Introduction
Osteosarcoma (OS) originates from the metaphyseal
areas of the long bones and is a frequently occurring disease in
children, adolescents, and young adults (1,2). The
risk of developing OS is highest among people under 25 years old
and second-highest in individuals aged 60–80 years (3,4).
Before the 1970s, the mortality rate was ~80% because surgery was
the only method for OS treatment. With advances in surgical
techniques and the emergence of chemotherapy, the disease-free
survival rate has improved substantially (5,6). In
response to tumor metastasis, radiotherapy has emerged as a more
efficient treatment (7,8). However, radiotherapy to treat OS is
limited by radioresistance (9,10).
Therefore, it is necessary to study the molecular mechanism of OS
radioresistance to improve the efficiency of OS radiotherapy.
Ionizing radiation can activate reactive oxygen species (ROS) and
cause oxidative damage to DNA bases. DNA double-strand breaks (DSB)
have a dual influence on tumor therapy; they can lead to cancer,
but can also kill cancer cells. The phosphorylation of histone H2AX
is a sensitive marker for DSB (11)
and H2AX contributes to DSB repair (12). The expression level of phospho-H2AX
(pH2AX) contributes to the detection of epithelial ovarian cancer
(13). γH2AX plays an important
role in the response to DSB and participates in the DSB repair
process mediated by DNA repair enzymes (14).
MicroRNAs (miRNAs) are short (18–24 nucleotides)
single-stranded non-coding RNAs; they cannot be translated into
proteins or oligopeptides. In some cases, miRNAs can
post-transcriptionally regulate gene expression by binding to the
target site in mRNA (15,16). Some studies have found that miRNAs
are involved in the process of tumor development. The
overexpression of miR-187 can inhibit colorectal cancer progression
by directly targeting CD276 (17). In OS, miR-489-3p is downregulated
and the overexpression of miR-489-3p can suppress OS progression by
targeting PAX3 (18). Recent
research has indicated that miRNAs are also involved in tumor
radioresistance. miR-185 can target ATR (rad3-related), which can
respond to DNA damage and DNA replication stress, enhancing
radiosensitivity in tumor cells (19). In esophageal squamous cell
carcinoma, the expression level of miR-98 is positively correlated
with radiosensitivity (20). In
cervical cancer cells, miR-424 can target aprataxin and the
overexpression of miR-424 can increase the cell sensitivity to
radiation (21).
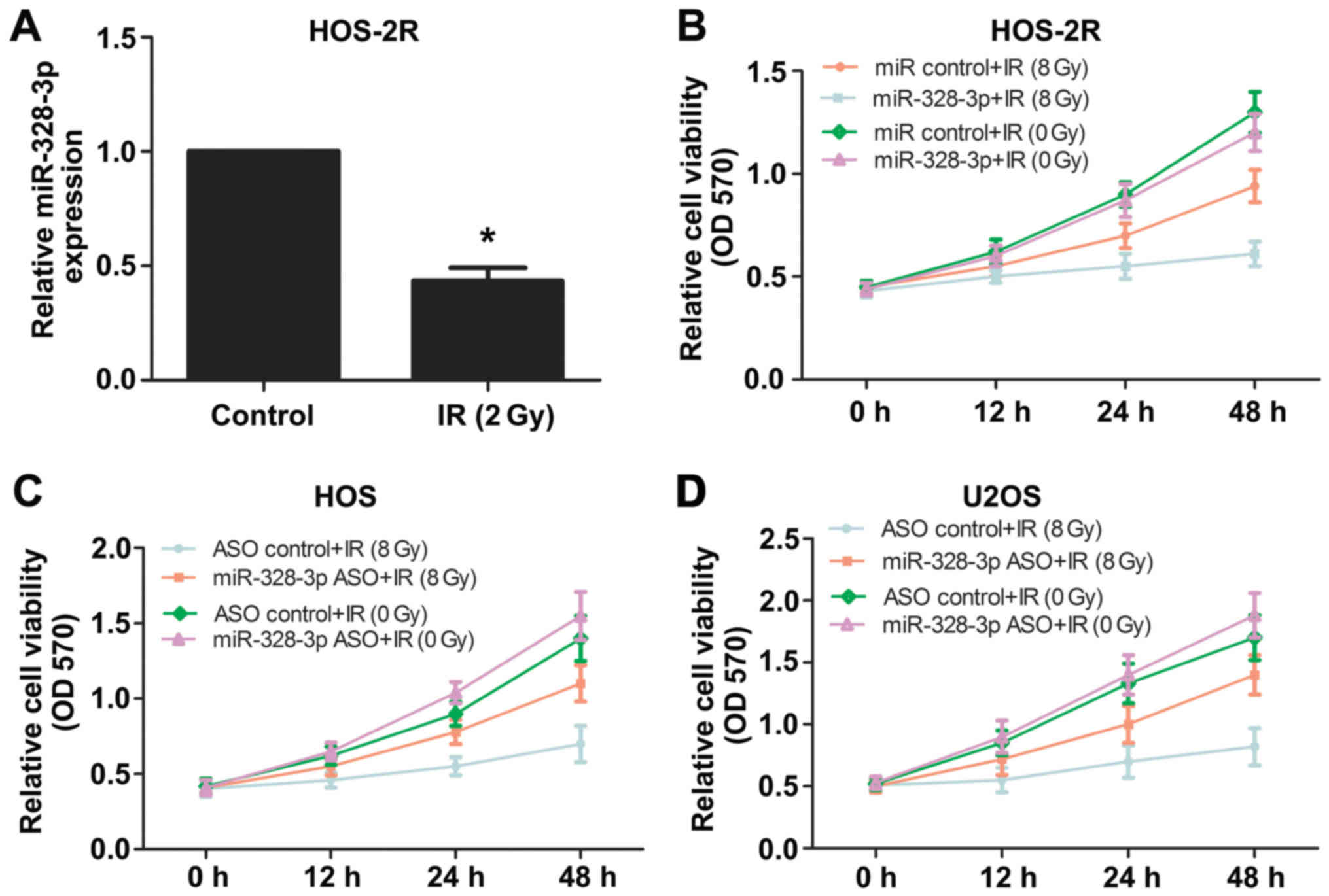
In our previous study, miR-328-3p expression was
distinctly reduced in a radiation-tolerant HOS cell line (HOS-2R)
(Fig. 1). We hypothesized that
miR-328-3p plays a role in the development of OS cells. In the
present study, we examined the correlation between miR-328-3p and
radioresistance in OS cells in vivo and in vitro. We
determined the regulatory role of miR-328-3p in OS radioresistance
by overexpressing or knocking out miR-328-3p in OS cell lines, and
we showed that miR-328-3p targets H2AX to enhance OS
radiosensitivity.
