Introduction
Several signaling pathways are crucial in
orchestrating cancer stem cell (CSC) activity. Dysregulation of
these pathways has been implicated in the maintenance and function
of CSCs. A study by Sunayama et al showed that simultaneous
blocking of phosphoinositide 3-kinase (PI3K)/AKT and extracellular
signal-regulated kinase (ERK1/2) signaling promoted glioblastoma
cancer stem-like cell (CSLC) differentiation, suppressing
tumorigenicity by activating FoxO3a, which is commonly
phosphorylated by AKT and ERK for signal transduction (1). Jacobsen et al reported that
deregulation of Foxo3a and NF-κB/Rel is associated with malignancy
in SCID temperature-sensitive form of Abelson mouse leukemia virus
pre-B cells (2). Our previous
studies demonstrated that FoxO3a inactivation and/or FoxM1
activation are essential for oncogenicity as well as stemness in
ovarian cancer stem-like cells (OVCSLCs) derived from established
ovarian cancer cell lines (3,4).
However, whether FoxO3a can integrate these pro-survival and
pro-inflammatory pathways to induce tumorigenicity in OVCSLCs
remains to be investigated.
Numerous epidemiological studies have substantiated
the efficient anticancer properties of dietary components in
vegetables and fruits (5). These
bioactive and non/low-toxic phytochemicals are considered promising
candidates for cancer intervention (5,6).
Genistein, 5,7,4′-trihydroxylisoflavone, a major soybean compound,
possesses antitumor properties (7,8).
However, genistein has poor bioavailability due to its low
solubility in both organic solvents and water (9). Notably, the introduction of
HCF2 or CF3 into the genistein molecule
improves the anticancer activities of genistein derivatives
(9). Furthermore, the newly
synthesized genistein derivative,
7-difluoromethoxyl-5,4′-di-n-octylygenistein (DFOG), was found to
induce apoptosis in ovarian and gastric carcinomas (3,4). DFOG
acts as an inhibitor of CSCs or tumor-initiating cells by the
activation of FoxO3a and/or inactivation of FoxM1 (3,4).
Nevertheless, whether DFOG inhibits oncogenicity in OVCSLCs by
activating FoxO3a and/or inactivating FoxM1 through targeting of
multiple pro-survival (AKT and ERK1/2) and pro-inflammatory (NF-κB)
pathways is unclear.
In the present study, we demonstrated that DFOG
suppressed in vitro spheroid and colony formation on soft
agar of OVCSLCs obtained from SKOV3 cells. Mechanistically, DFOG
exhibited effects similar to those of the PI3K inhibitor
(LY294002), MEK inhibitor (U0126) and NF-κB inhibitor (PDTC),
simultaneously. The suppressive activity of DFOG on spheroid
formation in serum-free medium (SFM) and colony formation on soft
agar were dependent on FoxO3a and FoxM1 protein levels. These
results indicate that DFOG may be potentially used for the
treatment of human ovarian cancer.
Materials and methods
Reagents
Invitrogen Life Technologies (Shanghai, China)
supplied Dulbeccos modified Eagles medium (DMEM) and DMEM/F12,
trypsin-EDTA, fetal bovine serum (FBS) and penicillin-streptomycin.
Monoclonal antibodies raised in mice against human anti-β-actin
were manufactured by Sigma-Aldrich (St. Louis, MO, USA) (catalog
no. A2066). Rabbit polyclonal antibodies targeting CD44, ALDH1,
CD133 and FoxO3a were obtained from Abcam Co. (Cambridge, MA, USA)
(catalog nos. ab24504, ab9883, ab19898 and ab53287, respectively).
Monoclonal antibodies against FoxM1 (C-20) and NF-κBp65 raised in
rabbits were manufactured by Santa Cruz Biotechnology, Inc.
(Beverly, MA, USA) (catalog nos. sc-502 and sc-8008). Primary
antibodies against phospho-AKT (Ser473), AKT, p-ERK
(Thr202/Tyr204), phospho-FoxO3a (Ser253) and ERK1/2 were
manufactured by Cell Signaling Technology (Danvers, MA, USA)
(catalog nos. #9171, #9272, #9101, #9466 and #9102).
The pHBad-U6-GFP, pHBad-U6-GFP-shFOXO3a and
pHBad-U6-GFP-shFOXM1 plasmid packaging adenoviral particles were
obtained from Hanbio Biotechnology Co. Ltd. (Shanghai, China) (2.0
ml, 1×1011 PFU/ml). Pyrrolidine dithiocarbamate,
ammonium salt (PDTC), LY294002 and U0126 were purchased from
Sigma-Aldrich.
Cell culture and sphere formation
assay
Human ovarian carcinoma SKOV3 cells were obtained
from the Chinese Academy of Sciences (Shanghai, China) and cultured
in DMEM containing 10% FBS.
Sphere formation was assessed in serum-free culture
medium containing antibiotics, growth factors, vitamin B27, and the
N2 supplement (Invitrogen), following instructions from the
manufacturer. Cell seeding was performed at 104
cells/well in 6-well ultra-low attachment plates (Corning, Corning,
NY, USA).
Spheroids were obtained by centrifugation (200 × g)
and trypsin-EDTA digestion, followed by mechanical disruption.
Single cells were washed and transferred into SFM for sphere
induction. Second-generation spheroids were used as ovarian cancer
stem-like cells (OVCSLCs).
Single cells with potential for transformation into
new spheroids were cultured at 1,000 cells/well in a 24-well plate,
to generate new spheroids. Tumor spheroids were counted in 6 day
cultures; the efficiency of spheroid formation was expressed as the
ratio of the total number of spheroids generated to that of SKOV3
cells seeded, multiplied by 100.
Colony formation assay
In the present study, soft agar was used. Medium
containing 0.7% agarose was added into a 6-well plate. Then,
104 cells were seeded/well in medium containing 0.4%
agarose (top layer), and incubated for 3 weeks. Routine colony
count was carried out on an inverted microscope (Olympus IX53;
Olympus, Tokyo, Japan). Three independent experiments were carried
out.
In vivo tumorigenicity
experiments
Balb/c-nu mice aged 4 weeks were purchased from the
Animal Institute of the Chinese Academy of Medical Science (CAMS).
All animal studies were performed in accordance with the standard
protocols approved by the Ethics Committee of The First Affiliated
Hospital of Jinan University and the Committee of Experimental
Animal Feeding and Management. Mice were randomly divided into 3
groups (4 mice/group) and maintained under standard conditions,
according to the standard protocols. Cells were suspended in serum
free-DMEM/Matrigel (BD Biosciences) mixture (1:1 volume). Each
recipient Balb/c-nu mouse was inoculated subcutaneously with
various numbers of SKOV3-derived OVCSLCs (1×103,
1×104 and 1×105 cells) in one flank and the
monolayer SKOV3 cells (1×104, 1×105 and
1×106) in the other, respectively. Tumorigenicity
experiments were terminated 1 month after cell inoculation.
Harvested tumors were imaged and weighed immediately. After that,
specimens from tumor tissue samples were fixed in 10% neutral
buffered formalin, processed in paraffin blocks, and sectioned. The
sections were stained with H&E and examined for the
histopathology.
