Introduction
In 2012, colorectal cancer (CRC) was the third most
common cancer in men (746,000 case, 10.0% of the total) and the
second most common cancer in women (614,000 cases, 9.2% of the
total) worldwide (1). The primary
treatment for early stage CRC is surgery; however, in more advanced
stages of CRC, adjuvant therapy is recommended to increase overall
survival rate and prevent recurrence as either local or distant
metastasis (2). However, adjuvant
chemotherapy harms normal cells, increases treatment resistance,
and causes side effects such as vomiting, nausea, diarrhea, and
alopecia (3). Several papers have
presented candidate drugs from natural products to overcome
multidrug resistance in cancer chemotherapy (4–7).
Especially, Belcaro et al showed that curcumin reduced
semi-quantitative evaluation of cancer chemotherapy side effects
including vomiting, nausea, diarrhea, and malnutrition (8). Thus, a promising effective and safe
strategy to enhance cancer treatment is the development of natural
compound-based cancer therapies.
Natural products have been mainly used as functional
foods in order to improve health through activation of
physiological functions. In some cases, these have been developed
as medicines for several diseases, including cancer, diabetes, and
hyperlipidemia (9–11). Among the various natural products,
mushrooms and ginsengs are good resources of natural medicines that
are non-toxic to normal cells but cytotoxic to cancer cells.
Phellinus linteus has been demonstrated to inhibit tumor
proliferation by decreasing expression of cyclin B1 and
cyclin-dependent kinases (CDK2, 4, and 6), induce apoptosis through
the activation of caspase-2, −3, and −7 and cleaved-PARP in various
cancer cells (12), and also
suppress tumor growth and pulmonary metastasis in C57BL/6 mice
intravenously injected with melanoma cells (13). Furthermore, Inonotus obliquus
(Chaga mushroom) has been reported to prevent cell proliferation
via the induction of G1 cell cycle arrest in HT-29 human colon
cancer cells (14). Other mushrooms
have been shown to be synergistically adjuvant by exerting
immunomodulatory and anti-inflammatory effects (15). Additionally, P. ginseng is
commonly used by itself or in combination with other medical
components. Several studies have demonstrated that the root of
P. ginseng has anti-inflammatory, anti-diabetes, and
anticancer activity (16–18). The previous studies mainly describe
a single effect of medicinal mushrooms or P. ginseng extract
in various cancer cells. However, anticancer effects in a
combination of several medicinal mushrooms and/or P. ginseng
extracts are still unclear.
Our study, which focused on the cell cycle and cell
death pathways, investigated whether a combination of seven
medicinal mushrooms and P. ginseng root extracts (Amex7)
enhanced anticancer effects in human colorectal cancer cells when
used at a concentration lower than that used in previous studies.
Our data may contribute to provide a scientific rationale for the
clinical use of the combination of medicinal mushrooms and P.
ginseng root extracts as an adjuvant compound in human
colorectal cancer.
Materials and methods
Reagents
Anti-Rad51 and anti-excision repair
cross-complementation group 1 (ERCC1) antibodies were purchased
from Abcam (Cambridge, MA, USA). Cyclin B1, cyclin A2, cyclin D1,
cleaved-PARP (Asp214), phospho-p53 (Ser15), phospho-cdc2 (Tyr15),
LC3A/B, sequestosome 1 (SQSTM1)/p62 and cyclin-dependent kinase 2
(CDK2) antibodies were obtained from Cell Signaling Technology
(Beverly, MA, USA). Anti-cyclin-dependent kinase 4 (CDK4) and
anti-cyclin-dependent kinase 6 (CDK6) were obtained from Bethyl
(Montgomery, TX, USA). Cyclin E, Bcl-2, Bcl-xL, Ku70,
Ku80, anti-p53, survivin, DNA-PKcs, glyceraldegyde-3-phosphate
dehydrogenase (GAPDH), horseradish peroxidase (HRP)-conjugated goat
anti-rabbit and anti-mouse IgG antibodies were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-Histone H2AX
(γ-H2AX, Ser139) was provided by Millipore (Billerica, MA, USA).
Alexa Fluor® 488 goat anti-mouse IgG (H+L) and Alexa
Fluor 594 goat anti-rabbit IgG (H+L) secondary antibodies were
purchased from Invitrogen (Carlsbad, CA, USA). Roswell Park
Memorial Institute (RPMI)-1640 medium, fetal bovine serum (FBS),
and antibiotics (penicillin and streptomycin) were obtained from
Welgene (Daegu, South Korea).
