Introduction
Malignant melanoma (MM) is a type of highly
malignant tumor that primarily develops in adults over the age of
30 years, and is associated with a high rate of mortality (1). The morbidity rate of MM, which has
gradually increased over recent years, particularly in younger
generations, accounts for ~2% of all malignant tumors, and ranks
third among skin malignancies (6–20% of skin malignancies)
(2). MM can occur on any region of
the skin, with skin regions exposed to the highest levels of
friction at the extremities being the most vulnerable, and is
characterized by early metastasis, aggressive invasion and poor
prognosis (3). Current treatment
strategies for MM typically involve surgery followed by biotherapy,
radiotherapy and/or chemotherapy. However, these methods have to
date yielded unsatisfactory clinical effects on the prognosis and
mortality rate of patients with MM (4).
Due to the development of molecular biology
techniques over recent years, research into the genetics and
molecular biology of MM, including its genesis, development and
metastasis, has made great progress, which may aid in improving
clinical treatment. However, there has been little research into
the effects and underlying mechanisms of microRNAs (miRNAs/miRs) in
MM (5,6).
miRNAs are types of small non-coding RNAs of 18–25
nucleotides in length that have recently been associated with MM
(6). miRNAs serve important
regulatory roles in the normal physiological functioning of the
body by regulation of target gene expression, which can influence
protein expression. Notably, under certain conditions, increased or
decreased expression of specific target proteins can result in cell
functional changes and tumorigenesis (7). The expression profiles of miRNAs
greatly differ between tumor cells and normal tissue cells
(8). Increased, decreased or
depleted expression levels of certain miRNAs can be observed in the
majority of tumor cells of various types, which is an important
mechanism underlying changes in the proliferation and growth of
tumor cells (9). Therefore, in the
present study miR-138 was selected as an miRNA detected in MM, and
its putative target genes and function were predicted. Preliminary
investigations into the molecular mechanisms of miR-138 in the
proliferation and growth of MM were also conducted.
Autophagy (from the Greek for ‘self-eating’) is a
cell phenomenon in which the intracellular lysosome engulfs and
degrades damaged or redundant organelles and mutant proteins
(10). The autophagy-related genes
are critical factors that regulate the process of autophagy
(10). The primary functions of
autophagy are to eliminate biological waste materials within the
body and to promote metabolism by degradation of damaged organelles
under stress conditions, such as hunger, which aids in the
self-protection of cells and promotes cell survival (11). However, excessive autophagy
decreases cell survival by promoting autophagic cell death
(11).
Numerous signal transduction pathways are associated
with autophagy, including PI3K/AKT/mTOR, Ras/PKA and Jnk (12). Each pathway promotes or inhibits the
process of autophagy through various mechanisms (13). At present, the autophagy-associated
signaling pathway PI3K/AKT/mTOR has been the most widely studied
(13). Cell autophagy is regulated
by mTOR, and is mainly activated by inhibition of the PTEN protein.
The PI3K/AKT/mTOR signal transduction pathway can act against the
process of cell autophagy, which disrupts cellular homeostasis and
proliferation; however, PI3K/AKT/mTOR signaling may also increase
the survival of tumorigenic cells by preventing autophagic
programmed cell death, which facilitates tumor metastasis (14).
Phosphoinositide-dependent kinase l (PDK1) is a type
of serine/threonine protein kinase with a molecular weight of 63
kDa, and contains a PH domain that binds with the inositol
triphosphate (PIP3) product of PI3K, which enables PDK1 to target
the cell membrane and activate AKT. Meanwhile, other substrates of
PDK1, with an inability to target the cell membrane or lack of a PH
domain, can also directly bind with PDK1 to become activated
(15). PDK1 itself can be activated
by bivalent polymerization and cross phosphorylation (16). PDK1 serves as an upstream kinase of
AKT, and catalyzes the phosphorylation of Thr308 within the
activation loop of AKT, stimulating a >30-fold increase in AKT
activity. Such activation is PIP3- or PIP2-dependent, thus giving
rise to the name PDK1 (17). As a
common link between multiple important signaling pathways in cells,
PDK1 is widely expressed in many human tissues, and plays a
critical role in a number of tumor processes, including tumor cell
growth, protection against cell apoptosis, stimulation of the
epithelial-mesenchymal transition (EMT) and tumor angiogenesis
(17). The present study, aimed to
elucidate the clinical significance of miR-138 in patients with MM,
as well as its underlying mechanisms in MM cells.
Materials and methods
Patients and blood samples
The present study was approved by the Ethics
Committee of Shangqiu First People's Hospital (Shangqiu, China),
and written informed consent was obtained from all the patients.
Whole blood samples from 5 patients with MM and 6 healthy
volunteers were collected from the Department of Dermatology,
Shangqiu First People's Hospital. Serum was isolated after
centrifugation at 2,000 × g for 10 min at room temperature. A
follow-up of all patients was conducted every 3 months by telephone
or reexamination at the clinic, and the overall survival (OS) and
disease-free survival (DFS) rates of the MM patients with high and
low miR-138 expression were subsequently determined.
Cell lines and culture
The human MM cell line A2058 was purchased from the
Cell Bank of Central South University (Shanghai, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C and 5% CO2.
RNA isolation and RT-qPCR
Total RNA was extracted from serum and cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.). cDNA was
synthesized from total RNA using a MiRNA Reverse Transcription kit
(Qiagen, Inc., Valencia, CA, USA) under the following conditions:
16°C for 30 min, followed by 42°C for 30 min and enzyme
inactivation at 85°C for 5 min. The expression of miR-138 was
subsequently assessed using a 7500 Fast Real Time PCR System
(Thermo Fisher Scientific, Inc.). The PCR cycling conditions were
95°C for 5 min, followed by 40 cycles of denaturation at 95°C for
30 sec and 60°C for 45 sec. The relative expression of miRNA was
determined using the 2−∆∆Cq method.
Transfection
miR-138 mimics and negative control miRNAs were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). A2058
cells were transfected with 100 ng of miR-138 mimics or negative
control miRNA using Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.), in accordance with the manufacturer's instructions. At 6 h
after transfection, a subgroup of cells was treated with 100 nM of
LY294002 (Selleck Chemicals, Shanghai, China) for 48 h to inhibit
PI3K.
Cell proliferation and apoptosis
assays
At 48 h after transfection, cells were plated into a
96-well plate (10,000 cells/well), and 20 µl of MTT reagent (5
mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well
and incubated for 4 h. The supernatant was removed and 100 µl
dimethyl sulfoxide DMSO (Beyotime Institute of Biotechnology,
Haimen, China) was added and incubated for 20 min. The absorbance
was detected using an ELx800 absorbance microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 492 nm.
In addition, at 48 h after transfection, the cells
were plated into a 6-well plate (1×106 cells/well) and
washed 3 times with phosphate-buffered saline (PBS). Cells were
then stained using an Annexin V-FITC/PI kit (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). The rates of apoptosis were assessed
using a flow cytometer (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blot analysis and caspase-3
activity assay
At 48 h after transfection, cells were lysed in cold
RIPA buffer and protein concentration was determined using a BCA
protein assay kit (both from Beyotime Institute of Biotechnology).
