Introduction
Prolactin (PRL) is a peptidic hormone that has been
shown to play multiple biological functions. In addition to its
clear role in lactation, PRL participates in some cellular
processes such as proliferation, growth and differentiation
(1). PRL can modulate the immune
system, being involved in alterations of both cellular and humoral
responses (2,3). For instance, PRL is able to influence
different immunological processes such as proliferation (4), antigen presentation (5) and immunoglobulin production (6).
Human PRL, besides being expressed in the pituitary
gland, is independently and differentially expressed by different
tissues such as breast, prostate, endometrium and immune cells
(7).
Several studies have described the modulating
effects of PRL involving disturbances in chronic inflammatory
processes such as autoimmune diseases (8). Low doses of PRL are able to induce the
production of proinflammatory cytokines such as TNF-α, IL-1β, IFN-γ
in murine peritoneal macrophages; however, higher doses of PRL
(1,000 ng/ml) induced IL-10 synthesis with significant inhibition
of proinflammatory cytokine production in the same cells (9,10). One
study showed that proinflammatory cytokines induced in vitro
PRLR expression in pulmonary fibroblasts (11).
Increased PRL levels have been shown in autoimmune
diseases, such as systemic lupus erythematosus (SLE), rheumatoid
arthritis (RA), systemic sclerosis and Sjögrensindrome (12–14).
Proinflammatory cytokines have the ability to induce pituitary PRL
secretion (15), which in turn may
contribute to circulating pool of the hormone. Several studies have
focused on the blockade of proinflammatory cytokines to prevent
autoimmune diseases in murine models (16–18),
which may be a possible therapeutic strategy.
The PRL protein can undergo post-translational
modifications that include glycosylation, phosphorylation,
polymerization and proteolytic cleavage, which can influence its
biological activities (19).
Pituitary PRL is a 23 kDa polypeptidic hormone; however, other
forms of PRL called macroprolactin (over 100 kDa) and big PRL
(40–60 kDa) have been identified in human serum (19,20).
The 60 kDa PRL corresponds to the big PRL form, which is a dimer
that may be an aggregate of glycosylated subunits (25 kDa)
(21), and this modification may be
involved in mechanisms of biosynthesis, secretion, and clearance of
the hormone (19).
There is also evidence of the association between
PRL/PRLR and tumorigenesis in clinical samples. A previous study of
our research group showed the relation of high PRLR expression and
malignant laryngeal tumors (22).
In a previous study we provided evidence that the
cell lines and tissues of cervical cancer synthesize a 60 kDa PRL
variant unlike the non-tumorigenic keratinocytes (HaCaT) (23,24).
This PRL variant has been previously identified in peripheral blood
mononuclear cells (PBMC) from patients with SLE and THP-1 monocytes
(24,25).
The diverse activities of PRL are mediated by PRLR,
and this complex may activate several signaling pathways including
Jak-STAT (26,27), MAPK (28) and PI3K (29,30).
However, in a previous study, our group reported that STAT3 is an
important transcription factor for recombinant hPRL (rec-hPRL) to
carry its effects (31).
There is an association between PRL and its
receptor's overexpression with the development of different types
of cancer such as breast, laryngeal, prostate, colon and cervical
cancer (22,23,32–36).
The role of circulating PRL in tumorigenesis, mainly in breast
cancer, has been a topic of debate for more than 20 years since
various epidemiological studies were unable to reach unified
conclusions on the correlation between circulating PRL levels and
risk for cancer (37,38). However, there is no evidence that
this 60 kDa PRL, which may be acting in an autocrine manner, may
cooperate with the development of cancer.
Cervical cancer is still a major public health
problem and the third most common type of cancer in women worldwide
(39). Persistent high-risk human
papillomavirus infection is the main risk factor for cervical
cancer; however, there are other important cofactors for this
outcome. Previous results of our group show that a long PRLR
isoform is mainly expressed in cervical cancer tissues (40), and its overexpression is associated
to cell survival by inhibition of apoptosis (23). Moreover, we found the presence of a
60 kDa PRL isoform that is present in cervical cancer cells
(23).
There are no studies that evaluate the biological
effect of PRL produced by tumor cells and the different responses
due to the existence of different isoforms. The aim of the present
study was to obtain the 60 kDa PRL produced by cervical cancer
cells and to evaluate some aspects of its functionality. We tested
whether the 60 kDa PRL is bioactive in Nb2 cells and its effect on
apoptosis in cervical cancer cells. In addition, we assessed its
impact on the induction of proinflammatory cytokines in THP-1
macrophages.
Materials and methods
Reagents
Rec-hPRL (L-4021) and
3-(4,5-dimetylthiazol-2-yl)-2,5-diphenyltetrazolium reagent (MTT)
were obtained from Sigma−Aldrich® (St.
Louis, MO, USA). Polyvinylidene difluoride (PVDF) membranes,
enhanced chemiluminescence (ECL), and western blotting detection
kit (WBKLS0500) were purchased from Merck Millipore (EMD Millipore
Corporation Billerica, MA, USA) as well as Amicon® Ultra
0.5 ml centrifugal filters. Monoclonal antibody anti-PRL (E-9)
sc-48383 was obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Dulbecco's modified Eagle's medium (DMEM), DMEM
advanced, charcoal stripped fetal bovine serum (FBS) and
antibiotic/antimicotic were purchased from Gibco Life Technologies
(Carlsbad, CA, USA). Polyclonal antibodies anti-pSTAT3 (Ser 727),
sc-13564, anti-bcl-xl (H-5) sc-8392 and anti-bcl-2 (C-2) sc-7382
were obtained from Santa Cruz Biotechnology. Polyclonal antibody
anti-survivin AF886 was purchased from R&D Systems (R&D
Systems, Inc., Minneapolis, MN, USA). Inhibitor for JAK/STAT
signaling pathway α-cyano-(3,4-dihydroxy)-N-benzylcinnamide (AG490)
was dissolved in dimethyl sulfoxide (DMSO) and stored at −20°C as
recommended by the manufacturer. Phorbol 12.myristate 13-acetate
(PMA) was obtained by Sigma (P8139). µMACS™ Protein G
MicroBeadsMultiMACS™ Protein G kit was provided by MiltenyiBiotec.
Finally, multiple cytokine magnetic bead MIHCYTOMAG-60K-05 kit was
manufactured by MILLIPLEX® MAP.
Cell culture
Cervical cancer derived cells (HeLa, SiHa and
C-33A), non-tumorigenic immortalized keratinocytes (HaCaT), as a
negative control, THP-1 monocitic cell line, and Nb2 rat
lymphoma-derived cells were used for stimulation with PRL assays.