Transduction of shFOXO3a and
shFOXM1
SKOV3 cell-derived OVCSLCs were plated into 24-well
culture plates at 40–50% confluency, and incubated overnight. Then,
the cells were transduced with the pHBad-U6-GFP or
pHBad-U6-GFP-shFOXO3a or pHBad-U6-GFP-shFOXM1 plasmid packaging
adenoviral particles using an enhanced infection solution (ENi.s;
cat. no. REVG0002; GeneChem, Shanghai, China). Following 4 h of
transduction, DMEM with 10% fetal calf serum (FCS) was added to
replace the transduction medium; this was followed by 48 h of
incubation before gene and protein level assessments.
Western blot analysis
Cells lysis was performed according to published
protocols (10). Monoclonal
anti-β-actin, anti-NF-κBp65, anti-FoxM1, anti-phospho-AKT (Ser473)
antibodies, and polyclonal anti-CD44, anti-ALDH1A1, anti-CD133,
anti-AKT, anti-p-ERK (Thr202/Tyr204), anti- ERK1/2,
anti-Phospho-FoxO3a (Ser253) antibodies were used as primary
antibodies, for overnight incubation at 4°C. Adequate horseradish
peroxidase (HRP) bound secondary antibodies were added for 1 h at
ambient temperature; visualization of specific protein bands was
carried out using enhanced chemiluminescence, with β-actin employed
for normalization.
Statistical analysis
Comparisons were conducted by two-tailed Student's
t-test. A P-value <0.05 indicated statistical significance.
Results
Spheroids reflect SKOV3 cell-derived
OVCSLCs
CSCs are mainly characterized by their capacity to
form 3-dimensional spheroids, and tumorsphere formation assay via
SFM culturing is widely used in their isolation and enrichment
in vitro. Under SFM culture conditions, most cancer cells
undergo apoptosis, whereas only a small proportion form
tumorspheres. These subpopulations of cancer cells are believed to
have CSC characteristics such as self-renewal ability and unlimited
differentiation. Our group and other investigators have
demonstrated that the spheroids of established ovarian cancer cell
lines and transplanted human ovarian cancer possess the
characteristics of OVCSLCs (3,4,11–14).
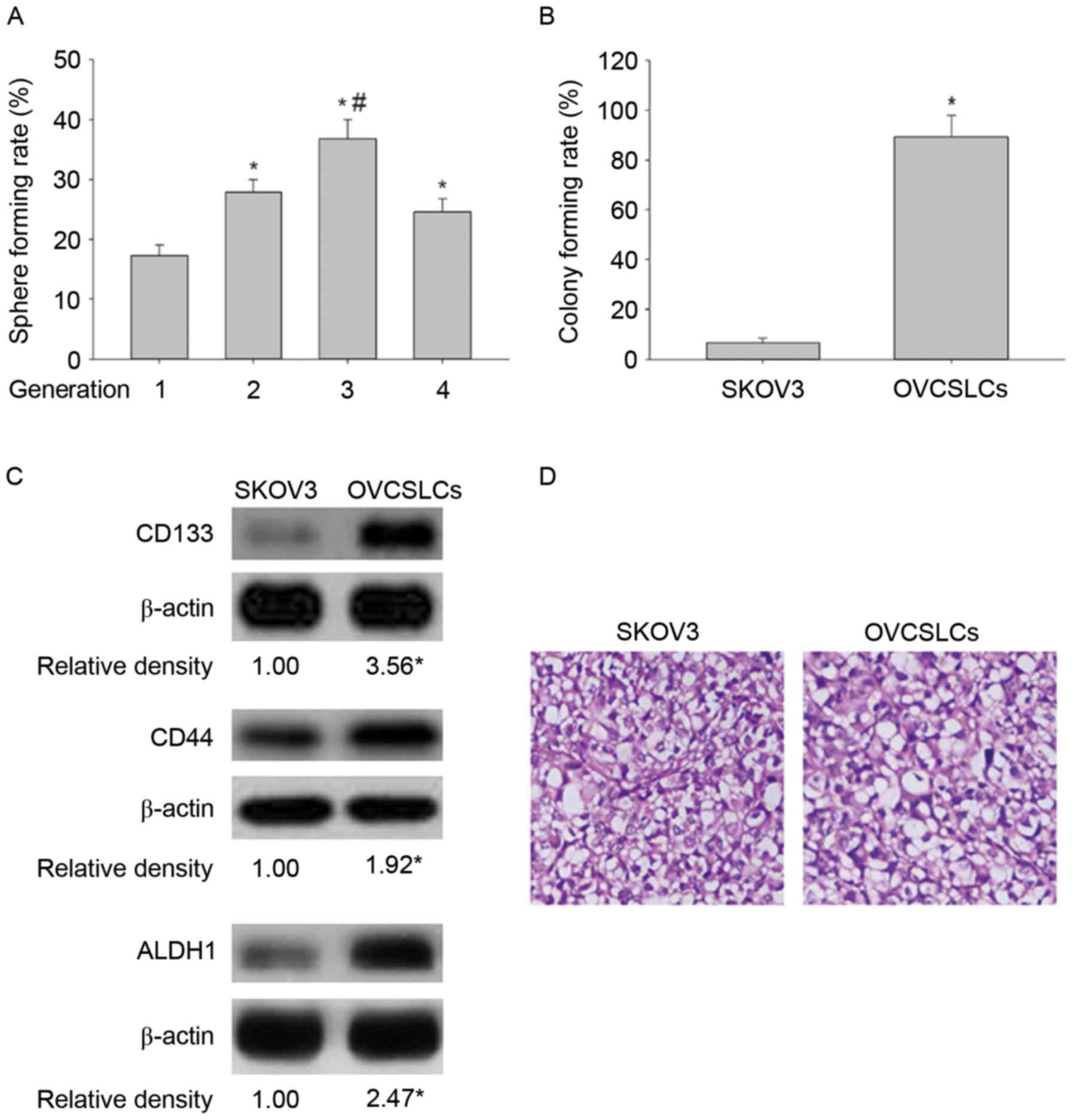
In the present study, we also identified the characteristics of
OVCSLCs in spheroids obtained from SKOV3 cells. We showed that the
second-generation spheroids had the highest self-renewal potential
(Fig. 1A). We further demonstrated
that second-generation spheroids had a higher colony formation rate
compared with cells in monolayer growth (Fig. 1B). In addition, the results obtained
using western blotting revealed that second-generation spheroids
had elevated amounts of CSC-related markers (CD133, CD44 and
ALDH1), compared with SKOV3 cells in monolayer growth (Fig. 1C). Significantly, second-generation
spheroids displayed more powerful carcinogenicity than cells of the
SKOV3 cell line in vivo (Fig.
1D and Table I). These results
demonstrated that spheroids derived from the SKOV3 cell line
possessed OVCSLC properties, such as higher oncogenicity in
vitro and in vivo, and overexpression of ‘stemness’
biomarkers. For this reason, second-generation spheroids were used
as OVCSLCs in the subsequent experimental studies.
 | Table I.Tumorigenicity experiments of OVCSLCs
and SKOV3 cells in BALB/c-nu mice. |
Table I.
