Introduction
Hepatocellular carcinoma (HCC), a common malignancy
worldwide, is associated with persistently increasing incidence and
mortality rates (1). Patients with
early stage HCC are indicated for multiple treatments including
surgical resection, liver transplantation or local ablation, and
are associated with a better prognosis. However, the sole strategy
for patients with intermediate and advanced HCC is chemotherapy to
inhibit tumor progression and these patients are associated with a
poor prognosis (2). Thus,
developing methods to facilitate the diagnosis of early stage HCC
can improve the prognosis of these patients. However, the detection
of existing tumor biomarkers is unable to meet the demand (3). Thus, we need to identify new tumor
biomarkers for facilitating the early detection of HCC.
RAB34 is a gene that encodes a protein
belonging to the RAB family of proteins, which are small GTPases
involved in protein transport. Wang and colleagues found that RAB34
is involved in the repositioning of lysosomes and the activation of
micropinocytosis by interacting with Rab-interacting lysosomal
protein (RILP) (4–6). Further study by Starling et al
showed that the formation of the RAB34-PILP complex is regulated by
folliculin under the condition of nutrients (7). In addition, Speight and Silverman
found that RAB34 is also regulated by Hmunc13, which is a cytosolic
diacylglycerol (DAG)-binding protein (8). Alloatti et al showed that RAB34
plays an important role in the topological re-organization of
lysosomes during a transient phase after TLR4 engagement in the
maturation of dendritic cells (9).
A previous study showed that Rab34/Munc13-2 plays an important role
in alternative Rab7-independent phagosome maturation. This result
uncovered a deeper mechanism of RAB34 in regulating phago-lysosome
fusion (10). In addition to these
findings, Wang et al demonstrated that overexpression of
RAB34 is related to the progression of glioma grade and is
associated with the poor prognosis of high-grade glioma patients
(11). Based on these findings, we
aimed to ascertain whether RAB34 plays a vital role in the
prognosis of HCC patients.
In the present study, we first found that RAB34 was
overexpressed in HCC tissues, and was correlated with tumor size
and tumor grade. Further overall survival analysis showed that
patients with high expression of RAB34 consistently had poor
prognosis. Then, we used siRNA to study the function of RAB34 in
HCC cell lines. We found that suppression of RAB34 led to a
decrease in the cell proliferation rate and migration ability.
Further study showed that RAB34 regulated the progression of the G1
phase in the cell cycle and epithelial to mesenchymal transition.
In conclusion, we found that RAB34 plays a vital role in the
progression of HCC and may be a new biomarker for assessing the
prognosis of HCC patients.
Materials and methods
Patients, tissue samples and
follow-up
All cancer and adjacent tissue samples were obtained
from 79 HCC patients who underwent surgery at the Nanjing Medical
University Affiliated Suzhou Hospital from September 1, 2012 to
September 1, 2013. All patients were diagnosed with HCC by imaging
and serological examination and accepted surgery without radiation
and chemotherapy. Fresh cancer and adjacent matched normal tissues
were collected and used for constructing paraffin blocks. Two
pathologists examined the cancer and the matched normal tissues.
The Medical Ethics Committee of The Affiliated Suzhou Hospital of
Nanjing Medical University approved the protocol, and written
informed consent was obtained from all participants. Clinical data
of all the HCC patients including age, sex, size of the tumor,
number of tumors, vessel invasion and tumor grade were collected.
Two students were responsible for the follow-up of these patients
every 3 months. All of these patients were followed up until the
follow-up termination date (July 31, 2016) or death. Overall
survival was calculated in months from the diagnosis until the date
of death, last known to be alive, or the study closing date.
Ethical approval
The Second Affiliated Hospital, Xi'an Jiaotong
University Ethics Committee approved the present study. Informed
consent was obtained from all individual participants included in
the study. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards.
Cell culture
HCC cell lines LM3, Huh7, SK and 7701 were purchased
from the Chinese Academy of Science Cell Bank (Shanghai, China).
All cells were maintained in minimum essential medium (MEM), and
supplemented with 10% fetal bovine serum (FBS) at 37°C in a
humidified atmosphere of 5% CO2.
Transfection of LM3 and Huh7 cell
lines with small interfering RNA
Human-specific RAB34 small interfering RNA (siRNA)
was designed, constructed and purified by Jima Biotech Co.
(Shanghai, China). siRNA targeting Rab34 had the following annealed
duplex: 5′-AAUCGUUCCAUCUCGAAGUCCACUC-3′ and
5-'GAGUGGACUUCGAGAUGGAACGAUU-3′. The transfection was conducted
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer's instructions.
Immunohistochemistry (IHC)
The 79 paired paraffin-embedded cancer and adjacent
tissues of HCC patients were sectioned (3 µm), deparaffinized in
xylene, and rehydrated in a series of graded alcohol dilutions.
Then, we used heat epitope retrieval for 20 min under a condition
of citrate salt solution. After incubation in 5% BSA for 30 min,
all tissues were incubated with a rabbit antibody to human RAB34
anti-RAB34 antibody (ab110821; dilution, 1/1,000; Abcam
Biotechnology, Cambridge, UK) overnight at 4°C. Slides were then
incubated with HRP at room temperature for 30 min and were
visualized using DAB as chromogen for 5–10 min. The method for IHC
scoring was as follows: 0, negative; 1, <30% positive tumor
cells; 2, 30–50% positive tumor cells; 3, 50–70% positive tumor
cells; and 4, >70% positive tumor cells. The intensity of the
dye color was graded as 0 (no color), 1 (light yellow), 2 (light
brown), or 3 (brown). The staining index was calculated as follows:
Staining index = staining intensity + tumor cell staining grade.