Materials and methods
Cell culture, irradiation, and
transfection
The human osteosarcoma cell line HOS was purchased
from the Cell Culture Center, Institute of Basic Medical Sciences
(Beijing, China). U2-OS cells were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). Cultures of the cell
lines were maintained in a humidified, 37°C, 5% CO2
incubator. McCoy's 5A Media (modified with Tricine) (Sigma, St.
Louis, MO, USA) with 10% FBS (Gibco, Grand Island, NY, USA) was
used for cell culture. The cells in exponential growth were exposed
to X-ray radiation at 2, 4 or 8 Gy at room temperature to measure
radioresistance in vitro. An X-ray machine (Faxitron RX-650;
Tucson, AZ, USA) with 100 kVp (0.835 Gy/min) was used for
irradiation.
Cells were transfected with miRNA mimics and a
control, i.e., miR-328-3p mimics, miR-328-3p ASO (anti-miRNA
oligos), or ASO control (RiboBio, Guangzhou, China). Additionally,
siRNA-H2AX and siRNA control were purchased from GeneChem
(Shanghai, China).
Establishment of radioresistant cell
lines
HOS cells (2×106) were cultured in
McCoy's 5A Media supplemented with 10% FBS. Ten minutes before
irradiation, the cells were replaced with fresh medium, then cells
were irradiated by an X-ray machine (0.835 Gy/min) with 2 Gy dose.
To obtain radioresistant cell population, a total dose of 44 Gy was
reached by 22 fractions of irradiation. Each time after
irradiation, the culture medium was replaced with fresh complete
medium. When the cell confluence reached >80%, the cells were
subcultured. Additionally, the next irradiation was performed when
the cell confluence reached 50%.
Quantitative real-time PCR
Total RNA was extracted from OS cell lines using
TRIzol LS reagent (Invitrogen, Carlsbad, CA, USA) and cDNA was
synthesized using the TaqMan miRNA Reverse Transcription kit
(Applied Biosystems, Waltham, MA, USA). Then, the cDNA was
amplified for 28 cycles in a PCR machine (Roche): 94°C for 30 sec;
annealing for 30 sec, 72°C for 30 sec. Quantitative real-time PCR
(qRT-PCR) analyses were performed with SYBR® Premix Ex
Taq™ (Takara, Japan) using a StepOne-plus Real-Time PCR System
(Applied Biosystems). The PCR cycling was as follows: 95°C for 2
min, 40 cycles of 95°C for 10 sec, 60°C for 20 sec, 72°C for 20
sec. The relative expression levels of miRNAs were calculated using
the 2−∆∆Ct method. U6 snRNA was used as internal
controls to normalize the expression levels of miRNAs. The qPCR
primers for miR-328-3p were as follows:
F-5′-TGCGGCTGGCCCTCTCTGCCC-3′; R-5′-CCAGTGCAGGGTCCGAGGT-3′. The
qPCR primers for U6 snRNA were as follows:
F-5′-TGCGGGTGCTCGCTTCGGCAGC-3′; R-5′-CCAGTGCAGGGTCCGAGGT-3′.
MTT assay
The
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to measure the viability of osteosarcoma cells
using the Cell Proliferation Kit I (Sigma), following the procedure
described in the kit manual. Cells were grown in 96-wells in a
final volume of 100 µl of culture medium per well in a humidified
37°C incubator. MTT labeling reagent was added to each well to
obtain a final concentration of 0.5 mg/ml. Samples were incubated
for 4 h in a humidified atmosphere. Then, 100 µl of solubilization
solution was added to each well, followed by incubation (37°C)
overnight. Absorbance of the samples was measured
spectrophotometrically using a microplate reader.
Western blot analysis
Cells were scraped from the wells after washing
twice with cold PBS. Total proteins were extracted using ice-cold
RIPA lysis buffer (Solarbio, Beijing, China) with a protease
inhibitor. Then, 40 µg of total proteins from each sample was
subjected to 12% SDS-PAGE and transferred to a nitrocellulose
membrane (Bio-Rad, Hercules, CA, USA). After samples were washed
and blocked with 5% non-fat milk, the membrane was incubated with
the primary antibodies (Abcam, UK). After they were washed in TBS,
the membrane was treated with a horseradish peroxidase-conjugated
secondary antibody (ICLLAB, USA). Proteins were observed by
chemiluminescence (Millipore, Billerica, MA, USA).
Survival colony formation assay
Cell suspensions were exposed to X-ray radiation;
then, 1×106 cells were seeded in 6-well plates and
cultured for 12 days. Detailed cultivation methods are described
above (Cell culture and irradiation). The number of colonies that
contained at least 50 cells was counted.
Apoptosis
Cells (1×107) were washed with cold PBS
and fixed in cold 70% ethanol overnight at −20°C. The Annexin
V-FITC Apoptosis Detection kit (Abcam) was used to perform
apoptosis assays following the manufacturer's instructions. The
samples were analyzed using the FACS III (BD Biosciences, Franklin
Lakes, NJ, USA).
Tumor xenograft assay
Female nude mice (BALB/c, 5 weeks old) were
purchased from Nanjing Biomedical Research Institute of Nanjing
University (Nanjing, China) and housed under SPF conditions. All
animal experiments were approved by the Institutional Animal Care
and Use Committee of Guizhou Provincial People's Hospital (Guizhou,
China).
The mouse tumor xenograft model was established;
1×107 HOS cells were suspended in 0.1 ml of PBS and
subcutaneously injected into the right thigh of the nude mice. Ten
days later, the mice were intratumorally injected with 5 nmol
miR-328-3p agomir or 5 nmol miR-NC agomir at days 12, 16, 20, 24,
28 and 32. Mice were exposed to 4 Gy of X-ray radiation after each
intratumoral injection. At day 34, the mice were sacrificed by
cervical dislocation. The tumor weight was measured using
electronic scales (Sartorius, Beijing, China), and the tumor volume
was calculated as length × (width)2/2.
Statistical analysis
One-way ANOVA was performed to determine the
statistical significance of differences between groups. The
numerical results are presented as means ± standard deviation.
Differences with a p-value of <0.05 were considered
statistically significant.