Tumorigenicity experiments of OVCSLCs
and SKOV3 cells in BALB/c-nu mice.
| Cellsa | Inoculum
amount | Tumor
incidence | Latency period
(days) |
|---|
| SKOV3 |
1×103 | 0/4 | – |
|
|
1×104 | 0/4 | – |
|
|
1×105 | 2/4 | 42 |
| OVCSLC |
1×103 | 3/4 | 25 |
|
|
1×104 | 4/4 | 16 |
|
|
1×105 | 4/4 | 8 |
DFOG inhibits the characteristics of
SKOV3 cell-produced OVCSLCs
Previous studies revealed the novel genistein
analogue DFOG induces apoptosis of various cancer cell lines
(9,15). DFOG inhibited the proliferation of
CSCs or tumor-initiating cells (3,4).
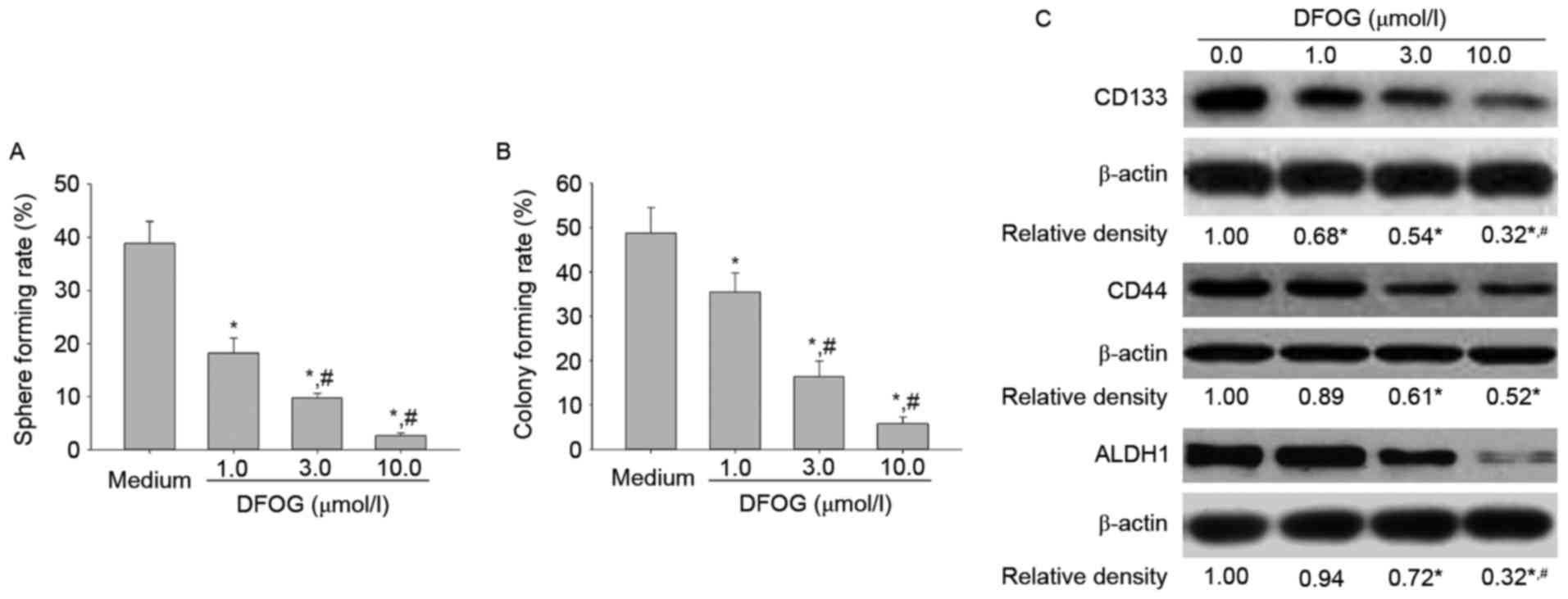
Therefore, we next sought to examine the inhibitory effects of DFOG
on stemness of OVCSLCs from SKOV3 cells. In the present study, we
found that DFOG dose-dependently reduced spheroid (Fig. 2A) and colony (Fig. 2B) formation rates. Furthermore, our
data provided evidence that DFOG displayed a
concentration-dependent downregulation of CSC-related proteins
(CD133, CD44 and ALDH1) (Fig. 2C).
The current findings suggest that DFOG can efficiently inhibit
stemness in OVCSLCs from SKOV3 cells.
DFOG reduces the phosphorylation
levels of AKT in OVCSLCs from SKOV3 cells
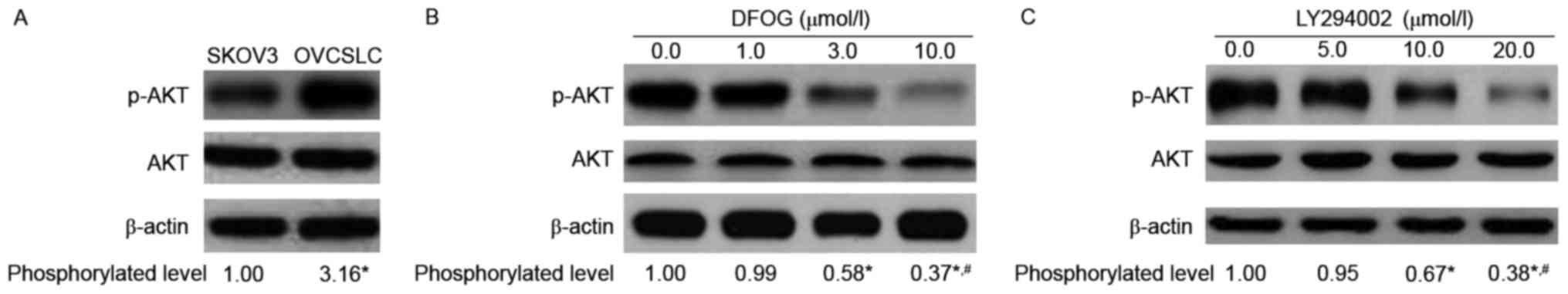
Numerous studies have reported that genistein
abolishes stemness in CSCs or CSLCs via inhibition of AKT
phosphorylation and activity (10,16).
We thus hypothesized that the novel genistein analogue
DFOG-inhibited spheroid and colony formation is associated with AKT
inactivation. To test the hypothesis, we used western blotting to
analyze the protein expression levels of p-AKT in cells treated
with DFOG or not. As expected, elevated amounts of phosphorylated
AKT in OVCSLCs were obtained compared with the levels in SKOV3
cells grown in monolayers (Fig.
3A). DFOG effects (Fig. 3B)
were similar to those of the PI3K inhibitor LY294002 (Fig. 3C), effectively and
concentration-dependently reducing the phosphorylation levels of
the AKT protein in SKOV3 cell-produced OVCSLCs.
