Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related death among women,
and is a major health concern for women around the world. There
were an estimated 1.7 million new cases (25% of all cancer cases in
women) and 0.5 million breast cancer deaths (15% of all
cancer-related deaths in women) globally in females in 2012
(1). In China, among the 5 most
commonly diagnosed cancers, breast cancer alone accounted for 15%
of the cases (2). Although there
are numerous adjuvant treatments with new drugs and systematic
treatment schedules to be applied in the clinic, metastasis, the
mainly cause of death in breast cancer patients partly due to the
lack of effective treatment, is still a huge barrier to overcome
for therapy (3). Thus, to identify
agents which can effectively inhibit breast cancer metastasis and
explore the related mechanisms may provide a solution for this
issue.
Epithelial-to-mesenchymal transition (EMT), which is
involved in embryonic development, reconstruction of wounded
tissues and fibrosis, plays an important role in tumor formation
and metastasis (4). EMT is a
process that guides the transformation of adhesive, non-mobile,
polar, epithelial-like tumor cells into cells with a mobile,
invasive, non-polar mesenchymal-like phenotype, which provides the
potential for tumor cells to migrate to distant sites and form
metastatic tumors. During this process, molecular biomarkers of
epithelial cells such as E-cadherin and claudin are downregulated
while markers of mesenchymal cells including vimentin and
N-cadherin are altered in an opposite way (5). A series of transcriptional factors
(TFs) have been identified as EMT regulators, directly or
indirectly repressing the encoding of E-cadherin. These TFs such as
ZEB, Snail, Slug, Twist, are regulated by various complex signaling
pathway networks such as Hedgehog (Hh), Wnt/β-catenin, Notch, or
transforming growth factor-β (TGF-β), by acting as isolated units
or cross-talking to provide tumor cells with an additional
mechanism with which to escape the effects of chemotherapy
(6).
In recent years, due to the resistance of tumor
cells to traditional therapy, dietary chemopreventive agents, as
additional treatment strategies against cancers, have received
increased attention for their excellent effects to suppress,
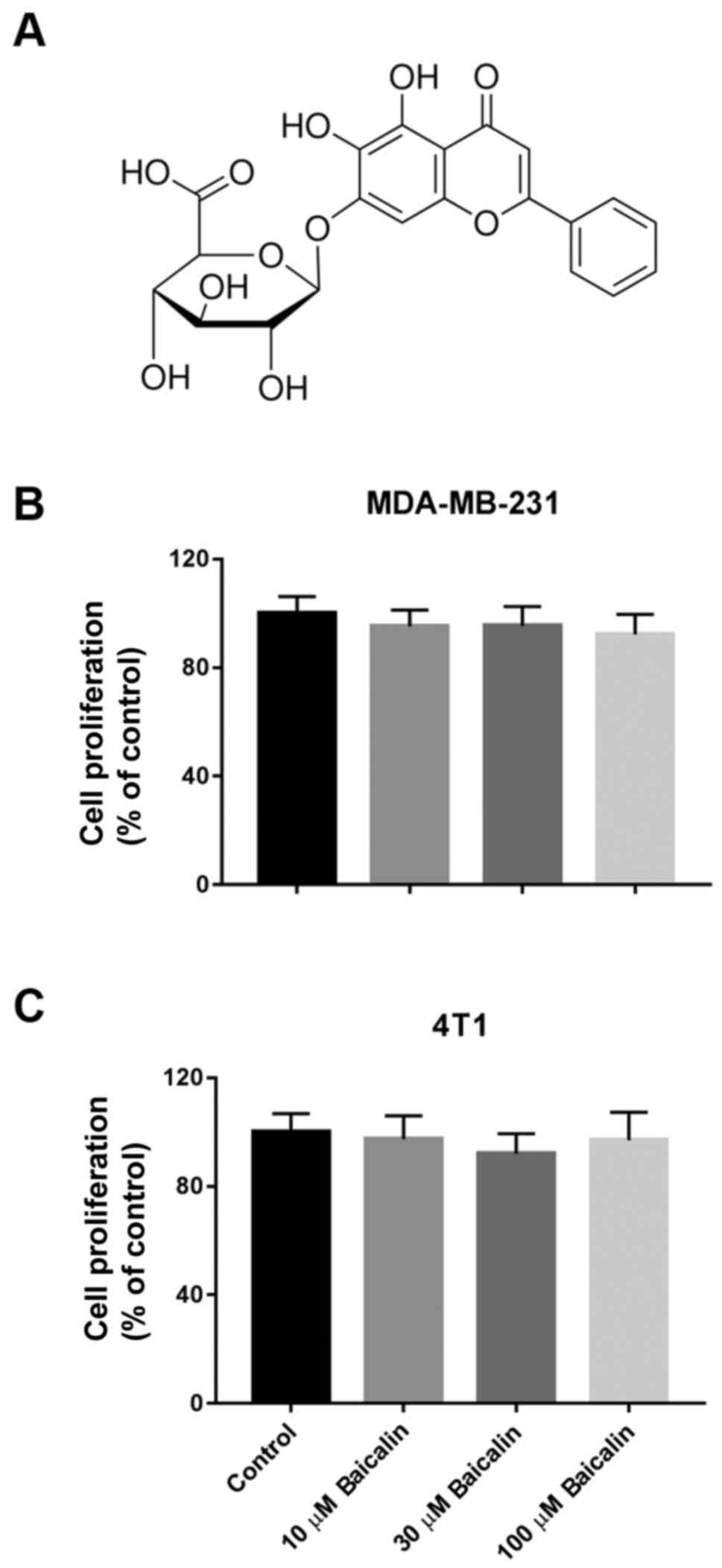
reverse, or retard the process of tumorigenesis (7). Baicalin (Fig. 1A), a flavonoid compound isolated
from the roots of Scutellaria baicalensis Georgi, has been
demonstrated to possess many different pharmacological actions
including antioxidant, anti-inflammation, anti-HIV-1 and antitumor
activity (8–12). In regards to the antitumor
activities, accumulating evidence reveals that baicalin exhibits
its function in a wide range of cancers such as hematological
malignancies, hepatic cancer, gallbladder carcinoma, lung cancer,
colorectal cancer, breast cancer and bladder cancer (13–19),
by inducing cell apoptosis, cycle arrest and autophagy, suppressing
cell proliferation and tumor growth, and inhibiting migration,
invasion and metastasis of cancer cells (15,18,20,21).
