Introduction
Osteosarcoma is the most common type of solid bone
cancer, mainly arising in children and young adults (1). The majority of osteosarcomas develop
in the long bones, most commonly in the distal femur and proximal
tibia (2). It is highly malignant
and invasive, with a metastatic rate of ~20% (3). Other common targets are the lung and
other bones in addition to long bones (4,5).
Numerous studies have reported that angiogenesis is closely related
to the development, invasion and metastasis of osteosarcoma
(6). Therefore, actively exploring
the mechanism of angiogenesis in osteosarcoma may help to enhance
understanding of the mechanisms of osteosarcoma occurrence and
progression and potentially aid in developing a new strategy for
anti-angiogenesis clinical tumor treatment.
Tumor angiogenesis involves degradation of the
extracellular matrix, endothelial cell (EC) migration,
proliferation, elongation and tube formation to form new vessels
(7). Pre-existing ECs, bone
marrow-derived endothelial progenitor cells (EPCs) and mesenchymal
stem cells have traditionally been regarded as the sources of ECs
in tumor angiogenesis (8,9). However, recent studies suggest that
tumor cells may differentiate into ECs in myeloma, lymphoma,
chronic myeloid leukemia (CML), glioblastoma, breast cancer and
neuroblastoma (10–15). However, whether the mechanism of
tumor angiogenesis in osteosarcoma is also different from regular
tumor vascular formation, remains an unanswered question. The aim
of the present study was to determine whether human osteosarcoma
MNNG/HOS cells can also transdifferentiate into ECs and acquire
endothelial markers in vitro, and whether they participate
in tumor vascularization in vivo.
For this purpose, the research is divided into two
parts: in vitro and in vivo. In vitro, we
cultured MNNG/HOS cells on Matrigel under hypoxic conditions, and
then investigated changes in the morphology and molecular phenotype
of the cells. In vivo, human osteosarcoma cells
(1×106 cells) that were cultured under conditions of
hypoxia were subcutaneously injected into nude mice, and then the
mice were sacrificed 49 days after inoculation. Tumor angiogenesis
in human MNNG/HOS osteosarcoma cells xenografted in nude mice was
confirmed using immunohistochemical staining with anti-human CD31
antibody. The results demonstrated that human MNNG/HOS osteosarcoma
cells can transdifferentiate into vascular endothelial cell-like
cells in vitro and in vivo.
Materials and methods
Cell lines and culture
The human osteosarcoma cell line MNNG/HOS was
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in essential medium MEM
+ 10% fetal bovine serum (FBS) (both from HyClone, Logan, UT, USA)
+ 100 U/ml penicillin/streptomycin in vitro.
Induced transdifferentiation of human
osteosarcoma cells
The MNNG/HOS cells were divided into 4 groups:
control, normoxia, hypoxia and preconditioning group. MNNG/HOS
cells in the control and preconditioning group were cultured in
essential medium. MNNG/HOS cells in the normoxia and hypoxia group
were cultured in endothelial differentiation medium consisting of
MEM/F-12 containing 10% FBS (HyClone), 1% N2 supplement (Gibco,
Carlsbad, CA, USA), human vascular endothelial growth factor (20
ng/ml), human recombinant epidermal growth factor (20 ng/ml) (both
from Invitrogen, Carlsbad, CA, USA), basic fibroblast growth factor
(10 ng/ml) and heparin (both from Gibco) in vitro. MNNG/HOS
cells in the control and normoxia group were cultivated in an
incubator at 37°C under conditions of normoxia (5% CO2,
95% air). MNNG/HOS cells in the hypoxia and preconditioning group
were cultivated in an incubator at 37°C under conditions of hypoxia
(1% O2, 5% CO2, 94% nitrogen). Four days
later, their appearance was observed.
Three-dimensional culture
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was dissolved at 4°C overnight. Ninety-six well plates were
prepared at 4°C for 60 min. Matrigel (30 µl) was poured onto the
96-well plates, and then the plates were placed in an incubator at
37°C with 5% CO2 for 30 min. After allowing 30 min for
Matrigel gel formation, MNNG/HOS cell (1×105 cells/200
µl) suspensions in the endothelial differentiation medium were
added to the Matrigel-coated wells. For the control and normoxia
group, the cells were cultivated at 37°C with 5% CO2 and
95% air. For the hypoxia and preconditioning group the cells were
cultivated at 37°C with 1% O2, 5% CO2 and 94%
nitrogen. Formation of tubular-like structures and networks was
periodically observed and imaged by inverted phase contrast
microscopy.
Isolation of RNA and RT-PCR
MNNG/HOS cells in each group were cultivated in
different medium under normoxia or hypoxia condition. Forty-eight
hours later, total RNA was extracted from each group using TRIzol
reagent and treated with DNase I (both from Invitrogen).
First-strand cDNA was synthesized from total RNA (5 µg) using a
ReverTra Ace kit (Toyobo Co., Ltd., Osaka, Japan) and an
oligo(dT)20 primer. First-strand cDNA was synthesized
from total RNA using a ReverTra Ace kit (Toyobo) and an
oligo(dT)20 primer. The target cDNA was amplified using
a combination of Taq DNA polymerase and the proofreading Pfu
DNA polymerase. The primers used were: CD31 forward (5′-ACA TGG CAA
CAA GGC TGT GTA-3′) and reverse (5′-CCT CAA ACT GGG CAT CAT
AAG-3′); CD34 forward (5′-CCA CTC GGT GCG TCT CTC TAG GAG C-3′) and
reverse (5′-TTG TCT CTG GAG TTG AAA CGT TGG C-3′); vWF forward
(5′-CTG AAG AGT CAT CGG GTC AAC TGT-3′) and reverse (5′-AGC ATG AAG
TCA TTG GCT CCG TTC T-3′); β-actin forward (5′-TTC TGT GGC ATC CAC
GAA ACT-3′) and reverse (5′-GAA GCA TTT GCG GTG GAC GAT-3′). PCR
amplification was carried out in a final volume of 25 µl of
reaction mixture. After amplification, 5 µl of the PCR products was
electrophoresed through a 1.5% agarose gel.
