Introduction
Renal cell carcinoma (RCC) is the second most
prevalent urologic malignancy in China only after bladder cancer
(1). It often arises without early
symptoms, thus 20–30% patients are diagnosed when distant
metastases already present (2).
Surgery is the current major therapeutic approach for RCC patients,
whereas, for patients with advanced RCC, who are not suitable for
surgery, or whose solitary kidney cannot be completely excised by
surgery, radiation and chemotherapy can be applied as alternative
treatments (3,4). However, clinical investigations report
that the sensitivity of RCC patients to radiation and chemotherapy
is extremely low, greatly restricting the clinical therapeutic
effects on advanced RCC patients (5). Thus, unraveling the molecular
mechanisms underlying the initiation and progression of RCC may
provide a better understanding of the therapy of RCC.
MicroRNAs (miRNAs) are usually 20–24 nucleotides in
length and non-coding small RNAs that transcriptionally and
post-transcriptionally mediate 60% of human protein coding gene
expression and participate in a variety of biological processes
including cell differentiation, proliferation and apoptosis
(6,7). miRNAs function as either oncogenes or
tumor-suppressor genes by binding to complementary sequences at
3′-untranslated regions (3′-UTRs) of target messenger RNAs in
diverse cancers (8,9). Among them, Li et al have
identified miR-21 as an oncogenic driver in RCC cells that
regulates cell invasion (10). Xu
et al have suggested that miR-203 could be a prognostic
marker and serves as a tumor suppressor in human RCC cells
(11). Recent studies have shown
that downregulation of miR-15a is involved in the tumorigenesis and
progression of several human types of cancer (12–14).
However, the role that miR-15a plays in the carcinogenesis of RCC
is still unclear.
Eukaryotic translation initiation factor 4E (eIF4E)
as an mRNA cap-binding protein is regulated via phosphorylation by
binding to eukaryotic initiation factor 4E binding proteins
(4E-BPs) (15). It is the most
efficient speed regulator for eukaryotic mRNA translation and plays
an important regulatory role in the initial phase of protein
synthesis (16). Overexpression of
eIF4E causes preferential translation of mRNAs containing excessive
secondary structures in their 5′-UTR that are normally
inefficiently translated, such as growth promoting proteins and
oncogenic proteins (17). Through
this mechanism, eIF4E overexpression in cancer cells is associated
with cancer-related events such as transformation, angiogenesis,
invasion and metastasis (18).
Accordingly, the aberrant expression of eIF4E is reported to be
closely related to the occurrence and development of several tumors
including RCC (19).
In the present study, the expression of miR-15a was
evaluated in the RCC tissue specimens, and the functions of miR-15a
and the mechanisms involved were also investigated. We demonstrated
that miR-15a expression was significantly downregulated in RCC
specimens when compared with that of adjacent normal tissues. Its
overexpression inhibited proliferation and invasion of RCC cells,
in association with blocking cell cycle progression and inducing
cell apoptosis by directly targeting eIF4E. These data strongly
demonstrated the tumor-suppressor role of miR-15a in the
development of human RCC.
Materials and methods
Specimens
Fresh biopsy specimens of RCC and normal renal
tissues from the incisal margin were collected from 40 patients
with RCC who underwent radical surgery at The Second Affiliated
Hospital of Xi'an Jiaotong University (Xian, China) from May 2011
to July 2012. None of the patients, aged 40–75 years (mean age,
58), had received any chemotherapy, radiotherapy or other adjuvant
therapy before surgery. Informed consent was obtained from all
patients, and the present study was approved by the Ethical Review
Committee of Xi'an Jiaotong University and complied with the
Declaration of Helsinki.
Cell culture and treatment
The human renal carcinoma cell lines (ACHN, 786-O,
769-P and OS-RC-2) and normal renal cell line HK-2 were obtained
from the China Center for Type Culture Collection (CCTCC; Shanghai,
China). The cells were cultured in Dulbeccos modified Eagles medium
(DMEM) supplemented with 10% (v/v) sterile newborn calf serum
(NCBS) and antibiotics (10 U/ml penicillin and 10 µg/ml
streptomycin). The cells were then incubated at 37°C in a
humidified chamber supplemented with 5% CO2. For
transfections, miR-15a and negative control mimics, pcDNA3.1-eIF4E
and negative control plasmids were synthesized by GenePharma
(Shanghai, China) and transfected into 769-P and OS-RC-2 cells
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer's instructions.
Cell proliferation assay
Cells were transfected with miR-15a mimics or NC for
48 h, and then ~4×103 cells were plated into each well
of a 96-well plate and incubated overnight. The medium was removed,
and Cell Counting solution [Cell Counting Kit-8 (CCK-8); Beyotime,
Jiangsu, China] was added to each well and incubated for 1 h. The
absorbance of solubilized dye was assessed at 450 nm with a
microplate reader (BioTech Instruments, Winooski, VT, USA) at 24-h
intervals for 5 continuous days.
Colony formation assay
After transfection with miR-15a or NC for 48 h,
769-P and OS-RC-2 cells were trypsinized and replaced into 6-well
plates for colony formation assay. After 5 days, the cells were
fixed in 4% formaldehyde, stained with crystal violet, and the
number of colonies (>50 cells) were counted.
Matrigel invasion assay
Cell invasion assay was performed using a Transwell
chamber (8-µm pore size). After transfection with miR-15a mimics or
NC for 48 h, 769-P and OS-RC-2 cells were diluted in serum-free
DMEM and plated in the top chamber, which was pre-coated with
Matrigel (BD Biosciences, San Jose, CA, USA) to assess cell
invasion. Meanwhile, DMEM containing 10% fetal bovine serum (FBS)
was added to the bottom chamber as the chemoattractant. After
incubation for 48 h, the migrated cells on the bottom chamber were
fixed in 4% paraformaldehyde and stained with crystal violet, and
counted under an inverted microscope (Olympus, Tokyo, Japan).