Results
Modulation of miR-328-3p alters the
radiosensitivity of osteosarcoma cells
HOS and U2OS are human osteosarcoma cell lines, we
used these two kinds of cells to explore the effect of miR-328-3p
on the radiosensitivity of osteosarcoma cells. In order to
investigate the relationship between miR-328-3p and
radiosensitivity in OS cells, we also established
radiation-tolerant HOS cell lines (HOS-2R) by X-ray (2 Gy).
miR-328-3p levels in HOS-2R cells were detected by qPCR and the
expression levels were downregulated compared with those in control
cells (Fig. 1A). To explore whether
the modulation of miR-328-3p affects the radiosensitivity of OS
cells, we also performed an MTT assay to determine the survival
rate of HOS-2R cells, HOS cells, and U2OS cells following
irradiation at 0 or 8 Gy. The overexpression of miR-328-3p reduced
HOS-2R cell viability following 8 Gy of X-ray, and there was no
significant difference between miRNA control and miR-328-3p mimic
groups following irradiation with 0 Gy (Fig. 1B). Then, we silenced miR-328-3p in
HOS and U2OS cells using antisense oligonucleotides (ASO). The
deletion of miR-328-3p increased the viability of HOS cells
following 8-Gy irradiation (Fig.
1C), similar to the results obtained for U2OS cells (Fig. 1D). These results confirmed that
miR-328-3p has a regulatory role in OS cell radiosensitivity.
miR-328-3p increases the rate of
apoptosis and reduces the proliferation ability of osteosarcoma
cells after ionizing radiation
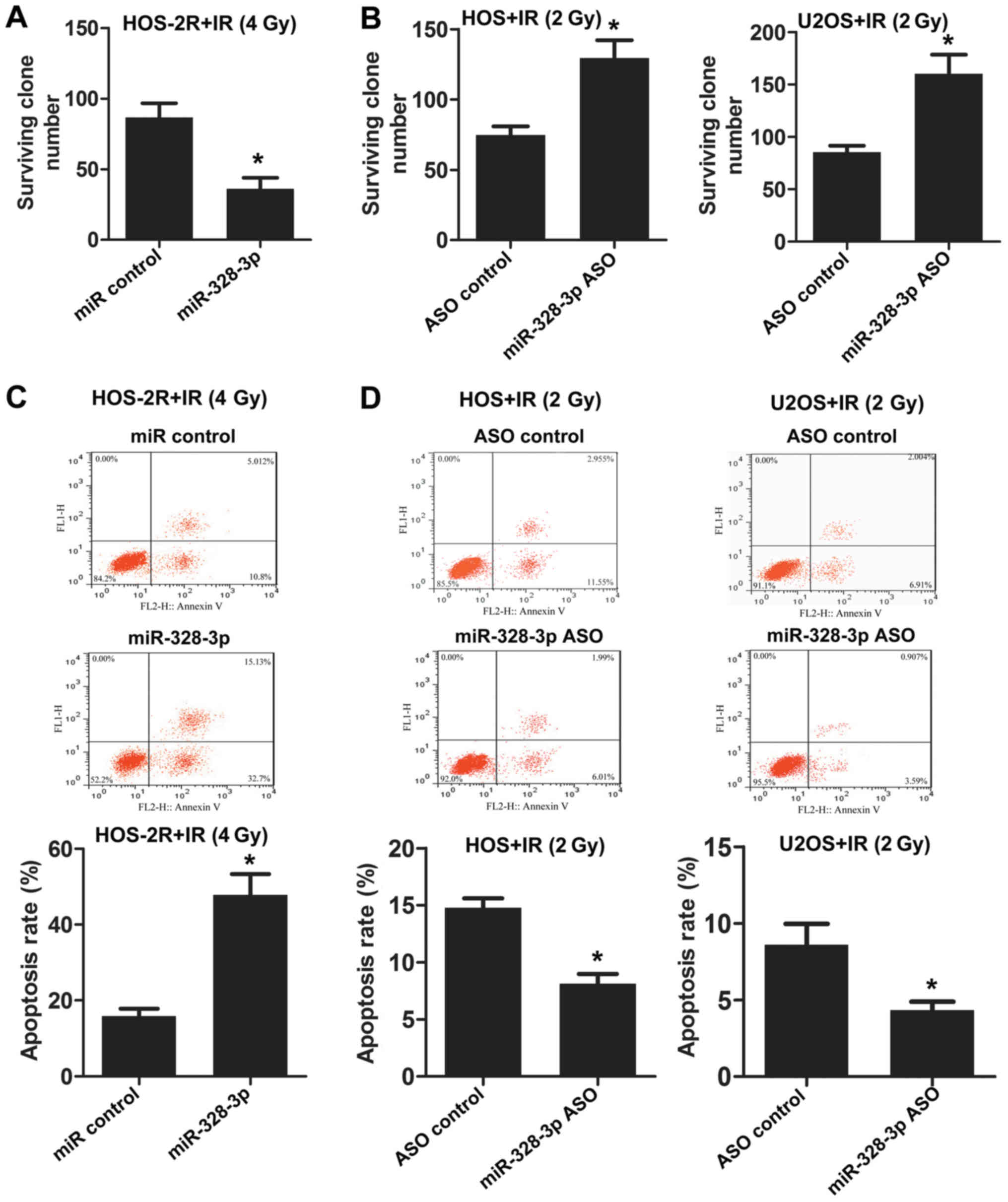
Based on the observation that the abnormal
expression of miR-328-3p alters the radiosensitivity of OS cells,
we examined whether miR-328-3p influences the apoptosis and
proliferation of OS cells. Radioresistant cell lines were
established under the condition of 2-Gy irradiation, so we need to
increase the dose of X-ray to 4 Gy. Compared to HOS-2R, HOS and
U2OS was more sensitive to irradiation. 2 Gy X-ray exposure was
enough to investigate the biological characteristics of HOS and
U2OS cells. miR-328-3p was overexpressed in HOS-2R cells, and the
number of surviving clones after X-ray exposure at 0 or 4 Gy was
counted. The surviving clone number for HOS-2R cells overexpressing
miR-328-3p decreased following irradiation at 4 Gy (Fig. 2A). Then, we inhibited the expression
of miR-328-3p in HOS and U2OS cells using miR-328-3p ASO. The
deficiency of miR-328-3p increased the survival rate after X-ray
exposure at 2 Gy, both in HOS and U2OS cells (Fig. 2B). Apoptosis was analyzed by flow
cytometry following miR-328-3p overexpression or knockout in
various cell types (including HOS-2R, HOS and U2OS cells). The
overexpression of miR-328-3p enhanced HOS-2R cell apoptosis after
exposure to 4 Gy (Fig. 2C). In HOS
and U2OS cells in which miR-328-3p was knocked out, apoptosis
decreased after exposure to 2 Gy (Fig.
