Introduction
Epithelial-mesenchymal transition (EMT) is a process
where cells lose epithelial phenotypes, decrease cell-cell
recognition and adhesion, and gain mesenchymal phenotypes and an
increased potential for metastasis (1). The EMT process is essential in
embryonic and breast development. In tumour progression and
metastasis, EMT processes are also important. Cells with a
mesenchymal phenotype show an increased potential of migration and
invasion, anoikis resistance, chemoresistance, radioresistance, and
stemness (1–3). E-cadherin loss is considered a
hallmark of EMT. In the EMT process, the expression of E-cadherin
is controlled by transcriptional and post-transcriptional
regulation. E-cadherin gene (CDH1) expression is modulated by
several transcription repressors, which are typically expressed in
mesenchymal cells, including Snail genes, Twist, Zeb genes and E47
(1,4). The zinc-finger transcriptional
repressor Snail is one of the genes frequently associated with EMT
(4,5). Previous studies indicated that Snail
may participate in the progression of breast cancer and other types
of cancer via the downregulation of CDH1 and the upregulation of
mesenchymal genes (4). It was also
reported that Snail participates in metastasis, cancer stemness,
recurrence, and resistance to chemotherapy and radiotherapy
(6–9).
In a previous study, we reported on the TUBO-P2J
spontaneous metastatic mouse breast cancer cell line, which is
derived from TUBO, a non-metastatic epithelial breast cancer cell
line (10). The TUBO-P2J cell line
showed a mesenchymal phenotype compared to TUBO, including a loss
of E-cadherin, downregulation of claudin 1 and occludin 1, and a
gain of vimentin, α-sm-actin and fibronectin. Various EMT
transcription factors (EMT-TF), including Snail, Twist, FoxC2 and
ZEB2, are also upregulated in TUBO-P2J cells. In addition, TUBO-P2J
cells showed increased resistance to chemotherapy and radiotherapy
(10,11). However, it is not clear which
factors are critical in the resistance to chemotherapy and
radiotherapy.
In this study, we attempted to define the role of
Snail in TUBO-P2J cells in the maintenance of mesenchymal cell
phenotypes, including the loss of E-cadherin, metastasis, stemness,
and resistance to chemotherapy and radiotherapy through a
knock-down of Snail with short hairpin RNA (shRNA). Our results
showed that Snail is not essential in the maintenance of
mesenchymal cell phenotypes, such as cell morphology, the loss of
E-cadherin, and resistance to radiation, but is essential for
metastasis, stemness and resistance to chemotherapy. In addition,
E-cadherin transcription was recovered by DNA methyltransferase
(DNMT) inhibitor (5-aza-2′-deoxycytidine, 5-aza-dC) alone, but
protein expression was detected only in Snail knock-down cells
treated with both 5-aza-dC and an HDAC inhibitor (SAHA).
Materials and methods
Cell culture, shRNA construct and
chemicals
The TUBO and TUBO-P2J cell lines were cultured in
vitro in high glucose DMEM (HyClone™; Thermo Fisher Scientific,
MA, USA) supplemented with heat-inactivated 10% foetal bovine serum
(FBS; Thermo Fisher Scientific) and 1% penicillin-streptomycin
(HyClone; Thermo Fisher Scientific); the cells were maintained
under 5% CO2. Snail shRNA constructs targeting the Mus
musculus sequences of Snai1 (NM_011427) were purchased from Sigma
(Sh1-sequence: GTACCGGATGTGTC TCCCAGAACTATTTCTCGAGAAATAGTTCTGGGAGAC
ACATTTTTTTG, Sh3-sequence: CCGG GATCTTCAACTG
CAAATATTGCTCGAGCAATATTTGCAGTTGAAGATCT TTTTG). 5-Aza-dC (DNA
methyltransferase inhibitor), SAHA (HDAC inhibitor), epirubicin and
doxorubicin was purchased from Sigma.
Generation of Snail knockdown stable
cell lines
The lentiviral transduction particles are produced
from a library of sequence-verified lentiviral plasmid vectors for
mouse snail gene (Sigma). Transduction of lentiviral particles were
performed according to the manufacturer's instructions. Transduced
cells were selected with 500 µg/ml of puromycin for 3–14 days.
Purimycin-resistant colonies and separated single cells were
picked. Each single cell was expanded to evaluate the knockdown
status of the Snail gene via PCR and western blotting.
Inhibition of DNA methyltransferase
and/or histone deacetylase
Cells (1×106/dish) were seeded on 10 cm
culture plate and incubated for 5 h for the cells to attach to the
dishes. 5-Aza-dc (2.5 µM) and/or SAHA (0.5 or 2 µM) were treated
and incubated at 37°C in a humid atmosphere of 5% CO2
for 3 or 7 days, then cells were subcultured with trypsinization to
prevent overgrowth.
Reverse transcription-PCR
Total RNA extracted from cultured cells was used as
a template for reverse transcriptase reactions. Aliquots of cDNA
were amplified using the mouse and human primer pairs (Table I). After an initial denaturation at
94°C for 5 min, the following was performed: 30 cycles of
denaturation at 94°C for 30 sec, annealing at 55–60°C for 30 sec,
and extension at 72°C for 30 sec. PCR was performed with a 2720
thermo cycler (Applied Biosystems, CA, USA). The reaction products
were analysed in 1.5% agarose gels.
 | Table I.PCR primer pairs of mouse and human
genes. |
Table I.
PCR primer pairs of mouse and human
genes.
| Genes | Species | NCBI no. | Forward
(5′-3′) | Reverse
(5′-3′) | Size (bp) |
|---|
| CDH1 | Human | NM_004360 |
ATTTTTCCCTCGACACCCGAT |
TCCCAGGCGTAGACCAAGA | 109 |
| Snail | Human | NM_005985 |
TCGGAAGCCTAACTACAGCGA |
AGATGAGCATTGGCAGCGAG | 140 |
| GAPDH | Human | NM_001256799 |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG | 197 |
| CDH1 | Mouse | NM_009864.2 |
CCATTTTCACGCGCGCTG |
CGCGAGCTTGAGATGGAT | 396 |
| DDR2 | Mouse | NM_022563.2 |
ATCACAGCCTCAAGTCAGTGG |
TTCAGGTCATCGGGTTGCAC | 116 |
| Snail | Mouse | NM_011427.2 |
CACACGCTGCCTTGTGTCT |
GGTCAGCAAAAGCACGGTT | 133 |
| Snail2 | Mouse | NM_011415.2 |
ATGCCCAGTCTAGGAAATCG |
TGATGACAACCAGGCATCAT | 551 |
| Foxc2 | Mouse | NM_013519.2 |
AACCCAACAGCAAACTTTCCC |
GCGTAGCTCGATAGGGCAG | 130 |
| Vimentin | Mouse | NM_011701.4 |
CGGCTGCGAGAGAAATTGC |
CCACTTTCCGTTCAAGGTCAAG | 124 |
| Fibronectin | Mouse | NM_010233.1 |
AGAGCAAGCCTGAGCCTGAAG |
TCGCCAATCTTGTAGGACTGACC | 192 |
| Twist | Mouse | NM_011658.2 |
GGACAAGCTGAGCAAGATTCA |
CGGAGAAGGCGTAGCTGAG | 146 |
| GAPDH | Mouse | NM_008084.2 |
TTCACCACCATGGAGAAGGC |
GGCATGGACTGTGGTCATGA | 250 |
Western blot assay
Cell lysates (40 µg/lane) were electrophoresed on
polyacrylamide-SDS gels and then transferred to polyvinylidene
fluoride membranes. Immunoblotting was performed by primary
antibodies. E-cadherin, Snail-1 and GAPDH antibodies were obtained
from Cell Signaling Technology (MA, USA) and estrogen receptor α
(ESR1) antibody was purchased from Santa Cruz (TX, USA).