High RAB34 expression was defined as a staining index score ≥4,
while low expression was defined as a staining index <4.
Western blot analysis
Total proteins were extracted from the harvested
cells using a protein extraction kit (Beyotime, Jiangsu, China).
All the proteins were degenerated by boiling, separated by SDS-PAGE
and transferred onto polyvinylidene difluoride (PVDF) membranes.
Then, the membranes were blocked by 5% milk. Next, the membranes
were incubated in the primary antibodies overnight at 4°C. Then,
after washing 3 times with Tris-buffered saline and Tween-20
(TBST), the membranes were incubated with the secondary antibody
(goat anti-rabbit) for 2 h in a 37°C incubator. Finally, all the
membranes were visualized using a chemiluminescence kit (Beyotime)
on a Bio-Rad imaging system. Primary antibodies used in the present
study were as follows: RAB34 (ab110821; dilution, 1/1,000; Abcam
Biotechnology), E-cadherin and N-cadherin [1:1,000 dilution, (EMT)
Antibody Sampler kit #9782; Cell Signaling Technology, Beverly, MA,
USA], CDK-2 (ab32147; dilution, 1/1,000), CDK4 (ab108357; dilution,
1/1,000) and cyclin B1 (ab32053; dilution, 1/1,000) and the
internal control was β-actin (dilution, 1:1,000) (all from Abcam
Biotechnology).
Cell viability and EDU assays
To detect the relative cell viability, LM3 and Huh7
cell lines were seeded into 96-well microplates at a density of
5,000 cells/well. Then, Cell Counting Kit-8 (CCK-8) assay (Dojindo,
Kumamoto, Japan) was performed according to the manufacturer's
instructions at 24, 48 and 72 h. Briefly, 10 µl of CCK-8 working
solution/100 µl of medium was added into the microplates and the
cells were incubated for 2 h. The optical density OD450 value was
determined using an MRX II microplate reader (DYNEX Technologies,
Chantilly, VA, USA). In addition, the detection of EDU was
conducted according to a previous study (12).
Cell cycle analysis
LM3 and Huh7 cells transfected with the RAB34 siRNA
or the negative control (NC) were trypsinized and washed 3 times
with pre-chilled phosphate-buffered saline (PBS), and resuspended
in 100 µl PBS at 1×106 cells/ml after 48 h of
transfection. For cell cycle analysis, samples of cells were fixed
in 75% ethanol overnight, and DNA was stained for cell cycle
analysis. RNA was removed by mixing 0.5 ml DNA Prep Stain (Coulter
DNA Prep Reagents kit; Beckman Coulter, Brea, CA, USA) in dark at
room temperature for 30 min. DNA content of stained cells was
measured with BD LSR II (BD Biosciences, Bedford, MA, USA). Each
histogram was constructed with data from at least 10,000 events.
The percentage of the cell population in each phase from the
experimental data was calculated using ModFit LT software (Verity
Software House, Inc., Topsham, ME, USA).
Cell migration assay
Migration assays were performed using Transwell
plates (Corning, Corning, NY, USA). After 48 h of transfection, LM3
and Huh7 cells transfected with RAB34 siRNA or the negative control
were trypsinized and collected. Then, 1×105 cells were
cultured in serum-free MEM on an insert coated without Matrigel
(migration assay; BD Biosciences) for 24 h. In the lower
compartment, medium was replaced with MEM complete medium. After
fixation and staining, cells on the bottom surface that invaded
across the membranes were counted and photographed. All experiments
were performed in triplicate.
Scratch wound healing assay
LM3 and Huh7 cells transfected with RAB34 siRNA or
the negative control were inoculated onto 6-well plates and
cultured at 37°C in a 5% CO2 cell incubator. After the
cells reached 70–80% confluence, cross lines were made using a
200-µl sterile pipette tip. The cells were washed 3 times with
sterile PBS to remove the scratched cells. The cells were
continuously cultured in serum-free culture medium. After 0 and 48
h, the cells were photographed. Cell migration distance = distance
at 0 h - distance at 48 h.
Statistical analysis
Data are presented as means ± standard deviation
(SD). For comparisons, the Student's t-test, paired-samples t-test,
and Fisher's exact test were performed as appropriate. Cumulative
recurrence and survival probabilities were evaluated using the
Kaplan-Meier method, and differences were assessed using the
log-rank test. All analyses were performed using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA). Differences were considered
significant at P<0.05.
