Introduction
Glioblastoma (GBM) is the most common primary
malignant brain tumour, and one of the most aggressive neoplasms,
considered incurable, and classified as grade IV by the World
Health Organization (WHO) (1).
Despite current standard multimodal therapy concepts (excision,
radiation and chemotherapy) 1-year survival reaches 37.2% and
5-year survival only 5.1% in unselected GBM patients across all age
groups (2). Incidence of GBM
increases with age, paralleled by increasingly worse prognosis
(2).
In the WHO classification 2016, which for the first
time also incorporates molecular parameters for tumour typing, GBM
harbouring mutations in the isocitrate dehydrogenase gene (IDH) and
those without (GBM, IDH-wildtype) are perceived as two very
different disease entities, differing in molecular alterations,
precursor cells and prognosis (1,3,4).
IDH-wildtype GBM is synonymous to primary GBM, arising de
novo predominantly in the older age group, and having a worse
prognosis. One of the hallmark alterations of this predominant
GBM-subtype (comprising ~90%) are high-level gene amplifications of
certain proto-oncogenes most commonly the epidermal growth factor
receptor (EGFR) gene. This results in EGFR overexpression, and as a
late event, constituitively activating mutations, mainly the
variant III (vIII) deletion (3).
IDH-mutant (secondary) GBMs arise from lower grade gliomas, affect
younger patients, and have a comparably longer survival (1,4,5). Most
of the IDH point mutations in GBM affect the amino acid arginine at
position 132 (R132H-IDH1), and can be visualized using
immunohistochemical staining (IHC) (6). In case of negative IHC, sequencing
should be applied in patients younger than 54 years of age in order
to safely exclude IDH-mutant GBM (1).
Treatment options for GBM are limited. Complete
resection is virtually impossible due to its highly infiltrative
growth. Resistance to chemotherapy and radiation result in the
inevitable treatment failure. Targeted therapies may permit
individualized treatment of different molecular GBM-subtypes.
Exploiting molecular alterations for targeted therapeutic
approaches has proved successful in other malignant neoplasms, e.g.
anti-EGFR tyrosine kinase inhibitors (TKI) in EGFR-dependent
non-small cell lung cancer (7).
EGFR displays its oncogenic potential also in GBM
(8,9). By dysregulation, amplification or
mutation, EGFR acts as an activator of signalling pathways,
stimulating cell proliferation, anti-apoptosis, angiogenesis,
invasion and metastasis (8,10). Despite this rationale, clinical
studies using TKI against EGFR have shown insufficient efficacy to
date (11). Multitarget approaches
may be needed.
Heat shock proteins (HSPs) are highly conserved
molecular chaperones, i.e. molecules enabling proper folding and
stabilization of their client proteins (12). HSPs belong to the cellular stress
response machinery e.g. to heat shock, hence the name, and are
categorized by approximate molecular weights. By stabilizing and
maturating oncogenic proteins, HSPs contribute to tumour
invasiveness, angiogenesis and metastasis, making them putative
therapeutic targets (12,13). EGFR and many molecules of the
downstream Akt/PI3K and MAPK pathways are important client proteins
of HSP90. In the last years, targeting HSP90 has emerged as
possible anticancer therapy, alone or in combination with other
drugs or radiotherapy (12,14,15).
Therefore, in the present study, we aimed to assess
the co-expression patterns and any prognostic significance of EGFR
and its chaperone HSP90 in the subgroup of GBM, IDH-wildtype.
Materials and methods
Patient cohort
The initial study cohort comprised all consecutive
patients diagnosed with GBM at the Institute of Pathology,
University of Bern, Switzerland, between 2000 and 2012 (16). All biopsies and resections were
performed in the Department of Neurosurgery, Inselspital,
University Hospital Bern, Switzerland. The clinicopathological data
were collected from pathology reports and a clinical database
(Swiss Glioma Network). This retrospective study was conducted
according to the REMARK guidelines (17,18),
and was approved by the Cantonal Ethics Commission of the Canton of
Bern (KEK 200/14), which waived the requirement for written
informed consent.
In total, we identified 449 patients with GBM in our
database. In 237 cases, sufficient formalin fixed and
paraffin-embedded (FFPE) tissue was available for construction of a
tissue microarray (TMA). Material availability was assessed on
hematoxylin and eosin stained slides and the corresponding paraffin
blocks. Thus, material originating from stereotactic biopsies was
insufficient for inclusion. To avoid confounding factors of
immunohistochemical analysis, tissue that had been frozen for
intraoperative assessment using cryosections before fixation in
formalin was excluded.