2D). These results confirmed that miR-328-3p inhibits
proliferation and promotes apoptosis in OS cells under radiation
conditions, and miR-328-3p enhances the radiosensitivity of OS.
miR-328-3p targets H2AX and inhibits
its expression
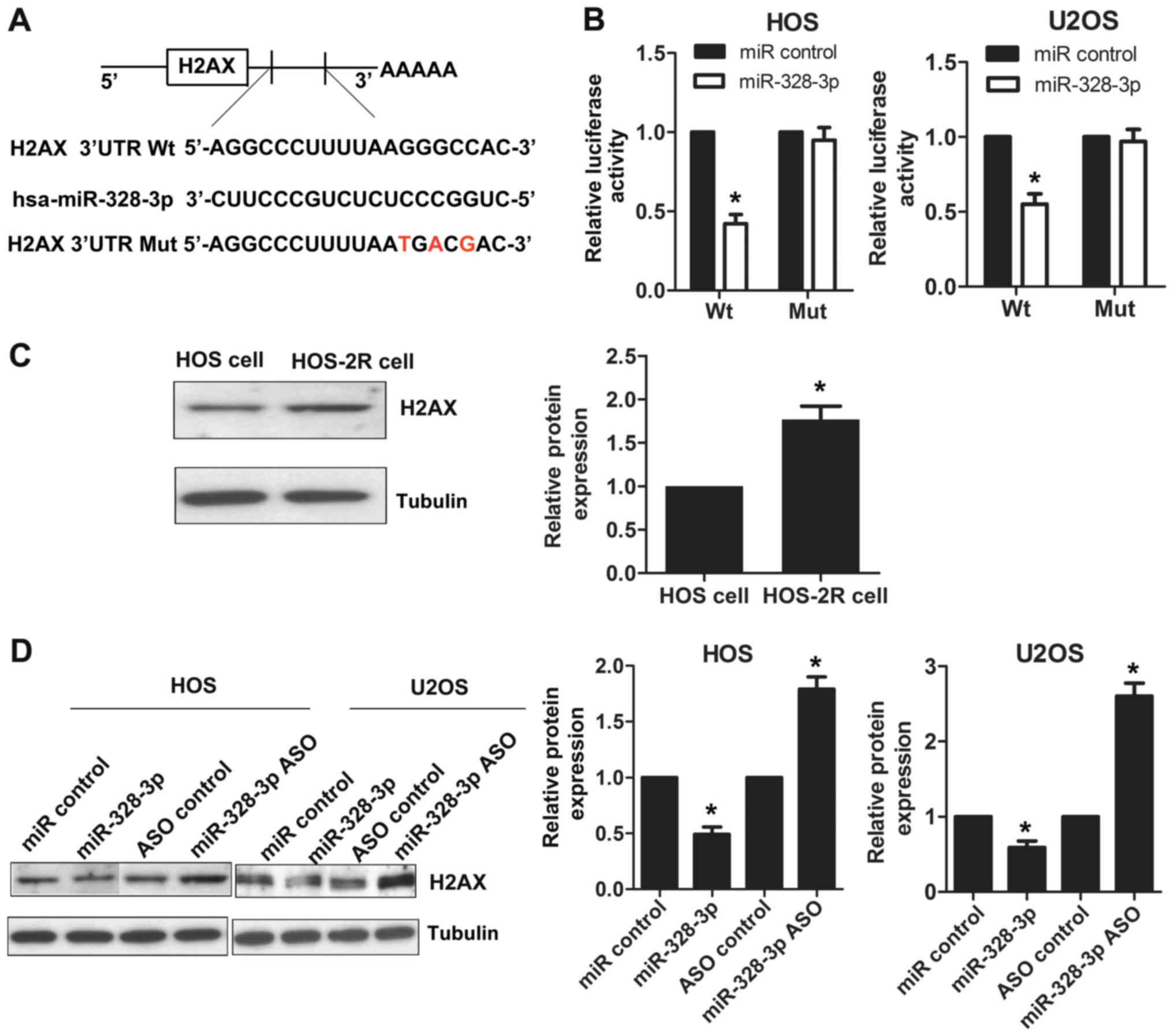
Using the bioinformatics tool TargetScan, we
determined that H2AX is a predicted target for miR-328-3p. As shown
in Fig. 3A, the 3′-UTR region of
H2AX contains a sequence that matches the specific binding
sequences of miR-328-3p. A luciferase reporter assay was used to
further prove the targeting of miR-328-3p to the H2AX
3′-UTR. The cotransfection of miR-328-3p mimics with the wild-type
vector reduced luciferase activity in HOS cells compared to that of
control-treated cells, and a similar trend was found in U2OS cells
(Fig. 3B). No statistically
significant differences in luciferase activity were observed for
cells cotransfected with miR-328-3p mimics and mutant vector
compared to control-treated cells (Fig.
3B). We also detected the expression levels of H2AX in
HOS-2R and HOS cells by western blotting, and HOS-2R cells had a
higher level of H2AX expression compared to that of HOS
cells (Fig. 3C), in contrast to the
miR-328-3p expression patterns (Fig.
1A). To explore the miR-328-3p regulatory effect on
H2AX, we used HOS and U2OS cell lines. We detected the
expression levels of H2AX by western blotting after
overexpressing or silencing miR-328-3p. We found that the
expression of H2AX was suppressed after miR-328-3p mimic
transfection in HOS cells. When miR-328-3p was knocked out using
miR-328-3p ASO, the expression of H2AX increased. A similar
phenomenon was observed in U2OS cells (Fig. 3D). These results showed that
miR-328-3p targets H2AX, and the modulation of miR-328-3p
affects the expression of H2AX.
