Introduction
The skin is both a physical barrier between the host
and the environment, as well as an immunological gatekeeper
destined to optimally fulfill the needs of the host. Thus, the
interactions between the external factors and the internal
environment of the skin significantly affect both skin homeostasis,
as well as the incidence of skin pathologies (1).
Comprising basal cell carcinoma (BCC) and squamous
cell carcinoma (SCC), together with a large number of rare tumors,
non-melanoma skin cancer (NMSC) is the most common form of cancer
worldwide, encompassing 95% of all cutaneous neoplasms and
approximately 40% of all malignancies (2,3).
Despite growing awareness and sun-protective measures, the
incidence of NMSC has markedly increased over the past decades
(4). Due to the current age shift
in the population, mainly in developed countries, the incidence of
NMSC may increase by an estimated 50% by the year 2030 (4,5).
BCC is the most frequently occurring form of skin
cancer (80% of all skin cancers), accounting for one third of all
cancers per year (6,7). While BCCs rarely exhibit an
unrestrained behavior, SCCs are more aggressive and metastasize
more frequently. SCCs represent over 20% of all cutaneous
malignancies, and 14% of these tumors metastasize and are
responsible for the majority of deaths due to NMSC (8–13).
Furthermore, SCC is the most frequently reported pathology of
penile cancer (>95%); this is a rare malignancy accounting for
0.24% of all neoplasms among males in the United States with a
significantly higher incidence (up to 20–30-fold greater) in areas
of Africa and South America, that usually arises from the
epithelium of the inner prepuce or the glans, and it exists in
several histological subtypes and shares a similar pathology with
SCC of other origins (14–16). As a direct consequence, NMSCs add a
considerable financial burden to healthcare systems, significantly
reducing the quality of life of patients and having a marked
socio-economic impact. Thus, continuous efforts are being made
towards the identification of novel etiopathogenic mechanisms.
NMSCs have many common environmental triggers, some
stimulating tumor development more than others. The major risk
factors for NMSCs are chronic exposure to ultraviolet radiation
(UVR), the thinning of the ozone layer due to pollution,
non-specific immune suppression, the increased lifespan, protein
patched homolog 1 (PTCH) gene mutations and fair skin (17). Human papilloma virus (HPV) infection
is an important risk factor for penile SCC; viral DNA has been
detected in 70–100% of penile intraepithelial neoplasia and in
30–40% of invasive cancer tissue samples (15). HPV infection (particularly by high
risk HPV types 16, 18, 31, 33, 35, 56 and 64) is nowadays
recognized as a major co-factor in penile SCC through oncogenes and
tumor suppressor genes (p53, pRb, p16) (16,18).
Nevertheless, it is now clear that apart from the HPV-induced
pathway (through which up to 50–80% of penile SCC cases arise), a
non-HPV-induced pathway represents a divergent molecular pathway
accounting for penile carcinogenesis related to several risk
factors, such as chronic inflamation and specific mediators
(16,19).
The fact that NMSC arises mostly on sun-exposed
areas (with the exception of penile SCC) has highlighted the
crucial role of UVR in the pathogenesis of NMSC. The effects of UVR
exposure include DNA alteration, epidermal inflammation and
hyperplasia, creating favorable conditions for the development of
cutaneous malignancies (20–22).
UVR is the principal cause of the occurrence of NMSC. Due to the
interaction of the external environment with the internal
mechanisms of the human organism, factors that influence the
functions of the human body also affect the appearance and/or
progression of precancerous lesions (23,24).
Thus, an underactive and poor performing immune system causes these
lesions to rapidly appear and develop, while a strong immune system
may prevent the appearance and development of NMSC (23,24).
The immunosuppressive-inducing effects of UVR are primarily cited
as conditioning a permissive tumor microenvironment for BCC and
SCC. Several studies have confirmed that the immune system has a
significant influence on the development of skin tumors, by
creating a microenvironment favorable for carcinogenesis. However,
these effects may be insufficient to induce carcinogenesis
(25–29). Moreover, various neuroendocrine
stimuli may potentiate and maintain a chronic state of
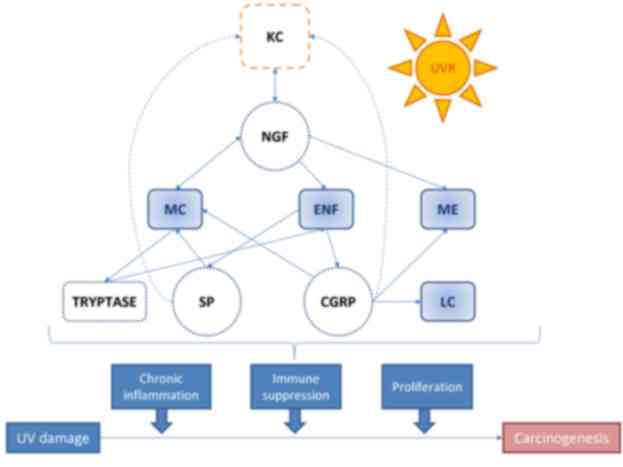
inflammation, promoting tumorigenesis (Fig. 1) (30–33).
Importantly, the function of the skin as a peripheral
neuroendocrine organ has been well established. Thus, signals of
cutaneous origin but related to the neuroendocrine system may
trigger cascades of responses addressed at maintaining local and
global homeostasis. The neuroendocrine function of the skin is
performed by cells of mesenchymal, neural crest, bone marrow and
epithelial origin that are compartmentally arranged into endocrine
units localized at the epidermis, dermis and adnexal structures.
These cells produce the respective hormones and express the
corresponding receptors, suggesting that the main interactive
mechanisms within the distinct cutaneous domains are of both
autocrine and paracrine capacity (34). Moreover, cutaneous nerve fibers
provide additional levels of communication with the release of
neuropeptides at dermal or epidermal levels. The true endocrine
component of the cutaneous neuroendocrine system is thus
represented by those humoral signals that can directly enter the
circulation (35). Therefore, the
skin can generate signals to produce rapid or slow responses at
local or systemic level. These signals counteract the environmental
insults and/or internal functions. Thus, the skin can be
characterized as a sensor for external or internal factors, which
are subsequently translated by the cutaneous neuroendocrine system
into humoral or neural signals and dispatched to local or distant
coordinating centers (35).
Various studies have suggested a link between
neuroendocrine factors and skin carcinogenesis in the two most
common types of NMSC, BCC and SCC, with the exception of penile
SCC, for which a paucity of specific data on their pathogenic role
exists. A complex interaction between nervous system and target
cells, such as keratinocytes, melanocytes, Langerhans cells (LCs),
mast cells, endothelial cells and inflammatory infiltrate cells has
been previously described in skin. This interaction appears to be
mediated by locally released neuroendocrine factors encompassing
classic neurotransmitters, neuropeptides and neurohormones
(36–39). The mechanisms through which
neuroendocrine factors influence mucocutaneous physiopathological
processes are extremely complex, involving the dysregulation of the
dynamic balance between the nervous, endocrine and immune systems
(40). While NMSCs are highly
immunogenic, the action of neuroendocrine factors, when combined
with other appropriate factors, such as UVRs, may result in either
the development of SCC or BCC (30,31).
The aim of this review was to provide an overview of
the neuroendocrine factors role in the initiation and/or promotion
of NMSC (with a focus on SCC and BCC) in relation to environmental
factor exposure.
Stress
Psychological stress is involved in the onset or
aggravation of numerous skin pathophysiological processes (37–39,41,42).
In addition, a significant body of evidence suggests that stress is
a crucial impact factor influencing the course of skin cancers
(38,43–45).
Neuroendocrine factor dysregulation, as observed in stress
reactions, may be involved in the modulation of tumorigenesis,
accelerating the development and progression, and suppressing the
regression of cancer.
Chronic stress
Alterations in the functionality of the immune
system and promotion of tumor development (46) have been proven to represent the
long-term effects of chronic stress exposure. Even without any
obvious impact on the general health status, a moderate but chronic
stress factor seems to lead to a substantial increase in skin
cancer susceptibility in affected individuals. Moreover, the
negative impact of stress factors on clinical, cellular and
molecular parameters adding up to immunosuppression can be observed
for months, even after the stressors have ceased their activity
(31).
Studying skin tumor development in rat models, Saul
et al (31) demonstrated
that stressed mice exposed to UVB radiation developed skin
malignancies in a shorter timeframe and that the stressed study
group reached 50% tumoral incidence earlier than the non-stressed
control group. Another study by Parker et al (47) demonstrated accelerated tumor
development in an extended chronic stress model, in which stress
factor administration began 2 weeks prior to UVR exposure.
Furthermore, as previously demonstrated, stressed mice exhibit
reduced values of interferon (IFN)-γ, CCL27/CTACK (expressed
predominantly in the skin and responsible for T cell recruitment)
(48), CD3ε (considered an
important indicator of T cell infiltration) and interleukin
(IL)-12p40 gene expression, as well as a reduced CD4+
cell count and an increased number of CD25+ suppressor
cells in peritumoral infiltrates. IFN-γ, which promotes tumor
recognition and destruction (49),
is an essential mediator of IL-12-related antitumoral effects
(50), and promotes tumor
suppression (51). The authors
hence concluded that chronic stress increases UVB-induced cutaneous
SCC in their rat model, primarily by suppressing type 1 cytokine
production and increasing T suppressor cell count (51). Furthermore, it has been shown that
basal corticosterone levels in stressed mice remain higher than
those in the control group for almost 7 months following the
cessation of stress factors (51).
Importantly, higher morning corticosterone levels indicate a
circadian rhythm disturbance, leading to immune function impairment
(52), accelerated tumor
progression (53–55) and increased mortality (56).
Due to its immunosuppressive effect, chronic stress
also creates a permissive environment for BCC tumorigenesis
(30,31). Transplant patients, as well as
patients receiving moderate doses of immunosuppressive therapies
are more susceptible to developing BCC than the general population
(57), since the immune system is
central to BCC appearance and progression. Fagundes et al
showed that patients with BCC who experienced a stressful event in
the past year or who had been maltreated in childhood had a poorer
antitumor immune response (30). Of
note, messenger RNA encoding for proteins expressed on immune
cells, such as CD3ε, CD68, CD25 and ICAM-1 are indicators of a
targeted anti-BCC immune response. Patients who reported early
childhood abuse had lower mRNA levels of CD3ε, CD68, CD25 and
ICAM-1, illustrating a tolerant microenvironment for tumor
development (30). The results from
the study by Saul et al concerning SCC in mouse models could
also be applicable to BCC, as both tumors are intensely immunogenic
(31).
Two isoenzymes regulate cortisol activity in the
skin, activating and, respectively, inactivating it:
11-β-hydroxysteroid dehydrogenase (11βHSDH)1 and 2 (32,58).