All the cell lines were obtained from American Type Culture
Collection (ATCC; University Boulevard Manassas, VA, USA) and were
grown with DMEM, DMEM advanced or RPMI-1640 medium supplemented
with 5% of charcoal stripped FBS, penicillin G, streptomycin and
amphotericin B.
PMA was used to differentiate THP-1 monocytes into
macrophages, and its concentration was 200 nM for 72 h.
Cells were cultured in a jacket-water incubator at
37°C with an atmosphere of 5% CO2. The cells were grown
to reach 80% confluence, thus they could be used for assays.
Western blotting
Protein (40 µg) from supernatants of cervical cancer
cells were mixed with loading buffer and denatured at 95°C for 5
min. Afterwards, they were loaded on 10% SDS polyacrylamide gels to
be resolved. Protein transference was carried out in PVDF membranes
(Bio-Rad Laboratories, Hercules, CA, USA). Blocking solution was
prepared with 5% of Blotting Grade Blocker (Bio-Rad Laboratories),
and membranes were incubated in this solution for 2 h. Solutions
with primary antibodies were prepared at a dilution of 1:500, and
membranes were kept overnight followed by the secondary antibody
solutions (diluted 1:5,000). The process was developed with a
chemiluminescence system (Immobilion, Merck Millipore). MicroChemi
6.0 was also used to reveal membranes and measure optical
density.
Immunoprecipitation with magnetic
beads
Immuno precipitation was carried out to isolate the
60 kDa isoform of PRL. Supernatants of different cervical cancer
cell cultures were tested by western blotting to evaluate whether
the isoform was present. Positive samples (250 µl) were mixed with
10 µl of anti-PRL and 50 µl of microbeads using µMACS™ Protein G
MicroBeadsMultiMACS™ Protein G kit. The mixture was kept on ice for
30 min, and then it was transferred into magnetic columns followed
by four rinse steps and a final one to detach the protein with an
acid glycine solution. After testing the correct PRL isolation, the
protein was quantified to proceed with stimuli.
Molecular weight cut-off
filtration
The PRL isolated from cervical cancer cell
supernatants was filtered to purify the 60 kDa isoform using
Amicon® Ultra 0.5 ml centrifugal filters setting up a
molecular weight cut-off at 50 kDa. Filtration conditions were
14,000 × g for 30 min for concentration spin and 1,000 × g for 2
min for reverse spin.
Quantification of 60 kDa PRL
Lowry method was used to measure the isolated and
purified 60 kDa PRL, having a bovine serum albumin standard curve
and reading samples by triplicate. The plates were read in an iMark
microplate reader Bio-Rad version 6.1.
Silver nitrate staining
Polyacrylamide gels (10%) were used to determine the
presence of PRL after isolation assays and to determine the correct
60 kDa isoform isolation after molecular weight cut-off filtration.
After electrophoretic running, a silver nitrate staining was
performed.
Nb2 cell proliferation assay
Nb2 cells were cultured with complete medium (10% of
horse serum and 10% of FBS) and 24 h before the assay cells were
transferred to maintenance medium (1% FBS). Finally, assay medium
(0.1% FBS) was used to carry out the test. Cells were grown for 60
h with no stimulus, rec-hPRL (200 ng/ml), or 60 kDa PRL (200
ng/ml). MTT (5 mg/ml) was added at a proportion of one tenth of
final medium volume and incubated for 4 h, according to the
protocol of the manufacturer. Blue formazan crystals were
solubilized with acidified isopropanol, and absorbances were read
at a wavelength of 570 nm in an Epoch Microplate Spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA).
Apoptotic assay (TUNEL assay)
The kit APO-BrdU (Invitrogen, Carlsbad, CA, USA) was
used to carry out TUNEL assay. Cells were grown in 8-well chamber
slides seeded with 5×104 cells/well, and treated with
etoposide alone or in combination with the 60 kDa PRL for 24 h and
incubated at 37°C. The slides were washed with PBS and fixed with
4% paraformaldehyde for 30 min at room temperature. Fixed cells
were washed and permeabilized using 0.2% Tween-20 for 10 min and
then incubated with terminal deoxynucleotidyl transferase and BrdU
for 1 h at 37°C. After rinsing with PBS, cells were treated with
Alexa Fluor 488 dye-labeled anti-BrdU antibody at 37°C for 30 min
and mounted with a glass coverslip. Staining of DNA fragmentation
was observed with ultraviolet fluorescent microscope (Carl Zeiss,
Jena, Germany) counting at least 200 cells/well. Semi-automatic
quantification was performed using the cell counter function of
Image-pro Plus 6.0 software. The color intensity of the positive
objects (green) was manually preset for each pattern (pixel/pixel)
based on the hue-saturation-intensity (HSI) histogram. The final
option of the ‘count/size’ command allows to obtain the mean
optical densities of 5 fields/sample.
Human cytokine magnetic bead panel
assay
Cytokines secreted by THP-1 cells differentiated
into macrophages were quantified using a multiple cytokine magnetic
bead kit (MILLIPLEX® MAP, MIHCYTOMAG-60K-05; Millipore)
following the instructions of the manufacturer. This kit was
targeted to TNF-α, IL-1β, IL-6, IL-12 and IL-10.
Statistical analysis
Data were analyzed using (GraphPad Prism software
(GraphPad version 6.01). Significant effects were determined using
ANOVA. Statistically significant differences were considered for
P-values <0.05.
Results
Isolation and purification of 60 kDa
PRL
After demonstrating the presence of 60 and 80 kDa
isoforms of PRL in total protein extracts from cervical cancer cell
lines HeLa, SiHa and C-33A (23),
it was decided to evaluate whether both isoforms were also present
in these cell culture supernatants. The 60 kDa variant of PRL was
present in all of the 18 samples of cervical cancer cell
supernatants evaluated; in contrast, the 80 kDa variant was absent
(Fig. 1A). Additionally, the
supernatants of non-tumorigenic immortalized keratinocytes (HaCaT)
were also evaluated and the 60 kDa variant was not found (data not
shown). Hence, compared with cervical cancer cell lines, HaCaT does
not produce the 60 kDa PRL variant, and do not express long
isoforms of PRLR (23).
