Introduction
Prostate cancer (PCa) is generally diagnosed with
malignant tumor and this is important in terms of mortality.
Approxiamtely 180,890 new cases are estimated for 2016 and more
than 26,120 PCa deaths will occur in the USA. PCa has exceeded lung
cancer in men accounting for 21% of all male malignancies. PCa is
also the second cause of death in men (1). Its incidence rates vary between
countries. Incidence rate of PCa in Asia is widely accepted to be
the lowest all over the world. However, it has gradually increased
recently (2). Aside from age and
race, the risk factor for PCa is a family history of the disease.
In addition, its greater prevalence in the west implicates
lifestyle and environmental risk factors (3). Currently, there is no reliable
prediction methods and effective clinical therapies for PCa.
However, similar to other human malignancies, clinical relevance of
genetics and molecular mechanism supporting PCa development and
progression remain poorly understood.
CR-1 is an EGF-CFC family member gene (4). CR-1 was primitively found from NTERA2
(5). The anchor protein CR-1
contains extracellular signal sequence, EGF-like domain and
CFC-motif, GPI attachment. CR-1 takes actively part in the
mediation of the embryonic development and malignant tumor
occurrence (6). CR-1 is an
obligatory co-receptor during embryogenesis (7). CR-1 is expressed at low level in
adults. In contrast, high levels of CR-1 are detected in many
cancer patients (8). CR-1 plays
important roles in promoting tumor cell progression, stimulating
cell proliferation and invasion between epithelial-mesenchymal
transition (EMT) (9,10).
Previous research has reported that different kinds
of cells in the embryonic development and progression of malignant
tumor share common signaling pathways. The study of Wechselberger
et al (11) revealed that
CR-1 might be a mediator of the effects of activated Wnt/β-catenin
pathway in hyperplasia and adenocarcinoma of mouse mammary gland
(12,13). Epithelial-mesenchymal transition
(EMT) can promote attached epithelial cells into individual
migration into the extracellular matrix (14). Prasad et al (15) reported that Wnt/β-catenin signaling
upregulated invasion of breast cancer cells. It contained
E-cadherin and GSK3β in implementing EMT. Wnt/β-catenin pathway
contributed to epithelial tissue inducing EMT to produce signal
transduction pathways. Thus, we hypothesized that CR-1 would
promote PCa occurrence by the Wnt/β-catenin pathway.
Now, we describe connection of CR-1 and EMT with PCa
tissues. We found an important role of CR-1 in PCa cells by the
Wnt/β-catenin pathway. Furthermore, CR-1 positively regulated
invasion, migration and EMT. These results shed light on the
mechanisms by which PCa occurs, develops and provides evidence for
CR-1 as a new biological marker contributing to clinical treatment
against PCa.
Materials and methods
Human prostate specimens
The present study on human prostate specimens was
approved by the Ethics Committee of the Second Hospital of Tianjin
Medical University. Specimens were obtained from patients with
signed informed consent for use in research. Patient tissues were
collected immediately and then were quickly frozen with liquid
nitrogen. Patients did not receive therapy before surgery. Prostate
cancer tissues were collected after radical prostatectomy at the
Department of Urology of the hospital. Adjacent normal tissues were
gained from the same patients. Hematoxylin and eosin (H&E)
staining sections were used to identify the tissues by
pathologists.
Cell culture
LNCap, PC-3 and RWPE-1 were maintained in our
laboratory. LNCap and PC-3 were cultivated in RPMI-1640 medium
(Gibco, Shanghai, China) with 10% fetal bovine serum (FBS). RWPE-1
was incubated in keratinocyte serum-free medium (K-SFM; Gibco). All
the cells were incubated at 37°C and 5% CO2.
Plasmid construction and cell
transfection
According to the shRNA design principle, four
suitable shRNA target sequences (sh1-4), from human CR-1 gene
GenBank accession no. NM_001174136.1, were synthesized,
respectively: sh-1, CCA TCAGGAATTTGCTCGTCC; sh-2, GCAAATTTCATGAC
CAGTAAA; sh-3, CCTAACTGAAAGATGATCATT; and sh-4
CCAAGGTCTTCTTAATATGTT. A small hairpin RNA (shRNA) of CR-1 exists
in psi-U6.1 vector. GeneCopoeia constructed plasmids with enhanced
green fluorescent protein (EGFP) and puromycin resistance (cat. no.
HSH060851-CU6; GeneCopoeia, Guangzhou, China). We transfected the
plasmid when PC-3 cells were 90% confluent. Lipofectamine 2000
(Invitrogen) was in strict accordance with the instructions of the
manufacturer. Fluorescence microscopy was used to observe the
transfection efficiency.
Immunohistochemical (IHC) staining.
Sixty PCa and 60 BPH tissues were collected from the Urology
Department
Xylene was used to deparaffinize and hydrate
paraffin-embedded sections (4 µm) followed by graded alcohols to
water; 0.01 M citrate for 10 min was used for antigen retrieval.
Then slowly cooled down. Primary antibodies, horseradish peroxidase
and DAB were then used, respectively (Beijing Zhongshan Golden
Bridge, Beijing, China). Finally, sections were counterstained and
dehydrated. Antibodies were as follows: anti-CR-1 (Sigma-Aldrich)
anti-β-catenin and anti-E-cadherin (Beijing Zhongshan Golden
Bridge).
Immunofluorescence (IF)
For detection of CR-1, E-cadherin and β-catenin
cells were put on the coverslips. Paraformaldehyde (4%), 0.1%
Triton X-100, primary antibodies and secondary antibodies were then
used. The secondary antibodies were labeled by conjugation for 90
min at RT in the dark. After washing, DAPI stained cells for 5 min.
After extensive washing, cells were mounted on glass slides with
antifade solution and the fluorescence was visualized by laser
scanning confocal microscopy using a 200x lens (FV1000; Olympus
Optical, Co., Ltd., Tokyo, Japan).
MTT assay
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
PC-3 was placed with 1×104 cells/well.
Lithium chlorides (Licl) have different concentrations at various
time intervals (12, 24 and 48 h). Next, each well was added with
MTT solution. Then, each well was put into dimethyl sulfoxide
(DMSO) and rotated at room temperature (RT). The absorbance value
was measured with microplate reader at 570 nm (BioTek Instruments,
Inc., Winooski, VT, USA).
RT-qPCR and RT-PCR assay
EasyPure® kit (TransGen Biotech, Inc.,
Beijing, China) was applied to extract total RNA. We applied
TransScript® SuperMix (TransGen Biotech) to synthesis of
cDNA. RT-qPCR was used with SYBR-Green Master Mix kit (Roche,
Basel, Switzerland) and GADPH was used as the internal reference.
Samples were denaturized at 95°C for 10 min, followed by 45 cycles
at 95°C for 15 sec, 60°C for 30 sec and 72°C for 15 sec. cDNA
template was amplified with 2X EasyTaq® PCR Mix
(TransGen Biotech) for RT-PCR. Reaction conditions were 94°C for 5
min, 35 cycles of: 94°C for 30 sec, 60°C for 30 sec and 72°C for 60
sec and 72°C for 10 min. Then, samples were electrophoresed with
2.5% agarose gel. The primers (Sangon Biotech Co., Ltd., Shanghai,
China) were designed as showen in Table
I. Each sample was run in triplicate.
 | Table I.Primer for RT-PCR and RT-qPCR. |
Table I.
