Introduction
The formation of new blood vessels from the existing
vasculature is essential to support tumoral development (1). Since neo-angiogenesis is a key event
in tumor progression, antiangiogenic agents are considered as an
alternative strategy in cancer treatment (2). In healthy tissues, the balance of
angiogenic activating and inhibiting factors determines the
transition of endothelial cells between a pro-angiogenic or a
quiescent stage (2). Vascular
endothelial growth factor (VEGF) and angiopoietins are some of the
main pro-angiogenic factors (3,4). VEGF
is one of the most important molecules stimulating tumoral
angiogenesis and an increased VEGF expression has been described in
different types of cancers, such as breast, brain, lung, urothelial
and gastrointestinal tract tumors (5). In mammary tumors, VEGF is released by
human breast cancer cells and binds VEGF receptors triggering
proliferation, growth, survival and migration of endothelial cells
(6–8). Angiopoietins are endothelial-produced
proteins which bind the tyrosine kinase receptor Tie2, modulating
vessel stability (9). Although four
angiopoietins (ANG-1 to ANG-4) have been described, ANG-1 and ANG-2
are the most widely studied. ANG-1 is a Tie2 receptor agonist
expressed in vascular mural cells and non-vascular normal and tumor
cells. It is a vascular stabilizing factor that stimulates
recruitment of pericytes and smooth muscle cells, collaborates to
maintain vascular integrity and quiescence and is also able to
promote angiogenesis (9,10). Contrary to ANG-1, ANG-2 behaves as
an antagonist of Tie2, blocking ANG-1-mediated phosphorylation of
Tie2 therefore reducing the interactions between endothelial and
perivascular support cells and extracellular matrix, decreasing
vascular integrity and causing vessel regression in the absence of
angiogenic factors, whereas it potentiates angiogenesis in the
presence of VEGF (9,11). ANG-2 is mainly produced by
endothelial cells and formed during vascular remodelling (11). A wide number of malignant tumors
show upregulation of both ANG-1 and ANG-2 angiopoietins, promoting
a shift in the ANG-1:ANG-2 ratio towards ANG-2 which in the
presence of VEGF is associated with tumor angiogenesis (12). Tie2 receptors bind directly to
angiopoietins, have strong tyrosine kinase activity, and are
selectively expressed in endothelial cells, although other cell
types including early hematopoietic cells and subsets of monocytes
also express Tie2 (13).
Angiogenesis is dependent on a dynamic equilibrium between the
production of VEGF and angiopoietins that must be both
quantitatively and temporally coordinated (14).
Melatonin, synthesized and released from the pineal
gland, has been demonstrated to have oncostatic actions in
hormone-dependent tumors (15–17).
Melatonin exerts oncostatic activity through several biological
mechanisms including: indirect effects of melatonin via the
hypothalamic-pituitary-reproductive axis, which results in the
downregulation of some of the hormones that may stimulate
proliferation of malignant cells, such as estrogenic compounds
produced by the gonads (18);
direct antiestrogenic molecular mechanisms that take place inside
epithelial cells of the mammary tissue (19,20);
antioxidant effects (21);
melatonin has been also implicated in both hemopoiesis and
enhancement of anticancer immunity (22); inhibition of telomerase in
epithelial malignant cells (23,24);
inhibition of fatty acid uptake and fat metabolic pathways
(25,26) and inhibition of angiogenesis
(27–29). With respect to its antiangiogenic
effects, in co-cultures of human breast cancer and endothelial
cells, melatonin was found to regulate the tumor microenvironment
through the downregulation of VEGF expression in human breast
cancer cells, which results in a decrease of the secretion of VEGF
and as a consequence, a reduction in the levels of VEGF around
endothelial cells (28–30). Melatonin strongly inhibits the
proliferation as well as the invasion/migration of endothelial
cells, disrupts tube formation and counteracts the VEGF-stimulated
tubular network assembly (29).
Melatonin also shows indirect antiangiogenic effects by inhibiting
various other tumor growth factors, such as IGF, EGF and ET-1,
which are strong mitogens and stimulators of cancer angiogenesis
(31). Neutralization of reactive
oxygen species which, during hypoxia, plays an important role in
stabilizing hypoxia-inducible factor HIF-α (32) is another indirect antiangiogenic
effect of melatonin.
Although a variety of factors can modulate
endothelial cell response, a complementary and coordinated action
of VEGF and angiopoietins during angiogenesis is required (9,33).
Since melatonin can modulate VEGF in tumor cells and has
antiangiogenic effects, in the present study, we aimed to ascertain
whether melatonin modulates in a coordinated action angiopoietins 1
and 2, their cognate Tie2 receptor and VEGF in vitro in
endothelial cell cultures. To accomplish this we used co-cultures
of human breast cancer cells (MCF-7) with human umbilical vein
endothelial cells (HUVECs).
Materials and methods
Cells and culture conditions
Human umbilical vein endothelial cells (HUVECs) were
purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA). They were maintained as monolayer cultures in
58.1 cm2 plastic culture plates in Vascular Cell Basal
Medium (VCBM) (ATCC) supplemented with Endothelial Cell Growth
Kit-BBE (ATCC) which consisted of 2% fetal bovine serum (FBS; PAA
Laboratories, Pasching, Austria), 0.2% bovine brain extract, 5
ng/ml rhEGF, 10 mM L-glutamine, 0.75 U/ml heparin sulfate, 1 µg/ml
hydrocortisone hemisuccinate, 50 µg/ml ascorbic acid, penicillin
(20 U/ml) and streptomycin (20 µg/ml) (Sigma-Aldrich, Madrid,
Spain) at 37°C in a humid atmosphere containing 5% CO2.
To avoid genetic mutation and low viability, no more than six
passages of HUVECs were used for the following experiments.
MCF-7 human breast cancer cells were purchased from
ATTC. They were maintained as monolayer cultures in 58.1
cm2 plastic culture plates in Dulbecco's modified
Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% FBS,
penicillin (20 U/ml) and streptomycin (20 µg/ml) at 37°C in a humid
atmosphere containing 5% CO2.
Non-malignant human mammary epithelial cell line
(MCF-10A) was purchased from ATTC. They were maintained as
monolayer cultures in 58.1 cm2 plastic culture plates in
DMEM/F12 supplemented with 5% horse serum, 0.5 µg/ml hydrocortisone
(all from Sigma-Aldrich), 20 ng/ml epidermal growth factor (R&D
Systems Europe Ltd., Abingdon, UK), 100 ng/ml cholera toxin, 10
µg/ml insulin (both from Sigma-Aldrich), penicillin (20 U/ml) and
streptomycin (20 µg/ml) at 37°C in a humid atmosphere containing 5%
CO2.