HRP-conjugated secondary antibodies were purchased from Jackson
Laboratories (Jackson ImmunoResearch, PA, USA).
Flow cytometry
To detect CD24 and CD44 expression, 2×105
cells were stained with 0.5 µg/ml of PE-Cy5-conjugated CD24 and
FITC-conjugated CD44 antibody (BioLegend) for 30 min at room
temperature in the dark. For aldehyde dehydrogenase (ALDH)
activity, cells were stained using the Aldefluor kit (Stem Cell
Technologies Inc., Vancouver, Canada) according to the
manufacturer's protocol. Stained cells were acquired with the BD
FACSCanto II cytometry system and analysed with FlowJo software
(version 10).
Migration and invasion assays
The migration and invasion assays were performed
using 8.0-µm pore size 24-well insert systems (BD Falcon) with 2
mg/ml of Matrigel coating (invasion) or not (migration). Then,
5×104 cells (migration) or 5×105 cells
(invasion) were added to the upper chamber and incubated for 4–6 h
(migration) or 72 h (invasion). After incubation, the upper surface
of the membrane was wiped with a cotton-tipped applicator to remove
residual cells. Cells in the bottom compartment were fixed and
stained with H&E. Cells in ten randomly selected fields at ×40
magnification were counted.
Soft agar colony-forming assay
Six-well plates were covered with a layer of 0.5%
agar in medium supplemented with 10% foetal bovine serum. Cells
were prepared in 0.3% agar and seeded in triplicate at 3 different
dilutions ranging from 1×103 to 5×105. The
plates were incubated at 37°C in a humid atmosphere of 5%
CO2 for 2 weeks. Each experiment was repeated at least 3
times. Colonies were photographed between 10 and 12 days at an
original magnification of ×40 under phase contrast.
Chemo-susceptibility test
The chemo-susceptibility was measured with an In
Vitro Toxicology assay kit (TOX6, Sigma). Briefly,
0.5–1×104 cells/well were seeded and attached in 96-well
culture plates. Indicated doses of chemo-drugs were administered
for 72 h. Cells were fixed in 10% trichloroacetic acid for 1 h at
4°C, stained with SRB for 15 min, and washed 3 times with 1% acetic
acid. The incorporated dye was solubilized with 10 mM Tris base, pH
8.8. Absorbance was spectrophotometrically measured at 565 nm using
an EL800 microplate reader (Bio-Tek Instruments, Winooski, VT,
USA).
Radiation susceptibility test
Cells were harvested with trypsin and suspended in
15 ml conical tubes at 1×104 cells/ml. After irradiation
with the indicated doses with X-ray generator
(RapidArc®; Varian Medical Systems Inc., Palo Alto, CA,
USA), cells were diluted 10-fold and seeded in 6-well plates. After
72 h, the cells were stained with 0.5% crystal violet for 10 min
and fixed with 4% paraformaldehyde. Colonies were counted under a
microscope. For in vivo radiation treatment, TUBO-P2J/NC
(1.5×105) and TUBO-P2J/shSnail (2×105) cells
were implanted subcutaneously and tumour tissues were locally
irradiated at 15-Gy with an X-ray generator under anaesthesia
(ketamine 90 mg/kg and xylazine 10 mg/kg).
In vivo animal study
All of the procedures involving animals were