TMA
A TMA was constructed as previously described:
slides were scanned, digitally annotated and punched using the TMA
Grand Master (3DHISTECH, Budapest, Hungary) (19). Whenever possible, 4 cores each
(diameter, 0.6 mm) derived from the: i) tumour centre; ii)
infiltration edge; and iii) distant non-neoplastic tissue from each
tumour were included.
Immunohistochemistry
The TMA blocks were sectioned at 3 µm, and stained
using an automated immunostainer Leica Bond III system (Leica
Biosystems, Newcastle, UK). Staining conditions including primary
antibodies and antigen retrieval were as following: mouse
monoclonal R132H-mutant IDH antibody, clone H09 (ref. DIA-H09;
Dianova, Hamburg, Germany), 1:50, Tris-EDTA buffer pH 9 at 95°C, 30
min; mouse monoclonal HSP90 antibody, clone S88 (ref. ab1429;
Abcam, Cambridge, UK), 1:25, citrate buffer pH 6 at 100°C, 30 min
and mouse monoclonal wildtype EGFR antibody, clone DAK-H1-WT (ref.
M7298; Dako, Gloustrup, Denmark), 1:50, Tris-EDTA buffer pH 9 at
95°C, 30 min. All secondary antibodies, the chromogen
(3,3′-diaminobenzidine) and the hematoxylin counter-stain were
included in the visualization system, Bond Polymer Refine Detection
(Leica Biosystems).
For scoring of immunohistochemical markers, we
assessed the staining intensity ranging from 1 (negative or
traces); 2 (weak); 3 (medium) to 4 (strong) for EGFR; and ranging
from 1 (negative or traces), 2 (weak) to 3 (strong) for HSP90,
according to published scoring systems for both EGFR (20) and HSP90 (21) on glioma cells (Fig. 1). The percentage of stained cells
was determined as follows: 1, ≤10%; 2, 11–50%; and 3, ≥50%. The
expression was assessed for every TMA core separately and the mean
across all cores was calculated to determine the final expression
intensity (EI) and an expression score (ES). ES was defined as the
product of EI and the percentages of positive cells.
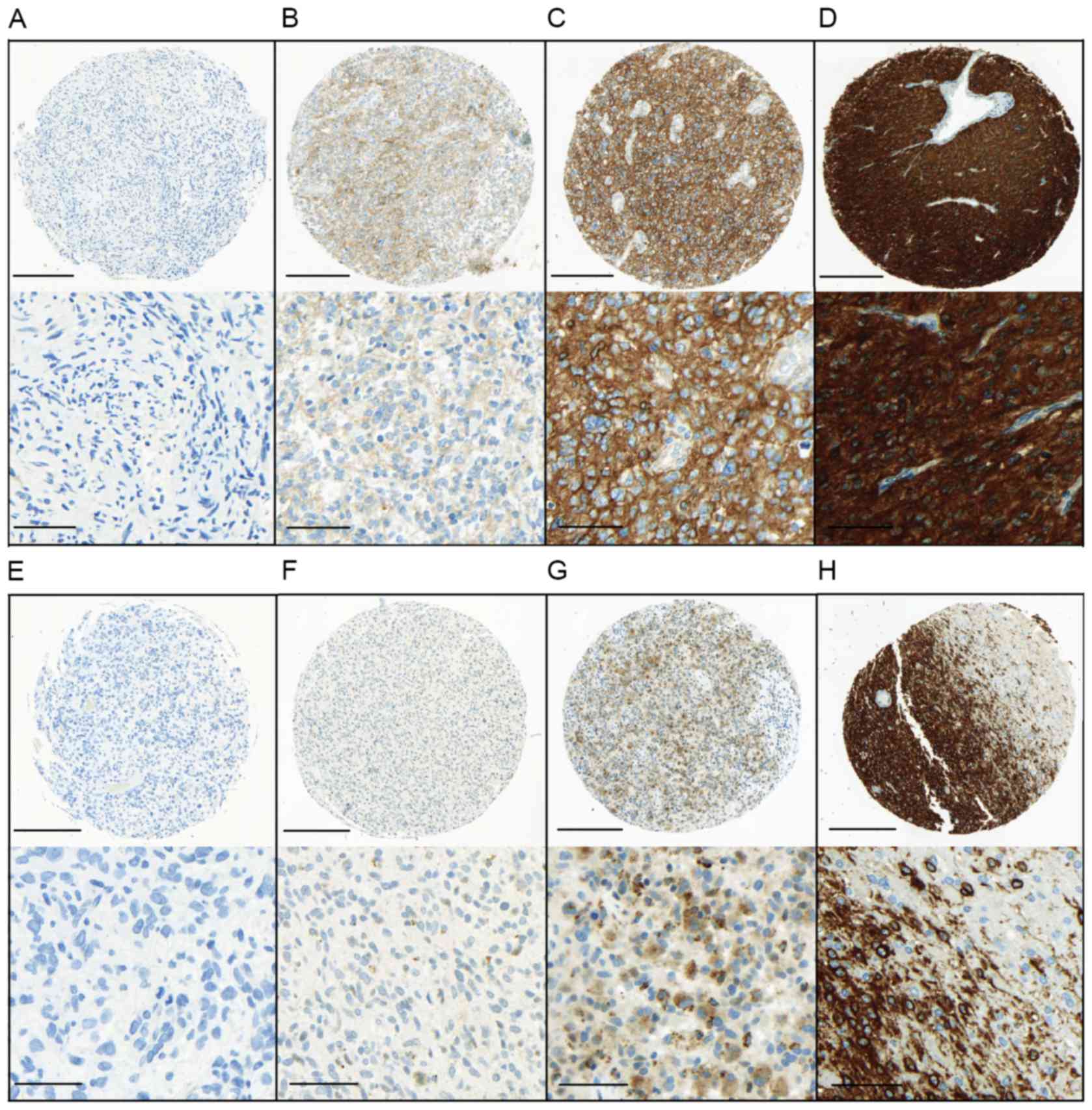 | Figure 1.Immunohistochemical staining
intensity for EGFR and HSP90. Upper panel, EGFR staining intensity
was evaluated as (A) negative or traces, (B) weak, (C) moderate or
(D) marked. Lower panel, HSP90 staining intensity was evaluated as
(E) negative or traces, (F) weak or (G) marked. (H) EGFR staining
pattern at the infiltration edge. All photomicrographs in the upper
panels were captured at an objective magnification of ×10 (scale