A total of 30–60 µg of protein was then separated using 8–10%
SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF)
membrane (Thermo Fisher Scientific, Inc.). The PVDF membrane was
blocked in 5% non-fat dried milk in TBST (Thermo Fisher Scientific,
Inc.) for 1–2 h, and then incubated with primary antibodies against
LC3, Bax, p62, PI3K, p-AKT, p-mTOR, p-PDK1 and GAPDH overnight at
4°C. After 3 washes with PBS, the PVDF membrane was incubated with
an anti-rabbit secondary antibody for 1 h at room temperature.
Immunolabelled bands were detected using an ECL Western Blotting
kit (Beyotime Institute of Biotechnology) and analyzed using
Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
In addition, a total of 5 µg of protein was used to
assess caspase-3 activity using a caspase-3 activity kit (Beyotime
Institute of Biotechnology), and the absorbance was assessed using
an ELx800 absorbance microplate reader at 405 nm.
Fluorescence microscopy
At 48 h after transfection, A2058 cells
(1×103 cells/ml) were seeded onto cell chamber slides
and washed 3 times with PBS. The cells were then fixed with 4%
formaldehyde at 4°C for 30 min and stained with anti-LC3 (1:200;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
The cells were then incubated with an anti-rabbit secondary
antibody (1:1,000; Bio-Rad Laboratories, Inc.) at room temperature
for 3 h, and subsequently stained with DAPI at room temperature for
30 min. The expression of the LC3 protein was visualized by
fluorescence microscopy using an AxioScope A1 Microscope (Zeiss
GmbH, Jena, Germany).
Statistical analysis
All data are expressed as the mean ± SD. Statistical
analysis of differences was performed using one-way analysis of
variance (ANOVA) and P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-138 expression in MM patients and
cells
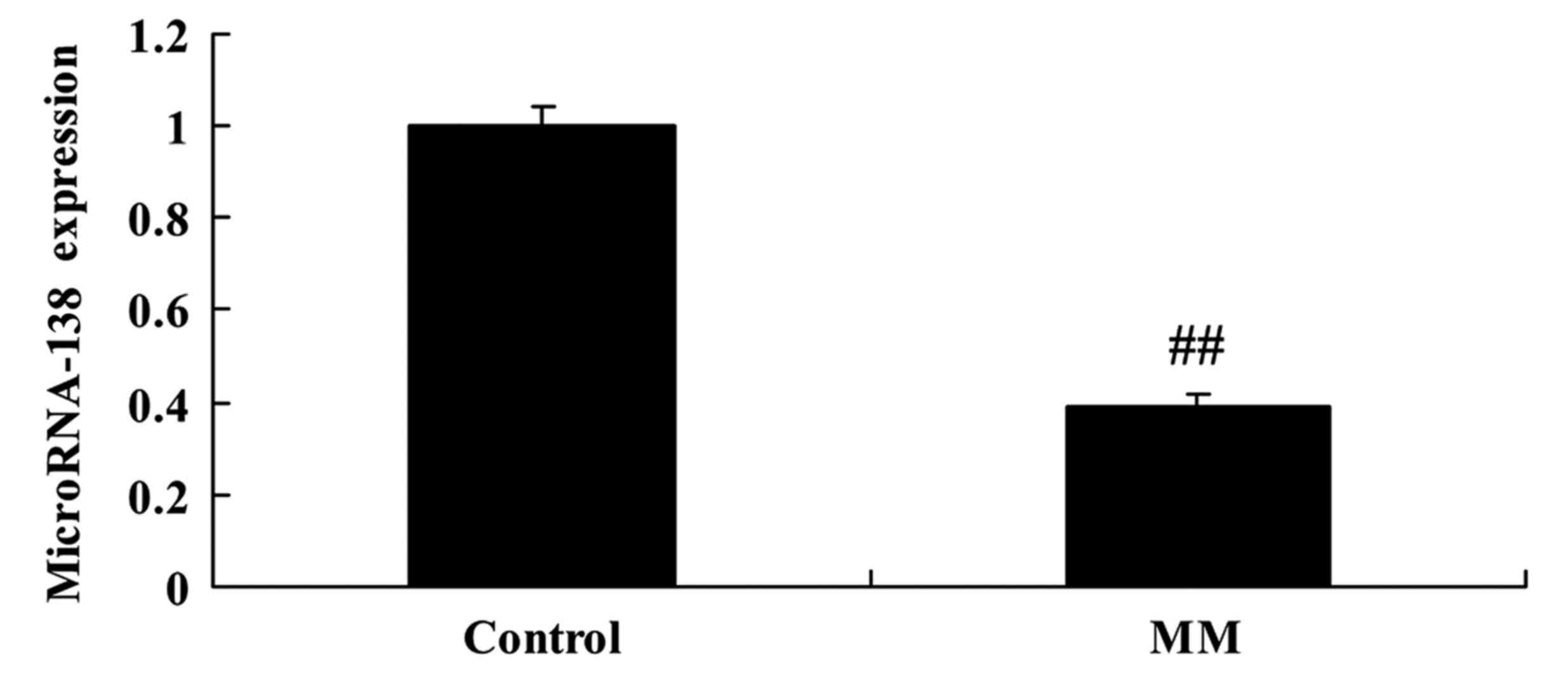
Firstly, we analyzed the expression of miR-138 in
patients with MM using RT-qPCR. In patients with MM, we found that
miR-138 expression was significantly downregulated when compared
with healthy control subjects (Fig.
1).
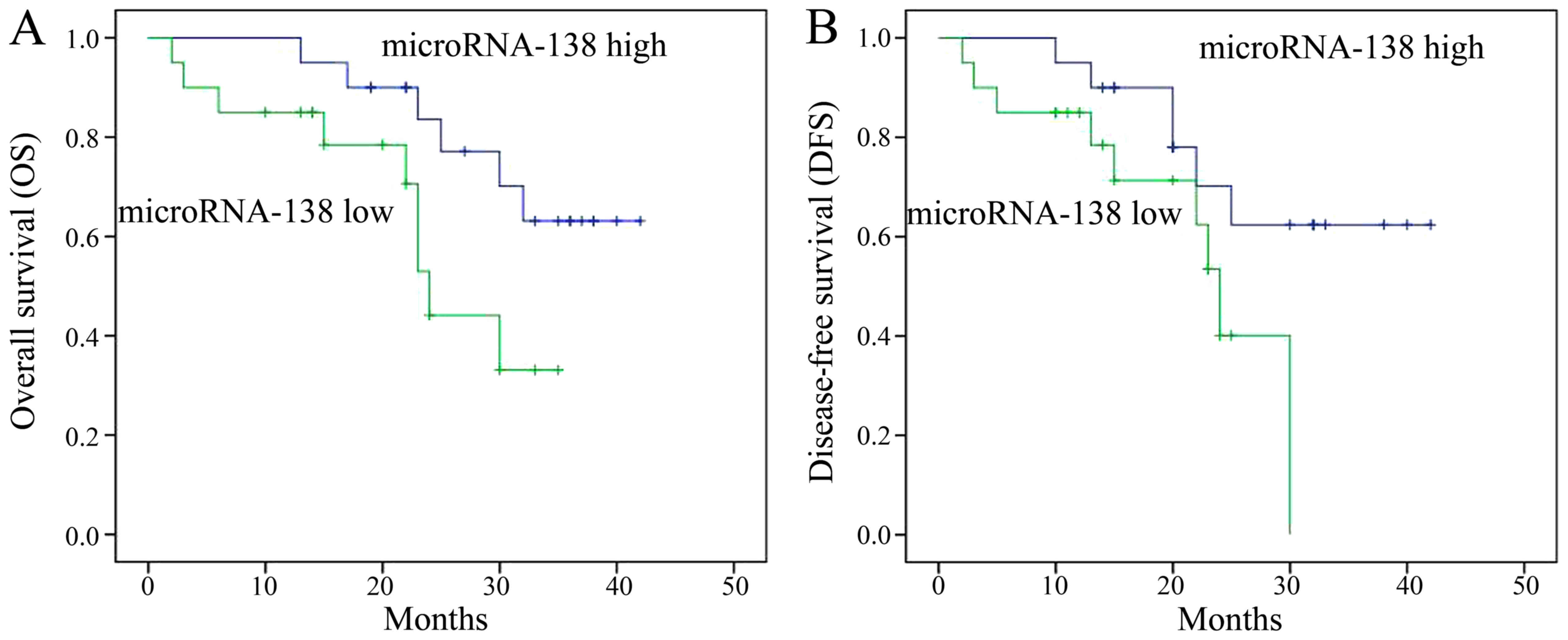
OS and DFS rates are associated with
miR-138 expression
Next, the OS and DFS rates of MM patients with high
and low miR-138 expression were determined. The OS and DFS rates of
MM patients with high miR-138 expression were markedly lower than
those of patients with low miR-138 expression (Fig. 2).
