Introduction
Human melanoma is one of the most aggressive
cutaneous and mucosal cancers, and is characterized by different
genetic alterations that are dependent upon body site and the
extent of ultraviolet exposure (1).
The incidence of cutaneous melanoma has been increasing rapidly,
although, relatively few new chemotherapeutic agents have been
approved. This contrasts with the use of checkpoint immune therapy
for melanoma which has had a significant impact on melanoma
outcomes. The treatment of in-transit and satellite melanoma
metastases are challenging and the treatment options for these
cutaneous and subcutaneous lesions, including surgical excision,
radiotherapy, electro-chemotherapy and cryotherapy, have suboptimal
response rates (2).
Methionine enkephalin (MENK), an inhibitory growth
factor, binds to opioid growth factor receptor (OGFR) in a
selective manner, upregulating cyclin-dependent inhibitory kinases,
and thus potentially regulating tumor cell replication. MENK is
produced in an autocrine or paracrine manner and is modulated by
the cells themselves or the extracellular matrix (3,4). MENK,
known as an endogenous opioid peptide, was found to be an important
inhibitor of human cancer cell proliferation (5,6). There
are 3 MENK receptors that were identified more than 3 decades ago:
specifically the µ-, δ- and κ-opioid receptor (OR). The inhibitory
effects of MENK on cell replication were first reported from the
developing brains of rats (7,8), then,
in tissue culture studies using mouse and human neuroblastoma cell
lines (9–12). MENK inhibits DNA synthesis and cell
replication of normal cells and tissues (13,14),
human tumor cells (15), and
bacteria (16). The main function
of MENK is to upregulate inhibitory kinases in the cell cycle
process. The activity of MENK can be regulated by the potent and
long-acting opioid receptor antagonist naltrexone (NTX), resulting
in increased DNA synthesis and cell division.
At present, there is no published data examining the
response of murine melanoma cell lines and tumors by MENK. We
investigated the mechanism underlying the therapeutic effect of
MENK on human melanoma, and confirmed that MENK can inhibit the
progression, aggression and metastasis of melanoma cells. Based on
our studies reported herein, we conclude that MENK provides a
potential therapeutic strategy for the treatment of neoplasia and
other immunosuppressive conditions, and we may further identify the
cytotoxic activity and mechanisms of action of melanoma regulation,
both in vivo and in vitro.
Materials and methods
Cell culture
B16 mouse melanoma cells were provided by No. 1
Hospital, China Medical University (Shenyang, China), and cultured
in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal calf serum (FCS;
Biological Industries, Kibbutz Beit Haemek, Israel), 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were cultured at 37°C
in 5% CO2, and cells in the exponential phase of growth
were used for subsequent experiments.
Reagents
MENK (≥97% purity) was provided by Penta Biotech,
Inc. (Union City, CA, USA). Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) kits were purchased from Takara
Bio, Inc. (Otsu, Japan). TRIzol® was obtained from
Invitrogen; Thermo Fisher Scientific, Inc. Propidium iodide (PI),
dimethyl sulfoxide and thymidine were purchased from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany).
MENK co-culture with B16 cells
B16 cells were treated with various concentrations
of MENK (0, 2.5, 5, 10, 12.5 and 15 mg/ml in RPMI-1640) for various
times (24, 48, 72 and 96 h). Cell viability was evaluated by MTS
assays and FCM.
Tumor model and MENK
administration
Syngenic mice (C57Bl/6) at 6–8 weeks of age were
injected with B16 melanoma cells at a subcutaneous site. C57BL/6
mice (18–21 g) were obtained from Slac Laboratory Animals Co. Ltd.
(Shanghai, China). All experiments with animals were conducted in
pathogen-free conditions according to the guidelines of the NIH
Guide for the Care and Use of Laboratory Animals. B16 cells
(2.0×106/ml) were subcutaneously injected into C57BL/6
mice (0.2 ml/mouse). Twenty-four hours after tumor cell injection,
the mice were divided into 3 groups (10 mice/group). Mice in the
MENK group were injected (i.p.) with MENK (20 mg/kg; ≥97 purity;
Penta Biotech, Inc.) once a day, consecutively for 30 days
(17). Mice in the MENK and NTX
groups were injected with NTX at 10 mg/kg by i.p. injection and 30
min later with MENK using the same schedule of administration. Mice
in the vehicle control group received normal saline (NS) only. The
mice were observed daily and when tumors became palpable, the
shortest/longest diameters of the tumors were measured with a
Vernier caliper every 3 days. Final tumor volume (mm3)
(V) was calculated via the following formula: V = (shortest
diameter)2 × (longest diameter) × 0.5 (18).
Cell growth and cell cycle
analysis
MTS assay was carried out to determine the optimal
concentration and incubation time for MENK. B16 cells were
collected as target cells and seeded into 96-well plates
(3×103/well). The B16 proliferation in each well was
determined 3 h following plating by measuring the optical density
at 570 nm using a bichromatic microplate reader.
The effect of MENK on B16 cell cycle was analyzed
using FCM. B16 cells were seeded in 6-well plates for 48 h
(5×105/well). The cells were trypsinized, washed with
phosphate-buffered saline (PBS), and fixed in 70% ethanol. Prior to
flow cytometric analysis, fixed cells were stained with 0.5 mg/ml
PI in PBS containing 50 mg/ml RNase A. Cells were acquired on a
flow cytometer and the data were analyzed using ModiFit LT™
software version 4.0 (BD Biosciences, Franklin Lakes, NJ, USA).
RT-qPCR analysis of MENK-associated
opioid receptors
The mRNA expression levels of µOR (MOR), δOR (DOR)
and κOR (KOR) were detected by RT-qPCR. B16 cells
(3×106/well) were divided into 3 groups: MENK (12.5
mg/ml), MENK+NTX 1 mg/ml; and RPMI-1640 medium and cultured for 48
h. Total RNA was extracted using TRIzol, and cDNA was synthesized
using reverse transcriptase. Aliquots of cDNA were used as the
template for qPCR reactions containing primers for MOR, DOR, KOR or
β-actin. The primers were synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. and had the following oligonucleotide sequences:
forward, 5′-TGCTCCTGGCTCAACTTGTCC-3′ and reverse,
5′-GCGTGCTAGTGGCTAAGGCATCTG-3′ for MOR; forward,
5′-CCATCCACATCTTCGTCATCGTCTG-3′ and reverse,
5′-TCGTCCAGGAAGGCGTAGAGAAC-3′ for DOR; forward,
5′-TCTCCCAGTGCTTGCCTTCTCC-3′ and reverse,
5′-TTGCGGTCTTCATCTTCGTGTATCG-3′ for KOR; and forward,
5′-TTCCAGCGTTCCTTCTTGGGTAT-3′ and reverse,
5′-GTTGGCATAGAGGTGTTTACGG-3′ for β-actin. The qPCR reactions were
performed using an Applied Biosystems 7500 Real-Time PCR System
(Thermo Fisher Scientific, Inc.) and SYBR® Premix Ex Taq
II. The cycling conditions were as follows: an initial denaturation
step at 95°C for 2 min, followed by 40 cycles of denaturation at
95°C for 15 sec and annealing at 60°C for 1 min. Data were
normalized to β-actin using the 2−ΔΔCq method (19).
