Introduction
Retinoblastoma (RB) is the most common intraocular
malignant tumor in childhood with a high mortality rate, especially
in developing countries (1,2). Although great efforts have been made
in the treatment of RB in recent years, the survival rate remains
poor mainly due to limitations in the early diagnosis of the
disease and the development of metastasis (3). Therefore, it is crucial to explore the
key molecular mechanisms involved in RB initiation and development
to identify new diagnostic markers and therapeutic targets.
MicroRNAs (miRNAs) are small (18–25 nucleotide
long), non-coding RNAs that regulate gene expression via binding to
the 3′-untranslated region (3′UTR) of target mRNAs, leading to mRNA
degradation or translational inhibition (4,5). It
has been demonstrated that miRNAs are involved in diverse
biological processes, including metabolic homeostasis, cell
proliferation and cell apoptosis (6,7).
Accumulating evidence shows that the altered expression of miRNAs
are involved in the initiation and progression of cancer (8), suggested that miRNAs can serve as
diagnostic markers and therapeutic targets in human cancers. miRNAs
have now been identified to serve as either tumor suppressors or as
oncogenes in RB by exerting effects on important regulatory
cellular pathways (9,10).
An accumulating body of evidence shows that
microRNA-29a (miR-29a) expression is dysregulated and plays crucial
roles in progression and development of multiple cancers (11–16).
Yet, the role and underlying mechanism of miR-29a in RB cells
remain unclear. The aims of the present study were to investigate
miR-29a expression and clinical significance, and determine the
biological function of miR-29a in RB and explore the possible
regulating mechanisms in RB cells.
Materials and methods
Human tissue samples and cell
lines
Twenty human RB specimens and 5 retina tissues were
obtained from patients with RB and ruptured globe at the Department
of Ophthalmology, the Second Hospital of Jilin University,
respectively. All patients had not received preoperative
radiotherapy and/or chemotherapy prior to enucleation. All tissues
were stored in liquid nitrogen until RNA isolation. This study was
approved by the Ethics Committee of Jilin University. Written
informed consent was obtained from each patient.
Two human RB cell lines (Y79 and SO-RB50) were
obtained from the Institute of Biochemistry and Cell Biology of the
Chinese Academy of Sciences (Shanghai, China). All cells were
maintained at 37°C in a humidified 5% CO2 atmosphere in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, and 100 mg/ml
streptomycin.
Plasmid, microRNA mimic, and
transfection
Full-length STAT3 (without the 3′UTR) was amplified
by PCR and inserted into the eukaryotic expression vector pcDNA3.1
(+) (Invitrogen). The STAT3 3′UTR target site for miR-29a was
amplified by PCR, and subcloned into the pGL3-control vector
(Ambion, Austin, TX, USA) and named WT-STAT3-3′UTR. Quick-Change
Mutagenesis kit (Stratagene, Heidelberg, Germany) was used for
mutagenesis of the miR-29a target-site in the STAT3 3′UTR, and was
referred to as MT-STAT3-3′UTR. The miR-29a mimic or corresponding
negative control (miR-NC) were chemically synthesized by GenePharma
Co. (Shanghai, China). For the transfection experiments,
2×105 RB cells were seeded in a 6-cm dish, and cultured
in RPMI-1640 with 10% FBS up to 70–80% confluence. Then
transfection was performed using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's
instructions.
RNA extraction and quantitative
real-time PCR (qRT-PCR)
Total RNA was isolated from tissue samples and cell
lines by the TRIzol reagent (Invitrogen) following the
manufacturer's protocol. For miR-29a expression analysis, total RNA
was reverse transcribed to cDNA using the microRNA reverse
transcription kit (Takara, Dalian, China), and then were quantified
with SYBR miRNA detection assays (Takara) under an Applied
Biosystems 7500 HT system (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) using primers for U6 and miR-29a (Applied Biosystems). For
STAT3 mRNA detection, total RNA was reverse transcribed to
cDNA using the Prime-Script RT reagent kit (Takara), and then were
quantified using SYBR Premix Ex Taq (Takara). The primers for STAT3
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used in this
study were described previously (17). U6 and GAPDH were used as control for
miR-29A and STAT3 mRNA, respectively using the
2−ΔΔCT method.
Cell proliferation, cell cycle
distribution, apoptosis, migration and invasion analyses
3-(4,5-Dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay was performed to
determine cell proliferation. Briefly, 5×103 transfected
cells were seeded into a 96-well plate, and cultured for 24–72 h.
At the indicated time, 20 µl MTT solution (5 mg/ml, Sigma-Aldrich,
St. Louis, MO, USA) was added to each well and additionally
cultured for 4 h. The MTT solution was removed and 150 µl dimethyl
sulfoxide (DMSO, Sigma-Aldrich) was added to each well. Optical
density (OD) was detected at the wavelength of 570 using a
Benchmark Plus™ microplate spectrometer (Bio-Rad, Hercules, CA,
USA).
For cell cycle and apoptosis assays, the RB cells
were harvested 48 h after transfection, and washed with PBS. Then
5×104 cells were resuspended in 500 µl of binding buffer
containing 5 µl of Annexin V-fluorescein isothiocyanate (FITC) and
5 µl of propidium iodide (PI). Cell cycle arrest was detected using
FACS-Calibur™ flow cytometer (BD Biosciences, San Jose, CA, USA),
and was analyzed by CellQuest software (BD Biosciences). Cell
apoptosis was determined using Annexin V-FITC Apoptosis Detection
kit (KeyGEN, Shanghai, China) according to the instructions of the
manufacturer. The data were analyzed with FlowJo v5.7.2 software
(BD Biosciences).
For cell migration, the transfected cells were
seeded into 24-well dishes and cultured to near (>80%)
confluence. Then an artificial homogeneous wound was created with a
sterile pipette tip onto the monolayer, and cultured for 24 h.
Cells were imaged using inverted Nikon Eclipse TS100 phase-contrast
microscope (Tokyo, Japan) at 0 and 24 h after the wounding. Wound
closure was determined using Nikon NIS-Element Basic Research v3.2
software (Tokyo, Japan).
Transwell chamber assay was performed to analyze
cell invasion. Matrigel was employed to pre-coat the membrane of
Transwells to simulate a matrix barrier for the invasion assay. The
transfected cells growing in the log phase were seeded on the upper
champers at a density of 2×105 cells/well. Medium with
10% FBS was added to the lower chamber to stimulate cell invasion.