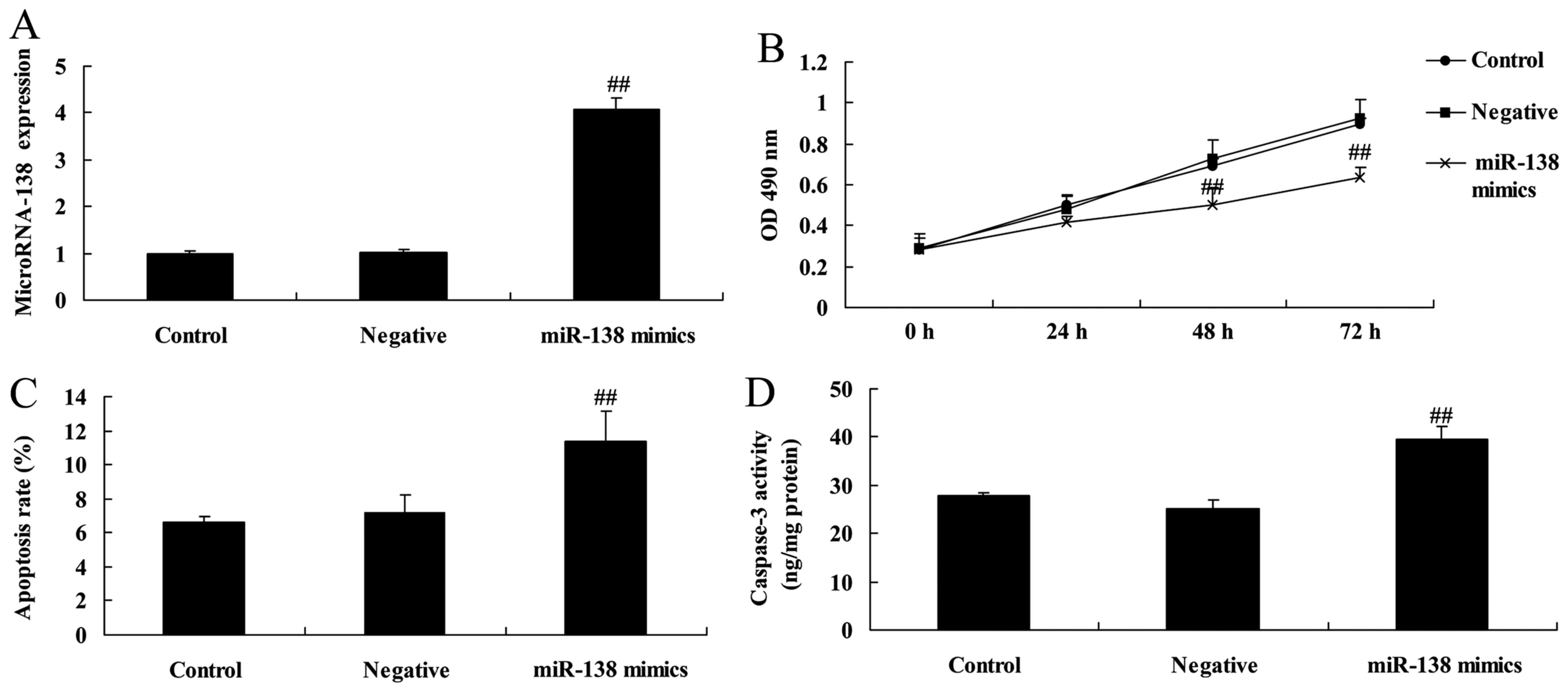
miR-138 overexpression inhibits
proliferation and induces apoptosis in A2058 cells
We investigated the effects of miR-138 on the growth
and apoptosis of MM cells. As shown in Fig. 3A, miR-138 mimics were transfected
into A2058 cells, which increased the expression of miR-138 in
A2058 cells when compared with cells transfected with negative
control mimics. Overexpression of miR-138 significantly inhibited
proliferation, induced apoptosis and increased caspase-3 activity
in A2058 cells when compared with the negative control group
(Fig. 3B-D).