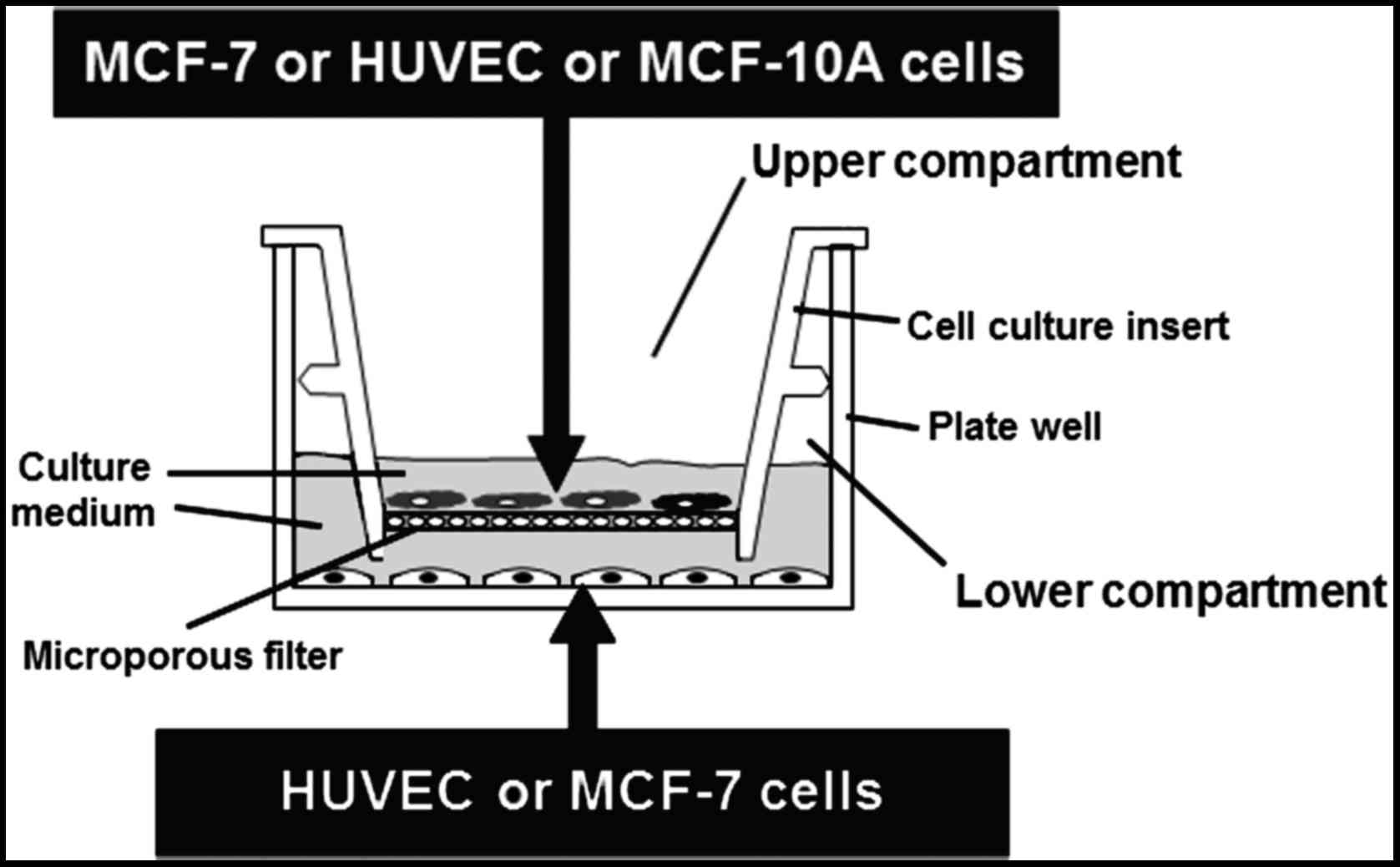
Co-culture of HUVECs and MCF-7 or
MCF-10A cells
HUVECs were co-cultured together with MCF-7 or
MCF-10A cells using Falcon 6-multiwell plates and Falcon cell
culture inserts. HUVECs were plated (50×104 cells/well)
on the bottom wells in VCBM supplemented with 2% FBS and incubated
overnight. At this time, MCF-7 cells (40×104 cells) or
MCF-10A cells (30×104 cells) were seeded on the
permeable membrane (0.45-µm) of the tissue-culture inserts in DMEM
supplemented with 10% FBS for 24 h. HUVECs and MCF-7 or MCF-10A
cells were cultured separately for 24 h to establish attachment.
After 24 h, MCF-7 or MCF-10A seeded inserts were moved over the
HUVEC cell cultures in the 6-well plates in fresh VCBM supplemented
with 2% FBS to create the hanging co-culture setup (Fig. 1). Due to the membrane pore size and
diffusional distance between cells within this setup, cell to cell
contact is prevented but paracrine signalling can occur between
endothelial cells in the 6-well plate and epithelial cells on the
insert. After 24 h, media were replaced with VCBM supplemented with
2% FBS containing melatonin (1 mM or 1 nM) or vehicle (ethanol) for
4 h to measure mRNA expression of angiogenic factors or for 72 h to
measure proliferation and ANG-1, ANG-2 and VEGF protein levels. At
the end of the experiment, media were collected, centrifuged to
remove particulates and subjected to measurement of ANG-1, ANG-2
and VEGF protein levels. Cells (HUVECs) in the bottom plate were
evaluated for proliferative indices by the MTT method and for
ANG-1, ANG-2, Tie2 and VEGF mRNA expression by RT-PCR. Since we
were only able to measure ANG-1, ANG-2, Tie2 and VEGF mRNA
expression of the cells that were in the lower compartment, in
other experiments MCF-7 cells were plated (80×104
cells/well) on the bottom wells and HUVECs (30×104) on
the permeable membrane of the tissue-culture inserts to be able to
measure ANG-1, ANG-2, Tie2 and VEGF mRNA expression by RT-PCR in
MCF-7 cells.
Measurement of cellular
proliferation
Since the reduction of tetrazolium salts is widely
accepted as a reliable way to examine cell proliferation, we used
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) method (34), reading
absorbance at 570 nm in a microplate reader (Multiskan RC 351;
LabSystems Vienna, VA, USA). MTT was obtained from Molecular Probes
Inc. (Eugene, OR, USA).
Measurement of ANG-1, ANG-2, Tie2 and
VEGF mRNA expression
Analysis of the ANG-1, ANG-2, Tie2 and VEGF mRNA
expression in HUVECs, MCF-7 and MCF-10A cells was carried out by
real-time reverse transcription RT-PCR after incubation of cells
with either 1 mM melatonin and/or estradiol 10 nM (Sigma-Aldrich)
and/or vehicle (ethanol) for 4 h. The total cellular RNA was
isolated from HUVECs or MCF-7 cells and purified using the
NucleoSpin RNA II kit (Machenery-Nagel, Düren, Germany) following
the manufacturer's instructions. Integrity of RNA was assessed by
electrophoresis in ethidium bromide-stained 1% agarose- Tris-borate
EDTA gels. The absorbance ratio A260nm/A280nm
was >1.8. For cDNA synthesis, 0.5 µg of total RNA was denatured
at 65°C for 10 min and reverse transcribed for 50 min at 45°C with
a cDNA synthesis kit (BioLine, London, UK) in a final volume of 20
µl in the presence of 500 ng of oligo(dT)12-18 primers.
Quantitative real-time PCRs were performed using the following set
of human ANG-1-specific primers: [5′-GAAGGGAACCGAGCCTATTC-3′
(forward) and 5′-AGGGCACATTTGCACATACA-3′ (reverse)]; ANG-2-specific
primers [5′-AAGAGAAAGATCAGCTACAGG-3′ (forward) and
5′-CCTTAGAGTTTGATGTGGAC-3′ (reverse)], Tie-2-specific primers
[5′-AAGACCTACGTGAATACCAC-3′ (forward) and
5′-GAAACAGAGGGTATACAGATG-3′ (reverse)]; and human VEGF 165-specific
primers [5′-ACCAAGGCCAGCACATAGG-3′ (forward) and
5′-ACGCTCCAGGACTTATACCG-3′ (reverse)] (Sigma Genosys Ltd.,
Cambridge, UK). As a control quantification, s14 mRNA was also
subjected to real-time RT-PCR using a set of specific primers
[5′-TCCTGCGAGTGCTGTCAGAG −3′ (forward) and
5′-TCACCGCCCTACACATCAAA-3′ (reverse)] (Sigma Genosys Ltd.). RT-PCRs
were performed in a MX3005P system (Stratagene, La Jolla, CA, USA)
using Brilliant® SYBR®-Green PCR Master Mix
(Applied Biosystems, Madrid, Spain) following the manufacturer's
instructions. Amplifications were performed for 40 cycles using the
following temperature profile: 60°C, 45 sec (annealing); 72°C, 30
sec (extension) and 95°C, 30 sec (denaturation). Each reaction was
run 9-fold by quadruplicate. Melting curves were performed to
verify that only a single product with no primer-dimers was
amplified. For the primers used there were no differences between
transcription efficiencies, and the fold-change in each sample was
calculated by the 2−ΔΔCt method (35).
Measurement of ANG-1, ANG-2 and VEGF
protein levels
In order to measure ANG-1, ANG-2 and VEGF protein
levels in cell co-culture media, samples were collected,
centrifuged and processed immediately. For the determination of
VEGF concentration in the HUVEC/MCF-7 cell co-culture media a human
VEGF Immunoassay kit (R&D Systems Europe Ltd.) was used. The
samples (in triplicate) were processed according to the supplier's
instructions. At the end of the procedure, absorbance was
determined at a wavelength of 450 nm, with corrections at 540 nm.