After 24 h of incubation, cells which migrated to the lower chamber
were fixed with paraformaldehyde for 5 min, and stained with 0.1%
crystal violet for 5 min. The images of cells were photographed
with a TS100 inverted microscope (Nikon) at ×200 magnification, and
the cell number was counted in five selected randomly fields per
membrane.
miRNA target prediction and luciferase
reporter assay
Three online databases for target site predictions
(TargetScan7.1, PicTar and miRDB) were used to predict the putative
targets of miR-29a. For the luciferase reporter assay, RB cells
were cotransfected with miR-29a mimic or miR-NC and WT-STAT3-3′UTR
or MT-STAT3-3′UTR reporter plasmid by Lipofectamine 2000. The cells
were lysed and luciferase activities were detected 48 h after
transfection using a Dual-Luciferase® Reporter Assay kit
(Promega, Madison, WI, USA) according to the manufacturer's
protocols. Renilla luciferase activity was normalized to
firefly luciferase activity.
Western blotting
RB cells and tissues were harvested and lysed with
RIPA buffer (Beyotime, Jiangsu, China), following by quantification
with the BCA protein assay kit (Pierce, Bonn, Germany). The
proteins were separated by 10% SDS-polyacrylamide gel
electrophoresis, and subsequently transferred to polyvinylidene
difluoride (PVDF) membranes (Merck, Millipore, Germany). The
membranes were blocked with 5% nonfat milk and then incubated with
the following primary antibodies overnight at 4°C: anti-STAT3
(1:1,000, cat. no. sc-293151), anti-Bcl-2 (1:1,000, cat. no.
sc-56015), anti-cyclin D1 (1:1,000, cat. no. sc-70899; all from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-MMP2
(1:500, cat. no. 40994; Cell Signaling Technology, Danvers, MA,
USA) or anti-GAPDH (1:3,000, cat. no. sc-365062; Santa Cruz
Biotechnology, Inc.). The blots were washed with PBST and incubated
with horseradish peroxidase (HRP)-conjugated corresponding
secondary antibody for 2 h at room temperature. Protein bands were
observed using an enhanced chemiluminescence system (Thermo Fisher
Scientific, Inc.) and exposed to X-ray film. GAPDH was used as a
control.
Xenograft tumor model
BALB/c-nu mice (5–6 weeks of age and weighing 20–25
g) were purchased from the Experimental Animal Center of Jilin
University, and their care was in accordance with institutional
guidelines. Stable cell lines with high expression of miR-29a were
established by transfecting RB cells with the miR-29a mimic. Mice
were inoculated subcutaneously with 2×106 Y79 cells
expressing miR-NC (Y79/miR-NC cells) in the left dorsal flank and
2×106 Y79 cells expressing miR-29a (Y79/miR-29a cells)
in the right dorsal flank. Tumor volume was determined once every
week from the first injection until sacrifice according to the
following formula: V = (LxW2/2 (where V is the volume;
L, length; W, width of tumor). On day 35 day, the mice were
sacrificed, and the tumors were stripped, and the wet weight of
each tumor was determined. Tumor tissues were stored at −80°C for
further analysis.
Statistical analysis
SPSS statistical software for Windows version 19
(SPSS, Chicago, IL, USA) and GraphPad Prism 5.01 (GraphPad
Software, Inc, San Diego, CA, USA) software were used for
statistical analysis. All data are represented as the mean ± SD
(standard deviation) from at least three independent experiments.
Comparisons between the groups were analyzed with two-tailed
Student's t-test or one-way ANOVA. Spearman's rank correlation
analysis was performed to analyze correlation between miR-29a and
STAT3. A P<0.05 was considered to indicate a statistically
significant result.
Results
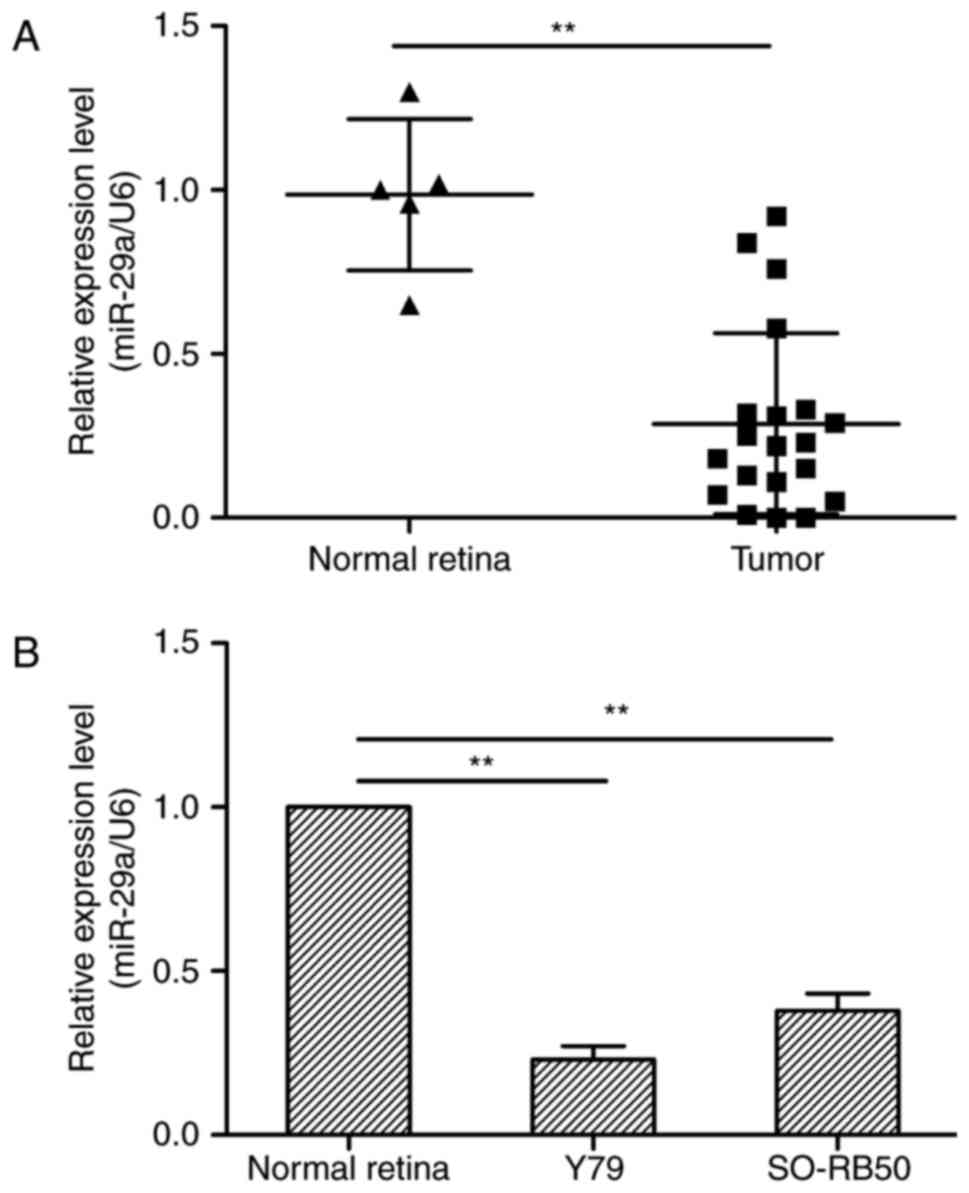
miR-29a is downregulated in RB tissues
and RB cell lines
To investigate the expression status of miR-29a in
RB and RB cell lines, real-time RT-PCR (qRT-PCR) was conducted.
