Introduction
Pyruvate kinase converts phosphoenolpyruvate to
pyruvate, catalyzing the rate-limiting step of glycolysis (1). The M1 isoenzyme of pyruvate kinase
(PKM1) is found in adult tissues, but the M2 isoenzyme of pyruvate
kinase (PKM2) is a spliced variant found in embryonic stem cells
(2) and cancer cells (3,4). It
has been recently demonstrated that expression of PKM2 contributes
to maintain the pluripotency of embryonic stem cells under hypoxic
condition (2). The expression of
PKM2 in cancer cells is well known to shift an aerobic glycolysis,
a phenomenon known as Warburg effect (3–5).
Beyond the metabolic function, PKM2 expression in cancer cells is
also well known to regulate tumor formation and growth by being
instrumental in gene transcription (6,7). Many
studies have demonstrated that the enhanced expression of PKM2
associates with therapeutic resistance and contributes to poor
prognosis of cancers (8,9). However, it is still necessary to
further understand how PKM2 regulates the cell biological property
of cancer cells.
The heterogeneity of cancer cells is generally
accepted, and a stem cell-like subpopulation, known as ‘cancer stem
cells’ (CSCs) has been identified in various types of malignant
tumors (10,11). Although lacking of consensus on the
definition and markers for identification, CSCs are widely
recognized as a subpopulation among cancer cells that keeps the
properties of self-renewal and tumor initiation (10,11).
CSCs are also well known to play key roles in the tumor therapeutic
resistance, metastasis and recurrence (12). Therefore, CSCs are considered to be
promising targets for the treatment of cancers.
Normal tissue specific stem cells are considered as
the main origin of cancers (13,14),
and the CSCs are thought to have inherited, at least partly, the
characterization of normal tissue specific stem cells. Actually,
the identification of CSCs has simply shared markers of
hematopoietic stem cells, including the most popularly used cell
surface markers of CD44 and CD133 (15–17).
The expression of either CD44 or CD133 in cancer cells has also
been previously demonstrated to contribute to therapeutic
resistance (18,19). As CD44 positive lung cancer cells
were shown to have stem-like properties in a previous study
(17), we used CD44 as lung cancer
stem cell biomarker in the present study. Considering the
relatively low oxygen microenvironment of stem cell niche (20,21),
we herein examined the hypothesis that cancer cells might enhance
the expression of glycolytic enzyme PKM2 to facilitate the
phenotype of CSCs, which thereby change the cell biological
properties in resistance to chemotherapy, radiotherapy and various
stresses.
Materials and methods
Immunohistological analysis on the
expression of PKM2 and CD44 in human lung cancer tissues
By using surgical resected tissue samples from 5
patients of lung cancer (3 slides per sample), we investigated the
expression and co-localization of PKM2 and CD44 in lung cancer
cells. Briefly, 5-µm paraffin tissue sections were mounted on
polylysine-coated slides. The slides were deparaffinized in xylene,
cleared in a graded ethanol series and heat-treated for 30 min in
10 mM citrate buffer pH 7.0 in a microwave at 98°C for antigen
retrieval. After blocking with Blocking One Histo (Nacalai Tesque,
Inc., Kyoto, Japan), slides were stained with primary antibody
specific against PKM2 (#4053S; Cell Signal Technology, Danvers, MA,
USA) for 1 h at room temperature, and then followed by
PE-conjugated secondary antibody. Slides were washed and then
incubated with FITC-conjugated rabbit anti-human CD44 antibody
(R&D Systems, Minneapolis, MN, USA). Nuclei were labelled with
DAPI. Informed consent was obtained from each patient, and the
study protocol was approved by the Ethics Committee of Jiangxi
Cancer Hospital.
Human lung cancer cells and cell
culture
The A549 human lung cancer cells were maintained in
RPMI-1640 basic medium supplemented with 10% fetal bovine serum
(FBS), in a humidified atmosphere of 95% air and 5% CO2
at 37°C.
Immunocytochemistry
To confirm the relationship between an enhanced
expression of PKM2 and the phenotype of CSCs, we performed double
immunostaining with PKM2 and CD44. Briefly, cells cultured on
4-well culture slides were fixed in 1% formaldehyde for 10 min.
After blocking with 2% bovine serum albumin (BSA), the cells were
incubated with the rabbit specific anti-human PKM2 polyclonal
antibody (#4053S; Cell Signal Technology) for 1 h at room
temperature and then followed by PE-conjugated secondary antibody.
Culture slides were washed and then were incubated with
FITC-conjugated rabbit anti-human CD44 antibody (R&D Systems).
Cell nuclei were stained with DAPI. The positively stained cells
were observed under fluorescence microscope with 200-fold
magnification.