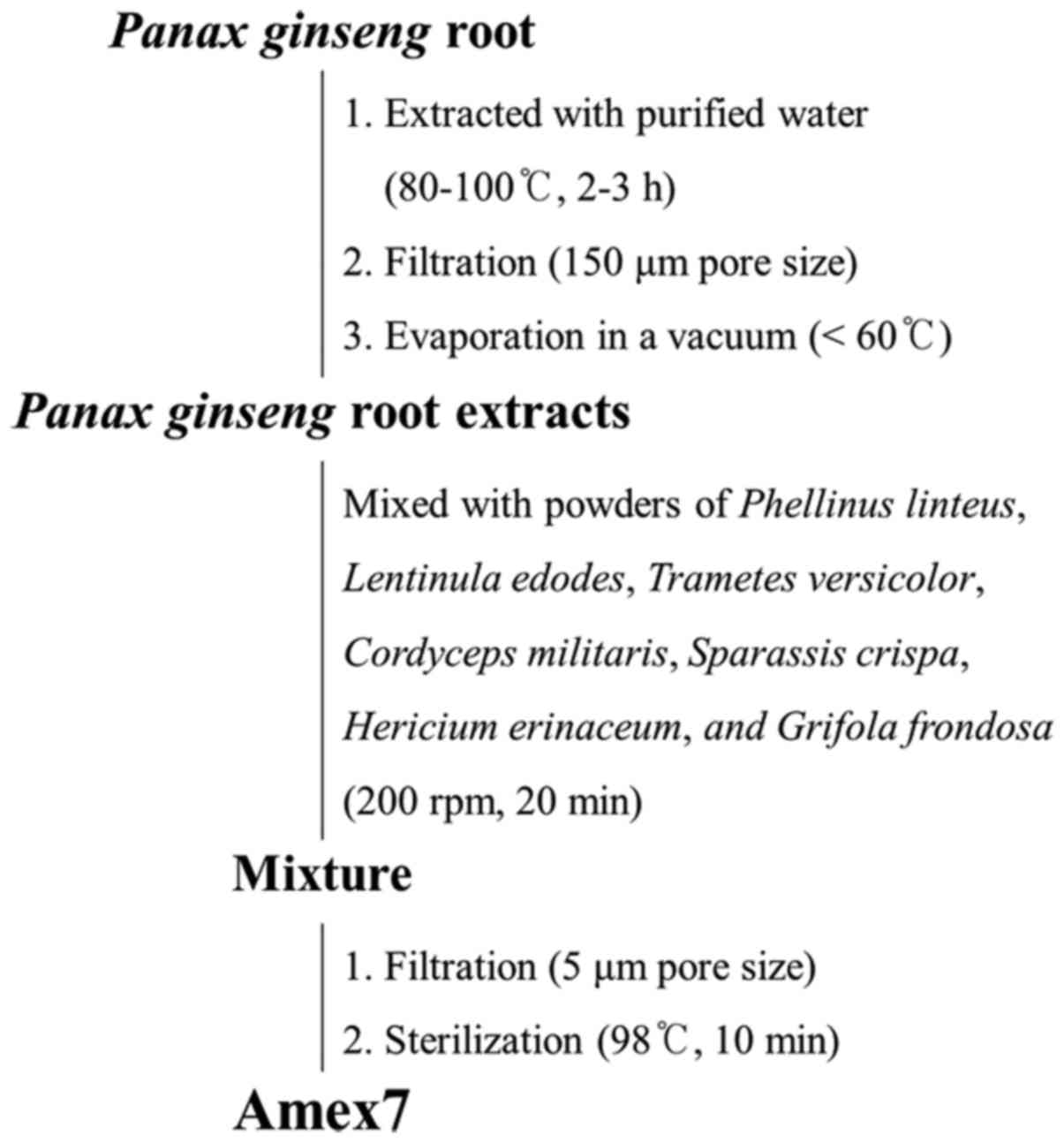
Preparation of the combined mixture of
various mushrooms and P. ginseng root extracts (Amex7)
The combined mixture of seven mushrooms and P.
ginseng root extracts (also called Amex7) used in this study
was obtained from Han Kook Shin Yak Pharmaceutical Co., Ltd. In the
first step, P. ginseng root was extracted with purified
water at 80–100°C for 2–3 h. The water extract from P.
ginseng root was filtered through a 150-µm pore size, and then
evaporated in a vacuum below 60°C. In the second step, the extract
of P. ginseng was mixed with extract powders of Phellinus
linteus, Lentinula edodes, Trametes versicolor, Cordyceps
militaris, Sparassis crispa, Hericium erinaceum, and Grifola
frondosa at 200 rpm for 20 min. The mixture was filtered
through a 5-µm pore size and subsequently sterilized at 98°C for 10
min. Fig. 1 shows the preparation
process of Amex7 and Table I lists
the combined ratio of several compounds in Amex7.
 | Table I.Combined ratio of several compounds
in Amex7. |
Table I.
Combined ratio of several compounds
in Amex7.
| Compound | Combination
percentage (%) | Volume (ml) |
|---|
| Phellinus
linteus |
1.340 |
1.005 |
| Lentinula
edodes |
0.400 |
0.300 |
| Trametes
versicolor |
0.400 |
0.300 |
| Cordyceps
militaris |
0.400 |
0.300 |
| Sparassis
crispa |
0.400 |
0.300 |
| Hericium
erinaceum |
0.400 |
0.300 |
| Grifola
frondosa |
0.400 |
0.300 |
| Panax
ginseng root |
0.130 |
0.098 |
| Pure water |
81.464 | 61.098 |
| Others |
14.666 | 10.999 |
| Total | 100.000 | 75.000 |
Cell lines and cell culture
HT-29 human colorectal cancer cells were obtained
from the Korean Cell Line Bank (Seoul, South Korea). HT-29 cells
were cultured in RPMI-1640 medium supplemented with 10% FBS, 100
U/ml penicillin, and 100 µg/ml streptomycin and incubated in an
atmosphere of 5% CO2 at 37°C.
Drug treatment
Amex7, a combined mixture of several medicinal
mushrooms and P. ginseng extracts, was kindly provided by
Han Kook Shin Yak Co., Ltd. For in vivo experiments, Amex7
was freshly mixed with drinking water at 1.25, 6.25, and 12.5
ml/kg. For in vitro experiments, Amex7 was suspended at a
concentration of 1, 2, 4, 8, and 16% in culture medium.
Tumor xenografts in Balb/c nude
mice
Balb/c nude mice (5-week-old males) were obtained
from Nara Biotech Co. (Seoul, South Korea) and maintained in a
laminar airflow cabinet under specific pathogen-free conditions.
Mice xenografted with HT-29 cells were obtained by subcutaneous
inoculation of 3×106 HT-29 cells into the right hind
leg. After tumor implantation, we monitored the condition of the
animals twice daily and prepared analgesics to minimize animal
suffering. When the tumor attained a volume of approximately
180–200 mm3, the mice were randomly divided into four
groups (n=10): a) control, b) 1.25 ml/kg, c) 6.25 ml/kg, and d)
12.5 ml/kg. The tumor volume (V) was calculated using the standard
formula: V (mm3) = π/6 × (shortest diameter)2
× (longest diameter). Mice were euthanized by CO2
inhalation when the average tumor volume of the control group
reached 2,000 mm3.
Cell count assay
Cells were seeded in 60-mm culture dishes at
5×104 per well and cultured overnight. Then, the cells
were treated with serial concentrations of Amex7 (0, 1, 2, 4, 8,
and 16%) for 6, 12, and 24 h. At the indicated time points, cells
were washed with phosphate buffered saline (PBS), harvested, and
immediately stained with 0.4% trypan blue. Viable cells were
quantified using a hemocytometer under an inverted microscope. Cell
viability of control group was set at 100% and the effects of Amex7
on cell viability were expressed relative to the control group.
Cell morphology
Cells were seeded at a density of 3×106
cells/dish in a 100π dish and incubated for 24 h. The cells were
treated with 4% Amex7 and incubated for a further 6, 12, and 24 h.
The cells were observed by light microscopy (×200) (Motic AE31
microscope, Ted Pella Inc., Redding, CA, USA).