Expression of miR-29a was lower in the 20 RB tissues and 2 RB cell
lines than that observed in the 5 normal retinal tissues
(P<0.05; Fig. 1), suggesting
that the downregulation of miR-29a may be involved in human RB
tumorigenesis.
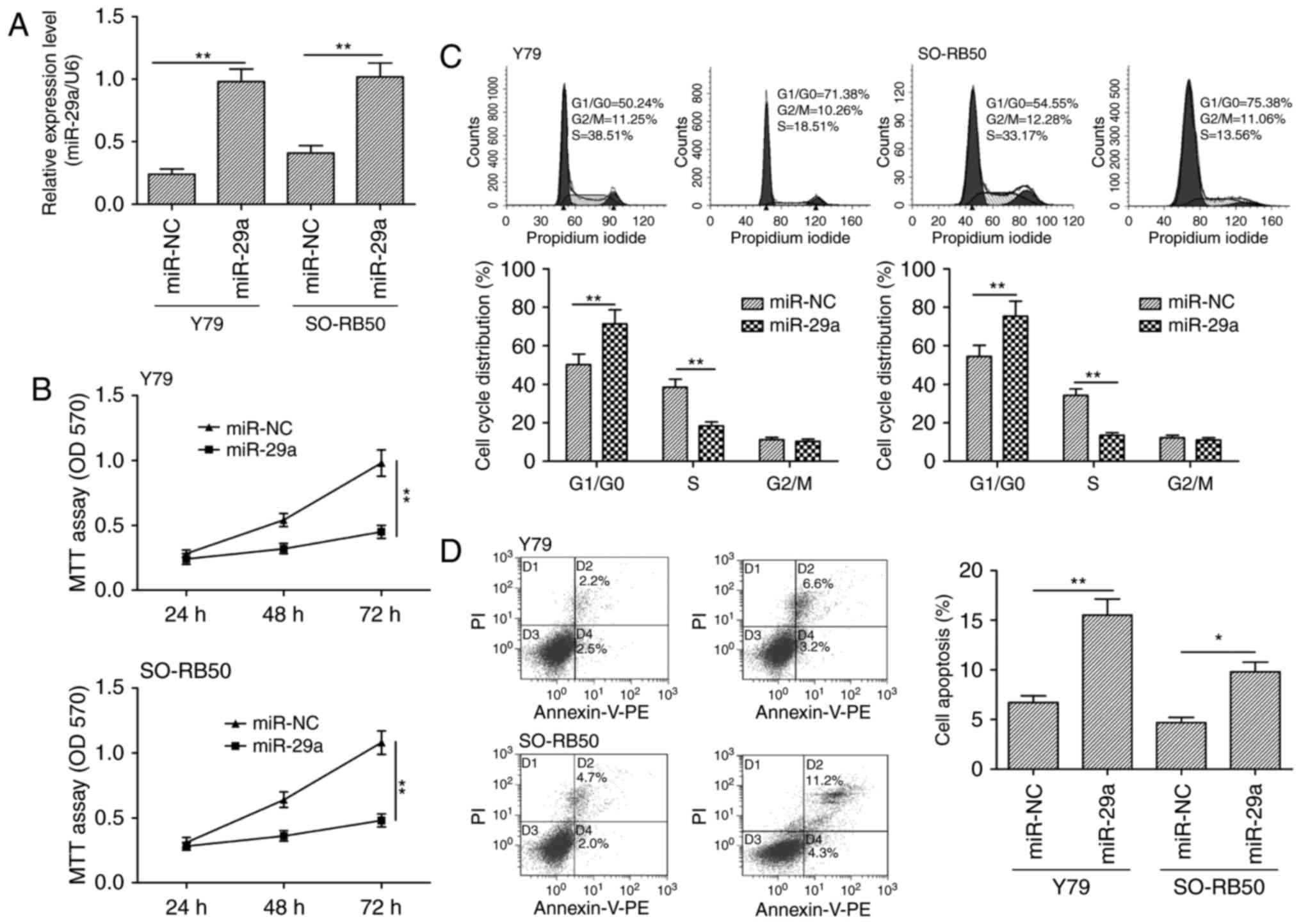
Overexpression of miR-29a inhibits
cell proliferation and promotes apoptosis in RB cells
To assess the biological effects of miR-29a on RB
cells, Y79 and SO-RB50 cells were transiently transfected with
miR-29a mimic and miR-NC, and then cell proliferation, cell cycle
distribution and apoptosis were determined. qRT-PCR confirmed
miR-29a overexpression in Y79 and SO-RB50 cells (Fig. 2A). The MTT assay showed that
restoration of miR-29a expression in Y79 and SO-RB50 cells
significantly inhibited cell proliferation (Fig. 2B). Since cell proliferation is
closely associated with cell cycle arrest, we investigated the
effect of miR-29a on the cell cycle. FACS analysis showed that
transfection of the Y79 and SO-RB50 cells with the miR-29a mimic
significantly increased the proportion of cells in the G0/G1 phase
and reduced the proportion of S phase cells compared to the cells
transfected with miR-NC (Fig. 2C).