The purification of CD44+
CSCs
The CD44+ CSCs were purified by using the
Magnetic Cell Sorting system (autoMACS; Miltenyi Biotec, Inc.,
Auburn, CA, USA) (22). Briefly,
single cell suspension of A549 cells was incubated with anti-human
CD44 antibody (Miltenyi Biotec) for 30 min. After washing,
CD44+ cells were separated by passing a MACS column. The
purity of the CD44+ CSCs collected by the autoMACS was
~95%.
Knockdown of PKM2
To further understand the biological roles of PKM2
expression in cancer cells, we knocked down the expression of PKM2
in CD44+ CSCs by PKM siRNA (L-006781-00-0005; GE
Healthcare Dharmacon, Inc., Lafayette, CO, USA). The control group
was transfected with a negative control siRNA (Thermo Fisher
Scientific/Dharmacon). Briefly, cells were seeded in 6-well plates
or 4-well chamber slides (for immunostaining), and transient
transfections were then performed with DharmaFECT-1 transfection
reagent according to THE manufacturer's protocol. The efficiency on
PKM2 siRNA knockdown was confirmed by western blot analysis as
described below.
Western blot analysis
The expression level of PKM2 in CD44+
CSCs was measured by western blot analysis as previously described
(23). Briefly, the total protein
was purified from cells, separated using SDS-PAGE gels, and then
were transferred to nitrocellulose membranes. After blocking, the
membranes were incubated with rabbit polyclonal antibody specific
against PKM2 (#4053S; Cell Signal Technology) or goat monoclonal
antibody against β-actin (Cell Signal Technology), followed by the
appropriate horseradish peroxidase-conjugated secondary antibodies.
The expression was visualized using an enhanced chemiluminescence
detection kit.
Spheroid formation assay
We further examined the characterization of CSCs by
spheroid formation assay. Briefly, 1×103
CD44+ CSCs were seeded in 60 mm low cell binding dish
(Nunc, Roskilde, Denmark). After 7 days of culture, the formation
of spheres was observed under phase-contrast microscopy and the
total number of spheroids in each culture dish was counted.
Clonogenic assay
For clonogenic assay, we seeded CD44+
CSCs into 6-well plates at a density of 100 cells/well (24). After an overnight incubation, the
cells were exposed to the indicated doses of γ-rays, and the
formation of colonies was quantified 7 days after irradiation.
Colonies with >50 cells were counted under a microscope.
Assessment of anticancer drug
sensitivity
To evaluate the anticancer drug sensitivity, cells
were seeded in 96-well culture plates at a density of
5×103 cells/well and cultured overnight. Cells were then
treated with various concentrations of cisplatin (CDDP). After 48
h, cell survival and proliferation was evaluated by the MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. Briefly, MTT were added to medium for 4 h, and the formation
of formazan from MTT in viable cells was stopped by adding lysis
buffer 24 h after the addition of MTT. The absorbance of formazan
was measured at 570 nm using a microplate reader (Thermo Scientific
Multiskan FC).
Estimation of the tolerance to FBS and
oxygen depletion stresses
To evaluate the resistance of cells to FBS
deprivation and hypoxic stress, cells were seeded in 96-well
culture plates at a density of 5×103 cells/well and
maintained in a low FBS (1% FBS) culture medium or low oxygen
condition (1% O2) for 24 h. The survival and
proliferation of cells was then measured by the MTT assay as
described above.
Statistical analysis
All results are presented as the means ± SD. The
statistical analysis was done by one-way ANOVA followed by the
Bonferroni post hoc test among groups, or by the unpaired t-test
between two groups (SPSS II; SPSS, Inc., Chicago, IL, USA).
Differences were considered significant at P<0.05.