Apoptosis analysis
The induction of apoptosis was analyzed by FCM.
Apoptosis was assessed by labeling B16 cells with Annexin
V-fluorescein isothiocyanate (FITC) and PI (BD Biosciences)
according to the manufacturer's protocol. The samples were acquired
on a FACSCalibur flow cytometer (BD Biosciences) and analyzed by
ModiFit LT software version 4.0 or WinMDI version 2.9 (The Scripps
Research Institute, La Jolla, CA, USA).
Transwell invasion assay
The invasive ability of cells was determined by
Transwell assay. The 8-µm pore polycarbonate filters were coated
with extracellular matrix (50 µg/filter; Sigma-Aldrich; Merck
Millipore); 500 µl (2×105) cells were added to the upper
chamber and 500 µl RPMI-1640 with 10% FCS was pipetted into the
lower chamber. The non-invasive cells in the upper chamber were
gently wiped-off 16 h later. The cells that penetrated to the lower
chamber were stained with crystal violet, imaged and counted. Each
experiment was performed in triplicate.
Tumor morphology
Tumor tissues from each mouse were fixed in 4%
paraformaldehyde at 4°C for histological assessment. After
fixation, the tissues were dehydrated with a graded series of
ethanol and embedded in paraffin. Thin sections (4-µm) prepared
from the paraffin-embedded tissues were mounted on glass slides,
and deparaffinized with xylene. Then, tissues were mounted on glass
slides and stained with H&E for light microscopic examination.
For morphological studies, 3 randomly selected sections were
photographed using a ×100 objective lens (Olympus BX-51) locating
the tumor as described previously (21) (Olympus, Tokyo, Japan).
Analysis of macrophages and Th
lymphocyte subsets by FCM
Splenocytes were separated and tested for membrane
phenotypes CD4, CD8, CD3 and NK cells by FCM. Splenocytes were
prepared as previously described. The digested and processed
splenocytes were filtered by a 200-micron nylon cell strainer (BD
Biosciences) in PBS containing 2% FCS to obtain single-cell
suspensions and red blood cells were lysed. Isolated splenocytes
were placed in 1 ml of staining buffer at 1×106
cells/ml. The following antibodies were utilized: anti-CD3 (APC),
anti-CD4 (PE), anti-CD8 (FITC) and anti-NK (PE).
All cells were analyzed using FACSCalibur (BD
Biosciences). The percentage of positive cell staining for each
antibody was determined by comparing test samples with an isotype
control. Data were analyzed by FCS Express Version 3 software.
ELISA assay for cytokines
The production of cytokines, IL-2, TNF-α and IFN-γ
in the sera obtained by retinal orbital bleed was measured using
ELISA kits from eBioscience (San Diego, CA, USA).
Statistical analysis
All experiments were performed in triplicate at a
minimum. Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). Groups were compared using
one-way analyses of variance followed by a post hoc test S-N-K.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MENK inhibits B16 cell
proliferation
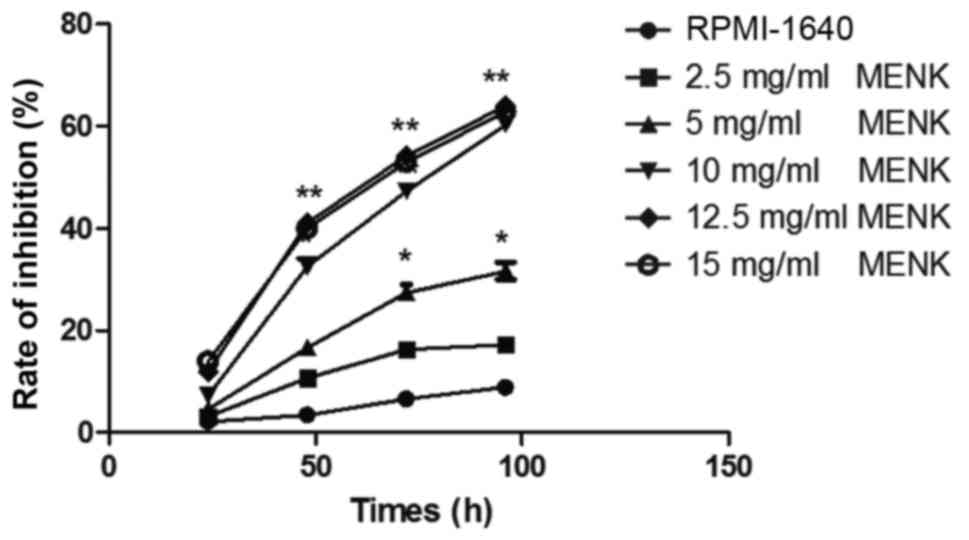
The highest extent of B16 inhibition was observed at
concentrations of MENK >10 mg/ml. As compared to the inhibition
observed in the control group following 48 h of incubation, cell
proliferation in the 12.5 mg/ml MENK-treated group was reduced by
41.06% (P<0.001; Fig. 1). The
viability of B16 cells treated with MENK was reduced in a dose- and
time-dependent manner.