Cell cycle analysis
Cells were treated with 4% Amex7, incubated for 6,
12, and 24 h, harvested, stained with propidium iodide (1 mg/ml)
according to the manufacturer's protocol, and then analyzed using a
FACScan flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA).
A minimum of 10,000 cells were counted for each sample and data
analysis was performed using the CellQuest software.
Immunoblotting
The cells were lysed in radioimmunoprecipitation
assay (RIPA) buffer and the proteins were separated using sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membranes. The membranes blots were
blocked with 5% (v/v) skim milk in PBS with 0.1% Tween-20,
incubated with the indicated primary (1:1,000 dilution) and
secondary antibodies (1:1,000 dilution), developed using enhanced
chemiluminescence (ECL) immunoblotting substrate (Pierce, Rockford,
IL, USA), and imaged with the ImageQuant LAS-4000 mini (GE
Healthcare, Fairfield, CT, USA). The signal intensity of the bands
was measured with the Multi Gauge image analysis software
(Fujifilm, Tokyo, Japan).
Immunofluorescence and confocal
microscopy
HT-29 cells were seeded on coverslips and grown
overnight. After treatment with 4% Amex7 for 6, 12, and 24 h, the
cells were washed and fixed with 4% paraformaldehyde for 10 min at
room temperature. The cells were washed again, permeabilized in
PBS/0.4% Triton X-100 for 10 min at room temperature, and blocked
in PBS/4% FBS for 1 h at room temperature. The coverslips were
stained with primary anti-γ-H2AX (1:500 dilution) antibody,
incubated overnight at 4°C, and then stained with Alexa Fluor 488
Goat anti-Mouse IgG (H+L) secondary antibody and DAPI for 1 h at
room temperature. The coverslips were washed and mounted in
VectaMount™ mounting medium (Vector Laboratory, Inc., Burlingame,
CA, USA).
Ethics statement
All animal protocols and studies were approved by
the Institutional Animal Care and Use Committee (IACUC) of the
Korean Institute of Radiological and Medical Sciences (KIRAMS
2015-0058).
Statistical analysis
Results from the in vivo study are expressed
as the mean ± standard error of the mean (SEM) and those from the
in vitro study were plotted as the mean ± standard deviation
(SD). The statistical analysis was performed using a Student's
t-test and one-way ANOVA followed by Tukey's HSD test using the
statistical package for the social sciences (SPSS) software
(version 23.0; Chicago, IL, USA). The level of statistical
significance was set at a p<0.05.
Results
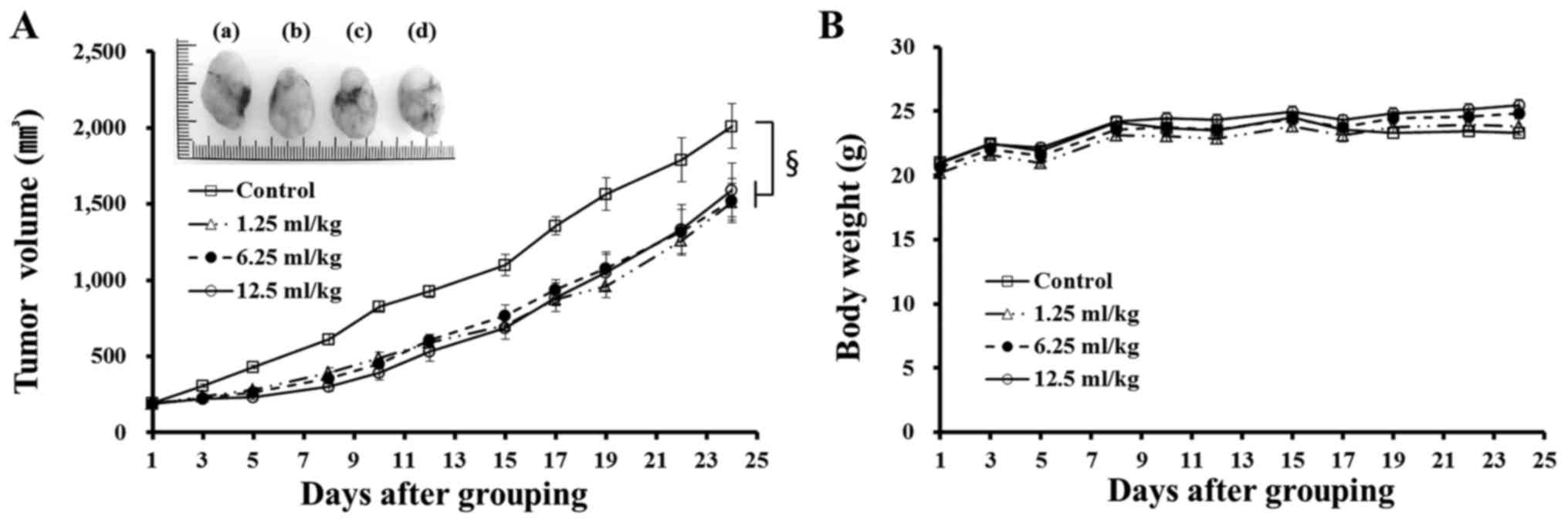
Amex7 significantly inhibits tumor
growth in HT-29 human colorectal cancer xenografts
We evaluated the in vivo anticancer effect of
Amex7 at various concentrations (1.25, 6.25, and 12.5 ml/kg) in
mice xenografted with HT-29 cells. When the mean tumor volume in
the control group reached approximately 2,000 mm3, the
tumor volume in the mice treated with Amex7 at 1.25, 6.25, and 12.5
ml/kg was 46.7±1.5, 46.4±1.2 and 43.9±1.8% smaller, respectively,
than the control (Fig. 2A). A
significant concentration-independent anticancer effect
(p<0.001) of Amex7 was observed in the mouse model. Moreover,
there were no marked changes in body weight between the control and
Amex7 treatment groups during the administration period of Amex7
(Fig. 2B).