Results
Co-expression of PKM2 and CD44 in lung
cancer cells from surgically resected tissue samples
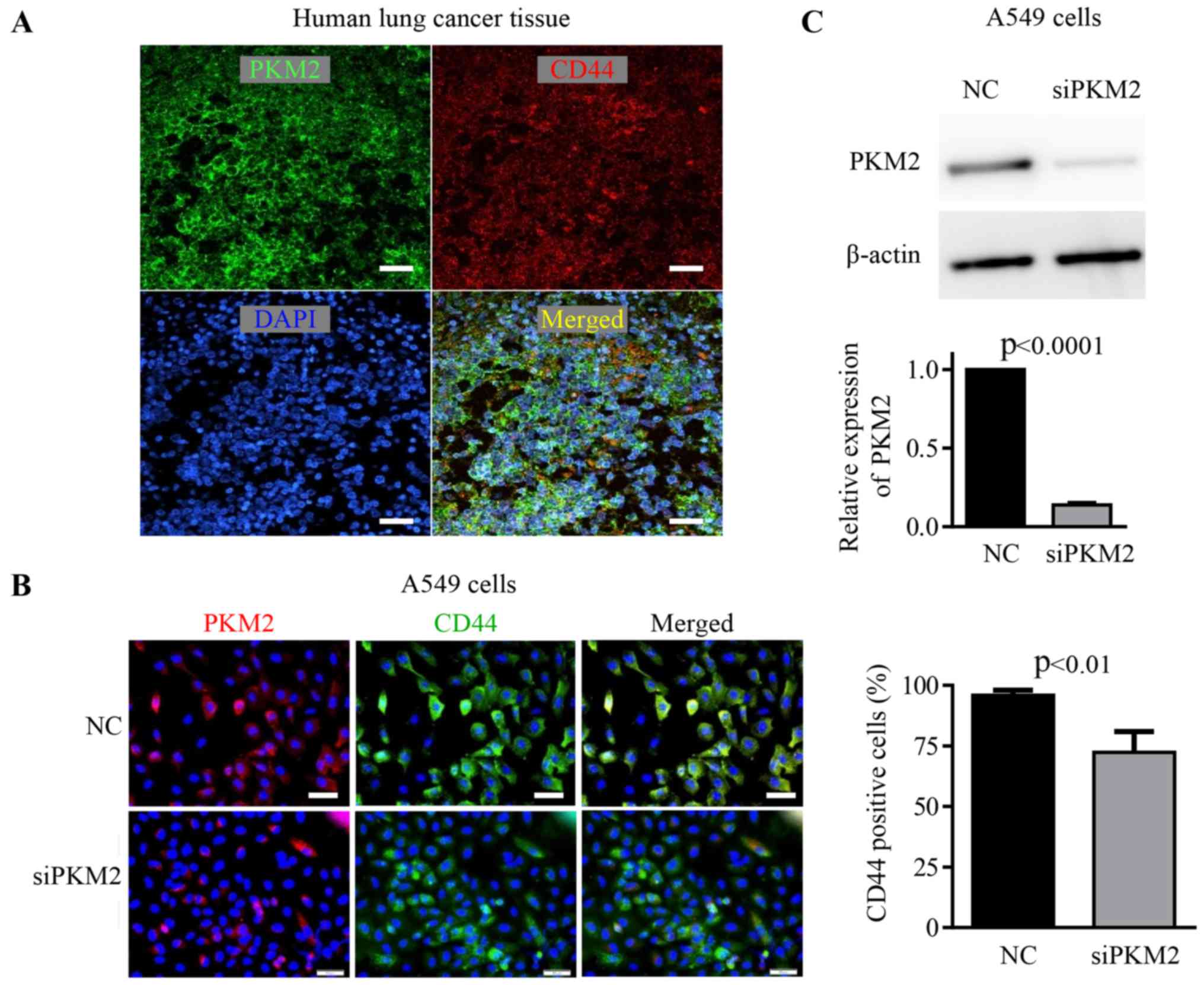
By using lung cancer tissue samples from patients,
we could clearly observe that some lung cancer cells positively
expressed CD44 (Fig. 1A).
Furthermore, these CD44+ cancer cells were also highly
expressed with PKM2 (Fig. 1A),
supported the likely relationship between PKM2 expression and
cancer stem cell phenotype.
Enhanced expression of PKM2 is closely
associated with the phenotype of CSCs
PKM2 was highly expressed in ~80% of the A549 lung
cancer cells. Over 70% of these A549 cells also positively
expressed CD44, a marker popularly used for identifying CSCs. We
noted that the enhanced expression of PKM2 was specially observed
in the cells that also strongly expressed CD44 (Fig. 1B).
To confirm the directly relationship between PKM2
expression and the biological property of CSCs, we purified
CD44+ CSCs for further studies. We knocked down the
expression of PKM2 in CD44+ CSCs by siRNA, and then
observed how the decrease of PKM2 expression could change the
phenotype of CD44+ CSCs. The siRNA knockdown clearly
decreased the expression of PKM2 in CD44+ CSCs (Fig. 1B and C), and also resulted in a
slightly decrease in the expression of CD44 in cells (Fig. 1B).