bar, 200 µm). Detailed images of the staining are shown under each
sample (objective magnification, ×40; scale bar, 50 µm). EGFR,
epidermal growth factor receptor; HSP90, heat schock protein
90. |
IDH sequencing and assessment of MGMT
promoter methylation
DNA was extracted from tissue sections containing at
least 70% tumour cells using the Qiagen EZ1 tissue kit (Qiagen,
Hombrechtikon, Switzerland) according to the manufacturer's
instructions. The mutational status of the IDH1 and IDH2 genes was
assessed by pyrosequencing as previously described (22,23).
MGMT promoter methylation was determined using a primer
extension-based quantitative assay as already reported (24).
Statistical analysis
Descriptive and comparative statistical analyses
were performed using IBM SPSS Statistics 23 (IBM Corp., Armonk, NY,
USA). We applied Spearman's Rho test for correlation analysis, and
evaluated associations between staining patterns and categorical
parameters using simple cross tabs (χ2-test or Fisher's
exact test). For binded samples, we used the Wilcoxon test.
Survival analysis was performed by log-rank test (univariate) and
Cox regression analysis (multivariate). The significance level was
set at P<0.05.
Results
Patient cohort
In total, 449 patients with GBM were initially
identified, with a male to female ratio of 271 (60.4%) and 178
(39.6%), and a median age at surgery of 61 years (range 21-88).
The TMA sub-collective comprised of 237 patients,
136 (57.4%) men and 101 (42.6%) women. Age at the time of surgery
ranged from 24–85 years (median 59 years) (Table I), comparable to the initial cohort
(data not shown). Using immunohistochemistry, 23/237 tumours (9.7%)
exhibited evidence of R132H-IDH1 mutations. Sequencing of IDH1 and
IDH2 was performed on all 28 IHC-negative tumours from patients
younger than 54 years of age at diagnosis, as recommended by the
WHO 2016, but revealed no additional IDH-mutant tumours (1). Finally, 214/237 (90.3%) cases were
GBM, IDH-wildtype. The study cohorts are juxtaposed in Table I. MGMT methylation data was
available in 106 cases of the TMA cohort.
 | Table I.Clinicopathological parameters and
the expression of EGFR/HSP90 in the total TMA-cohort and the GBM,
IDH-wild-type sub-cohort (study cohort). |
Table I.
Clinicopathological parameters and
the expression of EGFR/HSP90 in the total TMA-cohort and the GBM,
IDH-wild-type sub-cohort (study cohort).