miR-328-3p mediates the
radiosensitivity of osteosarcoma cells by targeting H2AX
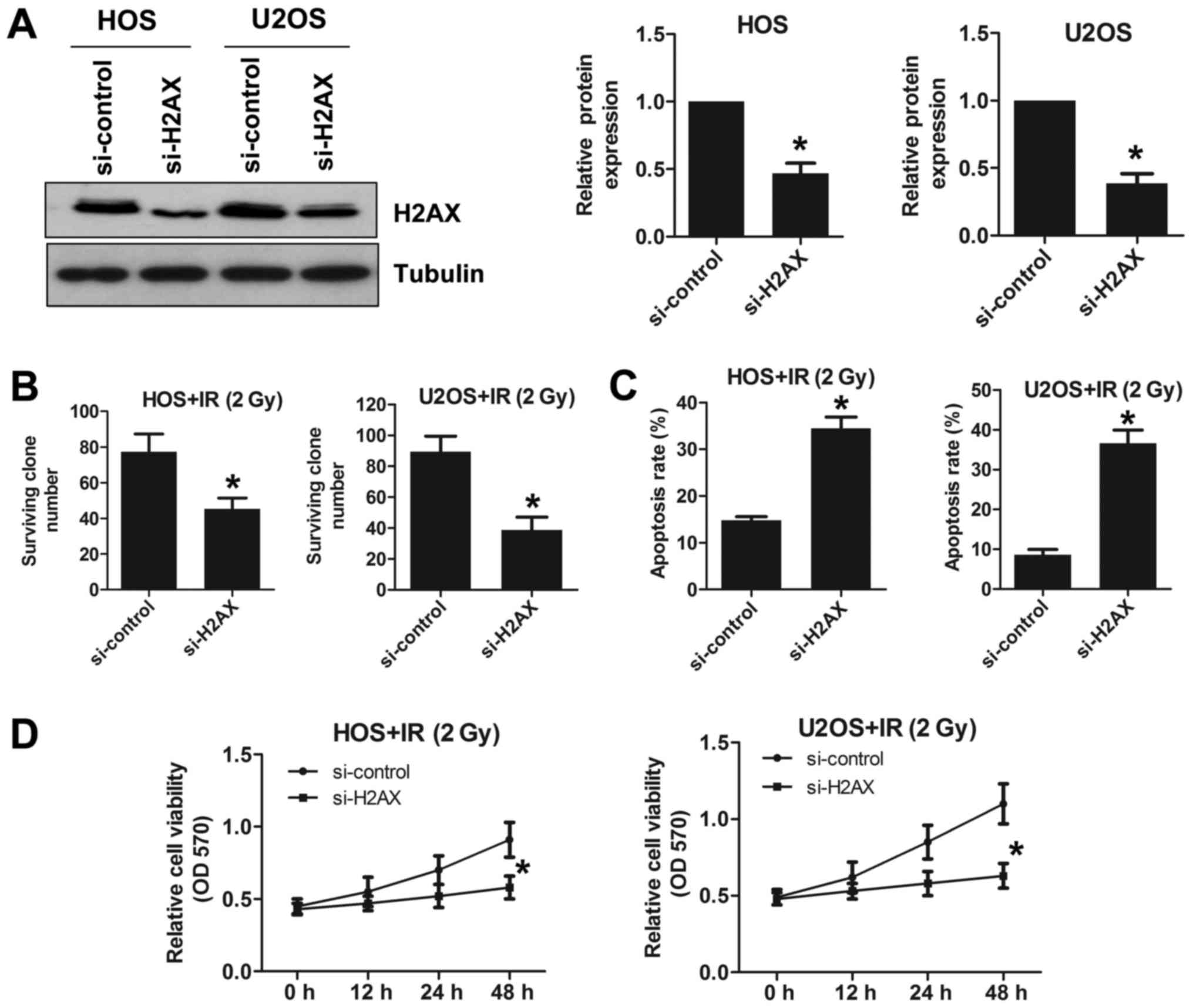
We knocked out H2AX using H2AX siRNA
in the HOS and U2OS cell lines. We verified the knockout efficiency
by western blotting (Fig. 4A). The
survival rate of H2AX-deficient HOS cells was reduced
following irradiation at 2 Gy compared to that of sham-treated
cells (Fig. 4B). A similar trend
was also found in U2OS cells (Fig.
4B). To explore the effect of H2AX expression changes on
apoptosis in OS cell lines, we examined apoptosis using a flow
cytometry assay. Under radiation conditions of 2 Gy, H2AX
deletion increased apoptosis in both HOS and U2OS cells (Fig. 4C). We also found that deleting
H2AX reduced cell viability following irradiation at 2 Gy in
both HOS and U2OS cells (Fig. 4D).
Taken together, these results showed that deleting H2AX
increases the radiosensitivity of OS cell lines.
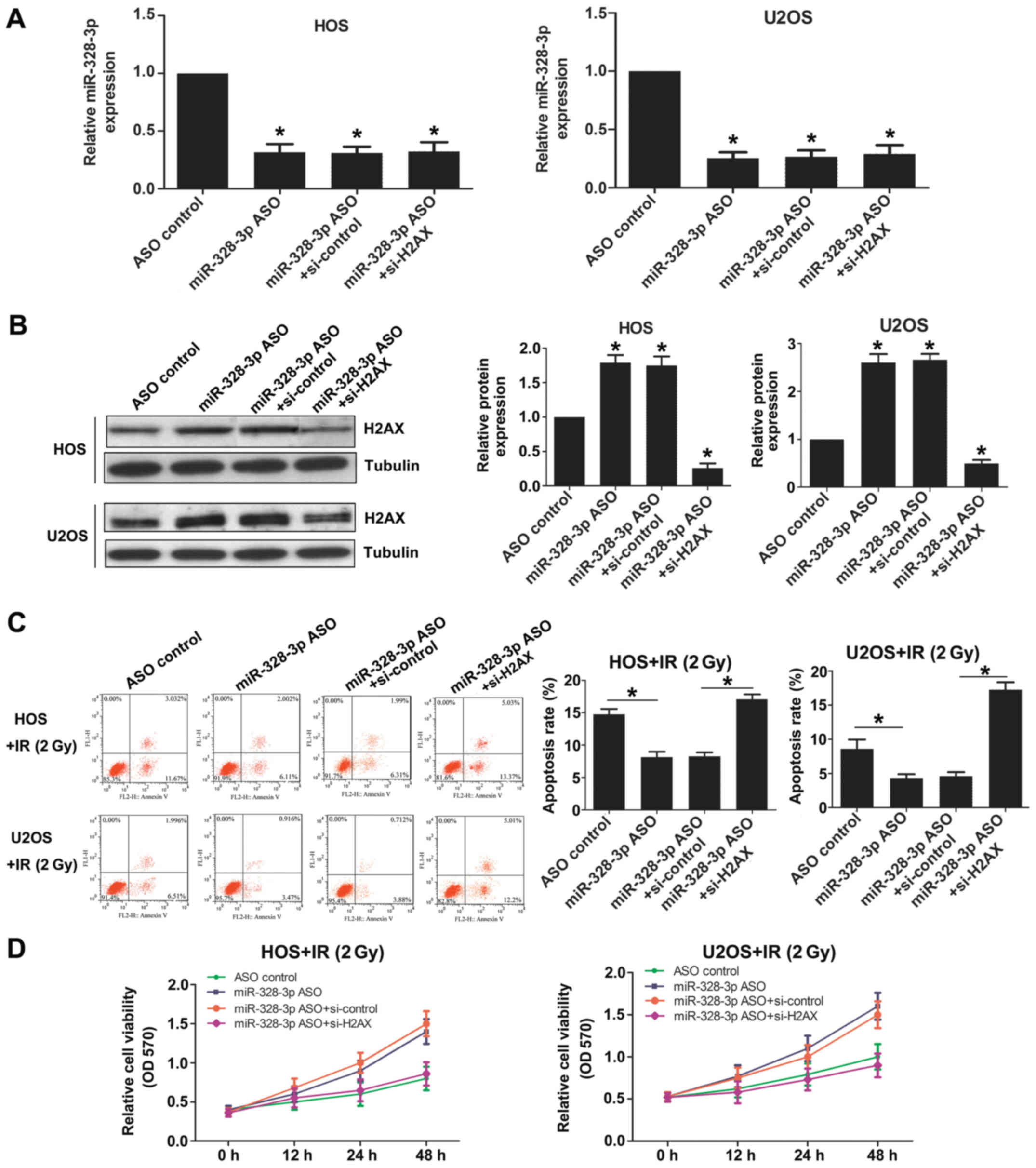
To further study the function of miR-328-3p targeted
to H2AX, we detected the cell sensitivity to radiation by
western blotting. Cells were transfected with ASO con (control),
miR-328-3p ASO, miR-328-3p ASO+siRNA con or miR-328-3p
ASO+H2AX siRNA, respectively. The expression levels of
miR-328-3p were reduced in miR-328-3p ASO transfected, miR-328-3p
ASO and siRNA con cotransfected, and miR-328-3p ASO and H2AX
siRNA cotransfected HOS cells, similar to the results observed in
U2OS cells (Fig. 5A). The
expression levels of H2AX increased after silencing