For the determination of ANG-1 and ANG-2 concentration in the
HUVEC/MCF-7 cell co-culture media we used a Human Angiopoietin-1 or
−2 Immunoassay kit (R&D Systems Europe Ltd.) following the
supplier's instructions.
Statistical analysis
Data are expressed as the mean ± standard errors of
the mean (SEM). Statistical differences between groups were
analyzed using one way analysis of variance (ANOVA), followed by
the Student-Newman-Keuls test. Results were considered as
statistically significant at P<0.05.
Results
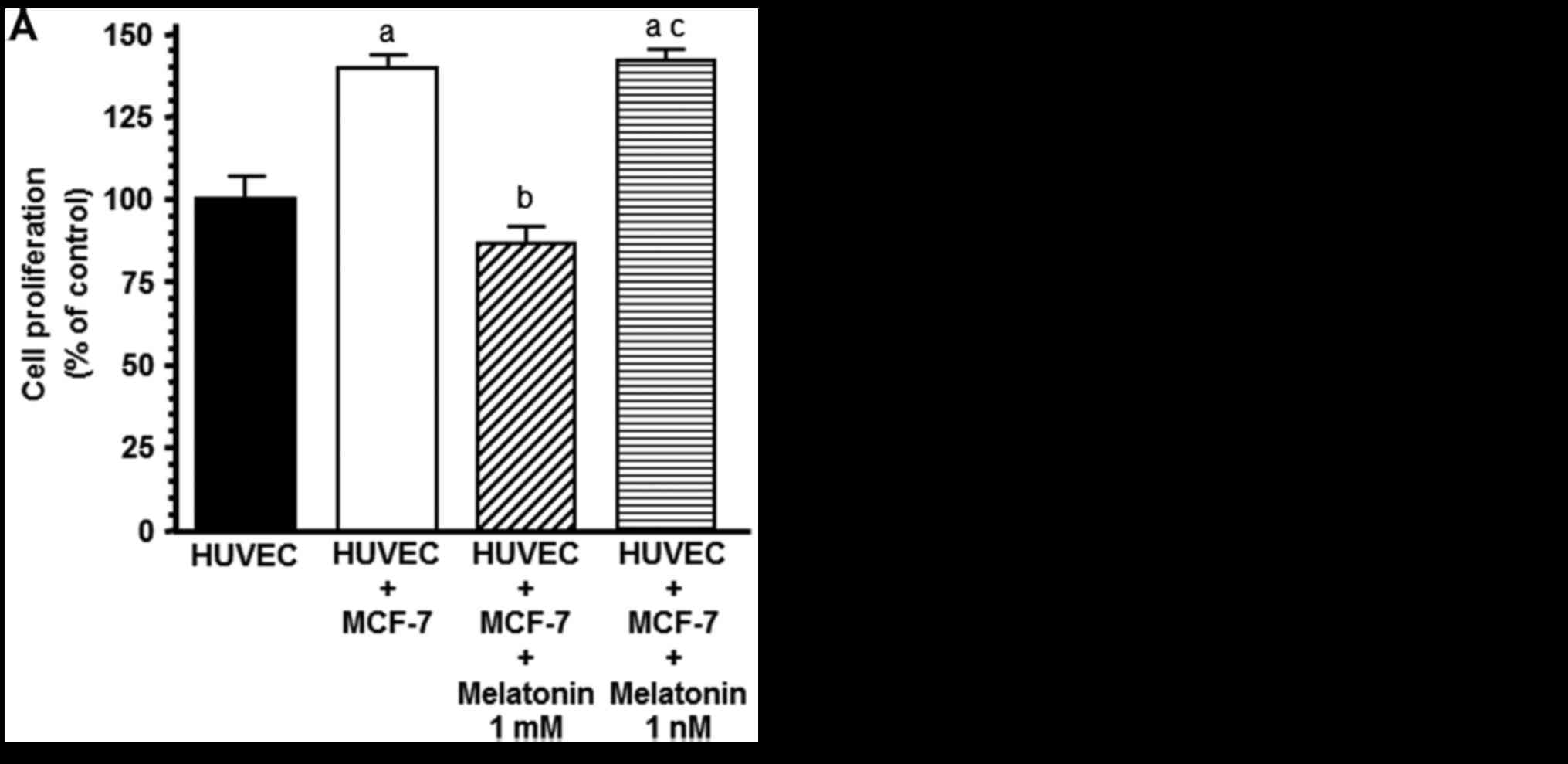
Melatonin counteracts the stimulatory
effect on HUVEC proliferation induced by the presence of tumoral
cells
Since reciprocal growth factor exchange between
endothelial and breast cancer cells within the tumor
microenvironment may directly stimulate neovascularization, we
firstly employed co-cultures of HUVECs (lower compartment of the
chamber) and MCF-7 (upper compartment of the chamber) cells to
investigate whether the presence of malignant epithelial cells
affects the growth of the endothelial cells. Indeed, we observed
that the presence of breast cancer cells promoted an increase in
HUVEC proliferation (P<0.01) and 1 mM melatonin prevented this
stimulatory effect (Fig. 2A). Since
melatonin at physiological concentrations did not affect cell
proliferation of endothelial cells, we used 1 mM concentration of
melatonin in the following experiments. The presence of
non-malignant breast epithelial cells in the co-cultures did not
promote an increase in HUVEC proliferation and melatonin had no
effect (Fig. 2B).
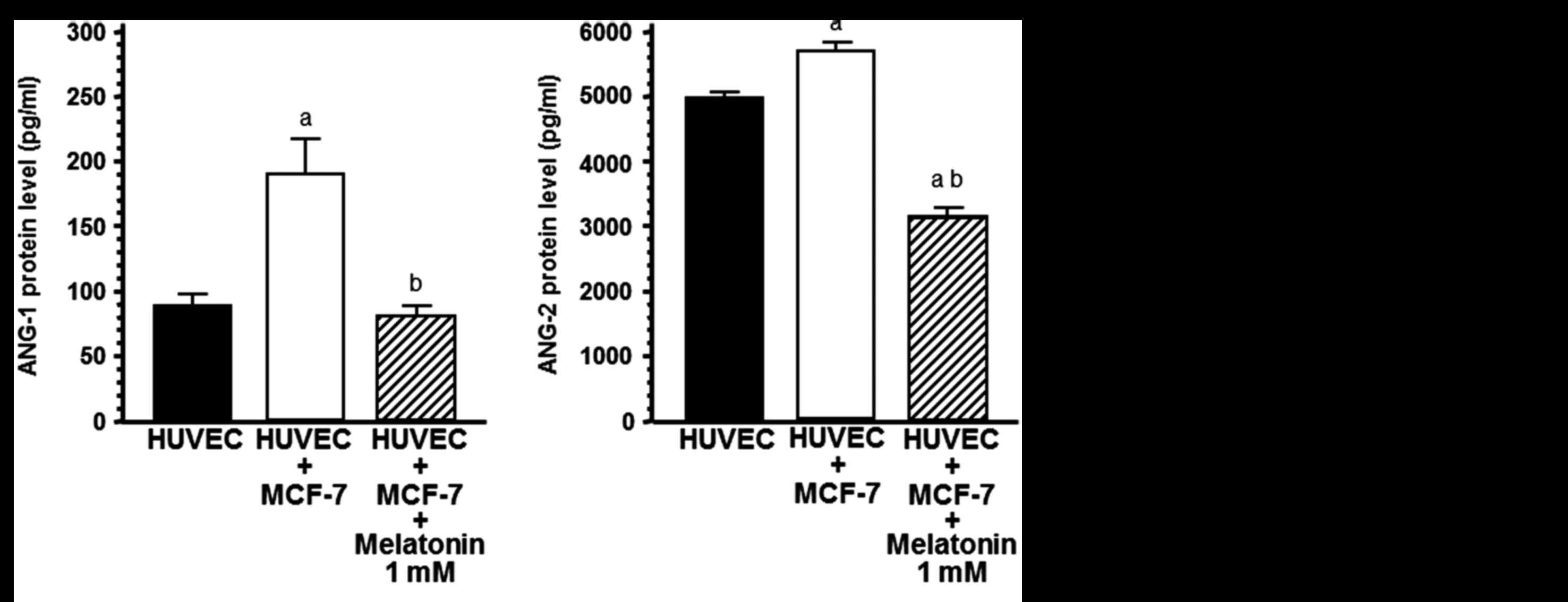
Effects of melatonin on protein levels
of angiogenic factors
With the aim of determining whether the increase in
HUVEC proliferation could be due to the release of angiogenic
factors, such as ANG-1, ANG-2 and VEGF, we measured ANG-1, ANG-2
and VEGF concentrations in the cell co-culture media. The presence
of breast cancer cells in the upper compartment of the chamber
significantly increased the concentrations of ANG-1, ANG-2 and VEGF
(P<0.001) in the co-culture media, whereas the addition of 1 mM
melatonin decreased the concentration of ANG-1, ANG-2 and VEGF and
counteracted the stimulatory effect induced by the presence of
tumoral cells (P<0.001) (Fig.