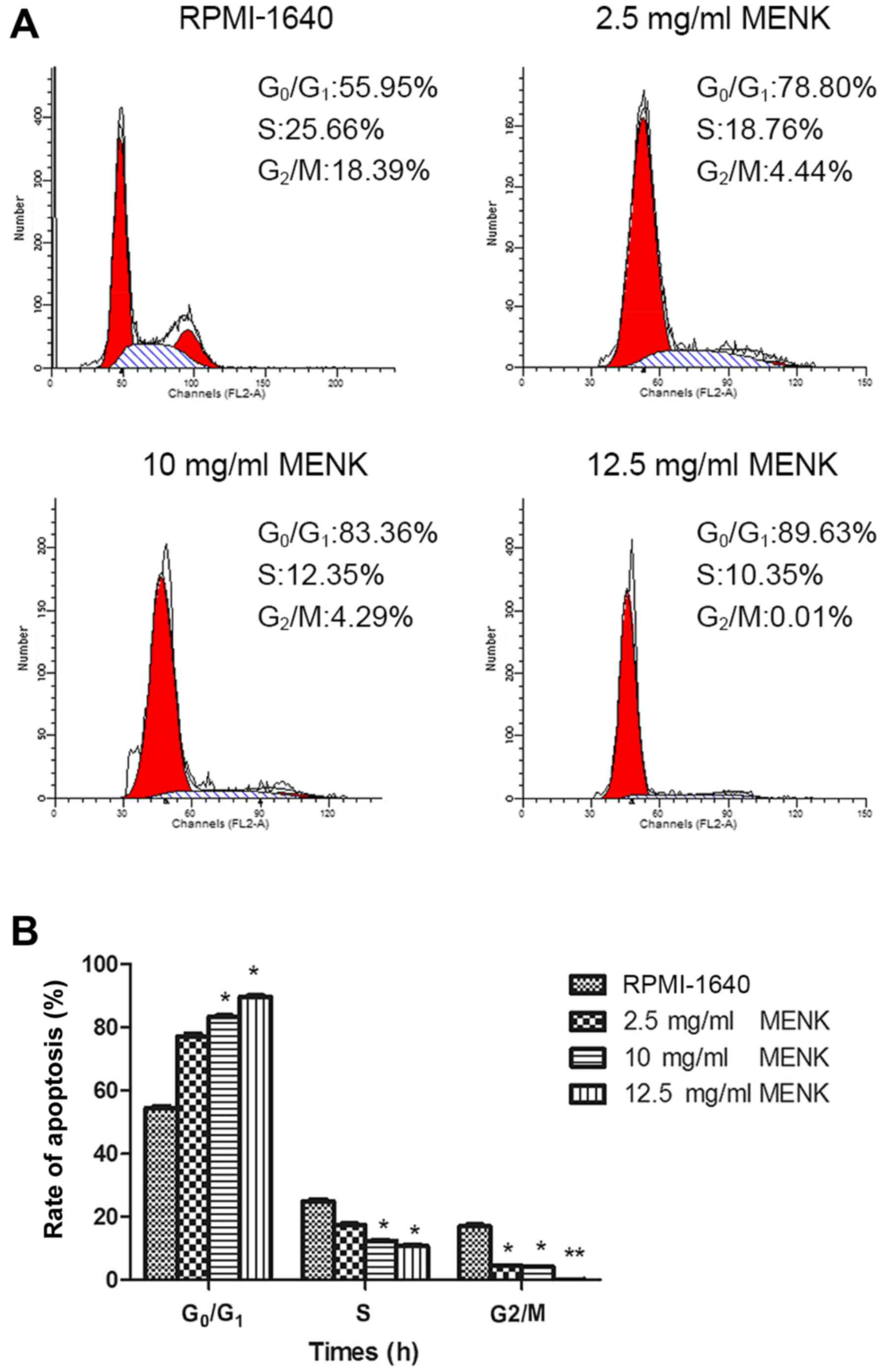
MENK induces cell cycle arrest
MENK reduced B16 cell proliferation and restricted
them to the G0-G1 phase of the cell cycle (Fig. 2A). The percentage of B16 cells in
the G0/G1 phase cultured with 12.5 mg/ml MENK at 48 h was 89.63%,
as compared to 55.95% in control group (P=0.015; Fig. 2B). Furthermore, the percentage of
cells in the S phase cultured with 12.5 mg/ml MENK was decreased to
10.35% compared to 25.66% of the control cells (P=0.004). The
percentage of B16 cells in the G2/M phase following treatment with
12.5 mg/ml MENK for 48 h was 0.01% compared with 18.39% in the
control cells (P=0.24).
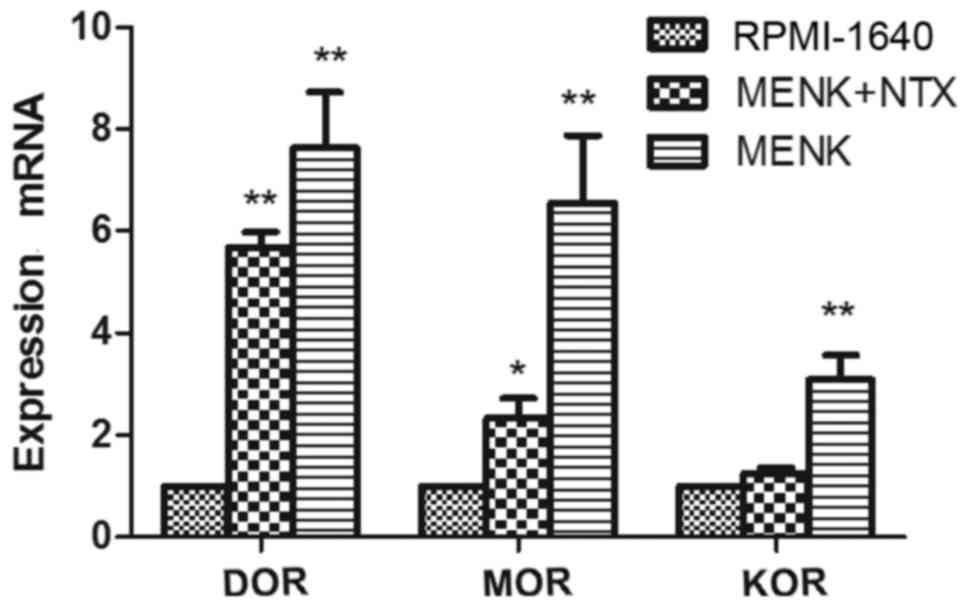
Culture of B16 cells with MENK
increases mRNA opioid receptor expression
Following treatment with 12.5 mg/ml MENK, mRNA
transcription of MOR, DOR and KOR by B16 cells was determined by
RT-qPCR (Fig. 3). Their expression
levels were upregulated post-treatment with MENK and the expression
levels were blocked by NTX. Expression levels of DOR, MOR and KOR
were 6.68-, 5.46- and 2.81-fold increased, compared to the control
group. These differences reached statistical significance for DOR
(P<0.001) and MOR (P=0.001). This increased expression of all 3
ORs was attenuated by NTX (P=0.004).