Although research has reported the protective activities of
baicalin in malignancies, its underlying detailed mechanisms and
its relationship with EMT remain unclear. Furthermore, the upstream
intracellular-signaling cascades of metastatic breast cancer cells
with high malignancy remain indeterminate.
MDA-MB-231 is an estrogen-independent fibroblastic
human triple-negative breast cancer cell line lacking the estrogen
receptor (ER), progesterone receptor (PR) and human epidermal
growth factor receptor (HER)-2 (22). 4T1 is a mouse mammary cancer cell
line, possessing the characteristic of resistance to 6-thioquanine
and metastasizes from the primary tumor to multiple distant sites
spontaneously including lymph nodes, blood, liver and lung
(23). In the present study, we
selected these two highly aggressive breast cancer cell lines to
investigate the potential effects and mechanisms of baicalin on the
metastasis of breast cancer in vitro and in vivo.
Materials and methods
Reagents and antibodies
Baicalin (purity >99%) was obtained from Zelang
Medical Technology Co. (Nanjing, China). Crystal violet was
purchased from Sigma (St. Louis, MO, USA). Vectastain ABC kit and
liquid DAB+ Substrate Chromogen system for immunohistochemistry
were purchased from Vector Laboratories Inc. (Burlingame, CA, USA)
and Dako (Carpinteria, CA, USA), respectively. RPMI-1640 medium,
Dulbecco's modified Eagle's medium (DMEM), trypsin-EDTA,
phosphate-buffered saline (PBS) and penicillin/streptomycin were
the products of Hyclone Laboratories Inc. (Los Angeles, CA, USA).
Fetal bovine serum (FBS) was purchased from Gibco (Grand Island,
NY, USA). Antibodies used in this study were anti-E-cadherin,
anti-claudin, anti-vimentin, anti-N-cadherin, anti-β-catentin,
anti-Snail and anti-Slug from Cell Signaling Technology (New
England Biolabs, Ipswich, MA, USA); anti-Ki67 (rabbit monoclonal),
secondary antibody (rabbit monoclonal IgG) from Abcam (Cambridge,
UK); anti-GAPDH from Santa Cruz Biotechnology (Santa Cruz, CA,
USA); anti-F-actin-Green 488, and Alexa Fluor 594 (goat-anti-rabbit
IgG) from Molecular Probes (Oregon, Eugene, OR, USA).
Cell lines and culture
Human breast cancer cell line MDA-MB-231 and mouse
mammary cancer cell line 4T1 were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM
and RPMI-1640 medium containing 10% FBS and 1%
penicillin/streptomycin, respectively. Both of the cell lines were
cultured in a humidified atmosphere with 5% CO2 at
37°C.
Cell viability assay
Cell viability was determined by crystal violet
assays. MDA-MB-231 and 4T1 breast cancer cells were harvested with
0.05% trypsin-EDTA and seeded in 24-well plates (Corning, MA, USA)
at a density of 6×105/well and 1×106/well,
respectively. Four hours later, the cells were treated with
different concentrations of baicalin (10, 30, or 100 µM) dissolved
in fresh medium or vehicle, and incubated for another 72 h. Then
cells were fixed with 10% formaldehyde solution and stained with
0.25% crystal violet. After being washed with PBS, crystal violet
was dissolved and the absorbance was read at 570 nM using a
microplate reader (ELx800; BioTek, Winooski, VT, USA).
Wound healing assay
MDA-MB-231 and 4T1 breast cancer cells were seeded
in 6-well plates (Corning, MA, USA) at a density of
2×105/well. Scrapes were made over the cells by a
sterile toothpick until cells formed a confluent monolayer. After
scraping, the cells were washed with PBS twice, and then replaced
with fresh medium with or without various concentrations of
baicalin and continued to culture. The width of the wound was
monitored and photographed using a microscope (Nikon, Tokyo, Japan)
at 100-fold magnification until the wound of the control group
healed or almost healed.
Transwell migration assay
The migration assay was performed in 24-well
Transwell chambers (Corning, MA, USA). Briefly, complete DMEM with
10% FBS with or without various concentrations of baicalin was
added into the lower chambers, and MDA-MB-231 (105) and
4T1 breast cancer (6×104) cells suspended in serum-free
DMEM were added into the upper chambers. After incubation for 18 h,
the cells were fixed with 10% formaldehyde solution and stained
with 0.25% crystal violet. Then the non-migration cells on the
upper chamber were wiped off and the migrated cells in the lower
chamber were photographed under a microscope (Nikon) at 100-fold
magnification.
Immunofluorescence (IF) analysis
MDA-MB-231 and 4T1 breast cancer cells were seeded
in 24-wells and treated with 100 µM baicalin for 24 h. After being
fixed with 4% paraformaldehyde and permeabilized with 0.05% Triton
X-100 at room temperature, the cells were incubated with primary
antibodies anti-vimentin (1:100) or anti-Slug (1:50) overnight at
4°C. After a further wash with PBS, the cells were incubated with
Alexa Fluor 594-conjugated secondary antibody (dilution 1:500) for
1 h. Then, anti-F-actin-Green 488 (dilution 1:500) was added to
stain the cytoskeleton and the nuclei were stained by DAPI. Images
were captured at ×200 under a fluorescence microscope (Nikon).
Immunohistochemical (IHC)
analysis
Tissues were fixed, dehydrated, embedded and cut
into 5-µm sections in accordance with standard procedures. After
deparaffinization, rehydration, antigen retrieval and blockage, the
sections were incubated with primary antibodies overnight at 4°C.