In addition, we found that restoration of miR-29a in Y79 and
SO-RB50 cells significantly increased the cell apoptosis rate
(Fig. 2D).
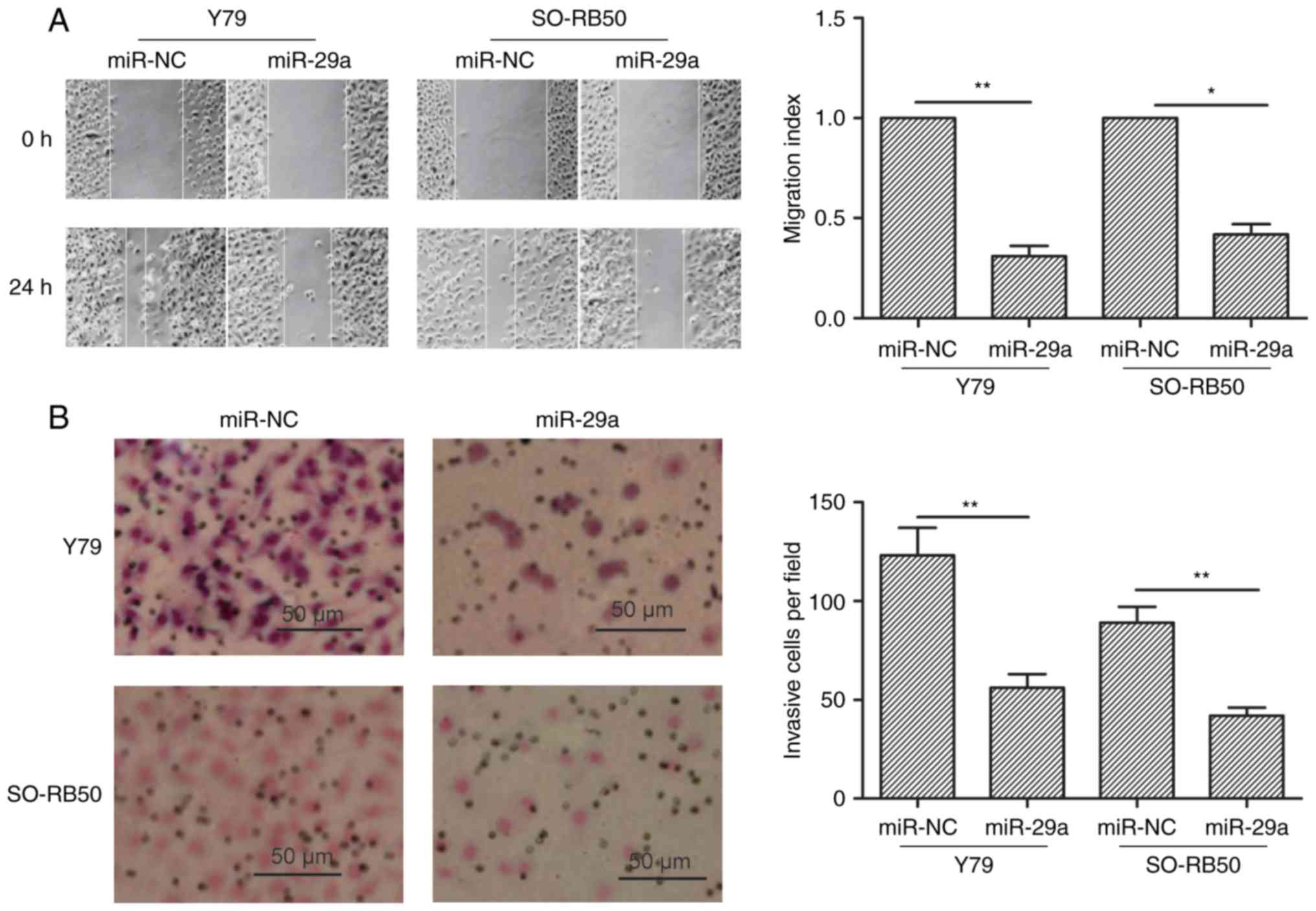
miR-29a inhibits the migration and
invasion of RB cells
To study the effect of miR-29a on migration and
invasion abilities, wound healing and invasion chamber assays were
performed in Y79 and SO-RB50 cells transfected with miR-29a or
miR-NC, respectively. It was found that restoration of miR-29a in
RB cells significantly decreased migration (Fig. 3A) and invasion (Fig. 3B) capacity.
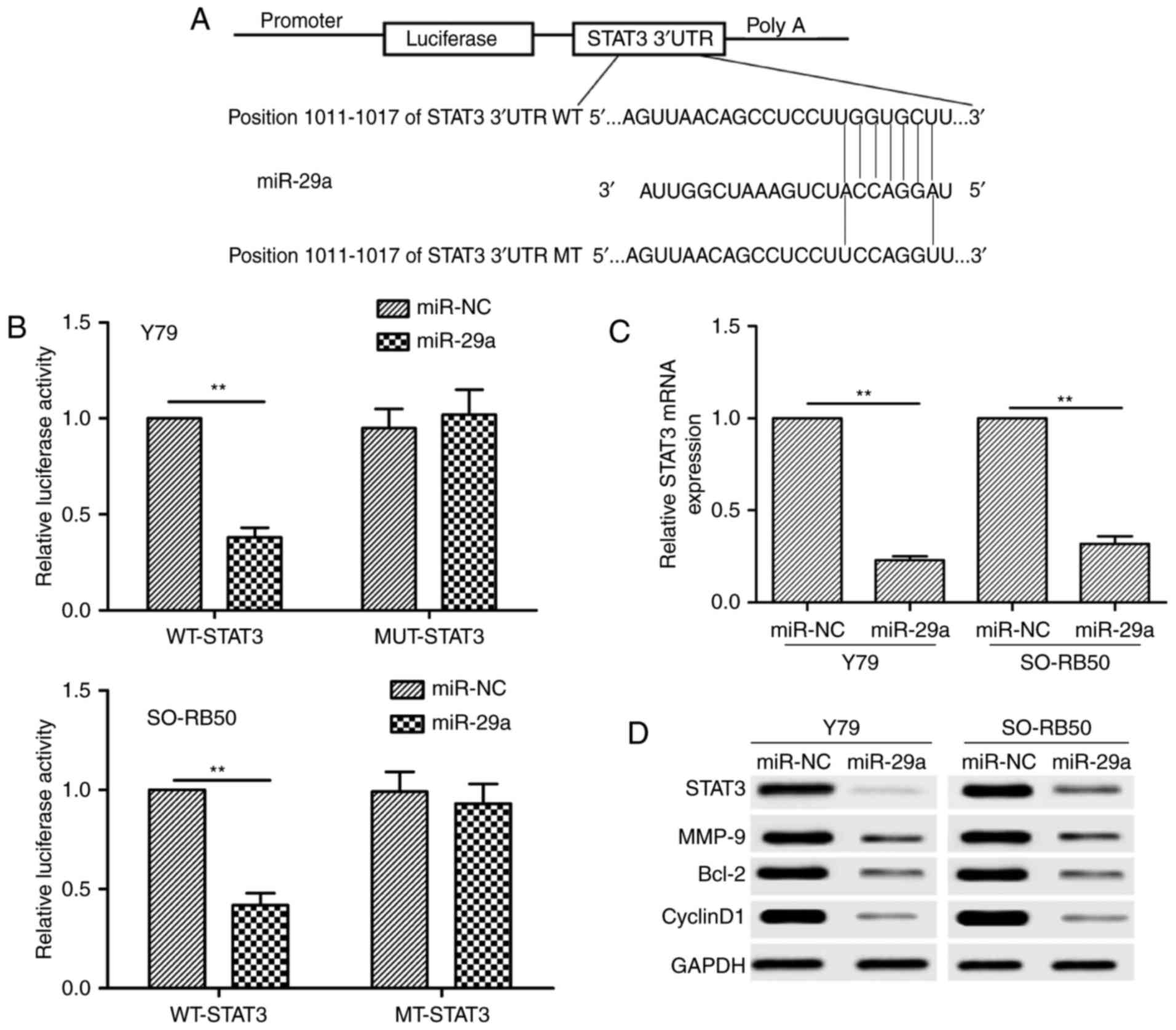
STAT3 is a direct target gene of
miR-29a in RB cells
Three miRNA databases (Targetscan, Pictar and
Miranda) were used to identify a putative miR-29a-binding site.
Among the candidate targets, the 3′UTR of human STAT3 contains a
putative region that matches to the seed sequence of miR-29a
(Fig. 4A). To confirm whether
miR-29a directly binds to STAT3, luciferase activity assay was
performed. It was found that miR-29a overexpression markedly
reduced the luciferase activity of the WT-STAT3-3′UTR, but
not the MT-STAT3-3′UTR in Y79 and SO-RB50 cells (Fig. 4B). To further confirm the effect of