Primer for RT-PCR and RT-qPCR.
| Gene | Forward primer | Reverse primer | Product (bp) |
|---|
| CR-1 |
5-GGAATTTGCTCGTCCATCTC-3 |
5-ACCGTGCCAGCATTTACAC-3 | 307 |
| β-catenin |
5-GCAACCAAGAAAGCAAGCTC-3 |
5-GCTGAACAAGAGTCCCAAGG-3 | 303 |
| E-cadherin |
5-AAACAGGATGGCTGAAGGTG-3 |
5-TCTTGGCTGAGGATGGTGTA-3 | 295 |
| c-myc |
5-CCGAGGAGAATGTCAAGAGG-3 |
5-ACGCACAAGAGTTCCGTAGC-3 | 270 |
| β-actin |
5-CTCTTCCAGCCTTCCTTCCT-3 |
5-ACTCCTGCTTGCTGATCCAC-3 | 304 |
Western blotting
For analyzing the expression of related proteins,
standard methods were performed as previously described (16). β-catenin, E-cadherin, c-myc, GSK-3β
and p-GSK-3β (1:500; Bioworld Technology, Inc., St. Louis Park, MN,
USA) were used for the primary antibody; GADPH was used as loading
control (1:1,000; Bioworld Technology).
Wound healing test
PC-3 was incubated up to 90% confluence and
transfected in 6-well plates. After 48 h, cells were scraped with a
pipette tip. Then, the cells were further incubated for 12 and 24
h. Wounded regions were photographed at ×40 amplification using
computer-aided microscopy (Nikon Eclipse Ti-U; Nikon, Tokyo,
Japan). Captured image were obtained 0, 12 and 24 h after the
wounding. The distance the cells migrated was measured and analyzed
using FV10-ASW 4.0 Viewer cell image analysis software
(Olympus).
Transwell invasion assay
Assay was implemented using Transwell inserts
(Corning, Inc., Corning, NY, USA) with Matrigel matrixes (BD
Biosciences, San Jose, CA, USA) in 24-well plates. A total of
1×105/ml transfected PC-3 cells were added into the
apical chamber and RPMI-1640 was added into basolateral chamber.
Cells were then cultured for 24 h. Inserts were scraped with cotton
swabs and fixed in paraformaldehyde. In addition, then they were
added with crystal violet. The PC-3 cells on permeable membrane
were distinguished using microscope with a camera.
Statistical analysis
We used the GraphPad Prism 5.0 and the SPSS 16.0. We
established the statistically significant difference making use of
two-tailed Student's t-test, Chi-square test and McNemar's
statistical test. P<0.05 and P<0.01 were deemed statistically
significant.
Results
CR-1 is highly expressed and
correlated with EMT in PCa tissues
We elected 60 PCa and 60 BPH patient tissues for
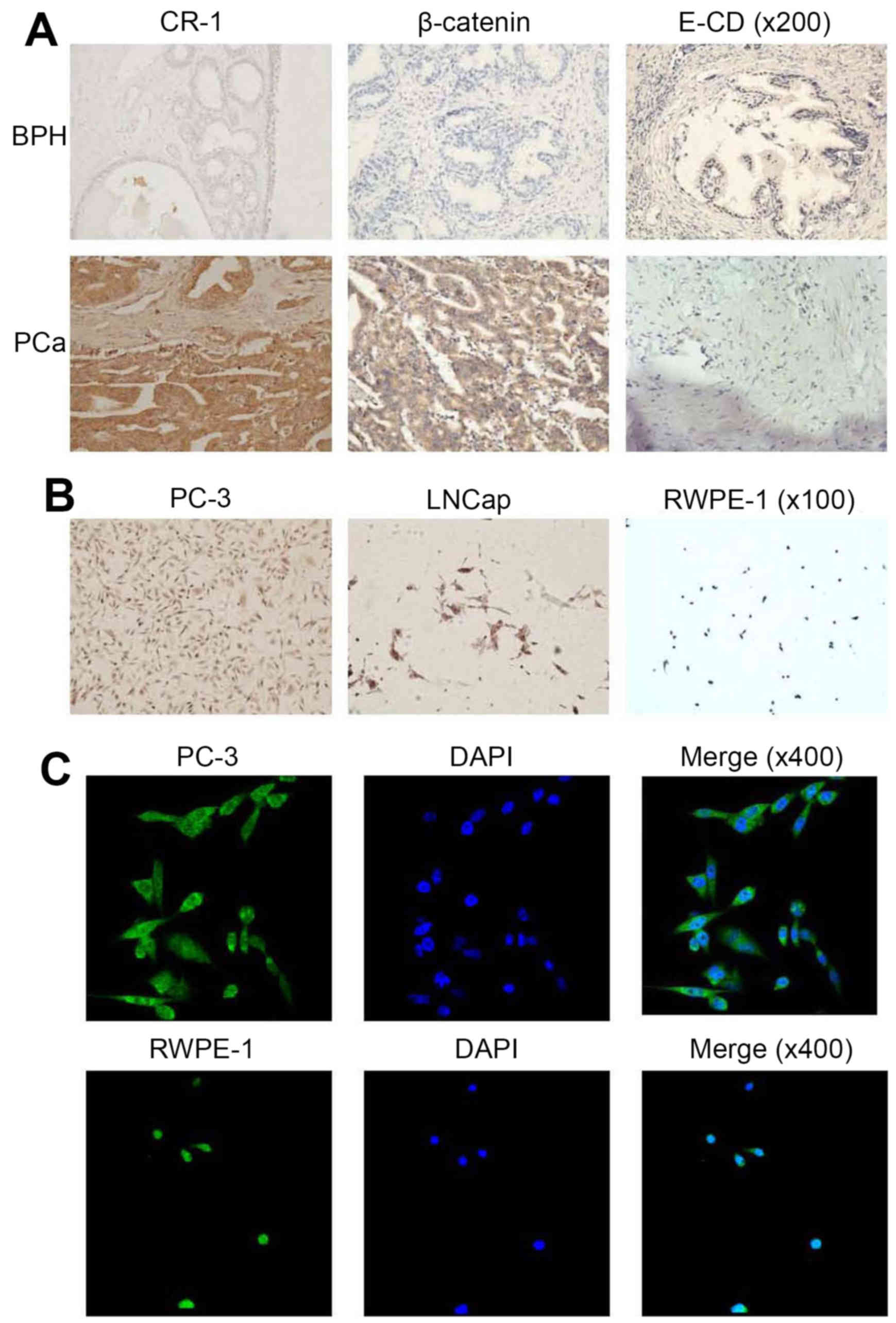
immunohistochemistry. In BPH tissues, CR-1 expression was low at
levels; however, CR-1 expression was high in PCa tissues. Thus,
CR-1 was overexpressed in PCa tissues (Fig. 1A). CR-1 expression in PC-3 cells was
the highest of all the cells (Fig.
1B). In our studies, immunohistochemistry staining showed that
CR-1 was predominantly localized to the cytoplasm of PCa cells.