Then the sections were blocked the endogenous peroxidase with
hydrogen peroxide and incubated with the biotinylated goat
anti-rabbit secondary antibodies (1:100). After signal
amplification with avidin and horseradish peroxidase
(HRP)-conjugated biotin, the sections were counterstained with DAB
to visualize the nuclei. Finally, the sections were mounted and
imaged.
Protein isolation and western blot
analysis
Cells were lysed in cell lysis buffer containing
various enzyme-protecting agents to collect total proteins. The
concentration of protein was determined by the BCA kit. Total
proteins (40 µg) were separated on 10% SDS gel and transferred onto
polyvinylidene fluoride (PVDF) membranes. After blocked with 5% BSA
in Tris-buffered saline (TBS) containing 0.1% Tween-20, the
membranes were incubated with the primary monoclonal antibody at
4°C overnight. The membranes were washed with TBST three times,
followed by incubation with the secondary antibodies labeled with
horseradish peroxidase (HRP). Finally, the membranes were
visualized with an enhanced chemiluminescent system.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA from cancer cells was extracted by using
the RNA isolation kit according to the manufacturer's instructions,
and cDNA was synthesized using PrimeScript RT reagent kit with 1 µg
total RNA. qPCR was performed by using the PCR kit according to the
instructions. The following primer sequences were used: E-cadherin
sense, 5′-TCCTGGGCAGAGTGAATTTTGAAGA-3′ and antisense,
5′-AAACGGAGGCCTGATGGGG-3′; claudin sense,
5′-CCTCCTGGGAGTGATAGCAAT-3′ and antisense,
5′-GGCAACTAAAATAGCCAGACCT-3′; vimentin sense,
5′-TACAGGAAGCTGCTGGAAGG-3′ and antisense,
5′-ACCAGAGGGAGTGAATCCAG-3′; N-cadherin sense,
5′-AGCCAACCTTAACTGAGGAGT-3′ and antisense,
5′-GGCAAGTTGATTGGAGGGATG-3′; Snail sense,
5′-TCGGAAGCCTAACTACAGCGA-3′ and antisense,
5′-AGATGAGCATTGGCAGCGAG-3′; Slug sense, 5′-GGGGAGAAGCCTTTTTCTTG-3′
and antisense, 5′-TCCTCATGTTTGTGCAGGAG-3′; and GAPDH sense,
5′-TGTTGCCATCAATGACCCCTT-3′ and antisense,
5′-CTCCACGACGTACTCAGCG-3′. Relative quantification was achieved by
normalization to GAPDH.
Xenograft model
Six- to eight-week-old female BALB/c mice were
obtained from the Laboratory Animal Center of Chongqing Medical
University and housed in a specific pathogen-free (SPF) laboratory
environment. BALB/c mice were injected subcutaneously with
1×106 4T1 cells into the bilateral gluteal region. One
week after inoculation, the mice were divided randomly into a
sham-treated group and a baicalin-treated group. The former
received PBS while the latter received 100 mg/kg baicalin in an
intraperitoneal injection approach every 3 days. After 6 weeks, all
mice were sacrificed under anesthesia, the tumors and lungs were