3).
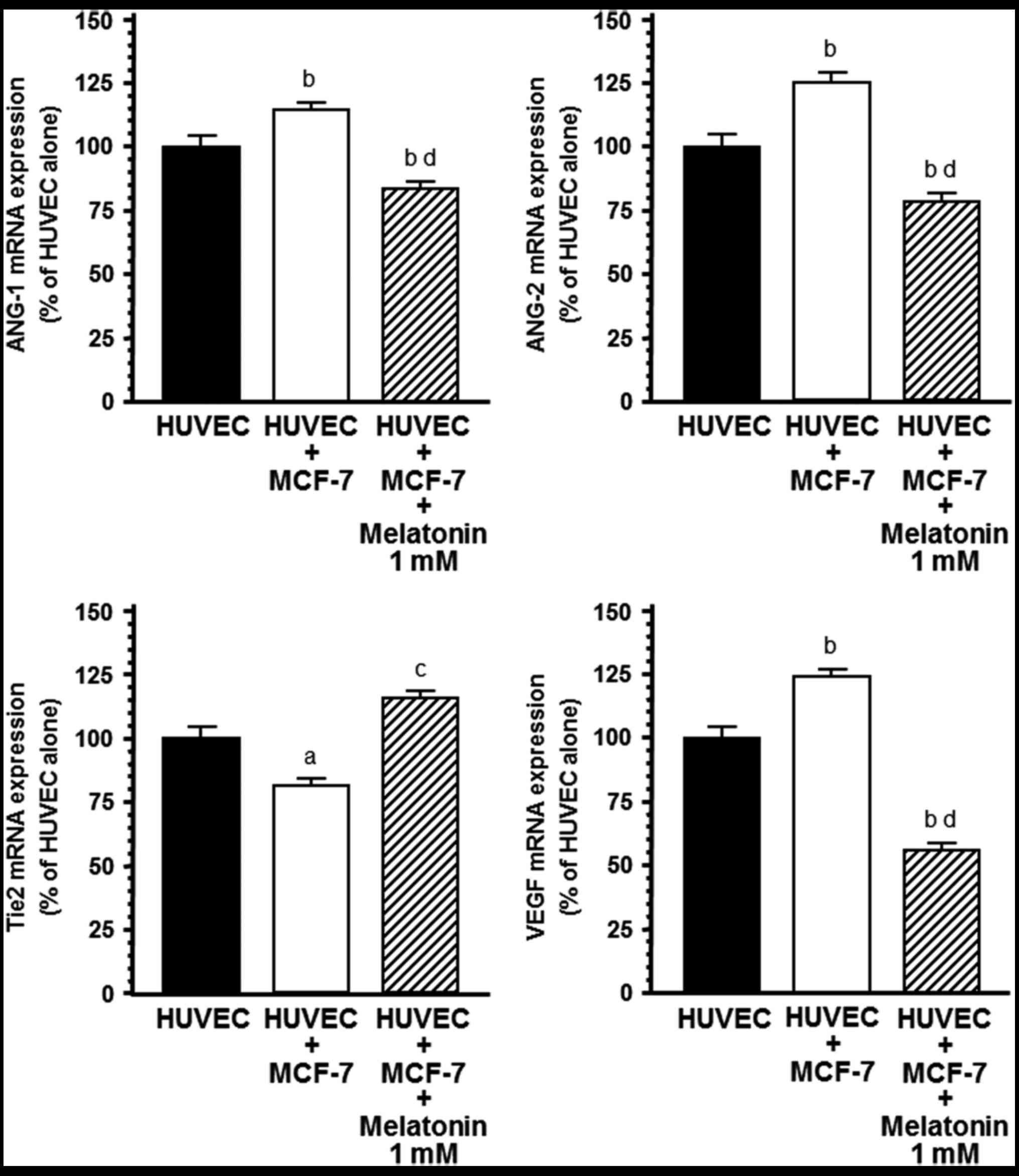
Effects of melatonin on mRNA
expression of angiogenic factors
With the aim of determining whether this inhibitory
effect of melatonin on ANG-1, ANG-2 and VEGF production was due to
a downregulation of ANG-1, ANG-2 and VEGF mRNA expression, total
RNA was isolated. RT-PCR was performed using specific primers for
human ANG-1, ANG-2 and VEGF and gene s14 as housekeeping. mRNA
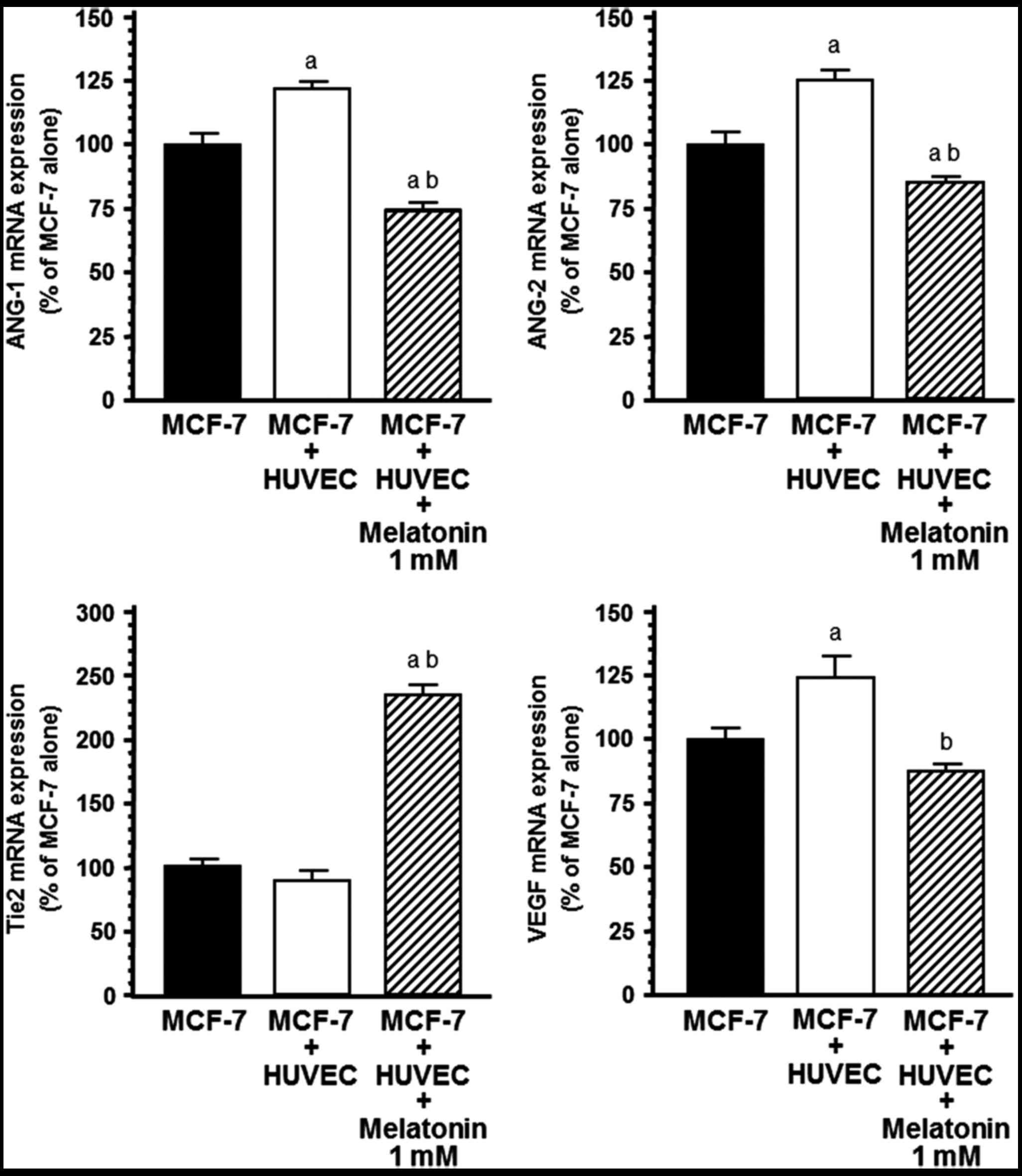
expression of angiogenic factors in endothelial cells was
significantly influenced by co-culture with human breast cancer
cells (Fig. 4). ANG-1, ANG-2 and
VEGF mRNA expression was significantly (P<0.001) upregulated
during the HUVEC/MCF-7 co-culture relative to the HUVEC
monoculture. The addition of melatonin (1 mM) to the co-culture
downregulated ANG-1, ANG-2 and VEGF mRNA expression in endothelial
cells, showing a 30% reduction in ANG-1, 50% downregulation in
ANG-2 and 70% reduction in VEGF mRNA expression (Fig. 4). Melatonin induced a higher
decrease in ANG-2 than ANG-1 and shifted ANG-1/ANG-2 balance in
favor of ANG-1. The presence of breast cancer cells also decreased
Tie2 mRNA expression, the specific tyrosine kinase receptor Tie2 of
ANG-1 and ANG-2 in endothelial cells. This effect was significantly
counteracted by the addition of 1 mM melatonin (Fig. 4).