Cell cycle assay
After transfection with the miR-15a mimics or NC for
36 h, 769-P and OS-RC-2 cells were trypsinized and collected. For
cell cycle analysis, the cells were fixed with ice-cold ethanol at
4°C overnight. Then, the cells were centrifuged at 1,000 rpm for 5
min at 4°C, and the pellets were treated with 50 µg/ml propidium
iodide (PI) and 100 µg/ml RNase A at room temperature in the dark
for 30 min. Cell cycle distribution was then analyzed using a flow
cytometric system (FACS; BD Biosciences).
Cell apoptosis assay
Cell apoptosis was analyzed using Annexin V/PI
apoptosis detection kit (Beyotime, Shanghai, China) according to
the manufacturer's instructions. After transfection with the
miR-15a mimics or NC for 36 h, 769-P and OS-RC-2 cells were
trypsinized and centrifuged at 1,000 rpm for 5 min, and then
resuspended with 1X binding buffer, the cells were then incubated
with 1 µl Annexin V-FITC and 5 µl PI at room temperature in the
dark for 5 min. Cell apoptosis was assessed by flow cytometry
(FACS).
Western blotting
After 36 h transfection, 769-P and OS-RC-2 cells
were treated with RIPA lysis buffer for 40 min on ice. The protein
concentration was determined using a BCA protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA), as described in the
manufacturer's manual. Lysate proteins (40 µg) were subjected to
10% SDS-PAGE and electrophoretically transferred to polyvinylidene
difluoride (PVDF) membranes. After blotting, the membranes were
blocked with 5% fat-free dry milk for 1 h and then incubated with
the specific primary antibody against eIF4E, cyclin D1, c-Myc,
MMP3, Bcl-2, P13K, p-AKT, mTOR or β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) for 2 h at 37°C. After rinsing,
membranes were hybridized with HRP-conjugated secondary antibodies
for 1 h at 37°C. The protein bands were detected using a
quantitative gel and a Western Blot Imaging System (FluorChem Q;
Alpha Innotech, San Leandro, CA, USA).
RNA isolation and quantitative
real-time PCR
Total RNA was extracted from tissue samples and cell
lines using TRIzol (Invitrogen) according to the manufactuer's
protocol. RNA was reversely transcribed into cDNA using a Reverse
Transcirption kit (Takara, Dalian, China). Quantitative real-time
PCR (qRT-PCR) analysis was performed using a SYBR-Green PCR Master
Mix (Takara) on an ABI 7900HT PCR System (Applied Biosystems,
Foster City, CA, USA). The special primers used for miR-15a, eIF4E,
U6 and β-actin were synthesized from GenScript Co., Ltd. (Nanjing,
China). The relative expression levels of the genes were quantified
using the 2−ΔΔCt method. U6 and β-actin were used as the
endogenous controls for normalization.
Bioinformatics and luciferase
assay
An online miRNA database (http://www.microrna.org/microrna/home.do) was used to
predict the potential target gene binding site of miR-15a. The
wild-type and mutant pGL3-eIF4E 3′-UTR were constructed and
co-transfected with the miR-15a mimics or NC into 769-P and OS-RC-2
cells using Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions. After 36 h of transfection, the
luciferase reporter activity was assesed using the
Renilla-Firefly Dual-Luciferase Assay kit (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Firefly luciferase activity was normalized to
Renilla luciferase activity.
Immunohistological staining
All 40-paired fresh specimens were fixed with 10%
formalin and embedded in paraffin using the standard protocol. The
slides were then deparaffinized in xylene and subsequently
rehydrated by a graded ethanol series ending in deionized water.
Any endogenous peroxidase was blocked by treatment with 3% hydrogen
peroxide for 15 min, followed by 3 rinses of 5 min each in
deionized water. Antigen retrieval was performed by placing slides
in 1X citrate buffer for 15 min at 100°C (in a microwave oven). The
rabbit anti-human eIF4E primary antibody (diluted 1:150; Cell
Signaling Technology Inc., Beverly, MA, USA) was left on tissue
samples overnight at 4°C in a humidified chamber. The secondary
antibody (ZSGB-BIO Co., Ltd., Beijing, China) was then applied to
all sections and incubated for 30 min at room temperature. Finally,
the sections were developed with 3,3′-diaminobenzidine for 5 min at
room temperature, and then counterstained with hematoxylin. After
staining, the sections were dehydrated through a graded series of
ethanol washes, cleared in xylene and cover-slipped. Positive
reactivity was detected with a diaminobenzidine kit (ZSGB-BIO Co.,
Ltd.).
Statistical analysis
Where necessary, data are expressed as the means ±
SD, and one-way ANOVA coupled with a Student's t-test were
performed to compare differences between experimental groups using
SPSS 11.5 statistical software (SPSS, Inc., Chicago, IL, USA).
Spearman's correlation analysis was applied to assess the
correlation between eIF4E expression and miR-15a in RCC tissues.
Each experiment was repeated at least 3 times. The criterion for
statistical significance was denoted as P<0.05.