In vivo xenograft experiments
Five-to-six week-old male BALB/c nude mice were
obtained from the Chinese Academy of Medical Sciences. Animals were
housed in laminar flow cabinets under specific pathogen-free
conditions. For the xenograft experiments, mice were randomized
into 4 groups, each with 5 mice. MNNG/HOS cells (1×106
cells) in each group were respectively subcutaneously injected into
the nude mice. After inoculation, the size of the subcutaneous
tumors was closely monitored. The tumor volume (V) was calculated
by the formula, V = 0.5(L × W2) mm3; the
longest axis diameter (L) and the greatest transverse diameter (W)
were estimated with external calipers every 7 days. The mice were
sacrificed 49 days after inoculation, and the tumors were weighed
and harvested for further evaluation by immunohistochemistry. The
mice were sacrificed and cared for according to the ethical
guidelines of the Animal Experimental Ethics Committee of Fujian
Medical University. None of the mice died during the whole
experimental process.
Immunofluorescence
MNNG/HOS cells were plated onto glass coverslips in
12-well plates and cultivated according to the different conditions
of each group. Four days later, the above-mentioned cells were
washed with phosphate-buffered saline (PBS) (pH 7.4) and fixed with
4% paraformaldehyde for 20 min at 37°C and washed 3 times with PBS.
Following permeabilization with 0.3% Triton X-100 for 5 min at room
temperature, the cells were blocked with 1% bovine serum albumin
(BSA) for 20 min at room temperature. The washed cells were
incubated overnight in the dark at 4°C with anti-human CD31 (1:200;
rabbit anti-human), anti-human CD34 (1:200; mouse anti-human), and
anti-human von Willebrand factor (vWF) (1:200; rabbit anti-human)
(all from Abcam, Cambridge, MA, USA) antibodies. The cells were
stained with PE-conjugated anti-mouse antibodies or FITC-conjugated
anti-rabbit antibodies (1:200; Abcam). Cell nuclei were
counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Sigma).
All fluorescence images were captured using an Olympus BX51
fluorescence microscope (Olympus BX51; Olympus Corp., Tokyo,
Japan).
Immunohistochemistry