Significant upregulation of ANG-1, ANG-2 and VEGF
mRNA expression occurred also in MCF-7 cells during co-culture with
endothelial cells relative to MCF-7 monoculture (Fig. 5). The addition of melatonin 1 mM to
the co-culture significantly (P<0.001) downregulated ANG-1,
ANG-2 and VEGF mRNA expression and upregulated Tie2 mRNA expression
in breast cancer cells (Fig.
5).
The expression of the angiogenic factors in
endothelial cells, in the presence of non-malignant MCF-10A breast
epithelial cell line was also assessed. ANG-1, ANG-2, Tie2 and VEGF
mRNA expression levels were not modified during the HUVEC/MCF-10A
co-culture in comparison to the HUVEC monoculture (Fig. 6). The addition of melatonin (1 mM)
to the co-culture only upregulated ANG-2 mRNA expression in the
endothelial cells (Fig. 6).
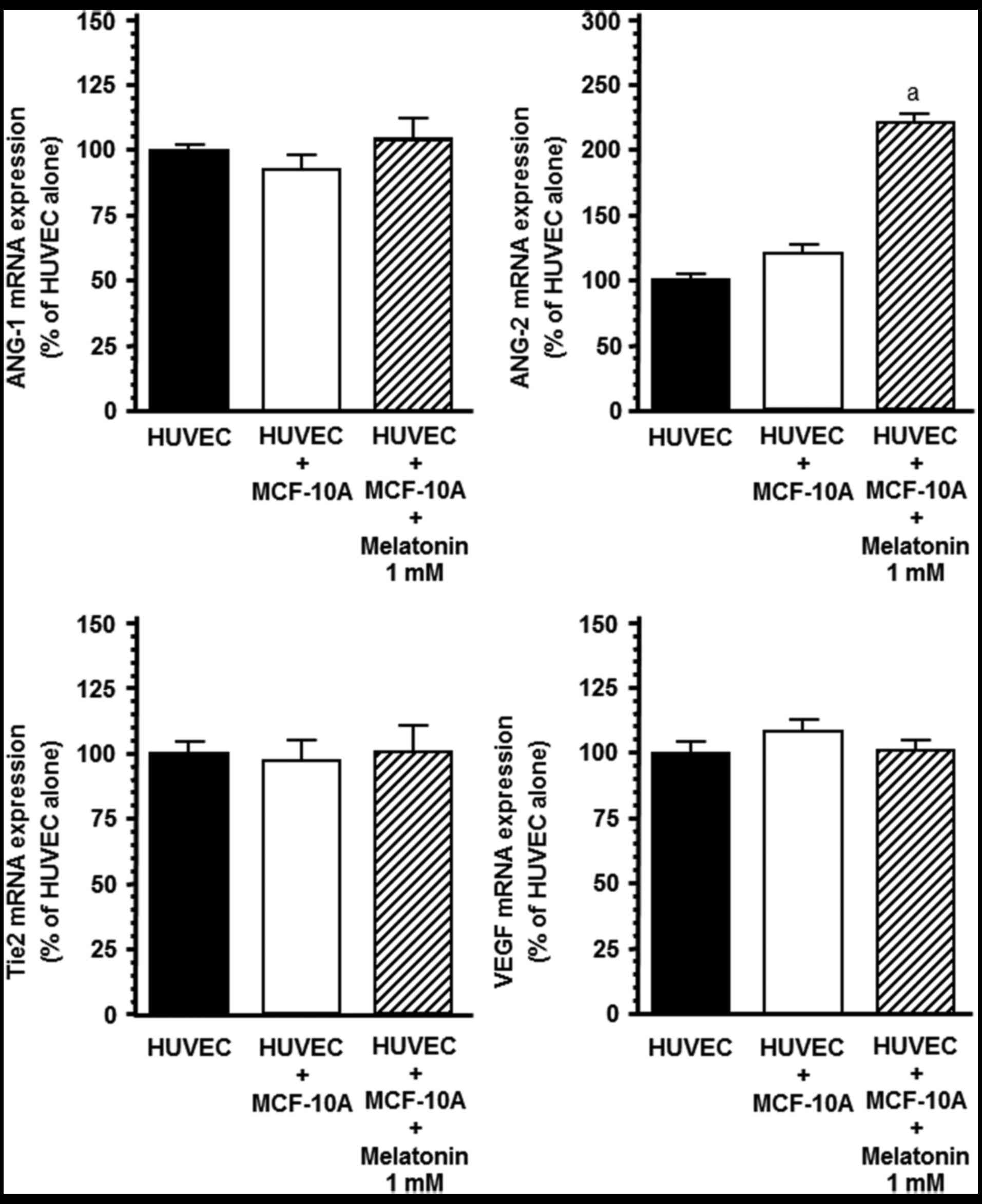 | Figure 6.Effects of melatonin (1 mM) on ANG-1,
ANG-2, Tie2 and VEGF mRNA expression in the HUVEC co-culture with
MCF-10A cells. HUVECs were plated (50×104/well) on the
bottom wells in VCBM supplemented with 2% FBS and incubated
overnight. Then, MCF-10A (30×104) cells were seeded on
the permeable membrane (0.45 µm) of the tissue-culture inserts in
DMEM/F12 supplemented with 5% horse serum, 0.5 µg/ml
hydrocortisone, 20 ng/ml epidermal growth factor, 100 ng/ml cholera
toxin and 10 µg/ml insulin for 24 h. Media were then replaced with
VCBM supplemented with 2% FBS containing melatonin (1 mM) or
vehicle (ethanol) for 4 h. Total mRNA was isolated from cells and
reverse transcribed. cDNA was subjected to RT-PCR using specific
primers for ANG-1, ANG-2, VEGF, Tier or s14. Data are expressed as
the percentage of the control group, cultures of only HUVECs (mean
± SEM). aP<0.001 vs. other groups. |
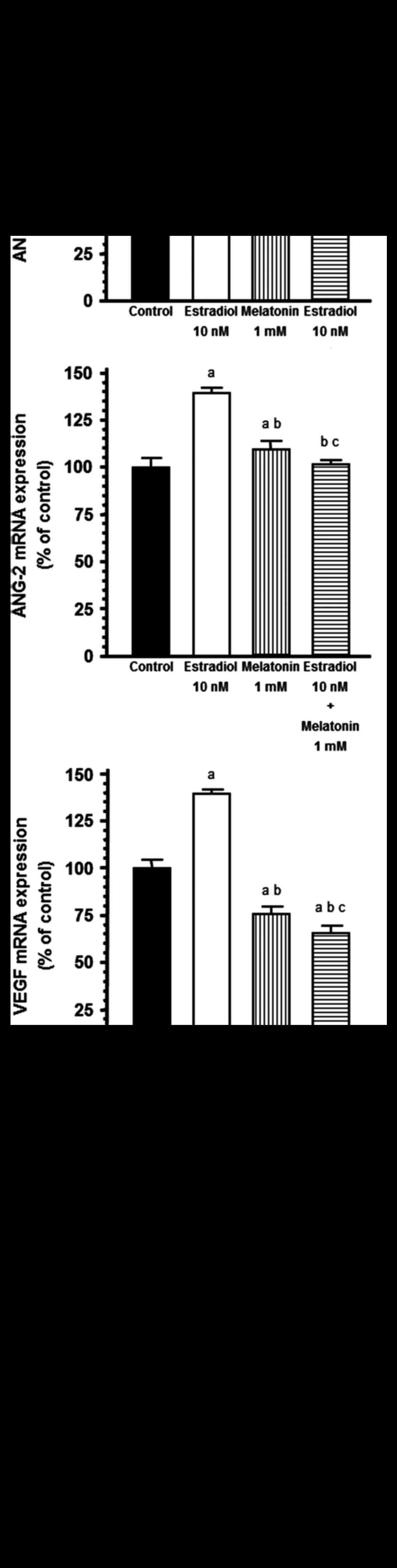
Estradiol (10 nM), added to both compartments of the
multi-well plate, increased ANG-1, ANG-2 and VEGF mRNA expression
in HUVECs and melatonin (1 mM) significantly (P<0.001)
counteracted this effect (Fig.