In BCC, 11βHSDH1 is decreased, while its counterpart is increased
(32). Of note, 11βHSDH2 is
non-existent in healthy skin, but its expression is increased in
proliferating basal cells in BCC and seborrheic keratosis (SK). One
hypothesis is that local cortisol activation via 11βHSDH1 in
keratinocytes plays a role in controlling local stress and repeated
stimulation (32). 11βHSDH1 levels
are low in BCC regardless of tumor differentiation, and may in fact
induce cellular proliferation (33). Inhibition of 11βHSDH1 has been
reported to activate epidermal cells hyperproliferation in murine
models (58). Moreover, Terao et
al found that 11βHSDH2 was increased in BCC and SK, but not in
SCC (33). According to these
authors, 11βHSDH1 and 2 may modulate intracellular glucocorticoid
levels, subsequently influencing keratinocyte proliferation,
differentiation and inflammation (33).
Chronic stress induces cellular mediated immune
suppression (59), which plays a
major role in NMSC-related tumoral surveillance. A reduction in the
peritumoral CD4 lymphocyte population has been reported during
chronic stress exposure, thus supporting the hypothesis according
to which SCC susceptibility is mediated through the suppression of
T cell activity (60,61). Other studies have shown that
long-term stress suppresses another tumor, eliminating NK cells,
which leads to tumor metastasis in experimental models (62,63).
Acute stress
As opposed to chronic stress, which acts as an
immunosuppressant, acute stress reactions during immune activation
or antigen exposure induce a redistribution of circulating immune
cells towards organs, such as the skin, subcutaneous tissue and
local lymph nodes (59,64,65),
thus enhancing both innate and acquired immunity (59,65–70).
This augmented immune response can also increase antitumoral
activity against immunoresponsive malignancies, during tumoral
immunotherapy or during surgery.
Due to the immunogenicity of SCC, studies have been
carried out starting from the hypothesis that acute stress prior to
UVR exposure would enhance the immune response and potentiate
resistance to SCC development. Dhabhar et al (71) studied the immunomodulatory effects
of short-term stress exposure in a murine model and found that the
exposed to acute stress study group exhibited an increased
expression of chemokine related genes, such as CTACK/CCL27, RANTES,
IL-12 and IFN-γ for up to 8 months, larger numbers of infiltrating
T cells (both CD8+ and CD4+) and a reduced
SCC incidence at 4 months. These results suggest that acute stress
increases chemokine gene expression and T cell trafficking
following UVR exposure, while it also enhances cellular
immunity.
Considering the relevance of these findings in
relation to the discovery of novel mechanisms of antitumoral
response activation, the intrinsic immune and non-immune mechanisms
through which short-term stress during UVR exposure reduces future
tumor burden need to be further elucidated.
Neuroactive mediators
Neurotransmitters
Whereas some studies suggest that neuroactive
molecules influence the risk for developing malignancies
(initiation phase), others have suggested that neuroactive
compounds also affect progression, once disease is established.
Under stressful conditions, sympathetic nerve fibers
release additional epinephrine and norepinephrine (NE), which have
been previously shown to increase IL-6 and reactive oxygen species
production, thereby promoting cancer cell survival and
proliferation (72,73). Moreover, in vitro studies
have proven that cancer cell survival and proliferation can be
successfully inhibited by incubation with adrenergic receptor (AR)
antagonists (74,75).
Following skin injury, basal keratinocytes migrate
into the wound to initialize re-epithelialization, critical in
wound repair and barrier function rehabilitation. Keratinocytes
express high levels of β-ARs, without expressing other types of
ARs, and they are also capable of synthesizing epinephrine
(76,77).
One explanation for the relative lack of studies and
therefore knowledge in this particular area, is probably the
difficulty in preserving and demonstrating neurotransmitter
expression in cutaneous NMSC skin biopsies coupled with the
inexistence of adequate antibodies for such conditions of
fixation.
As early as the 1950′s Winkelmann (78) reported the presence of nerve fibers
in close proximity to BCC cells; however, this author did not
believe in the existence of a solid functional association between
these nerve fibers and BCC proliferation. Current evidence
indicates that several tumor cell lines express β-ARs and that
catecholamine hormones may influence tumorigenesis via these
receptors (79–85). Catecholamines are able to modulate
the expression of vascular endothelial growth factor (VEGF) and
matrix metalloproteinases (MMPs); thus, they can regulate various
facets of tumor progression, such as proliferation, angiogenesis
and metastasis (79,86–89).
MMPs and tissue inhibitors of metalloproteinases
(TIMPs) are known for their role in extracellular matrix
remodelling in both physiological and pathological processes, and
several cytokines possess the ability to influence their expression
(90). When collagenolytic MMPs are
activated, they degrade the peritumoral matrix, thereby aiding in
BCC and SCC microenvironment remodelling (91). Yang and Eubank (92) studied the effect of neuroendocrine
alteration on MMP and TIMP expression. Using a blister chamber
wound model on UVB-exposed human forearm skin, they reported that
the activation of the hypothalamic-pituitary-adrenal (HPA) and
sympathetic-adrenal medullary (SAM) axes indeed modulate MMP
levels. Their results revealed a positive correlation between NE
plasma levels and MMP-2 levels, and a negative association between
plasmatic cortisol levels and MMP-2 levels. These data indicate
that the release of NE associated with the sympathetic nervous
system can modulate MMP levels (93). Dumas et al (94) also reported an increased expression
of MMP-9 and MMP-2 in SCC versus BCC, which in conjunction with the
reduced expression of type IV collagen, led the authors to conclude
that this could be a possible explanation to the significantly
enhanced aggressive behavior of SCC when compared to BCC (95).
A recent study by Peterson et al investigated
the role of the nervous system in somatic cancer (96). The authors examined the role of
cutaneous nerve fibers in a model of spontaneous BCC following the
genetic deletion of PTCH1 and found that tumors developed mainly in
touch domes and bulge regions of hair follicles, although not in
the interfollicular epidermis. In their study, they disclosed the
presence of mRNA for the three hedgehog ligands in the dorsal root
ganglia, the location of sensory neurons that project to the skin,
and thus hypothesized that the loss of sensory-derived signaling
mediators, which inhibit the hedgehog pathway may be a driving
factor for carcinogenesis.
There is also evidence supporting the direct
involvement of peripheral nerve fibers in cutaneous epithelial cell
homeostasis in reports showing the progressive loss of Merkel cells
following denervation (97,98). BCC, an epidermal tumor, is also
lined with Merkel cells.
In a previous study, PTCH1 wild-type mice had
considerably fewer Merkel cells and significantly fewer tumor cells
in touch dome-derived tumors in contrast to the sham-lesioned
contralateral side, five weeks after denervation (96). Based on this observation, the
authors suggested denervation as a potential therapeutic tool, not
only for BCC, but also for Merkel cell malignancies (96). Nevertheless, other authors have
suggested that, even though Merkel cell tumors display phenotypes
similar to native Merkel cells, they still arise from skin stem
cells, and as such are susceptible to influence from cutaneous
innervation (99). The one
compelling finding of this previous study was that surgical or
chemical ablation of nerve fibers significantly reduced or slowed
BCC progression.
Bernabé et al (72) examined the effects of stress
hormones on SCC cell lines (SCC9, SCC15 and SCC25) and found that
not only can stress-related mediators (NE and isoproterenol)
enhance the production of the pro-angiogenic cytokine, IL-6, in
these cell lines, but that these cells are also capable of
producing IL-6 by themselves and, without stimulation, these levels
being already detectable at 1 h. The authors noted that
concentrations of IL-6 secreted by these cells, even by
non-stimulated cells, are within the range expected to have
biological activity. Similarly to the effects of IL-6 expression,
in this study, treatment with NE at physiological stress levels (10
µM) induced direct proliferation in SCC9 and SCC15 cell lines,
demonstrating constitutive expression of β1- and β2-ARs in the
tested cell lines (72).
Another neurotransmitter-related enzyme,
acetylcholinesterase (AChE) is found in sites where acetylcholine
(Ach) acts as a neurotransmitter (e.g., cholinergic synapses or
myoneural junctions). Furthermore, this enzyme has frequently been
used as a marker for Ach activity. Iyengar, demonstrated AChE
activity on the melanocyte cell membrane in hyperpigmented skin
lesions such as pigmented BCC and lichen planus (100), but the study did not associate
AchE/Ach with any specific function or mechanism involved in
carcinogenesis.
Neuropeptides
When skin is exposed to harmful stimuli, including
UVR, the unmyelinated c-fibers and myelinated Ad-fibers of sensory
nerves release various neuropeptides (101,102), most importantly calcitonin
gene-related peptide (CGRP), substance P (SP) (103,104) and nerve growth factor (NGF),
thereby initiating an inflammatory process comprising cutaneous
vessel dilation, increased vascular permeability, plasmatic
extravasation and edema (105,106) which may promote tumorigenesis. The
most important neuropeptides are discussed below:
i) Calcitonin gene-related
peptide
CGRP is a 37-amino-acid neuropeptide which exists in
two isoforms (a- and b-) differing by a single amino-acid, and is
widely expressed in both the central and peripheral nervous systems
(107). CGRP-containing nerve
fibers in the skin are closely associated with LCs (108). Apart from being a potent
vasodilator and immunomodulator, CGRP increases cAMP levels in T
cells, participates in chemotaxis, inhibits proliferation, and
inhibits the production of IL-2 and the expression of TNF-α, TNF-β
and IFN-γ. It stimulates IL-10 and downregulates IL-1β expression
in macrophages. Furthermore, it also impedes antigen presentation
by LCs, as shown in murine models (102,108,109), hindering a crucial step in
anti-tumoral immune response initiation.
Studying the effects of UVB exposure on rat skin,
Gillardon et al (110)
demonstrated that an inflammatory dose of UVB caused the release of
SP and CGRP from terminals of cutaneous sensory nerve fibers,
leading to a temporary decrease in the skin's content of
neuropeptides during the first 6 to 12 h following exposure.
Nevertheless, as the authors pointed out, the level of CGRP
increased again and the cutaneous content of CGRP exceeded the
control levels at 48–72 h post-UVR exposure. This suggests an
increase in the synthesis and transport of neuropeptides into the
area of UVR-induced inflammation (111). Niizeki et al found similar
immunosuppressive effects by intradermal injections of CGRP and
acute UVR exposure, which could both be prevented by pre-treatment
of mice with a specific CGRP antagonist, namely CGRP 8–37 (112). Furthermore, the authors reported a
reduced number of cutaneous LCs after CGRP or UVR exposure. This
suggests a possible impact in skin carcinogenesis (113).
During the identification of intracellular
mechanisms significant for the transduction of the mitogenic
message of neuropeptides, much of the research has focused on cAMP,
mainly due to the demonstration of increased cAMP synthesis and its
association with the early events following the mitogenic
stimulation of various cell systems (114).
Takahashi et al (115) demonstrated the stimulation of cAMP
formation by CGRP in an in vitro study using primary
cultured normal human epidermal keratinocytes (NHEKs) and a human
SCC cell line, HSC-1. The authors noted that CGRP induced a rapid
and notable increase in the intracellular accumulation of cAMP in
both cell lines. CGRP also significantly enhanced DNA synthesis and
the growth of HSC-1 cells in a dose-dependent manner. Intriguingly,
the increase in intracellular cAMP content following stimulation
with CGRP was only 6-fold in the HSC-1 cell line compared to an
8-fold increase noted in the NHEKs. This result was attributed by
the authors to different levels of receptors expressed on the cell
membrane.