approved by the INJE University College of Medicine Institutional
Animal Care and Use Committee. All of the mice (BALB/C, 6–8 weeks
of age) used in this study were purchased from Orient Bio (Taejun,
Korea). To evaluate the tumour formation ability and growth,
1×105 or 1×104 cells were subcutaneously
implanted on the backside of the mice. Tumour development was
followed every day, and tumour sizes were measured two times per
week. Tumour volumes were measured along three orthogonal axes (x,
y, and z) and calculated as tumour volume = (xyz)/2. To analyse
experimental metastasis, 2×104 cells were intravenously
injected through the tail vein and lung tissues were collected at
day 19.
Statistics
Differences between groups were analysed using an
unpaired t-test. Error bars represent ± SD. All statistical
analyses were conducted using Graph-Pad Prism Version 4.0 (GraphPad
Software). Unless specified, statistically significant differences
of p<0.05, 0.01, and 0.001 are noted.
Results
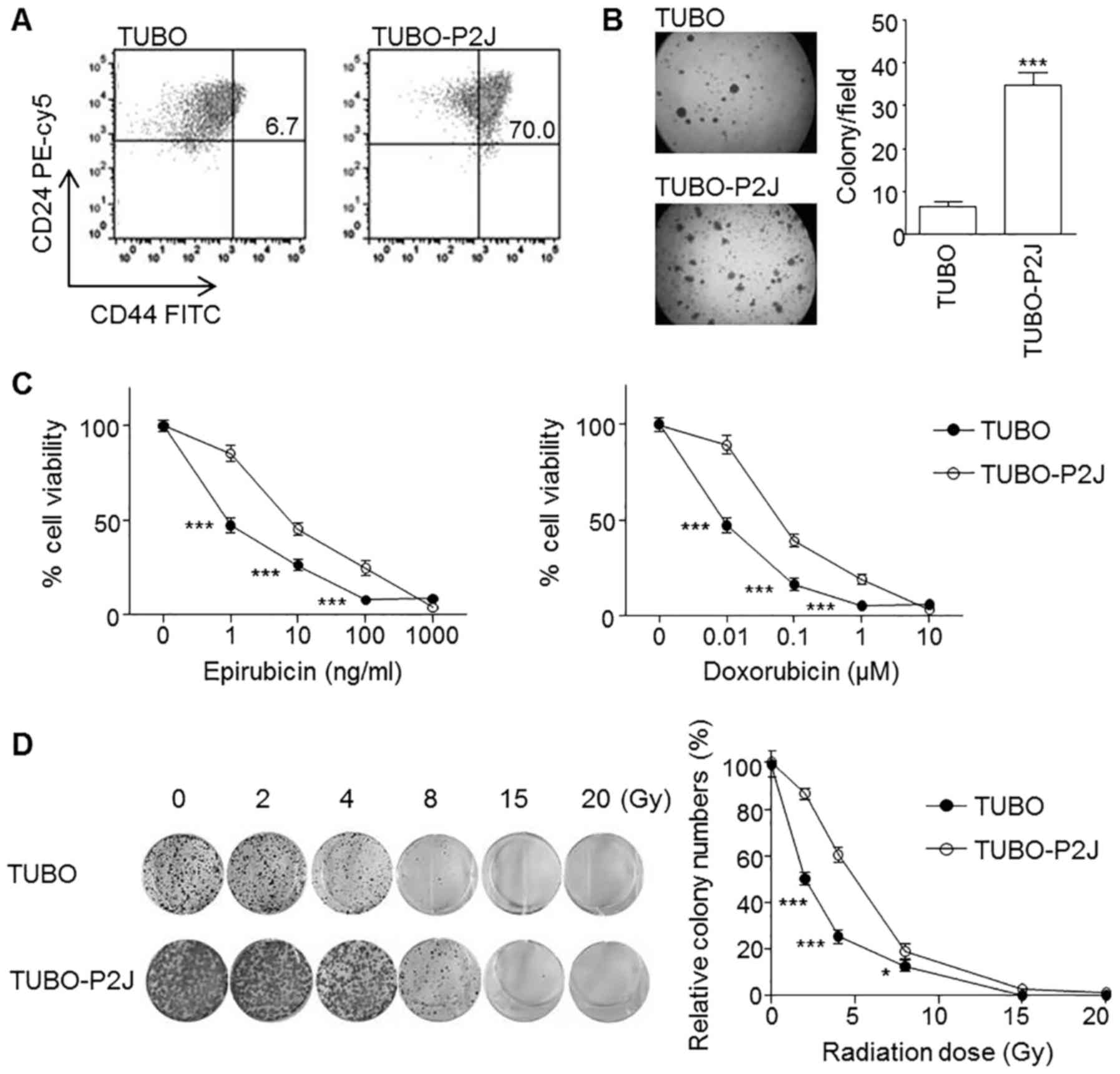
TUBO-P2J displays a cancer stem cell
phenotype and resistance to chemotherapy and radiotherapy
Previously we reported on the the highly metastatic
mouse breast cancer cell line TUBO-P2J, derived from the
non-metastatic TUBO cell line (10). TUBO cells were morphologically
epithelial in nature. In contrast, TUBO-P2J cells were
spindle-shaped mesenchymal-type cells. TUBO-P2J cells showed a gain
of Snail and loss of E-cadherin. In flow cytometry analysis and
soft agar colony assays, the TUBO-P2J cell line was characterized
as stem cell-like with a prominent CD44high population
(Fig. 1A) and 5 times higher colony
number (Fig. 1B) than TUBO cells.
Chemo and radiation susceptibility tests revealed that TUBO-P2J
cells were more resistant to chemotherapy and radiation therapy
(Fig. 1C and D). Snail expression
levels were associated with poor prognosis phenotypes, including
metastatic potential, soft agar colony formation capacity and
resistance to chemotherapy and radiotherapy.
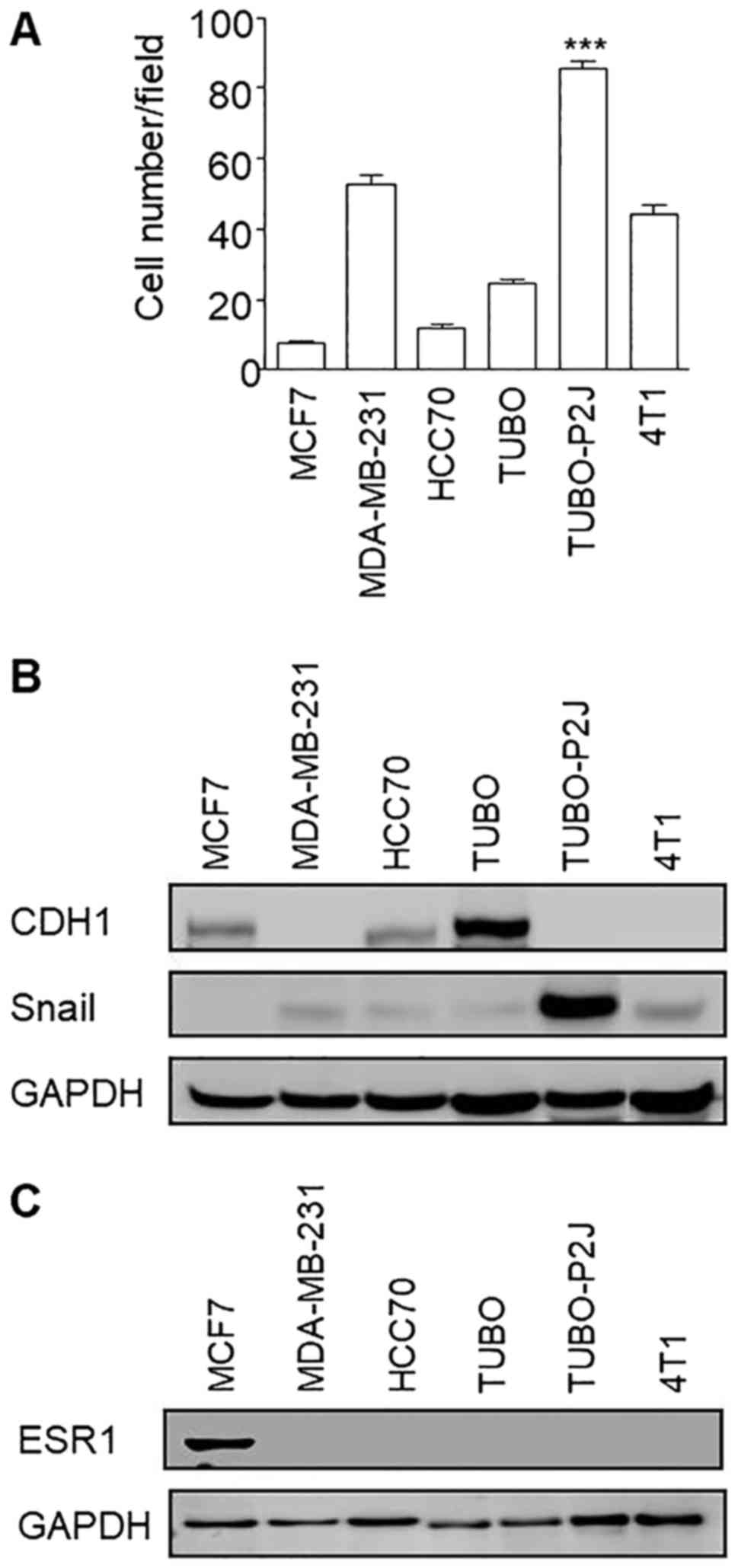
Before Snail silencing, we compared the migration
potentials of TUBO-P2J cell line with other breast cancer cell
lines, such as MCF7, MDA-MD-231, HCC70, 4T1, and original TUBO cell
lines (Fig. 2A). The migration
activity of TUBO-P2J was higher than that of human breast cancer
MDA-MB-231, which is a well-known cell line with a highly
metastatic potential. TUBO-P2J cell line also showed the highest
level of snail expression among the tested breast cancer cell lines
(Fig. 2B). In addition, TUBO-P2J
cell line did not express estrogen receptor α1 (ESR1) similarly to
MDA-MB-231, triple-negative breast cancer cell line (Fig. 2C). Even though TUBO-P2J is a mouse
tumour cell line, it is derived spontaneously in vivo and
could be exactly compared with parent TUBO cells which showed less
malignant phenotypes, including metastatic potential, stemness,
chemoresistance and radioresistance (10). Based on these data, we chose the
TUBO-P2J cell line for Snail silencing experiments with efficient
lentiviral transfection system.