For immunohistochemistry, the xenograft of mouse
tissues was cut into 4-µm-thick paraffin sections. The tissue
sections were incubated with antibodies against human CD31 (1:50;
rabbit anti-human; Abcam). The specimens were then incubated with
the secondary antibody, an anti-rabbit peroxidase-labeled polymer
(1:50; ZSGB-BIO, Beijing, China). The images were captured with
light microscopy (1X71 inverted microscope; Olympus Corp.,
Tokyo).
Statistical analysis
Statistical calculations were performed using SPSS
20.0 and GraphPad software (GraphPad Software, San Diego, CA, USA).
The quantitative assays of the statistical analysis are expressed
as the means ± standard error of the mean. The independent-sample
t-tests or one-way analysis of variance were used to determine
statistical comparisons. The asterisks in the figures indicate
statistically significant differences between the samples
(P<0.05), while ‘NS’ indicates that there was no statistically
significant difference between samples.
Results
MNNG/HOS cells have morphological
features of VECs after transdifferentiation
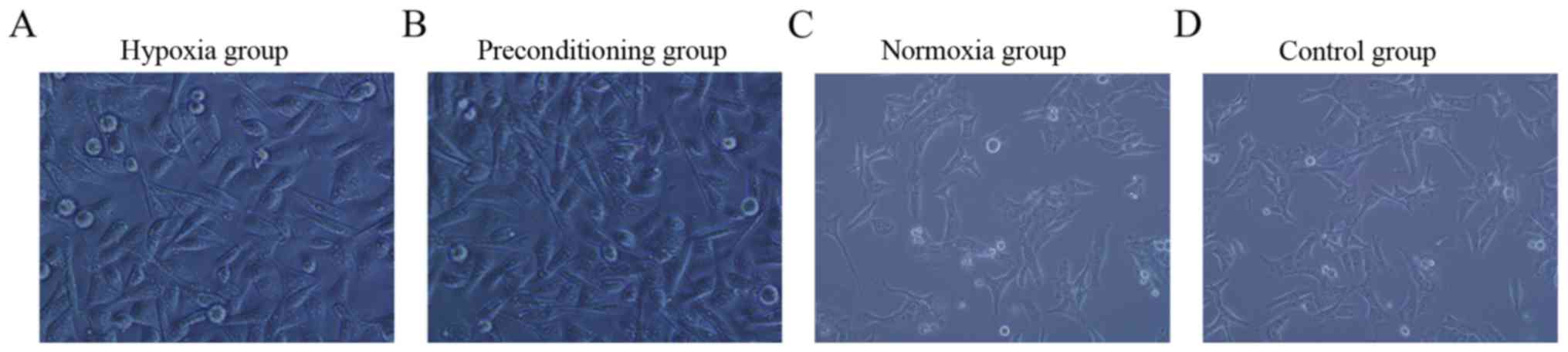
Four days after MNNG/HOS cells were cultured under
hypoxia conditions, the hypoxia and preconditioning group typically
developed a characteristic ‘flagstone’ appearance (Fig. 1A and B). However, for MNNG/HOS cells
cultured under normoxia conditions, the control and normoxia group
adhered to the culture dish rather than developing a ‘flagstone’
appearance (Fig. 1C and D). When we
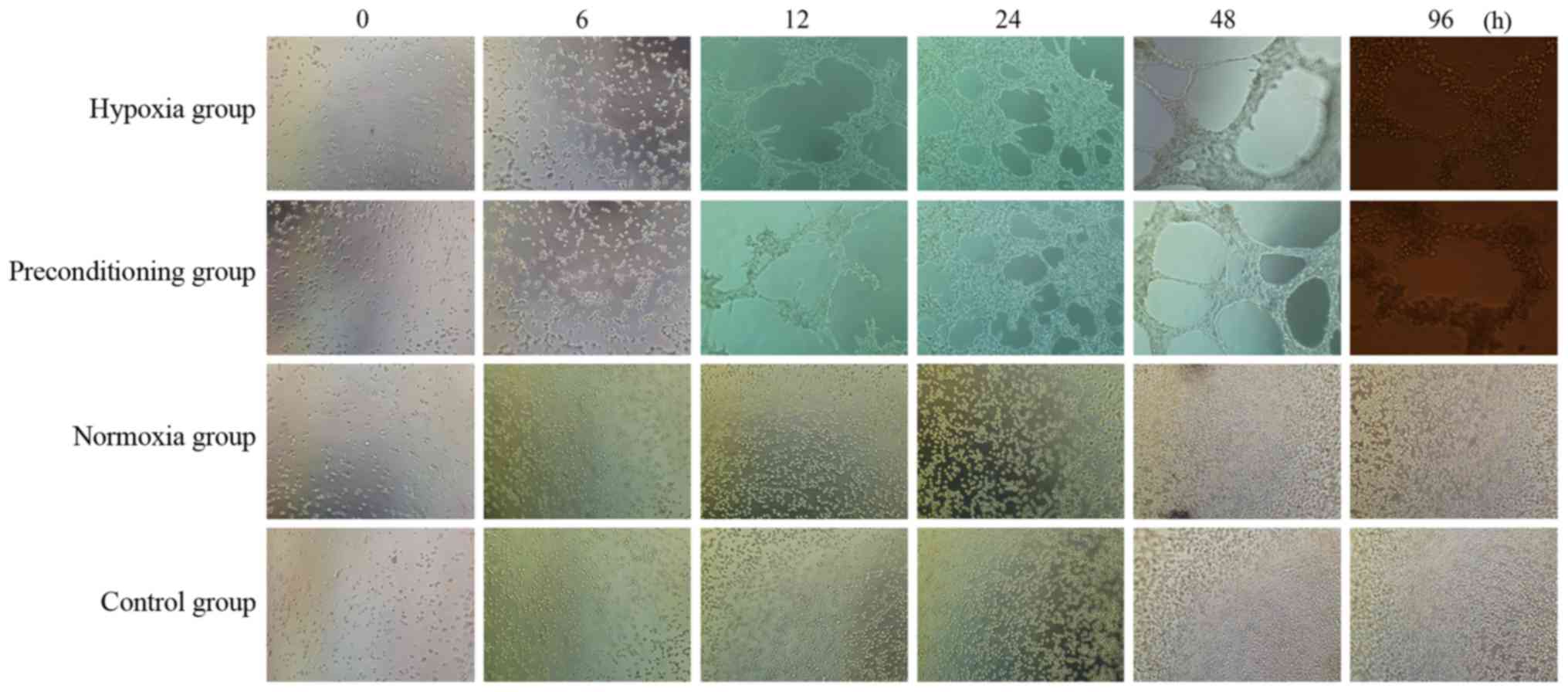
cultivated MNNG/HOS cells on Matrigel under hypoxia conditions, the
hypoxia and preconditioning group underwent a range of changes:
from a single or several cells (0 h), to intercellular connections
or discontinuous net-like connections (6 h), to continuous net-like
connections (12 h), to the significant increase in the number of
tubes (24 h), to a significant increase in the branch length (48
h). Vascular mimicry (VM) still existed at 96 h after seeding.
However, in cells cultivated on Matrigel under normoxia conditions,
the normoxia and control group did not form tube structures even 96
h after seeding (Fig. 2).