Immunofluorescence (IF) showed that CR-1 protein was expressed both
in the nucleus and cytoplasm, but mainly in the cytoplasm (Fig. 1C). CR-1 levels positively correlated
with β-catenin in the cytoplasm (P<0.05). CR-1 levels were
contrary to the E-CD levels (P<0.05) (Table II). Table III shows the relationship between
the CR-1 expression and the clinicopathological parameters.
Expression of CR-1 has no correlation with age; however,
overexpression of CR-1 and PSA (<4 vs. 4–10 vs. >10 ng/ml;
P<0.01), Gleason (≤7 vs. >7; P<0.05), clinical staging
(I–II vs. III–IV; P<0.05) and lymph node metastasis (no vs.
positive; P<0.05) had close relationships. We suggest that CR-1
was closely linked with PCa progression and relative EMT marker
protein.
 | Table II.Expression correlations between CR-1,
E-cadherin and β-catenin in PCa tissues for IHC. |
Table II.
Expression correlations between CR-1,
E-cadherin and β-catenin in PCa tissues for IHC.
|
|
|
CR-1*E-cadherin/β-catenin |
|---|
|
|
|
|
|---|
|
|
| E-cadherin | β-catenin |
|---|
|
|
|
|
|
|---|
|
|
| − | + | − | + |
|---|
| CR-1 |
|
|
|
|
|
| − | Count | 20 | 5 | 9 | 16 |
|
| % of total | 33 | 8 | 15 | 27 |
| + | Count | 16 | 19 | 4 | 31 |
|
| % of total | 27 | 32 | 6 | 52 |
| Total | Count | 36 | 24 | 13 | 47 |
|
| % of total | 60 | 40 | 21 | 79 |
 | Table III.Clinicopathological data of Cripto-1
in PCa. |
Table III.
Clinicopathological data of Cripto-1
in PCa.
| Clinical data | N | CR-1- (%) | CR-1+ (%) | P-value |
|---|
| Age at registration
(years) |
|
|
|
|
|
≥70 | 25 | 10 (40.00) | 15 (60.00) | 0.825 |
|
<70 | 35 | 15 (42.86) | 20 (57.14) |
|
| Serum PSA
(ng/ml) |
|
|
|
|
| <4 | 2 | 1 (50.00) | 1 (50.00) |
|
| 4–10 | 9 | 8 (88.89) | 1 (11.11) | 0.007a |
| >10 | 49 | 16 (32.65) | 33 (67.35) |
|
| Gleason score |
|
|
|
|
| <7 | 9 | 4 (44.44) | 5 (55.56) | 0.032b |
| 7 | 18 | 3 (16.67) | 15 (83.33) |
|
| >7 | 33 | 18 (54.55) | 15 (45.45) |
|
| Clinical stage |
|
|
|
|
| I–II | 27 | 16 (59.26) | 11 (40.74) | 0.012b |
| III–IV | 33 | 9 (27.27) | 24 (72.73) |
|
| Lymph node
metastasis |
|
|
|
|
| No | 15 | 10 (66.67) | 5 (33.33) | 0.023b |
| Yes | 45 | 15 (33.33) | 30 (66.67) |
|
Expression of mRNA and protein in
prostate cells and tissues
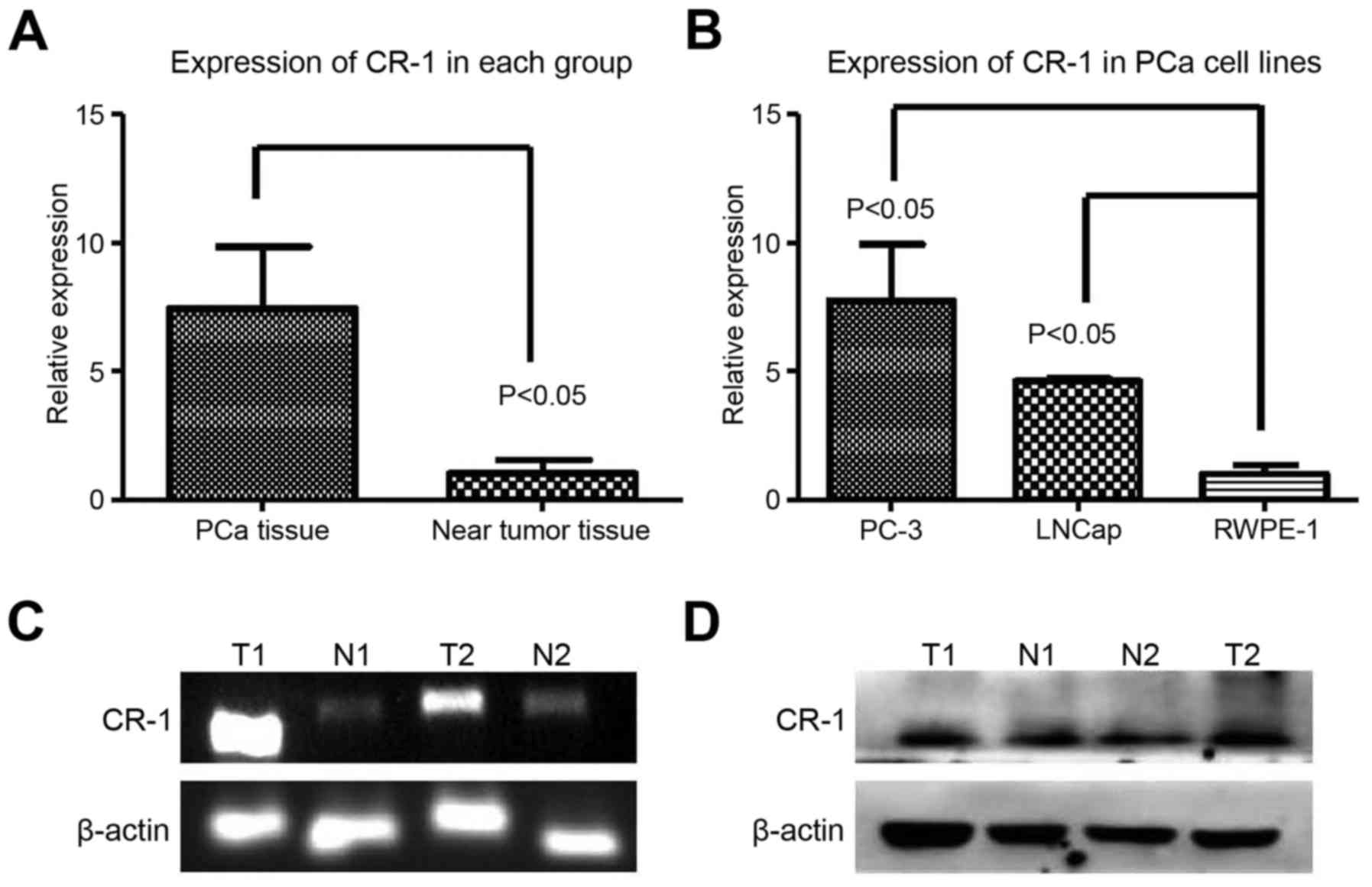
We used RT-PCR, RT-qPCR and western blot technology
to detect CR-1 in prostate cells and tissues. CR-1 level was shown
in the prostate specimens (PCa tissues, n=10; near tumor tissues,
n=10) using RT-PCR and RT-qPCR (Fig. 2A
and C). In comparison with the near tumor tissues, upregulated
CR-1 (P=0.0187) was displayed in PCa tissues (Fig. 2A, C and D). Expression of CR-1 was
higher in PC-3 cells (Fig. 2B).