Results
RAB34 is upregulated in human HCC
tissues and cell lines
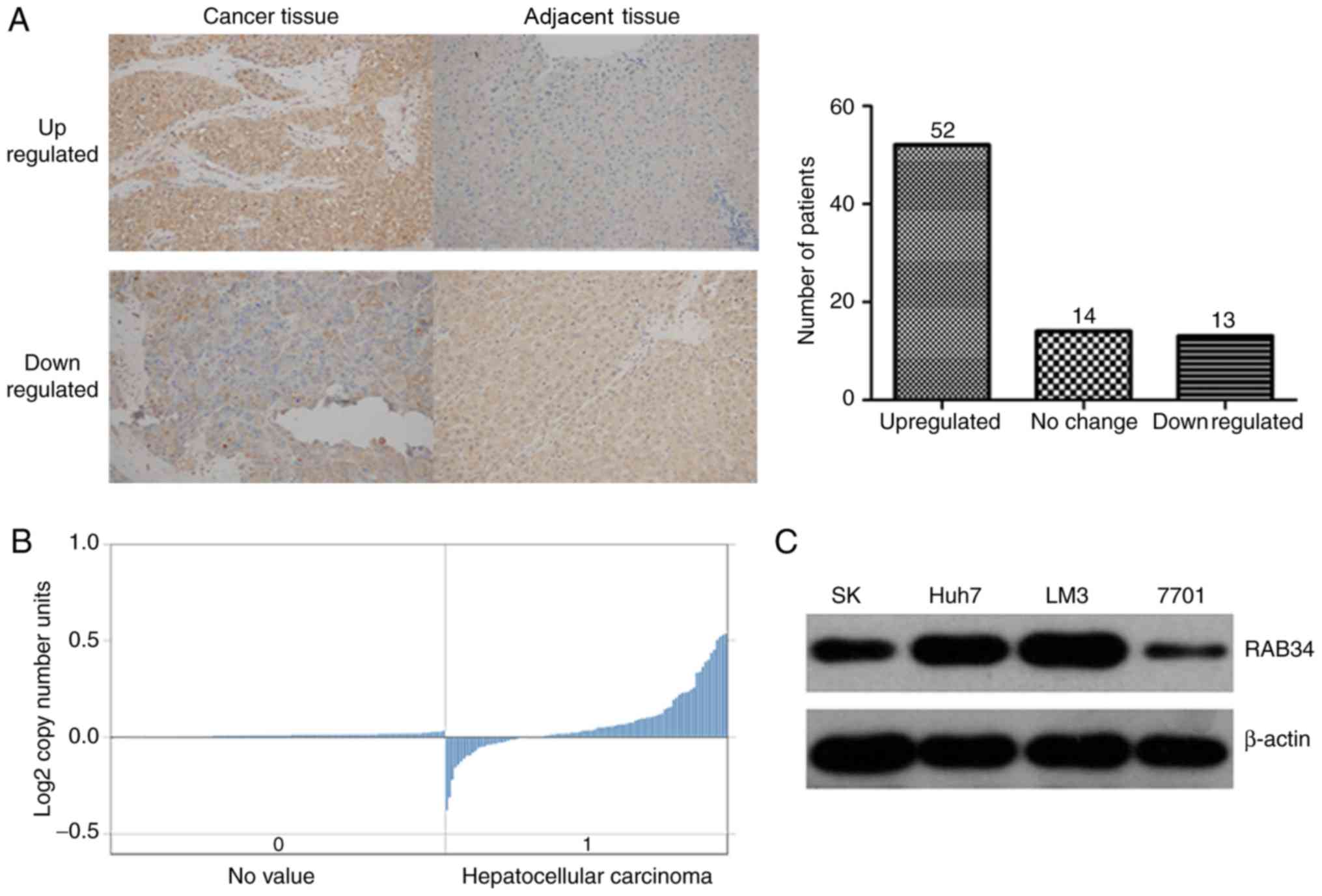
To investigate whether RAB34 plays an important role
in the initiation and progression of HCC, we detected the
expression level of RAB34 by IHC in 79 paired cancer and adjacent
tissues of HCC patients. The results showed that RAB34 was
overexpressed in almost all HCC tissues. By comparing with the
neighbor-cancer tissues, RAB34 was upregulated in 52 HCC tissues,
while downregulated in only 13 (Fig.
1A). To verify our findings, we searched Oncomine database
using the items (‘RAB34’ and ‘liver cancer vs. normal’) and found
the data of RAB34 copy no. in 212 TCGA liver tissues, which showed
that the RAB34 DNA copy no. was overexpressed in HCC (Fig. 1B). Furthermore, we compared the
RAB34 expression level among 3 HCC cell lines (Huh7, SK and LM3)
and a normal immortalized liver cell line (7701), and found that
RAB34 was overexpressed in the HCC cell lines, and the LM3 cell
line showed the highest expression (Fig. 1C). Such results showed that RAB34
was upregulated in HCC and may be associated with the initiation
and progression of HCC.