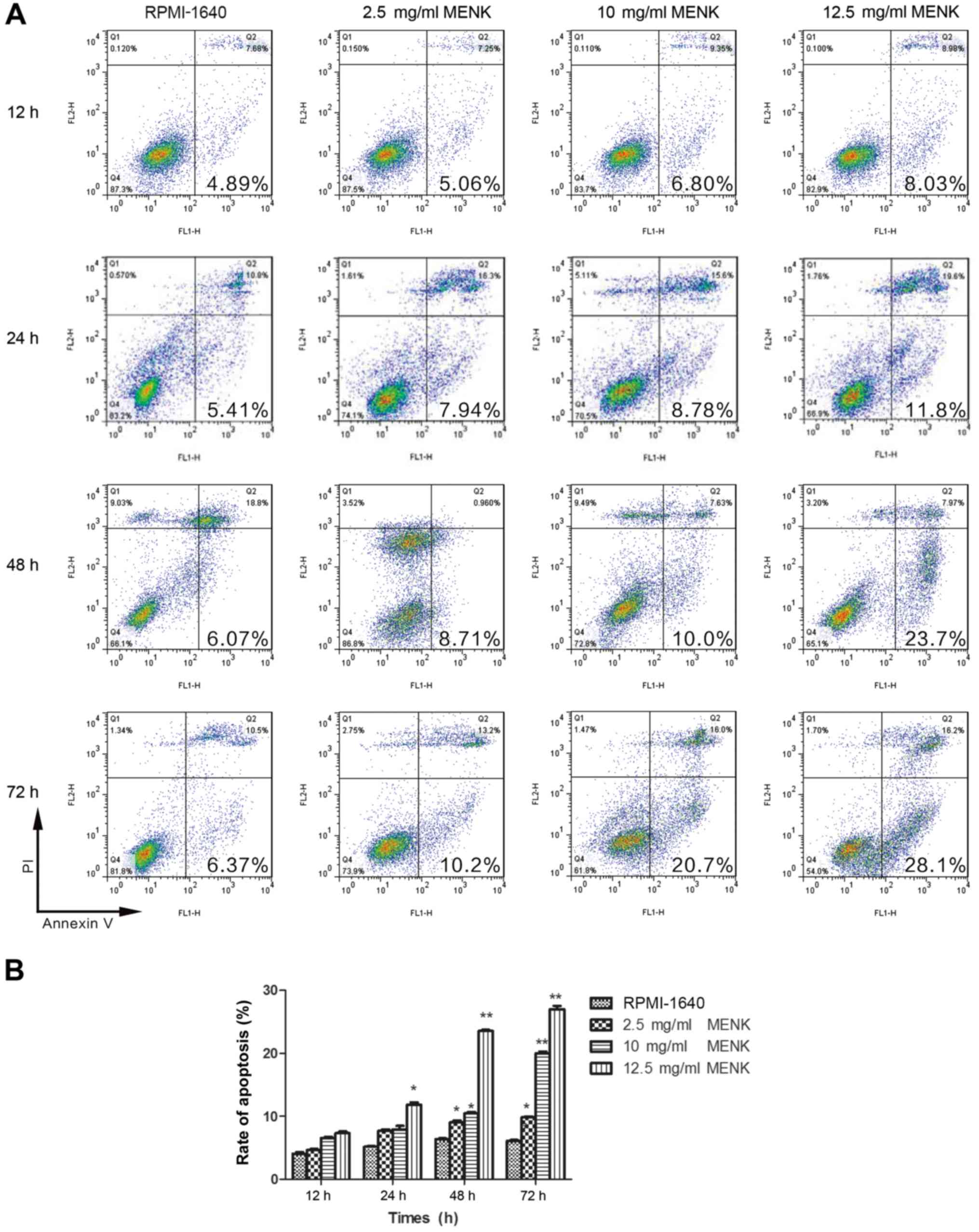
MENK induces apoptosis in vitro
Apoptosis of B16 cells was observed as assessed by
Annexin V staining. Following MENK treatment of B16 cells,
decreased viability was accompanied by alterations in cell
morphology. This was confirmed by Annexin V/PI staining. There was
a significant dose-dependent increase in Annexin
V+/PI− apoptotic cells following MENK
co-culture as compared to the control cells (Fig. 4A). Apoptosis appeared to peak at 48
h following addition of MENK to the culture. The apoptosis of B16
cells increased from 6.37% in the control group to 28.1% in the
12.5 mg/ml cultured MENK treatment group (P=0.001; Fig. 4B).
MENK inhibits B16 cell invasion
The invasion capacity of B16 cells was determined
using a Transwell assay. The number of cells that penetrated the
membrane was significantly decreased (5.46%) following culture with
5 mg/ml MENK (P=0.26) and by 31.18% following culture with 12.5
mg/ml MENK (P<0.001) as comparison to the control group
(Table I).
 | Table I.Methionine enkephalin (MENK) inhibits
B16 cell invasion. |
Table I.
Methionine enkephalin (MENK) inhibits
B16 cell invasion.
| Group | Cell number | P-value |
|---|
| Control | 95.65±6.71 |
|
| MENK (mg/ml) |
|
|
| 2.5 | 90.43±7.28 |
|
| 5 | 81.35±5.35 | 0.26 |
| 10 | 78.67±5.83 | <0.001 |
| 12.5 | 65.83±4.21 | <0.001 |
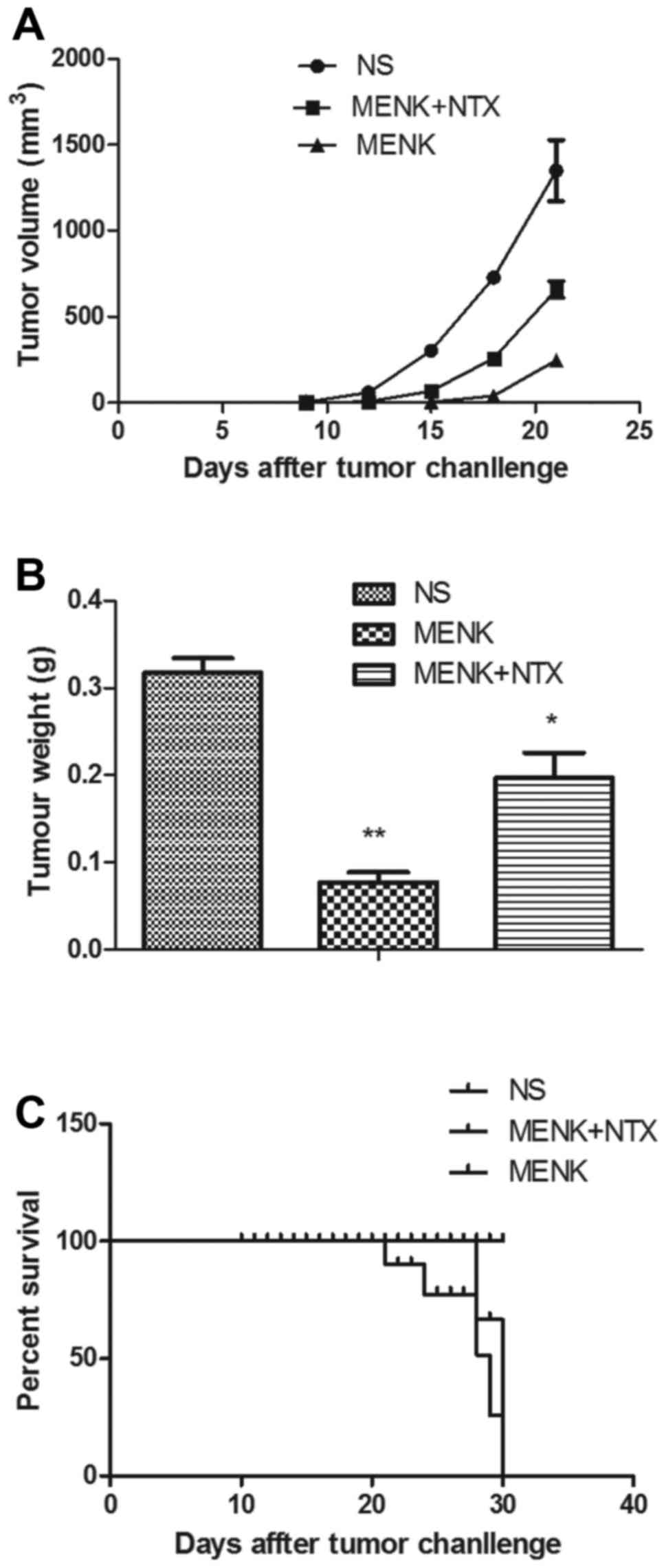
Tumor size and survival
The tumor volume and weight in the MENK-treated
group were significantly lower than those in the control and
MENK+NTX groups. Mean tumor volume and weight were significantly
different (P<0.01). MENK treatment significantly increased the
survival time relative to the control group and this impact on
survival was reversed by administration of NTX. The tumors in the
MENK group were suppressed during the treatment. In contrast, the
tumors in the vehicle control group grew quickly. The results
determined that after 30 days, tumor size and weight were
suppressed significantly in the MENK group (Fig. 5A and B; P<0.01) in comparison to
the control group. The survival rate in the MENK group was
significantly prolonged in comparison with that in the control
group (P<0.05), as shown in Fig.