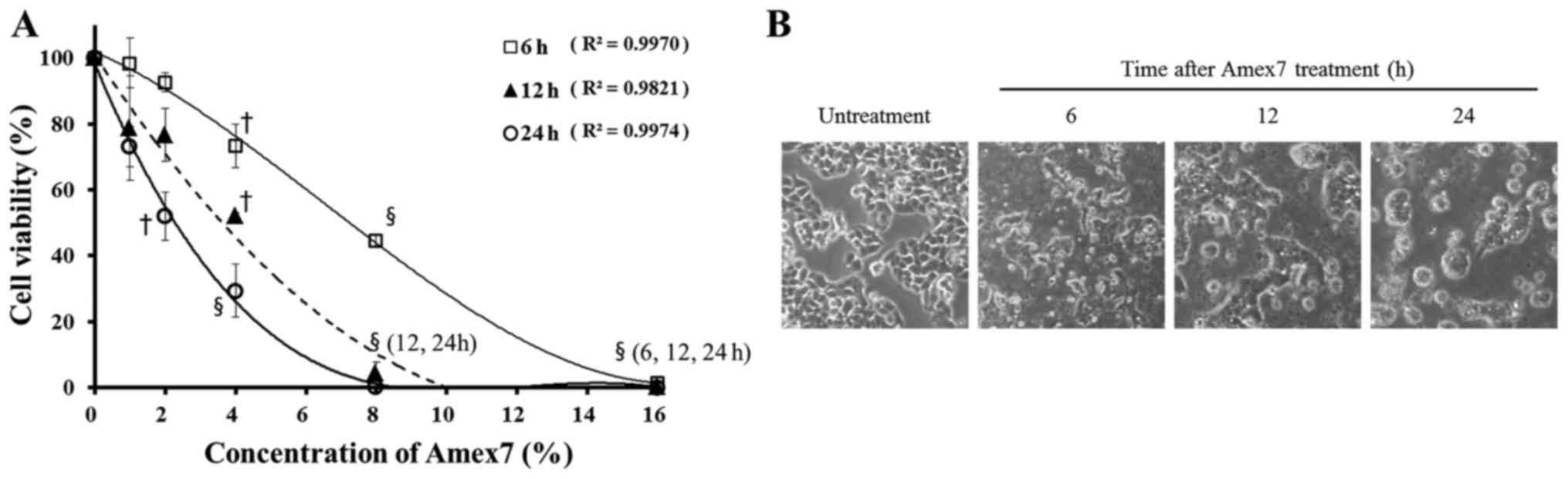
Amex7 decreases cell viability and
induces morphological changes in HT-29 human colorectal cancer
cells
To assess the anti-proliferative effect of Amex7 on
human colorectal cancer cells, we exposed HT-29 cells to serial
concentrations of Amex7 (0, 1, 2, 4, 8, and 16%) for 6, 12, and 24
h. By using cell count assay, Amex7 treatment resulted in 99, 93,
73, 45 and 1% cell viability for 6 h; 79, 77, 52, 4 and 0% cell
viability for 12 h; and 73, 52, 29, 0 and 0% cell viability for 24
h at 1, 2, 4, 8, and 16%, respectively (Fig. 3A). Furthermore, Amex7 changed cell
morphology and increased cell aggregations and cellular debris
compared with the control cells in time-dependent manner (Fig. 3B). Our data showed that Amex7
induces concentration- and time-dependent inhibition of HT-29 human
colorectal cancer cell lines.
Amex7 modulates cell cycle progression
in HT-29 human colorectal cancer cells
To determine whether the anti-proliferative effect
of Amex7 was associated with regulation of the cell cycle, we used
flow cytometry to analyze the cell cycle distribution of HT-29
cells in the presence of Amex7. Fig. 4A
and B show that Amex7 significantly decreased the proportion of
G0/G1 phase cells (p<0.001) and accumulation of G2/M phase cells
compared with the control (p<0.001). Furthermore, Amex7 markedly
increased the proportion of sub-G1 phase cells at 24 h, but not at
6 and 12 h after treatment of Amex7 compared with the control.
These results suggested that Amex7 inhibited cell proliferation by
inducing G2/M phase arrest and apoptosis.
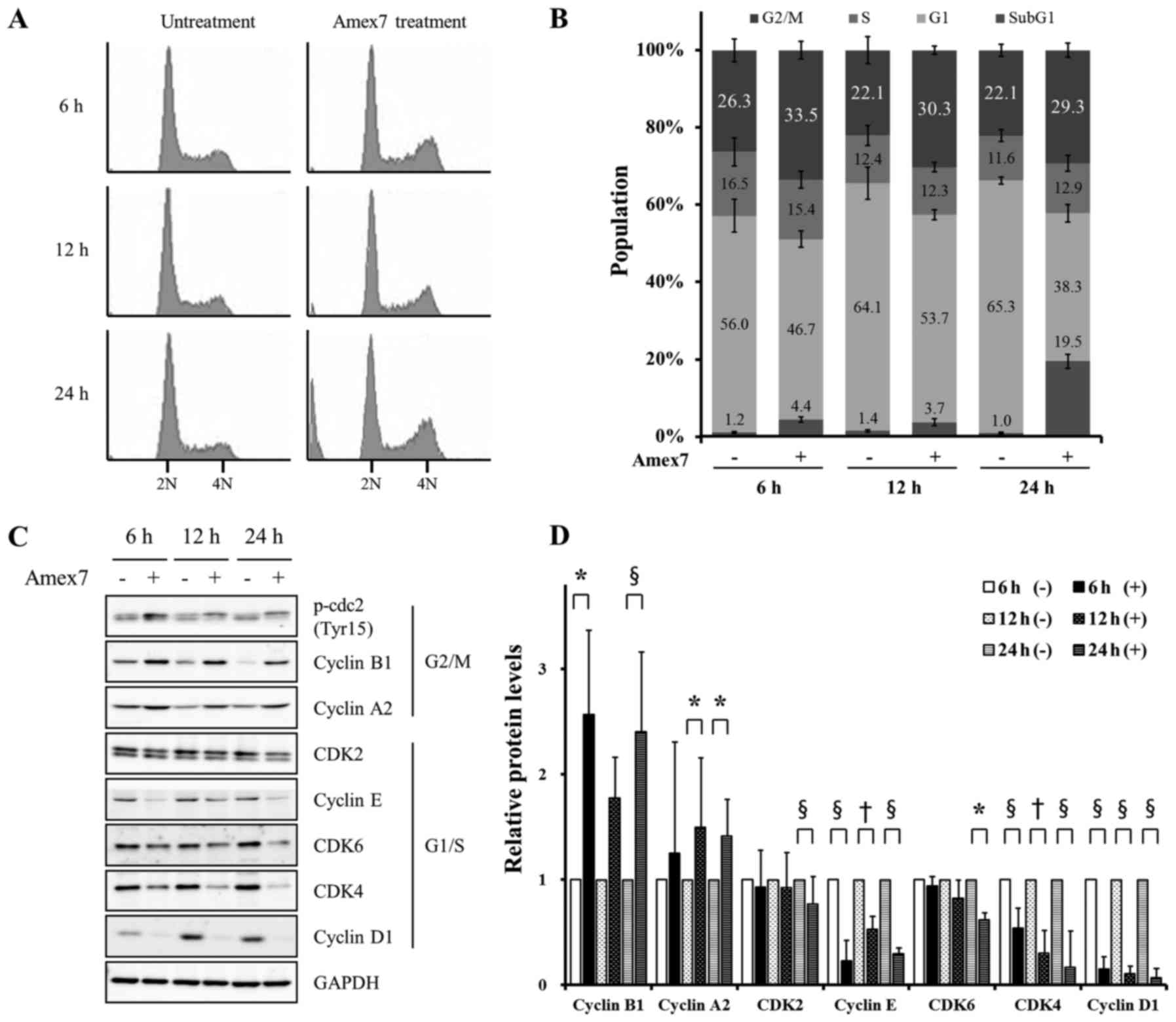 | Figure 4.Amex7 prevents cell cycle progression
by regulating cell cycle regulatory proteins. HT-29 cells were
treated with 4% Amex7 for 6, 12, and 24 h. (A) Cell cycle
distribution was analyzed by flow cytometry and (B) was quantified
using a FACScan flow cytometer. (C) Expression of G1/S checkpoint
regulators (cyclin D1, cyclin E, CDK2, CDK4, and CDK6) and G2/M
checkpoint regulators (cyclin A2, cyclin B1, and p-cdc2) were
measured by immunoblotting and (D) were quantified using Multi
Gauge V3.0 software in HT-29 cells. Densitometric quantification