Both the mRNA and protein levels of
vascular endothelial cell (VECs) markers (vWF, CD31 and CD34) are
increased after transdifferentiation
Since MNNG/HOS cells that were cultured under
hypoxia conditions were able to form tubular-like structures and
networks within Matrigel, we speculated that they may have the
ability to transdifferentiate into VEC-like cells and have
characteristics of VECs. Therefore, we examined the mRNA and
protein levels of vWF, CD31 and CD34, which serve as cellular
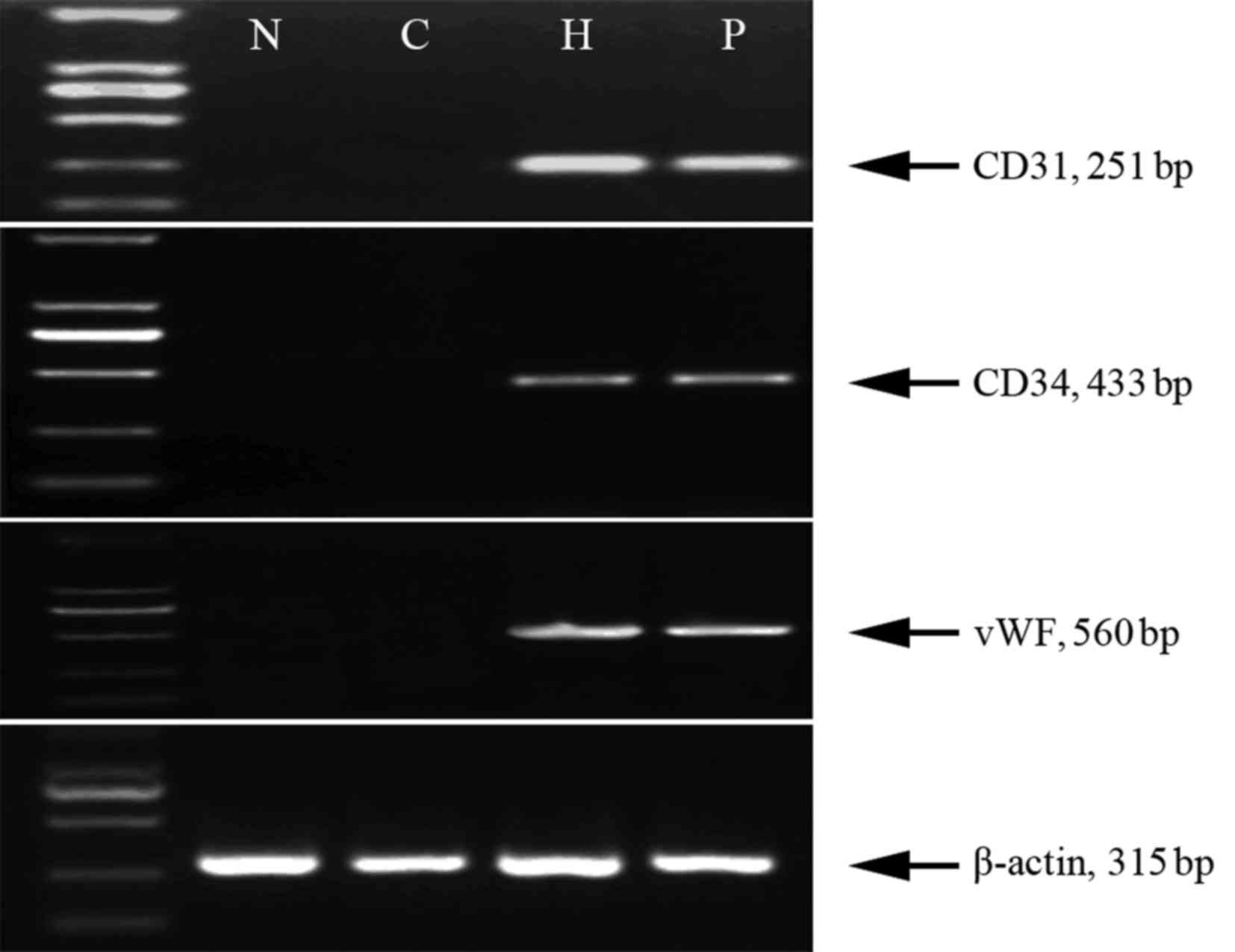
markers of VECs. The results showed that the level of mRNA of all
the 3 cellular markers had significantly increased after MNNG/HOS
cells were cultured under hypoxia conditions in the hypoxia and
preconditioning group (Fig. 3).
However, the levels of mRNA of all the 3 cellular markers were not
significantly different between the nornoxia and control group
(Fig. 3). In addition, to
investigate the proteins of the 3 cell surface markers of VECs in
osteosarcoma cells, we used immunofluorescent staining. Without
treatment, few MNNG/HOS cells showed positive immunofluorescent
staining for the 3 molecules (Fig.
4A-D). However, after exposure to hypoxic conditions, most
MNNG/HOS cells in the hypoxia and preconditioning groups showed
positive immunofluorescent staining for the 3 molecules (Fig. 4E-L). Few MNNG/HOS cells were
positive for the 3 molecules under the condition of normoxia, for
the normoxia and control group (Fig.
4M-T). The molecular change suggested that MNNG/HOS cells
exposed to hypoxia may have a VEC phenotype. The results were
consistent with the findings of RT-PCR.