Results
miR-15a is downregulated in human RCC
tissues
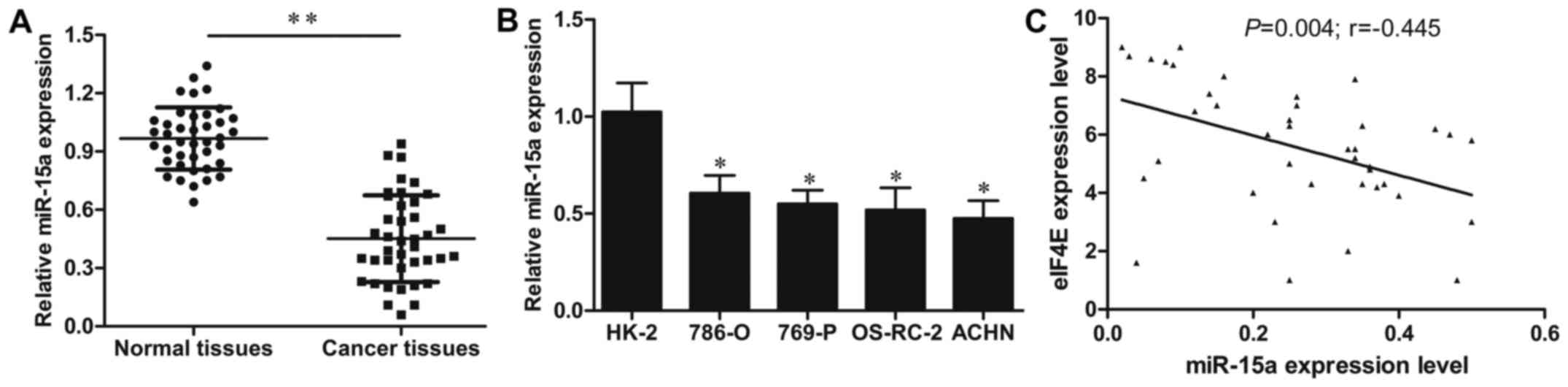
qRT-PCR method was performed to investigate the
expression levels of miR-15a in 40-paired cases of RCC and adjacent
normal tissues. As shown in Fig.
1A, the expression levels of miR-15a were significantly
decreased in RCC tissues compared to those in adjacent matched
normal tissues (P<0.01). Furthermore, the expression of miR-15a
was also detected in 4 human RCC cell lines ACHN, 786-O, 769-P,
OS-RC-2 and one normal renal cell line HK-2. The results revealed
that the expression level of miR-15a in ACHN, 786-O, 769-P and
OS-RC-2 cells was markedly decreased when compared to that in the
HK-2 cell line (all P<0.05; Fig.
1B). These findings revealed that miR-15a may be involved in
RCC progression.
miR-15a suppresses RCC cell
proliferation and invasion
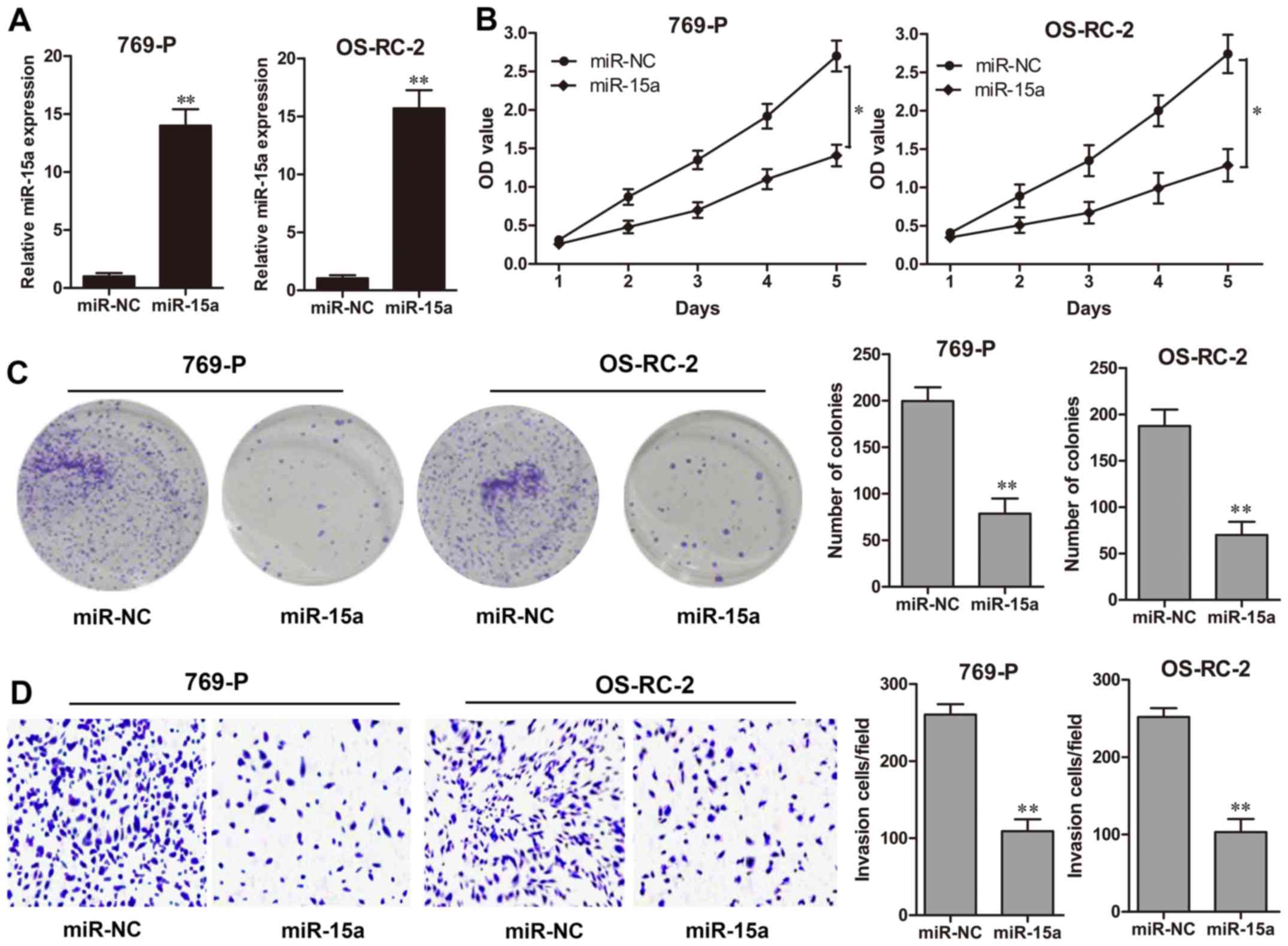
To determine the effect of miR-15a in RCC
development, miR-15a mimics were transfected into the RCC cell
lines 769-P and OS-RC-2 for 48 h, which exhibited a high
transfection efficiency (P<0.01, respectively; Fig. 2A). A CCK-8 assay revealed that
miR-15a significantly suppressed the cell growth rate of 769-P and
OS-RC-2 cells during the 5 days of detection (Fig. 2B). The colony formation assay also
revealed that the colony formation abilities of 769-P and OS-RC-2
cells transfected with the miR-15a mimics were significantly
decreased compared to those in the NC groups (P<0.01,
respectively; Fig. 2C). In
addition, a Matrigel invasion assay demonstrated that
overexpression of miR-15a markedly decreased the number of invasive
cells for 769-P and OS-RC-2 cells in comparison to the NC groups
(P<0.01, respectively; Fig.