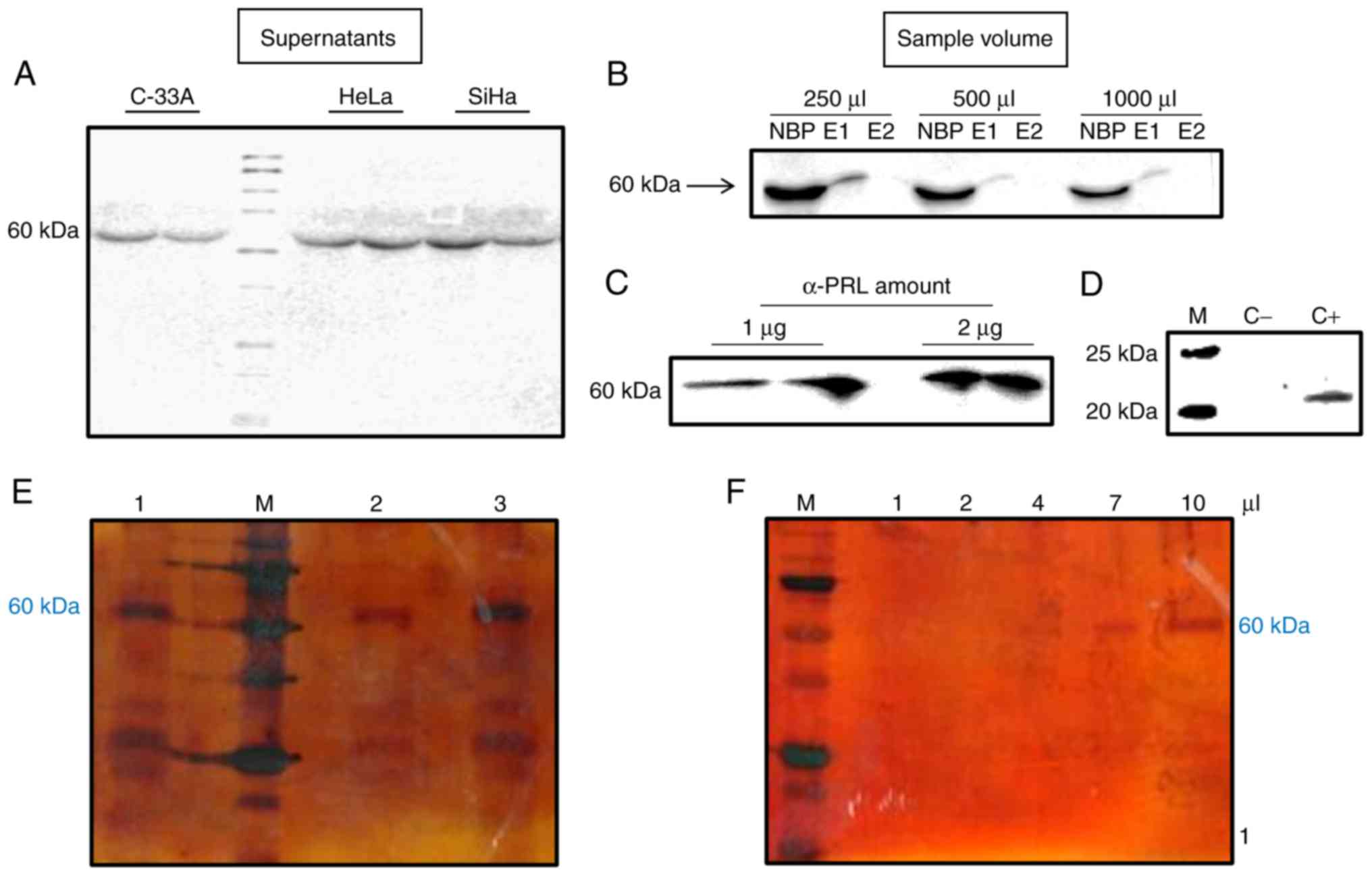 | Figure 1.Isolation of 60 kDa PRL from cervical
cancer cell supernatants. (A) Presence of 60 kDa PRL on
supernatants of HeLa, SiHa and C-33A by western blotting. (B and C)
To standarize optimal conditions for immunoprecipitation of 60 kDa
PRL different volumes of sample [250, 500 and 1,000 µl (B) and
amounts of α-PRL antibody (1 and 2 µg) (C) were tested by western
blotting (NBP, non-bound protein; E1, elution 1; E2, elution 2)].
(D) Polyacrylamide gel stained with silver nitrate showing the
presence of 60 kDa PRL after immunoprecipitation (1, 2 and 3, are 3
different samples of HeLa supernatants, M, molecular weight
marker). (E) Presence of a unique 60 kDa PRL band on a
polyacrylamide gel with different volumes after ultrafiltration of
immunoprecipitated supernatants. |
Due to its absence in HaCaT cell line, the PRL
isoform of 60 kDa was isolated only from cervical cancer cells
(particularly from HeLa) and was collected using magnetic bead
immunoprecipitation assays. After a second elution, PRL was not
recovered, which means that one rinse was enough to recover the
total immunoprecipitated PRL (Fig.
1B).
To identify the optimal conditions for the best
purification performance, different volumes of the supernatants
(250, 500 and 1,000 µl) and the antibody (1 and 2 µg) were assayed.
The highest amount of protein obtained was accomplished with 250 µl
of supernatant (Fig. 1B) and 2 µg
of antibody (Fig. 1C).
During the immunoprecipitation assays, positive and
negative controls were used to test the correct PRL isolation. In
Fig. 1D it is observed that when
using PBS buffer (negative control) there are no bands present,
which means no protein was recovered. In contrast, when using
rec-hPRL (positive control), we observed a band that shows
immunoprecipitation is carried out correctly.
To confirm the correct isolation of PRL and exclude
the presence of other interfering proteins, a silver staining assay
in polyacrylamide gels was performed, and we detected the presence
of PRL isoform of 60 kDa and other lower molecular weight bands,
which may be degraded proteins (Fig.
1E). These fragments were eliminated by molecular weight
cut-off filtration and the unique presence of the 60 kDa band was
observed by a silver staining assay in polyacrylamide gels
(Fig. 1F).