Overexpression of RAB34 leads to the
poor prognosis of HCC patients
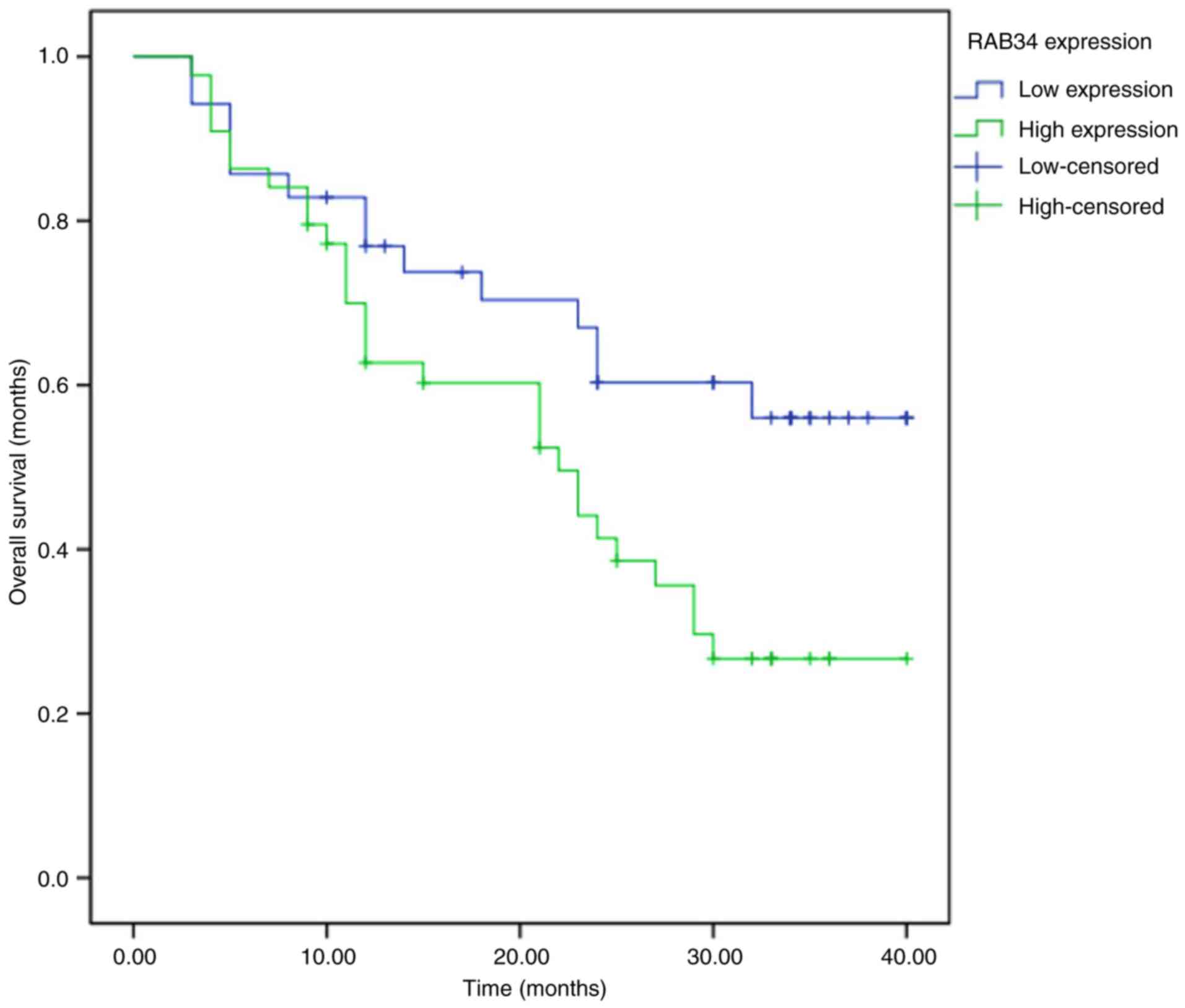
To investigate whether RAB34 expression is
associated with the prognosis of HCC patients, we divided the HCC
patients into two groups according to the expression of RAB34 in
cancer tissues (44 patients were high expression group while 35
patients were low expression group). The results of Kaplan-Meier
analysis showed that the patients with high expression of RAB34
were associated with poor prognosis when compared with those who
had low expression (P=0.026) (Fig.
2). In addition, we analyzed the relationship between RAB34
expression and clinicopathological features of HCC patients by
single-factor Chi-square test. We found a correlation between
expression of RAB34 and tumor size and tumor grade (Table I). Patients with high expression of
RAB34 consistently had large tumor size and poor tumor grade.
 | Table I.Relationship between RAB34 expression
and clinicopathologic features of the HCC patients. |
Table I.
Relationship between RAB34 expression
and clinicopathologic features of the HCC patients.
|
| All pts. | RAB34 expression |
|
|---|
|
|
|
|
|
|---|
| Variables | (n=79) | Higha | Lowb | P-valuec |
|---|
| Age (years) |
|
|
| 0.783 |
| ≤55 | 37 | 20 | 17 |
|
|
>55 | 42 | 24 | 18 |
|
| Gender |
|
|
| 0.822 |
| Male | 35 | 19 | 16 |
|
|
Female | 44 | 25 | 19 |
|
| Size of tumor
(cm) |
|
|
| 0.024 |
| ≤5 | 34 | 14 | 20 |
|
|