excised, weighed, counted for tumor nodules and fixed for further
analysis.
Statistical analysis
All data wre analyzed with SSPS 13.0. Data are
expressed as mean ± SD. Variances between groups were analyzed
using the Student's t-test and one-way analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant result.
Results
Baicalin does not affect the viability
of breast cancer cells
Previous studies have revealed that baicalin exerts
beneficial effects on inhibiting cell proliferation and inducing
apoptosis in various cancer types (24–26).
To evaluate whether baicalin affects the viability of breast cancer
cells, the crystal violet assay was performed in MDA-MB-231 and 4T1
cells. As shown in Fig. 1B and C,
the cell viability had no significant difference when the breast
cancer cells were treated with increasing concentrations of
baicalin up to 100 µM.
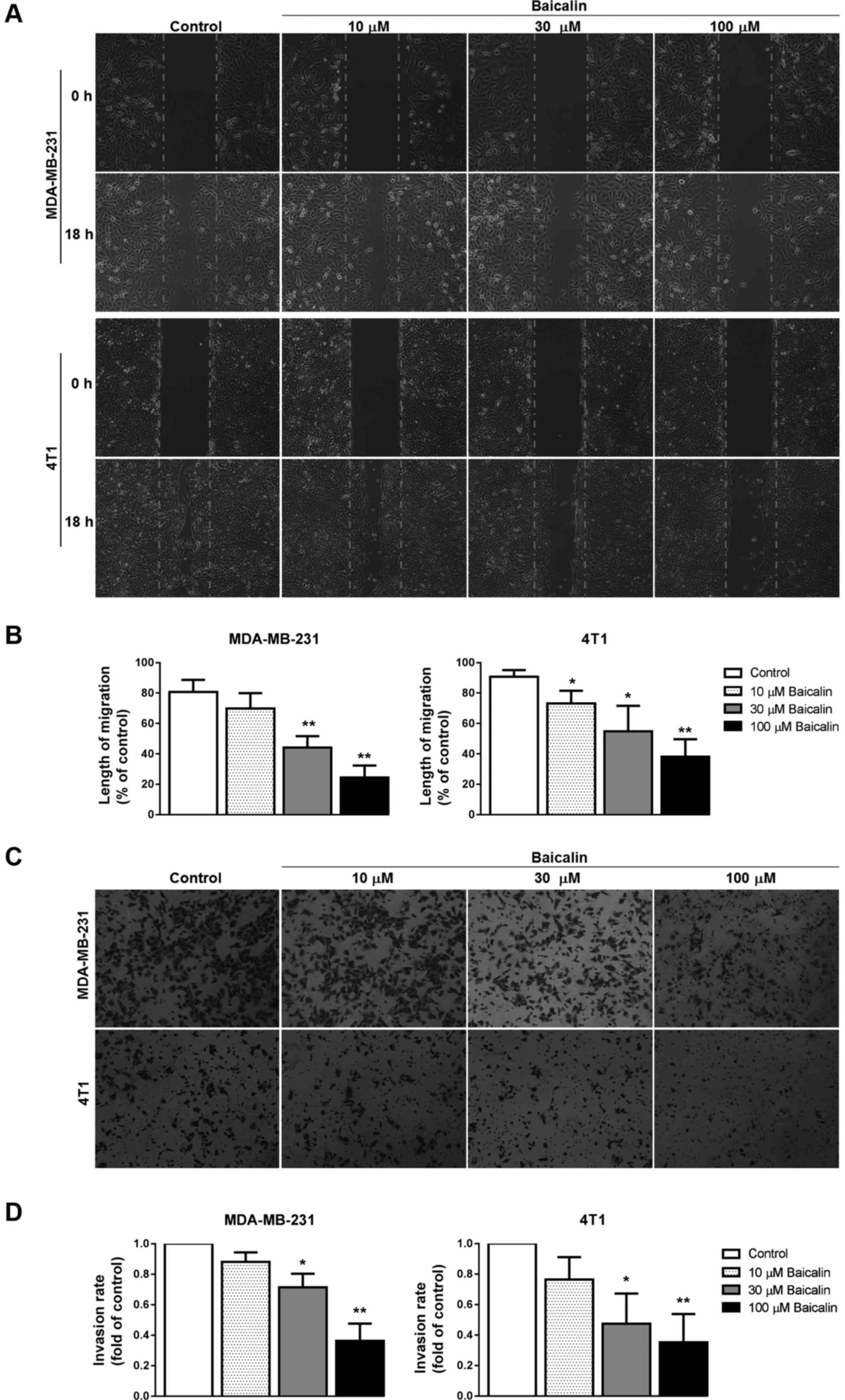
Baicalin suppresses breast cancer cell
migration and invasion
To determine whether baicalin has the potential to
inhibit breast cancer cell migration and invasion, wound healing
and transwell migration assays were performed using two highly
aggressive breast cancer cell lines MDA-MB-231 and 4T1. In the
wound healing assay, following pretreatment with baicalin (10, 30,
or 100 µM), the wound scratches in the baicalin-treated groups were
wider than those noted in the control group at 18 h, and had a
dose-dependent increasing trend (Fig.
2A and B), indicating that baicalin inhibited cell migration of
breast cancer cells. In the cell invasion experiment by Transwell
assay, results similar to those of the wound healing assay were
observed in the baicalin-treated breast cancer cells (Fig. 2C and D).
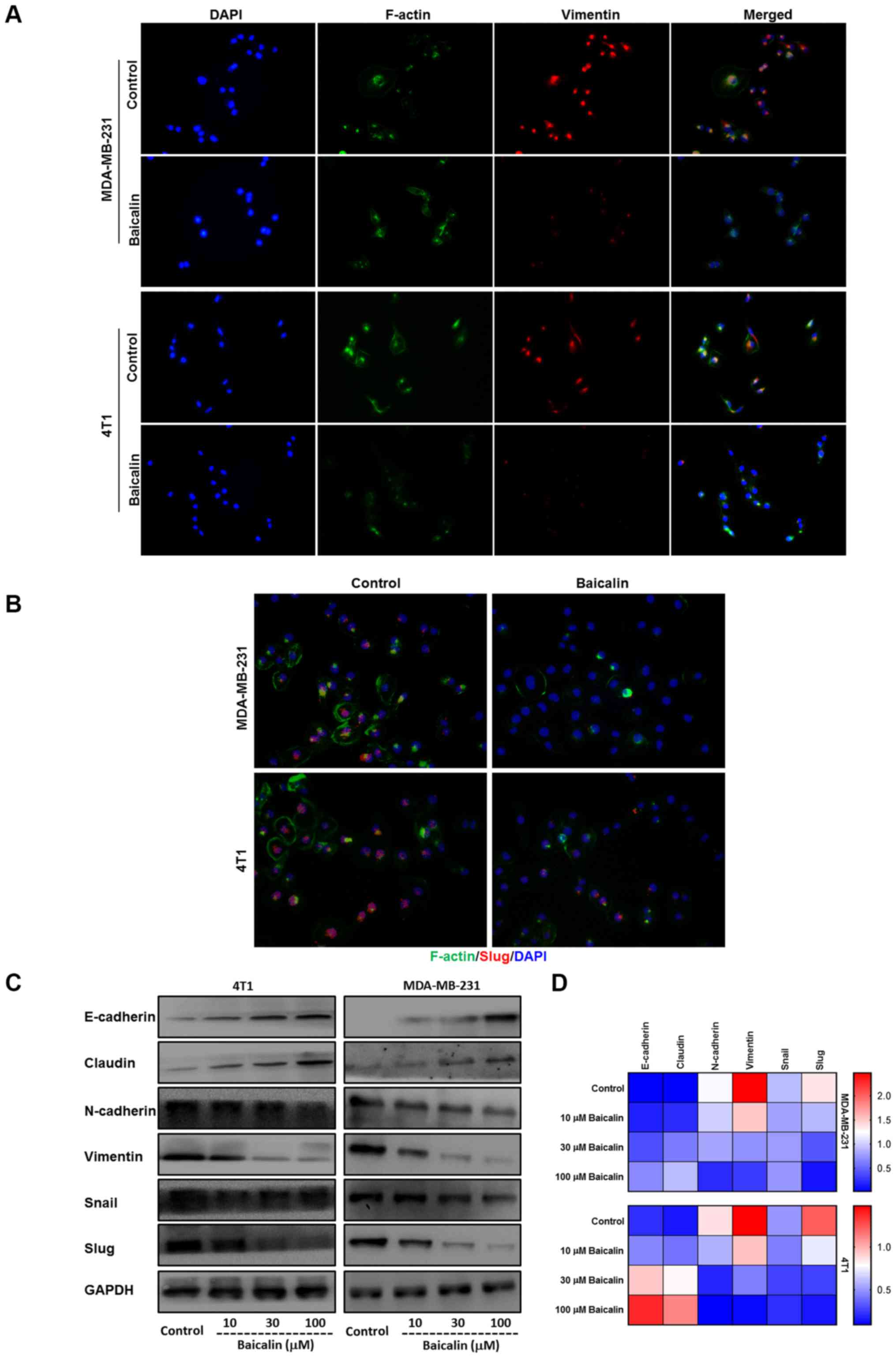
Baicalin reverses breast cancer cell
EMT process
It has been confirmed that EMT plays a major role in
tumor metastasis. In order to explore the relationship between
baicalin and EMT, we used varying concentrations of baicalin to
treat MDA-MB-231 and 4T1 breast cancer cells, respectively. IF
assay indicated that the mesenchymal marker vimentin was degraded
and the cytoskeletal protein F-actin was remolded after the two
breast cancer cell lines were treated with 100 µM baicalin
(Fig. 3A), and the expression of
Slug, one of the major EMT TFs that is usually maintained at a high
level in highly invasive breast cancer cells (27), was downregulated in the
baicalin-treated MDA-MB-231 and 4T1 cells (Fig. 3B). Furthermore, western blotting and
quantitative RT-PCR also showed that epithelial markers E-cadherin
and claudin were upregulated while mesenchymal markers N-cadherin
and vimentin and relative TFs Snail and Slug were downregulated in
a dose-dependent manner by baicalin in the two highly invasive
breast cancer cell lines (Fig. 3C and
D).