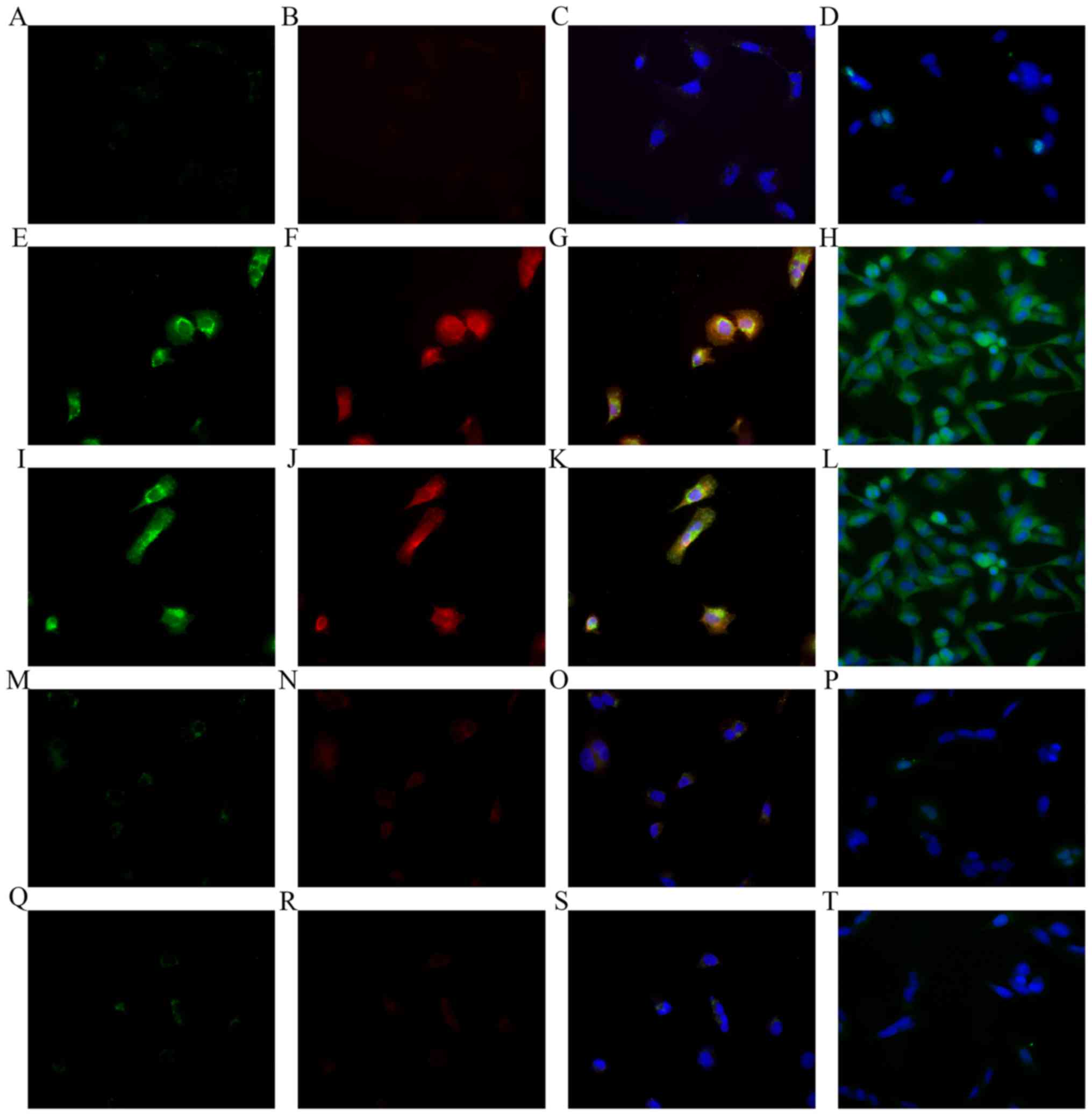 | Figure 4.Immunofluorescent staining performed
on MNNG/HOS cells before or after treatment. (A-D)
Immunofluorescent staining performed on MNNG/HOS cells without
treatment. (A) Magnification (×400), stained for CD31. (B)
Magnification (×400), for CD34. (C) Magnification (×400), the
merging of A and B. (D) Magnification (×400), for vWF. (E-H)
Immunofluorescent staining performed on MNNG/HOS cells exposed to
hypoxia conditions with endothelial differentiation medium. (E)
Magnification (×400), stained for CD31. (F) Magnification (×400),
for CD34. (G) Magnification (×400), the merging of E and F. (H)
Magnification (×400) for vWF. (I-L) Immunofluorescent staining
performed on MNNG/HOS cells exposed to hypoxia conditions with
essential medium. (I) Magnification (×400), stained for CD31. (J)
Magnification (×400), for CD34. (K) Magnification (×400), the
merging of G and H. (L) Magnification (×400), for vWF. (M-P)
Immunofluorescent staining performed on MNNG/HOS cells exposed to
normoxia conditions with endothelial differentiation medium. (M)
Magnification (×400), stained for CD31. (N) Magnification (×400),
for CD34. (O) Magnification (×400), the merging of E and F. (P)
Magnification (×400) for vWF. (Q-T) Immunofluorescent staining
performed on MNNG/HOS cells exposed to normoxia conditions with
essential medium. (Q) Magnification (×400), stained for CD31. (R)
Magnification (×400), for CD34. (S) Magnification (×400), the
merging of E and F. (T) Magnification (×400) for vWF. |
Tumorigenesis and transdifferentiation
into VEC-like cells in vivo
In order to examine the ability of MNNG/HOS cells to
form endothelial vessels in vivo, MNNG/HOS cells in each