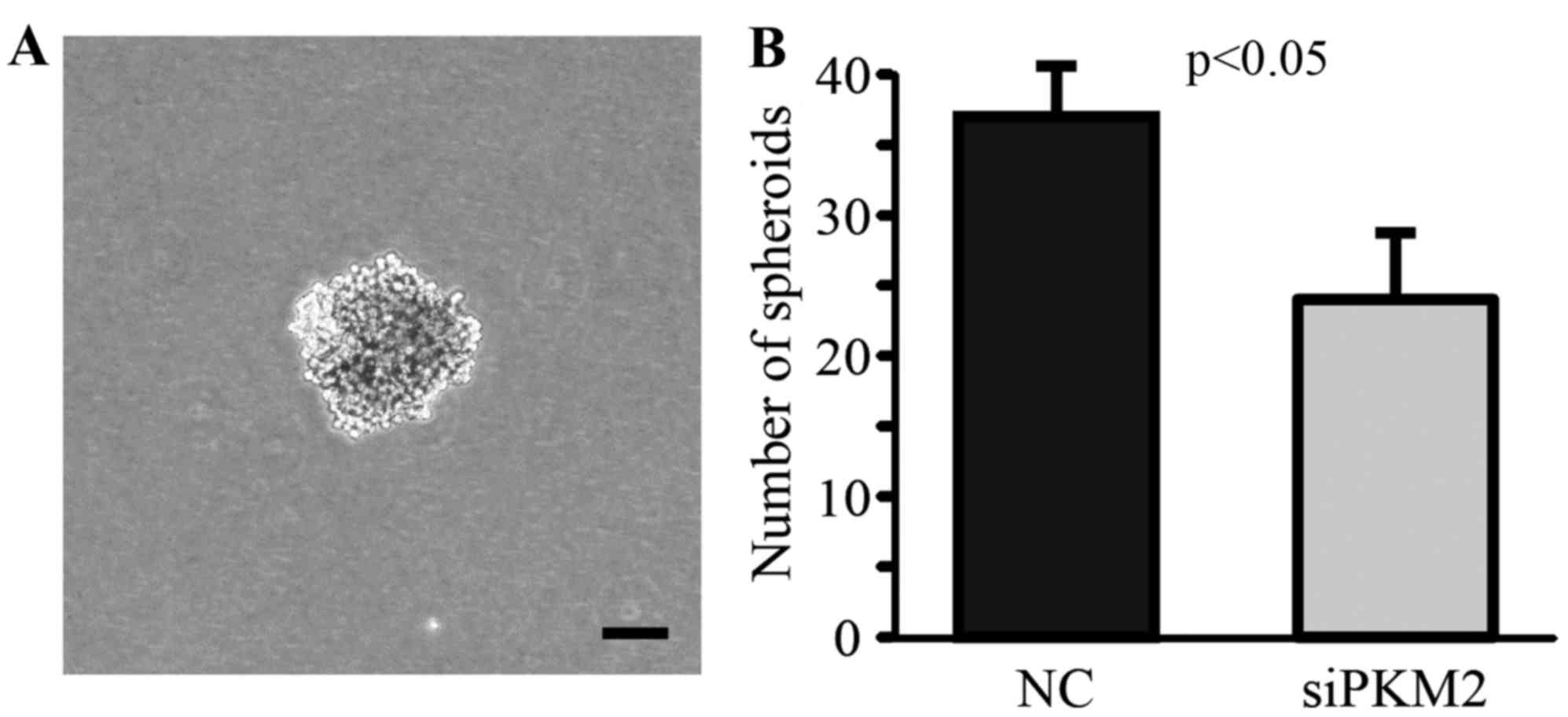
By using the spheroid forming assay, a widely
accepted in vitro method for the assessment of cancer stem
cell potency, we found that the knockdown of PKM2 significantly
decreased the property of CD44+ CSCs in forming spheroid
in vitro (Fig. 2). These
data suggest that the enhanced PKM2 expression directly associates
with cancer stem cell phenotype.
Enhanced PKM2 expression contributes
to the stress resistance of CSCs
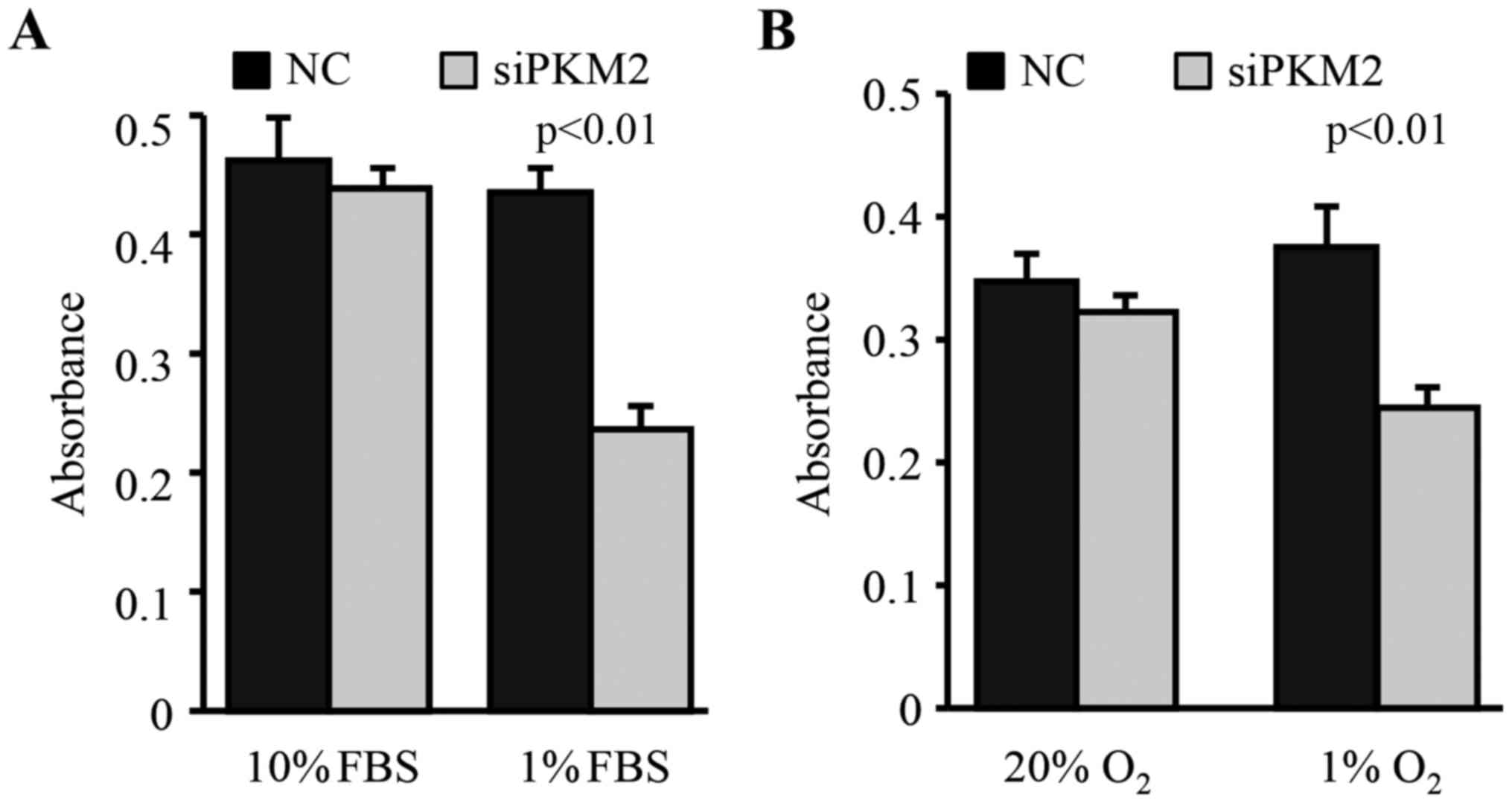
To test how the enhanced PKM2 expression in
CD44+ CSCs contributes to resistant to stresses, we
exposed cells to FBS deprivation and hypoxic conditions. We found
that the downregulation of PKM2 in CD44+ CSCs did not
change their survival in medium supplemented with 10% FBS and under
20% oxygen condition (Fig. 3).
However, the knockdown of PKM2 by siRNA significantly decreased the
survival of CD44+ CSCs under FBS deprivation (1% FBS)
and low oxygen (1% O2) conditions (Fig. 3).
Enhanced PKM2 expression contributes
to the therapeutic resistance of CSCs
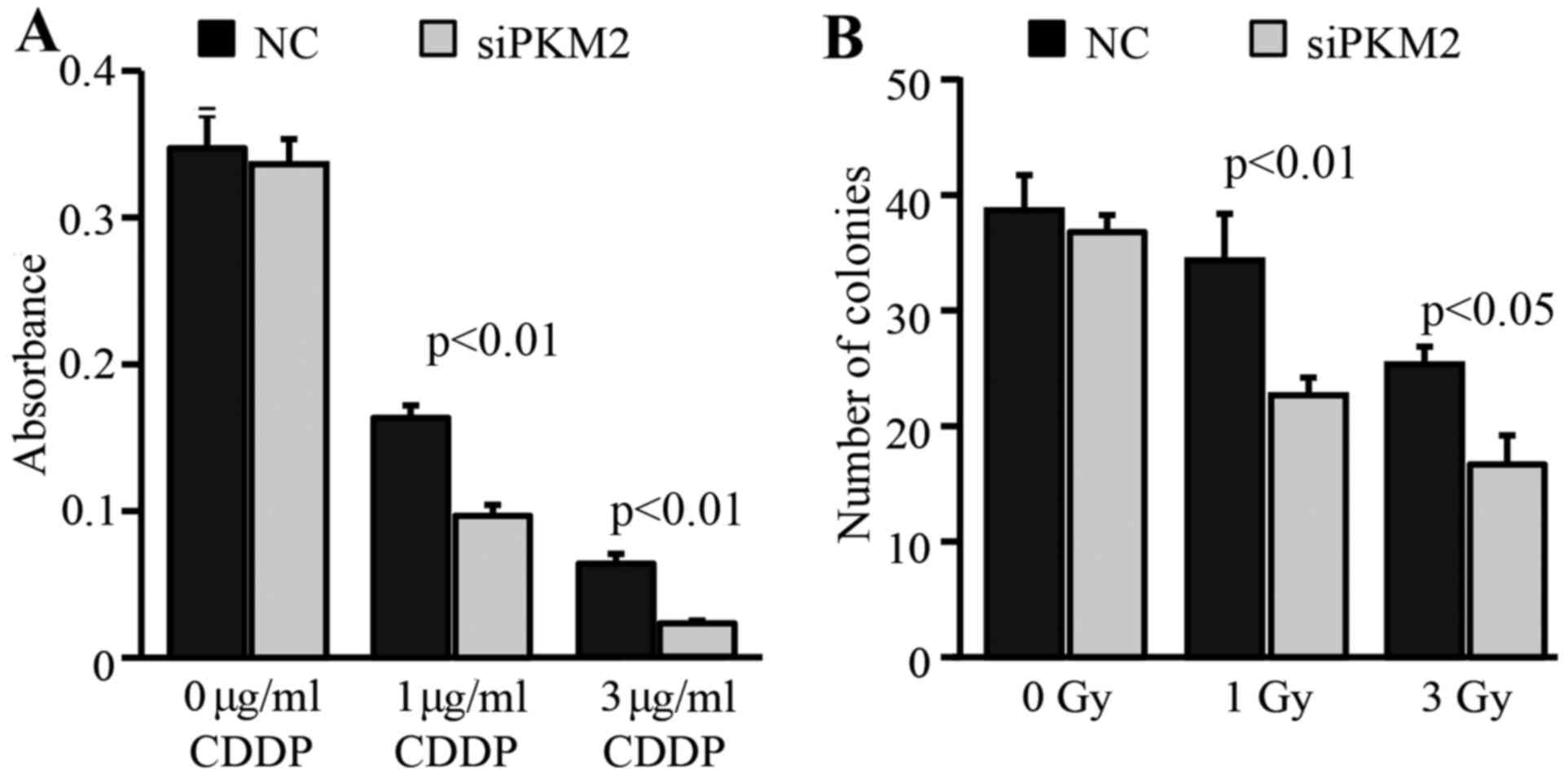
We also examined how the enhanced expression of PKM2
in CD44+ CSCs contributes to resistance to chemotherapy
and radiotherapy. The knockdown of PKM2 expression in