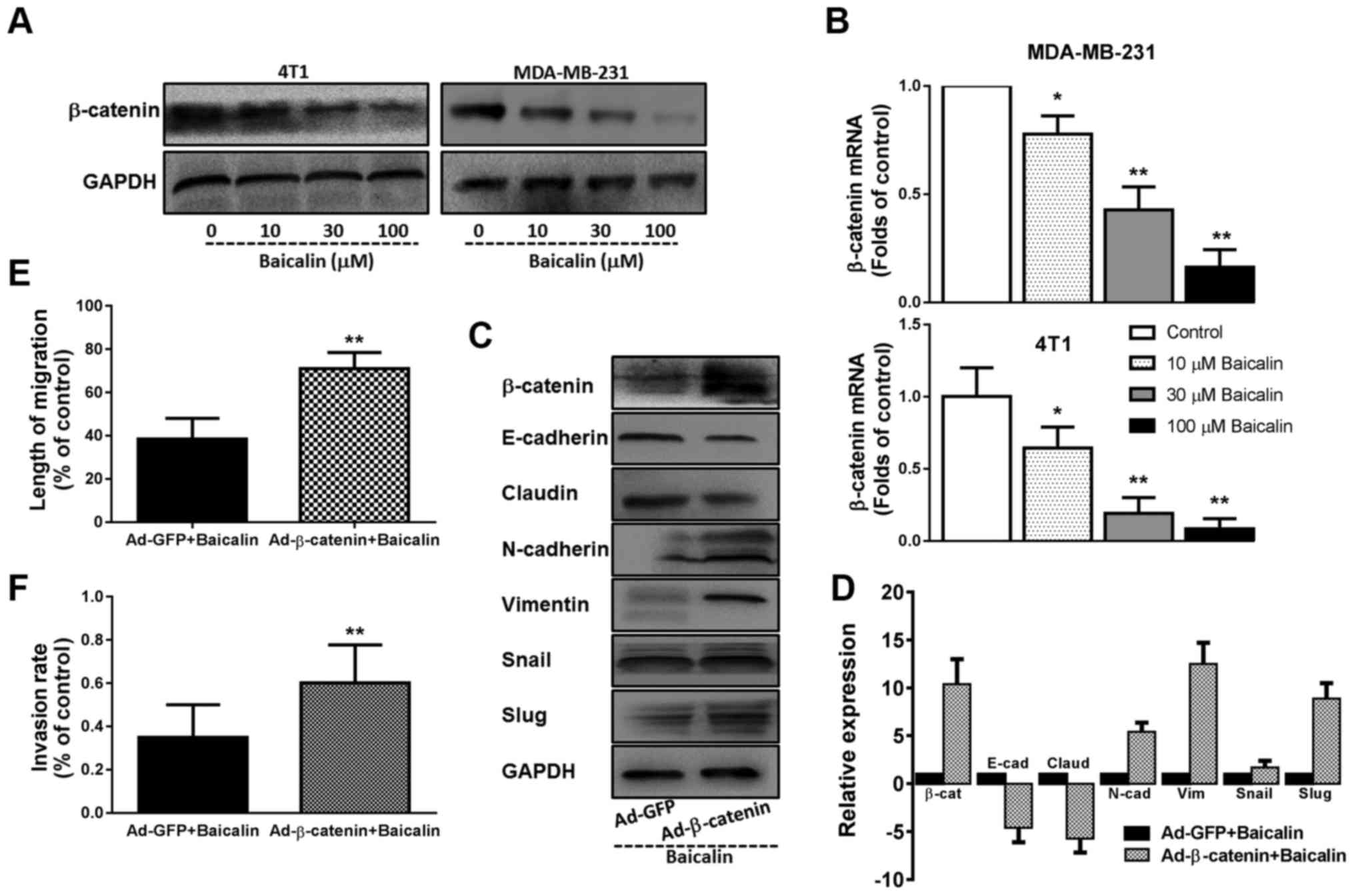
β-catenin contributes to the
beneficial effects of baicalin in regards to the metastasis and EMT
of breast cancer cells
Prior studies have demonstrated that baicalin can
inhibit breast cancer cell migration through the p38 MAPK signaling
pathway (18). Cancer metastasis
and EMT are complex processes which are influenced by numerous
signaling pathways through crosstalk (6). In view of the important status of
β-catenin in cancer metastasis and EMT, we first determined whether
baicalin affects the β-catenin signaling pathway. Western blotting
and quantitative RT-PCR analysis found that β-catenin was markedly
expressed in the MDA-MB-231 and 4T1 breast cancer cells, and
pretreatment with baicalin dose-dependently downregulated the
expression of β-catenin protein and mRNA (Fig. 4A and B). In addition, overexpression
of β-catenin in the baicalin-treated 4T1 cells by adenovirus vector
system, significantly blunted the role of baicalin in regards to
the expression levels of EMT-related molecules and TFs, returning
cells back to a mesenchymal type (Fig.