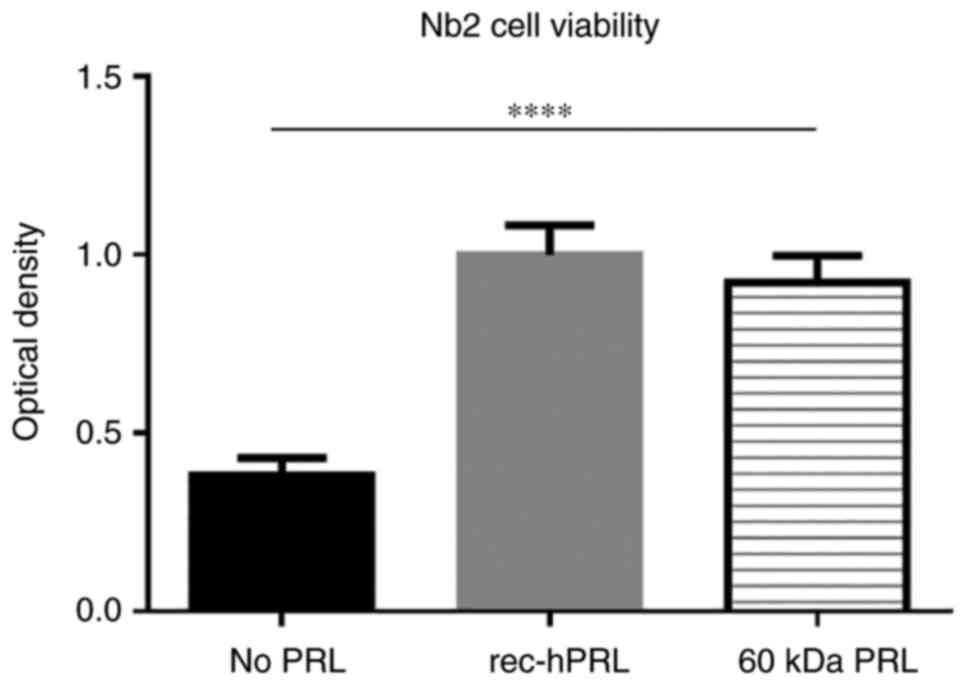
Bioactivity of 60 kDa PRL
Nb2 cell proliferation assay was used to determine
whether the autocrine 60 kDa PRL is bioactive. Nb2 cell
proliferation assay is the gold standard to evaluate PRL
bioactivity, based on the studies carried out by Noble et
al. In these studies, they used a broad panel of hormone
stimuli and identified that the only ones that induced
proliferation on the Nb2 cell line were the members of the PRL
family, particularly PRL, whose effects were observed to be in a
more sensitive manner (even to 1 ng/ml). This is the reason why we
decided to use this model to evaluate 60 kDa PRL bioactivity
(41,42).
Nb2 cells were stimulated either with rec-hPRL or 60
kDa PRL (200 ng/ml for 60 h), and then compared to non-stimulated
cells. The concentration of hormones and time of stimulus had been
standardized in previous studies published by our group (24).
As observed in Fig.
2, 60 kDa PRL strongly induced proliferation in Nb2 cells
almost in a 2-fold basis and this behavior is similar to that
observed with rec-hPRL.
Antiapoptotic effect of 60 kDa PRL in
cervical cancer cells
Our research group previously showed that rec-hPRL
has an important effect on cervical cancer cell survival by
inhibition of apoptosis (23) and
the optimal concentration of rec-hPRL and etoposide was tested in
previous studies for apoptosis assays (23,31).
This is the reason to evaluate whether the 60 kDa PRL has the same
capacity. The effect of the 60 kDa PRL on a model of apoptosis
induced by etoposide in cervical cancer cells was analyzed.
Treatment with etoposide (30 µg/ml) augmented the number of cells
with fragmented DNA in all cell lines after 24 h of incubation, as
expected. 60 kDa PRL (200 ng/ml) costimulus significantly decreased
the number of cells with DNA fragmentation. As observed in optical
analysis, HeLa, SiHa and C33A reduced its apoptotic cells when they
were stimulated with 60 kDa PRL (from 698 to 248.6 for HeLa, from
642 to 302.4 for SiHa, and from 695 to 407.6 for C33A; Fig. 3A). Representative images of
immunofluorescence from TUNEL assays in HeLa cell line are shown
(Fig. 3B).
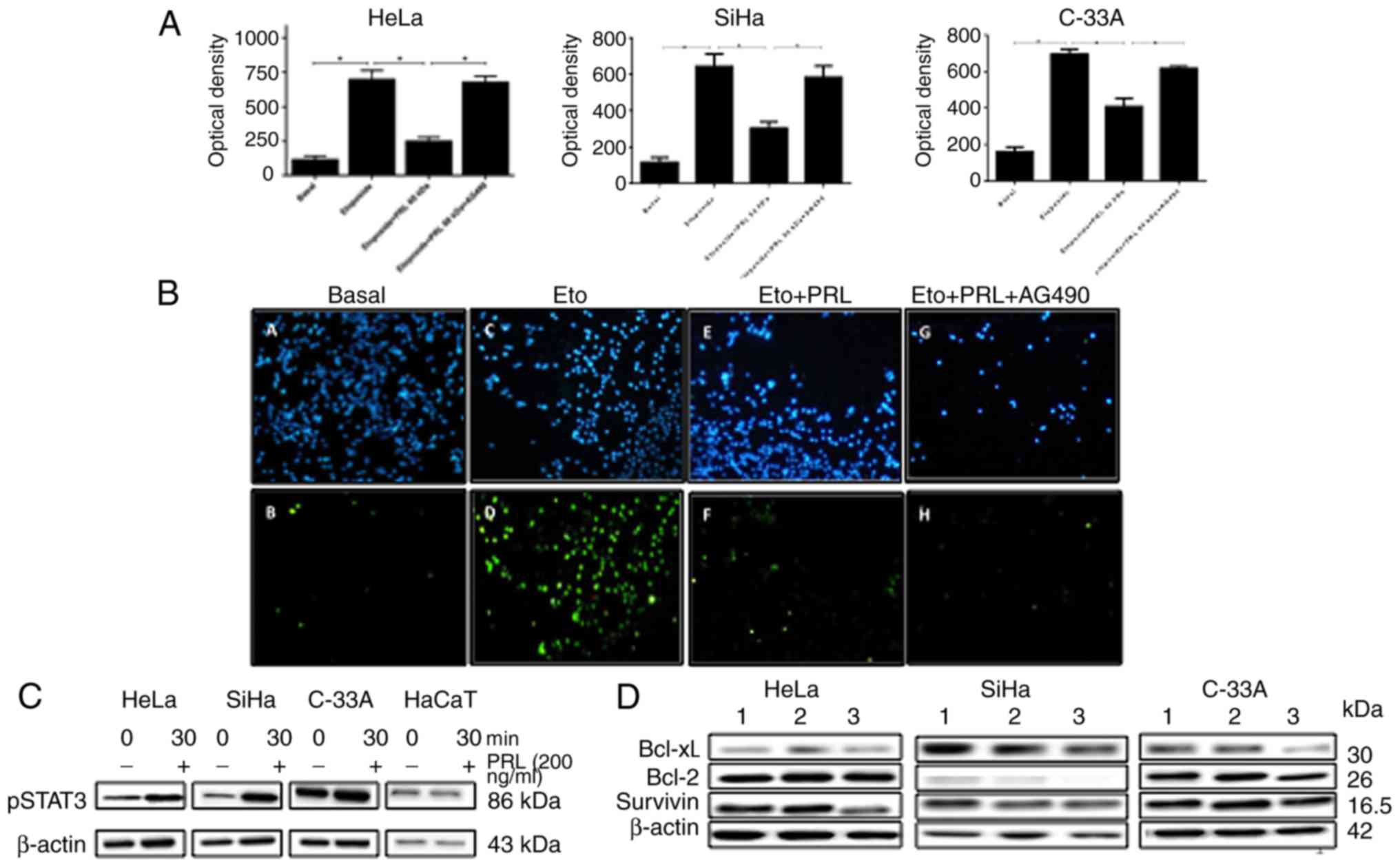 | Figure 3.Effect of 60 kDa PRL on apoptosis
induced by etoposide in cervical cancer cells. (A) HeLa, SiHa and
C-33A were stimulated with 60 kDa PRL for 24 h. Apoptosis was
measured by TUNEL assays and optical analysis was performed with
the software Image-pro Plus 6.0 (****P<0.0001, **P<0.01,
*P<0.05). (B) Representative images of immunostainning from
TUNEL assays in HeLa cells, apoptotic cells (green B, D, F, H) and
DAPI staining (blue A, C, E, G) are shown. (C) Phosphorylation of
STAT3 is shown after 30 min of stimulus with 60 kDa PRL in HeLa,
SiHa and C33A. (D) Expression of antiapoptotic proteins after
stimuli with 60 kDa PRL for 48 alone or in combination with AG490
in HeLa, SiHa and C-33A cells. 1, not stimulus; 2, with 60 kDa PRL;
3, with 60 kDa PRL plus AG490. |
Since rec-hPRL is able to induce phosphorylation of
STAT3, and this pathway is required for antiapoptotic effects of
PRL in cervical cancer cells (31),
we decided to evaluate whether autocrine 60 kDa PRL has the same
capacity. The 60 kDa PRL augmented STAT3 phosphorylation in HeLa,
SiHa and C33A; however, in HaCaT cells was not observed (Fig. 3C).