|
| TMA-cohort, total,
n=237 n (%) | TMA-cohort, GBM,
IDH-wild-type, n=214 n (%) |
|---|
| Sex |
|
|
|
Male | 136 (57.4) | 124 (57.9) |
|
Female | 101 (42.6) | 90 (42.1) |
| Age at operation,
(years), median (range) | 59 (24–85) | 60 (27–85) |
| Overall survival,
(months), median (95% CI) | 19.0 (14.1–23.9)
(n=102) | 17.0 (13.4–20.6)
(n=96) |
| MGMT methylation
(data available for n=106) |
|
|
|
Absent | 51 (48.1) | 51 (51) |
|
Present | 55 (51.9) | 49 (49) |
| EGFR/HSP90
expression |
|
|
|
EGFRlow/HSP90low | 77 (32.5) | 66 (30.8) |
|
EGFRlow/HSP90high | 57 (24.0) | 50 (23.4) |
|
EGFRhigh/HSP90low | 45 (19.0) | 43 (20.1) |
|
EGFRhigh/HSP90high | 58 (24.5) | 55 (25.7) |
Survival data were available for 102 patients of the
TMA cohort, among them 96 GBM, IDH-wildtype. For these patients,
additional information concerning post-operative treatment was
extracted from the database: 88 patients (85.4%) received
postoperative combined radiation and chemotherapy (temozolomide),
followed by bevacizumab in 44 patients. Seven patients (6.8%)
received radiation or chemotherapy only (3 only radiotherapy, 4
only chemotherapy), and 8 patients (7.8%) did not receive any type
of treatment.
Expression of EGFR and HSP90
The expression of EGFR and HSP90 was determined in
the tumour centre in all 237 cases. Additionally, the infiltration
zone was available for analysis in 161 cases and brain tissue
distant from the tumour in 167 cases. In 33 cases, tumour tissue of
recurrent disease was available, including the infiltration zone in
25 cases and brain tissue distant from the recurrent tumour in 18
cases. The expression levels evaluated as aforementioned ranged
from 0–4 for EGFR EI and from 1–12 for EGFR ES (Fig. 1). For HSP90, EI was observed from
0–3, and ES ranged from 1–9. Overall, there was a statistically
significant positive correlation between EGFR expression and HSP90
expression (EI, r=0.275, P<0.001; ES, r=0.333, P<0.001) (data
not shown).
Differences of EGFR and HSP90
expression within tumour regions
The expression of EGFR (EI and ES) was significantly
higher in the tumour centre, compared to the infiltration zone
(P=0.002; P<0.001) or distant brain tissue (P<0.001 each),
and higher in the infiltration zone compared to distant brain
tissue (P<0.001 each; Fig. 2).
Similar results were obtained for the recurrent tumours (data not
shown). For HSP90 the patterns were comparable, with higher levels
(EI and ES) in the tumour centre compared to the infiltration zone
(in trend, but failing to meet statistical significance for EI,
P=0.156; ES, P=0.007) and distant brain tissue (P<0.001 each),
and higher levels in the infiltration zone compared to distant
brain tissue (P<0.001 each; Fig.
2). Similarly, in recurrent tumours, higher HSP90 levels were
detected in the tumour, however with no differences between the
centre and the infiltration zone (data not shown).
Differences between the primary tumour
and recurrence
EGFR (ES) in the primary tumours were only in trend
higher than in recurrent tumours (P=0.05, data not shown), but this
was not observed for EI (P=0.523, data not shown). For HSP90 there
was no difference between the primary and recurrent tumours (EI,
P=0.485; ES, P=0.338).
Association of EGFR and HSP90
expression with clinicopathological parameters
IDH-wildtype GBM showed higher expression of EGFR
than IDH-mutant GBM (EI, P=0.001; ES P=0.005), but lower levels of
HSP90 (EI P=0.003; ES P=0.494). No significant association was
found for EGFR or HSP90 expression and age (cut-off, median), sex
or MGMT status.
Survival analysis was performed on the TMA cohort,
where survival data were available for 102 patients, among them 96
GBM, IDH-wildtype. Presence of IDH1 mutation (P<0.001) and
younger age (P=0.026) were associated with a significantly better
prognosis, without sex predilection (P=0.307). Lack of any
MGMT-methylation was associated with worse outcome (P=0.003).
Patients with combined postoperative radiation, temozolomide and
bevacizumab treatment showed a better outcome, followed by
radiation and chemotherapy, in contrast to radiation or
chemotherapy only and no treatment (P<0.001).
For the determination of a potential prognostic
impact of EGFR or HSP90 expression the median expression levels (EI
and ES) in the tumour centre were used as cut-off for the
discrimination into high and low expression (EGFR: EI, ≤3.25=low,
>3.25=high; ES, ≤9=low, >9=high; HSP90: EI, ≤2=low,
>2=high; ES, ≤3.25=low, >3.25=high). Low expression of EGFR
(ES) was noted in 134/237 GBM (56.5%), low expression of HSP90 (ES)
in 122/237 (51.5%). In the whole cohort, there was no association
between EGFR or HSP90 and patient outcome in univariate
analysis.