5C. The results showed that MENK could delay tumor growth and
significantly enhance the survival of mice bearing B16 tumors.
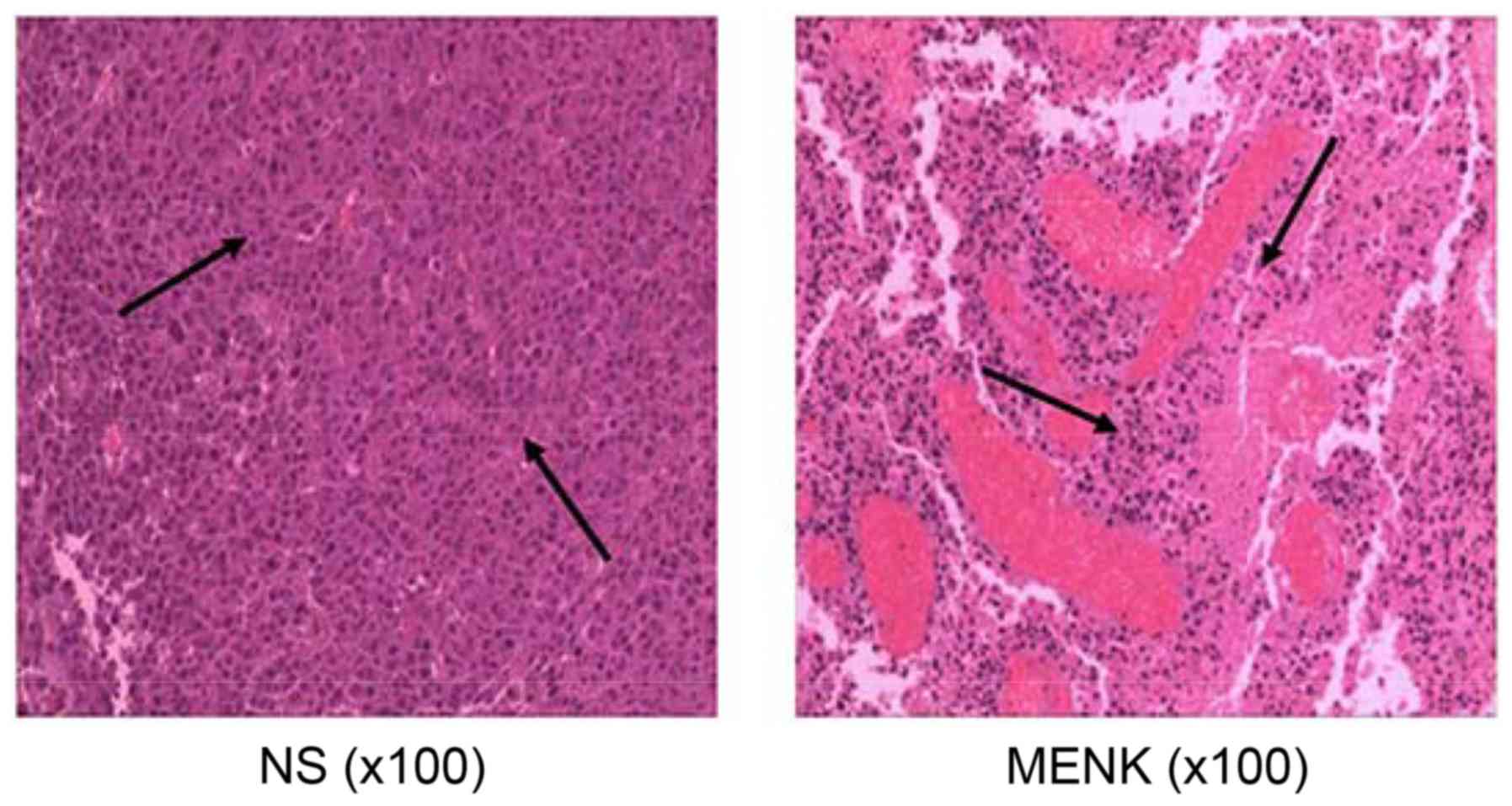
Histopathologic morphology of
tumors
Histopathologic analyses of control tumors, as
previously reported, have shown that B16 tumor cells grow as
diffused nests with a high frequency of mitotic cells, cellular
heterogeneity, with an epithelioid or spindle type, cell volume and
a large nuclear to cytoplasm ratio and varying nuclear morphology.
A significant increase in the nucleoli:nucleoplasm ratio is
observed, melanin is observed in the cytoplasm, less nuclear
pyknosis is observed with occasional sites of scattered focal
necrosis. The presence of necrosis in the MENK-treated tumors was
increased. In the treated B16 tumors they were significantly
reduced in size, had visible nuclear pyknosis, karyorrhexis,
necrosis and a rich cytoplasm and an increase in inflammatory cell
infiltration (Fig. 6).