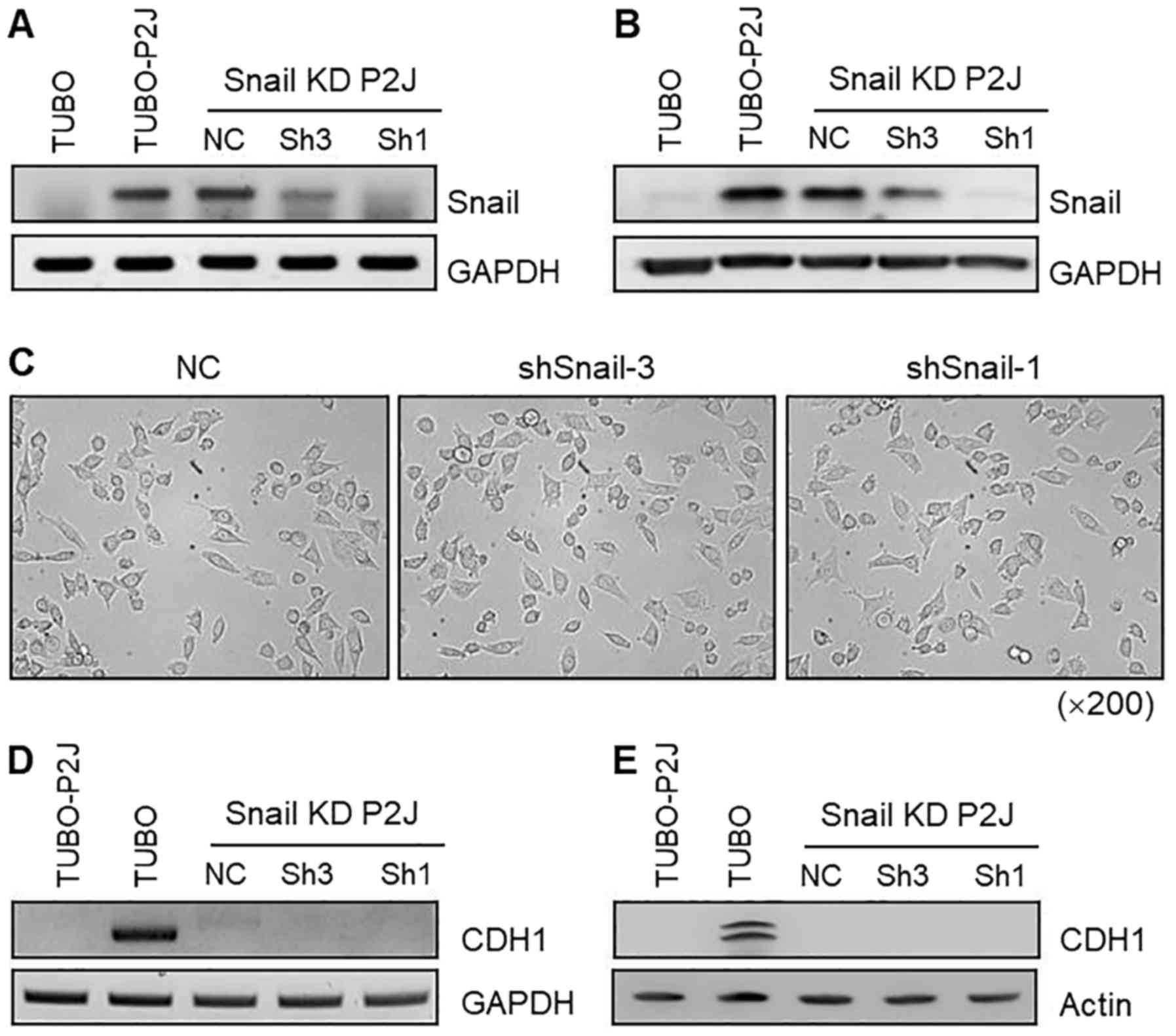
Downregulation of Snail does not
induce mesenchymal-epithelial transition in TUBO-P2J cells
To examine the role of Snail in maintaining the
mesenchymal phenotype and the suppression of E-cadherin, we
produced a stable Snail knockdown TUBO-P2J cell line
(TUBO-P2J/shSnail) by lentiviral transfection. Two independent
stable shSnail-expressing clones (Sh1 and Sh3, one clone from each
construct) and a stable negative control shRNA clone (NC) were
analysed for Snail mRNA expression. Sh1 and Sh3 showed 50–90%
downregulation of Snail mRNA, whereas the control clone did not
exhibit any significant reduction in Snail mRNA (Fig. 3A). Knockdown of Snail in TUBO-P2J
cells was confirmed with western blot analysis (Fig. 3B). Snail protein levels were
correlated with mRNA levels. The morphology of Snail knockdown
cells (Sh1 and Sh3) and control cells (NC) was not different, as
all of the cells showed a spindle-shaped mesenchymal phenotype
(Fig. 3C). Next, we evaluated the
expression of E-cadherin with RT-PCR and western blot analysis
(Fig. 3D and E). In TUBO-P2J cells,
Snail knockdown did not induce transcription of E-cadherin. Based
on Snail expression levels, we chose the Sh1 clone as
TUBO-P2J/shSnail for further experiments.