7).
Discussion
Tumor vascular neo-angiogenesis is an intrincate
dynamic process that has an important role in tumor ontogenesis and
progression. The VEGF pathway and more recently, ANG/Tie2 receptor
signaling are considered the main regulators of different
mechanisms of tumor vascularization (4,9–12).
VEGF synthesized in and secreted by cancer cells, plays a crucial
role in the progression and development of malignant mammary tumors
since VEGF stimulates vascular permeability and proliferation of
endothelial cells from contiguous blood vessels (36). Angiopoietin-1 (ANG-1) maintains the
integrity of vasculature and is expressed in perivascular cells
such as pericytes, vascular smooth muscle cells, fibroblasts and
tumor cells, whereas ANG-2 is mainly released by endothelial cells
only at the sites of vascular remodeling. Both ANG-1 and ANG-2 are
ligands of the Tie2 tyrosine kinase receptor presenting similar
affinities but antagonistic effects. The binding of ANG-1 triggers
a signal that finally induces vessel maturation and stabilizes
tumor vasculature. However, ANG-2 competes with ANG-1 for Tie2
binding, causing vessel regression in the absence of angiogenic
factors, such as VEGF, whereas it promotes angiogenesis in the
presence of VEGF (9,10,37,38).
Thus, the ratio of ANG-1 to ANG-2 is critical in balancing Tie2
signaling pathways and in regulating vascular homeostasis.
Angiopoietins seem to act in a complementary and coordinated manner
with VEGF, playing a later role in vascular development.
Melatonin exerts oncostatic effects through
different biological mechanisms (39–41).
The first description of the antiangiogenic properties of melatonin
came from a clinical study showing a decline in serum levels of
VEGF in cancer patients treated with this indoleamine (42). More recently it has been
demonstrated that melatonin exerts antiangiogenic actions mainly
through its inhibitory actions on VEGF expression and protein
levels (28,29,43,44).
The aim of the present study was to study whether melatonin may
modulate in a coordinated action the production of ANG-1 and ANG-2,
their cognate Tie2 receptor and VEGF in co-cultures of human
endothelial and breast cancer cells.
Our data, firstly, demonstrated that human breast
cancer cells can exert a potent influence on endothelial cells and
vice versa. The presence of breast cancer cells in the co-cultures
promoted an increase in HUVEC proliferation as well as an
upregulation of ANG-1, ANG-2 and VEGF mRNA expression in
endothelial cells in comparison to the HUVEC monocultures.
Additionally, the presence of tumor cells also induced the
downregulation of Tie2 mRNA expression in endothelial cells. This
pro-angiogenic response was not observed in co-cultures of
endothelial and non-malignant breast epithelial cells which
highlights an important difference in the reciprocal interactions
between endothelial and malignant and non-malignant breast
epithelial cells. The addition of melatonin at pharmacological
concentrations (1 mM) to the co-culture downregulated ANG-1, ANG-2
and VEGF mRNA expression in endothelial cells and counteracted the
reduction in Tie2 mRNA expression induced by the presence of the
tumor cells. Melatonin shifted the ANG-1/ANG-2 balance in favor of
ANG-1, since it induced a higher reduction in ANG-2 than ANG-1
expression. In addition, the presence of breast cancer cells
significantly increased the levels of ANG-1, ANG-2 and VEGF in the
co-culture media, whereas the addition of 1 mM melatonin decreased
the concentration of ANG-1, ANG-2 and VEGF and counteracted the
stimulatory effect triggered by the presence of tumoral cells. The
greatest melatonin inhibition of endothelial cell proliferation was
found with melatonin at a concentration of 1 mM as previously
demonstrated (43). The oncostatic
effects of melatonin on different cells have been related with
factors such as the concentration of melatonin in the cultures, the
time of exposure to this indolamine and the concrete
characteristics of the cells studied. In our study, only
pharmacological concentrations of melatonin had an inhibitory
effect on HUVEC proliferation. A potent inhibitory action of
melatonin at nanomolar concentrations on human breast cancer cell
proliferation has been previously shown (15,16,41,45).
However, melatonin at high doses is required to obtain antitumoral
effects in other types of normal cells and tumor cells (43,46–50).
It has been described that the melatonin concentration in the
cerebrospinal fluid is higher than that in blood since melatonin is
a highly lipophilic molecule which may easily cross the blood-brain
barrier (51). Additionally, it is
known that melatonin can become at least one thousand times more
concentrated in tumoral and adipose tissues of the breast (52). The fact that melatonin achieves high
concentrations in some tissues may justify why high levels of
melatonin are necessary to obtain some antitumoral effects of this
indolamine. Thus, we used this pharmacological concentration of
melatonin throughout our experimental study, since only this dose
of melatonin was effective in inhibiting the proliferation of
HUVECs.
Our results also demonstrated that the
pro-angiogenic activity of breast cancer cells, but not
non-malignant mammary epithelial cells, was significantly enhanced
by the presence of endothelial cells. The presence of endothelial
cells upregulated ANG-1, ANG-2, Tie2 and VEGF mRNA expression in
the MCF-7 cells and the addition of melatonin 1 mM to the
co-culture significantly downregulated ANG-1, ANG-2 and VEGF mRNA
expression and upregulated Tie2 mRNA expression in the breast
cancer cells. It has been described that the angiopoietin-Tie2
system has an autoregulation feedback system that modulates the
overall activity of the Tie2 system. ANG-1, but not ANG-2,
downregulates Tie2 mRNA expression (53). In our co-culture experimental
design, melatonin downregulated both ANG-1 and ANG-2 mRNA
expression. The lower levels of ANG-1 may explain the upregulation
of Tie2 mRNA expression induced by melatonin.
Since it is known that estrogens modulate
angiogenesis and little information is yet available regarding the
influence of estrogens on angiopoietins, we also aimed to study the
effects of estradiol on angiopoietin and VEGF mRNA expression in
endothelial cells with and without melatonin. Estradiol (10 nM)
increased ANG-1, ANG-2 and VEGF mRNA expression and melatonin (1
mM) significantly counteracted this effect. Melatonin is well known
for its oncostatic effects on estrogen-dependent breast tumors
mainly by two antiestrogenic mechanisms: interfering with estrogen
signaling pathways at the estrogen receptor level (17,19,20)
and regulating both the activity and expression of enzymes involved
in local estrogen biosynthesis in tumor cells and peritumoral
fibroblasts (40,41,45).