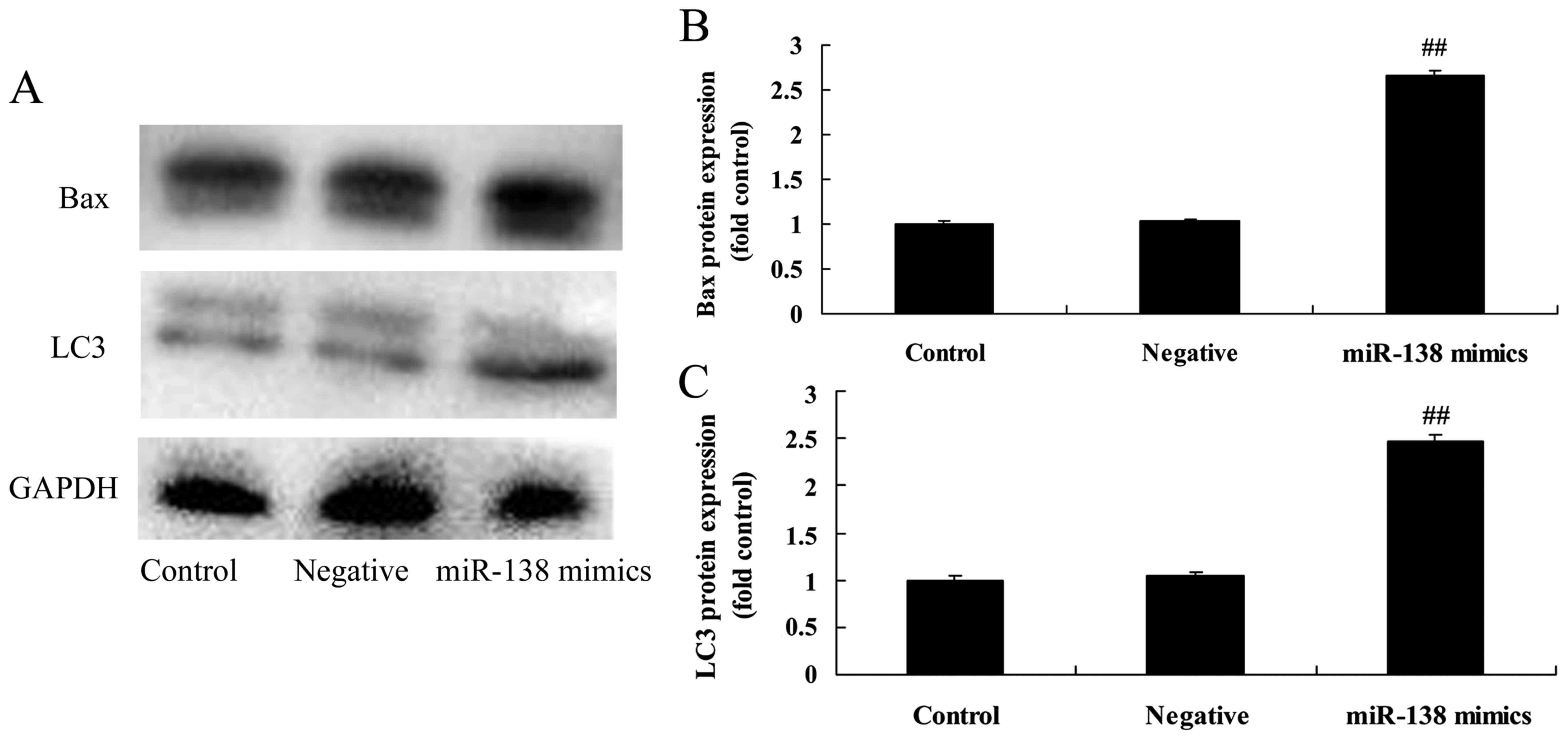
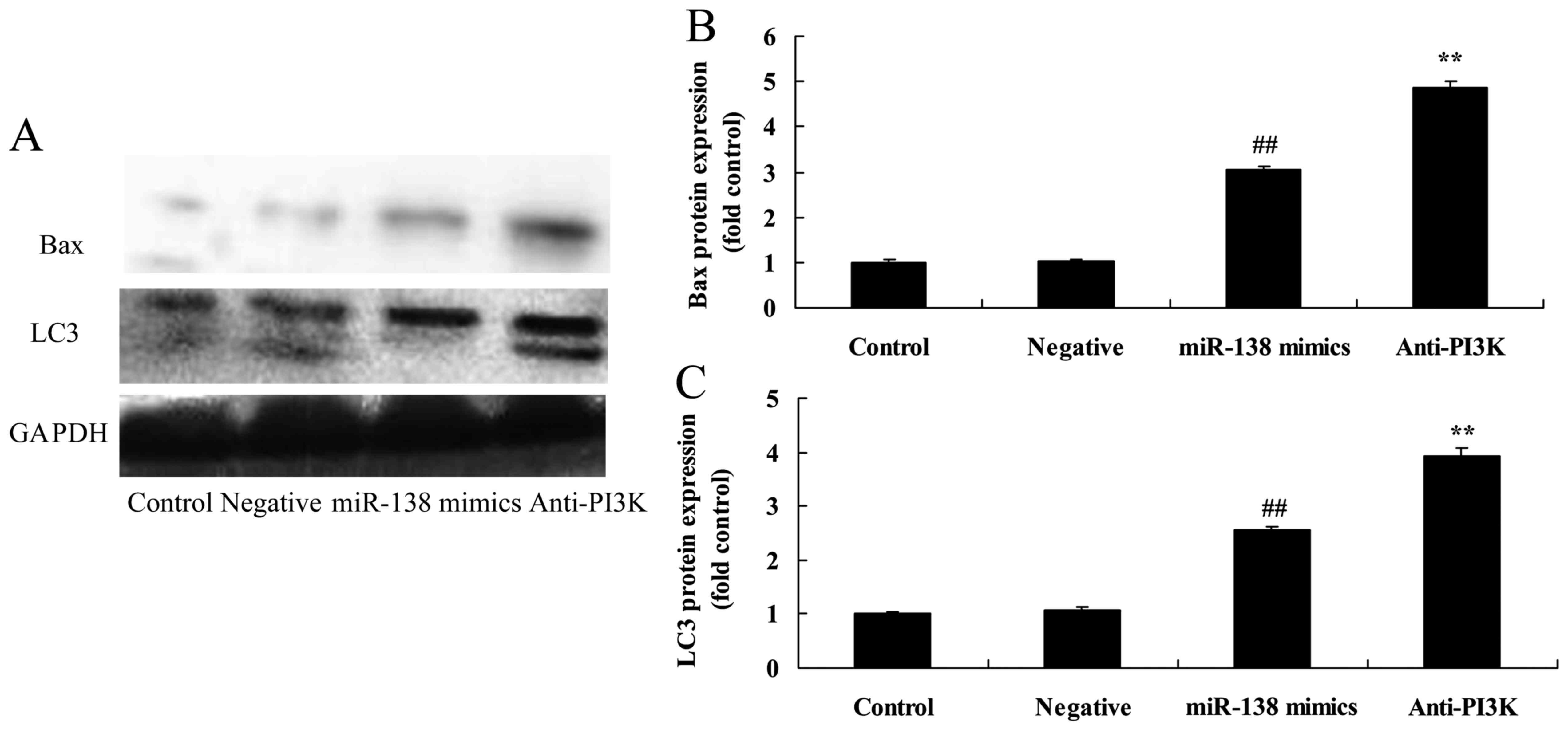
miR-138 overexpression induces LC3 and
Bax protein expression in A2058 cells
Subsequently, western blot analysis was performed to
evaluate the protein expression of LC3 and Bax. As shown in
Fig. 4, overexpression of miR-138
significantly induced LC3 and Bax protein expression in A2058 cells
compared with the negative control group.