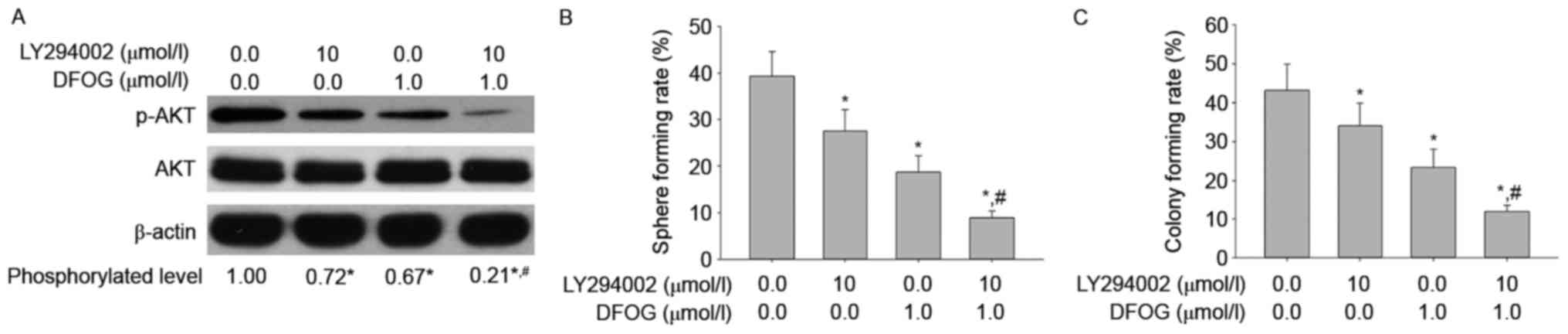
To confirm that the reduced AKT phosphorylation is
involved in oncogenicity maintenance in vitro in SKOV3
cell-produced OVCSLCs, we next sought to examine p-AKT levels, and
spheroid and colony forming capabilities in OVCSLCs treated with
DFOG (1 µmol/l) and/or LY294002 (10 µmol/l). We found that DFOG and
LY294002 cooperated to reduce the levels of AKT phosphorylation
(Fig. 4A), and attenuate spheroid
(Fig. 4B) and colony (Fig. 4C) forming capabilities in OVCSLCs
from the SKOV3 cell line. These results suggest that DFOG-inhibited
spheroid and colony formation may be associated with AKT
inactivation in OVCSLCs from SKOV3 cells.
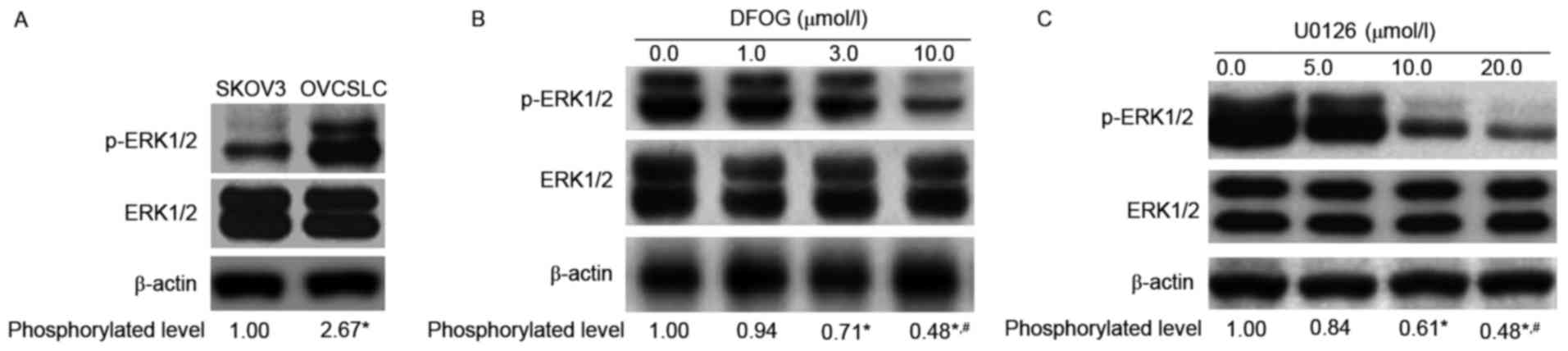
DFOG downregulates p-ERK1/2 expression
in OVCSLCs from SKOV3 cells
ERK kinase signaling is essential for cell
proliferation. In healthy cells, the activated signaling pathway
leads to progression from G1 to S phase, and is involved in the
inactivation of antiproliferative genes. Moreover, inhibition of
the ERK pathway reduces the development of CSCs (17). Increasing evidence demonstrates that
genistein inhibits self-renewal ability and CSC-related protein
expression in various CSCs or CSLCs via inhibition of ERK1/2
phosphorylation (18–21). We next sought to analyze whether
DFOG-inhibited spheroid and colony formation is associated with
reduced ERK1/2 protein phosphorylation. We found elevated levels of
phosphorylated ERK in OVCSLCs compared with the amounts obtained
for SKOV3 cells in monolayer growth (Fig. 5A). DFOG (Fig. 5B) showed similar effects to the MEK
inhibitor U0126 (Fig. 5C),
effectively concentration-dependently reducing the phosphorylation
levels of the ERK1/2 protein in OVCSLCs derived from SKOV3
cells.
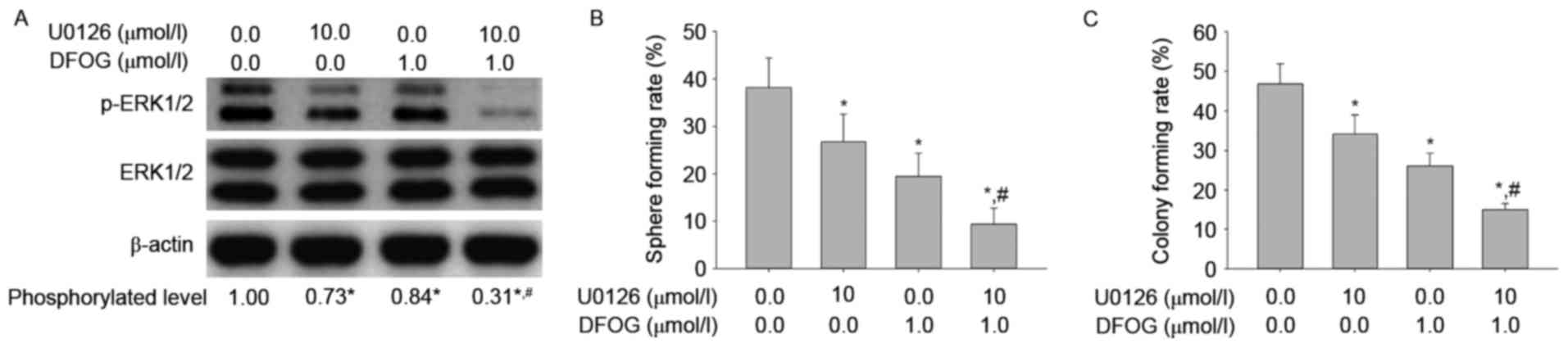
To determine how DFOG-related reduced ERK1/2 protein
phosphorylation affects oncogenicity maintenance in vitro in
SKOV3 cell-produced OVCSLCs, we next sought to examine p-ERK
levels, spheroid and colony forming capabilities in OVCSLCs treated
with DFOG (1 µmol/l) and/or U0126 (10 µmol/l). We showed that DFOG
and U0126 cooperated to reduce the levels of ERK1/2 protein
phosphorylation (Fig. 6A), and
attenuate spheroid (Fig. 6B) and
colony (Fig. 6C) forming
capabilities in OVCSLCs from the SKOV3 cell line. These results
suggest that DFOG-inhibited spheroid and colony formation may be
associated with inhibition of ERK1/2 protein phosphorylation in
OVCSLCs from SKOV3 cells.