>5 | 45 | 30 | 15 |
|
| Number of tumors |
|
|
| 0.143 |
|
Single | 47 | 23 | 24 |
|
|
Multiple | 32 | 21 | 11 |
|
| Vessel invasion |
|
|
| 0.115 |
|
Negative | 49 | 25 | 24 |
|
|
Positive | 30 | 19 | 11 |
|
| Grade |
|
|
| 0.026 |
| Well +
moderate | 32 | 13 | 19 |
|
| Poor | 47 | 31 | 16 |
|
Suppression of RAB34 inhibits the
proliferation of HCC-LM3 and Huh7 cell lines
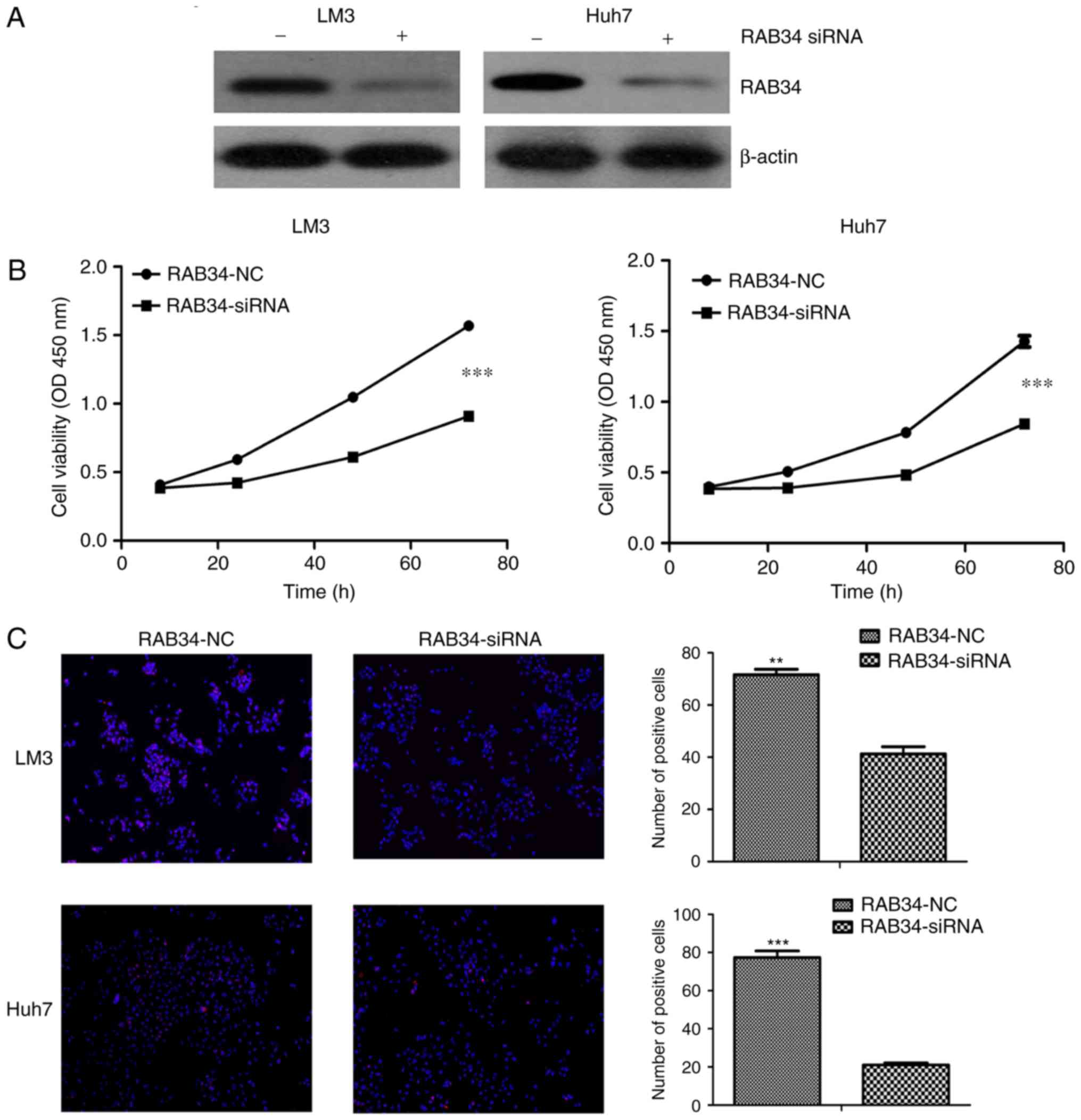
As expression of RAB34 in Huh7 and HCC-LM3 cells was
relative higher than that noted in the SK cell line, and SK was
derived from ascites, Huh7 and HCC-LM3 cells were deemed suitable
for our further study. We used RAB34 siRNA to suppress the
expression of RAB34 in the HCC-LM3 and Huh7 cell lines, and the
efficiency of knockdown was verified by western blotting (Fig. 3A). Then, we used CCK-8 assay to
compared cell viability under condition of RAB34 siRNA and negative
control, and found thtat the cell lines with RAB34 siRNA had lower
cell viability than that noted in the negative controls (Fig. 3B). However, such a phenomenon may be
induced by cell apoptosis. Thus, we detected the cell proliferation
rate by EDU assay following transfection with the siRNA and
negative control. The results showed that suppression of RAB34
markedly decreased the cell proliferation rate (Fig. 3C). Thus, we concluded that RAB34
regulated the proliferation of HCC-LM3 and Huh7 cell lines, and
these results corroborated the results of the clinical sample
analysis.