In IDH-wildtype GBM, low expression of EGFR (ES) was
noted in 116/214 GBM (54.2%), and low expression of HSP90 (ES) in
109/214 (50.9%). High expression of EGFR (ES) was in trend
associated with longer survival in univariate analysis but failed
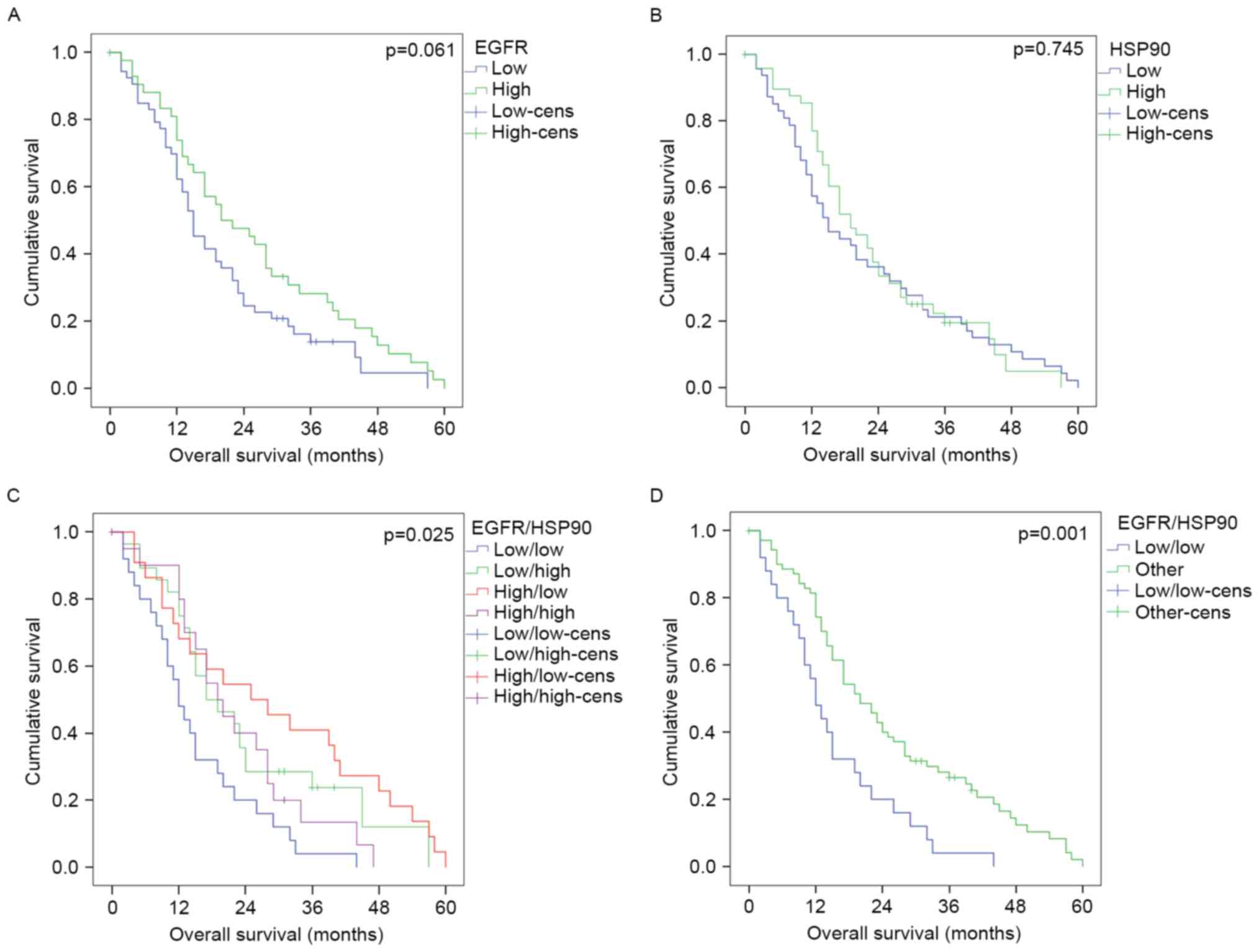
to meet statistical significance (P=0.061, Fig. 3A; not significant for EI, P=0.640).
HSP90 expression alone was not prognostic (EI, P=0.614; ES,
P=0.745, Fig. 3B). However, a
combination of EGFR and HSP90 expression strongly discriminated
between different prognostic groups, with
EGFRlow/HSP90low tumours (n=66) showing the
worst prognosis in contrast to
EGFRhigh/HSP90high tumours (n=55) or mixed
tumours (n=93, P=0.001; Table I,
Fig. 3C and D). In a multivariate
model including the other relevant prognostic factors, age, MGMT
status, and postoperative treatment, data on which were available
as complete dataset for 76 patients,
EGFRlow/HSP90low vs.