There are many conflicting in vivo and in
vitro reports concerning the increase in the levels of cAMP in
proliferating keratinocytes. Some authors have reported that the
mediators increasing intracellular cAMP stimulate keratinocyte
proliferation (116,117), while others have observed an
inhibitory effect of cAMP analogs on keratinocyte growth (118–122). It is important to take into
account that none of the cAMP analogs or agents used to increase
cAMP levels in those studies function specifically to increase
intracellular cAMP levels (114).
Thereby these results may be subjected to certain skepticism.
ii) Substance P
SP is an inflammatory molecule belonging to the
tachykinin family, found in primary sensory neurons and afferent
vagal sensory nerve fibers together with CGRP (123) Its cellular effects are mediated
through neurokinin-1 receptor (NK1-R), NK2-R and NK3-R of which
NK1-R has the highest binding affinity to SP (124). SP has been shown to be involved in
local blood flow and vascular permeability. It induces lymphocyte
proliferation and chemotaxis, increases immunoglobulin production,
activates macrophages and favors the secretion of several
pro-inflammatory cytokines, including IL-1, IL-6 and TNF-α. As a
consequence, among other important processes, SP has been found to
be involved in the regulation of sensorial perception and stress
responses (125,126).
Over the past few years, we have witnessed an
increasing interest in the role of SP in tumor development and
progression. Indeed, it has been identified as a potent mitogen for
certain epithelial premalignant lesions and cancer cellular lines,
such as melanoma, glioma, retinoblastoma and neuroblastoma
(127–131). Hence, the SP/NK-1R system may have
a role to play in NMSC development, since SP may indeed be a
universal mitogen in NK-1R-expressing tumor cell types.
Staniek et al (132) noted that at high concentrations
(104 and 105 M), SP was able to inhibit in vitro epidermal
cell reaction responses by acting on LCs and T cells. As stated
above, cutaneous sensory nerves contain SP and a considerable
increase in SP immunoreactivity is detected following UVR exposure
(133). Thus, there is indirect
evidence suggesting that SP plays a role in UV-induced inflammatory
reactions (134).
When SP binds to NK-1R, it activates certain members
of the mitogen-activated protein kinase intracellular signaling
cascade, including extracellular signal regulated kinases 1 and 2
(ERK 1/2). These molecules then translocate to the nucleus and
induce cell proliferation and protect the cell from apoptosis
(135).
Brener et al (136) investigated the presence and
distribution of SP and NK-1R in SCC and their association with
tumor proliferation. The authors demonstrated for the first time
the expression of SP and NK-1R in SCC, and reported a strong
expression of both proteins, particularly SP, in all tissue
compartments of the analyzed tumor samples. They also reported a
direct and significant correlation between SP and NK-1R expression
in different tumor tissue compartments, thus suggesting that SP
overexpression is accompanied by an increase in the expression of
NK-1R molecules, a phenomenon also demonstrated by other authors
(135).
According to Weinstock et al (137), SP can also stimulate cell
proliferation through the transactivation of EGFR. The connection
between these functions and the SP/NK-1R interactions has led some
authors to suggest that SP/NK-1R may be associated with both cancer
development and progression (135).
iii) Nitric oxide
Nitric oxide synthase (NOS) (along with CGRP and SP)
is located in visceral afferent neurons in the lower thoracic tract
dorsal root ganglion (138), and
has been incriminated in neurogenic inflammation in murine models
(139). This early observation led
to the investigation of the effect of NOS on immunoreactivity in
UVR-exposed skin and UV-induced vasodilation (134). In the study by Gillardon et
al (110), it was concluded
that NO released from nerve endings following UVR exposure promoted
post-liminary local immune suppression. Moreover, the authors noted
the far from negligible contribution of inducible NOS from
activated macrophages and neutrophils. Indeed, the inducible form
of NOS (iNOS) is the isoform most consistently associated with
neoplasia. When produced by immune cells, iNOS in turn leads to the
production of large amounts of NO, pivotal in pathogen defense
responses, cytokine production and T helper lymphocyte expansion.
On the other hand, the upregulation of iNOS has been characterized
to act exclusively as a pathological mediator (140–145).
Rosbe et al (146) investigated the activity of NOS and
the presence of NO in human SCC. They found iNOS activity in
squamous epithelial cells throughout all the analyzed SCC tumor
tissue, with the enzyme activity being highest in surrounding
keratin pearls.
It has been found that iNOS is upregulated in
cutaneous SCC (147), but is
downregulated in skin BCC, probably contributing to the lack of
aggressive features displayed by the latter (148).
NO production by SCC cells is consistent with other
evidence that NO may be a promoter of local tumor growth and
metastasis by enhancing neovascularization. In two separate
studies, Andrade et al (149) and Maeda et al (150) found that NOS inhibitors were able
to reduce tumor blood flow. The morphology and sensitivity to
vasoactive agents of these newly formed blood vessels seemed to
differ from vessels in normal tissue, possibly explaining the
presence of iNOS instead of endothelial-NOS in mouse tumor
capillaries.
Connelly et al (151) studied the expression of iNOS in
tissue samples of SCC and compared them to samples of dysplasia and
normal controls. They reported 95% staining in SCC, 50% in
dysplasia and 0% in normal epithelial controls with positive
staining for iNOS only in SCC cells. They also detected a
significantly higher production of NO in SCC cells compared to
control cells. Their results localized iNOS to SCC tumor cells, and
not to the region of inflammation within the stroma, thereby
suggesting that the malignant cells are the source of iNOS.
One study also found plasma levels of NO compounds
in patients with BCC to be significantly increased when compared to
controls (152). The authors
hypothesized that increased plasma levels of NO in patients with
BCC are the result of accentuated hyperkeratinization,
hyperpigmentation, and keratinocyte proliferation and proposed that
this increased production of NO may operate as a growth stimulator,
having a potential mitogenic function, finally accelerating the
proliferation of this type of NMSC cells (152).
NO may also play a role in tumor metastasis via the
promotion of endothelial cell adhesion and vascular permeability
(153). Even though it was
hypothesized that tumor cells may use an iNOS-mediated mechanism to
adhere to the vascular endothelium and further penetrate vessel
walls, thus gaining access to distant sites, some authors (154) have found an inverse correlation
between NO and metastasis. Therefore, the complete role of NO in
cancer metastasis remains to be fully elucidated.
iv) Somatostatin (SST)
SST was first identified in 1973 as a growth
hormone release-inhibitor (155)
with its main functions involving the regulation of exo- and
endocrine secretions, the modulation of motor activity and the
inhibition of gastrin-stimulated gastric acid secretion (156). Several recent studies have
suggested that SST can act as a tumor suppressor gene, and perform
significant antitumor and antisecretory activities in various human
malignancies both in vivo and in vitro (156–158).
Through the inhibition of growth factors and
hormones, the diminishing of vascularization and immune system
regulation, SST seems capable of suppressing tumor growth in a
variety of cancers (159,160), including human pancreatic tumor
cells (MIA PaCa-2), breast cancer cells (MCF-7) and small cell lung
cancer cells (HCI-H69) (160–162). An epidermoid carcinoma cell line,
A431, possesses a very high level of EGF receptors and exhibits an
attenuation of cell proliferation in response to EGF (163). Mascardo and Sherline demonstrated
rapid centrosomal separation and cell growth stimulation by EGF in
mitogenically responsive cells (164).
Kamiya et al (165) examined the in vitro effects
of SST-14 and its octapeptide analogue, SMS 201–995, on the growth
of A431 cells. The authors found that both SS-14 and SMS 201–995
stimulated the growth of A431 cells, most probably affecting their
inositolphosphatase metabolism. Moreover, these authors
demonstrated the presence of high-affinity receptors for SS in A431
cells (165). Of note, SS-14 and
SMS 201–995 initiated a stimulatory effect in KB cells, another
line of epidermoid carcinoma cells, but not in the three tested
squamous carcinoma cell lines, HSC-2, −3 and −4. Even though these
cell lines resemble the A431 and KB cell lines, they responded with
a decrease in cell growth. It was concluded that the growth
regulatory mechanism in the respective cell lines differs with
respect to SST or that HSC cells may lack SST receptors (165).
As evidenced from the above, it has been well
established that neurogenic inflammation and other various
alterations of the microenvironment create a milieu conducive to
carcinogenesis. Intriguingly, stress alone can induce neurochemical
changes that promote cell proliferation and carcinogenesis;
however, there are also reports in which the use of other
neuromodulators appears to create a tumor suppressive environment.
This body of evidence thereby suggests that these neuroactive
mediators affect the odds of developing skin cancers through their
neuromodulatory effects.
Neurohormones
The skin's neuroendocrinological properties have
been previously demonstrated, the cutaneous organ being capable of
secreting thyroid-stimulating hormone, oxytocin, growth hormones,
and corticotropin-releasing hormone (CRH) (32).
The proopiomelanocortin (POMC) gene codes for the
synthesis of a protein known as POMC, a precursor polypeptide with
241 amino acid residues, which is then enzymatically cleaved into a
variety of biologically active peptides with different functions
throughout the body. Among these neuropeptides, are
adrenocorticotropin (ACTH), β-lipotropin (β-LPH), endorphins (α-,
β-, γ-endorphin) and melanotropins [α-, β-,
γ-melanocyte-stimulating hormone (MSH)] (166). These peptides bind to several
proteins in various regions of the body, triggering the activation
of several signaling pathways that control a number of important
functions. It is worthwhile mentioning that the production of POMC
is not limited to the pituitary gland, and it has been found in
various other tissues, including the skin (167). More precisely, α-MSH and ACTH can
also be produced by keratinocytes (168).
The existence of a local stress response system in
the skin, which is equivalent to the central HPA axis has been
previously revealed (34,169–172). It has also been confirmed that
locally synthesized CRH can activate local CRH receptors (CRHRs)
(34,172). The discovery of CRHR in the skin
suggests a potential CRH-induced neuroendocrine cutaneous pathway
(172–175).
There are studies performed almost 20 years ago,
incriminating CRH-POMC axis-related hormones in the pathology of
malignant melanoma (MM), SCC and BCC alike (176–179). Several studies have also suggested
that UVR, the main carcinogen involved in the pathogenesis of NMSC,
is capable of inducing the expression of CRH-POMC axis-related
neurohormones (170,180,181). Simultaneously, following UVR
exposure or metabolic alteration, corresponding receptors are
upregulated, such as melanocortin (MC)-1 and MC-5. Most receptors
of the MC-1 class, are located on the surface of keratinocytes, and
recognize MSH and ACTH (182). MSH
and ACTH act not only as skin pigmentation regulators, but also as
immunosuppressors (183) through
the inhibition of specific IL-1, TNF-α and IL-2 functions (184).
Kim et al (185) performed an immunohistochemical
analysis of CRH, ACTH and α-MSH expression in specimens from normal
skin, melanocytic naevi, SK, actinic keratoses (AK), Bowen's
disease, cutaneous T cell lymphoma (CTCL), BCC, SCC and MM. They
reported CRH, ACTH and α-MSH expression only in the upper epidermal
layers in the normal skin, melanocytic naevus, SK, AK, Bowen's
disease and CTCL samples. The expression of these neurohormones was
generally weak or undetectable in benign and precancerous lesions,
in CTCL and in normal controls. However, in the same study, BCC
samples exhibited an intermediate expression of CRH while MM (80%)
and SCC (70%) samples exhibited a strong immunoreactivity for CRH.