Thus, we used the PC-3 cell line for the next experiment in
PCa.
Expression of EMT relative protein
after CR-1 shRNA transfection in PC-3
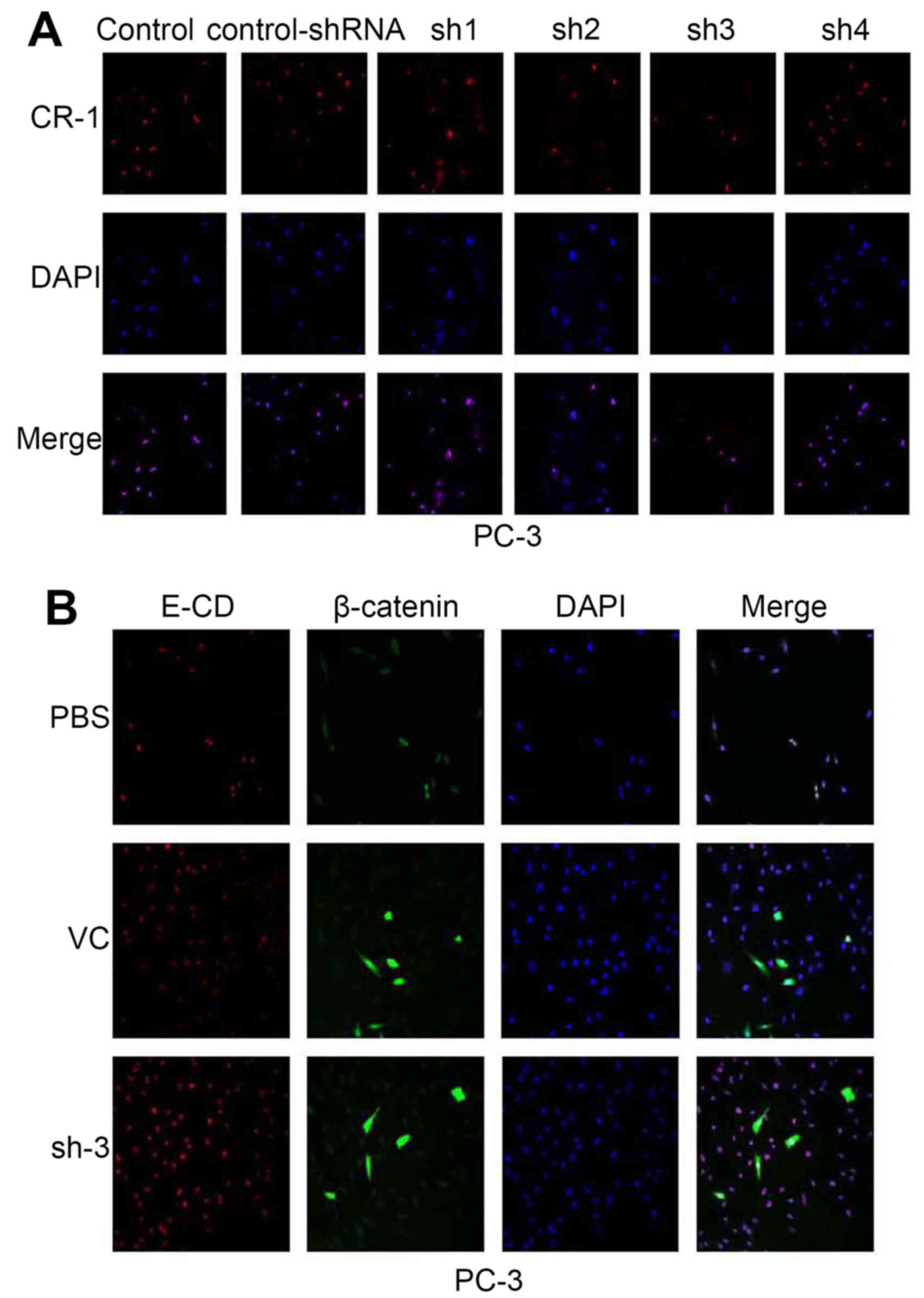
After PC-3 cell transfection with shRNA, CR-1
expression was downregulated. Expression of the CR-1-shRNA plasmid
vector (NC/CR-1-shRNA1-4) decreased red fluorescent protein (RFP)
was evaluated using scanning confocal microscopy in PC-3. We
counted transfection efficiency under a fluorescent microscope for
48 h after transfection. CR-1-shRNA-3 had the most preferable
interference effect (Fig. 4A).
CR-1-shRNA-3 was selected for the next experiments. β-catenin (a
Wnt signaling pathways marker) and E-cadherin (an epithelial
marker) was examined in cytoplasm by scanning confocal microscopy
(Fig. 4B). β-catenin was
prominently expressed at low level throughout the cytoplasm of PC-3
cells; whereas, a significantly enhanced E-CD was detected.