The antiapoptotic effect the autocrine 60 kDa PRL
exerted was abolished when Jak-STAT signaling pathway was inhibited
with AG490 inhibitor (Fig. 3A and
B). This behavior was similar on the three cervical cancer cell
lines used and similar to that observed in previous studies of our
group analyzing apoptosis of cervical cancer cells and STAT3
activation in response to rec-hPRL (31).
Since a similar behavior was observed regarding the
bioactivity and the antiapoptotic effect exerted by 60 kDa PRL
compared to rPRL in cervical cancer cells, we decided to evaluate
how the expression of antiapoptotic proteins was modulated.
In HeLa and C-33A cells, the expression of Bcl-xL
and Survivin slightly increased after stimuli with 60 kDa PRL; as
expected protein levels decreased when STAT3 pathway is blocked.
For Bcl-2 these changes were not observed. However, in SiHa the
expression of these antiapoptotic proteins were not modified
(Fig. 3D).
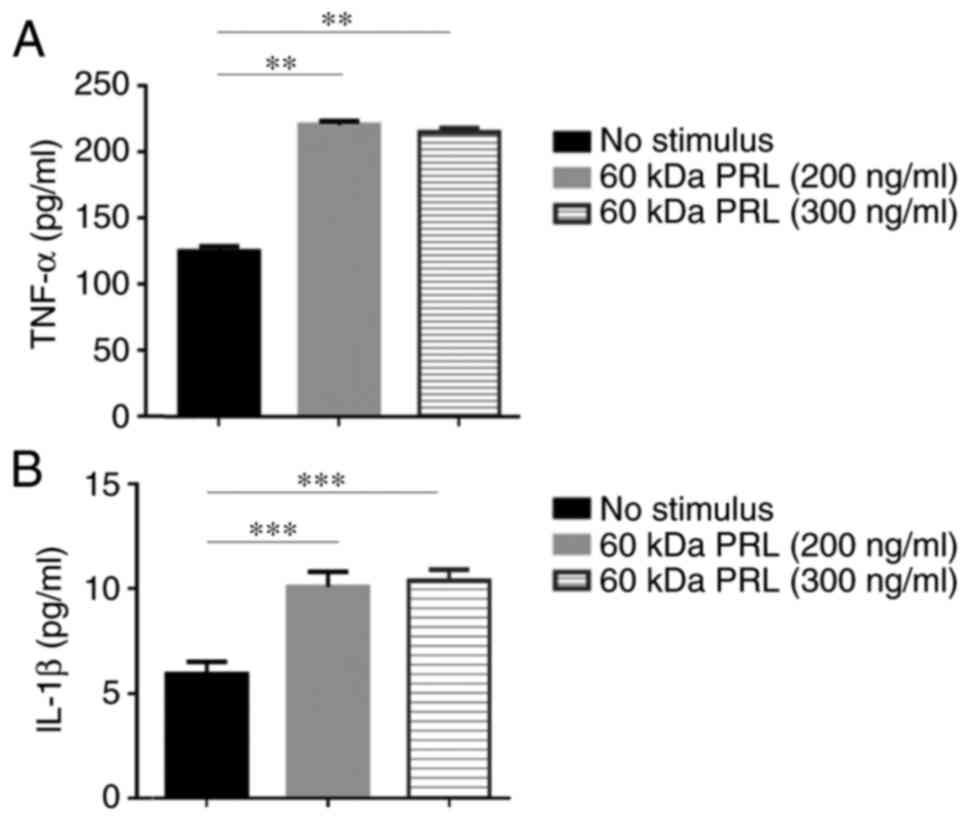
The 60 kDa PRL induces TNF-α and IL-1β
production in THP-1 macrophages
The THP-1 monocytes were differentiated into
macrophages. After 48 h of stimulation with the 60 kDa PRL (200 and
300 ng/ml), the concentration of cytokines on macrophage
supernatants was measured by MILLIPLEX® MAP,
MIHCYTOMAG-60K-05 kit. The 60 kDa PRL stimulus significantly
increased TNF-α (220.97 and 215.40 pg/ml, respectively) and IL-1β
(10.08 and 10.39 pg/ml, respectively) (Fig. 4). The production of IL-12, IL-6 and
IL-10 was not detected under the conditions set for this
assays.
Discussion
Prolactin (PRL) activates signaling pathways that
regulate cell proliferation, migration, differentiation and
apoptosis; therefore, it has been implicated in the etiology and
progression of cancer (1,37,43).
Even though pituitary gland is the main source of
this hormone, there are several cells and tissues that can produce
it such as brain, decidua, spleen, adipocytes and breast cancer
cells among others. In the same context, PRLR is also expressed,
which suggests an autocrine/paracrine mechanism (7).
However, opposite effects have been found on
prostate cancer cell lines: rec-hPRL, induces apoptosis in LNCaP
cells, but not in PC3 cells (44).
This demonstrates that PRL can modulate different actions depending
on the type of tumor.