2D).
miR-15a induces apoptosis and causes
G0/G1 cell cycle arrest in RCC cells
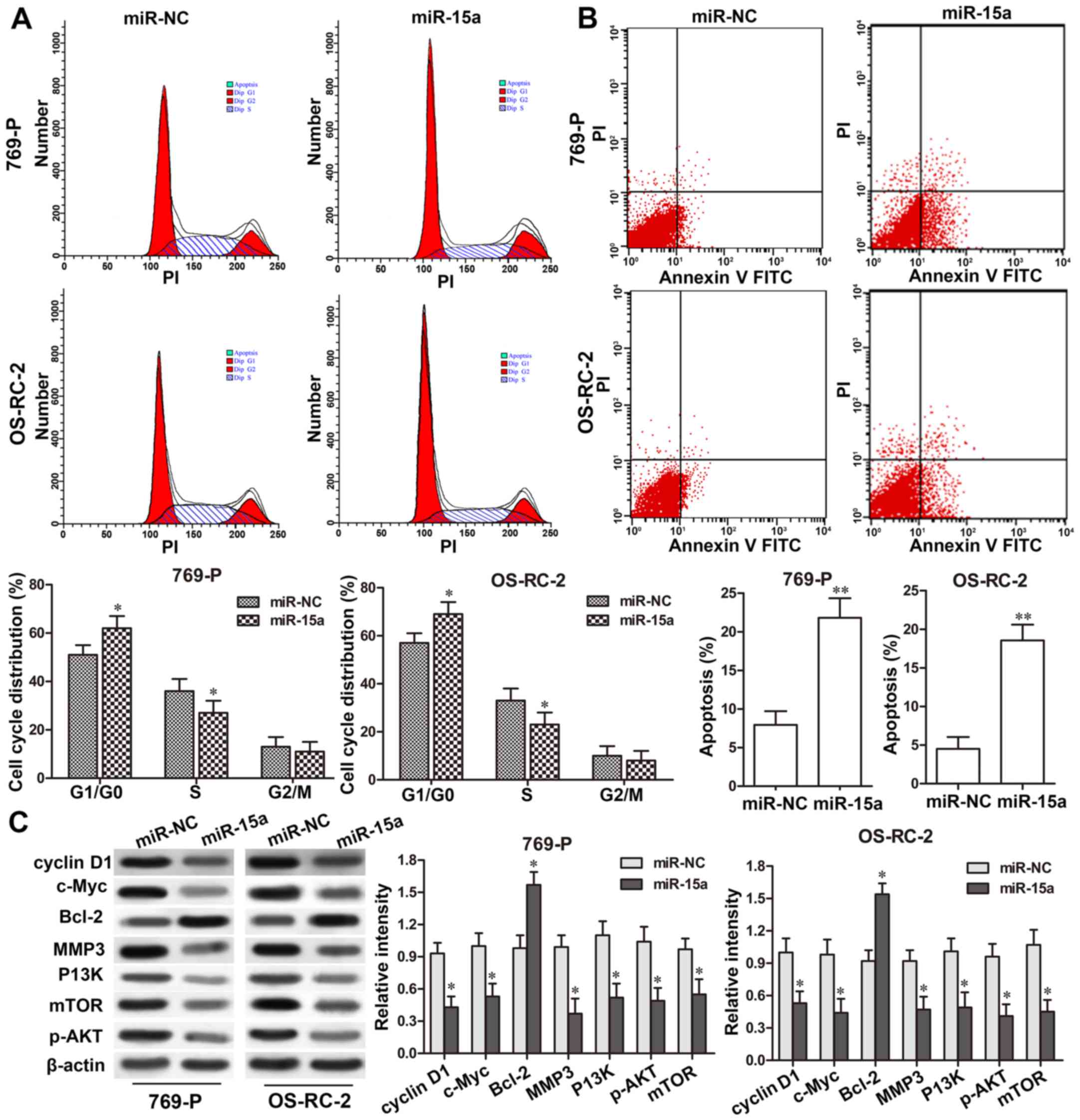
The cell cycle distribution in 769-P and OS-RC-2
cells after miR-15a-mimic transfection was determined using flow
cytometry. As shown in Fig. 3A,
miR-15a overexpression markedly increased the proportion of
G1/G0-phase cells in the 769-P and OS-RC-2 cells, when compared
with that of the NC groups (P<0.05, respectively); while it
decreased the percentage of S-phase cells in the 769-P and OS-RC-2
cells compared with that of the NC groups (P<0.05,
respectively). These findings indicated that miR-15a overexpression
could cause cell G1/G0-phase arrest and result in the decrease of
G1 to S phase transition. Furthermore, to evaluate the function of
miR-15a on RCC cell apoptosis, a flow cytometric assay was also
performed using Annexin V and PI staining. The results revealed
that transfection of 769-P and OS-RC-2 cells with miR-15a mimics
markedly induced cell apoptosis in comparison to the NC groups
(P<0.01; respectively, Fig. 3B),
indicating that miR-15a promoted apoptosis in RCC cells.