group were subcutaneously injected into nude mice (day 0) and
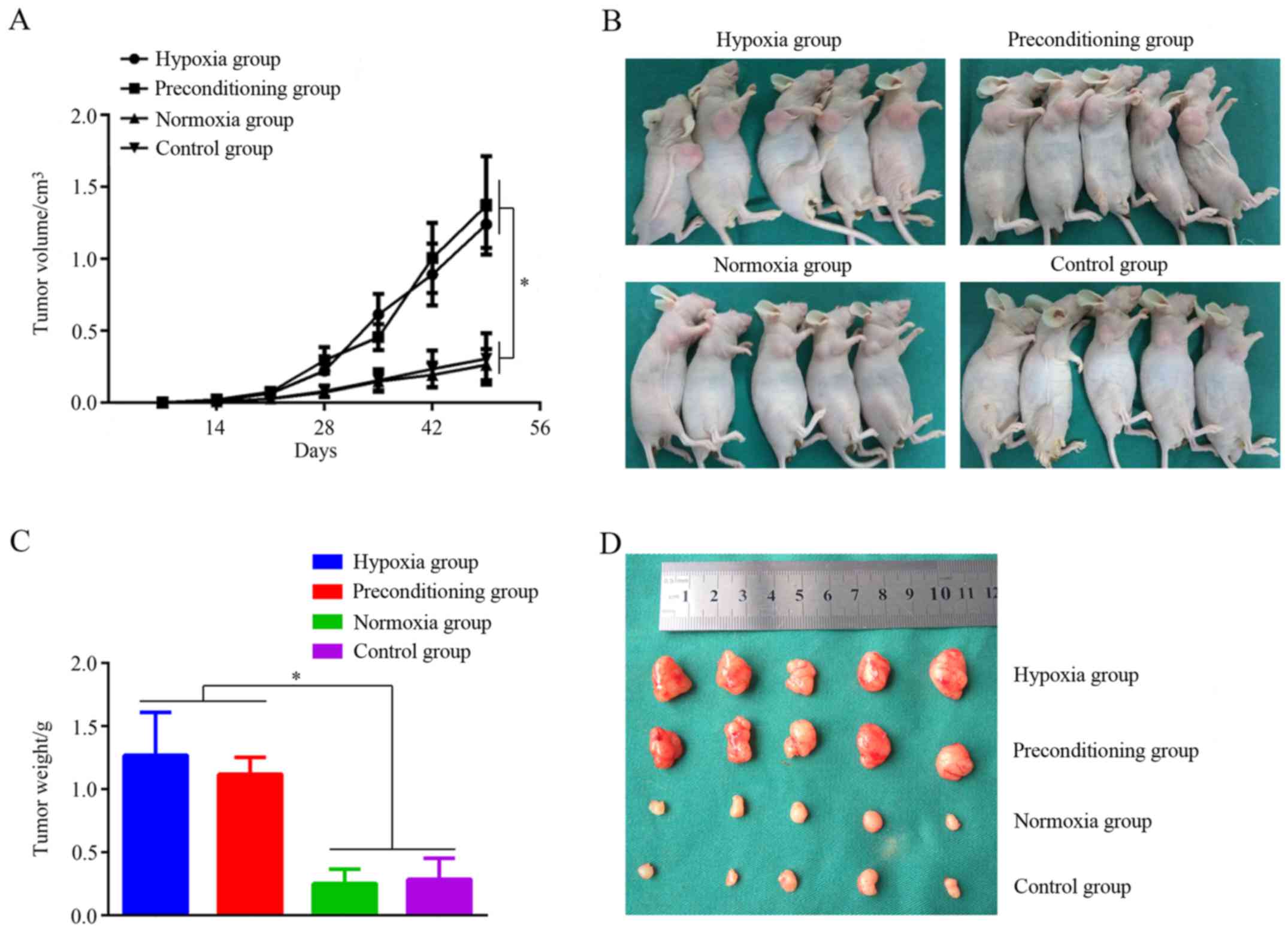
observed. The MNNG/HOS xenograft tumor volumes were calculated
every 7 days, and weighed on day 49. As shown in Fig. 5A, there was no significant
difference in the average volume of MNNG/HOS xenograft tumors among
the 4 groups for the first 14 days. However, the volume of MNNG/HOS
xenograft tumors in the hypoxia and preconditioning group were
significantly increased after the first 14 days, compared with the
control group (P<0.05). Nevertheless, there was no significant
difference in the average volume of MNNG/HOS xenograft tumors
between the normoxia and control group. The MNNG/HOS xenograft
tumors were weighed on day 49, and the hypoxia and preconditioning
group clearly produced larger tumors (Fig. 5B) and heavier tumors (Fig. 5C) compared with the control group.
However, there was no difference in the average volume and weight
of MNNG/HOS xenograft tumors between the normoxia and control
group. Morphologically, the MNNG/HOS xenograft tumors in the
hypoxia group and preconditioning hypoxia group clearly had a
greater number of vessel structures compared with those in the
control group (Fig. 5D). To study
the origins of these blood vessels within the MNNG/HOS xenograft
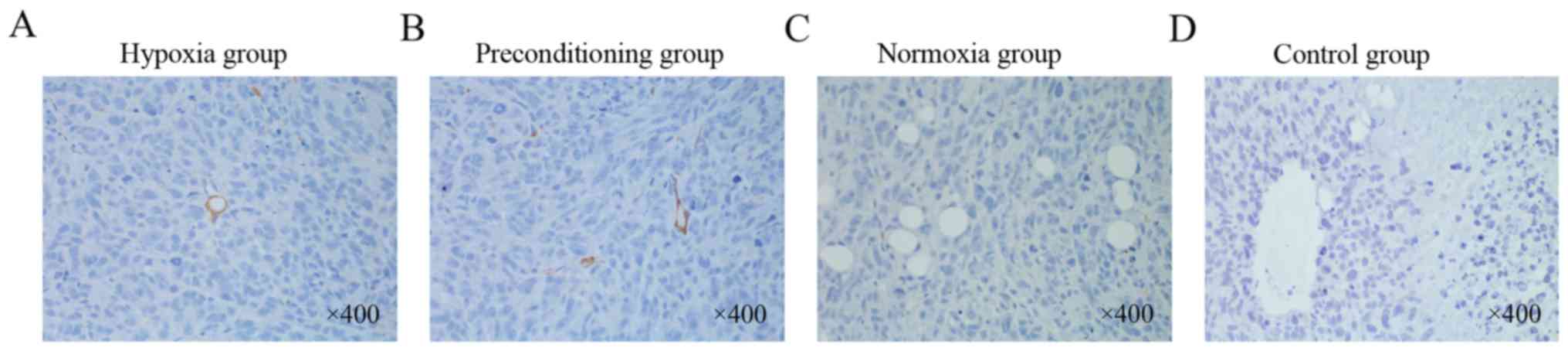
tumors in the 4 groups, we stained the tumor tissue with the
anti-human CD31 antibody to distinguish whether the blood vessels
originated from xenografted MNNG/HOS cells or from mouse VECs. The
immunofluorescence results showed that the anti-human CD31-stained
VECs were found only in MNNG/HOS xenograft tumors in the hypoxia
and preconditioning group, but could not be found in those in the
normoxia and control group (Fig.