Previous studies of our group demonstrated that
rec-hPRL induces apoptosis inhibition in cervical cancer cells
(23). Similar effects were
observed in ovarian carcinoma, where the rec-hPRL did not affect
proliferation, but it decreased apoptosis (45).
The molecular heterogeneity of PRL has been
previously described (46), and we
have provided evidence that the cell lines and tissues of cervical
cancer synthesize a 60 kDa PRL variant, unlike the non-tumorigenic
keratinocytes (HaCaT) that do not express this isoform (23,40).
This PRL variant has been previously detected in PBMCs from
patients with SLE and THP1 monocytes (25,32).
Consequently, we hypothesize that the expression of
an autocrine/paracrine loop of PRL may play an important role in
the tumoral microenvironment in cervical cancer that can lead to
changes in cell proliferation, apoptosis and immune response, among
other functions.
PRL is a pleiotropic hormone whose effects can
affect cellular processes that may favor the development of cancer.
Once the presence of PRL of 60 kDa was identified in the
supernatant of the 3 cell lines derived from cervical cancer, we
considered it may be important to determine whether the 60 kDa PRL
was bioactive and whether it was performing some functions in these
cells.
After the isolation of 60 kDa PRL from supernatants
of HeLa cells, its bioactivity was tested. In the present study, a
high proliferative bioactivity of the PRL of 60 kDa was
demonstrated on Nb2 cells. These results are in concordance with
the activity of PRL derived from PBMCs of SLE patients in the same
cells (25).
Unlike the study of Larrea et al, in which
stimuli were performed with the complete supernatant from PBMCs
(25), in the present study
purified 60 kDa PRL from cervical cancer cell supernatants was used
to avoid masked effects of other molecules contained in the culture
medium.
To prevent any interference due to hormones present
in the FBS, charcoal stripped serum was used and; moreover, we
corroborated that there was no presence of PRL or the levels are so
low they cannot be detected.
Subsequently, we determined that 60 kDa PRL
decreases apoptosis in HeLa, SiHa and C-33A cells, and it is able
to phosphorylate STAT3 in a similar way to that observed in
response rec-hPRL (31).
The high STAT3 expression has been related to many
types of cancer such as prostate (47), breast (48,49),
skin (50,51) and gliomas (52,53).
In a recent study of Shukla et al, a positive correlation
between STAT3 and increased E6/E7 expression as well as a
diminished p53/pRB were shown, which opens the possibility to focus
on therapeutic targets blocking STAT3. In the latter, curcumin is
proposed as a potential candidate (54). In the same regard, there are other
molecules proposed as STAT3 blockers such as SC99, proved in an
antimyeloma model with ability to induce apoptosis in a selective
STAT3 downregulation manner (55);
however, on cervical cancer there is only one study involving an
in vitro model where IL-37 inhibits STAT3 and supressed
proliferation of cervical cancer cells (56).
The latter effects may be carried out by expression
of antiapoptotic genes, such as bcl-2, bcl-xl and survivin. In
preliminary experiments we observed that in HeLa cells there was an
increase of Bcl-xL and survivin in response to etoposide, and these
proteins showed a decrease when 60 kDa PRL was added. Whereas in
SiHa cells, Bcl-xL and survivin do not show this tendence. These
results may be explained since it is well known that antiapoptotic
proteins are redundant in their functions (57–59),
and this is why it may occur that one or another protein is
expressed and in some cases both; however, more experiments must be
performed in order to conclude with more certainty.
It may be interesting to use siRNAs to block the
translation of 60 kDa PRL, however, it is necessary to characterize
first the mRNA sequence to design a specific probe to carry out
this assay. Nevertheless, this is a perspective our group is eager
to investigate in future studies.
Regarding the immune system in cervical cancer, it
is known that in early stages of precancerous cervical lesions
there is a proinflammatory cytokine profile in the cervical
microenvironment; however, in late phases, or cancer, this profile
is shifted to an anti-inflammatory one, characterized by
immunosuppressor cytokines (60,61).
One limitation of the present study was not blocking
the 60 kDa PRL. However, it is necessary to characterize first the
sequence of the protein, in order to use a specific antibody to
directly inhibit 60 kDa PRL. This is an ongoing project we are
currently working on.
We demonstrate that the 60 kDa PRL has the capacity
to induce production of proinflammatory cytokines (IL-1β and TNF-α)
in THP-1 macrophages at the concentration used. However, no IL-6,
IL-12 or IL-10 production was detected. Previous studies show that
rec-hPRL induces expression of TNF-α, IFN-γ, IL-12 and IL-1β in
THP-1 macrophages using the concentration 100 ng/ml, and, as for
the case of IL-10, its induction requires a higher concentration
(1,000 ng/ml) (9,10). Further analysis must be performed to
evaluate the complete immune role of the 60 kDa PRL on THP-1
macrophages or other cells.
In conclusion, we have shown that cervical cancer
cells synthesize and secrete a 60 kDa PRL isoform that can inhibit
apoptosis, and it can activate STAT3. In addition, this PRL form
induces IL-1β and TNF-α production in THP-1 macrophages. It may be
interesting to evaluate a broader panel of cytokines to establish
PRL autocrine/paracrine effects on the inflammatory response in
cervical cancer.
Acknowledgements
The present study was supported by grant from the
Consejo Nacional de Ciencia y Tecnología, Fondo SEP-CONACYT
CB-2013-01 (222205). The authors would like to thank Diana Jennifer
Carrillo Casillas and José David Ramos Solano for proof reading the
manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goffin V, Binart N, Touraine P and Kelly
PA: Prolactin: The new biology of an old hormone. Annu Rev Physiol.
64:47–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reber PM: Prolactin and immunomodulation.
Am J Med. 95:637–644. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freeman ME, Kanyicska B, Lerant A and Nagy
G: Prolactin: Structure, function, and regulation of secretion.
Physiol Rev. 80:1523–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Athreya BH, Pletcher J, Zulian F, Weiner
DB and Williams WV: Subset-specific effects of sex hormones and
pituitary gonadotropins on human lymphocyte proliferation in vitro.
Clin Immunol Immunopathol. 66:201–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matera L, Mori M and Galetto A: Galetto,
Effect of prolactin on the antigen presenting function of
monocyte-derived dendritic cells. Lupus. 10:728–734. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lahat N, Miller A, Shtiller R and Touby E:
Differential effects of prolactin upon activation and
differentiation of human B lymphocytes. J Neuroimmunol. 47:35–40.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ben-Jonathan N, Mershon JL, Allen DL and
Steinmetz RW: Extrapituitary prolactin: Distribution, regulation,
functions, and clinical aspects. Endocr Rev. 17:639–669. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Bellis A, Bizzarro A, Pivonello R,
Lombardi G and Bellastella A: Prolactin and autoimmunity.