It was thereby concluded that the CRH-POMC axis-related
neurohormones strongly correlated with cutaneous malignancies, and
that CRH appears to be a more specific biomarker for skin cancers
when compared to ACTH or α-MSH (185).
Luger et al (186) tested human normal keratinocytes
(HNKs) and A431 cells for their capacity to release α-MSH by
stimulating these cells with either the tumor promoter phorbol
myristate acetate (PMA), IL-1 or UVB radiation. The authors
reported the spontaneous release of low amounts of α-MSH by both
HNK and A431 cells, which was significantly increased by
stimulation with PMA, IL-1 or UVB. Indeed, α-MSH production by
epidermal cells occurred between 48 and 72 h following culture
initiation and among several other tested cytokines, only IL-1P
proved to be effective in stimulating α-MSH production by epidermal
cells. Moreover, α-MSH derived from epidermal cells was proven to
be biologically active (186).
Their results also showed that upon stimulation, normal and
malignant keratinocytes produced melanotropins able to suppress the
production and activity of the immunomodulating cytokine, IFN-γ
(186).
Arbiser et al (187) reported in vitro endothelial
cell chemotaxis stimulation and in vivo stimulation of tumor
growth and angiogenesis by CRH acting through CRHR. As a result,
the authors inferred that CRH directly stimulates angiogenesis via
the CRHRs present on endothelial cells and suggested that the
ectopic production of CRH in malignancies may promote
angiogenesis.
These aspects underline the fact that CRH-POMC
peptides directly participate in UV-related skin cancers. POMC
peptides are produced in the skin under UVB stimulation, and
therefore create a microenvironment favorable for NMSC progression
(177,188). Among the suggested mechanisms,
authors have cited immunosuppression, the inhibition of
antigen-presenting structures on keratinocytes, and cell
proliferation (177). It is
unknown whether CRH-POMC peptides initiate or simply promote skin
cancer pathogenesis (185), but
such peptides are most certainly associated with a higher rate of
tumor growth (187).
Cellular neuro-immune interactions
Numerous interactions have been identified linking
the nervous system to various immune cells, including mast cells,
neutrophils, macrophages and T cells (189,190). Mast cells however, appear to be
the key players in modulating stress-induced cutaneous inflammatory
reactions (38).
As early as the 1990s, it has been shown (191) that increased numbers of dermal
mast cells highly correlate with UVR-induced immune suppression,
aggravated by the fact that UVR exposure increases dermal mast cell
density (192,193). Ten years later, observational
studies reported that high densities of mast cells correlate with a
poor prognosis for various tumors, such as BCCs and SCCs, MM, colon
cancer and lymphoma (194). There
are in fact a number of studies that have shown mast cells to
accumulate around skin cancers and create permissive
microenvironments (26,195–197).
UVR exposure indirectly activates dermal mast
cells, supposedly through the activation of cutaneous nerve fibers,
which release mast cell-activating neuropeptides (198). Two mechanisms of mast cell
activation by means of UVR exposure are proposed: first a
photoinduced isomerization of a photo-receptor, trans-urocanic acid
(UCA) and second LC antigen-presenting dysfunction (188,199,200).
Thus, UCA is converted to cis-UCA in the stratum
corneum (195,196). Cis-UCA stimulates cutaneous
sensory nerves to release neuropeptides (SP and CGRP) (195,201) and therefore mast cell
degranulation. The other mechanisms refer to LC antigen-presenting
dysfunction due to ultrastructural modifications (195).
Furthermore, mast cell mediators function not only
as stimulators of angiogenesis, but also as immunosuppressors and
promoters of extracellular matrix destruction and mitosis (195,202). Tumor-associated mast cells are
recruited via chemokines released from tumor cells. BCCs release
stem cell factor that binds to the tyrosine-kinase receptor c-kit
on the mast cell surface, thereby increasing the mast cell numbers
around the tumor (197), and
subsequently increasing dendritic cells numbers, leading to
angiogenesis and collagenesis. Higher numbers of mast cells have
been reported around BCCs and melanomas than around SCCs; however,
the reason for this remains unclear (195). Mast cells are recruited
preferentially around BCCs, independent of inflammatory infiltrate,
with higher numbers observed around more aggressive tumors
(203). Moreover, patients with a
history of sporadic BCC have a high number of mast cells in
non-exposed skin, in contrast with control subjects (196). The factors that condition the
number of peritumoral mast cells in BCC are unclear; however, it
has been suggested that NGF, stem cell factor and VEGF may be
involved (196,204). NGF also facilitates histamine
release from mast cells (205).
Histamine is a known key mediator of UVB-induced immunosuppression
(196). It limits T helper-1
lymphocyte expansion, but not T helper-2 lymphocytes (206). It stimulates keratinocyte-produced
prostaglandin E2 that disrupts a cytokinic balance favoring IL-10,
an immunosuppressor, and IL-12 (207), an immunostimulant that can induce
DNA repair after UV damage (208).
It also increases the number of suppressor T lymphocytes that
produce IL-10, leading to immunosuppression via the apoptosis of
antigen-presenting cells (195).
Prostaglandin E2 can further stimulate angiogenic factor release
from mast cells (209). Creating a
paracrine loop, histamine further stimulates keratinocytes to
release NGF that in turn binds to mast cell surface to complete the
circle (205).
Apart from histamine, mast cells secrete many other
pro-angiogenic factors, such as heparin, TNF-α, TGF-β, IL-8 and
VEGF. Peritumoral mast cells are a major source of VEGF, which
leads to endothelial mitosis and vascular hyperpermeability. This
in turn permits the extravasation of other pro-angiogenic factors
in the extracellular matrix, and tumoral cytokines, such s TGF
alpha, a mitogenic polypeptide (195). All these cytokines stimulate VEGF
production further (210).
Angiogenesis must be supported by extracellular
matrix remodelling. Thus, mast cells release tryptase and chymase,
two serine proteases that initiate matrix degradation and turn over
(26,211). One study (211) showed that tryptase activity was
increased >2-fold in BCCs, while chymase-positive cells
exhibited partial enzymatic inactivity.
Some studies have reported a significant increase
in dermal mast cell degranulation, in a number of SP immunoreactive
nerve fibers and contacts between mast cells and these nerve
fibers, due to stress exposure (212,213). Stress-induced degranulation seems
to be dependent on CRH, but probably also involves SP and
neurotensin. As opposed to anaphylactic reactions, stress does not
provoke an explosive mast cell degranulation, but acts in a more
silent manner, primarily inducing intragranular changes (214). Căruntu et al (38) examined the effects of acute and
chronic stress on mast cell degranulation in glabrous and hairy
mouse skin. In their study, the authors reported an amplification
of mast cell degranulation in hairy skin due to short-term stress
exposure, effect that continued with prolonged exposure to stress.
By comparison, in glabrous skin, although acute stress intensely
stimulated mast cell degranulation, the phenomenon subsided
gradually as exposure to stress persisted (38).
The crosstalk between mast cells and sensory nerve
fibers by means of increased NGF, neuropeptides and various other
mediators, in photo-damaged skin, may thus influence the higher
prevalence of NMSCs in these individuals.
Conclusion and perspectives
In addition to the classic agents involved in the
pathogenesis of NMSC, such as chronic UVR exposure, the thinning of
the ozone layer through pollution, immunosuppression, an increased
lifespan, PTCH gene mutations and fair skin, a large number of
studies have incriminated various neuroactive factors in the
pathogenesis of NMSC. Furthermore, it has become obvious that the
intricate interaction between the peripheral nervous system and
cutaneous target cells (e.g., leukocytes, LCs and mast cells) is
mediated through locally released neuroendocrine factors including
catecholamines, SP, CGRP, SST, and neurohormones such as POMC and
its derived peptides, alpha-MSH and ACTH, all of which have been,
at one time or another, the subject of scientific dispute as to
their precise role in the pathogenesis of NMSC.
A key function of physiological mediators released
in short-term stress reactions may be to ensure that the proper
cells (e.g., leukocytes) are in the right place, at the right time,
and that they are activated appropriately to be able to respond to
the immune challenge posed by the stressor (e.g., pathogen invasion
of the host and UVR). Acute stress modulates immune cell
distribution as an adaptive response responsible for enhancing
immune surveillance and increasing the immune system's capacity to
respond to challenges in various compartments (skin, mucosa, and
epithelial linings of the gastrointestinal and genito-urinary
tracts) which constitute major defense barriers throughout the
body. Thereby, the current body of evidence suggests that
neurotransmitters and hormones released under acute stressful
situations may increase immune surveillance and augment immune
responses for potential or ongoing challenges.
Although acute stress seems to play a rather
protective role in the context of carcinogenesis for the
aforementioned reasons, chronic stress, acting through the plethora
of neuropeptides, neurohormones and cytokines involved, leads to
chronic immunosuppression and, as a result, promotes a favorable
environment for NMSC carcinogenesis. Further studies are needed in
order to elucidate the exact mechanisms mediating beneficial versus
harmful effects of stress mediated through the skin's
neuroendocrine system, in order to translate the findings from
bench to bedside. This field of research is very important,
considering that stress has become an ubiquitous aspect of life and
even though chronic stress, acting through the nervous, endocrine
and immune systems, is thought to be among the etiological factors
of various skin diseases (including NMSCs), acute stress is a
fundamental survival mechanism that may very well be harnessed for
immunoprotection. Additional studies are also required to define
the precise processes through which neuroactive molecules promote
or inhibit cutaneous carcinogenesis, as this could lead to the
development of more sophisticated and tailored treatment protocols,
as well as open new perspectives in skin cancer research, including
penile SCC, for which a paucity of specific data on the pathogenic
role of neuroendocrine factors exists.
Acknowledgements
This study was partly supported by a grant
PN-II-PT-PCCA-2013-4-1407 (Project 190/2014) financed by the
Executive Agency for Higher Education, Research, Development and
Innovation and by Young Researchers Grant 33891/2014 financed by
‘Carol Davila’ University of Medicine and Pharmacy, Bucharest,
Romania.
References
|
1
|
Freedberg IM, Eisen AZ, Wolff K, Austen
KF, Goldsmith LA and Katz S: Fitzpatrick's Dermatology in general
medicine. 6th. McGraw-Hil; New York, NY: 2003
|
|
2
|
Rubin AI, Chen EH and Ratner D: Basal-cell
carcinoma. N Engl J Med. 353:2262–2269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stern RS: Prevalence of a history of skin
cancer in 2007: Results of an incidence-based model. Arch Dermatol.
146:279–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rogers HW, Weinstock MA, Harris AR,
Hinckley MR, Feldman SR, Fleischer AB and Coldiron BM: Incidence
estimate of nonmelanoma skin cancer in the United States, 2006.