was normalized to GAPDH. (−) indicates the control group and (+)
indicates Amex7-treated group. Values are mean ± SD of four
determination. *p<0.05, †p<0.01,
§p<0.001 vs. each control group. |
Amex7 induces G2/M arrest by
regulating cell cycle regulatory proteins
To further confirm the reduction of cells in the
G0/G1 phase and the increase of cells in the G2/M phase, the
expression levels of cell cycle regulatory proteins such as cyclin
A2, cyclin B1, cyclin D1, cyclin E, phospho-cdc2 (Tyr15), CDK2,
CDK4, and CDK6 were examined by immunoblotting. At 6, 12, and 24 h
after Amex7 treatment, the expression levels of cyclin D1, cyclin
E, and CDK4, which are involved in G1/S progression, were
significantly decreased (p<0.01) and CDK6 protein level was
slightly decreased. Moreover, Amex7 markedly increased cyclin A2
and cyclin B1 protein levels, which are required for G2/M
progression (p<0.05), but did not change the expression level of
phospho-cdc2 (Tyr15) (Fig. 4C and
D). In summary, we showed that Amex7 regulated cell cycle
progression by significantly reducing G1/S phase-related proteins
and increasing G2/M phase-related proteins.
Amex7 induces apoptosis and autophagy
in HT-29 human colorectal cancer cells
In order to identify the induction of apoptosis by
Amex7, we observed the expression of p53, phospho-p53 (Ser15),
Bcl-2, Bcl-xL, survivin, and cleaved-PARP (Asp214) by
immunoblotting. At 12 and 24 h after treatment of Amex7,
cleaved-PARP protein (Asp214) was increased and anti-apoptotic
proteins, including Bcl-2, Bcl-xL, and survivin, were
decreased. In addition, Amex7 treatment for 12, 24 h reduced total
p53 protein level, whereas phosphorylated p53 at Ser15 (Fig. 5A and B). Next, to evaluate the
induction of autophagy by Amex7, we showed the conversion of the
autophagosome protein LC3A/B-I to LC3A/B-II and the expression of
SQTM1/p62 using immunoblotting. Starting 12 h after treatment of
Amex7, LC3A/B-II and SQTM1/p62 were consistently induced (Fig. 5C and D). These results suggested
that Amex7 induced apoptosis and autophagosome formation in human
colorectal cancer cells.
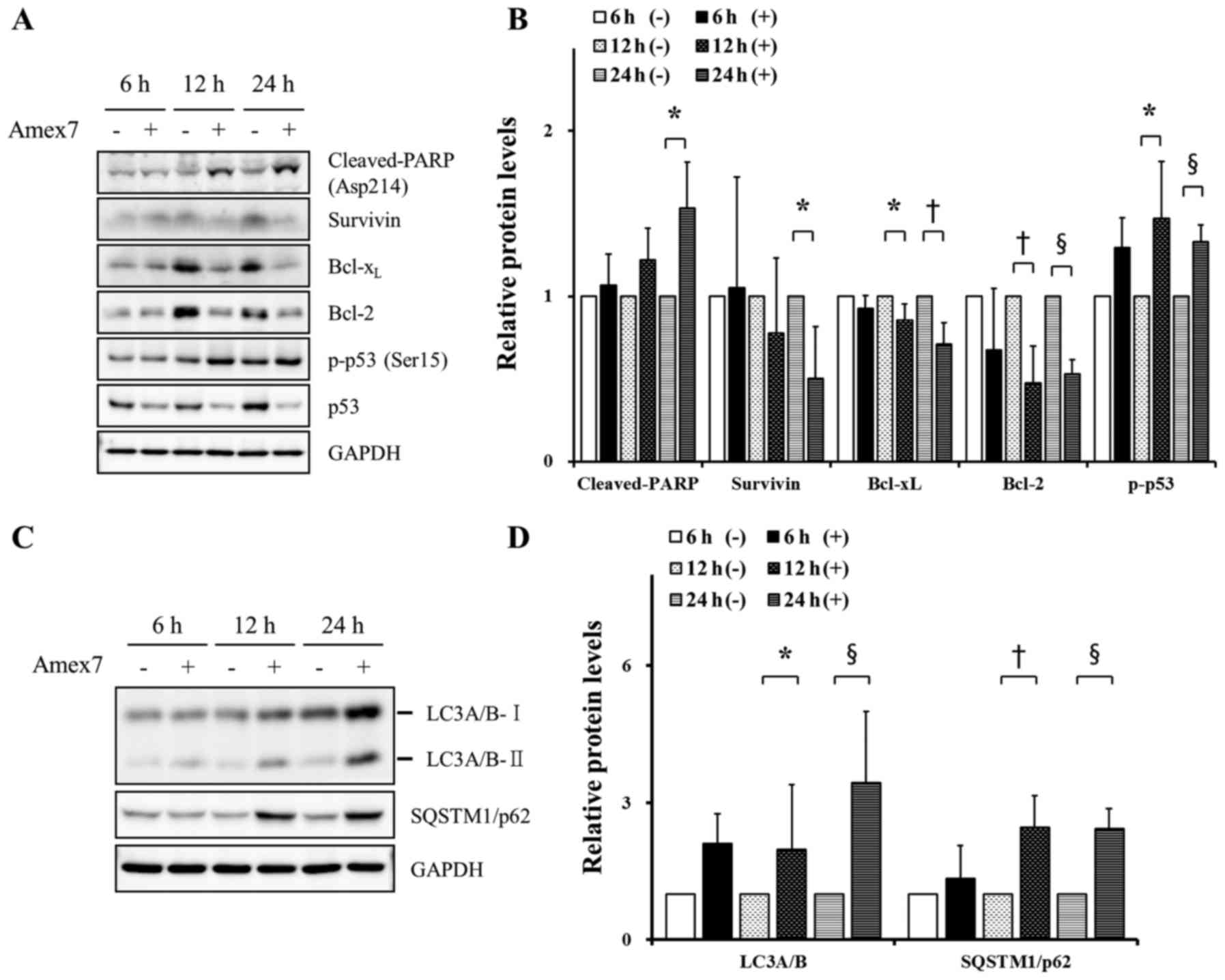 | Figure 5.Amex7 induces apoptosis and autophagy