This inhibitory action of melatonin counteracting the effect of
estrogens on angiopoietin and VEGF expression, could be included in
the oncostatic actions of melatonin interfering at different levels
in estrogen signaling pathways. There has been increasing evidence
that estrogens regulate angiopoietin expression; however, the
differential influence of estrogens on ANG-1 and ANG-2 mRNA
expression varies considerably between studies. In non-reproductive
rat tissues, estradiol increased ANG-2 mRNA expression whereas it
reduced ANG-1 mRNA expression (54). There is one study reporting an
inverse correlation of ANG-1 mRNA expression with the level of ERα
in breast cancer cell lines (55).
In our cultures, estradiol stimulated endothelial growth and
increased ANG-1, ANG-2 and VEGF mRNA expression while melatonin
decreased ANG-1, ANG-2 and VEGF mRNA expression and increased Tie2
mRNA expression. The overexpression of Tie2 induced by melatonin in
endothelial cells may lead to an increased vessel stabilization,
thereby making the vasculature less susceptible to pro-angiogenic
factors such as VEGF.
Sequential and complementary expression of ANG-1,
ANG-2 and VEGF has been described as crucial for successful
angiogenesis. Therefore, any interruption or disturbance in this
balanced expression may significantly affect the angiogenic
process. In the presence of VEGF, ANG-2 induces vascular sprouting
and disrupts the interaction between pericytes and endothelial
cells, promoting the destabilization of blood vessels and then
increasing VEGF stimulation. In contrast, in the absence of VEGF,
ANG-2 works as a suppressor that potentiates vessel regression
(37). Moreover, systemic
expression of ANG-2 has been described to increase metastasis and
specific blockade of ANG-2 reduces metastasis development (56). In preclinical studies, it was
demonstrated that the association of ANG-2 blockade with VEGF
blockade and with cytotoxic drugs produced significantly greater
inhibitory actions on tumor growth and angiogenesis than any agent
alone (57). Moreover, inhibition
of ANG-2 or VEGF reduced tumor growth but the inhibition of both
together slowed tumor growth even more and decreased the number of
endothelial sprouts to a degree greater than either inhibitor alone
(58). The use of ANG-2 blockers
reduced vessel sprouting, while anti-VEGF antibodies that work as
blockers of VEGF function caused vessel regression (58). Thus, high ANG-2 levels may interfere
with the efficacy of anti-VEGF therapy. In studies with mice, the
use of a specific anti-ANG-2 monoclonal antibody reduced lung
metastasis and lung lymph node metastasis from a non-small cell
carcinoma (59) and decreased
metastasis in spontaneous breast carcinoma, which may be explained
at least to a certain degree by reducing the pro-angiogenic action
of monocytes associated to tumors (59,60).
Recent studies suggest the benefits of antitumoral treatments that
target multiple antiangiogenic pathways, by acting on different
receptor tyrosine kinases, with the purpose of impairing tumor
neovascularization more efficiently than either inhibitor alone
(58). The fact that melatonin has
complementary actions and coordinates at the same time a
downregulation of angiopoietins with a reduction in VEGF could be
an effective therapeutic strategy for blocking tumor angiogenesis
and growth.
The present study was the first to demonstrate the
effect of melatonin on angiopoietins in human breast cancer and
endothelial cells. We found that the presence of breast cancer
cells increased endothelial cell proliferation and 1 mM melatonin
prevented this effect. ANG-1, ANG-2 and VEGF levels in co-culture
media and mRNA expression were upregulated and Tie2 mRNA expression
was downregulated in HUVECs and MCF-7 cells. Melatonin (1 mM)
downregulated ANG-1, ANG-2 and VEGF levels in co-culture media and
mRNA expression in both types of cells and upregulated Tie2 mRNA
expression in HUVECs. ANG-1, ANG-2, Tie2 and VEGF mRNA expression
were not modified during HUVEC/MCF-10A co-culture. Estradiol (10
nM) increased ANG-1, ANG-2 and VEGF mRNA expression in HUVECs and
melatonin (1 mM) counteracted this effect. Our findings suggest
that melatonin simultaneously coordinates downregulation of
angiopoietins with a reduction of VEGF which could be an important
action for blocking tumor angiogenesis. Further experiments are
necessary to clarify the mechanisms involved in the antiangiogenic
action of melatonin.
Acknowledgements
The present study was supported by grants from the
Spanish Economy and Competitiveness Ministry (SAF2013-42012-P,
SAF2016-77103-P), and from the Instituto de Investigación Sanitaria
Valdecilla (IDIVAL) (APG/12).
References
|
1
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bareschino MA, Schettino C, Colantuoni G,
Rossi E, Rossi A, Maione P, Ciardiello F and Gridelli C: The role
of antiangiogenetic agents in the treatment of breast cancer. Curr
Med Chem. 18:5022–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danza K, Pilato B, Lacalamita R, Addati T,
Giotta F, Bruno A, Paradiso A and Tommasi S: Angiogenetic axis
angiopoietins/Tie2 and VEGF in familial breast cancer. Eur J Hum
Genet. 21:824–830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senger DR, Van de Water L, Brown LF, Nagy
JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM and Dvorak HF:
Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer
Metastasis Rev. 12:303–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gu Q, Wang D, Wang X, Peng R, Liu J, Jiang
T, Wang Z, Wang S and Deng H: Basic fibroblast growth factor
inhibits radiation-induced apoptosis of HUVECs. I. The PI3K/AKT
pathway and induction of phosphorylation of BAD. Radiat Res.
161:692–702. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gingis-Velitski S, Zetser A, Flugelman MY,
Vlodavsky I and Ilan N: Heparanase induces endothelial cell
migration via protein kinase B/Akt activation. J Biol Chem.
279:23536–23541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fagiani E and Christofori G: Angiopoietins
in angiogenesis. Cancer Lett. 328:18–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao Y, Sonveaux P, Liu S, Zhao Y, Mi J,
Clary BM, Li CY, Kontos CD and Dewhirst MW: Systemic overexpression
of angiopoietin-2 promotes tumor microvessel regression and
inhibits angiogenesis and tumor growth. Cancer Res. 67:3835–3844.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tait CR and Jones PF: Angiopoietins in
tumours: The angiogenic switch. J Pathol. 204:1–10. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thurston G and Daly C: The complex role of
angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold
Spring Harb Perspect Med. 2:a0065502012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhadada SV, Goyal BR and Patel MM:
Angiogenic targets for potential disorders. Fundam Clin Pharmacol.
25:29–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hill SM and Blask DE: Effects of the
pineal hormone melatonin on the proliferation and morphological
characteristics of human breast cancer cells (MCF-7) in culture.
Cancer Res. 48:6121–6126. 1988.PubMed/NCBI
|
|
16
|
Cos S and Sánchez-Barceló EJ: Melatonin
and mammary pathological growth. Front Neuroendocrinol. 21:133–170.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blask DE, Sauer LA and Dauchy RT:
Melatonin as a chronobiotic/anticancer agent: Cellular,
biochemical, and molecular mechanisms of action and their
implications for circadian-based cancer therapy. Curr Top Med Chem.
2:113–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reiter RJ: The pineal and its hormones in
the control of reproduction in mammals. Endocr Rev. 1:109–131.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molis TM, Spriggs LL and Hill SM:
Modulation of estrogen receptor mRNA expression by melatonin in
MCF-7 human breast cancer cells. Mol Endocrinol. 8:1681–1690. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cos S, Blask DE, Lemus-Wilson A and Hill
AB: Effects of melatonin on the cell cycle kinetics and
‘estrogen-rescue’ of MCF-7 human breast cancer cells in culture. J
Pineal Res. 10:36–42. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allegra M, Reiter RJ, Tan DX, Gentile C,
Tesoriere L and Livrea MA: The chemistry of melatonin's interaction
with reactive species. J Pineal Res. 34:1–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fraschini F, Demartini G, Esposti D and
Scaglione F: Melatonin involvement in immunity and cancer. Biol
Signals Recept. 7:61–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leon-Blanco MM, Guerrero JM, Reiter RJ,
Calvo JR and Pozo D: Melatonin inhibits telomerase activity in the
MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res.