Pituitary. 8:25–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sodhi A and Tripathi A: Prolactin and
growth hormone induce differential cytokine and chemokine profile
in murine peritoneal macrophages in vitro: Involvement of p-38 MAP
kinase, STAT3 and NF-kappaB. Cytokine. 41:162–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripathi A and Sodhi A: Prolactin-induced
production of cytokines in macrophages in vitro involves JAK/STAT
and JNK MAPK pathways. Int Immunol. 20:327–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Corbacho AM, Macotela Y, Nava G, Eiserich
JP, Cross CE, Martínez de la Escalera G and Clapp C: Cytokine
induction of prolactin receptors mediates prolactin inhibition of
nitric oxide synthesis in pulmonary fibroblasts. FEBS Lett.
544:171–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jara LJ, Vera-Lastra O, Miranda JM, Alcala
M and Alvarez-Nemegyei J: Prolactin in human systemic lupus
erythematosus. Lupus. 10:748–756. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chikanza IC, Petrou P, Chrousos G,
Kingsley G and Panayi GS: Excessive and dysregulated secretion of
prolactin in rheumatoid arthritis: Immunopathogenetic and
therapeutic implications. Br J Rheumatol. 32:445–448. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El Miedany YM, Ahmed I, Moustafa H and El
Baddini M: Hyperprolactinemia in Sjogren's syndrome: A patient
subset or a disease manifestation? Joint Bone Spine. 71:203–208.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chikanza IC: Prolactin and
neuroimmunomodulation: in vitro and in vivo observations. Ann NY
Acad Sci. 876:119–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicoletti F, Di Marco R, Barcellini W,
Magro G, Schorlemmer HU, Kurrle R, Lunetta M, Grasso S, Zaccone P
and Meroni P: Protection from experimental autoimmune diabetes in
the non-obese diabetic mouse with soluble interleukin-1 receptor.
Eur J Immunol. 24:1843–1847. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nicoletti F, Zaccone P, Di Marco R,
Lunetta M, Magro G, Grasso S, Meroni P and Garotta G: Prevention of
spontaneous autoimmune diabetes in diabetes-prone BB rats by
prophylactic treatment with antirat interferon-gamma antibody.
Endocrinology. 138:281–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joosten LA, Helsen MM, van de Loo FA and
van den Berg WB: Anticytokine treatment of established type II
collagen-induced arthritis in DBA/1 mice: A comparative study using
anti-TNFalpha, anti-IL-1alpha/beta and IL-1Ra. Arthritis Rheum.
58:S110–S122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinha YN: Structural variants of
prolactin: Occurrence and physiological significance. Endocr Rev.
16:354–369. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sinha YN: Prolactin variants. Trends
Endocrinol Metab. 3:100–106. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Champier J, Claustrat B, Sassolas G and
Berger M: Detection and enzymatic deglycosylation of a glycosylated
variant of prolactin in human plasma. FEBS Lett. 212:220–224. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
González-Lucano LR, Muñoz-Valle JF,
Ascencio-Cedillo R, Domínguez-Rosales JA, López-Rincón G, Del
Toro-Arreola S, Bueno-Topete M, Daneri-Navarro A, Estrada-Chávez C
and Pereira-Suárez AL: Increased expression of the prolactin
receptor is associated with malignant laryngeal tumors. Exp Ther
Med. 3:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lopez-Pulido EI, Muñoz-Valle JF, Del
Toro-Arreola S, Jave-Suárez LF, Bueno-Topete MR, Estrada-Chávez C
and Pereira-Suárez AL: High expression of prolactin receptor is
associated with cell survival in cervical cancer cells. Cancer Cell
Int. 13:1032013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
López-Rincón G, Pereira-Suárez AL, Del
Toro-Arreola S, Sánchez-Hernández PE, Ochoa-Zarzosa A, Muñoz-Valle
JF and Estrada-Chávez C: Lipopolysaccharide induces the expression
of an autocrine prolactin loop enhancing inflammatory response in
monocytes. J Inflamm. 10:242013. View Article : Google Scholar
|
|
25
|
Larrea F, Martínez-Castillo A, Cabrera V,
Alcocer-Varela J, Queipo G, Cariño C and Alarcón-Segovia D: A
bioactive 60-kilodalton prolactin species is preferentially
secreted in cultures of mitogen-stimulated and nonstimulated
peripheral blood mononuclear cells from subjects with systemic
lupus erythematosus. J Clin Endocrinol Metab. 82:3664–3669. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goffin V, Binart N, Clément-Lacroix P,
Bouchard B, Bole-Feysot C, Edery M, Lucas BK, Touraine P, Pezet A,
Maaskant R, et al: From the molecular biology of prolactin and its
receptor to the lessons learned from knockout mice models. Genet
Anal. 15:189–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schindler C: Cytokines and JAK-STAT
signaling. Exp Cell Res. 253:7–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Das R and Vonderhaar BK: Involvement of
SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary
epithelial cells. Oncogene. 13:1139–1145. 1996.PubMed/NCBI
|
|
29
|
Domínguez-Cáceres MA, García-Martínez JM,
Calcabrini A, González L, Porque PG, León J and Martín-Pérez J:
Prolactin induces c-Myc expression and cell survival through
activation of Src/Akt pathway in lymphoid cells. Oncogene.
23:7378–7390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berlanga JJ, Gualillo O, Buteau H,
Applanat M, Kelly PA and Edery M: Prolactin activates tyrosyl
phosphorylation of insulin receptor substrate 1 and
phosphatidylinositol-3-OH kinase. J Biol Chem. 272:2050–2052. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ramírez de Arellano A, Lopez-Pulido EI,
Martínez-Neri PA, Estrada Chávez C, González Lucano R,
Fafutis-Morris M, Aguilar-Lemarroy A, Muñoz-Valle JF and
Pereira-Suárez AL: STAT3 activation is required for the
antiapoptotic effects of prolactin in cervical cancer cells. Cancer
Cell Int. 15:832015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López-Rincón G, Mancilla R, Pereira-Suárez
AL, Martínez-Neri PA, Ochoa-Zarzosa A, Muñoz-Valle JF and
Estrada-Chávez C: Expression of autocrine prolactin and the short
isoform of prolactin receptor are associated with inflammatory
response and apoptosis in monocytes stimulated with Mycobacterium
bovis proteins. Exp Mol Pathol. 98:517–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yonezawa T, Chen KH, Ghosh MK, Rivera L,
Dill R, Ma L, Villa PA, Kawaminami M and Walker AM: Anti-metastatic
outcome of isoform-specific prolactin receptor targeting in breast
cancer. Cancer Lett. 366:84–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neradugomma NK, Subramaniam D, Tawfsik OW,
Goffin V, Kumar TR, Jensen RA and Anant S: Prolactin signaling
enhances colon cancer stemness by modulating Notch signaling in a
Jak2-STAT3/ERK manner. Carcinogenesis. 35:795–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goffin V, Hoang DT, Bogorad RL and
Nevalainen MT: Prolactin regulation of the prostate gland: A female
player in a male game. Nat Rev Urol. 8:597–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robertson FG, Harris J, Naylor MJ, Oakes
SR, Kindblom J, Dillner K, Wennbo H, Törnell J, Kelly PA, Green J
and Ormandy CJ: Prostate development and carcinogenesis in
prolactin receptor knockout mice. Endocrinology. 144:3196–3205.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clevenger CV, Furth PA, Hankinson SE and
Schuler LA: The role of prolactin in mammary carcinoma. Endocr Rev.