in HT-29 cells. Cells were treated with 4% Amex7 for 6, 12, and 24
h. To confirm the induction of apoptosis by Amex7, (A) cells from
all groups were immunoblotted with p53, phospho-p53 (Ser15), Bcl-2,
Bcl-xL, survivin, and cleaved-PARP (Asp214) and (B)
protein levels were quantified using Multi Gauge V3.0 software. In
addition, (C) to assess induction of autophagy by Amex7, protein
levels of SQTM1/p62 and LC3A/B-II were measured by immunoblotting
and (D) protein levels were quantified using Multi Gauge V3.0
software. Densitometric quantification was normalized to GAPDH. (−)
indicates the control group and (+) indicates the Amex7-treated
group. Values are mean ± SD of four determinations,
†p<0.01, §p<0.001 vs. each control
group. |
Amex7 enhances anticancer effect by
regulating DNA damage and repair proteins
To investigate the effect of Amex7 on the kinetics
of DNA damage and repair, we examined the immunofluorescent
staining for γ-H2AX: a marker for DNA strand breaks (DSBs) and
stained nuclear DNA with DAPI. The images were analyzed using two
(green/blue) fluorescence channels. At 24 h after treatment of
Amex7, we observed an increased number of γ-H2AX foci compared with
the control group (p<0.05) (Fig. 6A
and B). Next, to evaluate whether Amex7 could affect the DNA
repair pathway, we examined the expression of homologous
recombination (HR) repair proteins and non-homologous end joining
(NHEJ) repair proteins using immunoblotting. As shown in Fig. 6C and D, Amex7 treatment for 12 and
24 h significantly decreased HR repair proteins, including Rad51
and ERCC1, in HT-29 cells (p<0.05). However, Amex7 did not
affect NHEJ repair proteins such as the catalytic subunit of
DNA-PKcs, Ku70, and Ku80. These results showed that Amex7 enhanced
anticancer effects by increasing DNA damage and reducing HR repair
proteins.
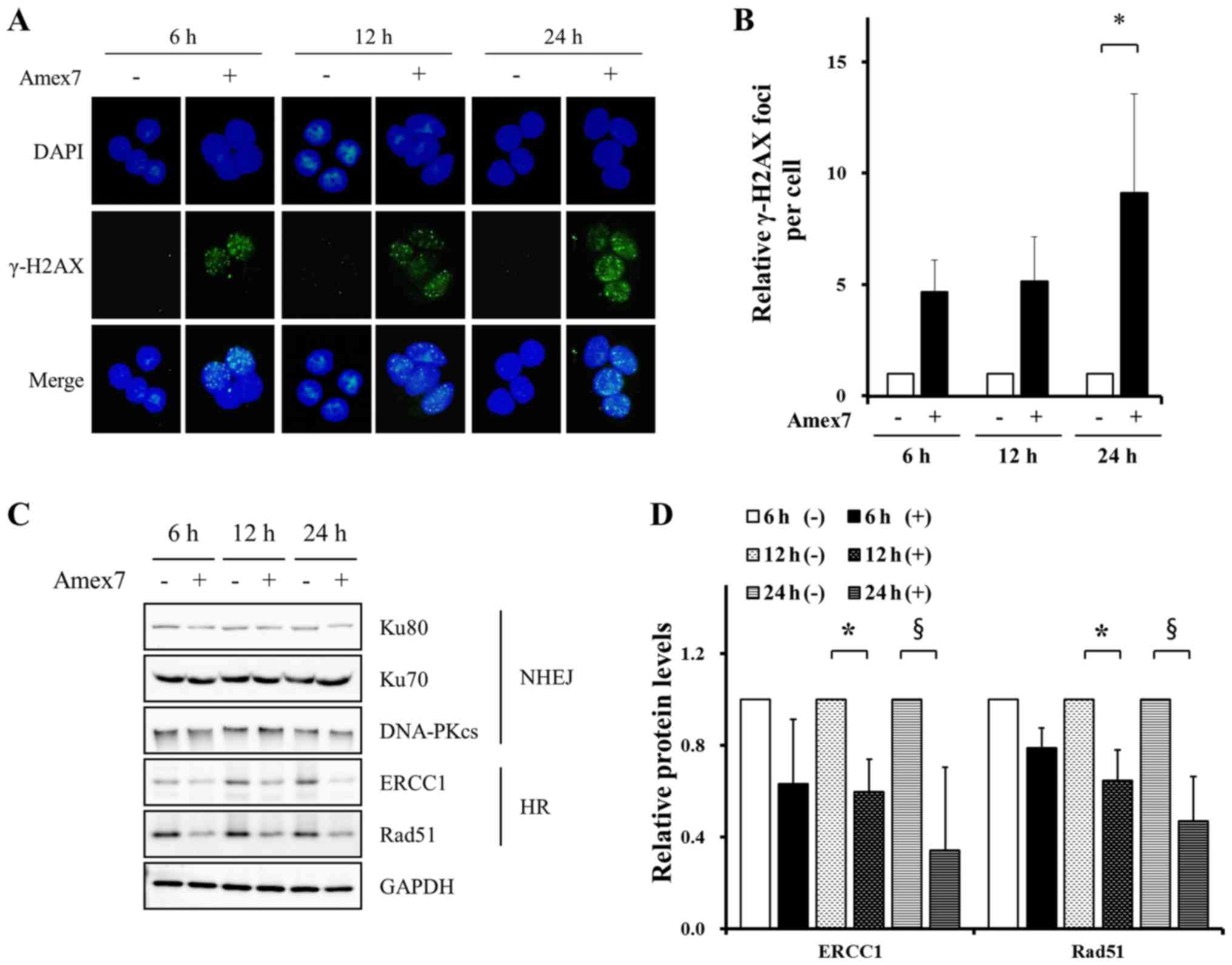 | Figure 6.Amex7 regulates DNA damage and repair
proteins. HT-29 cells were treated with 4% Amex7 for 6, 12, and 24
h. To observe DNA damage by Amex7, immunofluorescence staining was
performed on cells from all groups. (A) Fluorescence
photomicrographs show the merged images of cells stained with
γ-H2AX (green) and counterstained for the nucleus with DAPI (blue).
