Introduction
Osteosarcoma (OS) is a very common malignant bone
tumor especially in children and young adults (1), accounting for approximately 19% of the
malignant cancer in the bone. Although some advances have been
achieved in the treatment of OS including surgery, chemotherapy and
radiotherapy, the long-term prognosis of OS patients remains poor
(2,3). It is urgent to identify novel
biomarkers and therapeutic targets for OS which will greatly
improve the prognosis of OS patients. Therefore, it is of extreme
importance to clarify the molecular mechanisms underlying the
pathogenesis of OS.
MicroRNAs (miRNAs), a group of non-coding, short
(14–22 nucleotides in length) RNAs (4), play fundamental roles in human
diseases by interacting with 3′-UTR of targeted mRNA and inhibiting
the translation of targeted genes (5). They are involved in various biological
processes (6) including cell
differentiation, proliferation, apoptosis and movement. Moreover,
increasing evidence showed that by acting as either oncogenes or
tumor suppressors, deregulated expression or function of miRNAs is
a critical reason for the development and progression of a number
of human cancers (5,7–9)
including OS (10). Elucidating the
expression and function of specific miRNA in OS will potentially
lead to the identification of novel biomarkers and therapeutic
targets in OS. miR-92a that belongs to miR-17-92 cluster is
identified as a biomarker for pancreatic cancer, gastric cancer,
prostate cancer, colorectal cancer and hepatocellular carcinoma
(11–14). miR-92a facilitates the tumor growth
of human hepatocellular carcinoma by targeting F-box and WD repeat
domain-containing 7 (FBXW7) (14).
Otherwise, miR-92a promotes the metastasis of nasopharyngeal
carcinoma by targeting the phosphatase and tensin homolog
(PTEN)/AKT pathway (15). In OS,
Gougelet et al identified miR-92a as predictive tool, which
was overexpressed in good responders to ifosfamide (16). Upregulated miR-92a was observed in
OS cell line versus bone (17).
However, the expression of miR-92a and its function in OS remain
poorly disclosed.
In this study, we found that miR-92a expression was
significantly increased in OS tissues. Increased expression level
of miR-92a was correlated with malignant clinical features and
reduced survival rate. Functionally, miR-92a could inhibit the
tumor growth of OS by regulating cell proliferation, apoptosis and
cell cycle progression. In addition, PTEN was found to be the
direct downstream target of miR-92a. Furthermore, we confirmed that
miR-92a exerted its biological functions by modulating PTEN/AKT
signaling pathway in OS cells.
Materials and methods
Clinical tissues from OS patients
A total of 68 OS and 20 normal bone tissue samples
were obtained from Renji Hospital, School of Medicine, Shanghai
Jiaotong University during the period from January 2006 to December
2008. The demographic features and clinicopathologic data are shown
in Table I. All patients signed the
informed consents to participate in this study. The protocols and
consents were approved by the Ethics Committee of Shanghai Jiaotong
University and complied with the Declaration of Helsinki.
 | Table I.Correlation between the
clinicopathological characteristics and miR-92a expression in
osteosarcoma. |
Table I.
Correlation between the
clinicopathological characteristics and miR-92a expression in
osteosarcoma.
|
|
| miR-92a
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | n | High | Low | P-value |
|---|
| Gender |
|
|
| 0.324 |
| Male | 40 | 18 | 22 |
|
|
Female | 28 | 16 | 12 |
|
| Age (years) |
|
|
| 0.177 |
|
<24 | 49 | 22 | 27 |
|
| ≥25 | 19 | 12 | 7 |
|
| Clinical stage |
|
|
|
<0.001a |
| I | 27 | 8 | 19 |
|
| II | 34 | 20 | 14 |
|
|
III | 7 | 6 | 1 |
|
| T
classification |
|
|
| 0.003a |
| T1 | 26 | 7 | 19 |
|
| T2 | 42 | 27 | 15 |
|
| M
classification |
|
|
| 0.690 |
| M0 | 61 | 30 | 31 |
|
| M1 | 7 | 4 | 3 |
|
| Histology |
|
|
| 0.171 |
|
Conventional osteosarcoma | 58 | 31 | 27 |
|
|
Others | 10 | 3 | 7 |
|
| Histological
differentiation |
|
|
| 0.026a |
| G1 | 27 | 9 | 18 |
|
| G2 | 41 | 25 | 16 |
|
Cell lines and cell culture
The human OS cell lines used in this study were
MG-63 and U2OS. These OS cell lines were obtained from the American
Type Culture Collection (ATCC, Manassas, VA, USA). Cells were
cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with
10% fetal bovine serum (FBS, Gibco), streptomycin (100 µg/ml;
Sigma, St. Louis, MO, USA), and penicillin (100 U/ml, Sigma).
Cell transfection
The miR-92a mimics and scrambled vector, miR-92a
inhibitor and negative control inhibitor were obtained from
GenePharma (Shanghai, China). PTEN siRNA and scramble siRNA were
from Geneopenia (Guangzhou, China). Cell transfections in OS cells
were carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions.
RNA extraction and qRT-PCR
RNA from tissue samples and cell lines were
extracted using TRIzol (Ambion, Austin, TX, USA). Real-time PCR for
miR-92a was performed using TaqMan microRNA assays with specific
primers for miR-92a. The PCR amplification and the quantification
of the PTEN and GAPDH were performed with the ABI PRISM 7300
Sequence Detection System (Applied Biosystems, Foster City, CA,
USA) and the SYBR® Premix Ex Taq™ II kit (Takara Bio,
Shiga, Japan). The primers for PTEN, miR-92a, U6, GAPDH were from
GenePharma. U6 was used as internal control of miR-92a while GAPDH
was used as internal control of PTEN.
Cell proliferation assay
OS cells transfected with miR-92a mimic or inhibitor
were seeded in 96-well plates and were incubated with CCK-8 (10%
diluted in normal culture medium, Dojindo; Kumamoto, Japan) at 37°C
for 2 h. Absorbance was measured at 0, 24, 48, 72 and 96 h after
transfection at the wavelength of 490 nm.