RAB34 affects the proliferation rate
of HCC-LM3 and Huh7 cell lines by regulating progression of G1 cell
cycle phase
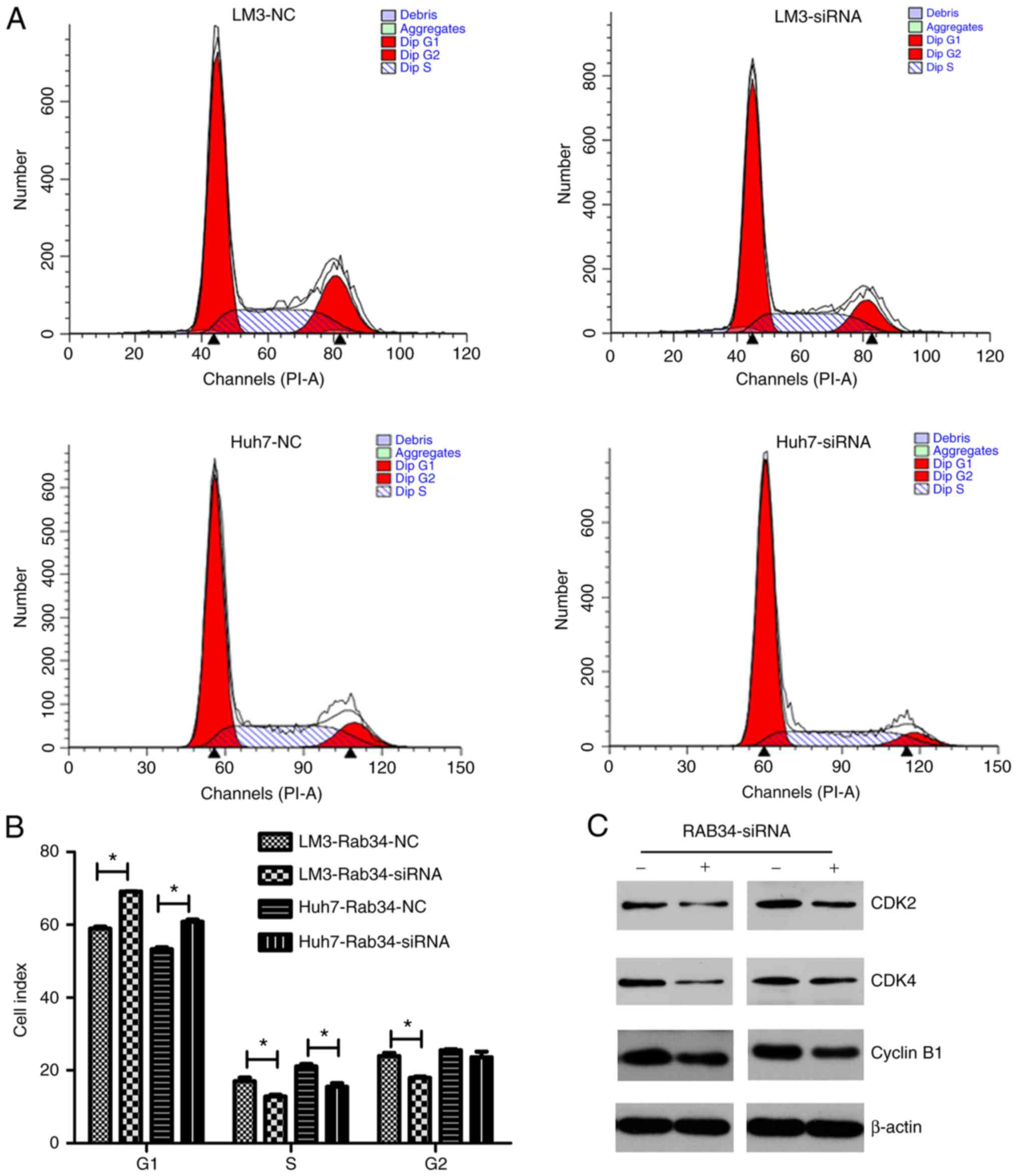
To study the mechanism of RAB34 in regulating the
proliferation rate of HCC cell lines, we used flow cytometry to
detect cell cycle change in the cells transfected with the RAB34
siRNA and negative control. The results were analyzed by Modfit
software and different cell cycle phases (G1, S and G2) were
distinguished (Fig. 4A). Then, we
used Graphpad software to compare the results of the two groups and
found that the cell number in the G1 phase in the siRNA group was
higher than that in the negative group, while the cell numbers in
the S and G2 phases were less than those in the negative group
(Fig. 4B). Thus, suppression of
RAB34 was found to lead to cell cycle G1 phase arrest. To further
verify our findings, we used western blotting to detect expression
of cell cycle-related proteins and found that CDK2, CDK4 and cyclin
B1 were all decreased in the RAB34 siRNA group (Fig. 4C). Thus, we concluded that RAB34
positively regulates progression of cell cycle G1 phase.
