
Mess AM and Miglino MA: Pilot study: Bone marrow stem cells as a
treatment for dogs with chronic spinal cord injury. Regen Med Res.
2:92014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong
MH, Yoon SH, Kim YS and Ahn Y: Mesenchymal stem cells reciprocally
regulate the M1/M2 balance in mouse bone marrow-derived
macrophages. Exp Mol Med. 46:e702014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wasnik S, Tiwari A, Kirkland MA and Pande
G: Osteohematopoietic stem cell niches in bone marrow. Int Rev Cell
Mol Biol. 298:95–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rong JU, Wen Z, Rong WU and Zhichun F:
Interaction between neural stem cells and bone marrow
derived-mesenchymal stem cells during differentiation. Biomed Rep.
3:242–246. 2015.PubMed/NCBI
|
|
20
|
Chang J, Rimando A, Pallas M, Camins A,
Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA and
Casadesus G: Low-dose pterostilbene, but not resveratrol, is a
potent neuromodulator in aging and Alzheimer's disease. Neurobiol
Aging. 33:2062–2071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCormack D and McFadden D: Pterostilbene
and cancer: Current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Xi WC and Wang F: The beneficial
effects of intracoronary autologous bone marrow stem cell transfer
as an adjunct to percutaneous coronary intervention in patients
with acute myocardial infarction. Biotechnol Lett. 36:2163–2168.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Choudhury SN, Hua X, Dai Z and Li Y:
Interaction of the oncogenic miR-21 microRNA and the p53 tumor
suppressor pathway. Carcinogenesis. 34:1216–1223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Wang L, Fatahi R, Kronenberg M,
Kalajzic I, Rowe D, Li Y and Maye P: Isolation of murine bone
marrow derived mesenchymal stem cells using Twist2 Cre transgenic
mice. Bone. 47:916–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robitaille N, Lacroix J, Alexandrov L,
Clayton L, Cortier M, Schultz KR, Bittencourt H and Duval M: Excess
of veno-occlusive disease in a randomized clinical trial on a
higher trigger for red blood cell transfusion after bone marrow
transplantation: A canadian blood and marrow transplant group
trial. Biol Blood Marrow Transplant. 19:468–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haghpanah V, Fallah P, Tavakoli R, Naderi
M, Samimi H, Soleimani M and Larijani B: Antisense-miR-21 enhances
differentiation/apoptosis and reduces cancer stemness state on
anaplastic thyroid cancer. Tumour Biol. Aug 20–2015.(Epub ahead of
print). PubMed/NCBI
|
|
27
|
Wang C, Sun H, Song Y, Ma Z, Zhang G, Gu X
and Zhao L: Pterostilbene attenuates inflammation in rat heart
subjected to ischemia-reperfusion: Role of TLR4/NF-κB signaling
pathway. Int J Clin Exp Med. 8:1737–1746. 2015.PubMed/NCBI
|
|
28
|
Cichocki M, Paluszczak J, Szaefer H,
Piechowiak A, Rimando AM and Baer-Dubowska W: Pterostilbene is
equally potent as resveratrol in inhibiting
12-O-tetradecanoylphorbol-13-acetate activated NκB, AP-1, COX-2,
and iNOS in mouse epidermis. Mol Nutr Food Res. 52 Suppl 1:S62–S70.
2008.PubMed/NCBI
|
|
29
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaman MS, Shahryari V, Deng G, Thamminana
S, Saini S, Majid S, Chang I, Hirata H, Ueno K, Yamamura S, et al:
Up-regulation of microRNA-21 correlates with lower kidney cancer
survival. PLoS One. 7:e310602012. View Article : Google Scholar : PubMed/NCBI
|