EGFRhigh/HSP90high/mixed was an independent
prognostic factor [n=76; hazard ratio (HR)=0.571; P=0.048, Table II]. This discrimination was not
detectable for EI (univariate analysis; P=1.00).
 | Table II.Multivariate analysis in
glioblastoma, IDH-wild-type (n=76). |
Table II.
Multivariate analysis in
glioblastoma, IDH-wild-type (n=76).
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|---|
| Parameter | HR | Min. | Max. | P-value |
|---|
| Age (median) | 0.839 | 0.497 | 1.418 | 0.513 |
| MGMT | 0.438 | 0.248 | 0.773 | 0.004 |
| Postoperative
treatment | 1.674 | 1.267 | 2.212 | <0.001 |
|
EGFRlow/HSP90low vs.
EGFRhigh/HSP90high/mixed | 0.571 | 0.328 | 0.996 | 0.048 |
Regarding the particular subgroup of
HSP90low GBM, IDH-wildtype, EGFR expression (ES) served
as a prognostic factor (Fig. 3C),
with a shorter survival of patients with low EGFR expression in
univariate analysis (P=0.003), and reached almost statistical
significance in a multivariate model encompassing age, MGMT status
and postoperative therapy [n=47; HR=0.465; 95% confidence interval
(CI) 0.201–1.077; P=0.074]. A prognostic value of EGFR was not
apparent in the subgroup of HSP90high tumours
(univariate analysis, P=0.617).
Discussion
We report a retrospective, single-institution study
on EGFR and HSP90 expression in GBM, IDH-wildtype. Despite a strong
biological rationale, to the best of our knowledge this marker
combination has not been investigated before in GBM. We revealed in
the present study a positive correlation between the expression of
both markers, and an independent prognostic value of EGFR and HSP90
co-expression, with worse prognosis in
EGFRlow/HSP90low tumours, apparent only in
the GBM IDH-wildtype sub-cohort.
The rate of IDH-mutated tumours in our total case
collection is comparable with published data (1,25).
From the total cohort of consecutive GBM operated/biopsied between
2000–2012 (n=449), tissue availability allowed further analysis in
237 patients, and 90.3% (n=214) were IDH-wildtype. Survival data,
which were available for 102 patients operated after 2007, after
introduction of temozolomide in standard treatment protocols,
showed that younger age, IDH1-mutations, MGMT methylation and
combined radiation and chemotherapy (temozolomide) were associated
with longer survival. This is also in line with previous studies,
clearly separating the IDH-wildtype and IDH-mutated subgroups
(9,25–27).
EGFR overexpression and amplification strongly
correlate in GBM (28), but their
prognostic value is still unclear (9,29–33).
Reasons for the heterogeneous results may be a lack of patient
selection according to e.g. IDH-status or treatment. The degree of
EGFR-amplification may also play a role, as depicted in a large
cohort of 532 GBM (unknown IDH-status). Temozolomide-treated
patients with tumours with highly-amplified and non-amplified EGFR
had a longer median survival than those with low/moderate-amplified
EGFR (34). We concentrated in the
present study on IDH-wildtype GBM, as they are known to often
harbour alterations in the EGFR gene. Accordingly, we found an
overall higher EGFR expression in IDH-wildtype than the IDH-mutant
tumours.
The prognostic value of EGFR expression was
demonstrated very recently by Delancre et al (35), who extensively characterized 59 GBM
using immunohistochemistry and targeted next generation sequencing
(Ampliseq Cancer Hotspot Panel, Ion Torrent) for assessment of
mutations (50 genes) and copy number variations (25 genes). Apart
from a predictive value of PTEN, they found high EGFR expression to
be associated with longer survival in the IDH-wildtype cohort (oral
presentation, 7th Belgian Week of Pathology, 2016). We revealed
only a statistical trend for the correlation of EGFR expression
with better outcome. In our significantly larger cohort, low
co-expression of EGFR and its chaperone HSP90 selected patients
with worse prognosis, and was independently significant in
multivariate models, highlighting the EGFR-HSP90 interaction.
The prognostic value of HSP90 expression is not
universal, but depends from the individual tumour entity analysed
(36). Only very few studies have
assessed HSP90 expression in GBM. Siegelin et al reported a
higher expression in tumour tissue compared to adjacent brain
tissue (21). Hermisson et
al found no correlation between HSP90 expression and
progression-free survival in a cohort of 24 IDH-unselected GBM
patients (37). We corroborated
both results.
EGFR and various downstream molecules are
client-proteins of HSP90, providing a rationale for co-expression
studies. We revealed that low co-expression was associated with
worse prognosis in IDH-wildtype GBM. Though unexpected, this result
suggests a variable reliance on the signalling pathway by
non-mutated EGFR. Notably, the expression of EGFR stratified most
pronounced among HSP90low tumours, whereas the
EGFRhigh phenotype was associated with longer survival.