Arch Dermatol. 146:283–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diffey BL and Langtry JA: Skin cancer
incidence and the ageing population. Br J Dermatol. 153:679–680.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lanoue J and Goldenberg G: Basal cell
carcinoma: A comprehensive review of existing and emerging
nonsurgical therapies. J Clin Aesthet Dermatol. 9:26–36.
2016.PubMed/NCBI
|
|
7
|
Renaud-Vilmer C and Basset-Seguin N: Basal
cell carcinomas. Rev Prat. 64:37–44. 2014.(In French). PubMed/NCBI
|
|
8
|
Alam M and Ratner D: Cutaneous
squamous-cell carcinoma. N Engl J Med. 344:975–983. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ch'ng S, Maitra A, Lea R, Brasch H and Tan
ST: Parotid metastasis - an independent prognostic factor for head
and neck cutaneous squamous cell carcinoma. J Plast Reconstr
Aesthet Surg. 59:1288–1293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson TM, Rowe DE, Nelson BR and Swanson
NA: Squamous cell carcinoma of the skin (excluding lip and oral
mucosa). J Am Acad Dermatol. 26:467–484. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowe DE, Carroll RJ and Day CL Jr:
Prognostic factors for local recurrence, metastasis, and survival
rates in squamous cell carcinoma of the skin, ear, and lip.
Implications for treatment modality selection. J Am Acad Dermatol.
26:976–990. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudolph R and Zelac DE: Squamous cell
carcinoma of the skin. Plast Reconstr Surg. 114:82e–94e. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weinberg AS, Ogle CA and Shim EK:
Metastatic cutaneous squamous cell carcinoma: An update. Dermatol
Surg. 33:885–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hakenberg OW, Compérat EM, Minhas S,
Necchi A, Protzel C, Watkin N, et al: European Association of
Urology: EAU guidelines on penile cancer: 2014 update. Eur Urol.
67:142–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spiess PE, Dhillon J, Baumgarten AS,
Johnstone PA and Giuliano AR: Pathophysiological basis of human
papillomavirus in penile cancer: Key to prevention and delivery of
more effective therapies. CA Cancer J Clin. 66:481–495. 2016.
View Article : Google Scholar
|
|
17
|
Cakir BÖ, Adamson P and Cingi C:
Epidemiology and economic burden of nonmelanoma skin cancer. Facial
Plast Surg Clin North Am. 20:419–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kayes O, Ahmed HU, Arya M and Minhas S:
Molecular and genetic pathways in penile cancer. Lancet Oncol.
8:420–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Protzel C and Spiess PE: Molecular
research in penile cancer-lessons learned from the past and bright
horizons of the future? Int J Mol Sci. 14:19494–19505. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berton TR, Pavone A and Fischer SM:
Ultraviolet-B irradiation alters the cell cycle machinery in murine
epidermis in vivo. J Invest Dermatol. 117:1171–1178. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oberyszyn TM: Non-melanoma skin cancer:
Importance of gender, immunosuppressive status and vitamin D.
Cancer Lett. 261:127–136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Voiculescu V, Calenic B, Ghita M, Lupu M,
Caruntu A, Moraru L, Voiculescu S, Ion A, Greabu M, Ishkitiev N, et
al: From normal skin to squamous cell carcinoma: A quest for novel
biomarkers. Dis Markers. 2016:45174922016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ratushny V, Gober MD, Hick R, Ridky TW and
Seykora JT: From keratinocyte to cancer: The pathogenesis and
modeling of cutaneous squamous cell carcinoma. J Clin Invest.
122:464–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stockfleth E, Ortonne JP and Alomar A:
Actinic keratosis and field cancerisation. Eur J Dermatol. 21:(Supp
1). 3–11. 2011.PubMed/NCBI
|
|
25
|
Lugović L, Situm M, Vurnek M and Buljan M:
Influence of psychoneuroimmunologic factors on patients with
malignant skin diseases. Acta Med Croatica. 61:383–389. 2007.(In
Croatian). PubMed/NCBI
|
|
26
|
Leon A, Ceauşu Z, Ceauşu M, Ardeleanu C
and Mehedinţi R: Mast cells and dendritic cells in basal cell
carcinoma. Rom J Morphol Embryol. 50:85–90. 2009.PubMed/NCBI
|
|
27
|
Calenic B, Greabu M, Caruntu C, Tanase C
and Battino M: Oral keratinocyte stem/progenitor cells: Specific
markers, molecular signaling pathways and potential uses.
Periodontol 2000. 69:68–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016.PubMed/NCBI
|
|
29
|
Neagu M, Constantin C, Dumitrascu GR, Lupu
AR, Caruntu C, Boda D and Zurac S: Inflammation markers in
cutaneous melanoma - edgy biomarkers for prognosis. Discoveries.
3:e382015. View Article : Google Scholar
|
|
30
|
Fagundes CP, Glaser R, Johnson SL,
Andridge RR, Yang EV, Di Gregorio MP, Chen M, Lambert DR, Jewell
SD, Bechtel MA, et al: Basal cell carcinoma: Stressful life events
and the tumor environment. Arch Gen Psychiatry. 69:618–626. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saul AN, Oberyszyn TM, Daugherty C,
Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S
and Dhabhar FS: Chronic stress and susceptibility to skin cancer. J
Natl Cancer Inst. 97:1760–1767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Terao M and Katayama I: Local
cortisol/corticosterone activation in skin physiology and
pathology. J Dermatol Sci. 84:11–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Terao M, Itoi S, Murota H and Katayama I:
Expression profiles of cortisol-inactivating enzyme,
11β-hydroxysteroid dehydrogenase-2, in human epidermal tumors and
its role in keratinocyte proliferation. Exp Dermatol. 22:98–101.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Slominski A and Wortsman J:
Neuroendocrinology of the skin. Endocr Rev. 21:457–487. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Slominski A: Neuroendocrine system of the
skin. Dermatology. 211:199–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zmijewski MA and Slominski AT:
Neuroendocrinology of the skin: An overview and selective analysis.
Dermatoendocrinol. 3:3–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Căruntu C, Grigore C, Căruntu A,
Diaconeasa A and Boda D: The role of stress in skin diseases.
Intern Med. 8:73–84. 2011.
|
|
38
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Căruntu C, Boda D, Musat S, Căruntu A,
Poenaru E, Calenic B, Savulescu-Fiedler I, Draghia A, Rotaru M and
Badarau AI: Stress effects on cutaneous nociceptive nerve fibers
and their neurons of origin in rats. Rom Biotechnol Lett.
19:95182014.
|
|
40
|
Arck P and Paus R: From the brain-skin
connection: The neuroendocrine-immune misalliance of stress and
itch. Neuroimmunomodulation. 13:347–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gupta MA and Gupta AK: Psychiatric and
psychological co-morbidity in patients with dermatologic disorders:
Epidemiology and management. Am J Clin Dermatol. 4:833–842. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Căruntu C, Ghita MA, Căruntu A and Boda D:
The role of stress in the multifactorial etiopathogenesis of acne.
Ro Med J. 58:98–101. 2011.
|
|
43
|
Sinnya S and De'Ambrosis B: Stress and
melanoma: Increasing the evidence towards a causal basis. Arch
Dermatol Res. 305:851–856. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sanzo M, Colucci R, Arunachalam M, Berti S
and Moretti S: Stress as a possible mechanism in melanoma
progression. Dermatol Res Pract. 2010:4834932010.PubMed/NCBI
|
|
45
|
de Vries E, Trakatelli M, Kalabalikis D,
Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D,
Ioannides D, Aquilina S, Apap C, et al: EPIDERM Group: Known and
potential new risk factors for skin cancer in European populations:
A multicentre case-control study. Br J Dermatol. 167:(Suppl 2).
1–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bulman A, Neagu M and Constantin C:
Immunomics in skin cancer - improvement in diagnosis, prognosis and
therapy monitoring. Curr Proteomics. 10:202–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Parker J, Klein SL, McClintock MK, Morison
WL, Ye X, Conti CJ, Peterson N, Nousari CH and Tausk FA: Chronic
stress accelerates ultraviolet-induced cutaneous carcinogenesis. J
Am Acad Dermatol. 51:919–922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Reiss Y, Proudfoot AE, Power CA, Campbell
JJ and Butcher EC: CC chemokine receptor (CCR)4 and the CCR10
ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte
trafficking to inflamed skin. J Exp Med. 194:1541–1547. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dighe AS, Richards E, Old LJ and Schreiber
RD: Enhanced in vivo growth and resistance to rejection of tumor
cells expressing dominant negative IFN γ receptors. Immunity.
1:447–456. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Voest EE, Kenyon BM, O'Reilly MS, Truitt
G, D'Amato RJ and Folkman J: Inhibition of angiogenesis in vivo by
interleukin 12. J Natl Cancer Inst. 87:581–586. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–1111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sephton SE, Dhabhar FS, Classen C and
Spiegel D: The diurnal cortisol slope as a predictor of immune
reactivity to interpersonal stress. Brain Behav Immun.
14:1282000.
|
|
53
|
Mormont MC and Lévi F: Circadian-system
alterations during cancer processes: A review. Int J Cancer.
70:241–247. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Filipski E, King VM, Li X, Granda TG,
Mormont MC, Liu X, Claustrat B, Hastings MH and Lévi F: Host
circadian clock as a control point in tumor progression. J Natl
Cancer Inst. 94:690–697. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu L and Lee CC: The circadian clock:
Pacemaker and tumour suppressor. Nat Rev Cancer. 3:350–361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sephton SE, Sapolsky RM, Kraemer HC and
Spiegel D: Early mortality in metastatic breast cancer patients
with absent or abnormal diurnal cortisol rhythms. J Natl Cancer
Inst. 92:994–1000. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wong CS, Strange RC and Lear JT: Basal
cell carcinoma. BMJ. 327:794–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Terao M, Murota H, Kimura A, Kato A,
Ishikawa A, Igawa K, Miyoshi E and Katayama I: 11β-Hydroxysteroid
dehydrogenase-1 is a novel regulator of skin homeostasis and a
candidate target for promoting tissue repair. PLoS One.
6:e250392011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dhabhar FS and McEwen BS: Acute stress
enhances while chronic stress suppresses cell-mediated immunity in
vivo: A potential role for leukocyte trafficking. Brain Behav
Immun. 11:286–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kripke ML: Ultraviolet radiation and
immunology: Something new under the sun - presidential address.
Cancer Res. 54:6102–6105. 1994.PubMed/NCBI
|
|
61
|
Granstein RD and Matsui MS: UV
radiation-induced immunosuppression and skin cancer. Cutis.
74:(Suppl). 4–9. 2004.PubMed/NCBI
|
|
62
|
Ben-Eliyahu S, Yirmiya R, Liebeskind JC,
Taylor AN and Gale RP: Stress increases metastatic spread of a
mammary tumor in rats: Evidence for mediation by the immune system.
Brain Behav Immun. 5:193–205. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ben-Eliyahu S: The promotion of tumor
metastasis by surgery and stress: Immunological basis and
implications for psychoneuroimmunology. Brain Behav Immun.
17:(Suppl 1). S27–S36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dhabhar FS, Miller AH, McEwen BS and
Spencer RL: Effects of stress on immune cell distribution. Dynamics
and hormonal mechanisms. J Immunol. 154:5511–5527. 1995.PubMed/NCBI
|
|
65
|
Dhabhar FS, Miller AH, McEwen BS and
Spencer RL: Stress-induced changes in blood leukocyte distribution.