Cell cycle assays
At 48 h after the transfection, OS cells were
collected and fixed overnight at 4°C with 80% ethanol. Then, cells
were incubated with propidium iodide (PI, Sigma) for 20 min at room
temperature. Then, flow cytometry assays for OS cell cycle were
carried out with a FACS Calibur (BD Biosciences, Bedford, MA,
USA).
Cell apoptosis assay
The apoptosis of OS cells after transfection were
examined with Annexin V/PI kit (BD Pharmingen, San Diego, CA, USA).
OS cells were incubated with Annexin V/PI at 37 °C for 30 min and
were subjected to a FACS Calibur (BD Biosciences) to evaluate the
apoptotic rate.
In vivo experiments
BALB/c nude mice were employed to perform nude mouse
xenograft model. MG-63 cells transfected with miR-92a inhibitors or
control cells were injected subcutaneously into the flank of the
nude mouse. Tumor volume was determined every 3 days by measuring
the length and width of the tumors and was calculated as tumor
volume = length × width × width/2. The animal protocols were
approved by the Institutional Animal Care and Use Committee of
Shanghai Jiaotong University.
Western blot analysis
Cellular proteins extracted from OS cells with RIPA
buffer were separated by SDS-PAGE (4–20%) and were then transferred
to PVDF membranes (Amersham, Buckinghamshire, UK). Membranes were
blocked with 5% milk/TBST and were incubated with following
antibodies at 4°C overnight: PTEN antibody (Abcam, Cambridge, MA,
USA), p-AKT antibody (Ser473, Cell Signaling Technology, Danvers,
MA, USA), AKT antibody (Cell Signaling Technology), mTOR (Cell
Signaling Technology), p-p27(Thr157, R&D Systems, Inc.,
Minneapolis, MN, USA), p-MDM2(Ser166, Cell Signaling Technology),
and GAPDH antibody (Proteintech, Chicago, IL, USA). Formed
immunocomplexes between the targeted protein and primary antibodies
were detected with secondary antibodies (GE Healthcare, Tokyo,
Japan) and were visualized using ECL Prime system (GE
Healthcare).
Luciferase reporter assay
Plasmids containing wild-type (wt) 3′-UTR of PTEN or
the mutant (mt) PTEN 3′-UTR and corresponding miRNA vectors were
transfected into U2OS cells using Lipofectamine 2000 based on
manufacturer's protocol. At 48 h after transfection, U2OS cells
were collected for measuring luciferase activity with the
luciferase reporter assay system (Promega, Madison, WI, USA).
Statistical analysis
The experiments in this study were repeated at least
three times. All data were collected and showed as mean ± SEM.
Statistical analysis including Pearson's Chi-squared test,
Student's t-test, ANOVA, Kaplan-Meier analysis, Log-rank test and
Spearman's correlation analysis were performed in this study with
Graphpad (GraphPad Software, Inc., San Diego, CA, USA). The
statistical significance level was set at P<0.05.
Results
Increased level of miR-92a in OS is
correlated with malignant clinicopathological features and poor
prognosis of patients
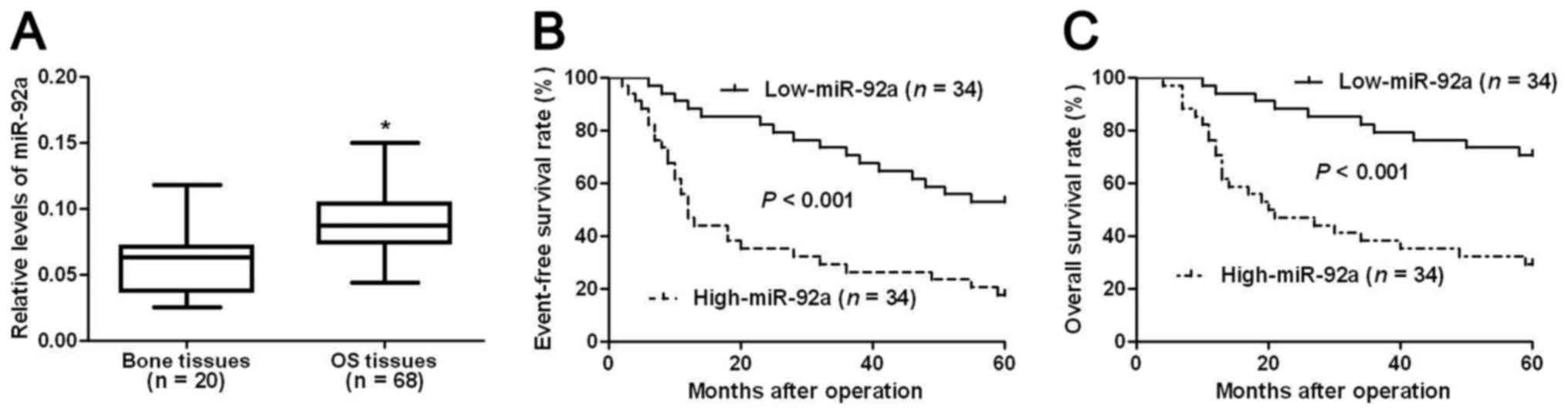
We first evaluated the expression level of miR-92a
in OS tissues. Compared with normal bone tissues, OS tissues showed
significantly increased level of miR-92a (P<0.05, Fig. 1A). This indicates that miR-92a
probably plays an oncogenic role in OS. Then, we investigated the
clinical significance and the prognostic value of miR-92a in OS. We
divided all OS patients into two groups (miR-92a low group and
miR-92a high group) using the median level of miR-92a expression as
a cutoff value. Clinical association analysis (Table I) showed that compared with patients
who had low expression level of miR-92a, patients with high level
of miR-92a showed advanced TNM stage (P<0.001) and high T
classification (P=0.003) and poor histological differentiation
(P=0.026), indicating that miR-92a can act as promising biomarkers
for OS patients.
miR-92a promotes proliferation and the
cell cycle process, and reduces apoptosis of OS cells
After confirming the elevated expression of miR-92a
in OS, we examined the biological functions of miR-92a in OS cells.