Expression of CR-1 and EMT markers in PC-3 prompted us to examine
whether CR-1 silencing could be in lost in mesenchymal and gained
in epithelial markers. To further determine if this transformation
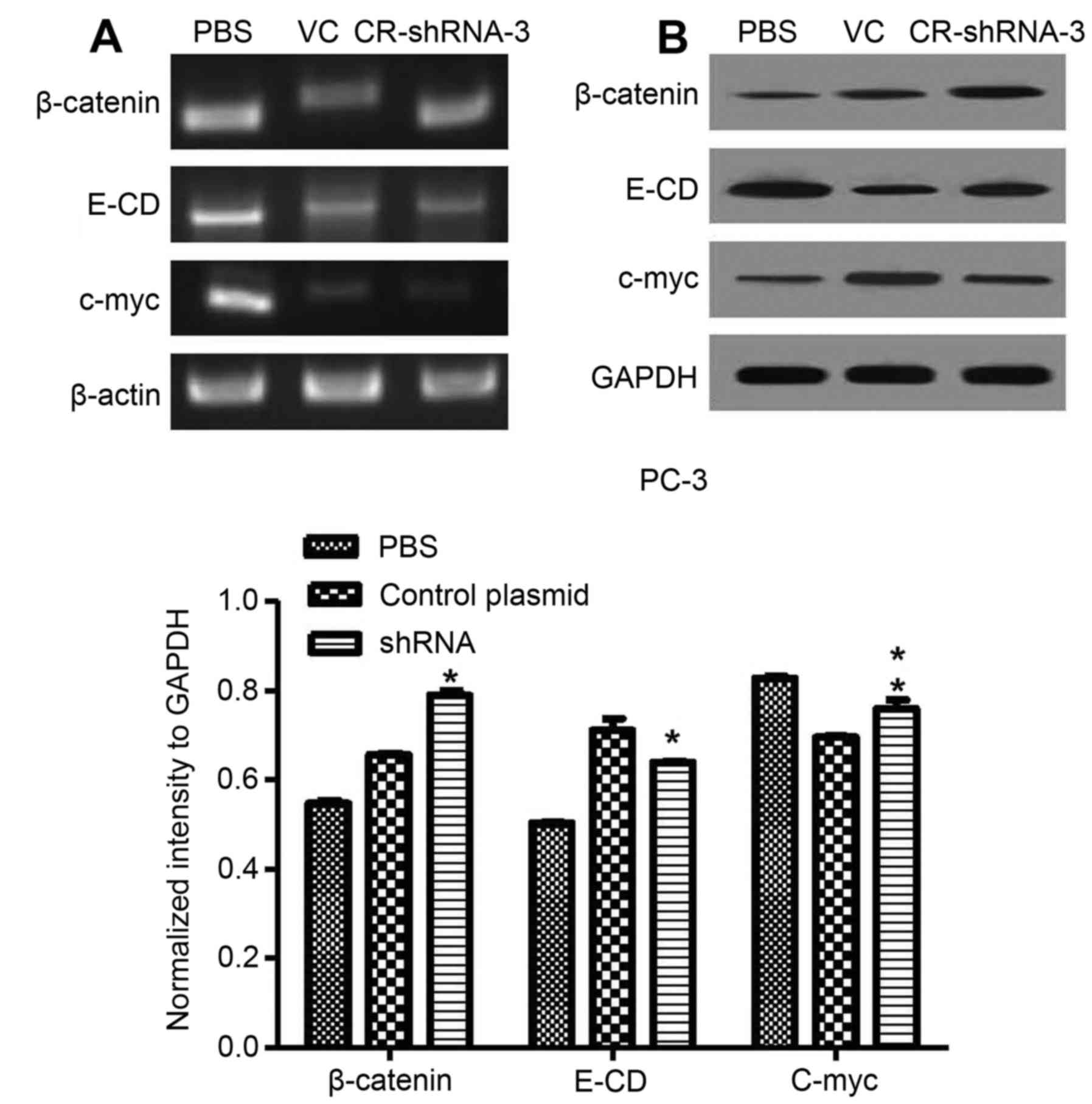
represented an EMT, RT-PCR and western blotting were used to show
the relationship between CR-1 and EMT. We found that c-myc was
markedly decreased by stable transfection with CR-1-shRNA-3. In
addition, β-catenin levels were increased in the membrane, while
E-cadherin expression levels were also higher in PC-3 CR-1-shRNA-3
cells (Fig. 5). Thus, our data
demonstrated that CR-1 likely plays a crucial part in mediating the
EMT in PC-3 cells.
Identification of the characteristic
protein and signal pathways associated with EMT in CR-1 silencing
PC-3 cells after adding Licl
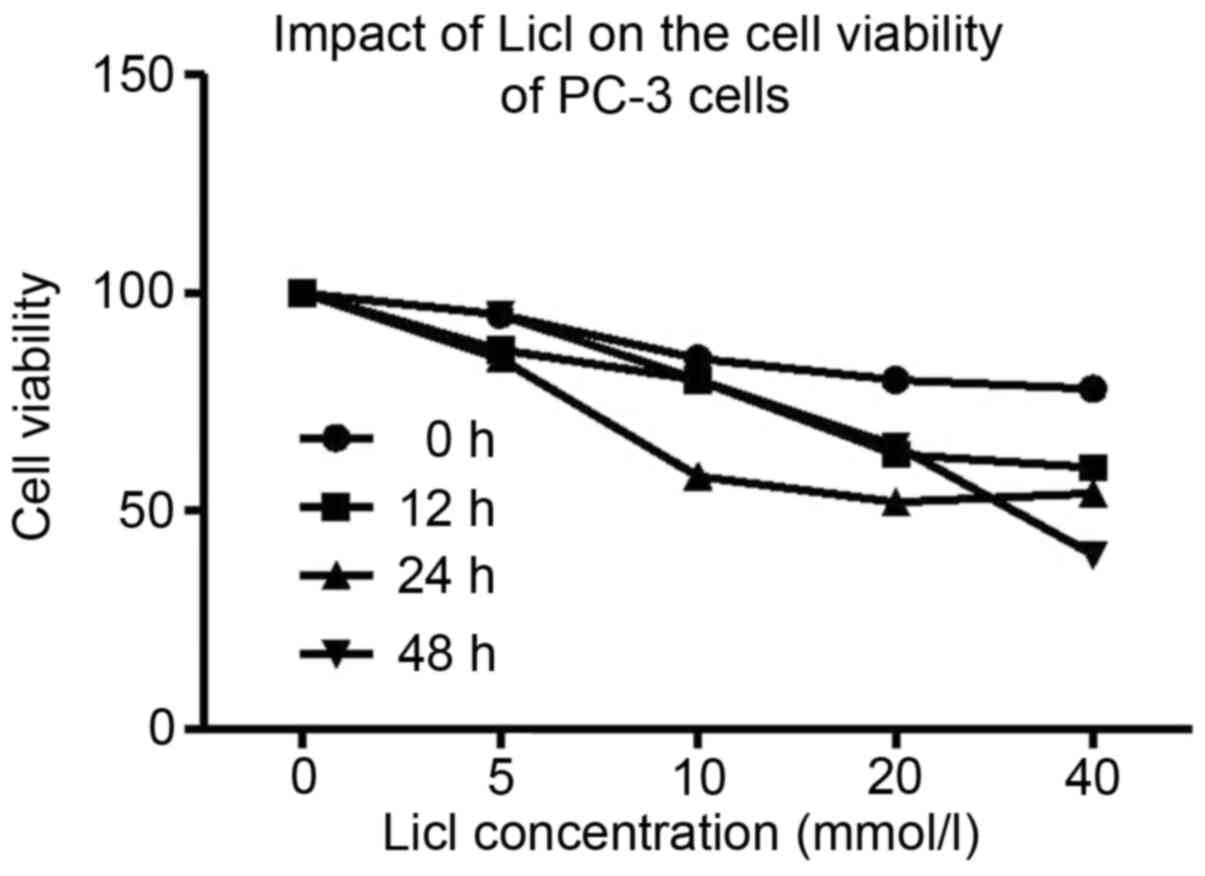
MTT assay demonstrated that after the treatment with
different concentrations of Licl, proliferation of PC-3 cell was
significantly decreased and cells appeared shrunken and non-viable.
Inhibiting effect of Licl was dose-dependent (Fig. 3). We adopted 40 mmol/l of Licl for
48 h to inhibit the tumor growth of PC-3 by Wnt/β-catenin pathway.