35:204–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martínez-Campa CM, Alonso-González C,
Mediavilla MD, Cos S, González A and Sanchez-Barcelo EJ: Melatonin
down-regulates hTERT expression induced by either natural estrogens
(17beta-estradiol) or metalloestrogens (cadmium) in MCF-7 human
breast cancer cells. Cancer Lett. 268:272–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blask DE, Dauchy RT, Sauer LA, Krause JA
and Brainard GC: Growth and fatty acid metabolism of human breast
cancer (MCF-7) xenografts in nude rats: Impact of constant
light-induced nocturnal melatonin suppression. Breast Cancer Res
Treat. 79:313–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blask DE, Dauchy RT and Sauer LA: Putting
cancer to sleep at night: The neuroendocrine/circadian melatonin
signal. Endocrine. 27:179–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alvarez-García V, González A,
Martínez-Campa C, Alonso-González C and Cos S: Melatonin modulates
aromatase activity and expression in endothelial cells. Oncol Rep.
29:2058–2064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Regulation of
vascular endothelial growth factor by melatonin in human breast
cancer cells. J Pineal Res. 54:373–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alvarez-García V, González A,
Alonso-González C, Martínez- Campa C and Cos S: Antiangiogenic
effects of melatonin in endothelial cell cultures. Microvasc Res.
87:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cos S, Alvarez-García V, González A,
Alonso-González C and Martínez-Campa C: Melatonin modulation of
crosstalk among malignant epithelial, endothelial and adipose cells
in breast cancer (Review). Oncol Lett. 8:487–492. 2014.PubMed/NCBI
|
|
31
|
Kajdaniuk D, Marek B, Kos-Kudła B,
Zwirska-Korczala K, Ostrowska Z, Buntner B and Szymszal J: Does the
negative correlation found in breast cancer patients between plasma
melatonin and insulin-like growth factor-I concentrations imply the
existence of an additional mechanism of oncostatic melatonin
influence involved in defense? Med Sci Monit. 8:CR457–CR461.
2002.PubMed/NCBI
|
|
32
|
Fandrey J and Genius J: Reactive oxygen
species as regulators of oxygen dependent gene expression. Adv Exp
Med Biol. 475:153–159. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lobov IB, Brooks PC and Lang RA:
Angiopoietin-2 displays VEGF-dependent modulation of capillary
structure and endothelial cell survival in vivo. Proc Natl Acad Sci
USA. 99:pp. 11205–11210. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang Y and Hyder SM: Proliferation of
endothelial and tumor epithelial cells by progestin-induced
vascular endothelial growth factor from human breast cancer cells:
Paracrine and autocrine effects. Endocrinology. 146:3632–3641.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: Dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Currie MJ, Gunningham SP, Han C, Scott PA,
Robinson BA, Harris AL and Fox SB: Angiopoietin-1 is inversely
related to thymidine phosphorylase expression in human breast
cancer, indicating a role in vascular remodeling. Clin Cancer Res.
7:918–927. 2001.PubMed/NCBI
|
|
39
|
Sánchez-Barceló EJ, Cos S, Fernández R and
Mediavilla MD: Melatonin and mammary cancer: A short review. Endocr
Relat Cancer. 10:153–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ:
Estrogen-signaling pathway: A link between breast cancer and
melatonin oncostatic actions. Cancer Detect Prev. 30:118–128. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cos S, González A, Martínez-Campa C,
Mediavilla MD, Alonso-González C and Sánchez-Barceló EJ: Melatonin
as a selective estrogen enzyme modulator. Curr Cancer Drug Targets.
8:691–702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lissoni P, Rovelli F, Malugani F, Bucovec
R, Conti A and Maestroni GJ: Anti-angiogenic activity of melatonin
in advanced cancer patients. Neuro Endocrinol Lett. 22:45–47.
2001.PubMed/NCBI
|
|
43
|
Cui P, Luo Z, Zhang H, Su Y, Li A, Li H,
Zhang J, Yang Z and Xiu R: Effect and mechanism of melatonin's
action on the proliferation of human umbilical vein endothelial
cells. J Pineal Res. 41:358–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dai M, Cui P, Yu M, Han J, Li H and Xiu R:
Melatonin modulates the expression of VEGF and HIF-1α induced by
CoCl2 in cultured cancer cells. J Pineal Res. 44:121–126. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cos S, Martínez-Campa C, Mediavilla MD and
Sánchez-Barceló EJ: Melatonin modulates aromatase activity in MCF-7
human breast cancer cells. J Pineal Res. 38:136–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alvarez-García V, González A,
Alonso-González C, Martínez- Campa C and Cos S: Melatonin
interferes in the desmoplastic reaction in breast cancer by
regulating cytokine production. J Pineal Res. 52:282–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
González A, Alvarez-García V,
Martínez-Campa C, Alonso-González C and Cos S: Melatonin promotes
differentiation of 3T3-L1 fibroblasts. J Pineal Res. 52:12–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cui P, Yu M, Luo Z, Dai M, Han J, Xiu R
and Yang Z: Intracellular signaling pathways involved in cell
growth inhibition of human umbilical vein endothelial cells by
melatonin. J Pineal Res. 44:107–114. 2008.PubMed/NCBI
|
|
49
|
García-Santos G, Antolín I, Herrera F,
Martín V, Rodríguez-Blanco J, del Pilar Carrera M and Rodríguez C:
Melatonin induces apoptosis in human neuroblastoma cancer cells. J
Pineal Res. 41:130–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sainz RM, Mayo JC, Tan DX, León J,
Manchester L and Reiter RJ: Melatonin reduces prostate cancer cell
growth leading to neuroendocrine differentiation via a receptor and
PKA independent mechanism. Prostate. 63:29–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Longatti P, Perin A, Rizzo V, Comai S,
Giusti P and Costa CV: Ventricular cerebrospinal fluid melatonin
concentrations investigated with an endoscopic technique. J Pineal
Res. 42:113–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Maestroni GJ and Conti A: Melatonin in
human breast cancer tissue: Association with nuclear grade and
estrogen receptor status. Lab Invest. 75:557–561. 1996.PubMed/NCBI
|
|
53
|
Hashimoto T, Wu Y, Boudreau N, Li J,
Matsumoto M and Young W: Regulation of tie2 expression by
angiopoietin - potential feedback system. Endothelium. 11:207–210.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ye F, Florian M, Magder SA and Hussain SN:
Regulation of angiopoietin and Tie-2 receptor expression in
non-reproductive tissues by estrogen. Steroids. 67:305–310. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Harfouche R, Echavarria R, Rabbani SA,
Arakelian A, Hussein MA and Hussain SN: Estradiol-dependent
regulation of angiopoietin expression in breast cancer cells. J
Steroid Biochem Mol Biol. 123:17–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Holopainen T, Saharinen P, D'Amico G,
Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M,
Heloterä H, et al: Effects of angiopoietin-2-blocking antibody on
endothelial cell-cell junctions and lung metastasis. J Natl Cancer
Inst. 104:461–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew
J, Reimer C, Wen S, Zhou JQ, Tabrizi M, Emery S, McDermott B, et
al: A human monoclonal anti-ANG2 antibody leads to broad antitumor
activity in combination with VEGF inhibitors and chemotherapy
agents in preclinical models. Mol Cancer Ther. 9:145–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hashizume H, Falcón BL, Kuroda T, Baluk P,
Coxon A, Yu D, Bready JV, Oliner JD and McDonald DM: Complementary
actions of inhibitors of angiopoietin-2 and VEGF on tumor
angiogenesis and growth. Cancer Res. 70:2213–2223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Leow CC, Coffman K, Inigo I, Breen S,
Czapiga M, Soukharev S, Gingles N, Peterson N, Fazenbaker C, Woods
R, et al: MEDI3617, a human anti-Angiopoietin 2 monoclonal
antibody, inhibits angiogenesis and tumor growth in human tumor
xenograft models. Int J Oncol. 40:1321–1330. 2012.PubMed/NCBI
|
|
60
|
Mazzieri R, Pucci F, Moi D, Zonari E,
Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et
al: Targeting the ANG2/TIE2 axis inhibits tumor growth and
metastasis by impairing angiogenesis and disabling rebounds of
proangiogenic myeloid cells. Cancer Cell. 19:512–526. 2011.
View Article : Google Scholar : PubMed/NCBI
|