Role of adrenal steroid hormones. J Immunol. 157:1638–1644.
1996.PubMed/NCBI
|
|
66
|
Dhabhar FS and McEwen BS: Enhancing versus
suppressive effects of stress hormones on skin immune function.
Proc Natl Acad Sci USA. 96:1059–1064. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dhabhar FS and Viswanathan K: Short-term
stress experienced at time of immunization induces a long-lasting
increase in immunologic memory. Am J Physiol Regul Integr Comp
Physiol. 289:R738–R744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Saint-Mezard P, Chavagnac C, Bosset S,
Ionescu M, Peyron E, Kaiserlian D, Nicolas JF and Bérard F:
Psychological stress exerts an adjuvant effect on skin dendritic
cell functions in vivo. J Immunol. 171:4073–4080. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Viswanathan K, Daugherty C and Dhabhar FS:
Stress as an endogenous adjuvant: Augmentation of the immunization
phase of cell-mediated immunity. Int Immunol. 17:1059–1069. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wood PG, Karol MH, Kusnecov AW and Rabin
BS: Enhancement of antigen-specific humoral and cell-mediated
immunity by electric footshock stress in rats. Brain Behav Immun.
7:121–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dhabhar FS, Saul AN, Daugherty C, Holmes
TH, Bouley DM and Oberyszyn TM: Short-term stress enhances cellular
immunity and increases early resistance to squamous cell carcinoma.
Brain Behav Immun. 24:127–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bernabé DG, Tamae AC, Biasoli ÉR and
Oliveira SHP: Stress hormones increase cell proliferation and
regulates interleukin-6 secretion in human oral squamous cell
carcinoma cells. Brain Behav Immun. 25:574–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lackovicova L, Banovska L, Bundzikova J,
Janega P, Bizik J, Kiss A and Mravec B: Chemical sympathectomy
suppresses fibrosarcoma development and improves survival of
tumor-bearing rats. Neoplasma. 58:424–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Coelho M, Moz M, Correia G, Teixeira A,
Medeiros R and Ribeiro L: Antiproliferative effects of β-blockers
on human colorectal cancer cells. Oncol Rep. 33:2513–2520.
2015.PubMed/NCBI
|
|
75
|
Liou SF, Lin HH, Liang JC, Chen IJ and Yeh
JL: Inhibition of human prostate cancer cells proliferation by a
selective alpha1-adrenoceptor antagonist labedipinedilol-A involves
cell cycle arrest and apoptosis. Toxicology. 256:13–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schallreuter KU, Lemke KR, Pittelkow MR,
Wood JM, Körner C and Malik R: Catecholamines in human keratinocyte
differentiation. J Invest Dermatol. 104:953–957. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pullar CE, Rizzo A and Isseroff RR:
beta-Adrenergic receptor antagonists accelerate skin wound healing:
Evidence for a catecholamine synthesis network in the epidermis. J
Biol Chem. 281:21225–21235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Winkelmann RK: Cutaneous nerves in
relation to epithelial tumors. J Invest Dermatol. 27:273–279. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lutgendorf SK, Cole S, Costanzo E, Bradley
S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M and Sood
AK: Stress-related mediators stimulate vascular endothelial growth
factor secretion by two ovarian cancer cell lines. Clin Cancer Res.
9:4514–4521. 2003.PubMed/NCBI
|
|
80
|
Lutgendorf SK, Johnsen EL, Cooper B,
Anderson B, Sorosky JI, Buller RE and Sood AK: Vascular endothelial
growth factor and social support in patients with ovarian
carcinoma. Cancer. 95:808–815. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Roy R, Zhang B and Moses MA: Making the
cut: Protease-mediated regulation of angiogenesis. Exp Cell Res.
312:608–622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tas F, Oguz H, Argon A, Duranyildiz D,
Camlica H, Yasasever V and Topuz E: The value of serum levels of
IL-6, TNF-alpha, and erythropoietin in metastatic malignant
melanoma: Serum IL-6 level is a valuable prognostic factor at least
as serum LDH in advanced melanoma. Med Oncol. 22:241–246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ugurel S, Rappl G, Tilgen W and Reinhold
U: Increased serum concentration of angiogenic factors in malignant
melanoma patients correlates with tumor progression and survival. J
Clin Oncol. 19:577–583. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Al-Wadei HAN, Plummer HK III and Schuller
HM: Nicotine stimulates pancreatic cancer xenografts by systemic
increase in stress neurotransmitters and suppression of the
inhibitory neurotransmitter gamma-aminobutyric acid.
Carcinogenesis. 30:506–511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Schuller HM, Al-Wadei HAN, Ullah MF and
Plummer HK III: Regulation of pancreatic cancer by
neuropsychological stress responses: A novel target for
intervention. Carcinogenesis. 33:191–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yang EV, Sood AK, Chen M, Li Y, Eubank TD,
Marsh CB, Jewell S, Flavahan NA, Morrison C, Yeh PE, et al:
Norepinephrine up-regulates the expression of vascular endothelial
growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in
nasopharyngeal carcinoma tumor cells. Cancer Res. 66:10357–10364.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang D, Ma QY, Hu HT and Zhang M:
β2-adrenergic antagonists suppress pancreatic cancer cell invasion
by inhibiting CREB, NFκB and AP-1. Cancer Biol Ther. 10:19–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016.PubMed/NCBI
|
|
91
|
Yucel T, Mutnal A, Fay K, Fligiel SE, Wang
T, Johnson T, Baker SR and Varani J: Matrix metalloproteinase
expression in basal cell carcinoma: Relationship between enzyme
profile and collagen fragmentation pattern. Exp Mol Pathol.
79:151–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang EV and Eubank TD: The impact of
adrenergic signaling in skin cancer progression: Possible
repurposing of β-blockers for treatment of skin cancer. Cancer
Biomark. 13:155–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang EV, Bane CM, MacCallum RC,
Kiecolt-Glaser JK, Malarkey WB and Glaser R: Stress-related
modulation of matrix metalloproteinase expression. J Neuroimmunol.
133:144–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dumas V, Kanitakis J, Charvat S, Euvrard
S, Faure M and Claudy A: Expression of basement membrane antigens
and matrix metalloproteinases 2 and 9 in cutaneous basal and
squamous cell carcinomas. Anticancer Res. 19:(4B). 2929–2938.
1999.PubMed/NCBI
|
|
95
|
Lupu M, Caruntu C, Ghita MA, Voiculescu V,
Voiculescu S, Rosca AE, Caruntu A, Moraru L, Popa IM, Calenic B, et
al: Gene expression and proteome analysis as sources of biomarkers
in basal cell carcinoma. Dis Markers. 2016:98312372016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Peterson SC, Eberl M, Vagnozzi AN, Belkadi
A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA
and Wong SY: Basal cell carcinoma preferentially arises from stem
cells within hair follicle and mechanosensory niches. Cell Stem
Cell. 16:400–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
English KB, Kavka-Van Norman D and Horch
K: Effects of chronic denervation in type I cutaneous
mechanoreceptors (Haarscheiben). Anat Rec. 207:79–88. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nurse CA, Macintyre L and Diamond J: A
quantitative study of the time course of the reduction in Merkel
cell number within denervated rat touch domes. Neuroscience.
11:521–533. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tilling T and Moll I: Which are the cells
of origin in merkel cell carcinoma? J Skin Cancer. 2012:6804102012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Iyengar B: Modulation of melanocytic
activity by acetylcholine. Acta Anat (Basel). 136:139–141. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Scholzen T, Armstrong CA, Bunnett NW,
Luger TA, Olerud JE and Ansel JC: Neuropeptides in the skin:
Interactions between the neuroendocrine and the skin immune
systems. Exp Dermatol. 7:81–96. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Seiffert K and Granstein RD: Neuropeptides
and neuroendocrine hormones in ultraviolet radiation-induced
immunosuppression. Methods. 28:97–103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Holzer P: Local effector functions of
capsaicin-sensitive sensory nerve endings: Involvement of
tachykinins, calcitonin gene-related peptide and other
neuropeptides. Neuroscience. 24:739–768. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Richardson JD and Vasko MR: Cellular
mechanisms of neurogenic inflammation. J Pharmacol Exp Ther.
302:839–845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Roosterman D, Goerge T, Schneider SW,
Bunnett NW and Steinhoff M: Neuronal control of skin function: The
skin as a neuroimmunoendocrine organ. Physiol Rev. 86:1309–1379.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zaidi M, Moonga BS, Bevis PJ, Bascal ZA
and Breimer LH: The calcitonin gene peptides: Biology and clinical
relevance. Crit Rev Clin Lab Sci. 28:109–174. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hosoi J, Murphy GF, Egan CL, Lerner EA,
Grabbe S, Asahina A and Granstein RD: Regulation of Langerhans cell
function by nerves containing calcitonin gene-related peptide.
Nature. 363:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Asahina A, Hosoi J, Grabbe S and Granstein
RD: Modulation of Langerhans cell function by epidermal nerves. J
Allergy Clin Immunol. 96:1178–1182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gillardon F, Moll I, Michel S, Benrath J,
Weihe E and Zimmermann M: Calcitonin gene-related peptide and
nitric oxide are involved in ultraviolet radiation-induced
immunosuppression. Eur J Pharmacol. 293:395–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Seike M, Ikeda M, Morimoto A, Matsumoto M
and Kodama H: Increased synthesis of calcitonin gene-related
peptide stimulates keratinocyte proliferation in murine
UVB-irradiated skin. J Dermatol Sci. 28:135–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Niizeki H, Alard P and Streilein JW:
Calcitonin gene-related peptide is necessary for ultraviolet
B-impaired induction of contact hypersensitivity. J Immunol.
159:5183–5186. 1997.PubMed/NCBI
|
|
113
|
Legat FJ and Wolf P: Photodamage to the
cutaneous sensory nerves: Role in photoaging and carcinogenesis of
the skin? Photochem Photobiol Sci. 5:170–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sjöholm A: Intracellular signal
transduction pathways that control pancreatic β-cell proliferation.
FEBS Lett. 311:85–90. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Takahashi K, Nakanishi S and Imamura S:
Direct effects of cutaneous neuropeptides on adenylyl cyclase
activity and proliferation in a keratinocyte cell line: Stimulation
of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY
through G protein-coupled receptors. J Invest Dermatol.
101:646–651. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Green H: Terminal differentiation of
cultured human epidermal cells. Cell. 11:405–416. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Green H: Cyclic AMP in relation to
proliferation of the epidermal cell: A new view. Cell. 15:801–811.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yoshikawa K, Takeda J, Nemoto O, Halprin
KM and Adachi K: Activation of cAMP-dependent protein kinase in
epidermis by the compounds which increase epidermal cAMP. J Invest
Dermatol. 77:397–401. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Elgjo K: Epidermal chalone and cyclic AMP:
An in vivo study. J Invest Dermatol. 64:14–18. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Delecluse C, Fukuyama K and Epstein WL:
Dibutyryl cyclic AMP-induced differentiation of epidermal cells in
tissue culture. J Invest Dermatol. 66:8–13. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Voorhees JJ, Duell EA and Kelsey WH:
Dibutyryl cyclic AMP inhibition of epidermal cell division. Arch
Dermatol. 105:384–386. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Marks F and Grimm W: Diurnal fluctuation
and -adrenergic elevation of cyclic AMP in mouse epidermis in vivo.