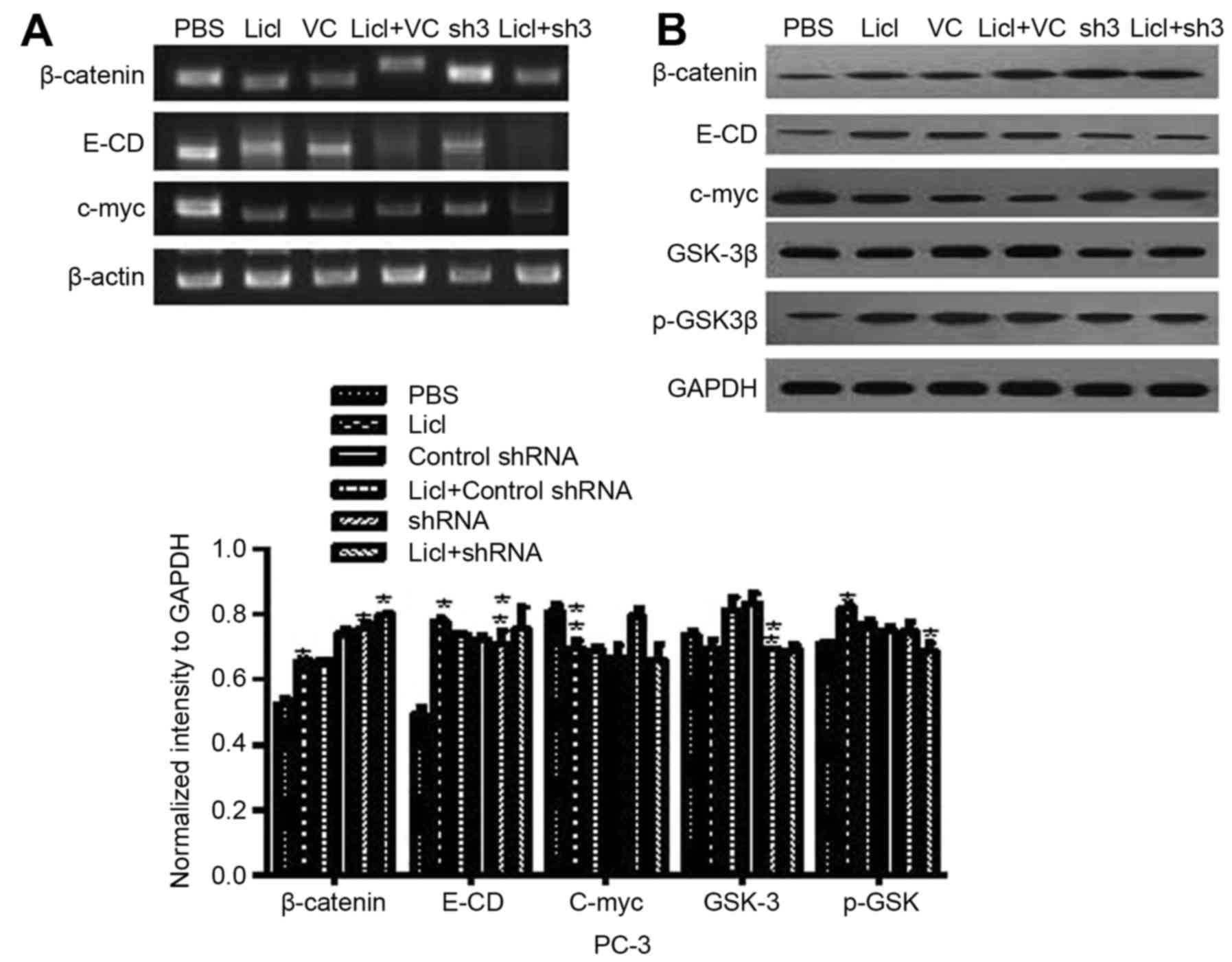
To explore which pathways were activated during EMT mediated by
CR-1, we adopted 5 differentially expressed genes regulated by CR-1
silencing in PC-3 cells after adding Licl. As shown in Fig. 6 we also especially focused on the
relationship between CR-1 and Wnt/β-catenin pathway. Licl
(Wnt/β-catenin pathway inhibitors) was pretreated in CR-1 silenced
PC-3. Licl upregulated E-cadherin, β-catenin and p-GSK-3β in CR-1
silenced PC-3 cell lines. In accordance with mRNA, western blotting
revealed that the effects of CR-1 on enhancing EMT phenotype were
eliminated in Licl pretreated groups. GSK-3β and c-myc were
downregulated (Fig. 6). We
demonstrated that CR-1 acted by Wnt/β-catenin pathway inducing
EMT.
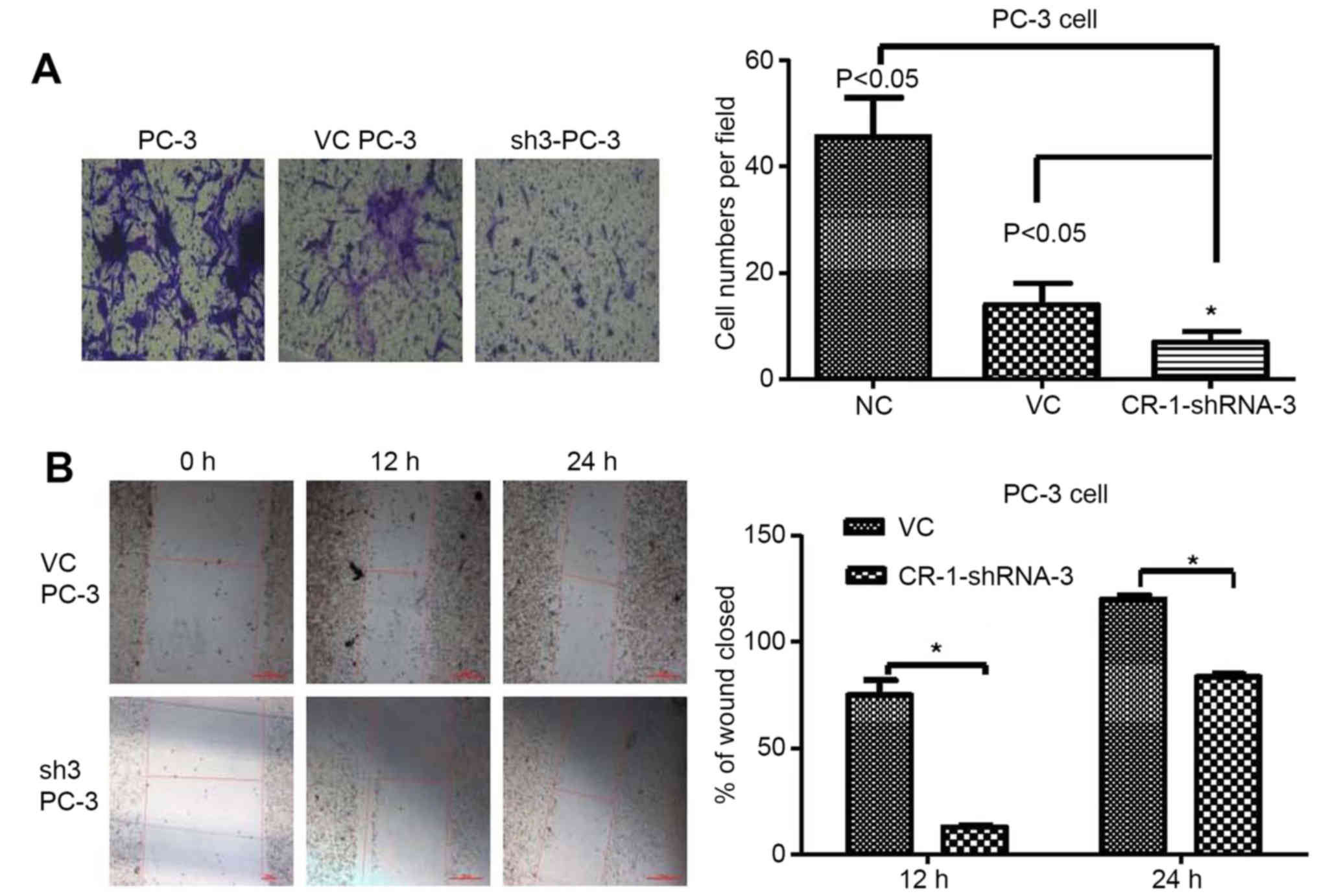
To investigate the invasion effects of
silencing CR-1 in PC-3
CR-1 silencing (CR-1-shRNA-3) and vector control
(VC) cells from PC-3 were established. Transwell assay revealed
that silencing CR-1 strongly suppressed the invasive ability in
PC-3 compared to non-transfected PC-3 or CR-1-VC-shRNA-3 cells
(both P<0.05) (Fig. 7A). High
expression of CR-1 was proved to strengthen invasiveness in PC-3
cells. In addition, wound healing assay showed that silencing CR-1
decreased healing speed of the scratch in transfected cells. Wound
closure rate of CR-1-shRNA-3-PC3 cells was significantly less than
that of non-transfected PC-3 cells and CR-1-VC-shRNA-3-PC3 cells
(both P<0.05). No obvious differences in the wound closure rates
between CR-1-VC-shRNA-3-PC3 cells and non-transfected PC-3 cells
were detected (Fig. 7B).