CD44+ CSCs significantly decreased their survival under
different concentrations of CDDP (Fig.
4A). Similarly, clonogenic assay showed that the knockdown of
PKM2 expression also significantly decreased the colony formation
capacity of CD44+ CSCs after exposure to 1 or 3 Gy γ-ray
(Fig. 4B).
Discussion
PKM2 is generally known to be expressed at high
levels in tumors, which functionally contribute to the rapidly
growth of cancer cells even under a relative hypoxic
microenvironment (3–6,25).
However, PKM2 is not necessary for tumor cell proliferation, and
heterozygous PKM2 mutations have been found in human tumors
(26). It has also been implied
that the inactive state of PKM2 is associated with the
proliferating cell population within tumors, but non-proliferating
tumor cells require active pyruvate kinase (26). This suggests that the variable of
PKM2 expression supports the different metabolic requirements of
proliferating and non-proliferating tumor cells. Considering the
heterogeneity of tumor cells (10,11),
the relative quiescent state of cancer stem cells may well match to
these non-proliferating tumor cells with high pyruvate kinase
expression. Actually, our data from both clinical lung cancer
tissue samples and a human lung cancer cell line have clearly shown
an enhanced expression of PKM2 in these cancer cells that
positively expressed CD44, a popular marker for CSCs in various
types of cancers.
To further confirm the role of PKM2 on the
biological property of cancer stem cells, we knocked down the PKM2
expression in CD44+ CSCs. In agreement with a previous
study (27), the PKM2 knockdown in
CD44+ CSCs resulted in a modest impairment of cell
proliferation under general culture conditions. Cell apoptosis is
rarely observed in these CD44+ CSCs by TUNEL staining,
thus, we did not regularly evaluate cell apoptosis in this study.
Notably, the PKM2 knockdown in CD44+ CSCs significantly
decreased the capacity of spheroids formation in vitro and
the resistant to FBS deprivation and hypoxic stress, but
significantly increased the sensitivity to chemotherapy and
radiotherapy. All of these data confirmed the direct relationship
between the enhanced expression of PKM2 and the biological property
of cancer stem cells. As PKM1 and PKM2 are different splicing
products of PKM gene (exon 9 for PKM1 and exon 10 for PKM2)
(28), it is impossible to
specifically knock down PKM2. Although cancer cells are known to
mainly express PKM2, the transfection with PKM siRNA in the present
study may also change the expression of PKM1 and probably results
in some other off-target effects.