4C and D). Simultaneously, the inhibitory effects of baicalin
on the migration and invasion of highly invasive breast cancer
cells were also reversed with the overexpression of β-catenin
(Fig. 4E and F).
Baicalin suppresses the metastasis of
breast cancer cells in vivo
Finally, we constructed a xenograft metastasis tumor
model of 4T1 breast cancer cells to investigate the effects of
baicalin on breast cancer metastasis in vivo. As shown in
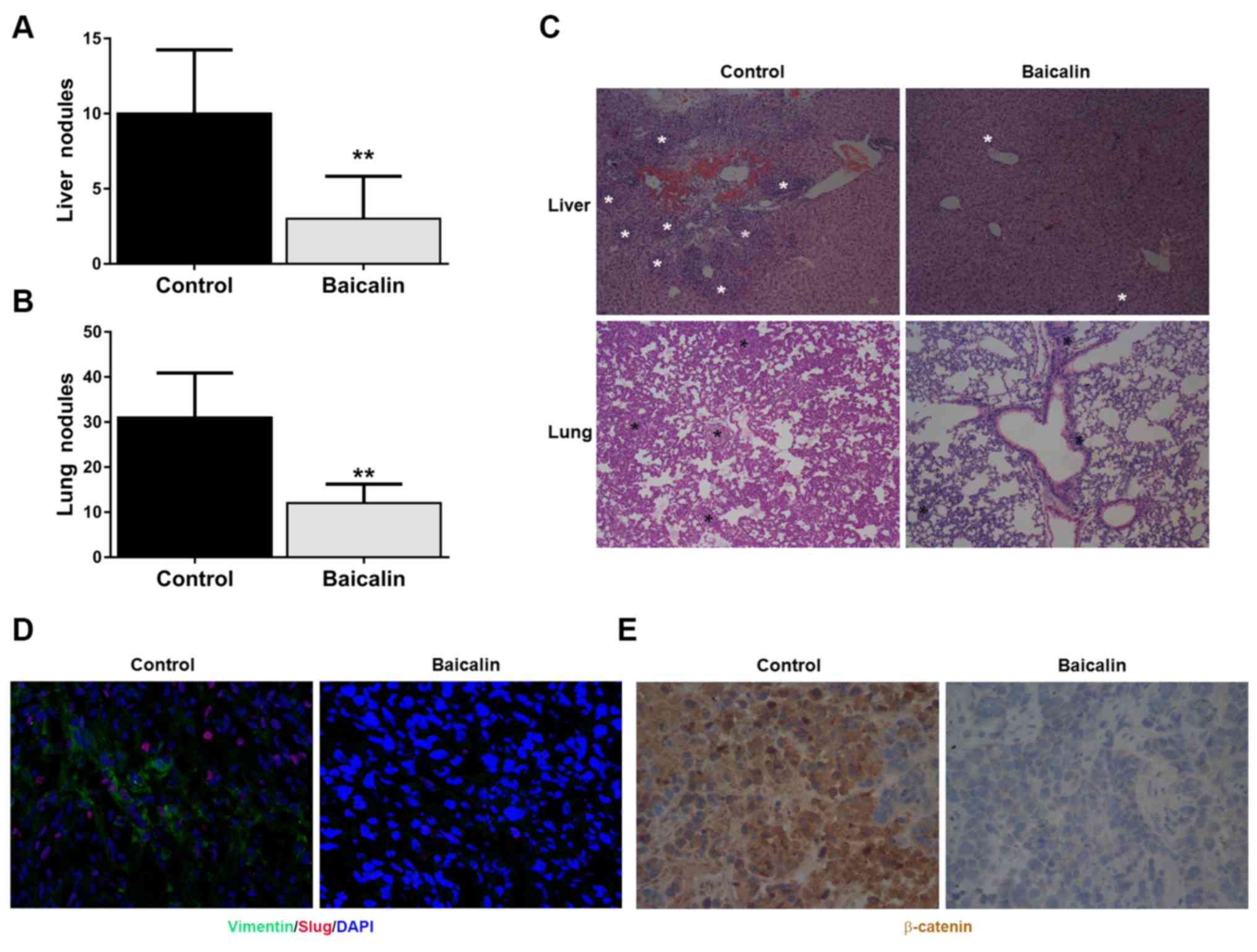
Fig. 5A and B, we found that the
numbers of metastatic nodules on the surface of the liver and lung
in the baicalin-treated group were less than these numbers in the
control group. H&E staining of the liver and lung also
indicated that baicalin reduced the metastatic lesions of breast
cancer cells in the liver and lung tissues (Fig. 5C). Similar to the in vitro
experiment, the expression levels of mesenchymal marker vimentin
and EMT-activating TF Slug in the orthotopic tumor tissues were
downregulated by baicalin (Fig.
5D). Finally, immunohistochemistry showed that baicalin
inhibited β-catenin expression in the orthotopic tumor tissues
(Fig. 5E).
Discussion
Although the mortality rate of breast cancer is
decreasing along with more efficacious adjuvant treatments and
systematic treatment schedules, the mortality rate is still high
and breast cancer is the leading cause of cancer-related mortality
among middle-age women (28). As is
known, the main cause of cancer death is not the primary tumor but
its metastasis to distal sites and metastasis-related diseases.
Partly due to ineffective therapy or therapeutic resistance, the
incidence of metastasis is becoming more and more frequent, which
causes a high percentage of recurrence and poor prognosis for
patients. In the present study, we investigated the potential
effect of baicalin on the inhibition of metastasis in breast cancer
cells and elucidated the underlying molecular mechanisms. Our
findings revealed that baicalin effectively inhibited the migration
and invasion of breast cancer cell lines MDA-MB-231 and 4T1, and
suppressed the lung and liver metastasis of breast cancer xenograft
tumors derived from 4T1 breast cancer cells, suggesting that
baicalin may serve as a rational therapeutic strategy to suppress
the metastasis of breast cancer.