6). These results from the in vivo studies further
support the hypothesis that hypoxia plays an important role in
inducing MNNG/HOS cell transdifferentiation into VEC-like
cells.
Discussion
Hypoxia strongly stimulates an increase in the
formation of new blood vasculature (16). In the present study, we examined
whether MNNG/HOS cells can transdifferentiate into VEC-like cells
both morphologically and functionally under the condition of
hypoxia. We observed transdifferentiation of MNNG/HOS cells into
VEC-like cells by cultivating the MNNG/HOS cells under hypoxia
conditions with or without VEGF (an important angiogenic factor),
and expression of several molecular markers of VECs (CD31, CD34 and
vWF). When MNNG/HOS cells were cultured on Matrigel under hypoxia
conditions, they gradually formed tubular-like structures. In
addition, when MNNG/HOS cells were inoculated subcutaneously into
BALB/c nude mice, blood vasculature derived from the MNNG/HOS cells
was observed in xenograft tumors of the hypoxia group and the
preconditioning group.
With sufficient blood supply, tumor cells can
survive, proliferate and metastasize (17). Angiogenesis is an extremely
important process facilitating the growth and metastasis of
osteosarcoma (18). It also has
become a major focus in tumor treatment (19). Angiogenesis is closely associated
with the formation of vascular endothelial cells (VECs) (20). It is widely accepted that bone
marrow-derived circulating endothelial precursors (CEPs) play a
critical role in tumor-associated angiogenesis, the main source of
the VECs (21). However, a recent
study showed that bone marrow-derived CEPs do not contribute to the
vascular endothelium (22).
Moreover, recent studies suggest the possibility of tumor-derived
endothelial cells in several malignant neoplasms, such as myeloma,
breast cancer and neuroblastoma (10–14).
In addition, research has shown that hypoxia can induce
glioblastoma cells to transdifferentiate into VECs (15). Therefore, the transdifferentiation
of MNNG/HOS cells into VEC-like cells under the condition of
hypoxia is plausible.
Hypoxia plays a very important role in causing tumor
angiogenesis (23), which is
significantly correlated to tumor progression and poor outcome
(24). Transcriptional responses to
hypoxia are commonly regulated by hypoxia-inducible factors (HIFs).
HIFs are composed of two subunits, HIF-α (an oxygen regulated
subunit) and HIF-β. Yet, the traditionally accepted mechanism has
been that hypoxia can activate VEGF, resulting in stimulation of
endothelial cell proliferation, migration and assembly, and
promotion of vessel sprouting and branching (25,26).
In the present study we found that MNNG/HOS cells can
transdifferentiate into VEC-like cells under hypoxia conditions
with or without VEGF. We also provide evidence that hypoxia can
induce MNNG/HOS cells to transdifferentiate into VEC-like cells
(Fig. 3), which may be a novel
mechanism of neovascularization in human osteosarcoma tumors.
However, the mechanism of MNNG/HOS cell
trans-differentiation into VEC-like cells under hypoxia conditions
remains unclear. Research has shown that under hypoxic conditions,
the malignant tumor cells can dedifferentiate into stem cell-like
cells, such as neuroblastoma and breast cancer (27,28).
Moreover, recent research indicates the possibility of cancer stem
cell differentiation into endothelial cells in some malignant
tumors, such as glioma and ovarian tumors (29,30).
Therefore, we think that the possible mechanism of MNNG/HOS cell
transdifferentiation into VEC-like cells under hypoxia conditions
may be that MNNG/HOS cells differentiate into stem cell-like cells
under the condition of hypoxia, and then cancer stem cell-like
cells differentiate into endothelial cells. This hypothesis
deserves further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 31571292).
References
|
1
|
Xu G, Kuang G, Jiang W, Jiang R and Jiang
D: Polydatin promotes apoptosis through upregulation the ratio of
Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin
signaling in human osteosarcoma cells. Am J Transl Res. 8:922–931.
2016.PubMed/NCBI
|
|
2
|
Cole HA, Ohba T, Ichikawa J, Nyman JS,
Cates JM, Haro H, Schwartz HS and Schoenecker JG: Micro-computed
tomography derived anisotropy detects tumor provoked deviations in
bone in an orthotopic osteosarcoma murine model. PLoS One.
9:e973812014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren Z, Liang S, Yang J, Han X, Shan L,
Wang B, Mu T, Zhang Y, Yang X, Xiong S, et al: Coexpression of
CXCR4 and MMP9 predicts lung metastasis and poor prognosis in
resected osteosarcoma. Tumour Biol. 37:5089–5096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu B, Yang H, Servaes S and Zhuang H:
Solitary retroperitoneal metastasis as the initial site of the
relapse of osteosarcoma revealed by FDG PET/CT. Clin Nucl Med.