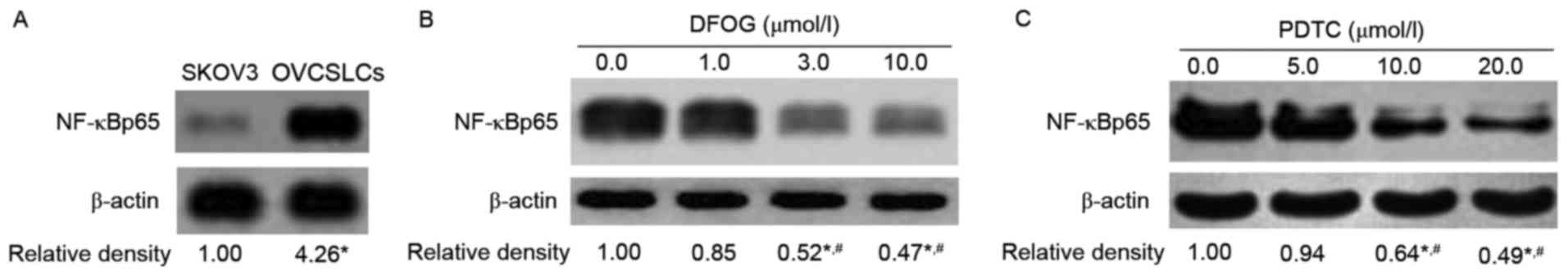
DFOG decreases NF-κB activity in
OVCSLCs from SKOV3 cells
Recent studies showed that genistein and its
derivatives inhibit the proliferation and invasion of various
cancer cells or cancer stem-like cells by inhibiting NF-κB nuclear
translocation via IκB signaling (19,22,23).
We next sought to assess whether DFOG-inhibited spheroid and colony
formation is associated with the inhibition of NF-κB activity. The
results obtained by western blot analysis demonstrated an
upregulation of NF-κBp65, an active fragment of NF-κB, in OVCSLCs
compared with cells in monolayer growth derived from the SKOV3 cell
line (Fig. 7A). Both DFOG (Fig. 7B) and PDTC (Fig. 7C), a NF-κB inhibitor,
dose-dependently reduced the expression levels of NF-κBp65.
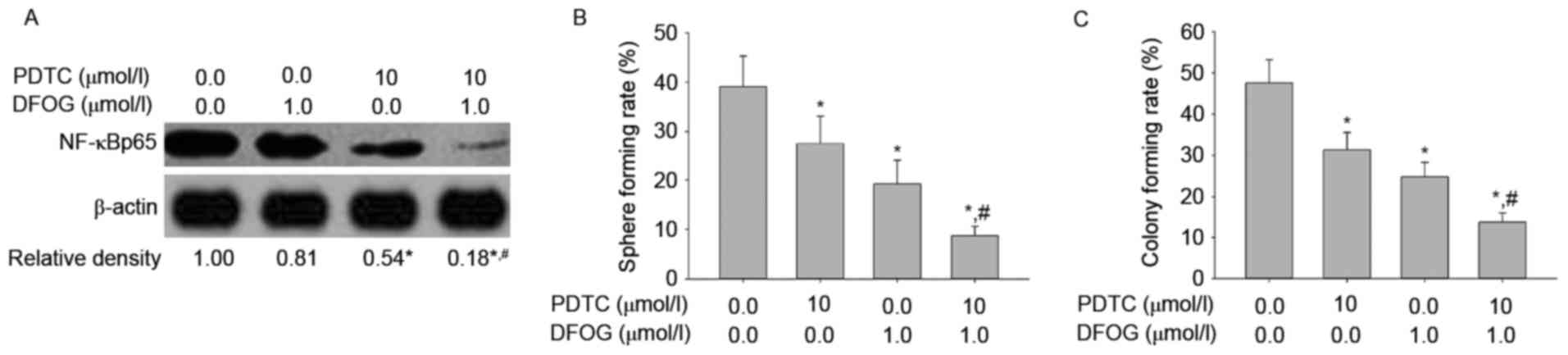
To examine the role of DFOG-related inhibition of
NF-κB activity in oncogenicity maintenance in vitro in SKOV3
cell-produced OVCSLCs, we next sought to quantify NF-κBp65 levels,
and spheroid and colony forming capabilities in OVCSLCs treated
with DFOG (1 µmol/l) or PDTC (10 µmol/l) or both. We found that
DFOG and PDTC cooperated to reduce the levels of NF-κBp65
expression (Fig. 8A), and attenuate
spheroid (Fig. 8B) and colony
(Fig. 8C) forming capabilities in
OVCSLCs from the SKOV3 cell line. These results suggest that
DFOG-inhibited spheroid and colony formation may be associated with
the inhibition of NF-κB activity in OVCSLCs from SKOV3 cells.
Inhibitory effects of DFOG on
oncogenicity in vitro depend on FoxO3a expression in OVCSLCs from
SKOV3 cells
Since the FoxO3a function is closely associated with
OVCSLC oncogenicity inhibition related to suppressed AKT and/or ERK
and/or NF-κB pathway (1,24,25),
whether oncogenicity reduction by DFOG in vitro depended on
FoxO3a expression in OVCSLCs from SKOV3 cells was assessed.