Suppression of RAB34 inhibits the
migration of HCC cell lines by mesenchymal-epithelial
transition
Since suppression of RAB34 regulated the
proliferation rate of HCC cell lines by inhibiting cell cycle
progression, we aimed to ascertain whether it plays an important
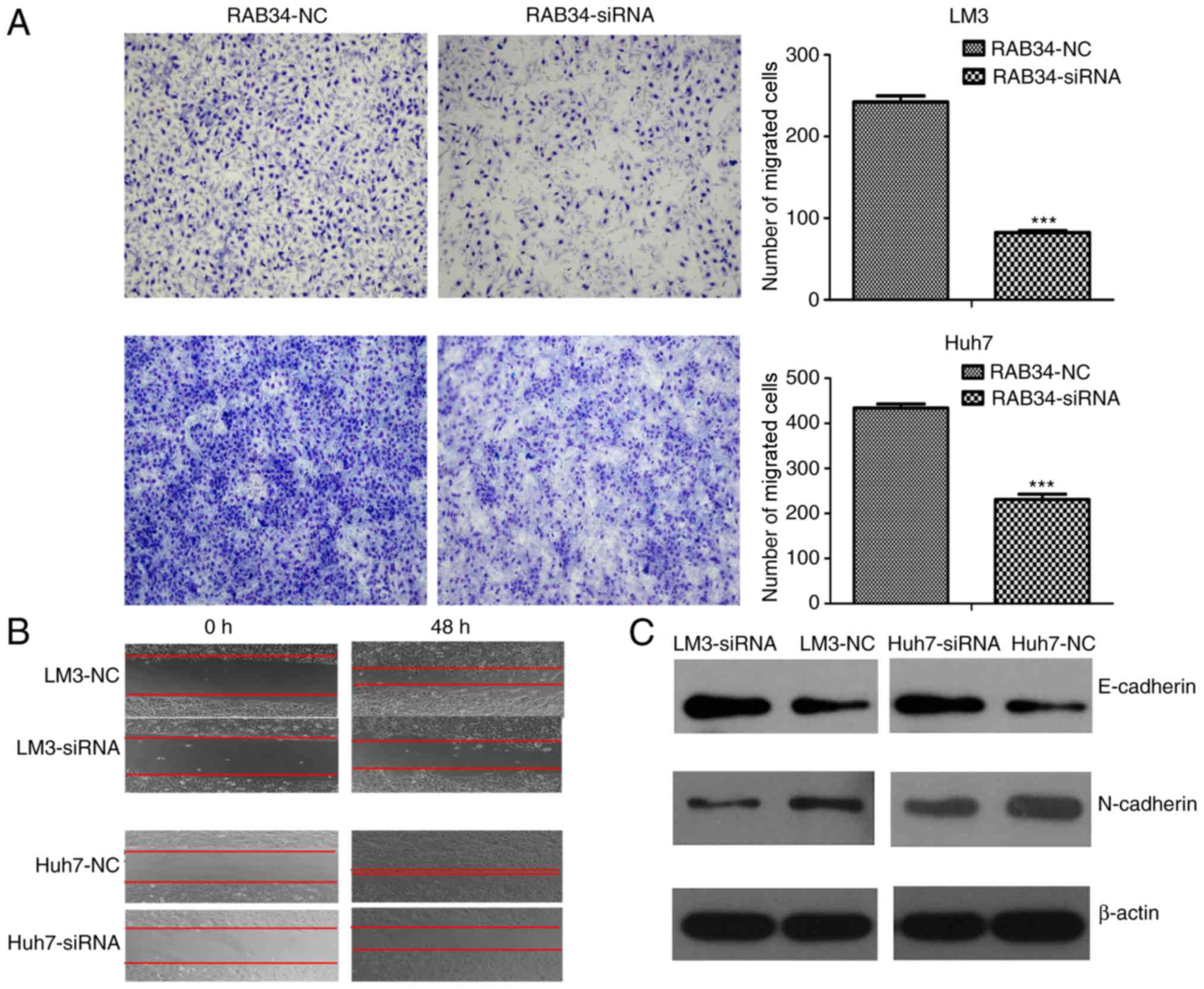
role in cell migration. We first used the Transwell assay to detect
cell migration change in the cells following transfection of RAB34
siRNA or negative control. We found that the number of migrated
cells in the siRNA groups was less than that noted in the negative
controls (Fig. 5A). To eliminate
the interference of cell proliferation, we used a scratch wound
healing assay to detect cell migration ability under a serum-free
environment and found the same result as above (Fig. 5B). Thus, suppression of RAB34
inhibited the ability of cell migration. To study the mechanism of
such a phenomenon, we used western blotting to detect expression of
E-cadherin and N-cadherin in the RAB34 siRNA and negative groups.
The results showed that suppression of RAB34 led to higher
expression of E-cadherin and lower expression of N-cadherin when
compared to the negative controls (Fig.
5C). Thus, we concluded that suppression of RAB34 inhibited
cell migration by mesenchymal-epithelial transition.
Discussion
The Ras superfamily is a protein superfamily of
small GTPases, which are divided into 5 main families (Ras, Rho,
Ran, Rab and Arf) based on their structure, sequence and function
(13,14). Numerous studies have shown that
vesicle trafficking and exocytosis are important in tumorigenesis,
which suggests that abnormal expression of RAB family proteins
plays a vital role in the initiation and progression of tumors
(15–17). In addition, numerous studies have
shown that the dysregulation of the RAB family of proteins could be
tumorigenic or tumor suppressive depending on different tumor
microenvironments. Tong and colleagues found that RAB25 had low
expression in esophageal squamous cell carcinoma and was an
important tumor suppressor with both anti-invasive and -angiogenic
abilities (18). Dong et al
analyzed the overall survival of 148 HCC patients and found that
expression of Rab27A/B was correlated with clinicopathological
characteristics and poor prognosis in HCC (19). In addition, a recent study found
that RAB34 is associated with the progression of glioma grade and
leads to poor prognosis in high-grade glioma patients (11). While the function of RAB34 in HCC
was unclear, we aimed to ascertain whether RAB34 plays an important
role in the progression of HCC.
Firstly, we found that RAB34 was overexpressed in
HCC cancer tissues by IHC detection and Oncomine database analysis.
Then, we found that expression of RAB34 was correlated with tumor
size and tumor grade and patients with high expression of RAB34
consistently had a poor prognosis. Further study showed that
suppression of RAB34 regulated the proliferation rate of HCC cell
lines by inhibiting progression of the G1 phase, and inhibited the
migration ability by promoting progression of an epithelial cell
phenotype.
In 2011, Hanahan and Weinberg wrote a study called
‘Hallmarks of Cancer: The Next Generation’. They showed 6 hallmark
capabilities of cancer and the ability of immortal proliferation
and migration were included (20).