Collectively, these data indicated that CR-1 stimulated PCa cell
invasiveness.
Discussion
In the present study, high expression levels of CR-1
were observed in PCa which is consistent with previous studies.
Reports illustrated that CR-1 upregulated due to the EGF-CFC family
members (4,17). Gene expressions have shown an
important role of CR-1 by Wnt/β-catenin pathway in PCa cells. CR-1
positively regulated proliferation, invasion and migration leading
to EMT. These findings provided strong evidence that elevated CR-1
promotes metastatic ability, EMT and stages of tumor progression
via Wnt/β-catenin pathway in PC-3, which further supported that
CR-1 could be a therapeutic target in PCa.
Since the role of CR-1 promotes tumor progression
via signaling pathway in tumor cells, some studies have focused on
CR-1 in colon, breast, gastric cancer, hepatocellular carcinoma,
pancreatic and bladder cancer (12,18–20).
It was found that CR-1 stimulated cell proliferation and EMT
(21). Bianco et al
(22) showed that CR-1 activated
the ras/raf/MAPK pathway and suppressed epithelial cells. Shukla
et al (23) reported that
CR-1 was overexpressed in mouse skin carcinogenesis. Xu et
al (24) found that the serum
CR-1 levels were extremely high in lung cancer patients. Another
study also found the same conclusion (11). Here, IHC showed that CR-1 expressed
mainly in PCa cytoplasm, which was in accordance with the report of
Gong et al in breast cancer (25). Furthermore, CR-1 had a positive
correlation with the patient PSA level, Gleason, clinical stage and
lymph node metastasis.
A correlation between CR-1 and EMT marker was also
found in PCa. In the EMT process, downregulation of E-cadherin can
cause cellular changes in different aspects (26,27).
Our present findings indicated that CR-1 silencing resulted in
marked changes in the reduction of mesenchymal proteins and the
appearance of epithelial proteins. E-cadherin levels increased in
CR-1 silenced PC-3 cells with a concomitant increase in β-catenin
expression in membranes. In part, CR-1 could regulate EMT by
activating Wnt/β-catenin pathway in PC-3 cells. We found that
silencing of CR-1 led to β-catenin c-myc and GSK-3β downregulation.
Zhong et al (28) indicated
that overexpressed CR-1 led to lower expression of E-CD in gastric
cancer patients. CR-1 and E-cadherin show potential to become
valuable markers for diagnosing and monitoring of gastric cancer.
Similarly, activated CR-1 induced EMT in HC-11 and Eph4 cells, in
which E-cadherin was downregulated (29). The accumulated data strongly
suggested that silencing of CR-1 might regulate the process of EMT
through downregulation of GSK-3β to activation of E-cadherin. This
agrees with Strizzi et al (9) who demonstrated that CR-1 was well
correlated with EMT marker proteins.
In the present study, we found that after adding
Licl, proliferation of PC-3 cells was decreased. Cell inhibition
rates were time- and concentration-dependent. Hou et al
(30) also reported that Licl
disrupted DNA replication and suppressed PCa cell growth, which was
in accordance with our research. Furthermore, some other evidence
reported that Licl was a direct inhibitor of GSK-3β which induced
GSK-3β N-terminal phosphorylation (31). Licl was used to pretreat CR-1
silencing PC-3 cells. Our results found that the levels of GSK-3β
and c-myc were substantially inhibited by CR-1 silencing after
adding Licl, whereas, it increased the levels of β-catenin,
p-GSK-3β and E-CD by means of Wnt/β-catenin pathway, compared with
the IF control groups. Our data suggested that CR-1 activated by
Wnt/β-catenin induced EMT in PC-3.
In addition, we applied the Transwell assay to
observe cell invasiveness after CR-1 silencing. Silencing CR-1 by
shRNA interference attenuates the CR-1-stimulation-induced
migration and invasion. Results from cells in vitro invasion
indicated that the number of cells that penetrated the basal
membrane from silenced CR-1 group was significantly less than
vector control group. Loss of CR-1 suppressed invasion of PCa
cells. After downregulation of CR-1 expression, the cell invasive
ability and proliferation rate in PC-3 cells was significantly
decreased. Similarly, Huang and colleagues (32) reported that CR-1 interference
significantly reduced the migratory activity of cells, suggesting
that CR-1 enhanced cell invasiveness in esophageal squamous cell
carcinoma. Wu et al (33)
also reported the same conclusion in nasopharyngeal carcinoma. In
brief, CR-1 stimulated PCa cell invasiveness via the activation of
Wnt/β-catenin pathway.
Our key finding is that activated CR-1 can increase
the proliferation and invasion of PCa cells. Levels of EMT relative
proteins were changed in CR-1 silenced PC-3 when Wnt/β-catenin
signaling pathway was activated. This result indicated that CR-1
activated Wnt/β-catenin signaling pathway and promoted the
occurrence of EMT in PCa. CR-1 may serve as a new biological marker
for clinical treatment against PCa.
Acknowledgements
The present study was supported by grants (81472416)
from the National Natural Science Foundation of China and grants
(20140122) from the Tianjin Municipal Education Commission.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Attard G, Parker C, Eeles RA, Schröder F,
Tomlins SA, Tannock I, Drake CG and de Bono JS: Prostate cancer.