The expression of CD44 in cancer stem cells has been
previously demonstrated to associate with the resistance to
oxidative stress and chemotherapy (29). PKM2 expression in cancer cells is
also well known to contribute to tumor progression and therapeutic
resistance (3–7,30). A
recent study has reported that the ablation of CD44 expression in
hypoxic cancer cells significantly increases the expression of PKM2
but decreases glucose uptake, which results in an enhanced
sensitivity to anticancer drugs (21). In the present study, we simply used
CD44 as a marker for the identification/purification of cancer stem
cells. An enhanced expression of PKM2 was observed in these
CD44+ CSCs, but the role of CD44 expression on the
resistance to drugs was not investigated. Therefore, the direct
regulatory relationship between PKM2 and CD44 is still
questionable. This study is largely limited by using a single cell
line. We attempted to isolate CD44+ cells from several
other lung cancer cell lines, such as NCI-H1975, NCI-H1299 and
NCI-H1437 cells. However, the purified CD44+ cells from
these cell lines showed very poor stability on the expression of
CD44 during the culture process. Further studies in other cancer
cell lines will be needed to confirm our findings.
Although the clear relationship between the enhanced
expression of PKM2 and the biological property of cancer stem
cells, it is unclear how the PKM2 expression facilitates the
biological property of cancer stem cells. Beyond the function in
regulating anabolic metabolism, PKM2 is also known to serve as
transcriptional co-activators (4–7). A
recent study has demonstrated that the stimulation of
epithelial-mesenchymal transition results in the nuclear
translocation of PKM2 in colon cancer cells (31). Furthermore, it has been found that
the phosphorylated PKM2 can translocate into the nucleus to serve
as a co-activator of β-catenin to induce c-Myc expression (32). As the expression of c-Myc and
β-catenin has been demonstrated to associate with normal tissue
stem cells, pluripotent stem cells, and cancer stem cells (33–35),
it is possible that PKM2 facilitates the biological property of
cancer stem cells through the induction of β-catenin and c-Myc.
The data from the present study clearly indicate
that the enhanced expression of PKM2 in lung cancer cells likely
associated with the biological properties of cancer stem cells,
including the resistant to various stresses and therapies.
Therefore, selective targeting of PKM2 may change the biological
property of cancer stem cells, which provides a new strategy for
treatment of cancer.
Acknowledgements
The present study was supported by the Jiangxi
Provinces Program of the Preponderant Team Building in Science and
Technology Innovation (no. 20161BCB24011), the Jiangxi Province
Sailing Project, the National Natural Science Foundation of China
(no. 81560382); and a grant-in-aid from the Ministry of Education,
Science, Sports, Culture and Technology, Japan.
References
|
1
|
Heiden MG Vander, Lunt SY, Dayton TL,
Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G,
Cantley LC, Metallo CM, et al: Metabolic pathway alterations that
support cell proliferation. Cold Spring Harb Symp Quant Biol.
76:325–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christensen DR, Calder PC and Houghton FD:
GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic
culture of human embryonic stem cells. Sci Rep. 5:175002015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iqbal MA, Gupta V, Gopinath P, Mazurek S
and Bamezai RN: Pyruvate kinase M2 and cancer: An updated
assessment. FEBS Lett. 588:2685–2692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamada M, Suematsu M and Saya H: Pyruvate
kinase M2: Multiple faces for conferring benefits on cancer cells.
Clin Cancer Res. 18:5554–5561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo W and Semenza GL: Emerging roles of
PKM2 in cell metabolism and cancer progression. Trends Endocrinol
Metab. 23:560–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y, Li X, Yang W, Hawke DH, Zheng Y,
Xia Y, Aldape K, Wei C, Guo F, Chen Y, et al: PKM2 regulates
chromosome segregation and mitosis progression of tumor cells. Mol
Cell. 53:75–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Yang P and Li Z: The multifaceted
regulation and functions of PKM2 in tumor progression. Biochim
Biophys Acta. 1846:285–296. 2014.PubMed/NCBI
|
|
8
|
Li SL, Ye F, Cai WJ, Hu HD, Hu P, Ren H,
Zhu FF and Zhang DZ: Quantitative proteome analysis of multidrug
resistance in human ovarian cancer cell line. J Cell Biochem.
109:625–633. 2010.PubMed/NCBI
|
|
9
|
Wang Y, Zhang X, Zhang Y, Zhu Y, Yuan C,
Qi B, Zhang W, Wang D, Ding X, Wu H, et al: Overexpression of
pyruvate kinase M2 associates with aggressive clinicopathological
features and unfavorable prognosis in oral squamous cell carcinoma.
Cancer Biol Ther. 16:839–845. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valent P, Bonnet D, Wöhrer S, Andreeff M,
Copland M, Chomienne C and Eaves C: Heterogeneity of neoplastic
stem cells: Theoretical, functional, and clinical implications.