40:892–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung TM, Cuong TD, Kim JA, Tae N, Lee JH
and Min BS: Cassaine diterpene alkaloids from Erythrophleum fordii
and their anti-angiogenic effect. Bioorg Med Chem Lett. 24:168–172.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao C, Su Y, Zhang J, Feng Q, Qu L, Wang
L, Liu C, Jiang B, Meng L and Shou C: Fibrinogen-derived
fibrinostatin inhibits tumor growth through anti-angiogenesis.
Cancer Sci. 106:1596–1606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shojaei F, Wu X, Zhong C, Yu L, Liang XH,
Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al: Bv8
regulates myeloid-cell-dependent tumour angiogenesis. Nature.
450:825–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tammela T, Zarkada G, Wallgard E,
Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M,
Schomber T, Peltonen R, et al: Blocking VEGFR-3 suppresses
angiogenic sprouting and vascular network formation. Nature.
454:656–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Streubel B, Chott A, Huber D, Exner M,
Jäger U, Wagner O and Schwarzinger I: Lymphoma-specific genetic
aberrations in microvascular endothelial cells in B-cell lymphomas.
N Engl J Med. 351:250–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gunsilius E, Duba HC, Petzer AL, Kähler
CM, Grünewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J and
Gastl G: Evidence from a leukaemia model for maintenance of
vascular endothelium by bone-marrow-derived endothelial cells.
Lancet. 355:1688–1691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rigolin GM, Fraulini C, Ciccone M, Mauro
E, Bugli AM, De Angeli C, Negrini M, Cuneo A and Castoldi G:
Neoplastic circulating endothelial cells in multiple myeloma with
13q14 deletion. Blood. 107:2531–2535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soda Y, Marumoto T, Friedmann-Morvinski D,
Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari
S, et al: Transdifferentiation of glioblastoma cells into vascular
endothelial cells. Proc Natl Acad Sci USA. 108:pp. 4274–4280. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bussolati B, Grange C, Sapino A and
Camussi G: Endothelial cell differentiation of human breast tumour
stem/progenitor cells. J Cell Mol Med. 13:309–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pezzolo A, Parodi F, Corrias MV, Cinti R,
Gambini C and Pistoia V: Tumor origin of endothelial cells in human
neuroblastoma. J Clin Oncol. 25:376–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffith CK and George SC: The effect of
hypoxia on in vitro prevascularization of a thick soft tissue.
Tissue Eng Part A. 15:2423–2434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren K, Yao N, Wang G, Tian L, Ma J, Shi X,
Zhang L, Zhang J, Zhou X, Zhou G, et al: Vasculogenic mimicry: A
new prognostic sign of human osteosarcoma. Hum Pathol.
45:2120–2129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng N, Gao S, Guo X, Wang G, Cheng C, Li
M and Liu K: Silencing of VEGF inhibits human osteosarcoma
angiogenesis and promotes cell apoptosis via VEGF/PI3K/AKT
signaling pathway. Am J Transl Res. 8:1005–1015. 2016.PubMed/NCBI
|
|
19
|
Chen F, Chen L, He H, Huang W, Zhang R, Li
P, Meng Y and Jiang X: Up-regulation of microRNA-16 in glioblastoma
inhibits the function of endothelial cells and tumor angiogenesis
by targeting Bmi-1. Anticancer Agents Med Chem. 16:609–620. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DeCicco-Skinner KL, Henry GH, Cataisson C,
Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L,
O'Neill RC, Morin A, et al: Endothelial cell tube formation assay
for the in vitro study of angiogenesis. J Vis Exp.
91:e513122014.
|
|
21
|
Bertolini F, Shaked Y, Mancuso P and
Kerbel RS: The multifaceted circulating endothelial cell in cancer:
Towards marker and target identification. Nat Rev Cancer.
6:835–845. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Purhonen S, Palm J, Rossi D, Kaskenpää N,
Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL and Salven P:
Bone marrow-derived circulating endothelial precursors do not
contribute to vascular endothelium and are not needed for tumor
growth. Proc Natl Acad Sci USA. 105:pp. 6620–6625. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Sun B, Liu Y, Zhang D, Liu Z, Zhao
X, Gu Q, Han C, Dong X, Che N, et al: Linearly patterned programmed
cell necrosis induced by chronic hypoxia plays a role in melanoma
angiogenesis. J Cancer. 7:22–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Li GY, Zhu JY, Huang DB, Zhou HC,
Zhong W and Ji CS: Overexpression of AGGF1 is correlated with
angiogenesis and poor prognosis of hepatocellular carcinoma. Med
Oncol. 32:1312015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uluer ET, Inan S, Ozbilgin K, Karaca F,
Dicle N and Sanci M: The role of hypoxia related angiogenesis in
uterine smooth muscle tumors. Biotech Histochem. 90:102–110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhaskara VK, Mohanam I, Rao JS and Mohanam
S: Intermittent hypoxia regulates stem-like characteristics and
differentiation of neuroblastoma cells. PLoS One. 7:e309052012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Axelson H, Fredlund E, Ovenberger M,
Landberg G and Påhlman S: Hypoxia-induced dedifferentiation of
tumor cells - a mechanism behind heterogeneity and aggressiveness
of solid tumors. Semin Cell Dev Biol. 16:554–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Dong J, Huang Q, Lou M, Wang A and
Lan Q: Endothelial cell transdifferentiation of human glioma stem
progenitor cells in vitro. Brain Res Bull. 82:308–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang S, Xiang T, Huang S, Zhou J, Wang Z,
Xie R, Long H and Zhu B: Ovarian cancer stem-like cells
differentiate into endothelial cells and participate in tumor
angiogenesis through autocrine CCL5 signaling. Cancer Lett.
376:137–147. 2016. View Article : Google Scholar : PubMed/NCBI
|