To further validate the effect of miR-15a on RCC
cell proliferation, apoptosis and invasion-related proteins, the
expression levels of cyclin D1, c-Myc, Bcl-2, MMP3 were detected in
769-P and OS-RC-2 cells with miR-15a-mimic transfection. Western
blot assay revealed that overepxression of miR-15a downregulated
the expression of cyclin D1, cyclin E, Bax, c-Myc and MMP3 (all
P<0.05), but upregulated the expression of Bcl2 (P<0.05;
Fig. 3C) significantly. In
addition, the P13K/AKT/mTOR signaling pathway was also detected,
and the results revealed that the expression levels of P13K, mTOR
and phosphorylated (p)-AKT were significantly decreased in 769-P
and OS-RC-2 cells with miR-15a-mimic transfection compared to those
in the NC groups (all P<0.05; Fig.
3C).
eIF4E serves as a target of
miR-15a
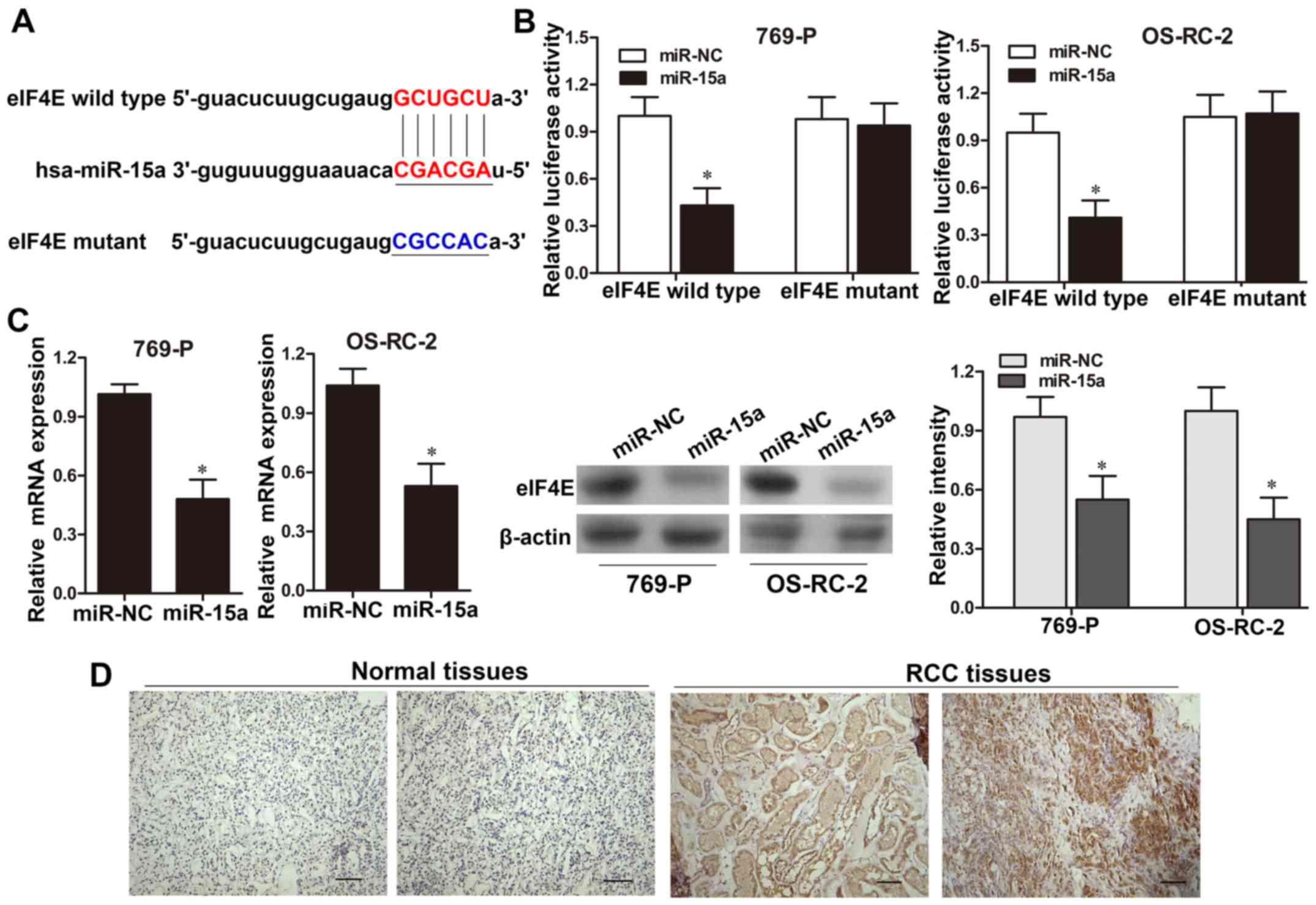
Using bioinformatic analysis (microRNAs database),
we found that the 3′-UTR of eIF4E possessed a putative target for
miR-15a (Fig. 4A). A luciferase
reporter assay was applied to determine the functional binding site
of miR-15a. The results revealed that the luciferase reporter
activity was significantly decreased in 769-P and OS-RC-2 cells
co-transfected with the pGL3-eIF4E-wild-type 3′-UTR and miR-15a
mimics, when compared with those co-tranfected with NC (P<0.05,
Fig. 4B). However, no significant
change was detected in 769-P and OS-RC-2 cells co-transfected with
pGL3-eIF4E-mutant construct (Fig.
4B). Moreover, after transfection 769-P and OS-RC-2 cells with
miR-15a or NC, qRT-PCR and western blot analysis showed that eIF4E
expression was significantly decreased in the miR-15a-mimic groups
compared with that of the NC groups at both the mRNA and protein
levels (P<0.05, respectively; Fig.
4C). These results revealed that miR-15a may directly bind to
the 3′-UTR region of eIF4E and suppress eIF4E expression. To
validate this hypothesis, we then examined the eIF4E expression
levels in 40 cases of RCC by immunohistochemical staining and
qRT-PCR assays. The results from immunohistochemical staining
revealed that eIF4E was barely detectable in normal renal tissues,
while it exhibited positive nuclear immunostaining in 26 out of 40
cancer tissues (Fig. 4D).