A compelling body of evidence has confirmed that EMT
is not only a crucial morphogenic process normally activated during
embryogenesis and reconstruction of wounded tissues, but also plays
an important role during the metastasis of cancers, allowing
dynamic and reversible transition from adhesive, non-mobile,
epithelial-like phenotype to mobile, invasive mesenchymal-like
phenotype, which is utilized by cancer cells to promote their
capabilities of local invasion and distant metastasis (29). Indeed, EMT exhibits an essential
manifestation of molecular changes, namely loss of epithelial
markers E-cadherin and claudin and gain of mesenchymal markers
vimentin and N-cadherin (30).
Previous studies have elucidated that EMT is associated with
clinicopathological features, resistance to apoptosis, evasion of
the immune response and poor prognosis of breast cancer (31). Based on its anti-migratory
potential, we aimed to ascertain whether baicalin has an influence
on EMT in highly aggressive breast cancer cell lines. Our study
found that MDA-MB-231 and 4T1 cancer cells acquired epithelial
features and lost mesenchymal phenotype at the same time following
treatment with baicalin, indicating that baicalin could effectively
reverse EMT in highly aggressive breast cancer cells. A large
number of previous studies have shown that Slug is an important
transcription factor of EMT, and promotes the process of EMT and
cancer metastasis (32,33). Thus, we analyzed the expression and
nuclear translocation of Slug, and found that the expression and
nuclear translocation of Slug was decreased after intervention of
baicalin. All these data confirmed that the effect of baicalin on
the migration of breast cancer cells resulted from the reversion of
EMT.
To date, numerous studies have demonstrated that
multiple signaling pathways participate in the regulation of EMT,
cell migration, invasion and tumor metastasis, including the
Wnt/β-catenin, NF-κB and p38MAPK signaling pathways (18,34–36).
Among them, the aberrant activation of the Wnt/β-catenin signaling
pathway has been found in many human malignancies. In terms of the
canonical Wnt/β-catenin signaling pathway, β-catenin is a key
component as a TF. Upon Wnt ligand engagement, β-catenin is
activated and disrupted, followed by its translocation from the
cytoplasm into the nucleus, where it facilitates the transcription
of genes involved in EMT, such as Twist, Snail, Slug, which repress
the expression of E-cadherin, influencing cell junction and
polarity (37). Previous studies
have reported that overexpression and hyperactivation of β-catenin
are observed in aggressive basal-like breast cancer, and are
positively correlated with poor patient clinical outcome (38–40).
Therefore, β-catenin may be a novel therapeutic target with which
to overcome breast cancer metastasis and improve the prognosis of
breast cancer patients. In the present study, we found that
β-catenin was robustly expressed in MDA-MB-231 and 4T1 breast
cancer cells which was dose-dependently inhibited by baicalin at
the protein and mRNA levels. Using a xenograft mouse in vivo
model, baicalin markedly downregulated the expression of β-catenin
in primary tumor tissues. Furthermore, overexpression of β-catenin
by an adenovirus vector system markedly blunted the suppressive
effects of baicalin on metastasis and EMT in breast cancer cells,
suggesting that these beneficial effects of baicalin may be
involved in the downregulation of β-catenin signaling.
In conclusion, our experimental data showed that
baicalin exhibited significant effects on the suppression of
migration and metastasis in highly aggressive breast cancer both
in vitro and in vivo, which may be through reversal
of the EMT process and downregulation of β-catenin expression.
Therefore, application of baicalin in conjunction with currently
conventional adjuvant treatments may provide a novel therapeutic
strategy for patients with metastatic breast cancer.
Acknowledgements
This research was supported by grants from the
National Natural Science Foundation of China (no. 81472475). We
thank Dr Jianfei Guo, James Winkle College of Pharmacy, University
of Cincinnati, for the discussion and proofreading of the
manuscript.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arnedos M, Vicier C, Loi S, Lefebvre C,
Michiels S, Bonnefoi H and Andre F: Precision medicine for
metastatic breast cancer - limitations and solutions. Nat Rev Clin
Oncol. 12:693–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samatov TR, Tonevitsky AG and Schumacher
U: Epithelial-mesenchymal transition: Focus on metastatic cascade,
alternative splicing, non-coding RNAs and modulating compounds. Mol
Cancer. 12:1072013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drasin DJ, Robin TP and Ford HL: Breast
cancer epithelial-to-mesenchymal transition: Examining the
functional consequences of plasticity. Breast Cancer Res.
13:2262011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takebe N, Warren RQ and Ivy SP: Breast
cancer growth and metastasis: Interplay between cancer stem cells,
embryonic signaling pathways and epithelial-to-mesenchymal
transition. Breast Cancer Res. 13:2112011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan MH and Ho CT: Chemopreventive effects
of natural dietary compounds on cancer development. Chem Soc Rev.
37:2558–2574. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng-Fei L, Fu-Gen H, Bin-Bin D,
Tian-Sheng D, Xiang-Lin H and Ming-Qin Z: Purification and
antioxidant activities of baicalin isolated from the root of
huangqin (Scutellaria baicalensis gcorsi). J Food Sci
Technol. 50:615–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burnett BP, Jia Q, Zhao Y and Levy RM: A
medicinal extract of Scutellaria baicalensis and Acacia
catechu acts as a dual inhibitor of cyclooxygenase and
5-lipoxygenase to reduce inflammation. J Med Food. 10:442–451.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li BQ, Fu T, Gong WH, Dunlop N, Kung H,
Yan Y, Kang J and Wang JM: The flavonoid baicalin exhibits
anti-inflammatory activity by binding to chemokines.
Immunopharmacology. 49:295–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li BQ, Fu T, Dongyan Y, Mikovits JA,
Ruscetti FW and Wang JM: Flavonoid baicalin inhibits HIV-1
infection at the level of viral entry. Biochem Biophys Res Commun.