miR-328-3p in both HOS and U2OS cells (Fig. 5B). The deficiency of miR-328-3p
decreased apoptosis after 2-Gy X-ray exposure in both HOS and U2OS
cells (Fig. 5C). The effect of
miR-328-3p knockout on reducing apoptosis was weakened after
knocking out miR-328-3p and H2AX simultaneously (Fig. 5C). The deficiency of miR-328-3p
increased the viability of both HOS and U2OS cells after 2-Gy X-ray
irradiation (Fig. 5D). The enhanced
cell viability after miR-328-3p knockout was weakened after
knocking out miR-328-3p and H2AX simultaneously (Fig. 5D). These results showed that
miR-328-3p targets H2AX and thereby miR-328-3p can
participate in the regulation of OS radiosensitivity.
Overexpression of miR-328-3p enhances
the radiosensitivity of osteosarcoma in mice
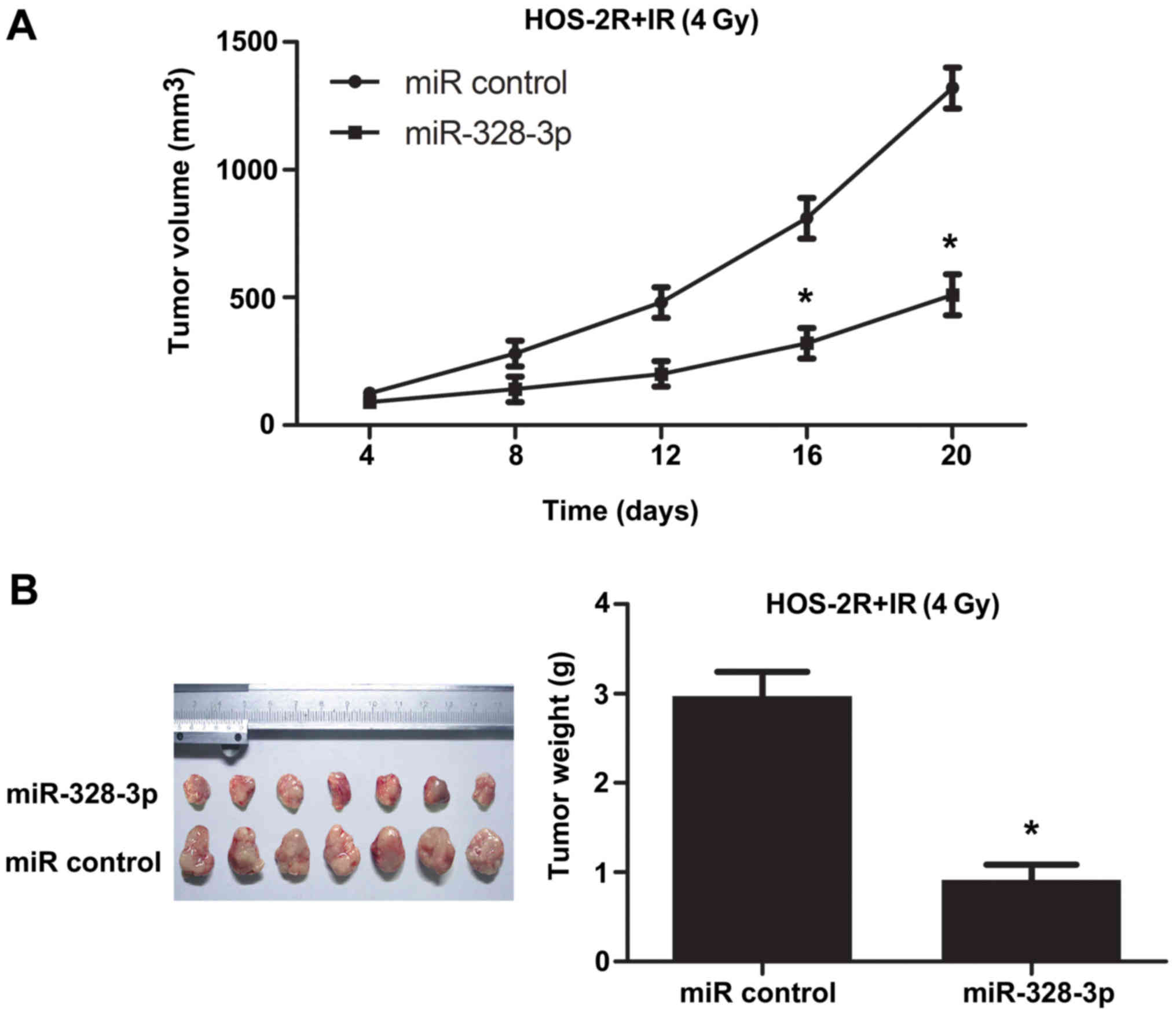
A xenograft tumor model was examined to further
study the effect of miR-328-3p on the radiosensitivity of OS in
vivo. To test tumor growth following irradiation at 4 Gy, we
subcutaneously injected miRNA control transfected HOS-2R cells or
miR-328-3p transfected HOS-2R cells into the right thigh of nude
mice. In the miRNA con-transfected HOS-2R group (control group),
tumor growth was checked. After irradiation at 4 Gy, tumors
continued to grow in the control group. However, tumors in the
miR-328-3p-transfected HOS-2R group grew substantially slower
compared to tumor growth in the control group (Fig. 6). These data showed that miR-328-3p
can promote growth restraint in OS after irradiation at 4 Gy in
mice.