Increased proliferation, decreased apoptosis and deregulated cell
cycle progression were critical for the malignant growth of cancer
cells. Therefore, we examined whether miR-92a could promote the
progression of OS by regulating these cellular functions. We first
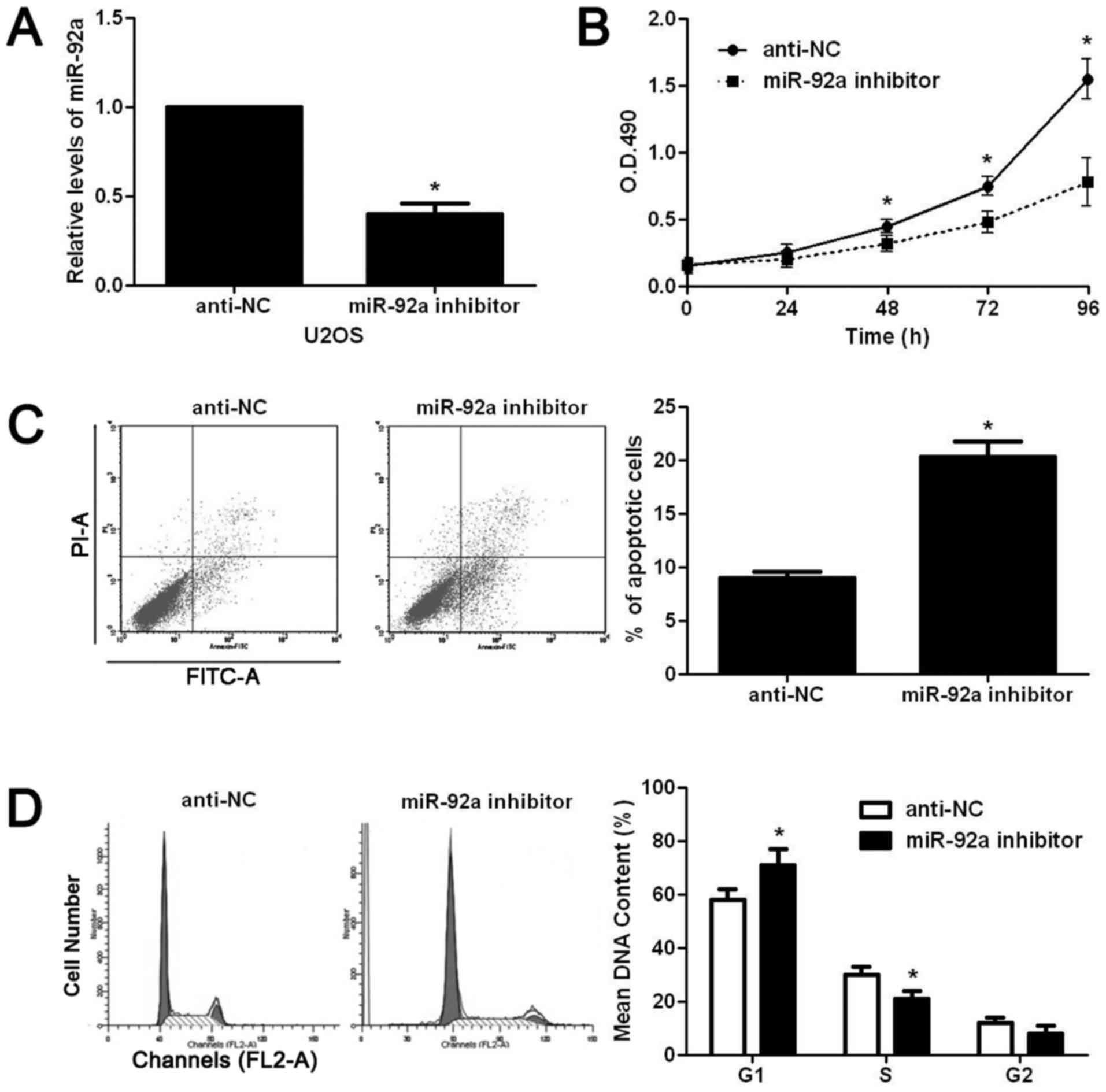
downregulated miR-92a expression level in U2OS cells with miR-92a
inhibitor. As shown in Fig. 2A,
miR-92a inhibitor significantly reduced the expression level of
miR-92a in U2OS cells (P<0.05). Then, we used CCK8 assay to
examine the alteration of proliferation after miR-92a knockdown in
U2OS cells. miR-92a inhibition significantly reduced the
proliferative ability of U2OS cells (P<0.05, Fig. 2B). The flow cytometry assay showed
that miR-92a inhibition significantly increased the apoptotic rate
of U2OS cells (P<0.05, Fig.
2C).