276:534–538. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J
and Zhou J: Exploring therapeutic potentials of baicalin and its
aglycone baicalein for hematological malignancies. Cancer Lett.
354:5–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Pei M and Li L: Baicalin induces
apoptosis in hepatic cancer cells in vitro and suppresses tumor
growth in vivo. Int J Clin Exp Med. 8:8958–8967. 2015.PubMed/NCBI
|
|
15
|
Shu YJ, Bao RF, Wu XS, Weng H, Ding Q, Cao
Y, Li ML, Mu JS, Wu WG, Ding QC, et al: Baicalin induces apoptosis
of gallbladder carcinoma cells in vitro via a
mitochondrial-mediated pathway and suppresses tumor growth in vivo.
Anticancer Agents Med Chem. 14:1136–1145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX,
Sun J, Lv YB, Wu X and Dong JC: Flavonoid components in
Scutellaria baicalensis inhibit nicotine-induced
proliferation, metastasis and lung cancer-associated inflammation
in vitro. Int J Oncol. 44:1561–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang BL, Chen HJ, Chen YG, Gu YF, Zhang
SP, Lin Q and Sun Y: Inhibitory effects of baicalin on orthotopic
xenografts of colorectal cancer cells that are deficient in a
mismatch repair gene in nude mice. Int J Colorectal Dis.
28:547–553. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC,
Lin MW, Hsu YM, Lee MR, Amagaya S, Huang WW, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Morgan WA, Sanchez-Medina A and
Corcoran O: The ethanol extract of Scutellaria baicalensis
and the active compounds induce cell cycle arrest and apoptosis
including upregulation of p53 and Bax in human lung cancer cells.
Toxicol Appl Pharmacol. 254:221–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Wan JY, Zhang L and Min S:
Synthetic RGDS peptide attenuates mechanical ventilation-induced
lung injury in rats. Exp Lung Res. 38:204–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pulaski BA and Ostrand-Rosenberg S: Mouse
4T1 breast tumor model. Curr Protoc Immunol: Chapter 20: Unit 20.2.
2001.doi: 10.1002/0471142735.im2002s39. View Article : Google Scholar
|
|
24
|
Chen WC, Kuo TH, Tzeng YS and Tsai YC:
Baicalin induces apoptosis in SW620 human colorectal carcinoma
cells in vitro and suppresses tumor growth in vivo. Molecules.
17:3844–3857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing
PDGFRβ-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 20:1252–1262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Y, Hu J, Zheng J, Li J, Wei T, Zheng
Z and Chen Y: Down-regulation of the PI3K/Akt signaling pathway and
induction of apoptosis in CA46 Burkitt lymphoma cells by baicalin.
J Exp Clin Cancer Res. 31:482012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grzegrzolka J, Biala M, Wojtyra P,
Kobierzycki C, Olbromski M, Gomulkiewicz A, Piotrowska A, Rys J,
Podhorska-Okolow M and Dziegiel P: Expression of EMT markers SLUG
and TWIST in breast cancer. Anticancer Res. 35:3961–3968.
2015.PubMed/NCBI
|
|
28
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nickel A and Stadler SC: Role of
epigenetic mechanisms in epithelial-to-mesenchymal transition of
breast cancer cells. Transl Res. 165:126–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bulfoni M, Gerratana L, Del Ben F,
Marzinotto S, Sorrentino M, Turetta M, Scoles G, Toffoletto B,
Isola M, Beltrami CA, et al: In patients with metastatic breast
cancer the identification of circulating tumor cells in
epithelial-to-mesenchymal transition is associated with a poor
prognosis. Breast Cancer Res. 18:302016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang LK, Pan SH, Chang YL, Hung PF, Kao
SH, Wang WL, Lin CW, Yang SC, Liang CH, Wu CT, et al:
MDA-9/Syntenin-Slug transcriptional complex promote
epithelial-mesenchymal transition and invasion/metastasis in lung
adenocarcinoma. Oncotarget. 7:386–401. 2016.PubMed/NCBI
|
|
33
|
Ganesan R, Mallets E and Gomez-Cambronero
J: The transcription factors Slug (SNAI2) and Snail (SNAI1)
regulate phospholipase D (PLD) promoter in opposite ways towards
cancer cell invasion. Mol Oncol. 10:663–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hino M, Kamo M, Saito D, Kyakumoto S,
Shibata T, Mizuki H and Ishisaki A: Transforming growth factor-β1
induces invasion ability of HSC-4 human oral squamous cell
carcinoma cells through the Slug/Wnt-5b/MMP-10 signalling axis. J
Biochem. 159:631–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH,
Way TD and Chen WJ: 3,5,4-Trimethoxystilbene, a natural
methoxylated analog of resveratrol, inhibits breast cancer cell
invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin
signaling cascades and reversal of epithelial-mesenchymal
transition. Toxicol Appl Pharmacol. 272:746–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chung H, Choi HS, Seo EK, Kang DH and Oh
ES: Baicalin and baicalein inhibit transforming growth
factor-β1-mediated epithelial-mesenchymal transition in human
breast epithelial cells. Biochem Biophys Res Commun. 458:707–713.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nowicki A, Sporny S and Duda-Szymańska J:
β-catenin as a prognostic factor for prostate cancer (PCa). Cent
European J Urol. 65:119–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chien AJ, Moore EC, Lonsdorf AS,
Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL
and Moon RT: Activated Wnt/beta-catenin signaling in melanoma is
associated with decreased proliferation in patient tumors and a
murine melanoma model. Proc Natl Acad Sci USA. 106:1193–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cuello-Carrión FD, Shortrede JE,
Alvarez-Olmedo D, Cayado-Gutiérrez N, Castro GN, Zoppino FC,
Guerrero M, Martinis E, Wuilloud R, Gómez NN, et al: HER2 and
β-catenin protein location: Importance in the prognosis of breast
cancer patients and their correlation when breast cancer cells
suffer stressful situations. Clin Exp Metastasis. 32:151–168. 2015.
View Article : Google Scholar : PubMed/NCBI
|