Nat New Biol. 240:178–179. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Wiesenfeld-Hallin Z, Hökfelt T, Lundberg
JM, Forssmann WG, Reinecke M, Tschopp FA and Fischer JA:
Immunoreactive calcitonin gene-related peptide and substance P
coexist in sensory neurons to the spinal cord and interact in
spinal behavioral responses of the rat. Neurosci Lett. 52:199–204.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Regoli D, Boudon A and Fauchére JL:
Receptors and antagonists for substance P and related peptides.
Pharmacol Rev. 46:551–599. 1994.PubMed/NCBI
|
|
125
|
Bang R, Sass G, Kiemer AK, Vollmar AM,
Neuhuber WL and Tiegs G: Neurokinin-1 receptor antagonists
CP-96,345 and L-733,060 protect mice from cytokine-mediated liver
injury. J Pharmacol Exp Ther. 305:31–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Catalioto RM, Criscuoli M, Cucchi P,
Giachetti A, Gianotti D, Giuliani S, Lecci A, Lippi A, Patacchini
R, Quartara L, et al: MEN 11420 (Nepadutant), a novel glycosylated
bicyclic peptide tachykinin NK2 receptor antagonist. Br J
Pharmacol. 123:81–91. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Esteban F, Muñoz M, González-Moles MA and
Rosso M: A role for substance P in cancer promotion and
progression: A mechanism to counteract intracellular death signals
following oncogene activation or DNA damage. Cancer Metastasis Rev.
25:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Muñoz M, Pérez A, Coveñas R, Rosso M and
Castro E: Antitumoural action of L-733,060 on neuroblastoma and
glioma cell lines. Arch Ital Biol. 142:105–112. 2004.PubMed/NCBI
|
|
129
|
Muñoz M, Rosso M, Pérez A, Coveñas R,
Rosso R, Zamarriego C and Piruat JI: The NK1 receptor is involved
in the antitumoural action of L-733,060 and in the mitogenic action
of substance P on neuroblastoma and glioma cell lines.
Neuropeptides. 39:427–432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Moles MA González, Mosqueda-Taylor A,
Esteban F, Gil-Montoya JA, Díaz-Franco MA, Delgado M and Muñoz M:
Cell proliferation associated with actions of the substance P/NK-1
receptor complex in keratocystic odontogenic tumours. Oral Oncol.
44:1127–1133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Moles MA González, Esteban F, Ruiz-Ávila
I, Montoya JA Gil, Brener S, Bascones-Martínez A and Muñoz M: A
role for the substance P/NK-1 receptor complex in cell
proliferation and apoptosis in oral lichen planus. Oral Dis.
15:162–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Staniek V, Misery L, Péguet-Navarro J,
Abello J, Doutremepuich JD, Claudy A and Schmitt D: Binding and in
vitro modulation of human epidermal Langerhans cell functions by
substance P. Arch Dermatol Res. 289:285–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Eschenfelder CC, Benrath J, Zimmermann M
and Gillardon F: Involvement of substance P in ultraviolet
irradiation-induced inflammation in rat skin. Eur J Neurosci.
7:1520–1526. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Benrath J, Eschenfelder C, Zimmerman M and
Gillardon F: Calcitonin gene-related peptide, substance P and
nitric oxide are involved in cutaneous inflammation following
ultraviolet irradiation. Eur J Pharmacol. 293:87–96. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Koon HW, Zhao D, Na X, Moyer MP and
Pothoulakis C: Metalloproteinases and transforming growth
factor-alpha mediate substance P-induced mitogen-activated protein
kinase activation and proliferation in human colonocytes. J Biol
Chem. 279:45519–45527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Brener S, González-Moles MA, Tostes D,
Esteban F, Gil-Montoya JA, Ruiz-Avila I, Bravo M and Muñoz M: A
role for the substance P/NK-1 receptor complex in cell
proliferation in oral squamous cell carcinoma. Anticancer Res.
29:2323–2329. 2009.PubMed/NCBI
|
|
137
|
Weinstock JV, Blum A, Walder J and Walder
R: Eosinophils from granulomas in murine schistosomiasis mansoni
produce substance P. J Immunol. 141:961–966. 1988.PubMed/NCBI
|
|
138
|
Vincent SR and Hope BT: Neurons that say
NO. Trends Neurosci. 15:108–113. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lippe IT, Stabentheiner A and Holzer P:
Participation of nitric oxide in the mustard oil-induced neurogenic
inflammation of the rat paw skin. Eur J Pharmacol. 232:113–120.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Gallo O, Masini E, Morbidelli L, Franchi
A, Fini-Storchi I, Vergari WA and Ziche M: Role of nitric oxide in
angiogenesis and tumor progression in head and neck cancer. J Natl
Cancer Inst. 90:587–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Thomsen LL, Lawton FG, Knowles RG, Beesley
JE, Riveros-Moreno V and Moncada S: Nitric oxide synthase activity
in human gynecological cancer. Cancer Res. 54:1352–1354.
1994.PubMed/NCBI
|
|
142
|
Thomsen LL, Miles DW, Happerfield L,
Bobrow LG, Knowles RG and Moncada S: Nitric oxide synthase activity
in human breast cancer. Br J Cancer. 72:41–44. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Nathan C: Inducible nitric oxide synthase:
what difference does it make? J Clin Invest. 100:2417–2423. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Song ZJ, Gong P and Wu YE: Relationship
between the expression of iNOS, VEGF, tumor angiogenesis and
gastric cancer. World J Gastroenterol. 8:591–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yagihashi N, Kasajima H, Sugai S,
Matsumoto K, Ebina Y, Morita T, Murakami T and Yagihashi S:
Increased in situ expression of nitric oxide synthase in human
colorectal cancer. Virchows Arch. 436:109–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Rosbe KW, Prazma J, Petrusz P, Mims W,
Ball SS and Weissler MC: Immunohistochemical characterization of
nitric oxide synthase activity in squamous cell carcinoma of the
head and neck. Otolaryngol Head Neck Surg. 113:541–549. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Brennan PA, Umar T, Smith GI, Lo CH and
Tant S: Expression of nitric oxide synthase-2 in cutaneous squamous
cell carcinoma of the head and neck. Br J Oral Maxillofac Surg.
40:191–194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Brennan PA, Umar T, Bowden J, Hobkirk A,
Spedding AV, Conroy B, Zaki G and Macpherson DW: Nitric oxide
synthase expression is downregulated in basal cell carcinoma of the
head and neck. Br J Oral Maxillofac Surg. 38:633–636. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Andrade SP, Hart IR and Piper PJ:
Inhibitors of nitric oxide synthase selectively reduce flow in
tumor-associated neovasculature. Br J Pharmacol. 107:1092–1095.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Maeda H, Noguchi Y, Sato K and Akaike T:
Enhanced vascular permeability in solid tumor is mediated by nitric
oxide and inhibited by both new nitric oxide scavenger and nitric
oxide synthase inhibitor. Jpn J Cancer Res. 85:331–334. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Connelly ST, Macabeo-Ong M, Dekker N,
Jordan RC and Schmidt BL: Increased nitric oxide levels and iNOS
over-expression in oral squamous cell carcinoma. Oral Oncol.
41:261–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Vural P, Erzengin D, Canbaz M and Selçuki
D: Nitric oxide and endothelin-1,2 in actinic keratosis and basal
cell carcinoma: Changes in nitric oxide/endothelin ratio. Int J
Dermatol. 40:704–708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Vidal MJ, Zocchi MR, Poggi A, Pellegatta F
and Chierchia SL: Involvement of nitric oxide in tumor cell
adhesion to cytokine-activated endothelial cells. J Cardiovasc
Pharmacol. 20:(Suppl 12). S155–S159. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Dong Z, Staroselsky AH, Qi X, Xie K and
Fidler IJ: Inverse correlation between expression of inducible
nitric oxide synthase activity and production of metastasis in
K-1735 murine melanoma cells. Cancer Res. 54:789–793.
1994.PubMed/NCBI
|
|
155
|
Brazeau P, Vale W, Burgus R, Ling N,
Butcher M, Rivier J and Guillemin R: Hypothalamic polypeptide that
inhibits the secretion of immunoreactive pituitary growth hormone.
Science. 179:77–79. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Jin Z, Mori Y, Hamilton JP, Olaru A, Sato
F, Yang J, Ito T, Kan T, Agarwal R and Meltzer SJ: Hypermethylation
of the somatostatin promoter is a common, early event in human
esophageal carcinogenesis. Cancer. 112:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Jackson K, Soutto M, Peng D, Hu T, Marshal
D and El-Rifai W: Epigenetic silencing of somatostatin in gastric
cancer. Dig Dis Sci. 56:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Mori Y, Cai K, Cheng Y, Wang S, Paun B,
Hamilton JP, Jin Z, Sato F, Berki AT, Kan T, et al: A genome-wide
search identifies epigenetic silencing of somatostatin,
tachykinin-1, and 5 other genes in colon cancer. Gastroenterology.
131:797–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Reubi JC and Laissue JA: Multiple actions
of somatostatin in neoplastic disease. Trends Pharmacol Sci.
16:110–115. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Scambia G, Panici P Benedetti, Baiocchi G,
Andreani C, Gaggini C, Giannelli S and Mancuso S: Growth inhibitory
effect of somatostatin (SS) on human breast cancer cells in
culture. J Steroid Biochem. 28:1651987. View Article : Google Scholar
|
|
161
|
Liebow C, Reilly C, Serrano M and Schally
AV: Somatostatin analogues inhibit growth of pancreatic cancer by
stimulating tyrosine phosphatase. Proc Natl Acad Sci USA.
86:2003–2007. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Taylor JE, Bogden AE, Moreau J-P and Coy
DH: In vitro and in vivo inhibition of human small cell lung
carcinoma (NCI-H69) growth by a somatostatin analogue. Biochem
Biophys Res Commun. 153:81–86. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Gill GN and Lazar CS: Increased
phosphotyrosine content and inhibition of proliferation in
EGF-treated A431 cells. Nature. 293:305–307. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Mascardo RN and Sherline P: Somatostatin
inhibits rapid centrosomal separation and cell proliferation
induced by epidermal growth factor. Endocrinology. 111:1394–1396.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Kamiya Y, Ohmura E, Arai M, Fujii T,
Hayakawa F, Ito J, Kawaguchi M, Tsushima T and Sakuma N: Effect of
somatostatin and its analogue on proliferation of human epidermoid
carcinoma cells in vitro. Biochem Biophys Res Commun. 191:302–307.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Burbach J and Wiegant V: Neuropeptides:
basics and perspectives. de Wied D: Elsevier; Amsterdam: pp.
45–103. 1990
|
|
167
|
Thody AJ, Ridley K, Penny RJ, Chalmers R,
Fisher C and Shuster S: MSH peptides are present in mammalian skin.