Thus, RAB34 may be a new therapeutic target for the clinical
treatment of HCC. As the cell cycle phase is important for cell
proliferation, each phase has its own checkpoint, and only passing
through the detection of this checkpoint can the cell proliferate
for the next generation (21,22).
Thus, we detected expression of various G1 proteins under a
condition of RAB34 suppression, and found that CDK2, CDK4 and
cyclin B1 were decreased. In contrast, epithelial-mesenchymal
transition (EMT) which is a reversible dynamic process during which
epithelial cells gradually adopt structural and functional
characteristics of mesenchymal cells, has gained much attention
regarding metastatic dissemination (23–25).
We used western blotting to observe the expression change of
E-cadherin and N-cadherin under condition of RAB34 siRNA. Notably,
we found that E-cadherin was upregulated and N-cadherin was
downregulated when RAB34 was suppression. In conclusion, we
demonstrated that RAB34 may become a new biomarker and therapeutic
target for HCC.
However, in consideration of the limitation of
experimental technology, we did not research the more detailed
mechanism involved in the function of RAB34 in HCC. Thus, further
clinical research is warranted to verify the clinical value of
RAB34. In a word, the present study demonstrated that RAB34 is
overexpressed in HCC and plays an important role in the progression
of HCC; patients with high expression of RAB34 have a poor
prognosis.
References
|
1
|
Finn RS: Current and future treatment
strategies for patients with advanced hepatocellular carcinoma:
Role of mTOR inhibition. Liver Cancer. 1:247–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Gilabert M, Bruix J and Raoul
JL: Treatment of intermediate-stage hepatocellular carcinoma. Nat
Rev Clin Oncol. 11:525–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galmiche A, Chauffert B and Barbare JC:
New biological perspectives for the improvement of the efficacy of
sorafenib in hepatocellular carcinoma. Cancer Lett. 346:159–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang T and Hong W: Interorganellar
regulation of lysosome positioning by the Golgi apparatus through
Rab34 interaction with Rab-interacting lysosomal protein. Mol Biol
Cell. 13:4317–4332. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang T, Wong KK and Hong W: A unique
region of RILP distinguishes it from its related proteins in its
regulation of lysosomal morphology and interaction with Rab7 and
Rab34. Mol Biol Cell. 15:815–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T and Hong W: Assay and functional
properties of Rab34 interaction with RILP in lysosome
morphogenesisMethods in Enzymology. Academic Press; New York, NY:
pp. 675–687. 2005, View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Starling GP, Yip YY, Sanger A, Morton PE,
Eden ER and Dodding MP: Folliculin directs the formation of a
Rab34-RILP complex to control the nutrient-dependent dynamic
distribution of lysosomes. EMBO Rep. 17:823–841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Speight P and Silverman M:
Diacylglycerol-activated Hmunc13 serves as an effector of the
GTPase Rab34. Traffic. 6:858–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alloatti A, Kotsias F, Pauwels AM, Carpier
JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U,
et al: Toll-like receptor 4 engagement on dendritic cells restrains
phago-lysosome fusion and promotes cross-presentation of antigens.
Immunity. 43:1087–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasmapour B, Gronow A, Bleck CK, Hong W
and Gutierrez MG: Size-dependent mechanism of cargo sorting during
lysosome-phagosome fusion is controlled by Rab34. Proc Natl Acad
Sci USA. 109:pp. 20485–20490. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang HJ, Gao Y, Chen L, Li YL and Jiang
CL: RAB34 was a progression- and prognosis-associated biomarker in
gliomas. Tumour Biol. 36:1573–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salic A and Mitchison TJ: A chemical
method for fast and sensitive detection of DNA synthesis in vivo.
Proc Natl Acad Sci USA. 105:pp. 2415–2420. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goitre L: The Ras superfamily of small
GTPases: The unlocked secretsRas Signaling: Methods and Protocols.
Trabalzini L and Retta SF: Humana Press; Totowa, NJ: pp. 1–18.
2014, View Article : Google Scholar
|
|
14
|
Wennerberg K, Rossman KL and Der CJ: The
Ras superfamily at a glance. J Cell Sci. 118:843–846. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan AM and Weber T: A putative link
between exocytosis and tumor development. Cancer Cell. 2:427–428.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wright PK: Targeting vesicle trafficking:
An important approach to cancer chemotherapy. Recent Patents
Anticancer Drug Discov. 3:137–147. 2008. View Article : Google Scholar
|
|
17
|
Chia WJ and Tang BL: Emerging roles for
Rab family GTPases in human cancer. Biochim Biophys Acta.
1795:110–116. 2009.PubMed/NCBI
|
|
18
|
Tong M, Chan KW, Bao JY, Wong KY, Chen JN,
Kwan PS, Tang KH, Fu L, Qin YR, Lok S, et al: Rab25 is a tumor
suppressor gene with antiangiogenic and anti-invasive activities in
esophageal squamous cell carcinoma. Cancer Res. 72:6024–6035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong WW, Mou Q, Chen J, Cui JT, Li WM and
Xiao WH: Differential expression of Rab27A/B correlates with
clinical outcome in hepatocellular carcinoma. World J
Gastroenterol. 18:1806–1813. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sears RC and Nevins JR: Signaling networks
that link cell proliferation and cell fate. J Biol Chem.
277:11617–11620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieto MA and Cano A: The
epithelial-mesenchymal transition under control: Global programs to
regulate epithelial plasticity. Semin Cancer Biol. 22:361–368.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|