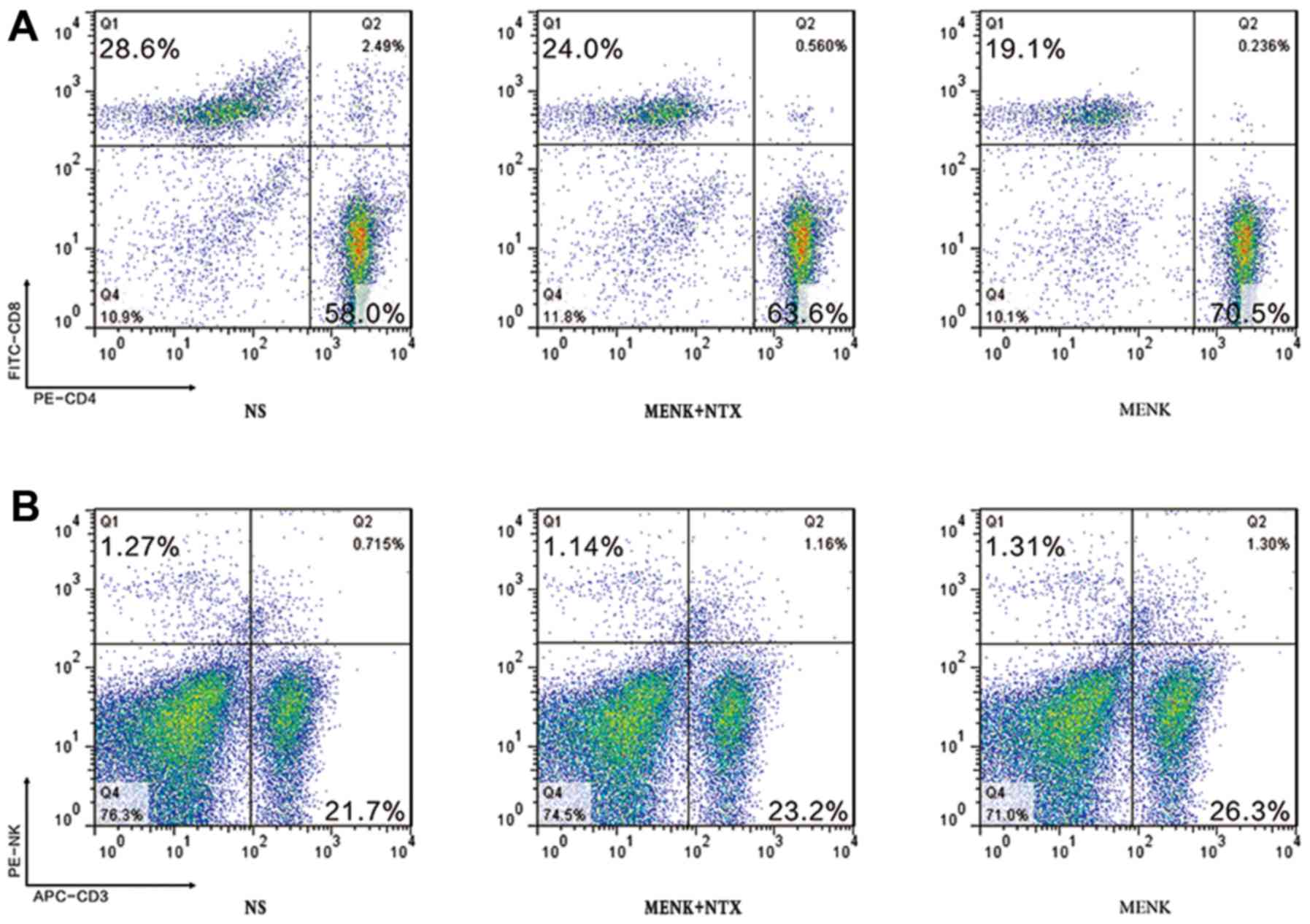
Analysis of T lymphocyte subsets by
FCM
The phenotypes of splenic T lymphocyte subsets were
studied using 3-color FCM. The percentage of CD3+
lymphocytes in the MENK group was markedly higher than that in the
control group, while the percentage of NK lymphocytes was not
significantly increased. The mice treated with MENK exhibited an
increased ratio of CD4+ to CD8+ T cells as
assessed by FCM, with a ratio of 2.03 in the control group, 3.69 in
the MENK-treated group and 2.65 in the MENK+NTX group. These data
indicate that MENK induced Th1 immunity effectively in the
tumor-bearing mice (Fig. 7).
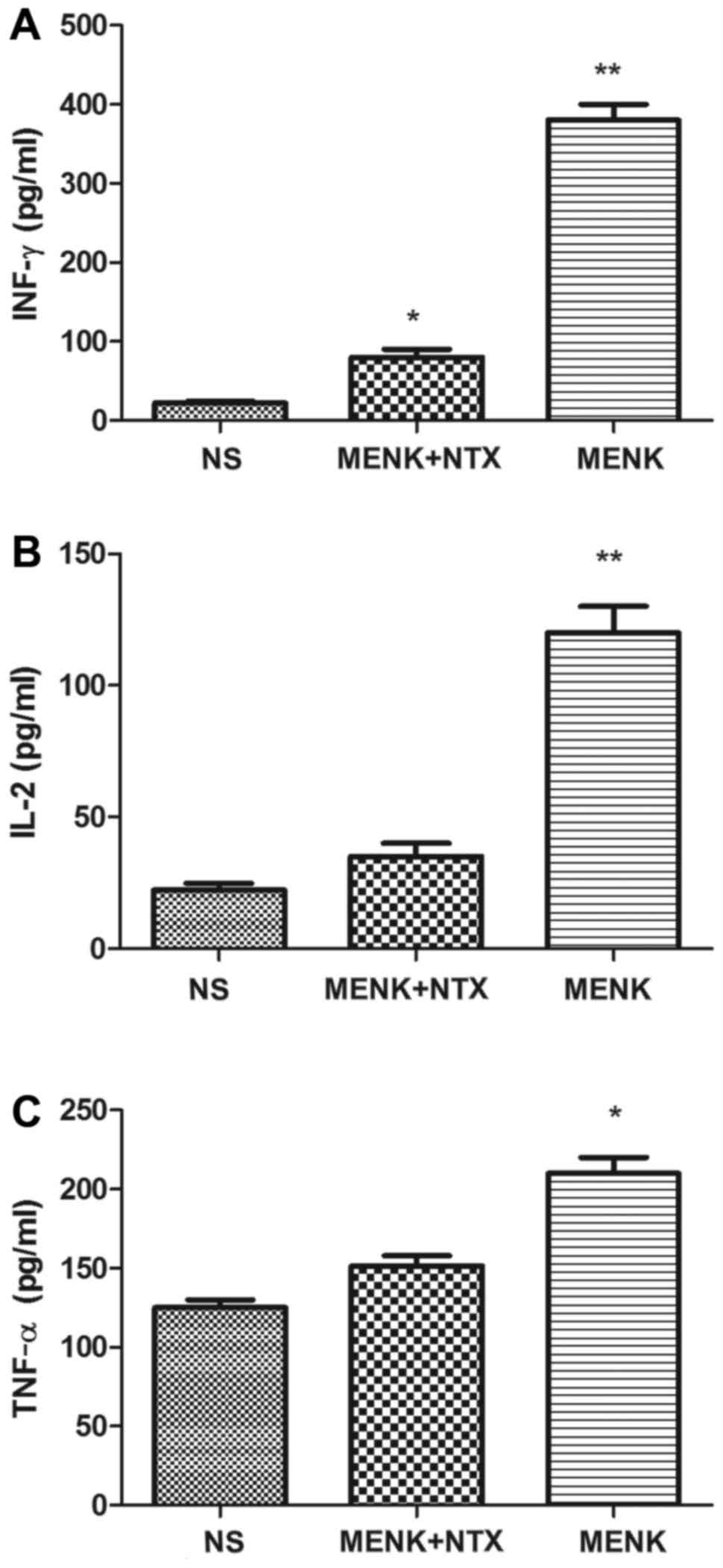
Effect of MENK administration on serum
cytokine levels
The cytokine levels in the serum of MENK-treated
mice were assessed by ELISA analysis and included IL-2, IFN-γ and
TNF-α levels. Twenty-four hours after the last injection, serum was
obtained from the retinal orbital plexus. In the MENK group all
cytokines were significantly increased (P<0.01) compared with
these levels in the NS and MENK+NTX groups (Fig. 8).
Discussion
Successful treatment of melanoma is a significant
challenge, and novel combinations of targeted drugs and
immunotherapies have recently provided encouraging outcomes, not
only for melanoma, but also for other solid tumors. In recent
years, cancer immunotherapy has become an important adjacent
therapy to traditional cancer therapies. By regulation of the
immune system, particularly immune cells within the tumor
microenvironment, the growth of some cancers can be controlled
(22).