Even though this result was not independent in multivariate
analysis, presumably due to the low number of cases, this points
towards a co-regulatory role of HSP90 with EGFR.
HSP90 has emerged as a potential target for
anticancer treatment (13). In GBM,
HSP90 inhibitors have shown anti-tumoural activity in vitro
and in xenograft experiments, as recently summarized (15). In the clinical setting, HSP90
inhibitors may have potential as additive compounds enhancing
response to radiotherapy or EGFR-targeted approaches (12,14,15).
The results of the present study may serve as a
rationale for targeting HSP90 in highly aggressive GBM,
IDH-wildtype. However, further studies are needed to elucidate the
biological background behind our results.
Acknowledgements
The authors gratefully acknowledge the Translational
Research Unit and the Molecular Pathology Laboratory of the
Institute of Pathology for excellent technical support. We thank
Nicole Soell, Department of Neurosurgery, for collecting clinical
and survival data.
References
|
1
|
Louis DN, Suvà ML, Burger PC, Perry A,
Kleihues P, Aldape KD, Brat DJ, Biernat W, Bigner DD, Nakazato Y,
et al: Glioblastoma, IDH-wildtype. In: WHO Classification of
Tumours of the Central Nervous System4th. Louis DN, Ohgaki H,
Wiestler OD and Cavenee WK: International Agency for the Research
on Cancer. Lyon: pp. 28–45. 2016
|
|
2
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 17
Suppl 4:iv1–iv62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohgaki H and Kleihues P: The definition of
primary and secondary glioblastoma. Clin Cancer Res. 19:764–772.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann C, Hentschel B, Wick W, Capper D,
Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch
T, et al: Patients with IDH1 wild-type anaplastic
astrocytomas exhibit worse prognosis than IDH1-mutated
glioblastomas, and IDH1 mutation status accounts for the
unfavorable prognostic effect of higher age: Implications for
classification of gliomas. Acta Neuropathol. 120:707–718. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Capper D, Weissert S, Balss J, Habel A,
Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H,
et al: Characterization of R132H mutation-specific IDH1 antibody
binding in brain tumors. Brain Pathol. 20:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke EE and Wu YL: EGFR as a pharmacological
target in EGFR-mutant non-small-cell lung cancer: Where do
we stand now? Trends Pharmacol Sci. 37:887–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wells A: EGF receptor. Int J Biochem Cell
Biol. 31:637–643. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohgaki H, Dessen P, Jourde B, Horstmann S,
Nishikawa T, Di Patre PL, Burkhard C, Schüler D, Probst-Hensch NM,
Maiorka PC, et al: Genetic pathways to glioblastoma: A
population-based study. Cancer Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roskoski R Jr: ErbB/HER protein-tyrosine
kinases: Structures and small molecule inhibitors. Pharmacol Res.
87:42–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Padfield E, Ellis HP and Kurian KM:
Current therapeutic advances targeting EGFR and EGFRvIII in
glioblastoma. Front Oncol. 5:52015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderwood SK and Gong J: Heat shock
proteins promote cancer: It's a protection racket. Trends Biochem
Sci. 41:311–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Combs SE, Schmid TE, Vaupel P and Multhoff
G: Stress response leading to resistance in glioblastoma-the need
for innovative radiotherapy (iRT) concepts. Cancers. 8:82016.
View Article : Google Scholar :
|
|
15
|
van Ommeren R, Staudt MD, Xu H and Hebb
MO: Advances in HSP27 and HSP90-targeting strategies for
glioblastoma. J Neuro Oncol. 127:209–219. 2016. View Article : Google Scholar
|
|
16
|
Sartori E: Zusammenstellung eines
Patienten- und Gewebekollektivs für die Untersuchung von Heat Shock
Proteinen und ErbB Rezeptoren in Glioblastomen. Master Thesis.
1–25. 2015.
|
|
17
|
McShane LM, Altman DG, Sauerbrei W, Taube
SE, Gion M and Clark GM: Statistics Subcommittee of the NCI-EORTC
Working Group on Cancer Diagnostics: Reporting recommendations for
tumor marker prognostic studies. J Clin Oncol. 23:9067–9072. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altman DG, McShane LM, Sauerbrei W and
Taube SE: Reporting Recommendations for Tumor Marker Prognostic
Studies (REMARK): Explanation and elaboration. PLoS Med.