Furthermore, the correlation between miR-15a and eIF4E mRNA
expression in 40 cases of RCC tissues was assessed by Spearman's
correlation analysis. As shown in Fig.
1C, a significant negative correlation was observed between the
mRNA levels of eIF4E and miR-15a expression (r=−0.445;
P<0.01).
eIF4E is involved in the regulation of
RCC cell proliferation and invasion by miR-15a
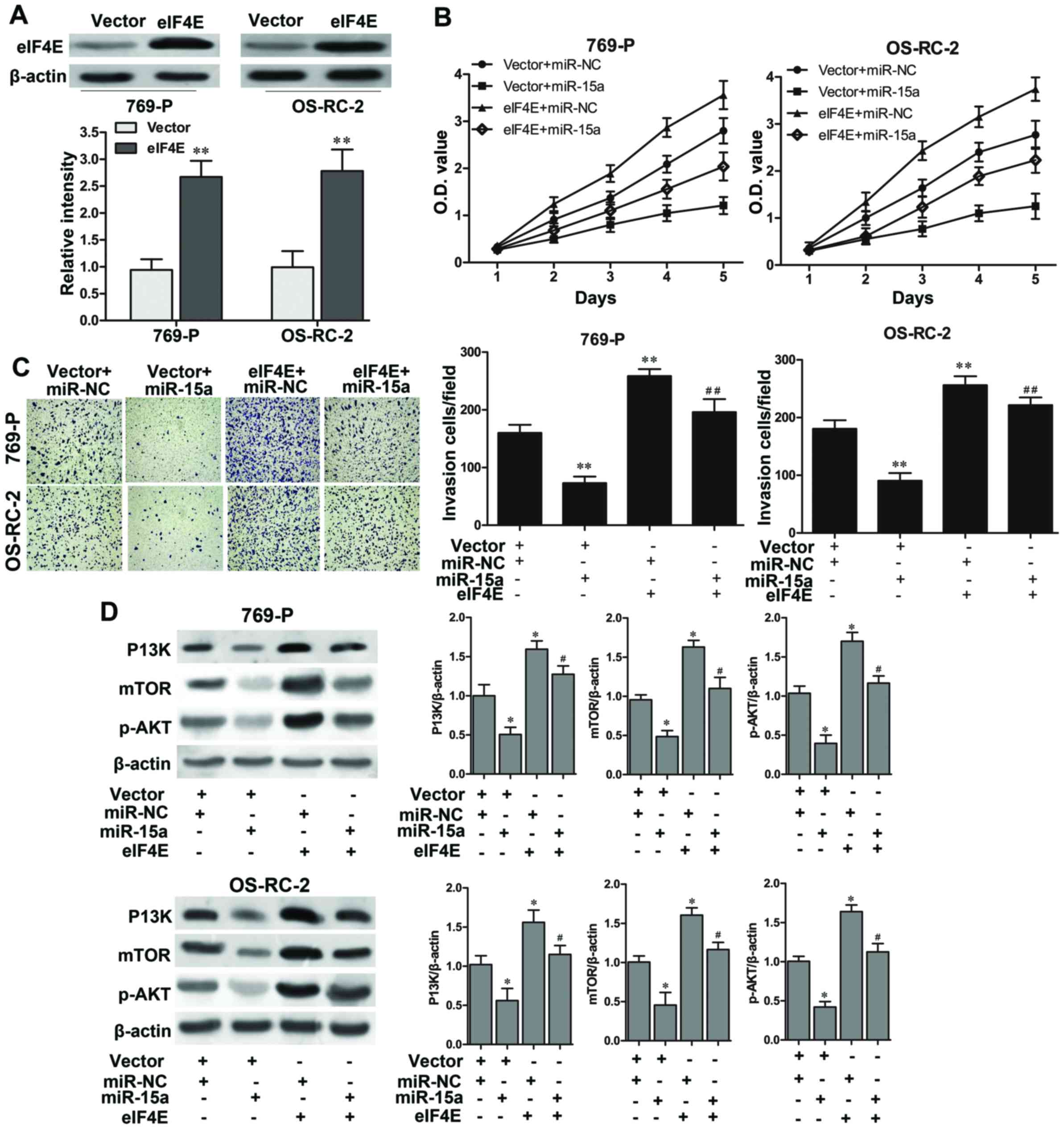
A rescue experiment was performed to further
determine that miR-15a suppressed the proliferation and invasion of
RCC cells by directly regulating eIF4E. As shown in Fig. 5A, eIF4E was significantly
overexpressed in 769-P and OS-RC-2 cells with eIF4E-plasmid
transfection (P<0.01, respectively). A CCK-8 assay revealed that
the cell growth rate was significantly enhanced in the 769-P and
OS-RC-2 cells with eIF4E overexpression, and partially reversed the
suppression of cell growth resulted from the miR-15a mimics
(Fig. 5B). Furthermore, a Matrigel
invasion assay also illustrated that overexpression of eIF4E
significantly promoted cell invasive abilities in 769-P and OS-RC-2
cells (P<0.01, respectively), and eIF4E markedly abolished the
inhibition of cell invasion caused by the miR-15a mimics (P<0.01
vs. the vector + miR-15a, respectively; Fig. 5C). Furthermore, the western blot
results also revealed that overexpression of eIF4E significantly
upregulated the expression of P13K, mTOR and p-AKT (all P<0.05),
and blocked the influence of miR-15a mimics on the activation of
the P13K/AKT/mTOR signaling pathway (all P<0.05 vs. the vector +
miR-15a; Fig. 5D). Collectively,
these findings demonstrated that eIF4E was involved in
miR-15a-induced inhibition of cell proliferation and invasion of
RCC cells which may be mediating by the activation of the
P13K/AKT/mTOR signaling pathway.