Discussion
Some miRNAs can regulate cancer cell
radiosensitivity to ionizing radiation, and downstream targets have
been identified (20–26). In our radiation-tolerant HOS cell
line (HOS-2R), miR-328-3p was downregulated. A previous study
showed that miR-328 can restrain proliferation and migration of
pulmonary arterial smooth muscle cells by targeting PIM-1 (27). In our study, we found a positive
correlation between miR-328-3p and OS radiosensitivity.
Overexpressed miR-328-3p in OS increases the rate of apoptosis and
reduces the proliferation ability after irradiation. miRNAs cannot
be translated into proteins or oligopeptides and they often combine
with the target points in mRNA to regulate gene expression and
further affect downstream pathways (15). miR-494-3p can inhibit the expression
of Bmil, which leads to cell aging and thus to increased
radiosensitivity of oral squamous cell carcinoma cells (28). In a study of radiation-resistant
tumor glioblastoma multiforme, Guo et al found that miR-26a
disturbs downstream pathways by inhibiting the expression of
ATM via its 3′-UTR (29). In
the SWI2/SNF2-family, helicase-like transcription factor (HLTF) is
an ATP-dependent chromatin remodeling enzyme related to DNA damage
repair. HLTF mRNA is a target of miR-145, by which miR-145
can enhance the radiosensitivity of cervical cancer cells (30). These studies show that miRNA can
take part in the development of cancer. Also, the regulated role of
miRNAs in OS has been revealed in previous studies, such as
miR-542-5p, miR-217, miR-182, miR-199a-3p and miR-153 related to
the OS progression (31–35).
These past results suggest that H2AX plays an
important role in DSB (11–13); H2AX is involved in
maintaining genome stability, and provides a platform for repair
factors to function (14,36–38).
Ionizing radiation can cause DSBs and, subsequently, H2AX
phosphorylation at Ser139 via ATM, generating γH2AX foci
(11,37). We found that H2AX was knocked
out, the survival rate and the viability of OS cells were both
reduced and the apoptosis was increased following irradiation at 2
Gy. The imbalance of H2AX probably perturbed the progress of
chromatin remodeling and DNA repair, thus affecting the radiation
tolerance of OS cells. The phosphorylation of H2AX can
promote genome stability and accelerated DSB repair (12,36).
γH2AX plays a critical role in the formation of
chromatin-remodeling complexes, and γH2AX also helps DNA
repair enzymes function effectively (14,38).
Liao et al found that STAT3 can promote the phosphorylation
of H2AX and enhance the expression of GADD45γ and MDC-1 after UV
exposure, and STAT3 reduces DNA damage caused by UV (39). The phosphorylation of H2AX promotes
NBS1 expression at damage sites, and the acetylation of histone
H2AX at Lys 5 is crucially important for the damaged
chromatin-specific binding of NBS1 (40).
We proved that miR-328-3p target to the H2AX
3′-UTR. Previous studies have shown that H2AX is regulated
by upstream miRNAs. For example, miR-138 directly targets the
histone H2AX to modulate the DNA damage response (41). Lin28 targets H2AX, and a
deficiency of Lin28 increases radiosensitivity in breast cancer
cells (42). H2AX has one
more upstream regulatory element: miR-328-3p. In addition, a
negative correlation was found between H2AX expression and
miR-328-3p level in HOS-2R cells. This tendency provided an
indirect proof that H2AX is a target of miR-328-3p.
One limitation of our study was the inability to
obtain clinical specimens for further verification of the effect of
miR-328-3p in humans. We tested xenograft tumor growth in mice. The
results are consistent with the experiments in vivo. In
general, miR-328-3p is a positive regulatory factor in OS
radiosensitivity by H2AX, and it is worthwhile to study the
function of miRNAs to treat cancer by regulating downstream
elements. miR-328-3p shows promise as a new potential therapy
target in OS.
Acknowledgements
This study was supported by The Youth Fund of
Guizhou Provincial People's Hospital (GZSYQN[2015]06) and the
National Natural Science Foundation of China (31660265).
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers PA and Gorlick R: Osteosarcoma.
Pediatr Clin North Am. 44:973–989. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer. 75 Suppl:203–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wittig JC, Bickels J, Priebat D, Jelinek
J, Kellar-Graney K, Shmookler B and Malawer MM: Osteosarcoma: A
multidisciplinary approach to diagnosis and treatment. Am Fam
Physician. 65:1123–1132. 2002.PubMed/NCBI
|
|
6
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwarz R, Bruland O, Cassoni A, Schomberg
P and Bielack S: The role of radiotherapy in oseosarcoma. Cancer
Treat Res. 152:147–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anderson PM, Wiseman GA, Erlandson L,
Rodriguez V, Trotz B, Dubansky SA and Albritton K: Gemcitabine
radiosensitization after high-dose samarium for osteoblastic
osteosarcoma. Clin Cancer Res. 11:6895–6900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonner WM, Redon CE, Dickey JS, Nakamura
AJ, Sedelnikova OA, Solier S and Pommier Y: GammaH2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bassing CH and Alt FW: H2AX may function
as an anchor to hold broken chromosomal DNA ends in close
proximity. Cell Cycle. 3:149–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei L, Hu Q, Peng J, Ruan J, Zou J, Huang
Q, Liu S and Wang H: Phospho-histone H2AX is a diagnostic and
prognostic marker for epithelial ovarian cancer. Int J Clin Exp
Pathol. 8:5597–5602. 2015.PubMed/NCBI
|
|
14
|
van Attikum H and Gasser SM: Crosstalk
between histone modifications during the DNA damage response.
Trends Cell Biol. 19:207–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Penna E, Orso F, Cimino D, Vercellino I,
Grassi E, Quaglino E, Turco E and Taverna D: miR-214 coordinates
melanoma progression by upregulating ALCAM through TFAP2 and
miR-148b downmodulation. Cancer Res. 73:4098–4111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZS, Zhong M, Bian YH, Mu YF, Qin SL,
Yu MH and Qin J: MicroRNA-187 inhibits tumor growth and invasion by
directly targeting CD276 in colorectal cancer. Oncotarget.