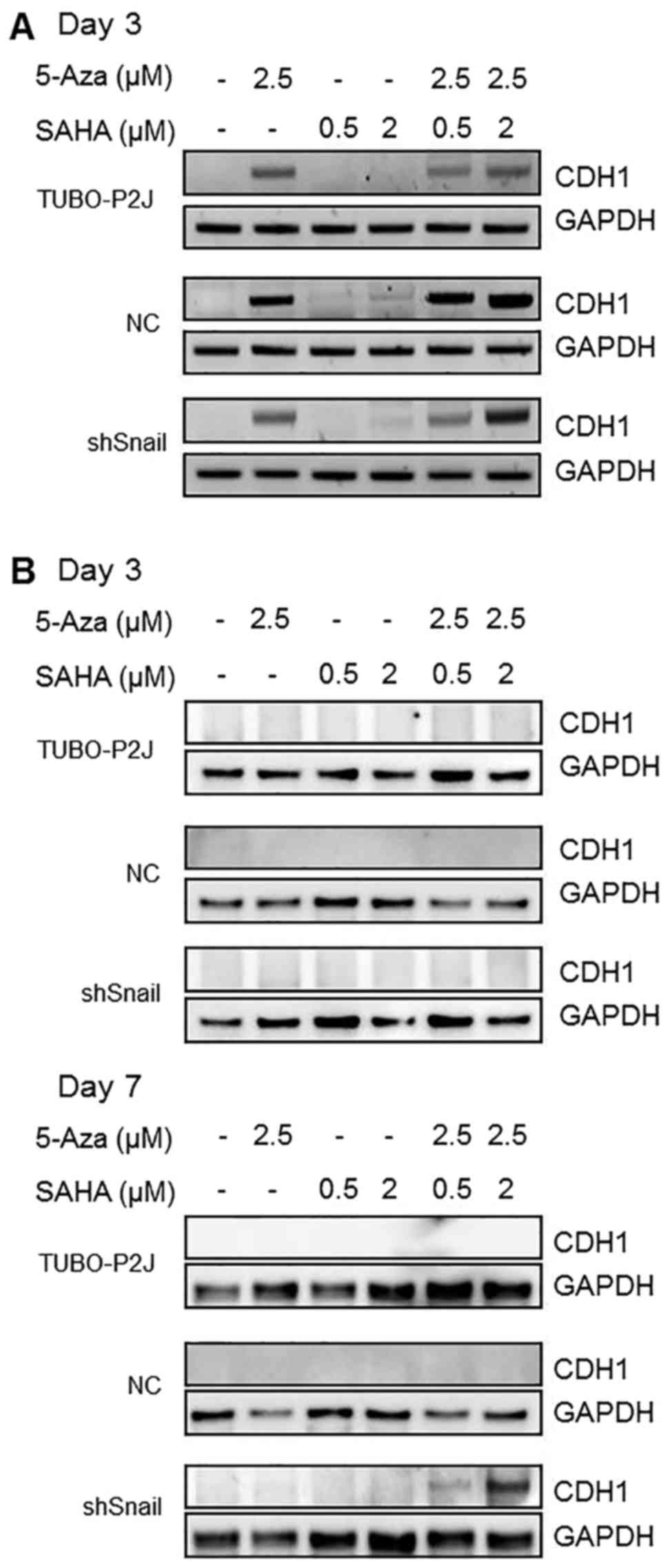
E-cadherin expression was controlled
by DNA methylation, histone deacetylation, and Snail in TUBO-P2J
cells
Lim et al (12) reported that Snail represses the
transcription of E-cadherin through binding to E-boxes of this gene
and inducing DNA methylation of its promoter by recruiting HDAC1
and DNMT. To evaluate the reason that E-cadherin was not expressed
in TUBO-P2J/shSnail cells, cells were treated with 5-aza-dC and/or
SAHA. By the third day of 5-azaC treatment, E-cadherin mRNA was
detected in all TUBO-P2J cells regardless of Snail knockdown
(Fig. 4A). However, E-cadherin
protein was detected only in shSnail cells by treatment of 5-azaC
and SAHA for 7 days (Fig. 4B).
These data suggest that Snail might suppress the expression of
E-cadherin through a translation step co-operating with HDAC in
TUBO-P2J cells.
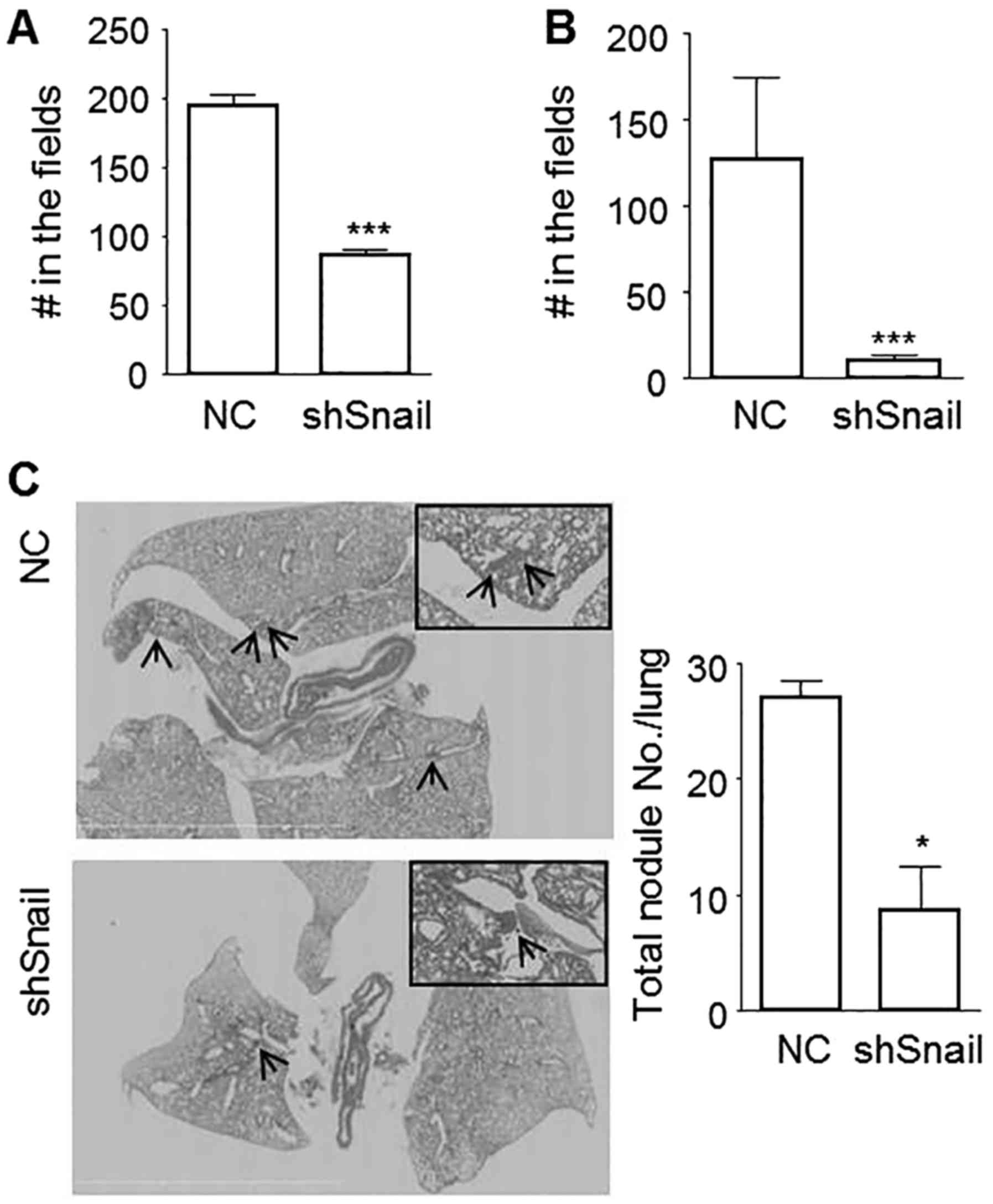
Snail increases metastatic potential
in TUBO-P2J cells
Because processes of EMT have been linked with
metastasis, we next evaluated the metastatic potential of PLKO and
Sh1 cells. By silencing Snail, migration was significantly
decreased from 195.3±7.5 (mean ± SE) cells/fields to 86.7±4.7
cells/fields (56% decrease) (Fig.
5A) and invasion was decreased even more from 127.1±47.6
cells/fields to 10.7±2.7 cells/fields (93% decrease) (Fig. 5B). An in vivo metastasis
assay using tail vein injection also showed that lung colony
numbers of shSnail cells were reduced to one-third of that of
control cells (Fig. 5C). These data
suggest that inactivation of Snail could reduce the metastatic
potentials of breast cancer cells without driving MET.