Cancer Res. 73:1037–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Islam F, Gopalan V, Smith RA and Lam AK:
Translational potential of cancer stem cells: A review of the
detection of cancer stem cells and their roles in cancer recurrence
and cancer treatment. Exp Cell Res. 335:135–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tomasetti C and Vogelstein B: Cancer
etiology. Variation in cancer risk among tissues can be explained
by the number of stem cell divisions. Science. 347:78–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Visvader JE: Cells of origin in cancer.
Nature. 469:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woodward WA and Sulman EP: Cancer stem
cells: Markers or biomarkers? Cancer Metastasis Rev. 27:459–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y and Wu PY: CD133 as a marker for
cancer stem cells: Progresses and concerns. Stem Cells Dev.
18:1127–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung EL, Fiscus RR, Tung JW, Tin VP,
Cheng LC, Sihoe AD, Fink LM, Ma Y and Wong MP: Non-small cell lung
cancer cells expressing CD44 are enriched for stem cell-like
properties. PLoS One. 5:e140622010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suda T, Takubo K and Semenza GL: Metabolic
regulation of hematopoietic stem cells in the hypoxic niche. Cell
Stem Cell. 9:298–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells
display stem-like features and are spared by cisplatin treatment.
Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamada M, Nagano O, Tateyama S, Ohmura M,
Yae T, Ishimoto T, Sugihara E, Onishi N, Yamamoto T, Yanagawa H, et
al: Modulation of glucose metabolism by CD44 contributes to
antioxidant status and drug resistance in cancer cells. Cancer Res.
72:1438–1448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo CY, Luo L, Urata Y, Goto S, Huang WJ,
Takamura S, Hayashi F, Doi H, Kitajima Y, Ono Y, et al: Sensitivity
and dose dependency of radiation-induced injury in hematopoietic
stem/progenitor cells in mice. Sci Rep. 5:80552015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li TS, Cheng K, Lee ST, Matsushita S,
Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR and Marbán E:
Cardiospheres recapitulate a niche-like microenvironment rich in
stemness and cell-matrix interactions, rationalizing their enhanced
functional potency for myocardial repair. Stem Cells. 28:2088–2098.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan C, Luo L, Goto S, Urata Y, Guo CY, Doi
H, Kitazato K and Li TS: Enhanced autophagy in colorectal cancer
stem cells does not contribute to radio-resistance. Oncotarget.
7:45112–45121. 2016.PubMed/NCBI
|
|
25
|
Christofk HR, Heiden MG Vander, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Israelsen WJ, Dayton TL, Davidson SM,
Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW,
et al: PKM2 isoform-specific deletion reveals a differential
requirement for pyruvate kinase in tumor cells. Cell. 155:397–409.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortés-Cros M, Hemmerlin C, Ferretti S,
Zhang J, Gounarides JS, Yin H, Muller A, Haberkorn A, Chene P,
Sellers WR, et al: M2 isoform of pyruvate kinase is dispensable for
tumor maintenance and growth. Proc Natl Acad Sci USA. 110:489–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dombrauckas JD, Santarsiero BD and Mesecar
AD: Structural basis for tumor pyruvate kinase M2 allosteric
regulation and catalysis. Biochemistry. 44:9417–9429. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc(−) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Papadaki C, Sfakianaki M, Lagoudaki E,
Giagkas G, Ioannidis G, Trypaki M, Tsakalaki E, Voutsina A,
Koutsopoulos A, Mavroudis D, et al: PKM2 as a biomarker for
chemosensitivity to front-line platinum-based chemotherapy in
patients with metastatic non-small-cell lung cancer. Br J Cancer.
111:1757–1764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamabe A, Konno M, Tanuma N, Shima H,
Tsunekuni K, Kawamoto K, Nishida N, Koseki J, Mimori K, Gotoh N, et
al: Role of pyruvate kinase M2 in transcriptional regulation
leading to epithelial-mesenchymal transition. Proc Natl Acad Sci
USA. 111:15526–15531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo
F, Lyssiotis CA, Aldape K, Cantley LC and Lu Z: ERK1/2-dependent
phosphorylation and nuclear translocation of PKM2 promotes the
Warburg effect. Nat Cell Biol. 14:1295–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benoit YD, Guezguez B, Boyd AL and Bhatia
M: Molecular pathways: Epigenetic modulation of Wnt-glycogen
synthase kinase-3 signaling to target human cancer stem cells. Clin
Cancer Res. 20:5372–5378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chappell J and Dalton S: Roles for MYC in
the establishment and maintenance of pluripotency. Cold Spring Harb
Perspect Med. 3:a0143812013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holland JD, Klaus A, Garratt AN and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Curr
Opin Cell Biol. 25:254–264. 2013. View Article : Google Scholar : PubMed/NCBI
|