Furthermore, cell cycle assay showed that miR-92a
inhibition prevented the cell cycle progression of U2OS cells as
suggested by significantly increased ratio of cells at G1 phase and
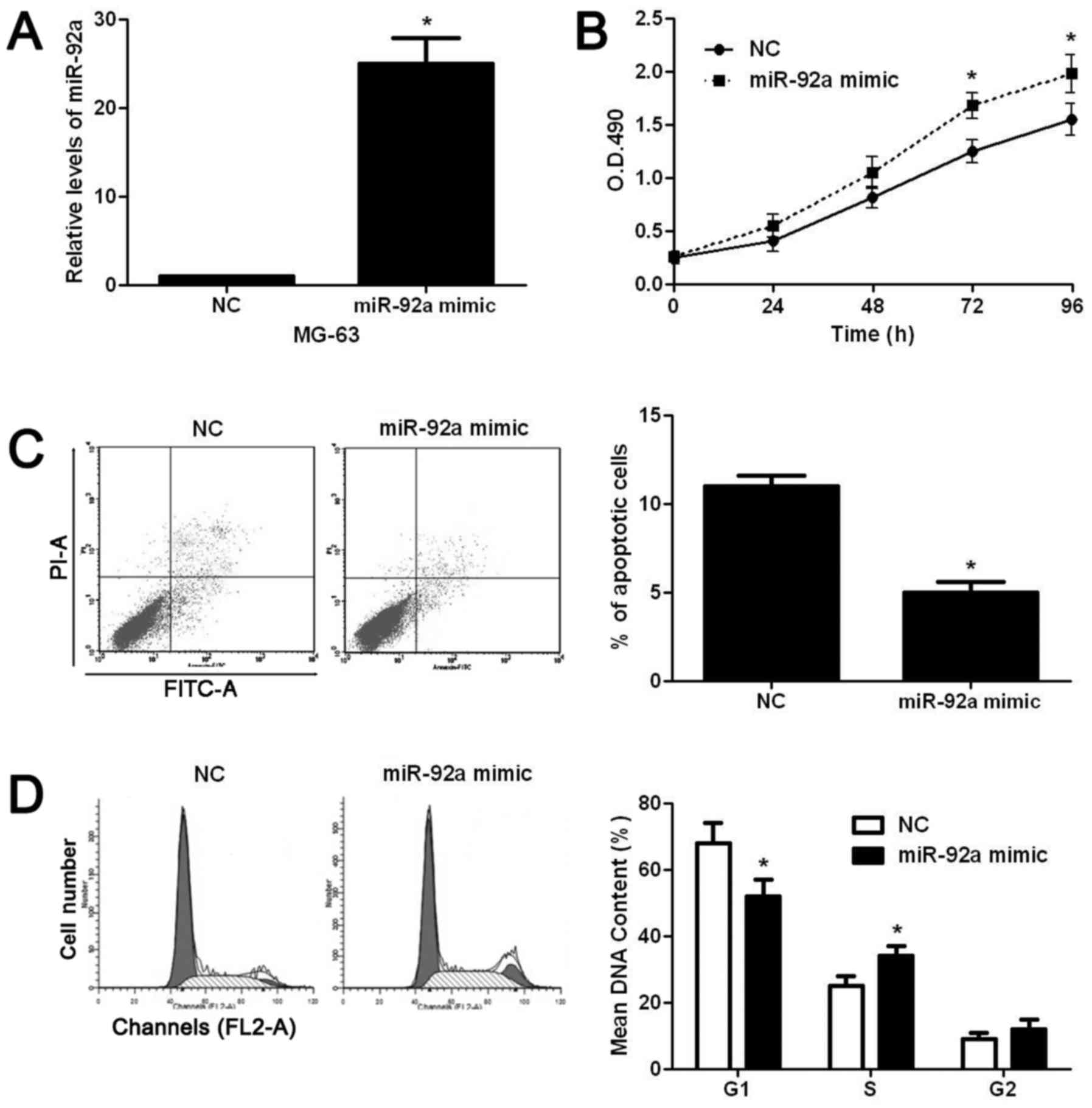
decreased ratio of cells at S phase (P<0.05, Fig. 2D). On the contrary, miR-92a mimic
significantly increased the level of miR-92a in MG-63 cells
(P<0.05, Fig. 3A). Subsequently,
miR-92a overexpression significantly increased the proliferation
(P<0.05, Fig. 3B), decreased
apoptosis (P<0.05, Fig. 3C), and
facilitated the cell cycle progression (P<0.05, Fig. 3D) of MG-63 cells. After confirming
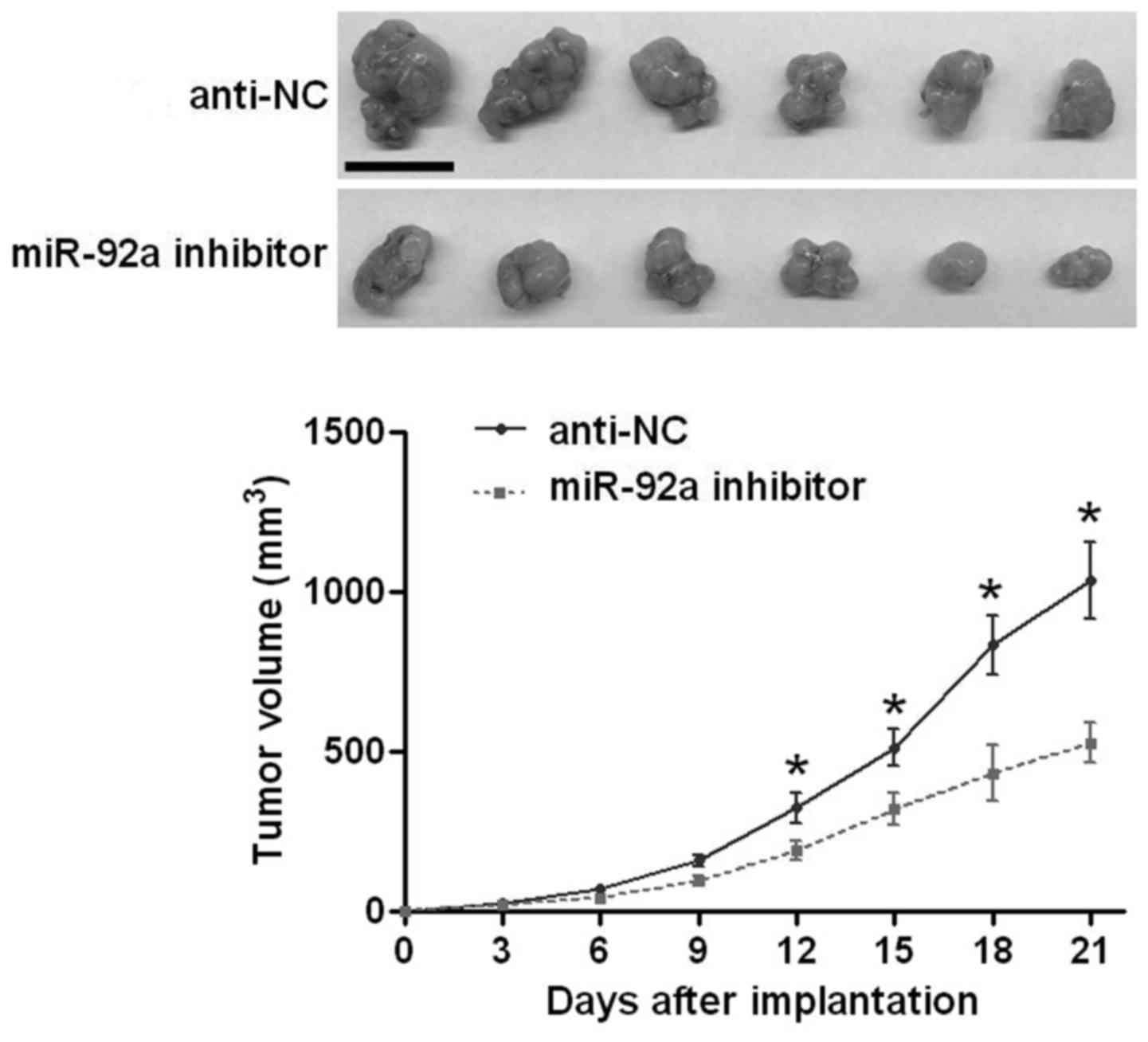
the functional effects of miR-92a in vitro, we performed
subcutaneous implantation model to test whether miR-92a could
affect the growth of U2OS cells in vivo. As shown in
Fig. 4, inhibiting miR-92a
significantly reduced the growth of U2OS cells in nude mice
(P<0.05, Fig. 4). The above
indicates that miR-92a can promote the growth of OS cells in
vitro and in vivo.