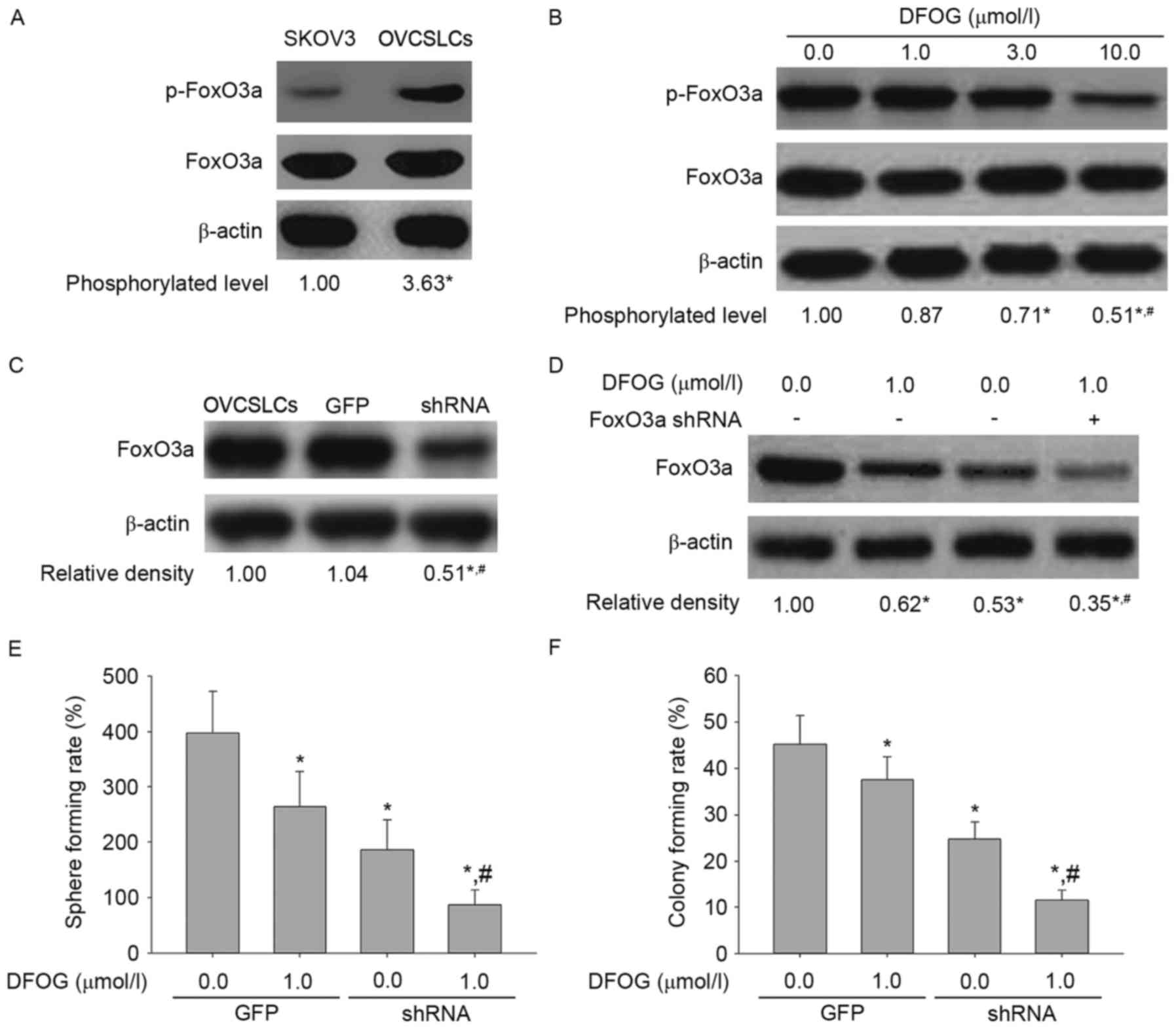
Fig. 9A shows elevated expression
levels of phosphorylated FoxO3a in OVCSLCs compared with these
levels in SKOV3 cells in monolayer growth. DFOG (Fig. 9B) effectively and
concentration-dependently reduced the phosphorylation levels of
FoxO3a in SKOV3 cell-produced OVCSLCs. In SKOV3 cell-produced
OVCSLCs, FoxO3a expression was knocked down by transduction with
the FOXO3a shRNA-expressing adenovirus (Fig. 9C). Our results revealed that
co-treatment with DFOG and FOXO3a shRNA reduced the expression
levels of FoxO3a (Fig. 9D), and
synergistically attenuated the spheroid (Fig. 9E) and colony (Fig. 9F) forming capabilities of OVCSLCs
from the SKOV3 cell line. Taken together, these data indicate that
inhibition of OVCSLC oncogenicity in vitro mediated by the
suppressive effects of DFOG on AKT and/or ERK and/or NF-κB pathways
requires FoxO3a expression.
Oncogenicity reduction by DFOG in
vitro requires FoxM1 in OVCSLCs from SKOV3 cells
A study by McGovern et al demonstrated
ectopically expressed FOXO3a downregulates FOXM1, while FOXO3a
silencing increases FOXM1 levels and rescues sensitive breast
cancer BT474 cells from gefitinib-related growth inhibition
(26). Our results suggested that
FoxM1 inhibition by FoxO3a activation induced apoptosis in ovarian
cancer cells (27). Furthermore,
previous studies demonstrated that FoxM1 inhibition results in
altered characteristics of OVCSLCs from SKOV3 cells (4). Importantly, Bao et al found
that genistein inhibits cell malignancy, in agreement with
decreased CD44 and EpCAM levels. Accordingly, we determined that
the inhibitory effects of DFOG on oncogenicity in vitro
depended on FoxM1 expression in OVCSLCs from SKOV3 cells.
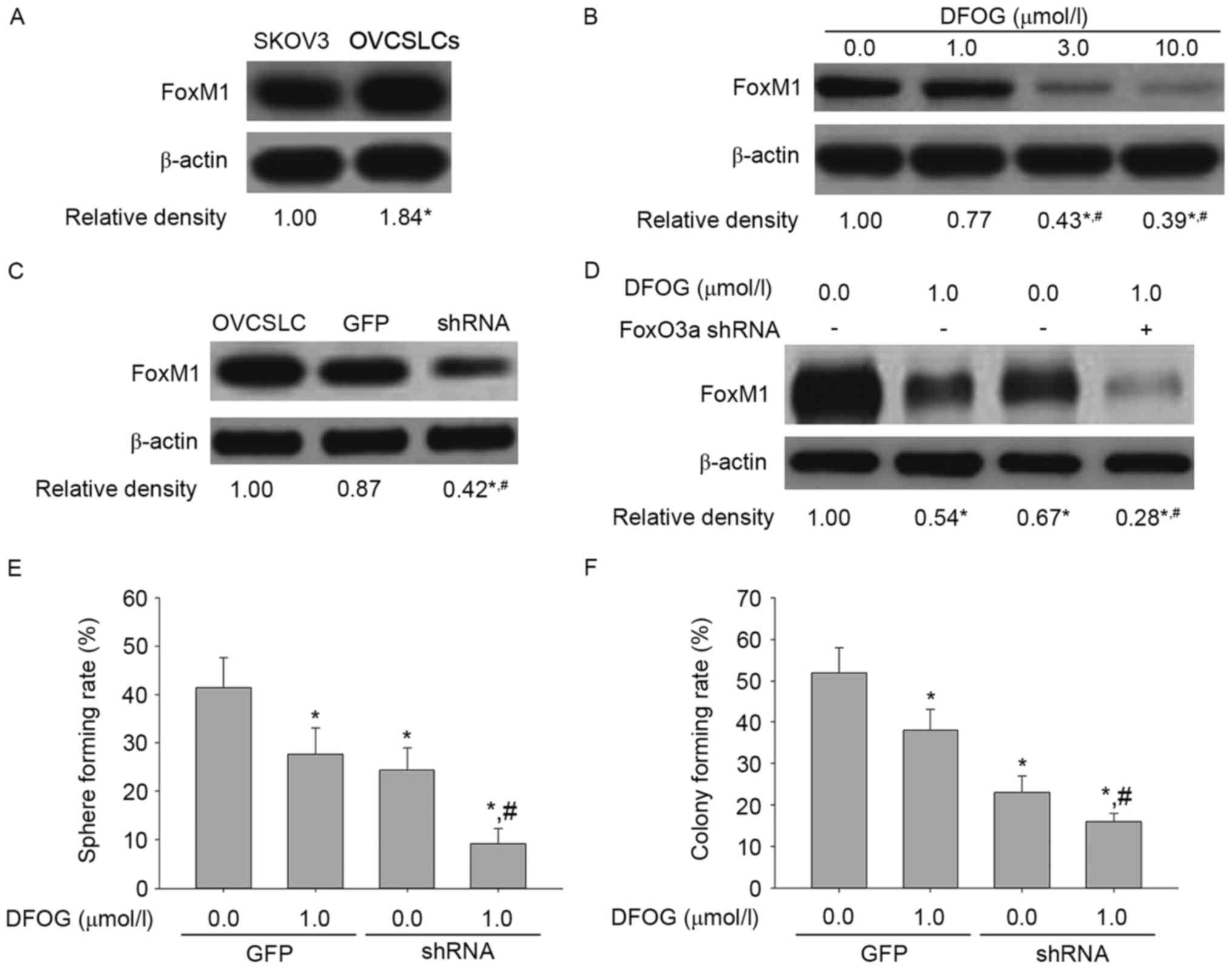
Fig. 10A shows
FoxM1 upregulation in OVCSLCs compared with the levels of SKOV3
cells in monolayer growth. DFOG (Fig.