miR-29a on STAT3 expression, we analyzed the STAT3 expression at
the mRNA and protein levels in Y79 and SO-RB50 cells transfected
with miR-29a mimic or miR-NC by qRT-PCR and western blot analysis,
respectively. We found that the STAT3 expression at the mRNA
and protein levels was decreased in the Y79 and SO-RB50 cells
transfected with miR-29a mimic compared with cells transfected with
miR-NC (Fig. 4C and D). We also
found that miR-29a overexpression also significantly inhibited
cyclin D1, Bcl-2 and MMP-9 expression, several downstream proteins
of STAT3, in the Y79 and SO-RB50 cells (Fig. 4D). These results indicate that STAT3
is a direct target of miR-29a in RB cells.
STAT3 expression is upregulated, and
inversely correlated with miR-29a expression in RB tissues
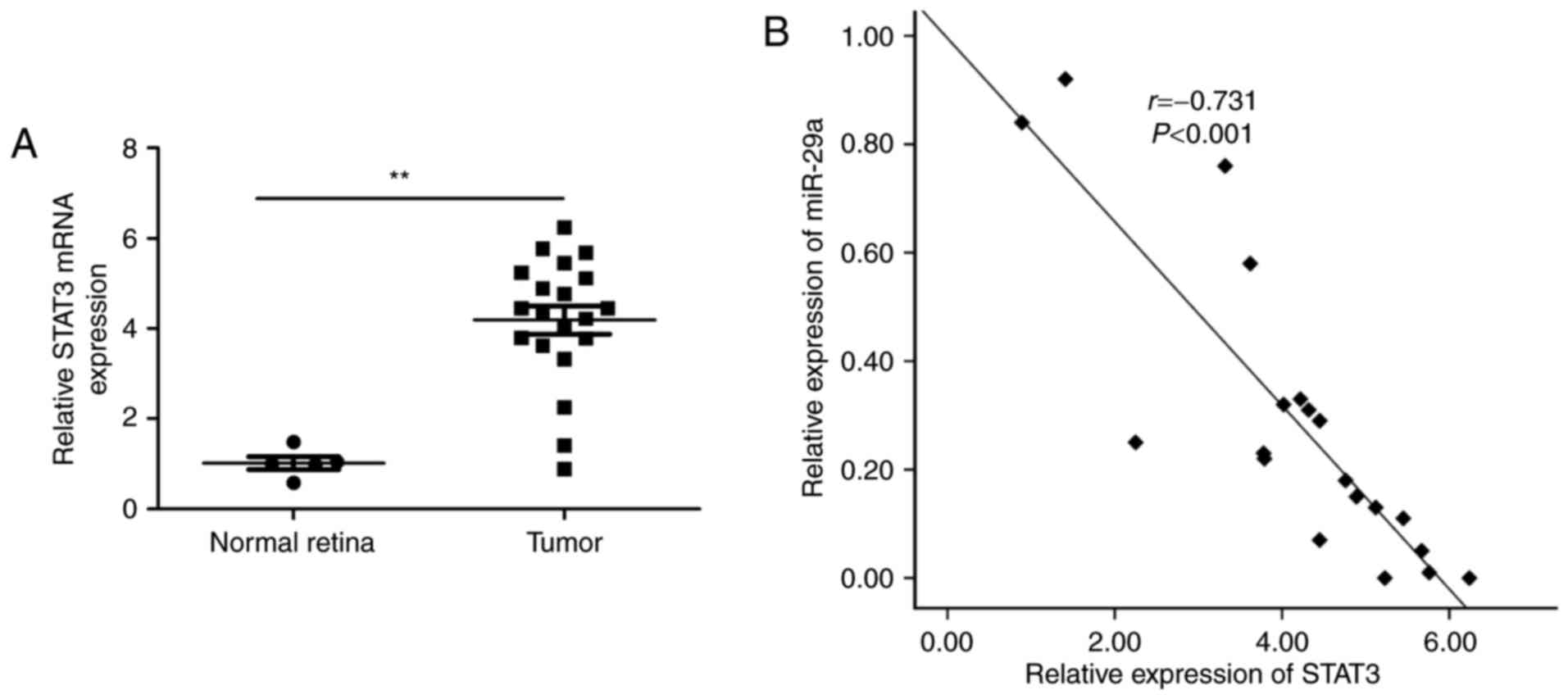
Next, we detected the STAT3 mRNA expression
in the 20 RB tissues and 5 normal retinal samples by qRT-PCR. We
found that RB tissues had a higher expression of STAT3 mRNA
relative to the normal retinal samples (Fig. 5A). In addition, a statistically
significant inverse correlation was found between the miR-29a level
and STAT3 mRNA level in RB tissues by Spearman's correlation
analysis (r=−0.731; P<0.001, Fig. 5B).