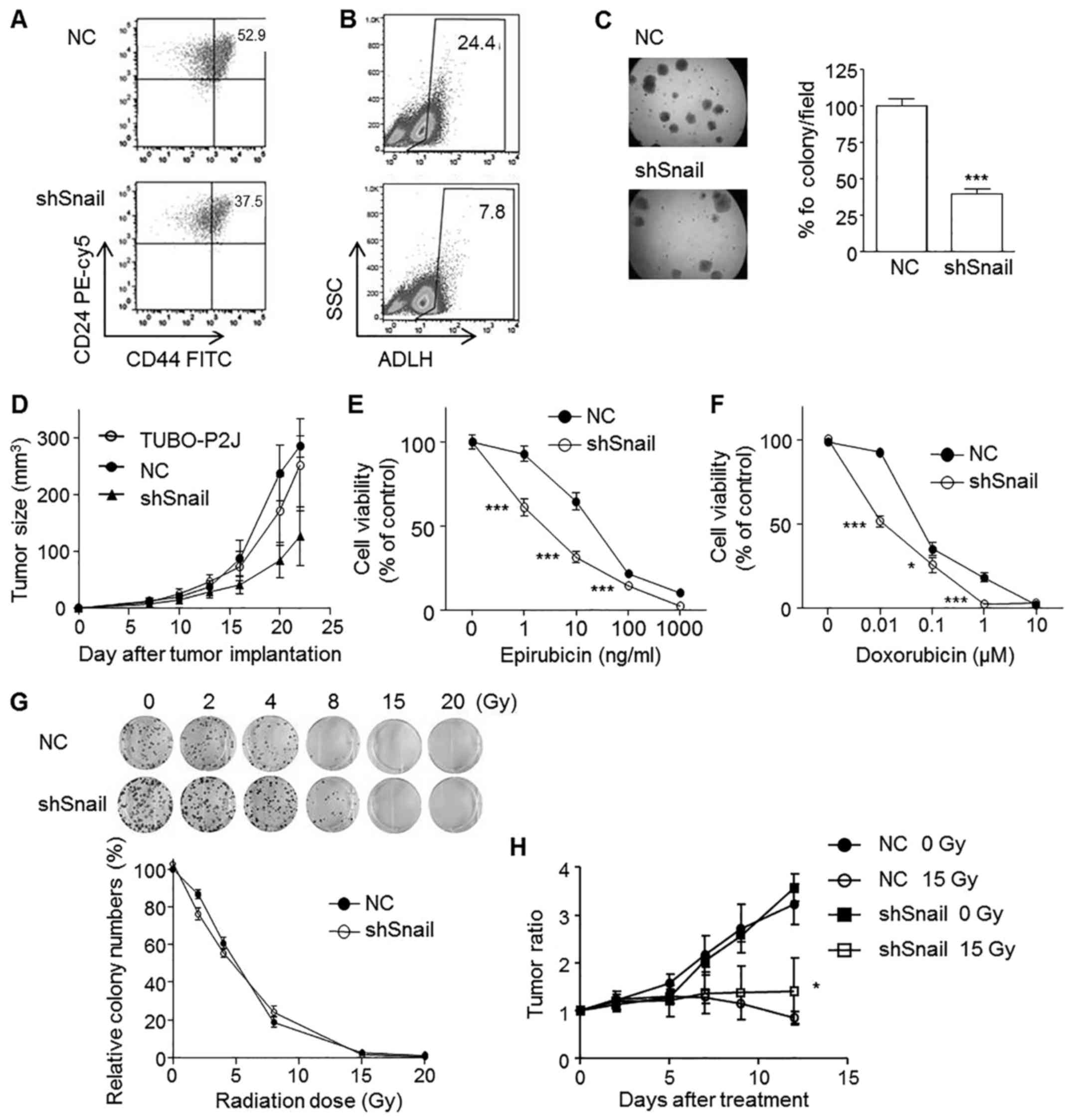
Snail maintains cancer stem cell-like
properties and in vivo tumourigenicity of TUBO-P2J cells
As Snail is expressed in CD44high
compared to CD44low cells, we examined whether Snail
could affect the expression of CD44, a breast cancer stem cell
marker. Snail silenced TUBO-P2J cells showed a slight decrease in
the CD44high population (37.5%) compared to their
control counterparts (52.9%) (Fig.
6A). Aldehyde dehydrogenase (ALDH) activity was also reduced
significantly by Snail silencing (Fig.
6B). Next, we used a soft agar colonization assay to evaluate
the role of Snail in anchorage-independent growth. Data showed that
the sizes of the colonies were not different with Snail silencing,
but the numbers were significantly reduced by 40% compared to that
of control cells (Fig. 6C). In
vivo tumourigenicity was tested with subcutaneous implantation
of TUBO-P2J, TUBO-P2J/NC, and TUBO-P2J/shSnail (1×104 or
1×105 cells/mouse). In all of the mice implanted with
parent TUBO-P2J and TUBO-P2J/NC cells, tumours developed at day 14,
but tumours developed in two mice out of seven at 30 days when
1×104 of TUBO-P2J/shSnail cells were implanted (Table II). Moreover, a decrease in tumour
size was observed in tumours established with Snail silenced cells
compared with those from parent and control cells (Fig. 6D). These in vitro and in
vivo data demonstrate that Snail has an important role in the
maintenance of breast cancer stem cell properties.
 | Table II.Incidence of tumour growth. |
Table II.
Incidence of tumour growth.
|
| Cell numbers
injected |
|---|
|
|
|
|---|
| Tumour cells | 105 | 104 |
|---|
| TUBO-P2J | 8/8 | 6/6 |
| NC | 7/7 | 6/6 |
| shSnail | 7/7 | 2/7 |
Snail increases chemoresistance but
not radioresistance
As Snail is expressed in chemo- and radioresistant
cells, TUBO-P2J, we examined whether Snail could induce chemo- and
radioresistance. Our data revealed that silencing Snail increased
the susceptibility to epirubicin and doxorubicin (Fig. 6E and F); however, susceptibility to
radiation therapy was not affected by Snail silencing (Fig. 6G and H). These results suggested
that Snail silencing might be a good strategy to overcome
chemoresistance.