The coordination of the endocrine and immune systems
maintains the stability of many host functions. Indeed, MENK may
contribute to immune responses by activating multiple types of
immune cells for tumors and viral infections, enabling them to
secrete various cytokines or killing target cells directly
(23,24). Opioid peptides and opioid receptors
have been found in a variety of human cancer tissues and to have a
direct influence on the growth of neural and non-neural cells and
tissues (25). Moreover, there are
interactions among immune cells which take place by the secretion
of a variety of cytokines. In our previous study, we demonstrated
that MENK alone or in combination with IL-2 or IFN-γ, respectively
strengthened the pathway between dendritic cells (DCs) and
CD4+ T cells, and induced maturation of DCs with higher
production of IL-12 (26). MENK was
found to inhibit the growth and induce apoptosis of A375 cells
(27). In the present study, we
made the unique discovery that MENK not only can inhibit the
proliferation and metastasis of B16 cells directly, but also can
indirectly kill B16 cells by stimulating lymphocyte subsets and
cytokine secretion to achieve the therapeutic goal. The importance
of this discovery cannot be emphasized sufficiently since it now
provides a direct clue to the solution for melanoma B16 treatment
with MENK.
The present study investigated the detailed
mechanisms underlying the effects of MENK on melanoma B16 cell
tumor-bearing mice. Our results conclude that: ii) the optimal
concentration of MENK to inhibit the progression, aggression and
metastasis of B16 cells was 12.5 mg/ml. ii) The expression of DOR,
MOR and KOR in B16 cells was upregulated post-treatment with MENK,
and the increased expression was attenuated by NTX. iii) MENK
retarded the growth of B16 cells, and restricted them to a G0-G1
portion of the cell cycle. iv) Apoptosis of B16 cells was observed.
v) The result of the Transwell assay also concluded that 12.5 mg/ml
MENK effectively inhibited B16 cell invasion. vi) Treatment of B16
primary tumors with MENK administration reduced tumor volume and
weight and prolonged survival relative to control-treated mice and
this therapeutic activity was blocked following the administration
of NTX. This is consistent with prior studies of MENK-increased
antitumor resistance in mice against the growth of B16 cells and
also increased survival time. vi) The histopathologic morphology of
tumors demonstrated significant differences between the MENK and NS
groups (8). Furthermore, in our
present study the mice treated with MENK exhibited increased
frequency of CD4+ and CD8+ T cells and ratio
as detected by FCM, with ratios of 2.03 in the control group, 3.69
in the MENK-treated group and 2.65 in the MENK+NTX group (Fig. 7). ix) MENK induced an increase in
IL-2, IFN-γ and TNF-α as assessed by ELISA. Therefore, MENK not
only inhibited the proliferation of B16 cells directly, but also
regulated B16 tumor growth by stimulating T cell frequency and
cytokine secretion.
The immune system includes a complex, diverse
network of leukocyte cells by forming a feedback loop regulated by
secreted cytokines. The secretion of IFN-γ may also entice B cells
to produce opsonizing antibodies, which aids in phagocytosis by the
macrophage and antibody-dependent, cell-mediated cytotoxicity of NK
cells (28). TNF-α is a
multi-functional cytokine, playing a pivotal role in cell
apoptosis, cell proliferation and tumorigenesis. A high level of
TNF-α could enhance the cytotoxic effect against cancer cells,
direct blockage of cancer cells, ischemic necrosis of tumors and
lysis of cancer cells (29). TNF-α
also can induce inflammation of capillary endothelium resulting in
tumor necrosis (30). In the
present study, MENK promoted the secretion of IFN-γ, TNF-α and
IL-2.
Intralesional IL-2 may be beneficial for the
treatment of intransit melanoma metastases. Previous studies used
lower concentrations (3 and 3.6 million units) with lower response
rates (50 and 62.5%) compared with the recent study by Shi et
al (31), in which higher-dose
IL-2 (up to 22 million units) combined with topical imiquimod and
tretinoin 0.1% cream resulted in a 100% complete response (32).
In response to immune system, antigen-presenting
cells such as DCs secrete a variety of cytokines by stimulating
CD4+ T cells to become differentiated into Th1, Th2 or
Th17 by a process known as polarization (33). Th1 cells are often considered as
pro-inflammatory factors, which could respond to IL-12, produce
IFN-γ and enhance the pathway of CD4+ T cell
proliferation and inflammation (34,35).
In the present study, the ratio of CD4+ to
CD8+ T cells was increased. It is also helpful to gain
deep insight into the immunological mechanisms of MENK for its
positive immune regulation. Based on the above findings, we may
consider this strategy for application in cancer therapy in the
future.
From the results of the present study we conclude
that MENK inhibits the growth and induces the apoptosis of B16
cells, resulting in a potential therapeutic mechanism. MENK
administration also enhanced immune function in tumor-bearing mice
and thus may act as an immunomodulator for patients with melanoma.
Therefore, MENK should be investigated as an adjuvant therapy for
melanoma patients in combination with chemotherapy. Additional
studies are needed to obtain deeper insight into the immunological
mechanisms of MENK and its immune regulatory activity. Further
studies are still needed to develop an optimal strategy for the
adjuvant use of MENK in the treatment of melanoma.
Acknowledgements
The present study was supported by the China Natural
Science Foundation (31670921 to F.S.), and the Liaoning Major
Science and Technology Platform for Institutions. The authors thank
their colleagues who contributed to the present study.
Glossary
Abbreviations
Abbreviations:
|
MENK
|
methionine enkephalin
|
|
MORs
|
µ-opioid receptors
|
|
DORs
|
δ-opioid receptors
|
|
KORs
|
κ-opioid receptors
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
MTS
|
5-(3-carboxymethoxyphenyl)-2-(4,5-dimethyl-thiazoly)-3
-(4-sulfophenyl) tetrazolium
|
References
|
1
|
Curtin JA, Fridlyand J, Kageshita T, Patel
HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, et
al: Distinct sets of genetic alterations in melanoma. N Engl J Med.
353:2135–2147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zagon IS, Sassani JW and McLaughlin PJ:
Reepithelialization of the human cornea is regulated by endogenous
opioids. Invest Ophthalmol Vis Sci. 41:73–81. 2000.PubMed/NCBI
|
|
3
|
Zagon IS, Sassani JW, Wu Y and McLaughlin
PJ: The autocrine derivation of the opioid growth factor,
[Met5]-enkephalin, in ocular surface epithelium. Brain
Res. 792:72–78. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolf IH, Richtig E, Kopera D and Kerl H:
Locoregional cutaneous metastases of malignant melanoma and their
management. Dermatol Surg. 30:244–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zagon IS and McLaughlin PJ: Naltrexone
modulates tumor response in mice with neuroblastoma. Science.
221:671–673. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zagon IS and McLaughlin PJ: Opioid
antagonists inhibit the growth of metastatic murine neuroblastoma.