(B) For quantitative analysis, γ-H2AX foci numbers were counted in
50 cells from randomly captured images. To investigate DNA repair
by Amex7, (C) cells from all groups were immunoblotted with Rad51,
ERCC1, DNA-PKcs, Ku70, and Ku80 and (D) protein levels were
quantified using Multi Gauge V3.0 software. (−) indicates the
control group and (+) indicates the Amex7-treated group. Values are
mean ± SD, *p<0.05, §p<0.001 vs. each control
group. |
Discussion
Among many functional foods, various types of
mushrooms and P. ginseng have been reported to possess
multiple biological effects, including immunomodulatory,
anticancer, anti-oxidative, and anti-inflammatory activity
(19–21). In particular, recent studies have
focused on the anticancer effect of a concentrated substance
extracted from a single medicinal mushroom or P. ginseng;
however, the biological activity of the combination of multiple
medicinal mushrooms and/or P. ginseng extracts is still
unclear. In our study, we investigated whether a combination of
seven medicinal mushrooms and P. ginseng root extracts
(Amex7), when used at lower concentration than in previous studies,
enhanced anticancer effects in human colorectal cancer cells,
focusing on multiple mechanisms for the anticancer effect.
Several studies have reported that medicinal
mushrooms, including C. militaris, P. linteus, and L.
edeodes inhibited tumor growth in various human cancer
xenografts (22–24), while in vivo anticancer
studies of P. ginseng root are inconclusive. Consistent with
previous studies, we confirmed that Amex7, a combined mixture of
medicinal mushrooms and P. ginseng root, markedly suppressed
tumor growth in HT-29 human colorectal cancer xenografts without a
loss of body weight (Fig. 2). Of
note, although the amount of extract of seven mushrooms and P.
ginseng root in Amex7 was much lower than that used in previous
studies, Amex7 significantly inhibited tumor growth in human
colorectal cancer xenografts. Therefore, our results indicated that
the mixture of a low-dose medicinal mushrooms and P. ginseng
root extracts enhanced anticancer effects without complication.
Next, we demonstrated the underlying anticancer
mechanisms of Amex7 in HT-29 cells. Many studies have reported that
the extract of medicinal mushrooms or P. ginseng root
resulted in cytotoxicity via cell cycle arrest and apoptosis in
various human cancer cells. As shown in Fig. 4, Amex7 induced G2/M arrest by
significantly reducing G1/S phase-related proteins (cyclin D1,
cyclin E, CDK2, CDK4, and CDK6) and increasing G2/M phase-related
proteins (cyclin A2 and cyclin B1), which was consistent with
previous studies (23,25). Especially, the present study showed
that Amex7 significantly altered the expression levels of cyclin
D1, cyclin E, CDK4, and cyclin B1 compared with the control cells.
Additionally, Amex7 induced sub-G1 accumulation, which was
suggestive of apoptosis, by reducing anti-apoptotic proteins
(Bcl-2, Bcl-xL, and survivin), increasing apoptotic
protein (cleaved-PARP protein (Asp214), and phosphorylating p53 at
Ser15 (Fig. 5A and B); these
changes were in agreement with previous reports (26,27).
Furthermore, we found that Amex7 induced autophagosome formation,
as evidenced by LC3A/B-II conversion and SQTM1/p62 accumulation
(Fig. 5C and D). Collectively,
these data provided that the mixture of medicinal mushrooms and
P. ginseng root extracts induced G2/M arrest, apoptosis, and
autophagy in human colorectal cancer cells.
An interesting observation in our study concerned
the effect of Amex7 on the kinetics of DNA damage and repair.
Starting early time after Amex7 treatment, Amex7 consistently
induced DNA damage (Fig. 6A and B)
and also suppressed the expression of DNA repair proteins,
including Rad51 and ERCC1, which play a key role in the DNA HR
repair pathway (Fig. 6C and D).
Therefore, these results suggested that Amex7 enhances cytotoxicity
in human colorectal cancer cells by inducing DNA damage and
inhibiting DNA repair.
In conclusion, the combined mixture of mushrooms and
P. ginseng root extracts inhibited tumor growth, increased
cytotoxicity via induction of G2/M arrest, and induced apoptosis
and autophagy. Moreover, it enhanced anticancer effect by impairing
DNA damage repair. Despite the low-dose of extracts, the mixture
enhanced the anticancer effect in human colorectal cancer cells.
Accordingly, we suggested a scientific rationale for the clinical
application of the mixture of medicinal mushrooms and P.
ginseng root extracts as an adjuvant compound in treatment of
human colorectal cancer that presented no safety concerns.