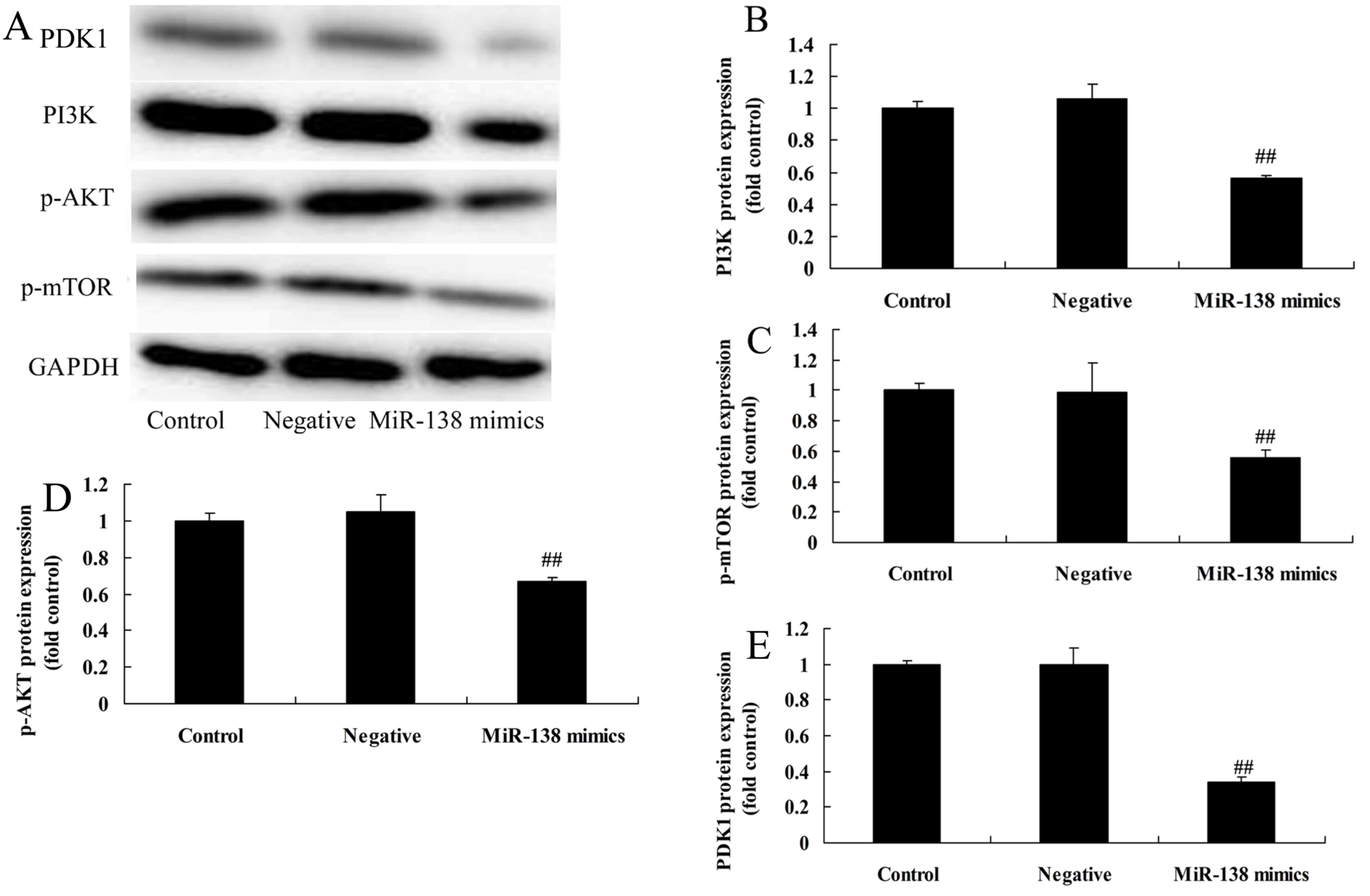
miR-138 overexpression suppresses
PI3K/PDK1/AKT/mTOR signaling in A2058 cells
To investigate the relationship between miR-138 and
the PI3K/PDK1/AKT/mTOR signaling pathway in MM, the effect of
miR-138 on the activation of PI3K/AKT/mTOR signaling in A2058 cells
was investigated by western blot analysis. Fig. 5 shows that miR-138 overexpression
significantly suppressed PI3K/AKT/mTOR signaling in A2058 cells
when compared with the negative control group.
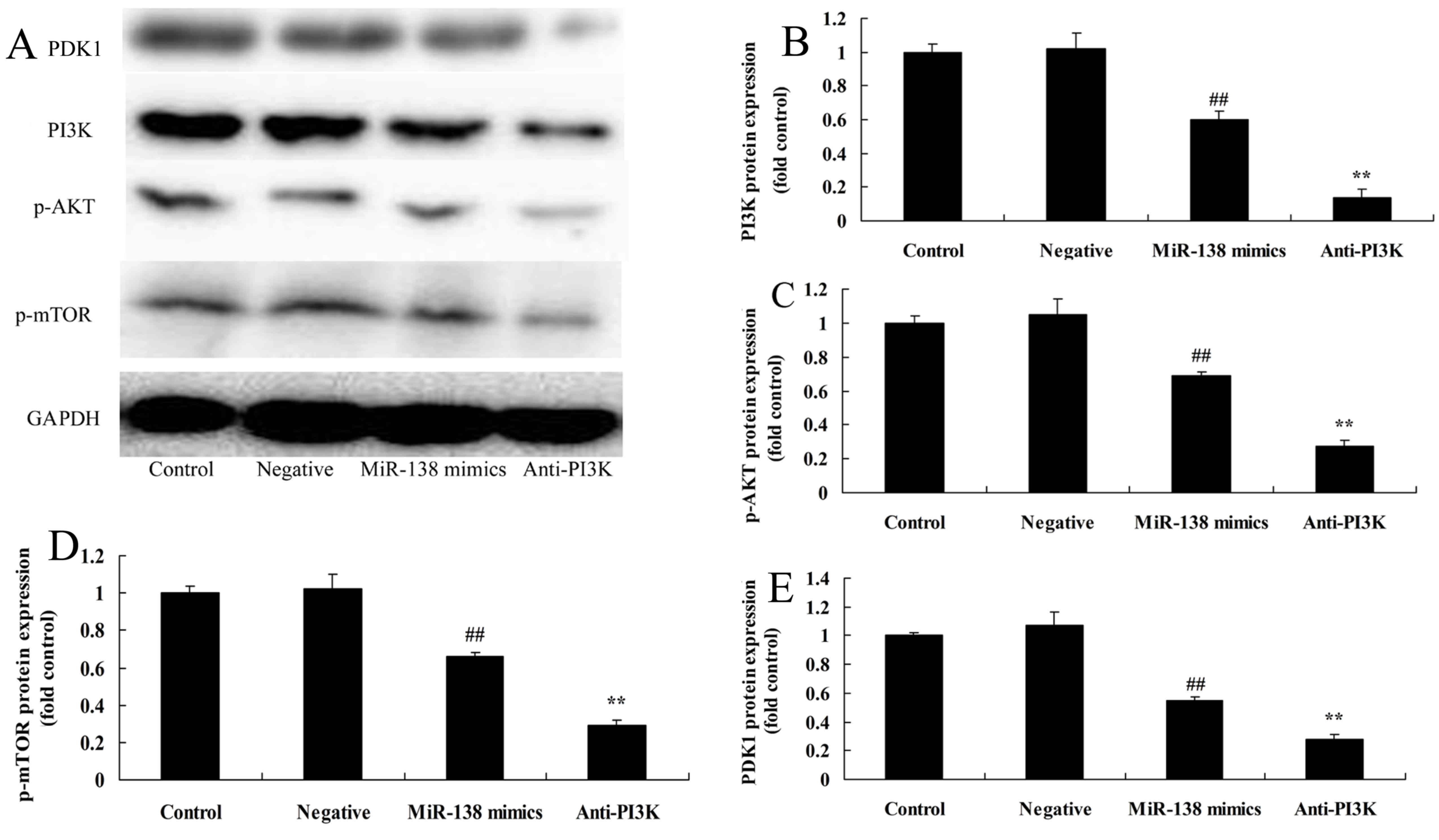
PI3K suppression enhances the effects
of miR-138 overexpression on PI3K/PDK1/AKT/mTOR signaling
The functions of miR-138 and the PI3K/PDK1/AKT/mTOR
signaling pathway in the regulation of A2058 cells were
investigated. As shown in Fig. 6,
the PI3K inhibitor LY294002 enhanced the inhibitory effects of
miR-138 overexpression on PI3K/AKT/mTOR signaling in A2058 cells,
relative to cells with only miR-138 overexpression.