STAT3 overexpression reverses the
effect of miR-29a on RB cells
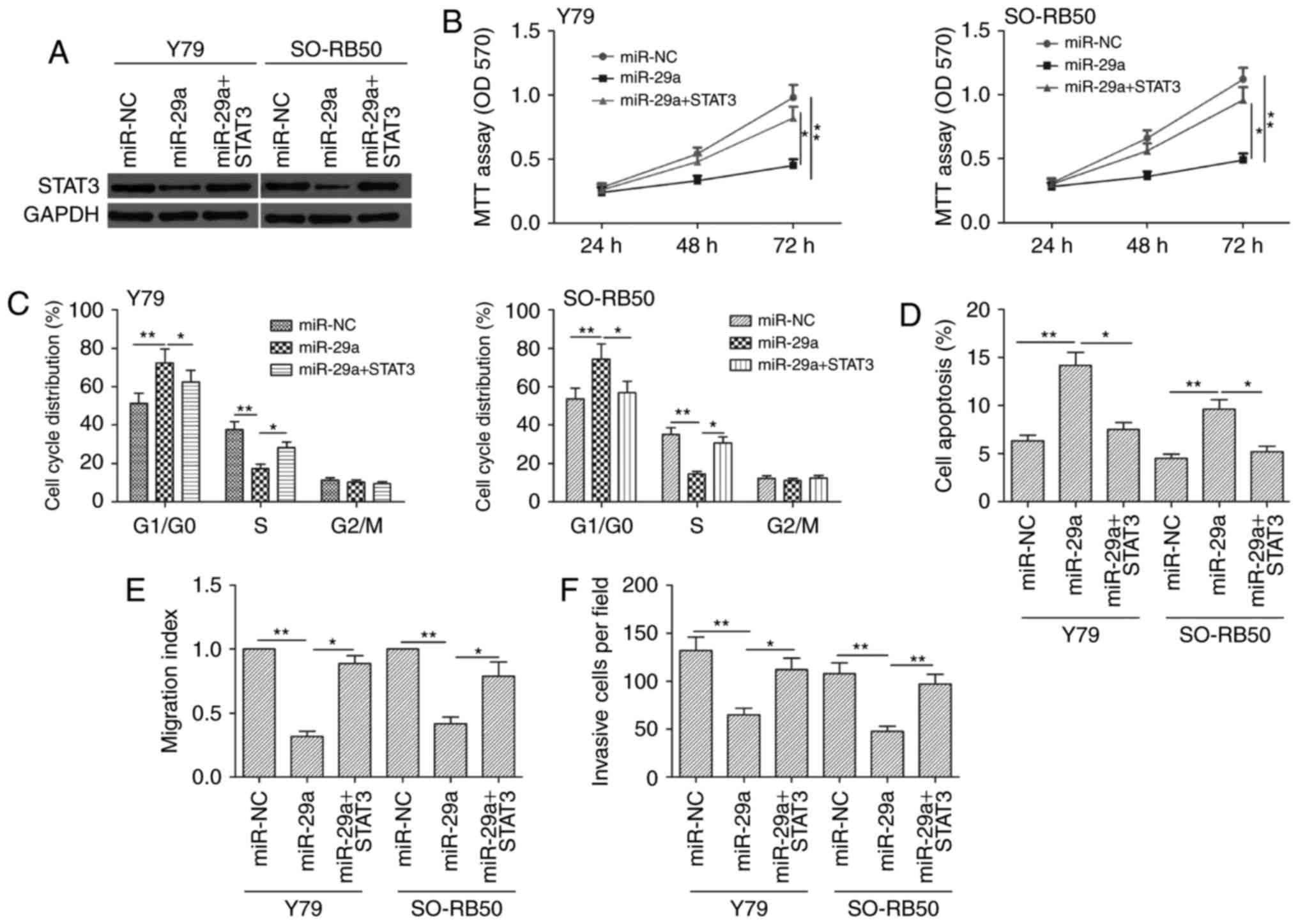
To further determine whether STAT3 is a
functional target of miR-29a in RB cells, we performed a rescue
experiment involving transfection of STAT3 plasmids (lack of 3′UTR)
into miR-29a-expressing RB cells. RB cells transfected with the
STAT3-overexpressing plasmid showed restoration of STAT3
expression, which was reduced via miR-29a overexpression (Fig. 6A). Moreover, STAT3 overexpression
reversed the effect of miR-29a on cell proliferation, cell cycle,
apoptosis, migration and invasion (Fig.
6B-F). Taken together, these results indicated that miR-29a
exerts its biological effect in RB by suppression of STAT3.