PTEN is a downstream target of miR-92a
in OS cells
To understand the underlying mechanisms for the
functional influence of miR-92a on OS cell proliferation, apoptosis
and cell cycle progression, we used public database (Targetscan) to
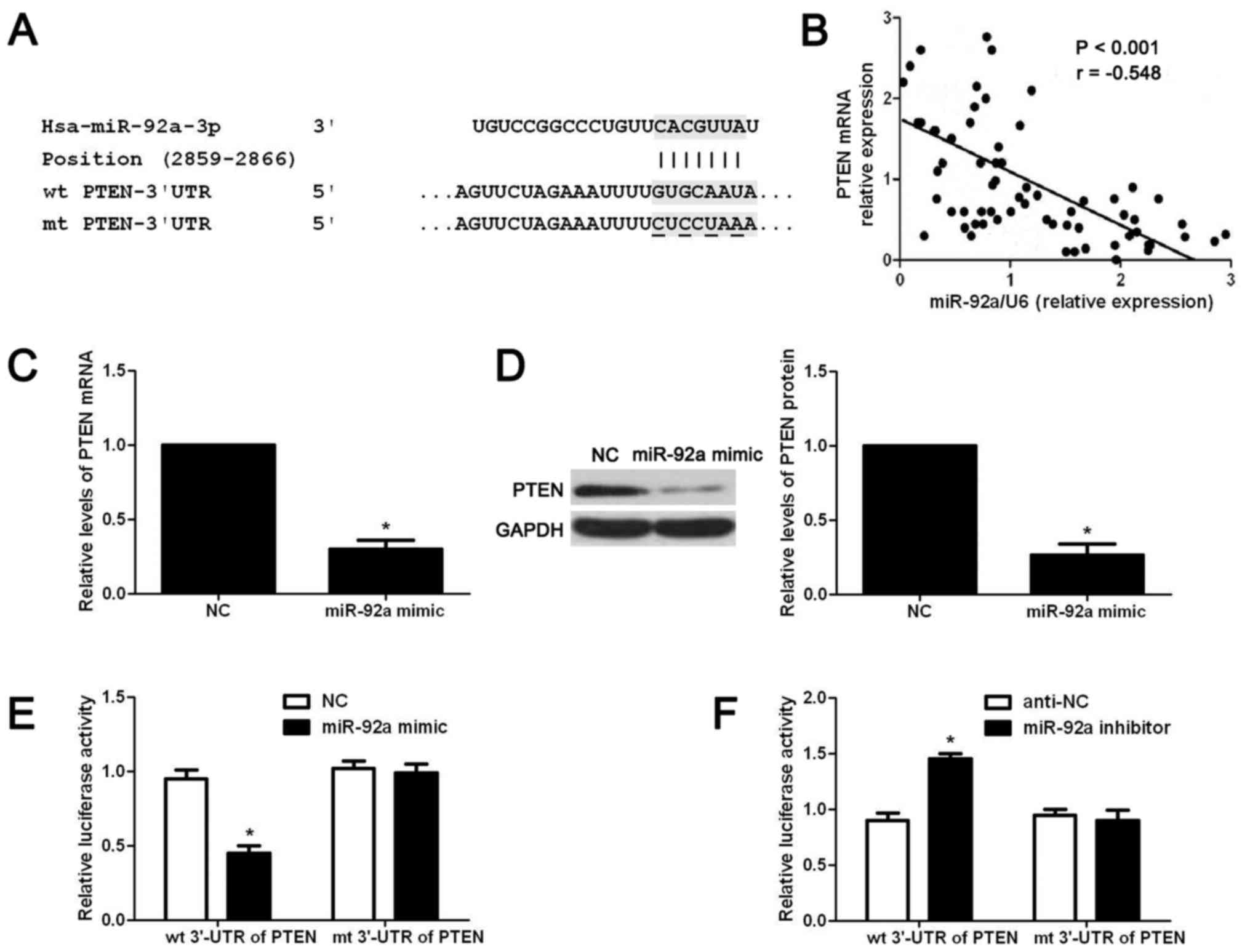
search for potential downstream targets of miR-92a. As shown in
Fig. 5A, the wt 3′-UTR of PTEN
contained potential binding sites for miR-92a, indicating PTEN was
a potential downstream target of miR-92a. Next, we examined the
correlation between the level of PTEN mRNA and miR-92a expression
in OS tissues. Our data indicated that the expression level of PTEN
mRNA was negatively correlated with miR-92a expression in OS
tissues (r= −0.548, P<0.05, Fig.
5B). Then, we examined whether miR-92a overexpression could
affect the expression level of PTEN mRNA and protein in OS cells.