Cancer Lett. 21:89–94. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zagon IS, Rhodes RE and McLaughlin PJ:
Distribution of enkephalin immunoreactivity in germinative cells of
developing rat cerebellum. Science. 227:1049–1051. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zagon IS and McLaughlin PJ: Endogenous
opioid systems regulate cell proliferation in the developing rat
brain. Brain Res. 412:68–72. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zagon IS and McLaughlin PJ: Endogenous
opioid systems, stress, and cancer. In: Enkephalins-Endorphins:
Stress and the Immune SystemPlotnikoff NP, Murgo AJ, Faith RE and
Good RA: Plenum Press; New York: pp. 81–100. 1986
|
|
10
|
Zagon IS, McLaughlin PJ, Goodman SR and
Rhodes RE: Opioid receptors and endogenous opioids in diverse human
and animal cancers. J Natl Cancer Inst. 79:1059–1065.
1987.PubMed/NCBI
|
|
11
|
Zagon IS and McLaughlin P: Endogenous
opioids and the growth regulation of a neural tumor. Life Sci.
43:1313–1318. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagon IS and McLaughlin PJ: Endogenous
opioid systems regulate growth of neural tumor cells in culture.
Brain Res. 490:14–25. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hauser KF, McLaughlin PJ and Zagon IS:
Endogenous opioids regulate dendritic growth and spine formation in
developing rat brain. Brain Res. 416:157–161. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McLaughlin PJ and Zagon IS: Modulation of
human neuroblastoma transplanted into nude mice by endogenous
opioid systems. Life Sci. 41:1465–1472. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zagon IS, Wu Y and McLaughlin PJ: Opioid
growth factor inhibits DNA synthesis in mouse tongue epithelium in
a circadian rhythm-dependent manner. Am J Physiol. 267:R645–R652.
1994.PubMed/NCBI
|
|
16
|
Zagon IS and McLaughlin PJ: An opioid
growth factor regulates the replication of microorganisms. Life
Sci. 50:1179–1187. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan F, Xia Y, Wang N, Meng J, Lu C, Meng
Y and Plotnikoff NP: Functional modulation of the pathway between
dendritic cells (DCs) and CD4+T cells by the
neuropeptide: Methionine enkephalin (MENK). Peptides. 32:929–937.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quidville V, Segond N, Pidoux E, Cohen R,
Jullienne A and Lausson S: Tumor growth inhibition by indomethacin
in a mouse model of human medullary thyroid cancer: Implication of
cyclooxygenases and 15-hydroxyprostaglandin dehydrogenase.
Endocrinology. 145:2561–2571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Gazzar A, Perco P, Eckelhart E, Anees
M, Sexl V, Mayer B, Liu Y, Mikulits W, Horvat R, Pangerl T, et al:
Natural immunity enhances the activity of a DR5 agonistic antibody
and carboplatin in the treatment of ovarian cancer. Mol Cancer
Ther. 9:1007–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Gao X, Yuan Z, Wang Z, Meng Y, Cao
Y, Plotnikoff NP, Griffin N and Shan F: Methionine enkephalin
(MENK) improves lymphocyte subpopulations in human peripheral blood
of 50 cancer patients by inhibiting regulatory T cells (Tregs). Hum
Vaccin Immunother. 10:1836–1840. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Chen W, Herberman RB, Plotnikoff NP,
Youkilis G, Griffin N, Wang E, Lu C and Shan F: Immunotherapy of
cancer via mediation of cytotoxic T lymphocytes by methionine
enkephalin (MENK). Cancer Lett. 344:212–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sassani JW, McLaughlin PJ and Zagon IS:
The Yin and Yang of the Opioid Growth Regulatory System: Focus on
Diabetes - The Lorenz E. Zimmerman Tribute Lecture. J Diabetes Res.
2016:97037292016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McLaughlin PJ, McHugh DP, Magister MJ and
Zagon IS: Endogenous opioid inhibition of proliferation of T and B
cell subpopulations in response to immunization for experimental
autoimmune encephalomyelitis. BMC Immunol. 16:242015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zagon IS, Donahue RN and McLaughlin PJ:
Opioid growth factor-opioid growth factor receptor axis is a
physiological determinant of cell proliferation in diverse human
cancers. Am J Physiol Regul Integr Comp Physiol. 297:R1154–R1161.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hua H, Lu C, Li W, Meng J, Wang D,
Plotnikoff NP, Wang E and Shan F: Comparison of stimulating effect
on subpopulations of lymphocytes in human peripheral blood by
methionine enkephalin with IL-2 and IFN-γ. Hum Vaccin Immunother.
8:1082–1089. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang DM, Wang GC, Yang J, Plotnikoff NP,
Griffin N, Han YM, Qi RQ, Gao XH and Shan FP: Inhibition of the
growth of human melanoma cells by methionine enkephalin. Mol Med
Rep. 14:5521–5527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue M, Sun H, Cao Y, Wang G, Meng Y, Wang
D and Hong Y: Mulberry leaf polysaccharides modulate murine
bone-marrow-derived dendritic cell maturation. Hum Vaccin
Immunother. 11:946–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Czura CJ and Tracey KJ: Tumor
necrosis factorThe Cytokine Handbook. 4th. Thomson AW and Lotze MT:
Academic Press; London, UK: pp. 837–860. 2003, View Article : Google Scholar
|
|
30
|
Motzer RJ, Hudes GR, Curti BD, McDermott
DF, Escudier BJ, Negrier S, Duclos B, Moore L, O'Toole T, Boni JP,
et al: Phase I/II trial of temsirolimus combined with interferon
alfa for advanced renal cell carcinoma. J Clin Oncol. 25:3958–3964.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi VY, Tran K, Patel F, Leventhal J,
Konia T, Fung MA, Wilken R, Garcia MS, Fitzmaurice SD, Joo J, et
al: 100% Complete response rate in patients with cutaneous
metastatic melanoma treated with intralesional interleukin (IL)-2,
imiquimod, and topical retinoid combination therapy: Results of a
case series. J Am Acad Dermatol. 73:645–654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leventhal JS, Odell ID, Imaeda S,
Maverakis E and King BA: Treatment of melanoma in-transit
metastases with combination intralesional interleukin-2, topical
imiquimod, and tretinoin 0.1% cream. JAAD Case Rep. 2:114–116.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fletcher JM, Lalor SJ, Sweeney CM, Tubridy
N and Mills KH: T cells in multiple sclerosis and experimental
autoimmune encephalomyelitis. Clin Exp Immunol. 162:1–11. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kebir H, Kreymborg K, Ifergan I,
Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N,
Becher B and Prat A: Human TH17 lymphocytes promote blood-brain
barrier disruption and central nervous system inflammation. Nat
Med. 13:1173–1175. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weir C, McNeill A, Hook S, Harvie M, La
Flamme AC, Le Gros G and Bäckström BT: Critical role of
preproenkephalin in experimental autoimmune encephalomyelitis. J
Neuroimmunol. 179:18–25. 2006. View Article : Google Scholar : PubMed/NCBI
|