miR-29a inhibits tumor growth in a
mouse model
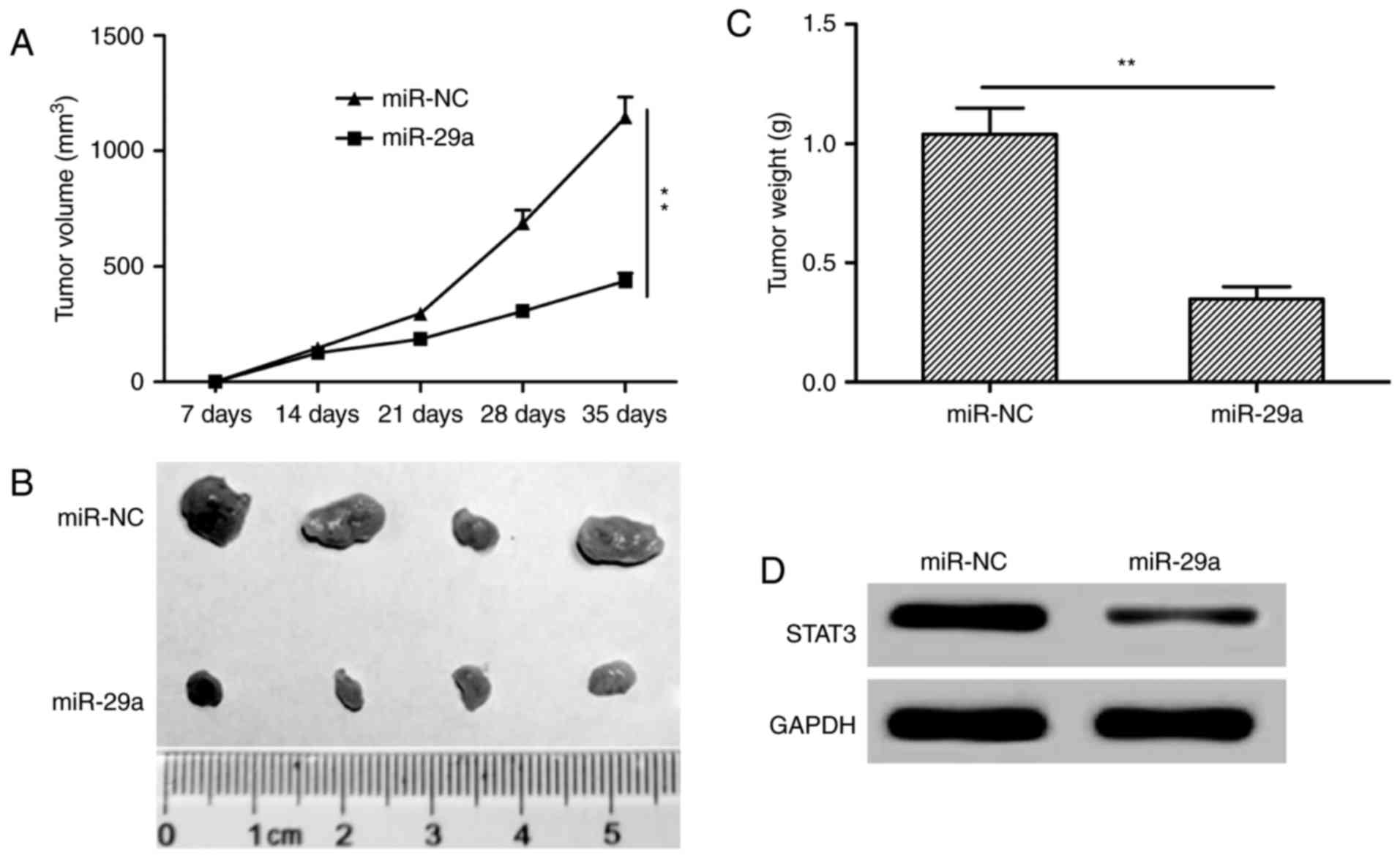
We assessed the in vivo therapeutic efficacy
of miR-29a in BALB mice. Y79 cells transfected with miR-29a
mimic or miR-NC were subcutaneously injected into the flank regions
of nude mice. We found that tumor growth was slower in the miR-29a
group compared to the miR-NC group (Fig. 7A). On day 35, the mice were
sacrificed, tumors were removed and weighed. The tumor size and
weight in the miR-29a group were significantly decreased compared
to these parameters in the miR-NC group (Fig. 7B and C). We also detected STAT3
protein expression in tumor tissues of nude mice, and found that
STAT3 expression was decreased in the miR-29a group (Fig. 7D). Taken together, these data showed
that miR-29a inhibits tumorigenicity in vivo.
Discussion
It is well documented that various microRNAs are
involved in the initiation and development of RB by acting as
oncogenes or tumor-suppressor genes (9,10). For
example, Liu et al reported that ectopic expression of
miR-124 significantly suppressed cell proliferation, colony
formation, migration and invasion, induced cell apoptosis in the RB
cells by repressing STAT3 (18). Wu
et al found that enforced expression of miR-204 in RB cells
inhibited proliferation and invasion in vitro and suppressed
tumor growth in vivo by targeting cyclin D2 and MMP-9
(19). Martin et al
demonstrated that overexpression of miR-449a and miR-449b in RB
cells significantly impaired proliferation and increased apoptosis
of tumor cells (20). Gui et
al showed that mIR-21 functions as an oncogene in RB via
regulation of the PTEN/PI3K/AKT pathway (21). In the present study, we found that
RB tissues and cell lines had higher expression of miR-29a compared
with that noted in normal retinal tissues. Function assays
demonstrated that restoration of miR-29a in RB cells impaired cell
proliferation, migration and invasion, and induced cell apoptosis
in vivo, as well as impaired tumor growth in vivo.
These results support the conclusion that miR-29a plays a crucial
role in RB progression.
miR-29a, located on chromosome 7q32 (22), has been reported in the literature
as playing a tumor suppressive role and exerting inhibitory effects
on cell proliferation, migration, and invasion in a subset of
cancer (12–16). However, the biological function and
underlying mechanism of miR-29a in RB have not been explored. Here,
our results showed that miR-29a expression in RB specimens was
significantly lower than that in normal retinal tissues.
Interestingly, the expression of miR-29a in human RB cell lines was
also downregulated compared to normal retinal tissues.
Subsequently, we found that overexpression of miR-29a in RB cells
inhibited cell proliferation and promoted cell apoptosis, as well
as decreased the migration and invasion capacity of RB cells in
vitro. Furthermore, we observed that miR-29a overexpression
decreased tumor growth in vivo. These results suggested that
miR-29a functios as a tumor suppressor in RB.
It is well documented that miRNAs exert their
biological function in cancer by regulating expression of their
downstream target genes (23).
Through three target prediction programs (TargetScan7.1, PicTar and
miRDB) putative miR-29a targets were predicted. Our analysis
suggests that STAT3 is a potential target of miR-29a. Subsequently,
STAT3 was identified as a potential functional target of miR-29a in
RB by luciferase assay, qRT-PCR and western blot analysis. STAT3
has been reported to be involved in the development and progression
of cancer by regulating cell proliferation, migration, invasion,
cell apoptosis and cell cycle arrest (24,25).