The results of qRT-PCR showed that miR-92a overexpression
significantly reduced the expression level of PTEN mRNA in MG-63
cells (P<0.05, Fig. 5C). Western
blot results showed that miR-92a overexpression significantly
reduced the PTEN protein level in MG-63 cells (P<0.05, Fig. 5D). Lastly, we performed luciferase
reporter assay to determine whether miR-92a regulated the
expression of miR-92a through directly interacting with 3′-UTR of
PTEN. As shown in Fig. 5E and F,
overexpression of miR-92a significantly reduced the luciferase
activity of wt 3′-UTR of PTEN (P<0.05, Fig. 5E) while miR-92a inhibition increased
that of wt 3′-UTR of PTEN (P<0.05, Fig. 5F). Furthermore, altering miR-92a
level in OS cells did not affect the luciferase activity of mt
3′-UTR of PTEN (Fig. 5E and F).
miR-92a plays an oncogenic role by
regulating PTEN/AKT pathway
PTEN, a versatile protein, inversely regulated
PI3K/AKT/mTOR signaling in cancer cells (18). Furthermore, PTEN/AKT pathway
mediated the phosphorylation of p27(Thr157), AKT(Ser473) and
MDM2(Ser166) (19). By regulating
the expression and phosphorylation of these proteins, PTEN plays a
tumor suppressive role in cancer cell proliferation, apoptosis and
movements (19). To further explore
the mechanisms involved in the function of miR-92a, OS cells that
were transfected with corresponding miRNA vectors were subjected to
western blot for PTEN, p-AKT(Ser473), AKT, mTOR, p-p27(Thr157) and
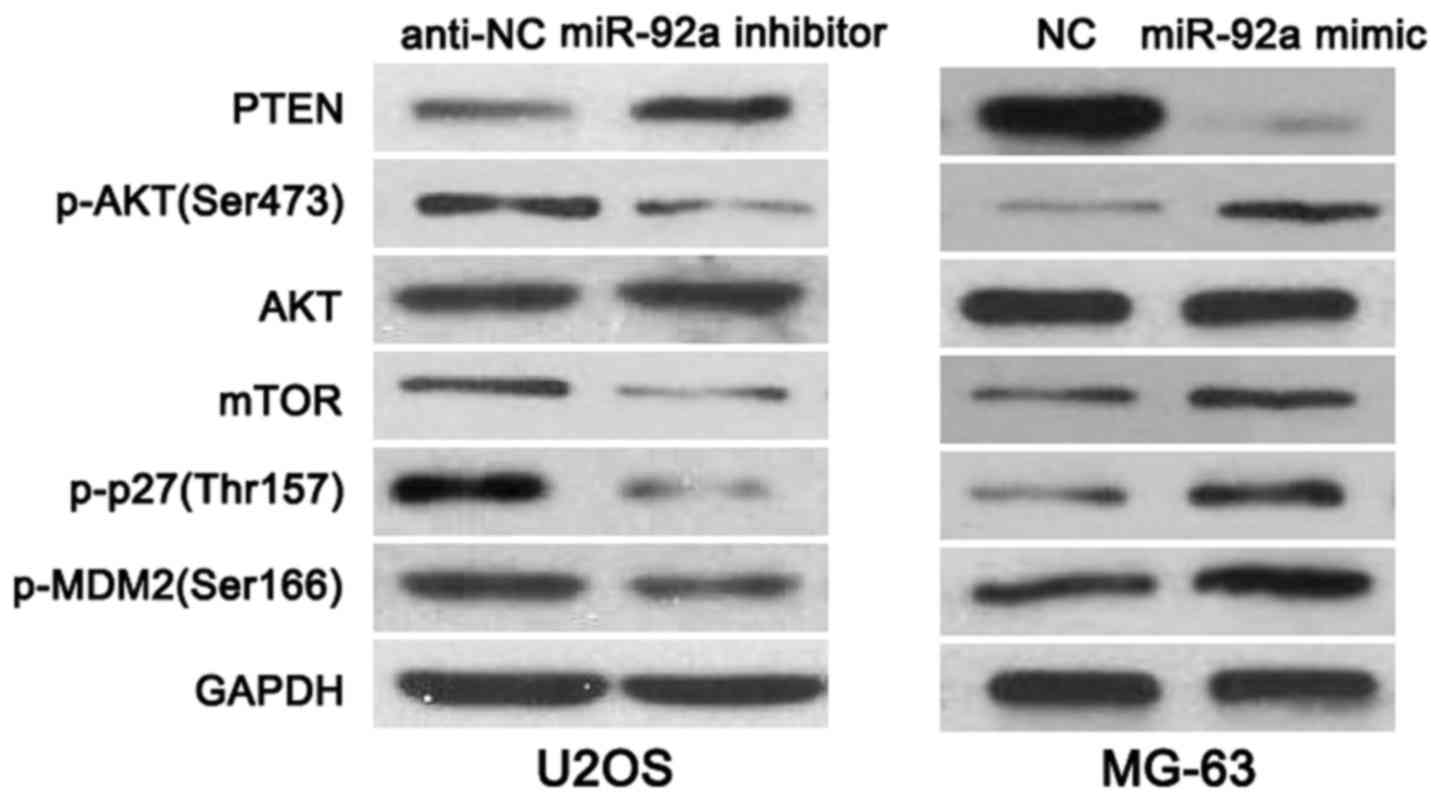
p-MDM2(Ser166) expression. As shown in Fig. 6, miR-92a knockdown increased the
level of PTEN and reduced the expression of p-AKT(Ser473), mTOR,
p-p27(Thr157) and p-MDM2(Ser166) in U2OS cells. While,
overexpressing of miR-92a decreased PTEN level and led to increased
expression of p-AKT(Ser473), mTOR, p-p27(Thr157) and p-MDM2(Ser166)
in MG-63 cells, thus indicating that miR-92a regulates PTEN/AKT
pathway in OS cells.