Discussion
Since RCC ususally presents with metastasis at the
time of diagnosis in adults, identifying valuable epigenetic
biomarkers that could better improve the accuracy of diagnosis and
treatment are urgently needed (20). Increasing evidence has demonstrated
that aberrant expression of various miRs contribute to the
pathogenesis and tumorigenesis of cancers through their
participation in many cellular processes, including cell
proliferation, invasion and angiogenesis (21). In the present study, we revealed
that miR-15a was significantly downregulated in RCC tissues and
cell lines. Ectopic expression of miR-15a suppressed RCC cell
proliferation and invasion in vitro. Furthermore, eIF4E was
identified as a direct and functional target of miR-15a and
partially reversed the miR-15a-induced suppression of RCC cell
proliferation and invasion. These data strongly revealed a possible
tumor-suppressor role for miR-15a in RCC development.
The downregualtion of miR-15a has been reported to
be associated with cell proliferation and metastases in several
types of cancer. Yang et al revealed that miR-15a acted as a
tumor suppressor in non-small cell lung cancer by inducing cell
apoptosis and inhibiting metastasis (12). Alderman et al found that
miR-15a inhibited the proliferation and invasiveness of melanoma
cells by directly targeting the CDCA4 gene (13). Li et al demonstrated that
ectopic expression of miR-15a regulated cell cycle arrest at the G1
phase and potentiated apoptosis in breast cancer cells (22). Consistent with the previous studies,
using CCK-8 proliferation and colony formation assays we found that
overexpresison of miR-15a significantly inhibited the cell growth
of RCC cells. Furthermore, the flow cytometric assay confirmed that
miR-15a mimic arrested RCC cell cycle by inhibiting the G1 and S
phase transition and induced cell apoptosis. Supplementary evidence
also revealed the inhibitory effect of miR-15a on RCC cell
metastases. Consequently, these findings demonstrated that miR-15a
served as a tumor suppressor in the progression of RCC cells, and
may be used as a potential therapeutic biomarker for RCC
treatment.
Previously, aberrant upregulation of eIF4E has been
found in many tumors, such as head and neck squamous cell
carcinoma, lung cancer, breast cancer, and thyroid cancer (23). Silencing of eIF4E revealed cell
proliferation and arrested the cell cycle at the G0/G1 phase, and
inhibition of eIF4E could decrease the invasion of cancer cells
(24). Furthermore, accumulating
studies reveal that overexpression of eIF4E is sufficient to
abrogate apoptosis and lead to chemoresistance and radio-resistance
in cancer cells (25,26). Recent studies reveal evidence that
eIF4E is maintained at levels in excess for normal development that
are hijacked by cancer cells to drive a translational program
supporting tumorigenesis, such as proliferation and
survival-promoting associated proteins cyclin D1, c-Myc, vascular
endothelial growth factor (VEGF), survivin and Bcl-2 (27,28).
These findings indicate that targeting the expression of eIF4E may
provide a potential molecular target for cancer therapy. For
example, eIF4E can be suppressed by some miRNAs. Li et al
demonstrated that microRNA-497 modulated gastric cancer cell
proliferation and invasion by suppressing eIF4E (29). Liu et al also pointed out
that miR-34c-3p functioned as a tumor suppressor by inhibiting
eIF4E in non-small cell lung cancer (30).
In the present study, bioinformatic analysis
determined eIF4E to be a putative target for miR-15a. The
luciferase assay using a reporter containing the wild-type miR-15a
binding sequence at the 3′-UTR of eIF4E mRNA indicated that the
luciferase activity could be significantly decreased by
overexpression of miR-15a. eIF4E participates in nuclear mRNA
export and translation of specific transcripts, which are necessary
for the promotion of cell proliferation, survival and metastases.
In the present study, eIF4E was found to be both overexpressed and
highly enriched in the nucleus of these specimens. Endogenous
expression of miR-15a could downregulate the expression of eIF4E
mRNA and protein levels in RCC cells. Furthermore, miR-15a levels
were found to be inversely correlated with mRNA expression of eIF4E
in RCC tissues, suggesting that miR-15a suppressed RCC development
by directly targeting endogenous eIF4E.
In the present study, ectopic expression of eIF4E in
RCC cells could partially rescue the inhibitory effect of miR-15a
overexpression on cell proliferation and invasion. Furthermore,
ectopic expression of eIF4E activated the PI3K/Akt/mTOR signaling
pathway, which was suppressed by miR-15a. It has also been
demonstrated that eIF4E is the crossing point of the PI3K/Akt/mTOR
signaling pathway and the Ras/Raf/MEK/ERK/Mnks signaling pathway
(15,31). The phosphoinositide 3-kinase/
protein kinase B/mammalian target of rapamycin (P13K/AKT/mTOR) is a
crucial and intensively explored intracellular signaling pathway in
tumorigenesis (32). PI3K signaling
leads to phosphorylation of Akt and the activation of protein
translation initiation via mTOR (upstream and downstream of mTOR),
a phenomenon which has been widely investigated in various types of
cancer and other aggressive diseases (33,34).
Activation of the P13K/AKT/mTOR signaling pathway mediated through
molecular aberrations such as eIF4E is instrumental in promoting
RCC development including cell proliferation, apoptosis and
migration as well as resistance to anticancer therapies (35,36).
Consequently, in the present study, we surmised that miR-15a
activated the P13K/AKT/mTOR signaling pathway and it downstream
factors through binding to eIF4E in RCC cells.
In summary, miR-15a expression was found to be
downregulated in RCC clinical specimens and cell lines.
Overexpression of miR-15a played a crucial role in suppressing cell
growth and invasion, causing cell cycle arrest and inducing cell
apoptosis in the progression of RCC by directly targeting eIF4E,
thus suggesting the potential of miR-15a to function as a tumor
suppressor and a therapeutic biomarker in RCC development.
Acknowledgements
The present study was supported by the Science and
Technique Project of Xi'an [SF1418 (1)].