10B) effectively and concentration-dependently reduced the
expression of the FoxM1 protein in OVCSLCs derived from SKOV3
cells. In SKOV3 cell-produced OVCSLCs, FoxM1 expression was knocked
down by transduction with FoxM1 shRNA-expressing adenovirus
(Fig. 10C). Our results also
revealed that co-treatment with DFOG and FOXM1 shRNA reduced the
expression levels of FoxM1 (Fig.
10D), and synergistically attenuated spheroid (Fig. 10E) and colony (Fig. 10F) forming capabilities in OVCSLCs
from the SKOV3 cell line. Taken together, these data indicate that
reduced OVCSLC oncogenicity in vitro by DFOG through
inhibitory effects on AKT and/or ERK and/or NF-κB pathways requires
FoxM1 expression.
Discussion
Cancer is the uncontrolled growth of cells. Numerous
signaling pathways regulate cell growth and proliferation (28). Various molecules are associated with
tumor development. For example, phosphorylation of AKT, activation
of ERK1 and ERK2, and NF-κBp65 proteins lead to cancer cell
development (24). In the present
study, we ascertained whether a newly synthesized potential
anticancer genistein analogue, DFOG, possesses inhibitory effects
on p-AKT and p-ERK1/2 expression, and NF-κB activity, as well as on
the suppression of oncogenicity in vitro in SKOV3-derived
OVCSLCs. SKOV3-derived spheroids were therefore used to demonstrate
the pharmacological effectiveness of the inhibitors. The other
drugs, including LY294002, U0126 and PDTC, whose chemopreventive
activities have been demonstrated in previous studies, were
compared to DFOG (29). Notably, in
the present study, we found that DFOG significantly reduced the
levels of phosphorylated AKT. Combination of DFOG with another
pharmacological inhibitor LY294002 led to a drastic decrease in
p-AKT expression. In addition, the target drug was capable of
downregulating phosphorylated ERK1 and ERK2 proteins in the ERK1/2
signaling pathway. However, DFOG compared with another tumor
inhibitor (U0126) was likely to be less effective in diminishing
the activation of key proteins involved in cell growth and
survival. Nevertheless, simultaneous application of the two
anticancer agents resulted in a significant inhibition of
phosphorylated ERK1 and ERK2 levels. Finally, the genistein
analogue DFOG demonstrated a high potential to decrease the level
of the NF-κBp65 protein, which is extremely important in
chemopreventive therapies against cancer development. However,
based on its antitumor potency, the novel genistein derivative DFOG
is considered an agent with activity against different types of
cancer (30).
The present study revealed the anticancer activity
of DFOG in OVCSLCs. Cancer stem cells (CSCs) are known to maintain
and facilitate the formation of cancers. In addition, CSLCs drive
drug resistance as well as recurrence or relapse (31). As ovarian cancer is resistant to
conventional chemotherapies, a new approach to target CSCs is
necessary. Compounds containing genistein can be applied against
CSCs (32). Thus, in the present
study, we determined how an anticancer analogue of genistein, DFOG,
affects OVCSLCs. A study by Roy et al (33) demonstrated that repressing PI3K/AKT
and MEK/ERK pathways induces FOXO transcription factors. After
transduction with the FOXO3a shRNA-expressing adenovirus, we found
that the combination of DFOG and FOXO3a shRNA downregulated FoxO3a,
synergistically attenuating spheroid and colony forming
capabilities of SKOV3 cell-produced OVCSLCs. To integrate the
abovementioned tight association of FoxO3a with OVCSLC oncogenicity
inhibition related to AKT and/or ERK and/or NF-κB pathway
suppression, our data showed that reduced OVCSLC oncogenicity in
vitro due to the suppressive effects of DFOG on AKT and/or ERK
and/or NF-κB pathways requires FoxO3a expression. These results
support the viewpoint of Sunayama et al (1) that FoxO3a likely has an important
function in controlling CSLCs via PI3K/AKT/mTOR and MEK/ERK
signaling, thereby implying that tools effectively targeting FoxO3a
induction may constitute a viable option for human carcinoma
treatment.
McGovern et al (26) assessed FOXM1 function and modulation
after gefitinib treatment, and demonstrated that gefitinib
downregulates FOXM1 through FOXO3a in breast carcinoma. Our
previous study demonstrated that DFOG acts as an inhibitor of CSCs
or tumor-initiating cells by activating FoxO3a and/or inactivating
FoxM1 (4). As demonstrated above,
combining DFOG and FOXM1 shRNA reduced the expression levels of
FoxM1, and synergistically attenuated the spheroid and colony
forming capabilities of OVCSLCs from the SKOV3 cell line. These
results indicated that reduced OVCSLC oncogenicity in vitro
by DFOG through inhibitory effects on AKT and/or ERK and/or NF-κB
pathways requires FoxM1 expression.
Overall, DFOG exerts anticancer activity by
targeting OVCSLCs. DFOG may be effective in reducing the expression
levels of cell regulatory proteins such as p-AKT, p-ERK1/2, and
NF-κBp65. Moreover, the novel genistein derivative prevented
spheroid and colony formation in OVCSLCs. These findings provide
evidence for DFOG potency in the inhibition of cancer progression.
Importantly, the reduced OVCSLC oncogenicity in vitro by
DFOG through inhibitory effects on AKT and/or ERK and/or NF-κB
pathways requires both FoxO3a and FoxM1 expression. Therefore, DFOG
may be used as a novel chemotherapeutic drug for ovarian
carcinoma.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (nos. 81301894 and 81302249), the
Guangzhou Science and Information Bureau Item (no. 201300000151),
the Guangdong Province Department of Science and Technology of
China (nos. 2014A020211028, 2014A020212609 and 2012B031800271) and
the Scientific Research Project for Medical College of Bureau of
Education of Guangzhou City (no. 1201410508).
References
|
1
|
Sunayama J, Sato A, Matsuda K, Tachibana
K, Watanabe E, Seino S, Suzuki K, Narita Y, Shibui S, Sakurada K,
et al: FoxO3a functions as a key integrator of cellular signals
that control glioblastoma stem-like cell differentiation and
tumorigenicity. Stem Cells. 29:1327–1337. 2011.PubMed/NCBI
|
|
2
|
Jacobsen EA, Ananieva O, Brown Ml and
Chang Y: Growth, differentiation, and malignant transformation of
pre-B cells mediated by inducible activation of v-Abl oncogene. J
Immunol. 176:6831–6838. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ning Y, Luo C, Ren K, Quan M and Cao J:
FOXO3a-mediated suppression of the self-renewal capacity of
sphere-forming cells derived from the ovarian cancer SKOV3 cell
line by 7-difluoromethoxyl-5,4′-di-n-octyl genistein. Mol Med Rep.