PTEN is required for the biological
functions of miR-92a in OS cells
To confirm whether PTEN was critical for the
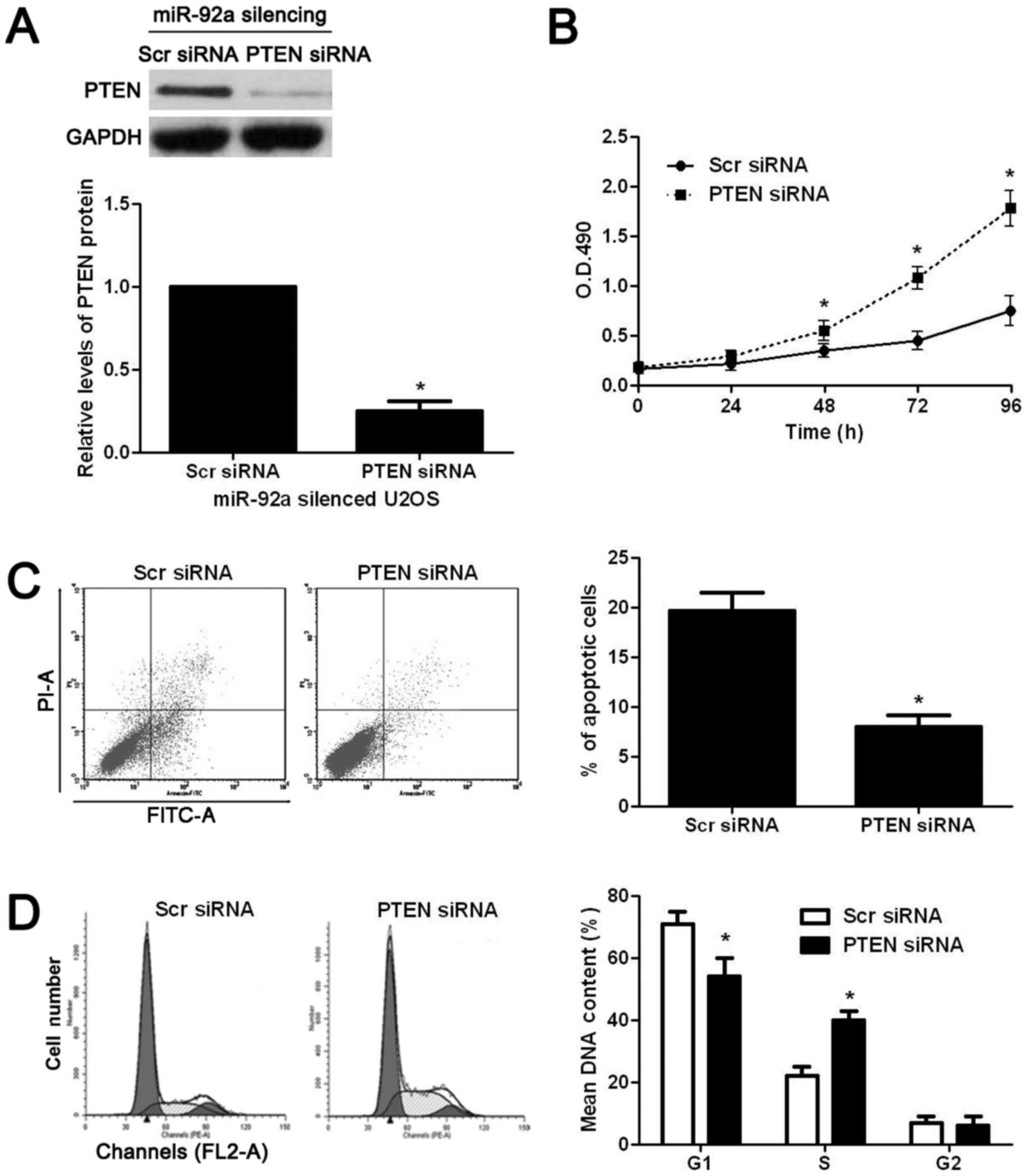
functional effects of miR-92a in OS cells, PTEN was knocked down by
a specific siRNA in miR-92a silenced U2OS cells. PTEN siRNA
significantly decreased the expression of PTEN in miR-92a silenced
U2OS cells (P<0.05, Fig. 7A).
Functional assays showed that knockdown of PTEN significantly
reversed the effects of miR-92a silencing on U2OS cells with
increased proliferation (P<0.05, Fig. 7B), decreased apoptosis (P<0.05,
Fig. 7C), and enhanced cell cycle
progression (P<0.05, Fig. 7D).
Thus, PTEN is not only a downstream target but also mediator of
miR-92a in OS.
Discussion
miR-92a was recently identified as a cancer-related
microRNA. Studies of nasopharyngeal carcinoma (15), colorectal cancer (20), rectal cancer (21), gastric cancer (22), non-small cell lung cancer(23), hepatocellular carcinoma (14), cervival cancer (24) and ovarian cancer (25) showed that miR-92a played critical
and different roles in the development and progression of these
cancers. The expression of miR-92a is increased in OS cell lines
compared to normal bone (17).
However, the clinical values and function of miR-92a in OS has not
been reported. Herein, we found that miR-92a was significantly
increased in OS tissues. Increased expression of miR-92a was
correlated with malignant clinical features and poor prognosis of
OS patients, demonstrating that miR-92a potentially acts as a
biomarker for OS.
miRNAs have been found to play various roles in
cancer cells including cell proliferation, apoptosis, and
metastasis and drug resistance (8).
In this study, functional assays showed that miR-92a could promote
the proliferation, inhibit apoptosis and contribute to cell cycle
progression of OS cells. In this way, miR-92a promoted the in
vivo growth of OS cells in nude mice. Therefore, we confirmed
that miR-92a promotes the tumor growth of OS by regulating cell
proliferation, apoptosis and cell cycle progression.
PTEN is a well-known tumor suppressor in human
cancers and could regulate different biological functions of cancer
cells including cell proliferation, apoptosis and metastasis
(26,27). In this study, we confirmed that
miR-92a could directly target PTEN in OS cells with the following
evidence: first, 3′-UTR of PTEN contained the complementary
sequences for miR-92a; second, the expression of PTEN mRNA was
negatively correlated with miR-92a level in OS tissues; third,
miR-92a reduced the expression of PTEN mRNA and protein in OS
cells; fourth, miR-92a inversely regulated the luciferase activity
of wt 3′-UTR of PTEN rather than mt 3′-UTR of PTEN. PTEN/AKT
pathway converts a subset of tumor suppressor proteins to oncogenic
proteins or vice versa through multiple pathways (19). Herein, we disclosed that miR-92a
regulated PTEN/AKT pathway and its downstream targets including
mTOR, p-p27(Thr157) and p-MDM2(Ser166). Furthermore, functional
assays confirmed that PTEN inhibition reversed the effects of
miR-92a silencing on OS cells growth. Altogether, miR-92a promotes
the tumor growth of OS probably by targeting the PTEN/AKT
pathway.
A previous study reported that ZEB2 mRNA was a PTEN
competitive endogenous RNA (ceRNA), which modulated PTEN protein
levels in a microRNA-dependent manner including miR-92a (28). ZEB2 has been reported to be
implicated in metastasis and epithelial-mesenchymal transition
(EMT) in OS cells (29).