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buonerba C, Di Lorenzo G and Sonpavde G:
Combination therapy for metastatic renal cell carcinoma. Ann Transl
Med. 4:1002016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Escudier B: Advanced renal cell carcinoma:
Current and emerging management strategies. Drugs. 67:1257–1264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Afriansyah A, Hamid AR, Mochtar CA and
Umbas R: Targeted Therapy for Metastatic Renal Cell Carcinoma. Acta
Med Indones. 48:335–347. 2016.PubMed/NCBI
|
|
6
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simonson B and Das S: MicroRNA
therapeutics: The next magic bullet? Mini Rev Med Chem. 15:467–474.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah MY, Ferrajoli A, Sood AK,
Lopez-Berestein G and Calin GA: MicroRNA therapeutics in cancer -
an emerging concept. EBioMedicine. 12:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer - a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Xin S, He Z, Che X, Wang J, Xiao X,
Chen J and Song X: MicroRNA-21 (miR-21) post-transcriptionally
downregulates tumor suppressor PDCD4 and promotes cell
transformation, proliferation, and metastasis in renal cell
carcinoma. Cell Physiol Biochem. 33:1631–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Gu M, Zhang K, Zhou J, Wang Z and Da
J: miR-203 inhibition of renal cancer cell proliferation, migration
and invasion by targeting of FGF2. Diagn Pathol. 10:242015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang T, Thakur A, Chen T, Yang L, Lei G,
Liang Y, Zhang S, Ren H and Chen M: MicroRNA-15a induces cell
apoptosis and inhibits metastasis by targeting BCL2L2 in non-small
cell lung cancer. Tumour Biol. 36:4357–4365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alderman C, Sehlaoui A, Xiao Z and Yang Y:
MicroRNA-15a inhibits the growth and invasiveness of malignant
melanoma and directly targets on CDCA4 gene. Tumour Biol.
37:13941–13950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Q, Li X, Li J, Kong X, Zhang J, Chen
L, Huang Y and Fang L: MiR-15a is underexpressed and inhibits the
cell cycle by targeting CCNE1 in breast cancer. Int J Oncol.
43:1212–1218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqui N and Sonenberg N: Signalling to
eIF4E in cancer. Biochem Soc Trans. 43:763–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sonenberg N: eIF4E, the mRNA cap-binding
protein: From basic discovery to translational research. Biochem
Cell Biol. 86:178–183. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Y, Polunovsky V, Bitterman PB and
Wagner CR: Cap-dependent translation initiation factor eIF4E: An
emerging anticancer drug target. Med Res Rev. 32:786–814. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Truitt ML, Conn CS, Shi Z, Pang X,
Tokuyasu T, Coady AM, Seo Y, Barna M and Ruggero D: Differential
requirements for eIF4E dose in normal development and cancer. Cell.
162:59–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campbell L, Jasani B, Griffiths DF and
Gumbleton M: Phospho-4e-BP1 and eIF4E overexpression
synergistically drives disease progression in clinically confined
clear cell renal cell carcinoma. Am J Cancer Res. 5:2838–2848.
2015.PubMed/NCBI
|
|
20
|
Stewart GD, O'Mahony FC, Powles T, Riddick
ACP, Harrison DJ and Faratian D: What can molecular pathology
contribute to the management of renal cell carcinoma? Nat Rev Urol.
8:255–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Xie XB, Chen Q, Pang GL, Luo W, Tu
JC, Zheng F, Liu SM, Han L, Zhang J-K, et al: MiRNA-15a mediates
cell cycle arrest and potentiates apoptosis in breast cancer cells
by targeting synuclein-γ. Asian Pac J Cancer Prev. 15:6949–6954.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Graff JR, Konicek BW, Carter JH and
Marcusson EG: Targeting the eukaryotic translation initiation
factor 4E for cancer therapy. Cancer Res. 68:631–634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan J, Shi F, Xu Z and Zhao M: Knockdown
of eIF4E suppresses cell proliferation, invasion and enhances
cisplatin cytotoxicity in human ovarian cancer cells. Int J Oncol.
47:2217–2225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Fan S, Koo J, Yue P, Chen ZG,
Owonikoko TK, Ramalingam SS, Khuri FR and Sun SY: Elevated
expression of eukaryotic translation initiation factor 4E is
associated with proliferation, invasion and acquired resistance to
erlotinib in lung cancer. Cancer Biol Ther. 13:272–280. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martineau Y, Azar R, Bousquet C and
Pyronnet S: Anti-oncogenic potential of the eIF4E-binding proteins.
Oncogene. 32:671–677. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsieh AC and Ruggero D: Targeting
eukaryotic translation initiation factor 4E (eIF4E) in cancer. Clin
Cancer Res. 16:4914–4920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu F, Wang X, Li J, Gu K, Lv L, Zhang S,
Che D, Cao J, Jin S and Yu Y: miR-34c-3p functions as a tumour
suppressor by inhibiting eIF4E expression in non-small cell lung
cancer. Cell Prolif. 48:582–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raught B and Gingras AC: eIF4E activity is
regulated at multiple levels. Int J Biochem Cell Biol. 31:43–57.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Shi H, Tang H, Fang Z, Wang J and
Cui S: miR-218 inhibits the invasion and migration of colon cancer
cells by targeting the PI3K/Akt/mTOR signaling pathway. Int J Mol
Med. 35:1301–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dancey JE: Therapeutic targets: MTOR and
related pathways. Cancer Biol Ther. 5:1065–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue
P, Fu H and Khuri FR: Activation of Akt and eIF4E survival pathways
by rapamycin-mediated mammalian target of rapamycin inhibition.
Cancer Res. 65:7052–7058. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ilic N, Utermark T, Widlund HR and Roberts
TM: PI3K-targeted therapy can be evaded by gene amplification along
the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis.
Proc Natl Acad Sci USA. 108:E699–E708. 2011. View Article : Google Scholar : PubMed/NCBI
|