9:1982–1988. 2014.PubMed/NCBI
|
|
4
|
Ning YX, Li QX, Ren KQ, Quan MF and Cao
JG: 7-difluoromethoxyl-5,4′-di-n-octyl genistein inhibits ovarian
cancer stem cell characteristics through the downregulation of
FOXM1. Oncol Lett. 8:295–300. 2014.PubMed/NCBI
|
|
5
|
Li Y, Wicha MS, Schwartz SJ and Sun D:
Implications of cancer stem cell theory for cancer chemoprevention
by natural dietary compounds. J Nutr Biochem. 22:799–806. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh J, Hlatky L, Jeong YS and Kim D:
Therapeutic effectiveness of anticancer phytochemicals on cancer
stem cells. Toxins. 8:pii: E199. 2016. View Article : Google Scholar :
|
|
7
|
Cunha-Rodrigues M, Portugal S, Prudêncio
M, Gonçalves LA, Casalou C, Buger D, Sauerwein R, Haas W and Mota
MM: Genistein-supplemented diet decreases malaria liver infection
in mice and constitutes a potential prophylactic strategy. PLoS
One. 3:e27322008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou HB, Chen JM, Cai JT, Du Q and Wu CN:
Anticancer activity of genistein on implanted tumor of human SG7901
cells in nude mice. World J Gastroenterol. 14:627–631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ning Y, Li Q, Xiang H, Liu F and Cao J:
Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein
via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep.
27:1857–1864. 2012.PubMed/NCBI
|
|
10
|
Montales MT, Rahal OM, Kang J, Rogers TJ,
Prior RL, Wu X and Simmen RC: Repression of mammosphere formation
of human breast cancer cells by soy isoflavone genistein and
blueberry polyphenolic acids suggests diet-mediated targeting of
cancer stem-like/progenitor cells. Carcinogenesis. 33:652–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chung H, Kim YH, Kwon M, Shin SJ, Kwon SH,
Cha SD and Cho CH: The effect of salinomycin on ovarian cancer
stem-like cells. Obstet Gynecol Sci. 59:261–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Latifi A, Luwor RB, Bilandzic M,
Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA,
Findlay JK, et al: Isolation and characterization of tumor cells
from the ascites of ovarian cancer patients: Molecular phenotype of
chemoresistant ovarian tumors. PLoS One. 7:e468582012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vermeersch KA, Wang L, Mezencev R,
McDonald JF and Styczynski MP: OVCAR-3 spheroid-derived cells
display distinct metabolic profiles. PLoS One. 10:e01182622015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Condello S, Morgan CA, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-Catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:2297–2308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang HL, Liu F, Quan MF, Cao JG and Lv Y:
7-difluoromethoxyl-5,4′-di-n-octylgenistein inhibits growth of
gastric cancer cells through downregulating forkhead box M1. World
J Gastroenterol. 18:4618–4626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Zou T, Wang S, Chen H, Su D, Fu X,
Zhang Q and Kang X: Genistein-induced differentiation of breast
cancer stem/progenitor cells through a paracrine mechanism. Int J
Oncol. 48:1063–1072. 2016.PubMed/NCBI
|
|
17
|
Yao Y, Li W, Wu J, Germann UA, Su MS,
Kuida K and Boucher DM: Extracellular signal-regulated kinase 2 is
necessary for mesoderm differentiation. Proc Natl Acad Sci USA.
100:12759–12764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Wan C and Luo Q, Huang Z and Luo
Q: Genistein-inhibited cancer stem cell-like properties and reduced
chemoresistance of gastric cancer. Int J Mol Sci. 15:3432–3443.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SD, Chen BC, Kao ST, Liu CJ and Yeh
CC: Genistein inhibits tumor invasion by suppressing multiple
signal transduction pathways in human hepatocellular carcinoma
cells. BMC Complement Altern Med. 14:262014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC
and Song YS: Genistein inhibits cell growth by modulating various
mitogen-activated protein kinases and AKT in cervical cancer cells.
Ann NY Acad Sci. 1171:495–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Blas E, Estañ MC, de Del Carmen Gómez
Frutos M, Ramos J, Del Carmen Boyano-Adánez M and Aller P: Selected
polyphenols potentiate the apoptotic efficacy of glycolytic
inhibitors in human acute myeloid leukemia cell lines. Regulation
by protein kinase activities. Cancer Cell Int. 16:702016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Lu P, Zhang W, Du Q, Tang J, Wang
H, Lu J and Hu R: GEN-27, a newly synthetic isoflavonoid, inhibits
the proliferation of colon cancer cells in inflammation
microenvironment by suppressing NF-κB pathway. Mediators Inflamm.
2016:28530402016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vazquez-Santillan K, Melendez-Zajgla J,
Jimenez-Hernandez LE, Gaytan-Cervantes J, Muñoz-Galindo L,
Piña-Sanchez P, Martinez-Ruiz G, Torres J, Garcia-Lopez P,
Gonzalez-Torres C, et al: NF-kappaB-inducing kinase regulates stem
cell phenotype in breast cancer. Sci Rep. 6:373402016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung Y, Park H, Zhao HY, Jeon R, Ryu JH
and Kim WY: Systemic approaches identify a garlic-derived chemical,
Z-ajoene, as a glioblastoma multiforme cancer stem cell-specific
targeting agent. Mol Cells. 37:547–553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dubrovska A, Kim S, Salamone RJ, Walker
JR, Maira SM, García-Echeverría C, Schultz PG and Reddy VA: The
role of PTEN/Akt/PI3K signaling in the maintenance and viability of
prostate cancer stem-like cell populations. Proc Natl Acad Sci USA.
106:268–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McGovern UB, Francis RE, Peck B, Guest SK,
Wang J, Myatt SS, Krol J, Kwok JM, Polychronis A, Coombes RC, et
al: Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in
breast cancer. Mol Cancer Ther. 8:582–591. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang L, Cao XC, Cao JG, Liu F, Quan MF,
Sheng XF and Ren KQ: Casticin induces ovarian cancer cell apoptosis
by repressing FoxM1 through the activation of FOXO3a. Oncol Lett.
5:1605–1610. 2013.PubMed/NCBI
|
|
28
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging. 3:192–222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smalley KS, Haass NK, Brafford PA, Lioni
M, Flaherty KT and Herlyn M: Multiple signaling pathways must be
targeted to overcome drug resistance in cell lines derived from
melanoma metastases. Mol Cancer Ther. 5:1136–1144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chahar MK, Sharma N, Dobhal MP and Joshi
YC: Flavonoids: A versatile source of anticancer drugs. Pharmacogn
Rev. 5:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakariassen PO, Immervoll H and Chekenya
M: Cancer stem cells as mediators of treatment resistance in brain
tumors: Status and controversies. Neoplasia. 9:882–892. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Li L, Jiao M, Wu D, Wu K, Li X,
Zhu G, Yang L, Wang X, Hsieh JT, et al: Genistein inhibits the
stemness properties of prostate cancer cells through targeting
Hedgehog-Gli1 pathway. Cancer Lett. 323:48–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|