Importantly, STAT3 exerts its biological role by regulating
multiple downstream gene, such as cyclin D1 (cell cycle related
gene), survivin, Bcl-xL, Bcl-2 (cell apoptosis-related gene), VEGF,
and MMP-2 and MMP-9 (cell invasion-related gene) (24–28).
Recently, a study showed that STAT3 expression was upregulated in
RB tissues, and that knockdown of STAT3 inhibited cell
proliferation in vitro, and suppressed tumor growth in
vivo (28,29). A recently study revealed that STAT3
was regulated by miR-124 in RB (18). In this study, we found that miR-29a
overexpression significantly inhibited STAT3 expression and
expression of its downstream genes cyclin D1, Bcl-2 and MMP-9. In
addition, we found that miR-29a was inversely correlated with
STAT3 mRNA in human RB tissues. Overexpression of STAT3
partially reversed the effects of miR-29a on RB cell proliferation,
cell cycle, apoptosis, migration and invasion. Our in vivo
study also confirmed that miR-29a suppressed tumor growth in nude
mice by repressing STAT3. These observations provide the first line
of evidence, to the best of our knowledge, that miR-29a exerts it
tumor suppressor role in RB via the regulation of STAT3.
In summary, our findings confirmed an inhibitory
effect of miR-29a in RB by demonstrating significantly impaired
proliferation, migration, invasion and promotion of apoptosis in
tumor cells by suppressing STAT3, suggesting that miR-29a could be
a potential therapeutic target for RB in the future.
Acknowledgements
The present study was funded bythe Finance
Department of Jilin Province (3D5177873429).
References
|
1
|
Shields CL and Shields JA: Retinoblastoma
management: advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jabbour P, Chalouhi N, Tjoumakaris S,
Gonzalez LF, Dumont AS, Chitale R, Rosenwasser R, Bianciotto CG and
Shields C: Pearls and pitfalls of intraarterial chemotherapy for
retinoblastoma. J Neurosurg Pediatr. 10:175–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malan-Müller S, Hemmings SM and Seedat S:
Big effects of small RNAs: A review of microRNAs in anxiety. Mol
Neurobiol. 47:726–739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y and Mei Q: miRNA signature
identification of retinoblastoma and the correlations between
differentially expressed miRNAs during retinoblastoma progression.
Mol Vis. 21:1307–1317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beta M, Venkatesan N, Vasudevan M,
Vetrivel U, Khetan V and Krishnakumar S: Identification and
insilico analysis of retinoblastoma serum microRNA profile and gene
targets towards prediction of novel serum biomarkers. Bioinform
Biol Insights. 7:21–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei YF, Lei Y and Liu XQ: MiR-29a promotes
cell proliferation and EMT in breast cancer by targeting ten eleven
translocation 1. Biochim Biophys Acta. 1862:2177–2185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Cui Y, Zhou Y, Zhou K, Qiao X, Li C
and Wang S: MicroRNA-29a plays a suppressive role in non-small cell
lung cancer cells via targeting LASP1. Onco Targets Ther.
9:6999–7009. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y,
Song C, Zhu S, Leng Y, Wang G, et al: MicroRNA-29a promotes
colorectal cancer metastasis by regulating matrix metalloproteinase
2 and E-cadherin via KLF4. Br J Cancer. 110:450–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Xiao YF, Tang B, Wu YY, Hu CJ, Xie
R, Yang X, Yu ST, Dong H, Zhao XY, et al: hTERT mediates gastric
cancer metastasis partially through the indirect targeting of ITGB1
by microRNA-29a. Sci Rep. 6:219552016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahati S, Xiao L, Yang Y, Mao R and Bao Y:
miR-29a suppresses growth and migration of hepatocellular carcinoma
by regulating CLDN1. Biochem Biophys Res Commun. 486:732–737. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Liu J, Li Q, Chen G and Yu X:
miR-29a suppresses growth and metastasis in papillary thyroid
carcinoma by targeting AKT3. Tumour Biol. 37:3987–3996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Li Y, Nian Y, Liu D, Dai F and
Zhang J: STAT3 is involved in miR-124-mediated suppressive effects
on esophageal cancer cells. BMC Cancer. 15:3062015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Hu C, Wang Y, Shi G, Li Y and Wu H:
miR-124 inhibits proliferation and invasion of human retinoblastoma
cells by targeting STAT3. Oncol Rep. 36:2398–2404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting Cyclin D2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin A, Jones A, Bryar PJ, Mets M,
Weinstein J, Zhang G and Laurie NA: MicroRNAs-449a and −449b
exhibit tumor suppressive effects in retinoblastoma. Biochem
Biophys Res Commun. 440:599–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gui F, Hong Z, You Z, Wu H and Zhang Y:
MiR-21 inhibitor suppressed the progression of retinoblastoma via
the modulation of PTEN/PI3K/AKT pathway. Cell Biol Int.
40:1294–1302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mott JL, Kurita S, Cazanave SC, Bronk SF,
Werneburg NW and Fernandez-Zapico ME: Transcriptional suppression
of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J
Cell Biochem. 110:1155–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Catela Ivkovic T, Voss G, Cornella H and
Ceder Y: microRNAs as cancer therapeutics: A step closer to
clinical application. Cancer Lett. 407:113–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chai EZ, Shanmugam MK, Arfuso F,
Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC,
et al: Targeting transcription factor STAT3 for cancer prevention
and therapy. Pharmacol Ther. 162:86–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masuda M, Suzui M, Yasumatu R, Nakashima
T, Kuratomi Y, Azuma K, Tomita K, Komiyama S and Weinstein IB:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with Cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.PubMed/NCBI
|
|
27
|
Kim BH, Yi EH and Ye SK: Signal transducer
and activator of transcription 3 as a therapeutic target for cancer
and the tumor microenvironment. Arch Pharm Res. 39:1085–1099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cafferkey C and Chau I: Novel STAT 3
inhibitors for treating gastric cancer. Expert Opin Investig Drugs.
25:1023–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jo DH and Kim JH, Cho CS, Cho YL, Jun HO,
Yu Y, Min JK and Kim JH: STAT3 inhibition suppresses proliferation
of retinoblastoma through down-regulation of positive feedback loop
of STAT3/miR-17-92 clusters. Oncotarget. 5:11513–11525. 2014.
View Article : Google Scholar : PubMed/NCBI
|