Acknowledgements
This study was supported by a grant of the Korea
Institute of Radiological and Medical Sciences (KIRAMS), funded by
Ministry of Science, ICT and Future Planning, Republic of Korea
(no. 1711045548).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim R: Treatment of Colorectal
CancerCleveland Clinic Foundation. Lyndhurst, OH: 2010
|
|
3
|
Safdie FM, Dorff T, Quinn D, Fontana L,
Wei M, Lee C, Cohen P and Longo VD: Fasting and cancer treatment in
humans: A case series report. Aging (Albany NY). 1:988–1007. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szakács G, Váradi A, Ozvegy-Laczka C and
Sarkadi B: The role of ABC transporters in drug absorption,
distribution, metabolism, excretion and toxicity (ADME-Tox). Drug
Discov Today. 13:379–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu CP, Ohnuma S and Ambudkar SV:
Discovering natural product modulators to overcome multidrug
resistance in cancer chemotherapy. Curr Pharm Biotechnol.
12:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loe DW, Deeley RG and Cole SP: Biology of
the multidrug resistance-associated protein, MRP. Eur J Cancer.
32A:945–957. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penson RT, Oliva E, Skates SJ, Glyptis T,
Fuller AF Jr, Goodman A and Seiden MV: Expression of multidrug
resistance-1 protein inversely correlates with paclitaxel response
and survival in ovarian cancer patients: A study in serial samples.
Gynecol Oncol. 93:98–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belcaro G, Hosoi M, Pellegrini L,
Appendino G, Ippolito E, Ricci A, Ledda A, Dugall M, Cesarone MR,
Maione C, et al: A controlled study of a lecithinized delivery
system of curcumin (Meriva®) to alleviate the adverse
effects of cancer treatment. Phytother Res. 28:444–450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang HP, Lee H, Oh TG, Lee KJ, Park SJ,
Chung MJ, Kim SU, Lee H, Park JC, Hong SP, et al: The use of health
functional foods in gastrointestinal cancer patients. Clin Nutr
Res. 2:19–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ballali S and Lanciai F: Functional food
and diabetes: A natural way in diabetes prevention? Int J Food Sci
Nutr. 63:(Suppl 1). 51–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alissa EM and Ferns GA: Functional foods
and nutraceuticals in the primary prevention of cardiovascular
diseases. J Nutr Metab. 2012:5694862012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sliva D: Medicinal mushroom Phellinus
linteus as an alternative cancer therapy. Exp Ther Med.
1:407–411. 2010.PubMed/NCBI
|
|
13
|
Lee HJ, Lee HJ, Lim ES, Ahn KS, Shim BS,
Kim HM, Gong SJ, Kim DK and Kim SH: Cambodian Phellinus
linteus inhibits experimental metastasis of melanoma cells in
mice via regulation of urokinase type plasminogen activator. Biol
Pharm Bull. 28:27–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HS, Kim EJ and Kim SH: Ethanol extract
of Inonotus obliquus (Chaga mushroom) induces G1 cell cycle
arrest in HT-29 human colon cancer cells. Nutr Res Pract.
9:111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guggenheim AG, Wright KM and Zwickey HL:
Immune modulation from five major mushrooms: Application to
integrative oncology. Integr Med (Encinitas). 13:32–44.
2014.PubMed/NCBI
|
|
16
|
Park JG, Kang WS, Park KT, Park DJ,
Aravinthan A, Kim JH and Cho JY: Anticancer effect of joboksansam,
Korean wild ginseng germinated from bird feces. J Ginseng Res.
40:304–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Yang WS, Yu T, Sung GH, Park KW,
Yoon K, Son YJ, Hwang H, Kwak YS, Lee CM, et al:
ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red
ginseng water extract. J Ethnopharmacol. 154:218–228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CH and Kim JH: A review on the
medicinal potentials of ginseng and ginsenosides on cardiovascular
diseases. J Ginseng Res. 38:161–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elbatrawy EN, Ghonimy EA, Alassar MM and
Wu FS: Medicinal mushroom extracts possess differential antioxidant
activity and cytotoxicity to cancer cells. Int J Med Mushrooms.
17:471–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin JY, Song JY, Yun YS, Yang HO, Rhee DK
and Pyo S: Immunostimulating effects of acidic polysaccharides
extract of Panax ginseng on macrophage function.
Immunopharmacol Immunotoxicol. 24:469–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wasser SP and Weis AL: Therapeutic effects
of substances occurring in higher Basidiomycetes mushrooms: A
modern perspective. Crit Rev Immunol. 19:65–96. 1999.PubMed/NCBI
|
|
22
|
Lee HH, Lee S, Lee K, Shin YS, Kang H and
Cho H: Anti-cancer effect of Cordyceps militaris in human
colorectal carcinoma RKO cells via cell cycle arrest and
mitochondrial apoptosis. Daru. 23:352015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song KS, Li G, Kim JS, Jing K, Kim TD, Kim
JP, Seo SB, Yoo JK, Park HD, Hwang BD, et al: Protein-bound
polysaccharide from Phellinus linteus inhibits tumor growth,
invasion, and angiogenesis and alters Wnt/β-catenin in SW480 human
colon cancer cells. BMC Cancer. 11:3072011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SE, Kim J, Lee YW, Yoo HS and Cho CK:
Antitumor activity of water extracts from Cordyceps
militaris in NCI-H460 cell xenografted nude mice. J Acupunct
Meridian Stud. 2:294–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shangguan WJ, Li H and Zhang YH: Induction
of G2/M phase cell cycle arrest and apoptosis by ginsenoside Rf in
human osteosarcoma MG-63 cells through the mitochondrial pathway.
Oncol Rep. 31:305–313. 2014.PubMed/NCBI
|
|
26
|
Youn MJ, Kim JK, Park SY, Kim Y, Kim SJ,
Lee JS, Chai KY, Kim HJ, Cui MX, So HS, et al: Chaga mushroom
(Inonotus obliquus) induces G0/G1 arrest and apoptosis in
human hepatoma HepG2 cells. World J Gastroenterol. 14:511–517.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Tian ZN, Cai JP, Chen KX, Zhang B,
Feng MY, Shi QT, Li R, Qin Y and Geng JS: Panax ginseng
polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1
pathway in human gastric cancer. Carbohydr Polym. 102:103–109.
2014. View Article : Google Scholar : PubMed/NCBI
|