Peptides. 4:813–816. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Schauer E, Trautinger F, Köck A, Schwarz
A, Bhardwaj R, Simon M, Ansel JC, Schwarz T and Luger TA:
Proopiomelanocortin-derived peptides are synthesized and released
by human keratinocytes. J Clin Invest. 93:2258–2262. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Slominski A and Mihm MC: Potential
mechanism of skin response to stress. Int J Dermatol. 35:849–851.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Slominski A, Wortsman J, Luger T, Paus R
and Solomon S: Corticotropin releasing hormone and
proopiomelanocortin involvement in the cutaneous response to
stress. Physiol Rev. 80:979–1020. 2000.PubMed/NCBI
|
|
171
|
Slominski AT, Botchkarev V, Choudhry M,
Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E,
et al: Cutaneous expression of CRH and CRH-R. Is there a ‘skin
stress response system?’. Ann N Y Acad Sci. 885:287–311. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Slominski A, Zbytek B, Pisarchik A,
Slominski RM, Zmijewski MA and Wortsman J: CRH functions as a
growth factor/cytokine in the skin. J Cell Physiol. 206:780–791.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Slominski A, Wortsman J, Pisarchik A,
Zbytek B, Linton EA, Mazurkiewicz JE and Wei ET: Cutaneous
expression of corticotropin-releasing hormone (CRH), urocortin, and
CRH receptors. FASEB J. 15:1678–1693. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Slominski A, Pisarchik A, Tobin DJ,
Mazurkiewicz JE and Wortsman J: Differential expression of a
cutaneous corticotropin-releasing hormone system. Endocrinology.
145:941–950. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Slominski A, Roloff B, Curry J, Dahiya M,
Szczesniewski A and Wortsman J: The skin produces urocortin. J Clin
Endocrinol Metab. 85:815–823. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Slominski A, Wortsman J, Mazurkiewicz JE,
Matsuoka L, Dietrich J, Lawrence K, Gorbani A and Paus R: Detection
of proopiomelanocortin-derived antigens in normal and pathologic
human skin. J Lab Clin Med. 122:658–666. 1993.PubMed/NCBI
|
|
177
|
Slominski A, Heasley D, Mazurkiewicz JE,
Ermak G, Baker J and Carlson JA: Expression of proopiomelanocortin
(POMC)-derived melanocyte-stimulating hormone (MSH) and
adrenocorticotropic hormone (ACTH) peptides in skin of basal cell
carcinoma patients. Hum Pathol. 30:208–215. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Slominski A: Identification of
beta-endorphin, alpha-MSH and ACTH peptides in cultured human
melanocytes, melanoma and squamous cell carcinoma cells by RP-HPLC.
Exp Dermatol. 7:213–216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Sato H, Nagashima Y, Chrousos GP,
Ichihashi M and Funasak Y: The expression of
corticotropin-releasing hormone in melanoma. Pigment Cell Res.
15:98–103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Slominski A and Pawelek J: Animals under
the sun: Effects of ultraviolet radiation on mammalian skin. Clin
Dermatol. 16:503–515. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Scholzen TE, Brzoska T, Kalden D-H,
O'Reilly F, Armstrong CA, Luger TA and Ansel JC: Effect of
ultraviolet light on the release of neuropeptides and
neuroendocrine hormones in the skin: Mediators of photodermatitis
and cutaneous inflammation. J Investig Dermatol Symp Proc. 4:55–60.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Huang CM, Elmets CA, van Kampen KR,
Desilva TS, Barnes S, Kim H and Tang DC: Prospective highlights of
functional skin proteomics. Mass Spectrom Rev. 24:647–660. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Mitsuma T, Matsumoto Y and Tomita Y:
Corticotropin releasing hormone stimulates proliferation of
keratinocytes. Life Sci. 69:1991–1998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Brown SL and Blalock JE: Neuroendocrine
Immune InteractionsImmunophysiology: the Rrole of cells
andcytokines in immunity and inflammation. Shevach JJOEM: Oxford
University Press; Oxford: pp. 306–319. 1990
|
|
185
|
Kim MH, Cho D, Kim HJ, Chong SJ, Lee KH,
Yu DS, Park CJ, Lee JY, Cho BK and Park HJ: Investigation of the
corticotropin-releasing hormone-proopiomelanocortin axis in various
skin tumours. Br J Dermatol. 155:910–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Luger TA, Schauer E, Trautinger F,
Krutmann J, Ansel J, Schwarz A and Schwarz T: Production of
immunosuppressing melanotropins by human keratinocytes. Ann N Y
Acad Sci. 680:(1 The Melanotro). 567–570. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Arbiser JL, Karalis K, Viswanathan A,
Koike C, Anand-Apte B, Flynn E, Zetter B and Majzoub JA:
Corticotropin-releasing hormone stimulates angiogenesis and
epithelial tumor growth in the skin. J Invest Dermatol.
113:838–842. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Chakraborty AK, Funasaka Y, Slominski A,
Ermak G, Hwang J, Pawelek JM and Ichihashi M: Production and
release of proopiomelanocortin (POMC) derived peptides by human
melanocytes and keratinocytes in culture: Regulation by ultraviolet
B. Biochim Biophys Acta. 1313:130–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Ferjan I and Lipnik-Štangelj M: Chronic
pain treatment: The influence of tricyclic antidepressants on
serotonin release and uptake in mast cells. Mediators Inflamm.
2013:3404732013. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Ton BH, Chen Q, Gaina G, Tucureanu C,
Georgescu A, Strungaru C, Flonta ML, Sah D and Ristoiu V:
Activation profile of dorsal root ganglia Iba-1 (+) macrophages
varies with the type of lesion in rats. Acta Histochem.
115:840–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Hart PH, Grimbaldeston MA, Swift GJ,
Jaksic A, Noonan FP and Finlay-Jones JJ: Dermal mast cells
determine susceptibility to ultraviolet B-induced systemic
suppression of contact hypersensitivity responses in mice. J Exp
Med. 187:2045–2053. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Kligman LH and Murphy GF: Ultraviolet B
radiation increases hairless mouse mast cells in a dose-dependent
manner and alters distribution of UV-induced mast cell growth
factor. Photochem Photobiol. 63:123–127. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Byrne SN, Limón-Flores AY and Ullrich SE:
Mast cell migration from the skin to the draining lymph nodes upon
ultraviolet irradiation represents a key step in the induction of
immune suppression. J Immunol. 180:4648–4655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Maltby S, Khazaie K and McNagny KM: Mast
cells in tumor growth: Angiogenesis, tissue remodelling and
immune-modulation. Biochim Biophys Acta. 1796:19–26.
2009.PubMed/NCBI
|
|
195
|
Ch'ng S, Wallis RA, Yuan L, Davis PF and
Tan ST: Mast cells and cutaneous malignancies. Mod Pathol.
19:149–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Grimbaldeston MA, Skov L, Finlay-Jones JJ
and Hart PH: Increased dermal mast cell prevalence and
susceptibility to development of basal cell carcinoma in humans.
Methods. 28:90–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Humphreys TR, Monteiro MR and Murphy GF:
Mast cells and dendritic cells in basal cell carcinoma stroma.
Dermatol Surg. 26:200–203; discussion 203–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Hart PH, Grimbaldeston MA, Swift GJ,
Hosszu EK and Finlay-Jones JJ: A critical role for dermal mast
cells in cis-urocanic acid-induced systemic suppression of contact
hypersensitivity responses in mice. Photochem Photobiol.
70:807–812. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Garssen J, Buckley TL and Van Loveren H: A
role for neuropeptides in UVB-induced systemic immunosuppression.
Photochem Photobiol. 68:205–210. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Wille JJ, Kydonieus AF and Murphy GF:
cis-urocanic acid induces mast cell degranulation and release of
preformed TNF-alpha: A possible mechanism linking UVB and
cis-urocanic acid to immunosuppression of contact hypersensitivity.
Skin Pharmacol Appl Skin Physiol. 12:18–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Khalil Z, Townley SL, Grimbaldeston MA,
Finlay-Jones JJ and Hart PH: cis-Urocanic acid stimulates
neuropeptide release from peripheral sensory nerves. J Invest
Dermatol. 117:886–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Neagu M, Constantin C and Longo C:
Chemokines in the melanoma metastasis biomarkers portrait. J
Immunoassay Immunochem. 36:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Honeyman JF: Psychoneuroimmunology and the
skin. Acta Derm Venereol. 96:38–46. 2016.PubMed/NCBI
|
|
204
|
Ribatti D and Crivellato E: Mast cells,
angiogenesis, and tumour growth. Biochim Biophys Acta. 1822:2–8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Kanda N and Watanabe S: Histamine enhances
the production of nerve growth factor in human keratinocytes. J
Invest Dermatol. 121:570–577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Hart PH, Grimbaldeston MA and Finlay-Jones
JJ: Sunlight, immunosuppression and skin cancer: Role of histamine
and mast cells. Clin Exp Pharmacol Physiol. 28:1–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Harizi H, Juzan M, Pitard V, Moreau JF and
Gualde N: Cyclooxygenase-2-issued prostaglandin e(2) enhances the
production of endogenous IL-10, which down-regulates dendritic cell
functions. J Immunol. 168:2255–2263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Schwarz A, Ständer S, Berneburg M, Böhm M,
Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J and Schwarz T:
Interleukin-12 suppresses ultraviolet radiation-induced apoptosis
by inducing DNA repair. Nat Cell Biol. 4:26–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Varricchi G, Galdiero MR and Marone G,
Granata F, Borriello F and Marone G: Controversial role of mast
cells in skin cancers. Exp Dermatol. 26:11–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Sawatsubashi M, Yamada T, Fukushima N,
Mizokami H, Tokunaga O and Shin T: Association of vascular
endothelial growth factor and mast cells with angiogenesis in
laryngeal squamous cell carcinoma. Virchows Arch. 436:243–248.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Diaconu NC, Kaminska R, Naukkarinen A,
Harvima RJ and Harvima IT: The increase in tryptase- and
chymase-positive mast cells is associated with partial inactivation
of chymase and increase in protease inhibitors in basal cell
carcinoma. J Eur Acad Dermatol Venereol. 21:908–915. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Hart PH, Townley SL, Grimbaldeston MA,
Khalil Z and Finlay-Jones JJ: Mast cells, neuropeptides, histamine,
and prostaglandins in UV-induced systemic immunosuppression.
Methods. 28:79–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Townley SL, Grimbaldeston MA, Ferguson I,
Rush RA, Zhang SH, Zhou XF, Conner JM, Finlay-Jones JJ and Hart PH:
Nerve growth factor, neuropeptides, and mast cells in
ultraviolet-B-induced systemic suppression of contact
hypersensitivity responses in mice. J Invest Dermatol. 118:396–401.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Singh LK, Pang X, Alexacos N, Letourneau R
and Theoharides TC: Acute immobilization stress triggers skin mast
cell degranulation via corticotropin releasing hormone,
neurotensin, and substance P: A link to neurogenic skin disorders.
Brain Behav Immun. 13:225–239. 1999. View Article : Google Scholar : PubMed/NCBI
|