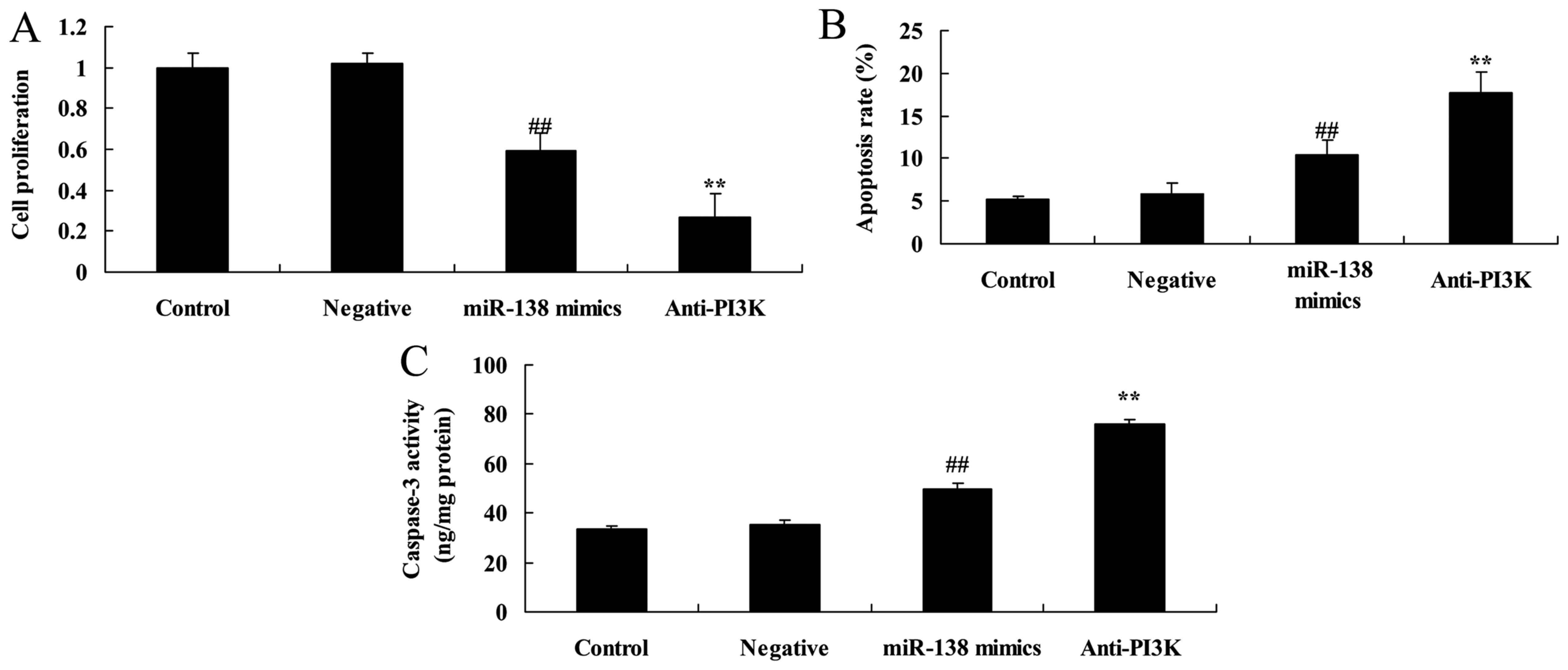
PI3K suppression enhances the effects
of miR-138 overexpression on the proliferation and apoptosis of
A2058 cells
We then evaluated in vitro whether PI3K was
involved in the effects of miR-138 on the proliferation and
apoptosis of A2058 cells. The data in Fig. 7 indicated that the PI3K inhibitor
significantly decreased cell proliferation while promoting
apoptosis and caspase-3 activity in A2058 cells following miR-138
overexpression, relative to the miR-138 overexpression-only cell
group.
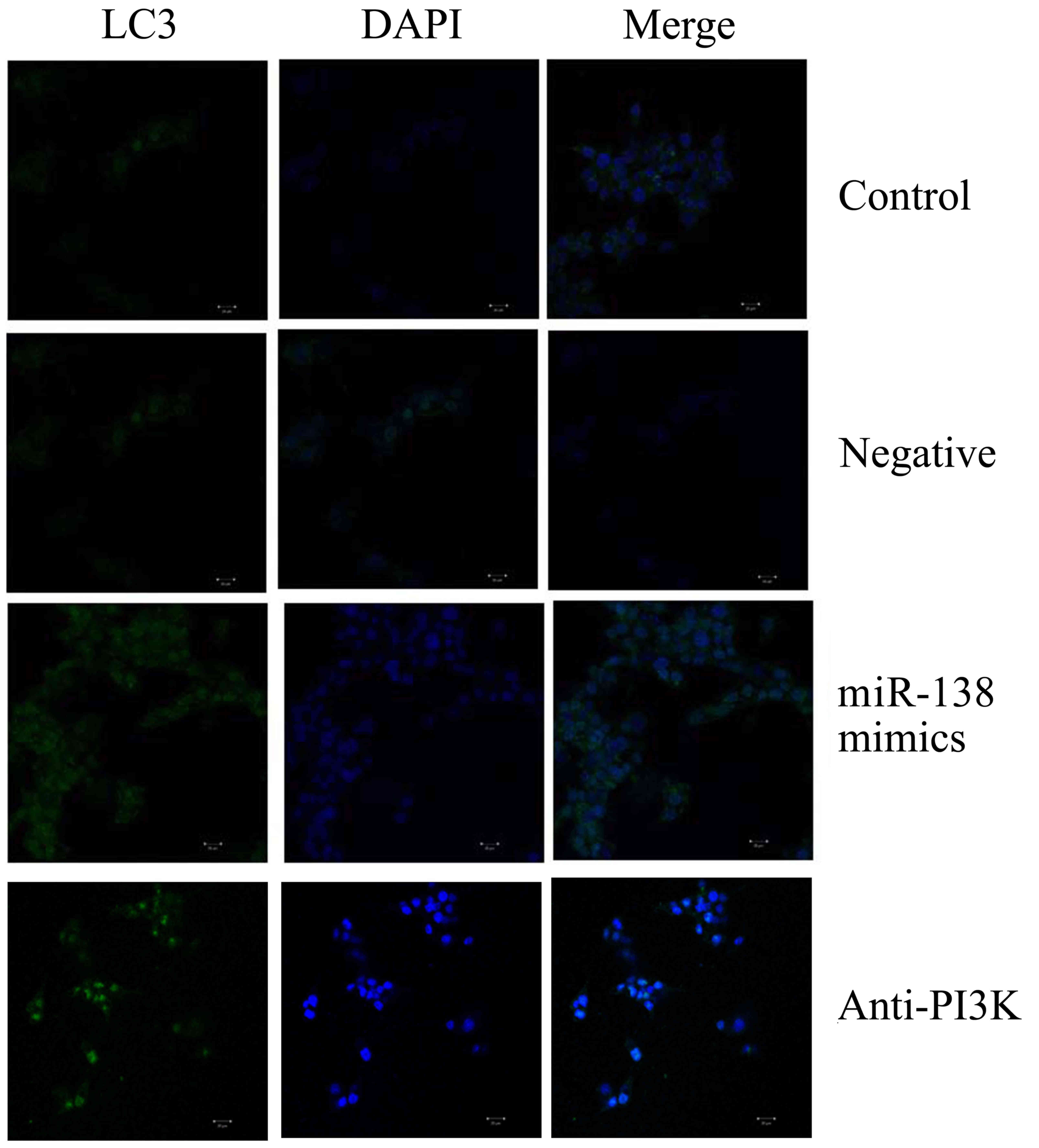
PI3K suppression enhances the effects
of miR-138 overexpression on LC3 and Bax protein expression in
A2058 cells
Furthermore, we determined whether PI3K was involved
in the effects of miR-138 on the protein expression of LC3 and Bax
in A2058 cells. We used laser confocal scanning microscopy to
analyze LC3 expression, and observed that PI3K suppression
significantly promoted LC3 protein expression in A2058 cells
following miR-138 overexpression, relative to the miR-138
overexpression-only cell group (Fig.