Furthermore, miR-130a exerted promoting effects on metastatic
behavior and EMT of OS cells through suppressing PTEN expression
(30). Thus, it is worth to
investigate crosstalk among ZEB2, PTEN and miR-92a in OS in further
study.
Herein, we found that miR-92a expression was
elevated in OS tissues and correlated with malignant
clinicopathological features and reduced survival of OS patients.
miR-92a played an oncogenic role in OS by promoting proliferation,
inhibiting apoptosis and contributed to cell cycle progression.
Mechanically, we showed that PTEN was a direct downstream target of
miR-92a in OS and miR-92a exerted its functional influence on OS
cells by regulating PTEN/AKT pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81370976 and 81400904) and
Medical-Engineering Joint Fund of Shanghai Jiaotong University
(nos. YG2014MS41 and YG2016MS53).
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Pediatric and Adolescent Osteosarcoma. Springer;
New York, NY: pp. 3–13. 2009, View Article : Google Scholar
|
|
2
|
Laux CJ, Berzaczy G, Weber M, Lang S,
Dominkus M, Windhager R, Nöbauer-Huhmann IM and Funovics PT: Tumour
response of osteosarcoma to neoadjuvant chemotherapy evaluated by
magnetic resonance imaging as prognostic factor for outcome. Int
Orthop. 39:97–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment - where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alvarez-Garcia I and Miska EA: MicroRNA
functions in animal development and human disease. Development.
132:4653–4662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartels CL and Tsongalis GJ: MicroRNAs:
Novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. MicroRNA Cancer Regulation. Springer;
pp. 1–20. 2013, View Article : Google Scholar
|
|
9
|
Shen J, Stass SA and Jiang F: MicroRNAs as
potential biomarkers in human solid tumors. Cancer Lett.
329:125–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumour Biol. 34:2093–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motoyama K, Inoue H, Takatsuno Y, Tanaka
F, Mimori K, Uetake H, Sugihara K and Mori M: Over- and
under-expressed microRNAs in human colorectal cancer. Int J Oncol.
34:1069–1075. 2009.PubMed/NCBI
|
|
14
|
Yang W, Dou C, Wang Y, Jia Y, Li C, Zheng
X and Tu K: MicroRNA-92a contributes to tumor growth of human
hepatocellular carcinoma by targeting FBXW7. Oncol Rep.
34:2576–2584. 2015.PubMed/NCBI
|
|
15
|
Zhang H, Cao H, Xu D and Zhu K:
MicroRNA-92a promotes metastasis of nasopharyngeal carcinoma by
targeting the PTEN/AKT pathway. Onco Targets Ther. 9:3579–3588.
2016.PubMed/NCBI
|
|
16
|
Gougelet A, Pissaloux D, Besse A, Perez J,
Duc A, Dutour A, Blay JY and Alberti L: Micro-RNA profiles in
osteosarcoma as a predictive tool for ifosfamide response. Int J
Cancer. 129:680–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong J, Li Z, Zhang Y, Li D, Zhang G, Luo
X, Jie Z, Liu Y, Cao Y, Le Z, et al: PRL-3 promotes the peritoneal
metastasis of gastric cancer through the PI3K/Akt signaling pathway
by regulating PTEN. Oncol Rep. 36:1819–1828. 2016.PubMed/NCBI
|
|
19
|
Xie Y, Naizabekov S, Chen Z and Tokay T:
Power of PTEN/AKT: Molecular switch between tumor suppressors and
oncogenes. Oncol Lett. 12:375–378. 2016.PubMed/NCBI
|
|
20
|
Zhou T, Zhang G, Liu Z, Xia S and Tian H:
Overexpression of miR-92a correlates with tumor metastasis and poor
prognosis in patients with colorectal cancer. Int J Colorectal Dis.
28:19–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pelossof R, Chow OS, Fairchild L, Smith
JJ, Setty M, Chen CT, Chen Z, Egawa F, Avila K, Leslie CS, et al:
Integrated genomic profiling identifies microRNA-92a regulation of
IQGAP2 in locally advanced rectal cancer. Genes Chromosomes Cancer.
55:311–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q, Yang Z, Wang F, Hu S, Yang L, Shi Y
and Fan D: MiR-19b/20a/92a regulates the self-renewal and
proliferation of gastric cancer stem cells. J Cell Sci.
126:4220–4229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao J, Fu W, Liao H, Dai L, Jiang Z, Pan
Y, Huang H, Mo Y, Li S, Yang G, et al: The regulatory and
predictive functions of miR-17 and miR-92 families on cisplatin
resistance of non-small cell lung cancer. BMC Cancer. 15:7312015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou C, Shen L, Mao L, Wang B, Li Y and Yu
H: miR-92a is upregulated in cervical cancer and promotes cell
proliferation and invasion by targeting FBXW7. Biochem Biophys Res
Commun. 458:63–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohyagi-Hara C, Sawada K, Kamiura S, Tomita
Y, Isobe A, Hashimoto K, Kinose Y, Mabuchi S, Hisamatsu T,
Takahashi T, et al: miR-92a inhibits peritoneal dissemination of
ovarian cancer cells by inhibiting integrin α5 expression. Am J
Pathol. 182:1876–1889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ortega-Molina A and Serrano M: PTEN in
cancer, metabolism, and aging. Trends Endocrinol Metab. 24:184–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012.PubMed/NCBI
|
|
28
|
Karreth FA, Tay Y, Perna D, Ala U, Tan SM,
Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al:
In vivo identification of tumor- suppressive PTEN ceRNAs in an
oncogenic BRAF-induced mouse model of melanoma. Cell. 147:382–395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Chen H, Zhao Y, Gao S and Cheng C:
H19 Functions as a ceRNA in promoting metastasis through decreasing
miR-200s activity in osteosarcoma. DNA Cell Biol. 35:235–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Yan D, Wu W, Zhu J, Ye W and Shu
Q: MicroRNA-130a promotes the metastasis and epithelial-mesenchymal
transition of osteosarcoma by targeting PTEN. Oncol Rep.
35:3285–3292. 2016.PubMed/NCBI
|