Lancet. 387:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saloman DS, Bianco C, Ebert AD, Khan NI,
De Santis M, Normanno N, Wechselberger C, Seno M, Williams K,
Sanicola M, et al: The EGF-CFC family: Novel epidermal growth
factor-related proteins in development and cancer. Endocr Relat
Cancer. 7:199–226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciccodicola A, Dono R, Obici S, Simeone A,
Zollo M and Persico MG: Molecular characterization of a gene of the
‘EGF family’ expressed in undifferentiated human NTERA2
teratocarcinoma cells. EMBO J. 8:1987–1991. 1989.PubMed/NCBI
|
|
6
|
Bianco C, Rangel MC, Castro NP, Nagaoka T,
Rollman K, Gonzales M and Salomon DS: Role of Cripto-1 in stem cell
maintenance and malignant progression. Am J Pathol. 177:532–540.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bianco C, Strizzi L, Normanno N, Khan N
and Salomon DS: Cripto-1: An oncofetal gene with many faces. Curr
Top Dev Biol. 67:85–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Castro NP, Rangel MC, Nagaoka T,
Salomon DS and Bianco C: Cripto-1: An embryonic gene that promotes
tumorigenesis. Future Oncol. 6:1127–1142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strizzi L, Bianco C, Normanno N, Seno M,
Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y,
Sanicola M, et al: Epithelial mesenchymal transition is a
characteristic of hyperplasias and tumors in mammary gland from
MMTV-Cripto-1 transgenic mice. J Cell Physiol. 201:266–276. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wechselberger C, Ebert AD, Bianco C, Khan
NI, Sun Y, Wallace-Jones B, Montesano R and Salomon DS: Cripto-1
enhances migration and branching morphogenesis of mouse mammary
epithelial cells. Exp Cell Res. 266:95–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wechselberger C, Strizzi L, Kenney N,
Hirota M, Sun Y, Ebert A, Orozco O, Bianco C, Khan NI,
Wallace-Jones B, et al: Human Cripto-1 overexpression in the mouse
mammary gland results in the development of hyperplasia and
adenocarcinoma. Oncogene. 24:4094–4105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strizzi L, Bianco C, Normanno N and
Salomon D: Cripto-1: A multifunctional modulator during
embryogenesis and oncogenesis. Oncogene. 24:5731–5741. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rangel MC, Karasawa H, Castro NP, Nagaoka
T, Salomon DS and Bianco C: Role of Cripto-1 during
epithelial-to-mesenchymal transition in development and cancer. Am
J Pathol. 180:2188–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prasad CP, Rath G, Mathur S, Bhatnagar D,
Parshad R and Ralhan R: Expression analysis of E-cadherin, Slug and
GSK3beta in invasive ductal carcinoma of breast. BMC Cancer.
9:3252009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Che Q, Bian Y, Zhou Q, Jiang F, Tong
H, Ke J, Wang K and Wan XP: Autocrine motility factor promotes
epithelial-mesenchymal transition in endometrial cancer via MAPK
signaling pathway. Int J Oncol. 47:1017–1024. 2015.PubMed/NCBI
|
|
17
|
Adamson ED, Minchiotti G and Salomon DS:
Cripto: A tumor growth factor and more. J Cell Physiol.
190:267–278. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bianco C, Normanno N, De Luca A, Maiello
MR, Wechselberger C, Sun Y, Khan N, Adkins H, Sanicola M,
Vonderhaar B, et al: Detection and localization of Cripto-1 binding
in mouse mammary epithelial cells and in the mouse mammary gland
using an immunoglobulin-cripto-1 fusion protein. J Cell Physiol.
190:74–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei B, Jin W, Ruan J, Xu Z, Zhou Y, Liang
J, Cheng H, Jin K, Huang X, Lu P, et al: Cripto-1 expression and
its prognostic value in human bladder cancer patients. Tumour Biol.
36:1105–1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JH, Wei W, Xu J, Guo ZX, Xiao CZ,
Zhang YF, Jian PE, Wu XL, Shi M and Guo RP: Elevated expression of
Cripto-1 correlates with poor prognosis in hepatocellular
carcinoma. Oncotarget. 6:35116–35128. 2015.PubMed/NCBI
|
|
21
|
Nagaoka T, Karasawa H, Castro NP, Rangel
MC, Salomon DS and Bianco C: An evolving web of signaling networks
regulated by Cripto-1. Growth Factors. 30:13–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bianco C, Castro NP, Baraty C, Rollman K,
Held N, Rangel MC, Karasawa H, Gonzales M, Strizzi L and Salomon
DS: Regulation of human Cripto-1 expression by nuclear receptors
and DNA promoter methylation in human embryonal and breast cancer
cells. J Cell Physiol. 228:1174–1188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shukla A, Ho Y, Liu X, Ryscavage A and
Glick AB: Cripto-1 alters keratinocyte differentiation via blockade
of transforming growth factor-beta1 signaling: Role in skin
carcinogenesis. Mol Cancer Res. 6:509–516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu CH, Wang Y, Qian LH, Yu LK, Zhang XW
and Wang QB: Serum Cripto-1 is a novel biomarker for non-small cell
lung cancer diagnosis and prognosis. Clin Respir J. Nov
25–2015.(Epub ahead of print) doi: 10.1111/crj.12414. View Article : Google Scholar
|
|
25
|
Gong YP, Yarrow PM, Carmalt HL, Kwun SY,
Kennedy CW, Lin BP, Xing PX and Gillett DJ: Overexpression of
Cripto and its prognostic significance in breast cancer: A study
with long-term survival. Eur J Surg Oncol. 33:438–443. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klauzinska M, Castro NP, Rangel MC, Spike
BT, Gray PC, Bertolette D, Cuttitta F and Salomon D: The
multifaceted role of the embryonic gene Cripto-1 in cancer, stem
cells and epithelial-mesenchymal transition. Semin Cancer Biol.
29:51–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ,
Du H, Zhang GG, Hu Y, Lu AP, Li JY, et al: Positive association of
up-regulated Cripto-1 and down-regulated E-cadherin with tumour
progression and poor prognosis in gastric cancer. Histopathology.
52:560–568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Strizzi L, Bianco C, Raafat A, Abdallah W,
Chang C, Raafat D, Hirota M, Hamada S, Sun Y, Normanno N, et al:
Netrin-1 regulates invasion and migration of mouse mammary
epithelial cells overexpressing Cripto-1 in vitro and in vivo. J
Cell Sci. 118:4633–4643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou CL, Zhang ZH, Huang DL and Sun AJ:
LiCl suppresses tumor growth and inhibits DNA replication in
prostate cancer. Zhonghua Bing Li Xue Za Zhi. 41:475–478. 2012.(In
Chinese). PubMed/NCBI
|
|
31
|
Zhang F, Phiel CJ, Spece L, Gurvich N and
Klein PS: Inhibitory phosphorylation of glycogen synthase kinase-3
(GSK-3) in response to lithium. Evidence for autoregulation of
GSK-3. J Biol Chem. 278:33067–33077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang C, Chen W, Wang X, Zhao J, Li Q and
Fu Z: Cripto-1 promotes the epithelial-mesenchymal transition in
esophageal squamous cell carcinoma cells. Evid Based Complement
Alternat Med. 2015:4212852015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Z, Li G, Wu L, Weng D, Li X and Yao K:
Cripto-1 overexpression is involved in the tumorigenesis of
nasopharyngeal carcinoma. BMC Cancer. 9:3152009. View Article : Google Scholar : PubMed/NCBI
|