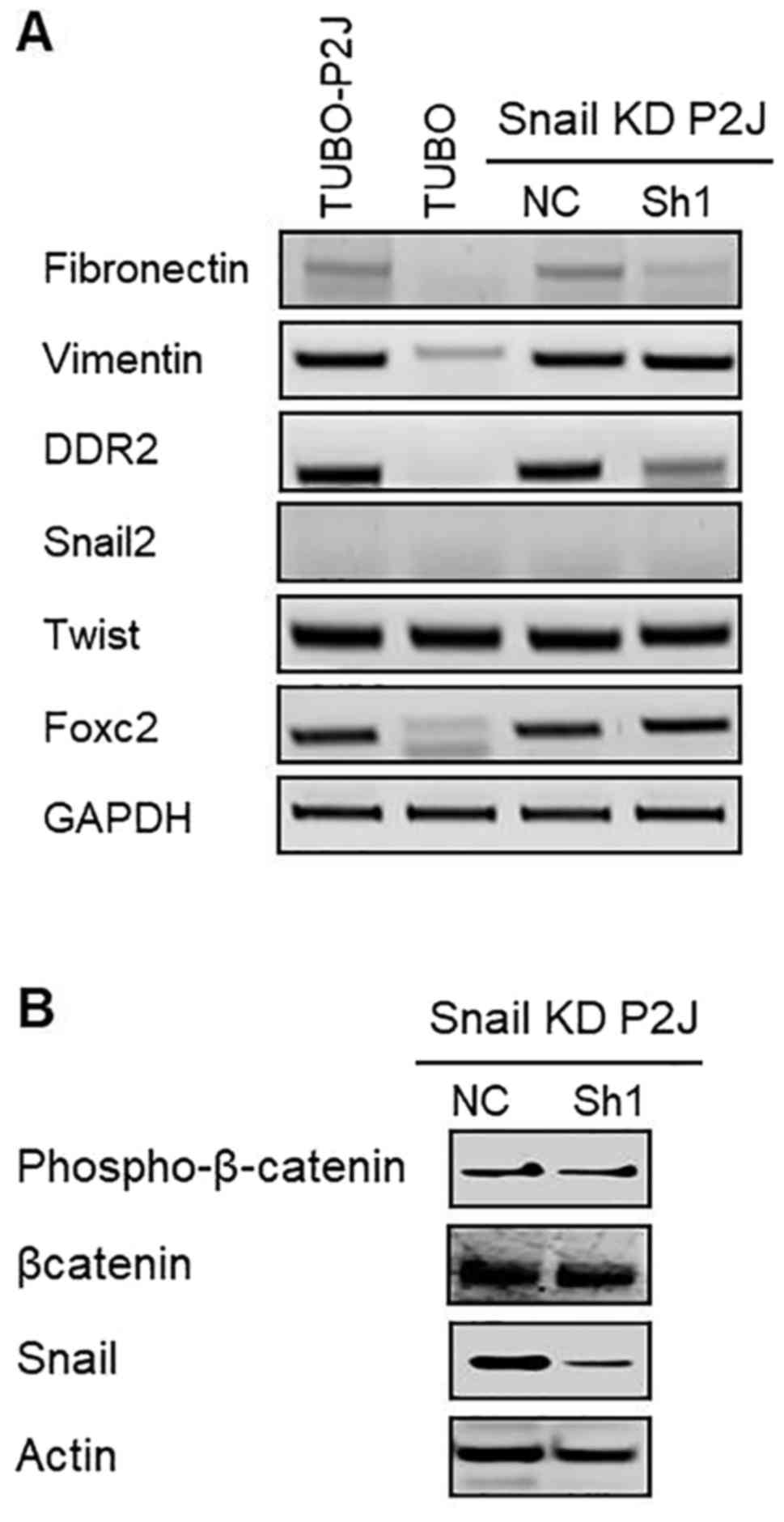
The expression of SLUG and Vimentin
and the β-catenin signaling are not changed by Snail silencing
To test whether the phenotype changes in Snail
silent cells were mediated by other EMT related genes, we evaluated
mRNA levels of EMT related genes such as SLUG (Snail2), vimentin,
fibrinectin, Twist, DDR2, and FoxC2 (Fig. 7A). Data revealed that silencing of
Snail reduced the expressions of fibronectin and DDR2 but did not
influence on the expressions of other EMT related genes including
vimentin, SLUG, and Twist. In addition, silencing of Snail did not
change the activation status of β-catenin (Fig. 7B). These data suggested that the
malignant phenotypes of TUBO-P2J cell lines which are increased
stemness, metastasis, and chemoresistance are mediated by the
increased expression of Snail and the role of Snail in this model
was not linked with the expression of vimentin and SLUG and
Wnt/β-catenin signaling pathway.
Discussion
This study demonstrates that Snail, one of the
master EMT genes in breast cancer cells, maintains metastatic
potential, clonogenicity, and drug resistance without restoration
of E-cadherin expression or morphological changes. In addition, our
results also revealed that Snail is required for tumour initiation
and growth in vivo. Our data suggested that inhibition of
Snail activity could be a good strategy to inhibit tumour
metastasis and to enhance the biological effects of anticancer
agents.
Loss of E-cadherin is a hallmark of the EMT process.
E-cadherin expression is controlled by various transcription
factors, Snail genes, Twist, Zeb genes and E47 (1,4). Snail
was discovered first and is the most important transcriptional
repressor of E-cadherin (6). Snail
binds to the E-box of the E-cadherin promoter through
C2H2-type zinc fingers and represses
transcription (13). Many reports
have demonstrated that silencing Snail can restore E-cadherin
expression in various cancer cells including breast cancer
(14–20). In TUBO-P2J cells, however, silencing
Snail did not restore the expression of E-cadherin. This finding
might be because: i) Snail sufficiently represses E-cadherin during
the initial stages of EMT while subsequent maintenance may require
cooperation of other transcription factors (21), or ii) Snail can play a role as a
mediator of epigenetic changes through recruiting histone
deacetylases (HDAC) and DNA methyltransferase (DNMT) to the
E-cadherin promoter (12,14). Though we cannot rule out the
possibility that other transcription factors repress the expression
of E-cadherin, epigenetic changes are a main regulator for
E-cadherin expression in TUBO-P2J cells. Interestingly, the
transcription of E-cadherin was restored by 5-aza-dC treatment
within 3 days regardless of the presence of Snail, but the
E-cadherin protein was detected only in TUBO-P2J/shSnail cells
treated with both 5-aza-dC and SAHA at day 7. These data suggested
that Snail may regulate E-cadherin expression at
post-transcriptional steps with HDAC. Further studies to define the
exact mechanisms of Snail in the post-transcriptional regulation of
E-cadherin and which types of HDAC participate would be
required.
In breast cancer, Snail expression has been detected
at the invasive area, coincidently with E-cadherin downregulation,
and has been associated with lymph node metastasis and recurrence
(17,22). Previous studies demonstrated that
Snail participates in the EMT-MET process, tumour metastasis,
cancer stemness, recurrence, and resistance to chemotherapy and
radiotherapy (6–9,23). In
these studies, however, the expression of Snail was linked with
E-cadherin expression and morphological changes, such as EMT or
MET. Therefore, it is not clear whether these poor prognostic
potentials are induced by Snail-induced EMT or by Snail expression
itself. In the metastatic potential, silencing Snail significantly
reduced in vitro migration and invasion and in vivo
lung colonization. These data revealed that Snail plays a role as a
central regulator of metastasis. For the breast cancer stem cell
markers, CD44 expression levels and ALDH activity were also
decreased by Snail silencing. In human breast cancer, various
markers, such as CD24, CD44, CD133, CD166 and ALDH1, were
identified as cancer stem cell markers, as cells with the phenotype
CD44+/CD24low/− and/or ALDH1+ are
most consistently associated with stem-like characteristics
(24–28). In our study, CD24 expression levels
were not different between epithelial non-metastatic TUBO cells and
mesenchymal metastatic TUBO-P2J cells and were not linked with
colony formation ability. In these mouse breast cancer cell lines,
CD44 expression levels were linked with colony formation ability
and in vivo tumour formation and growth and were regulated
by Snail expression. ALDH1 activities in TUBO and TUBO-P2J cells
were similar (data not shown); however, silencing Snail reduced
ALDH1 activity in TUBO-P2J cells. Though the reasons that CD24
expression was not linked with cancer stemness remain unknown, it
is clear that CD44 expression levels are closely related with
cancer stemness and are regulated by Snail expression. Regarding
resistance to chemo-drugs, while we tested only two anthracyline
drugs, our results are consistent with prior studies that suggested
that EMT is associated with an increased chemoresistance of cancer
cells (8). However, there are some
controversies regarding the link between EMT or Snail and
chemoresistance. Mezencev et al (23) reported that Snail-induced EMT in
MCF7 cells induced resistance to gemcitabine and mitomycin C, but
increased sensitivity to doxorubicin, methotrexate, cisplatin, and
5-fluorouracil. In our previous study, we showed that mesenchymal
TUBO-P2J cells were less sensitive to 12 breast cancer chemo-drugs
than parental epithelial TUBO cells (10). It is still difficult to generalize
whether Snail or EMT increase the resistance of breast cancer cells
to most chemo-drugs, but our results suggest the possibility that
Snail inhibition can reduce the resistance to some chemo-drugs. A
number of studies have reported that EMT and cancer stem cell-like
properties are also associated with radioresistance in breast
cancer cells (9,29–31).