8). Similarly, western blot analysis indicated that PI3K
suppression significantly increased LC3 and Bax protein expression
in A2058 cells following miR-138 overexpression, relative to the
miR-138 overexpression-only cell group (Fig. 9).
Discussion
MM is a type of highly malignant tumor that
predominantly occurs in adults over the age of 30 years, and is
associated with a high rate of mortality (18). The morbidity rate of MM, which has
gradually increased over recent years, particularly in younger
generations, accounts for ~2% of all malignant tumors, and ranks
third among skin malignancies (6–20% of skin malignancies)
(19). MM can occur on any region
of the skin, with skin regions exposed to friction at the
extremities being most vulnerable, and is characterized by early
metastasis, aggressive invasion and poor prognosis (20). Current treatment strategies for MM
typically involve surgery followed by biotherapy, radiotherapy
and/or chemotherapy, although these methods have to date yielded
unsatisfactory clinical effects on the prognosis and mortality rate
of patients (21). In the present
study the results revealed that miR-138 expression was
significantly downregulated in patients with MM and the MM cell
line A2058 when compared with normal controls. Furthermore, the OS
and DFS rates of MM patients with high miR-138 expression were
observably lower than those of patients with low miR-138
expression.
Cell autophagy can not only eliminate the metabolic
products of cells and abnormal intracellular proteins, but can also
degrade redundant or damaged organelles (11). Autophagy can be induced during early
newborn development, hypoxia, infection and hunger, and its
degradation products can provide materials and energy for cells,
thus maintaining cell homeostasis (22). However, mutant, damaged or aging
cells can undergo programmed cell death as a result of autophagy
(10). The present results revealed
that miR-138 overexpression significantly inhibited cell
proliferation, induced apoptosis, increased caspase-3 activity and
induced LC3 and Bax protein expression in A2058 cells. Stojcheva
et al (23) revealed that
miR-138 induced acquired alkylator resistance by the induction of
autophagy.
The PI3K/AKT signal transduction pathway is
principally regulated by the tumor suppressor genes PTEN and SHIP2.
PTEN and SHIP2 dephosphorylate PI(3,4,5)-P3 at
the 3′ and 5′ positions, respectively, and as a result, PI(3,4,5)-P3
becomes PI(3,4,5)-P2,
leading to inactivation of AKT (13). The tumor suppressor proteins (PTEN
and SHIP2), the proline-rich Akt substrate of 40 kDa (PRAS40), and
the tuberous sclerosis complex proteins (TSC1/2) are all negative
regulators of mTOR (24). The
phosphorylation of AKT can decrease this negative regulation, thus
verifying that AKT aids in suppressing the negative regulatory
factors of mTOR (25). However,
more than one signaling pathway (other than PI3K/AKT) is
responsible for the regulation of mTOR (26). Hunger and stress responses can
result in the dephosphorylation of the autophagy-related protein
Beclin-1, which inactivates mTOR and induces autophagy (26). AKT can promote the activation of
mTOR as an upstream effector molecule of mTORC1. In the present
study, miR-138 overexpression significantly suppressed the
PI3K/AKT/mTOR signaling pathway in A2058 cells.
As an upstream gene of AKT, PDK1 regulates the
activities of AKT and other downstream kinases of PI3K/AKT, which
enables PDK1 to regulate a series of biological responses in cells
by the PI3K/AKT signaling pathway (27). PDK1 is a serine/threonine kinase of
the AGC protein kinase family. As an important transduction
molecule of the PI3K signaling pathway, the binding of PDK1 with
PIP3 plays an important role in physiological processes, including
the activation of kinases (such as AKT), the control of cell
responses by regulating a large number of AGC protein kinase family
members, and cell metabolism, growth, proliferation and survival
(28). Research on PDK1 in recent
years has demonstrated the following: firstly, as a type of
regulatory kinase, PDK1 can activate 23 types of downstream
kinases; secondly, PDK1 can continuously phosphorylate substrates
due to its constant self-activation, which stimulates
conformational changes in the substrate to expose anchor points and
increase binding with PDK1, and this is an important mechanism by
which PDK1 controls a large number of AGC kinase activities;
thirdly, PDK1 is a type of single-copy sequence gene that is
applicable to genetic analysis, and research progress has been made
by use of the transgenic mouse model; and fourthly, inhibitors of
PDK1 may be useful in the treatment of diseases such as diabetes
and cancer, due to its key roles in numerous signal transduction
pathways (15,29,30).
In the present study, the PI3K inhibitor LY294002 suppressed
PI3K/AKT/mTOR signaling, inhibited proliferation, induced apoptosis
and caspase-3 activity, promoted LC3 and Bax protein expression,
and decreased PDK1 protein expression in A2058 cells following
miR-138 overexpression. Collectively these data suggest that
LY294002 may suppress the growth and metastasis of MM. Similarly,
Liu et al (31) reported
that miR-138 suppresses airway smooth muscle cell proliferation by
targeting PDK1 in the PI3K/AKT signaling pathway.
In conclusion, we demonstrated the clinical
significance of miR-138 in patients with MM through its targeting
of PDK1 expression in the PI3K/AKT/mTOR autophagy signaling
pathway. These results may provide insight into the mechanisms of
miR-138 as a therapeutic target for the treatment of MM.
References
|
1
|
Greene JM, Schneble EJ, Jackson DO, Hale
DF, Vreeland TJ, Flores M, Martin J, Herbert GS, Hardin MO, Yu X,
et al: A phase I/IIa clinical trial in stage IV melanoma of an
autologous tumor-dendritic cell fusion (dendritoma) vaccine with
low dose interleukin-2. Cancer Immunol Immunother. 65:383–392.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagaoka T, Kiyohara Y, Koga H, Nakamura A,
Saida T and Sota T: Modification of a melanoma discrimination index
derived from hyperspectral data: A clinical trial conducted in 2
centers between March 2011 and December 2013. Skin Res Technol.
21:278–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grob JJ, Amonkar MM, Karaszewska B,
Schachter J, Dummer R, Mackiewicz A, Stroyakovskiy D, Drucis K,
Grange F, Chiarion-Sileni V, et al: Comparison of dabrafenib and
trametinib combination therapy with vemurafenib monotherapy on
health-related quality of life in patients with unresectable or
metastatic cutaneous BRAF Val600-mutation-positive melanoma
(COMBI-v): Results of a phase 3, open-label, randomised trial.
Lancet Oncol. 16:1389–1398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohr P, Hauschild A, Trefzer U, Enk A,
Tilgen W, Loquai C, Gogas H, Haalck T, Koller J, Dummer R, et al:
Intermittent high-dose intravenous interferon alfa-2b for adjuvant
treatment of stage III melanoma: Final analysis of a randomized
phase III dermatologic cooperative oncology group trial. J Clin
Oncol. 33:4077–4084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varamo C, Occelli M, Vivenza D, Merlano M
and Lo Nigro C: MicroRNAs role as potential biomarkers and key
regulators in melanoma. Genes Chromosomes Cancer. 2016.PubMed/NCBI
|
|
6
|
Li JY, Zheng LL, Wang TT and Hu M:
Functional annotation of metastasis-associated microRNAs of
melanoma: A meta-analysis of expression profiles. Chin Med J.