7:44266–44276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q, Yang G and Qian Y: Loss of
MicroRNA-489-3p promotes osteosarcoma metastasis by activating
PAX3-MET pathway. Mol Carcinog. 56:1312–1321. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, He J, Su F, Ding N, Hu W, Yao B,
Wang W and Zhou G: Repression of ATR pathway by miR-185 enhances
radiation-induced apoptosis and proliferation inhibition. Cell
Death Dis. 4:e6992013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin YY, Chen QJ, Wei Y, Wang YL, Wang ZW,
Xu K, He Y and Ma HB: Upregulation of microRNA-98 increases
radiosensitivity in esophageal squamous cell carcinoma. J Radiat
Res (Tokyo). 57:468–476. 2016. View Article : Google Scholar
|
|
21
|
Wang X, Li Q, Jin H, Zou H, Xia W, Dai N,
Dai XY, Wang D, Xu CX and Qing Y: miR-424 acts as a tumor
radiosensitizer by targeting aprataxin in cervical cancer.
Oncotarget. 7:77508–77515. 2016.PubMed/NCBI
|
|
22
|
Zhang YH, Wang QQ, Li H, Ye T, Gao F and
Liu YC: miR-124 radiosensitizes human esophageal cancer cell TE-1
by targeting CDK4. Genet Mol Res. 15:2016.
|
|
23
|
Song L, Liu S, Zhang L, Yao H, Gao F, Xu D
and Li Q: MiR-21 modulates radiosensitivity of cervical cancer
through inhibiting autophagy via the PTEN/Akt/HIF-1alpha feedback
loop and the Akt-mTOR signaling pathway. Tumour Biol.
37:12161–12168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao A, Zhao Q, Zhou X, Sun C, Si J, Zhou
R, Gan L and Zhang H: MicroRNA-449a enhances radiosensitivity by
downregulation of c-Myc in prostate cancer cells. Sci Rep.
6:273462016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin SM, Xia Q, Zhang YQ, Sun AM, Shi YS,
Zheng L and Chen LH: miR-124 regulates radiosensitivity of
colorectal cancer cells by targeting PRRX1. Nan Fang Yi Ke Da Xue
Xue Bao. 36:1110–1116. 2016.(In Chinese). PubMed/NCBI
|
|
26
|
Chen S, Wang Y, Ni C, Meng G and Sheng X:
HLF/miR-132/TTK axis regulates cell proliferation, metastasis and
radiosensitivity of glioma cells. Biomed Pharmacother. 83:898–904.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian Z, Zhang L, Chen J, Li Y, Kang K, Qu
J, Wang Z, Zhai Y, Li L and Gou D: MiR-328 targeting PIM-1 inhibits
proliferation and migration of pulmonary arterial smooth muscle
cells in PDGFBB signaling pathway. Oncotarget. 7:54998–55011. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weng JH, Yu CC, Lee YC, Lin CW, Chang WW
and Kuo YL: miR-494-3p induces cellular senescence and enhances
radiosensitivity in human oral squamous carcinoma cells. Int J Mol
Sci. 17:E10922016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo P, Lan J, Ge J, Nie Q, Guo L, Qiu Y
and Mao Q: MiR-26a enhances the radiosensitivity of glioblastoma
multiforme cells through targeting of ataxia-telangiectasia
mutated. Exp Cell Res. 320:200–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye C, Sun NX, Ma Y, Zhao Q, Zhang Q, Xu C,
Wang SB, Sun SH, Wang F and Li W: MicroRNA-145 contributes to
enhancing radiosensitivity of cervical cancer cells. FEBS Lett.
589:702–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng DD, Yu T, Hu T, Yao M, Fan CY and
Yang QC: MiR-542-5p is a negative prognostic factor and promotes
osteosarcoma tumorigenesis by targeting HUWE1. Oncotarget.
6:42761–42772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen L, Wang P, Yang J and Li X:
MicroRNA-217 regulates WASF3 expression and suppresses tumor growth
and metastasis in osteosarcoma. PLoS One. 9:e1091382014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu J, Lv G, Zhou S, Zhou Y, Nie B, Duan H,
Zhang Y and Yuan X: The downregulation of miR-182 is associated
with the growth and invasion of osteosarcoma cells through the
regulation of TIAM1 expression. PLoS One. 10:e01211752015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Feng Y, Shen JK, Lin M, Choy E,
Cote GM, Harmon DC, Mankin HJ, Hornicek FJ and Duan Z: CD44 is a
direct target of miR-199a-3p and contributes to aggressive
progression in osteosarcoma. Sci Rep. 5:113652015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Niu G, Li B, Sun L and An C: MicroRNA-153
inhibits osteosarcoma cells proliferation and invasion by targeting
TGF-β2. PLoS One. 10:e01192252015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernandez-Capetillo O, Lee A, Nussenzweig
M and Nussenzweig A: H2AX: The histone guardian of the genome. DNA
Repair (Amst). 3:959–967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stucki M and Jackson SP: gammaH2AX and
MDC1: Anchoring the DNA-damage-response machinery to broken
chromosomes. DNA Repair (Amst). 5:534–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pinder JB, Attwood KM and Dellaire G:
Reading, writing, and repair: The role of ubiquitin and the
ubiquitin-like proteins in DNA damage signaling and repair. Front
Genet. 4:452013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao XH, Zheng L, He HP, Zheng DL, Wei ZQ,
Wang N, Dong J, Ma WJ and Zhang TC: STAT3 regulated ATR via
microRNA-383 to control DNA damage to affect apoptosis in A431
cells. Cell Signal. 27:2285–2295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikura M, Furuya K, Matsuda S, Matsuda R,
Shima H, Adachi J, Matsuda T, Shiraki T and Ikura T: Acetylation of
histone H2AX at Lys 5 by the TIP60 histone acetyltransferase
complex is essential for the dynamic binding of NBS1 to damaged
chromatin. Mol Cell Biol. 35:4147–4157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Huang JW, Li M, Cavenee WK,
Mitchell PS, Zhou X, Tewari M, Furnari FB and Taniguchi T:
MicroRNA-138 modulates DNA damage response by repressing histone
H2AX expression. Mol Cancer Res. 9:1100–1111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Yuan C, Lv K, Xie S, Fu P, Liu X,
Chen Y, Qin C, Deng W and Hu W: Lin28 mediates radiation resistance
of breast cancer cells via regulation of caspase, H2A.X and Let-7
signaling. PLoS One. 8:e673732013. View Article : Google Scholar : PubMed/NCBI
|