9:e10012162012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zlobec I, Koelzer VH, Dawson H, Perren A
and Lugli A: Next-generation tissue microarray (ngTMA) increases
the quality of biomarker studies: An example using CD3, CD8, and
CD45RO in the tumor microenvironment of six different solid tumor
types. J Transl Med. 11:1042013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guillaudeau A, Durand K, Pommepuy I,
Robert S, Chaunavel A, Lacorre S, DeArmas R, Bourtoumieux S, El
Demery M, Moreau JJ, et al: Determination of EGFR status in
gliomas: Usefulness of immunohistochemistry and fluorescent in situ
hybridization. Appl Immunohistochem Mol Morphol. 17:220–226. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegelin MD, Habel A and Gaiser T: 17-AAG
sensitized malignant glioma cells to death-receptor mediated
apoptosis. Neurobiol Dis. 33:243–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hewer E, Beck J, Murek M, Kappeler A,
Vassella E and Vajtai I: Polymorphous oligodendroglioma of Zülch
revisited: A genetically heterogeneous group of anaplastic gliomas
including tumors of bona fide oligodendroglial differentiation.
Neuropathology. 34:323–332. 2014.PubMed/NCBI
|
|
23
|
Leu S, von Felten S, Frank S, Vassella E,
Vajtai I, Taylor E, Schulz M, Hutter G, Hench J, Schucht P, et al:
IDH/MGMT-driven molecular classification of low-grade glioma is a
strong predictor for long-term survival. Neuro Oncol. 15:469–479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vassella E, Vajtai I, Bandi N, Arnold M,
Kocher V and Mariani L: Primer extension based quantitative
polymerase chain reaction reveals consistent differences in the
methylation status of the MGMT promoter in diffusely infiltrating
gliomas (WHO grade II–IV) of adults. J Neuro Oncol. 104:293–303.
2011. View Article : Google Scholar
|
|
25
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiu J, Piccioni D, Juarez T, Pingle SC, Hu
J, Rudnick J, Fink K, Spetzler DB, Maney T, Ghazalpour A, et al:
Multi-platform molecular profiling of a large cohort of
glioblastomas reveals potential therapeutic strategies. Oncotarget.
7:21556–21569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Filippini G, Falcone C, Boiardi A, Broggi
G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L,
Finocchiaro G, et al: Brain Cancer Register of the Fondazione IRCCS
(Istituto Ricovero e Cura a Carattere Scientifico) Istituto
Neurologico Carlo Besta: Prognostic factors for survival in 676
consecutive patients with newly diagnosed primary glioblastoma.
Neuro Oncol. 10:79–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horbinski C, Hobbs J, Cieply K, Dacic S
and Hamilton RL: EGFR expression stratifies oligodendroglioma
behavior. Am J Pathol. 179:1638–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith JS, Tachibana I, Passe SM, Huntley
BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW,
James CD, et al: PTEN mutation, EGFR amplification, and outcome in
patients with anaplastic astrocytoma and glioblastoma multiforme. J
Natl Cancer Inst. 93:1246–1256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shinojima N, Tada K, Shiraishi S, Kamiryo
T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, et
al: Prognostic value of epidermal growth factor receptor in
patients with glioblastoma multiforme. Cancer Res. 63:6962–6970.
2003.PubMed/NCBI
|
|
31
|
Simmons ML, Lamborn KR, Takahashi M, Chen
P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, et
al: Analysis of complex relationships between age, p53, epidermal
growth factor receptor, and survival in glioblastoma patients.
Cancer Res. 61:1122–1128. 2001.PubMed/NCBI
|
|
32
|
Batchelor TT, Betensky RA, Esposito JM,
Pham LD, Dorfman MV, Piscatelli N, Jhung S, Rhee D and Louis DN:
Age-dependent prognostic effects of genetic alterations in
glioblastoma. Clin Cancer Res. 10:228–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tini P, Pastina P, Nardone V, Sebaste L,
Toscano M, Miracco C, Cerase A and Pirtoli L: The combined EGFR
protein expression analysis refines the prognostic value of the
MGMT promoter methylation status in glioblastoma. Clin Neurol
Neurosurg. 149:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hobbs J, Nikiforova MN, Fardo DW,
Bortoluzzi S, Cieply K, Hamilton RL and Horbinski C: Paradoxical
relationship between the degree of EGFR amplification and outcome
in glioblastomas. Am J Surg Pathol. 36:1186–1193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Delancre C, Le Mercier M and Trepant A:
P04-Use of immunohistochemistry and next generation sequencing for
the classification of glioblastomas. 7th Belgian Week of Pathology.
2016.
|
|
36
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hermisson M, Strik H, Rieger J, Dichgans
J, Meyermann R and Weller M: Expression and functional activity of
heat shock proteins in human glioblastoma multiforme. Neurology.
54:1357–1365. 2000. View Article : Google Scholar : PubMed/NCBI
|