129:2484–2490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mannavola F, Tucci M, Felici C, Stucci S
and Silvestris F: miRNAs in melanoma: A defined role in tumor
progression and metastasis. Expert Rev Clin Immunol. 12:79–89.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jayawardana K, Schramm SJ, Haydu L,
Thompson JF, Scolyer RA, Mann GJ, Müller S and Yang JY:
Determination of prognosis in metastatic melanoma through
integration of clinico-pathologic, mutation, mRNA, microRNA, and
protein information. Int J Cancer. 136:863–874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aftab MN, Dinger ME and Perera RJ: The
role of microRNAs and long non-coding RNAs in the pathology,
diagnosis, and management of melanoma. Arch Biochem Biophys.
563:60–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng XX, Yao M, Zhang XD, Xu HX and Dong
Q: ER stress-induced autophagy in melanoma. Clin Exp Pharmacol
Physiol. 42:811–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lima RT, Sousa D, Paiva AM, Palmeira A,
Barbosa J, Pedro M, Pinto MM, Sousa E and Vasconcelos MH:
Modulation of autophagy by a thioxanthone decreases the viability
of melanoma cells. Molecules. 21:pii: E1343. 2016. View Article : Google Scholar
|
|
12
|
Caporali S, Alvino E, Lacal PM, Levati L,
Giurato G, Memoli D, Caprini E, Cappellini GC Antonini and D'Atri
S: Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating
effect of dabrafenib on the invasive behavior of melanoma cells
with acquired resistance to the BRAF inhibitor. Int J Oncol.
49:1164–1174. 2016.PubMed/NCBI
|
|
13
|
Peng Y, Li L, Huang M, Duan C, Zhang L and
Chen J: Angiogenin interacts with ribonuclease inhibitor regulating
PI3K/AKT/mTOR signaling pathway in bladder cancer cells. Cell
Signal. 26:2782–2792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Gao X, Deeb D and Gautam SC:
Oleanane triterpenoid CDDO-Me inhibits Akt activity without
affecting PDK1 kinase or PP2A phosphatase activity in cancer cells.
Biochem Biophys Res Commun. 417:570–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scortegagna M, Ruller C, Feng Y, Lazova R,
Kluger H, Li JL, De SK, Rickert R, Pellecchia M, Bosenberg M, et
al: Genetic inactivation or pharmacological inhibition of Pdk1
delays development and inhibits metastasis of
BrafV600E::Pten−/− melanoma. Oncogene.
33:4330–4339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Song R, Fang X, Wang L, Chen W, Tang
P, Yu B, Sun Y and Xu Q: SBF-1, a synthetic steroidal glycoside,
inhibits melanoma growth and metastasis through blocking
interaction between PDK1 and AKT3. Biochem Pharmacol. 84:172–181.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SJ, Kim TM, Kim YJ, Jang KT, Lee HJ,
Lee SN, Ahn MS, Hwang IG, Lee S, Lee MH, et al: Phase II trial of
nilotinib in patients with metastatic malignant melanoma harboring
KIT gene aberration: A multicenter trial of Korean Cancer
Study Group (UN10-06). Oncologist. 20:1312–1319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo J, Si L, Kong Y, Flaherty KT, Xu X,
Zhu Y, Corless CL, Li L, Li H, Sheng X, et al: Phase II,
open-label, single-arm trial of imatinib mesylate in patients with
metastatic melanoma harboring c-Kit mutation or
amplification. J Clin Oncol. 29:2904–2909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang R, Jing G, Lv J, Song H, Li C, Wang
X, Xia W, Wu Y, Ren G and Guo W: Interferon-α-2b as an adjuvant
therapy prolongs survival of patients with previously resected oral
muscosal melanoma. Genet Mol Res. 14:11944–11954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao H, Cai L, Moller M, Issac B, Zhang L,
Owyong M, Moscowitz AE, Vazquez-Padron R, Radtke F and Liu ZJ:
Notch1-WISP-1 axis determines the regulatory role of mesenchymal
stem cell-derived stromal fibroblasts in melanoma metastasis.
Oncotarget. 7:79262–79273. 2016.PubMed/NCBI
|
|
22
|
Ndoye A and Weeraratna AT: Autophagy - An
emerging target for melanoma therapy. F1000Res pii: F1000 Faculty
Rev-1888. 2016. View Article : Google Scholar
|
|
23
|
Stojcheva N, Schechtmann G, Sass S, Roth
P, Florea AM, Stefanski A, Stühler K, Wolter M, Müller NS, Theis
FJ, et al: MicroRNA-138 promotes acquired alkylator resistance in
glioblastoma by targeting the Bcl-2-interacting mediator BIM.
Oncotarget. 7:12937–12950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou Y, Li L, Li H, Tan Y, Li B, Wang K and
Du B: Berberine suppressed epithelial mesenchymal transition
through cross-talk regulation of PI3K/AKT and RARα/RARβ in melanoma
cells. Biochem Biophys Res Commun. 479:290–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J,
Sun Z, Qiao S and Song Z: Curcumin induces autophagy, inhibits
proliferation and invasion by downregulating AKT/mTOR signaling
pathway in human melanoma cells. Oncol Rep. 35:1065–1074.
2016.PubMed/NCBI
|
|
26
|
Wei BR, Michael HT, Halsey CH, Peer CJ,
Adhikari A, Dwyer JE, Hoover SB, El Meskini R, Kozlov S, Ohler Z
Weaver, et al: Synergistic targeted inhibition of MEK and dual
PI3K/mTOR diminishes viability and inhibits tumor growth of canine
melanoma underscoring its utility as a preclinical model for human
mucosal melanoma. Pigment Cell Melanoma Res. 29:643–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cordón-Barris L, Pascual-Guiral S, Yang S,
Giménez-Llort L, Lope-Piedrafita S, Niemeyer C, Claro E, Lizcano JM
and Bayascas JR: Mutation of the 3-phosphoinositide-dependent
protein kinase-1 (PDK1) substrate-docking site in the developing
brain causes microcephaly with abnormal brain morphogenesis
independently of Akt, leading to impaired cognition and disruptive
behaviors. Mol Cell Biol. pii: MCB.00230-16. 2016. View Article : Google Scholar
|
|
28
|
Guo JP, Coppola D and Cheng JQ: IKBKE
protein activates Akt independent of phosphatidylinositol
3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain
malignant transformation. J Biol Chem. 291:228532016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung Y, Yi YS, Yoo DS, Kim JH, Yang WS,
Lee J, Park KW, Kweon DH, Hong S and Cho JY:
8-(Tosylamino)quinoline inhibits tumour progression through
targeting phosphoinositide-3-kinase/Akt pathway. Pharmazie.
68:146–152. 2013.PubMed/NCBI
|
|
30
|
Medina JR: Selective
3-phosphoinositide-dependent kinase 1 (PDK1) inhibitors: Dissecting
the function and pharmacology of PDK1. J Med Chem. 56:2726–2737.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Yang K, Sun X, Fang P, Shi H, Xu J,
Xie M and Li M: MiR-138 suppresses airway smooth muscle cell
proliferation through the PI3K/AKT signaling pathway by targeting
PDK1. Exp Lung Res. 41:363–369. 2015. View Article : Google Scholar : PubMed/NCBI
|