However, it is not clear whether Snail regulates radioresistance in
breast cancer. Mezencev et al (23) showed that mesenchymal MCF7-Snail
cells are more sensitive to radiation that their parental cells.
Zhang et al (32)
demonstrated that radioresistance can be induced and maintained by
ZEB1, not by Twist and Snail, using gene expression and silencing
techniques. Our results also showed that silencing Snail without
E-cadherin restoration or morphological changes did not increase
the sensitivity to radiation. Considering cancer stem-like
properties were decreased by Snail silencing and there was no
detection of ZEB1 in TUBO-P2J cells (10), radioresistance might be regulated by
unknown factors in TUBO-P2J cells.
Based on this study, Snail is thought to be a
critical element in the machinery that maintains the metastatic
potential, stem-like properties and chemoresistance in mesenchymal
breast cancer. In addition, Snail inhibition would be a good target
for breast cancer treatment.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIP) (NRF-2015R1A2A2A01008394).
References
|
1
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: Concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y and Zhou BP: Snail: More than EMT.
Cell Adhes Migr. 4:199–203. 2010. View Article : Google Scholar
|
|
7
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Theys J, Jutten B, Habets R, Paesmans K,
Groot AJ, Lambin P, Wouters BG, Lammering G and Vooijs M:
E-cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song H, Kim TO, Ma SY, Park JH, Choi JH,
Kim JH, Kang MS, Bae SK, Kim KH, Kim TH, et al: Intratumoral
heterogeneity impacts the response to anti-neu antibody therapy.
BMC Cancer. 14:6472014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma SY, Song H, Park JH, Choi JH, Kim JH,
Kim KH, Park S, Park DH, Kang MS, Kwak M, et al: Addition of
anti-neu antibody to local irradiation can improve tumor-bearing
BALB/c mouse survival through immune-mediated mechanisms. Radiat
Res. 183:271–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim SO, Gu JM, Kim MS, Kim HS, Park YN,
Park CK, Cho JW, Park YM and Jung G: Epigenetic changes induced by
reactive oxygen species in hepatocellular carcinoma: Methylation of
the E-cadherin promoter. Gastroenterology. 135:2128–2140,
2140.e1-8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harney AS, Meade TJ and LaBonne C:
Targeted inactivation of Snail family EMT regulatory factors by a
Co(III)-Ebox conjugate. PLoS One. 7:e323182012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou W, Lv R, Qi W, Wu D, Xu Y, Liu W, Mou
Y and Wang L: Snail contributes to the maintenance of stem
cell-like phenotype cells in human pancreatic cancer. PLoS One.
9:e874092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olmeda D, Moreno-Bueno G, Flores JM, Fabra
A, Portillo F and Cano A: SNAI1 is required for tumor growth and
lymph node metastasis of human breast carcinoma MDA-MB-231 cells.
Cancer Res. 67:11721–11731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olmeda D, Montes A, Moreno-Bueno G, Flores
JM, Portillo F and Cano A: Snai1 and Snai2 collaborate on tumor
growth and metastasis properties of mouse skin carcinoma cell
lines. Oncogene. 27:4690–4701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang A, Chen G, Meng L, Wang Q, Hu W, Xi
L, Gao Q, Wang S, Zhou J, Xu G, et al: Antisense-Snail transfer
inhibits tumor metastasis by inducing E-cadherin expression.
Anticancer Res. 28A:621–628. 2008.
|
|
20
|
Smith BN, Burton LJ, Henderson V, Randle
DD, Morton DJ, Smith BA, Taliaferro-Smith L, Nagappan P, Yates C,
Zayzafoon M, et al: Snail promotes epithelial mesenchymal
transition in breast cancer cells in part via activation of nuclear
ERK2. PLoS One. 9:e1049872014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee K and Nelson CM: New insights into the
regulation of epithelial-mesenchymal transition and tissue
fibrosis. Int Rev Cell Mol Biol. 294:171–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blanco MJ, Moreno-Bueno G, Sarrio D,
Locascio A, Cano A, Palacios J and Nieto MA: Correlation of Snail
expression with histological grade and lymph node status in breast
carcinomas. Oncogene. 21:3241–3246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mezencev R, Matyunina LV, Jabbari N and
McDonald JF: Snail-induced epithelial-to-mesenchymal transition of
MCF-7 breast cancer cells: Systems analysis of molecular changes
and their effect on radiation and drug sensitivity. BMC Cancer.
16:2362016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abraham BK, Fritz P, McClellan M,
Hauptvogel P, Athelogou M and Brauch H: Prevalence of
CD44+/CD24−/low cells in breast cancer may
not be associated with clinical outcome but may favor distant
metastasis. Clin Cancer Res. 11:1154–1159. 2005.PubMed/NCBI
|
|
25
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: An enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Beça FF, Caetano P, Gerhard R,
Alvarenga CA, Gomes M, Paredes J and Schmitt F: Cancer stem cells
markers CD44, CD24 and ALDH1 in breast cancer special histological
types. J Clin Pathol. 66:187–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santisteban M, Reiman JM, Asiedu MK,
Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC,
Manjili MH, et al: Immune-induced epithelial to mesenchymal
transition in vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pajonk F, Vlashi E and McBride WH:
Radiation resistance of cancer stem cells: The 4 R's of
radiobiology revisited. Stem Cells. 28:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin H and Glass J: The phenotypic
radiation resistance of CD44+/CD24(−or low)
breast cancer cells is mediated through the enhanced activation of
ATM signaling. PLoS One. 6:e240802011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y,
Zhang J, Yuan J, Wang M, Chen D, Sun Y, et al: ATM-mediated
stabilization of ZEB1 promotes DNA damage response and
radioresistance through CHK1. Nat Cell Biol. 16:864–875. 2014.
View Article : Google Scholar : PubMed/NCBI
|