24:1–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tworoger SS and Hankinson SE: Prolactin
and breast cancer etiology: An epidemiologic perspective. J Mammary
Gland Biol Neoplasia. 13:41–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ascencio-Cedillo R, López-Pulido EI,
Muñoz-Valle JF, Villegas-Sepúlveda N, Del Toro-Arreola S,
Estrada-Chávez C, Daneri-Navarro A, Franco-Topete R, Pérez-Montiel
D, García-Carrancá A and Pereira-Suárez AL: Prolactin and prolactin
receptor expression in cervical intraepithelial neoplasia and
cancer. Pathol Oncol Res. 21:241–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gout PW, Beer CT and Noble RL:
Prolactin-stimulated growth of cell cultures established from
malignant Nb rat lymphomas. Cancer Res. 40:2433–2436.
1980.PubMed/NCBI
|
|
42
|
Noble RL, Beer CT and Gout PW: Evidence in
vivo and in vitro of a role for the pituitary in the growth of
malignant lymphomas in Nb rats. Cancer Res. 40:2437–2440.
1980.PubMed/NCBI
|
|
43
|
Perks CM, Keith AJ, Goodhew KL, Savage PB,
Winters ZE and Holly JM: Prolactin acts as a potent survival factor
for human breast cancer cell lines. Br J Cancer. 91:305–311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giuffrida D, Perdichizzi A, Giuffrida MC,
La Vignera S, D'Agata R, Vicari E and Calogero AE: Does prolactin
induce apoptosis? Evidences in a prostate cancer in vitro model. J
Endocrinol Invest. 33:313–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Asai-Sato M, Nagashima Y, Miyagi E, Sato
K, Ohta I, Vonderhaar BK and Hirahara F: Prolactin inhibits
apoptosis of ovarian carcinoma cells induced by serum starvation or
cisplatin treatment. Int J Cancer. 115:539–544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shoupe D, Montz FJ, Kletzky OA and
DiZerega GS: Prolactin molecular heterogeneity. Response to
thyrotropin-releasing hormone stimulation of concanavalin A-bound
and -unbound immunoassayable prolactin during human pregnancy. Am J
Obstet Gynecol. 147:482–487. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qin HR, Kim HJ, Kim JY, Hurt EM, Klarmann
GJ, Kawasaki BT, Duhagon Serrat MA and Farrar WL: Activation of
signal transducer and activator of transcription 3 through a
phosphomimetic serine 727 promotes prostate tumorigenesis
independent of tyrosine 705 phosphorylation. Cancer Res.
68:7736–7741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Clevenger CV: Roles and regulation of stat
family transcription factors in human breast cancer. Am J Pathol.
165:1449–1460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ling X and Arlinghaus RB: Knockdown of
STAT3 expression by RNA interference inhibits the induction of
breast tumors in immunocompetent mice. Cancer Res. 65:2532–2536.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan KS, Sano S, Kiguchi K, Anders J,
Komazawa N, Takeda J and DiGiovanni J: Disruption of Stat3 reveals
a critical role in both the initiation and the promotion stages of
epithelial carcinogenesis. J Clin Invest. 114:720–728. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pedranzini L, Leitch A and Bromberg J:
Stat3 is required for the development of skin cancer. J Clin
Invest. 114:619–622. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alvarez JV, Mukherjee N, Chakravarti A,
Robe P, Zhai G, Chakladar A, Loeffler J, Black P and Frank DA: A
STAT3 gene expression signature in gliomas is associated with a
poor prognosis. Transl Oncogenomics. 2:99–105. 2007.PubMed/NCBI
|
|
53
|
Abou-Ghazal M, Yang DS, Qiao W,
Reina-Ortiz C, Wei J, Kong LY, Fuller GN, Hiraoka N, Priebe W,
Sawaya R and Heimberger AB: The incidence, correlation with
tumor-infiltrating inflammation, and prognosis of phosphorylated
STAT3 expression in human gliomas. Clin Cancer Res. 14:8228–8235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shukla S, Mahata S, Shishodia G, Pandey A,
Tyagi A, Vishnoi K, Basir SF, Das BC and Bharti AC: Functional
regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis.
PLoS One. 8:e678492013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Z, Mao H, Du X, Zhu J, Xu Y, Wang S,
Xu X, Ji P, Yu Y, Cao B, et al: A novel small molecule agent
displays potent anti-myeloma activity by inhibiting the JAK2-STAT3
signaling pathway. Oncotarget. 7:9296–9308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang S, An W, Yao Y, Chen R, Zheng X, Yang
W, Zhao Y, Hu X, Jiang E, Bie Y, et al: Interleukin 37 expression
inhibits STAT3 to suppress the proliferation and invasion of human
cervical cancer cells. J Cancer. 6:962–969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Eichhorn JM, Alford SE, Sakurikar N and
Chambers TC: Molecular analysis of functional redundancy among
anti-apoptotic Bcl-2 proteins and its role in cancer cell survival.
Exp Cell Res. 322:415–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chao DT, Linette GP, Boise LH, White LS,
Thompson CB and Korsmeyer SJ: Bcl-XL and Bcl-2 repress a common
pathway of cell death. J Exp Med. 182:821–828. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Villunger A, Scott C, Bouillet P and
Strasser A: Essential role for the BH3-only protein Bim but
redundant roles for Bax, Bcl-2, and Bcl-w in the control of
granulocyte survival. Blood. 101:2393–2400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Paradkar PH, Joshi JV, Mertia PN, Agashe
SV and Vaidya RA: Role of cytokines in genesis, progression and
prognosis of cervical cancer. Asian Pac J Cancer Prev.
15:3851–3864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Goncalves MA and Donadi EA: Immune
cellular response to HPV: Current concepts. Braz J Infect Dis.
8:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|