Introduction
Head and neck squamous cell carcinoma (HNSCC)
describes a broad range of tumors that arise from the base of the
skull to the clavicles. The global incidence of HNSCC exceeds half
a million annually, making it the fifth most common cancer
worldwide (1). Surgery resection is
the classical treatment for HNSCC, with or without chemoradiation.
Despite advances in surgical techniques and the institution of
novel chemoradiation approaches, little improvement has been
achieved on the 5-year survival rate over the past 30 years
(2). Current treatment of HNSCC has
limited effectiveness in improving the survival rate, and
immunotherapy may be a promising strategy.
Epidermal growth factor receptor (EGFR) is a 170-kDa
transmembrane growth-regulating glycoprotein, which has an
intrinsic tyrosine-specific kinase activity. Ligand binding to EGFR
induces receptor dimerization and causes autophosphorylation and/or
cross-phosphorylation of several tyrosine residues, which in turn
initiate an intracellular signaling cascade, ultimately resulting
in increased proliferation and differentiation (3). Overexpression of EGFR has been
frequently observed in many malignancies, such as HNSCC, non-small
cell lung, breast, colon and pancreatic cancer (4–8). The
level of EGFR expression is believed to be associated with nodal
status and prognosis of these patients (9). Therefore, EGFR is considered to be an
attractive molecular target for cancer therapeutics. EGFR
monoclonal antibodies (mABs) and small-molecule EGFR tyrosine
kinase inhibitors (TKIs) are 2 major therapeutic agents that target
EGFR and have been demonstrated to be effective in prolonging
survival in HNSCC patients (10).
Dendritic cells (DCs), which are the most potent and competent
antigen-presenting cells (APCs), have the unique capability of
sensitizing naive T cells to protein antigens. The cytotoxic T
lymphocyte (CTL) responses elicited by DCs can kill the tumor cells
directly, whereas the mAbs and TKIs inhibit tumor growth mainly by
blocking the EGFR signal-transduction pathway. The ability of DCs
to present tumor antigens and thereby generate tumor-specific
immunity has been demonstrated in several clinical trials (11–13). A
previous study indicated that glutathione-S-transferase
(GST)-EGFR pulsing DCs could effectively elicit CTL activity and
prevent tumor progression in animal models (11).
Another possibility to enhance the potency of
DC-based immunotherapy is to silence the negative immunoregulatory
pathways. Cytokine signaling suppressor 1 (SOCS1) is a key
regulator of cytokine signaling that is important for maintaining
the balance of immune responses. This signaling pathway also plays
important roles in DC maturation through its negative cytokine
signaling feedback loops. SOCS1 has been discovered as a critical
inhibitory molecule in cytokine response and antigen presentation
by DCs, regulating the magnitude of both innate and adaptive
immunity (14). Previous studies
have suggested that SOCS1-deficient DCs induced higher naive T cell
activation (15) and stronger
Th1-type responses both in vitro and in vivo in
inflammatory disease and systemic autoimmunity (16). Reducing the expression of SOCS1
facilitated an effective immune response against HNSCC (17).
In the present study, we postulated that
immunotherapy using SOCS1-silenced DCs pulsed with EGFR may be an
effective approach and provide a novel strategy for HNSCC
treatment. In the present study, SOCS1-silenced DCs pulsed with the
GST-EGFR fusion protein were used to trigger CTL activity in a
mouse model, and the preventive and therapeutic antitumor effects
on Hep-2 cells were observed in vitro by a lactate
dehydrogenase (LDH) release assay.
Materials and methods
Cell culture
The Hep-2 human laryngeal carcinoma cell line, was
obtained from the Chinese PLA General Hospital. Cells were cultured
in RPMI-1640 (Gibco Life Technologies, Carlsbad, CA, USA),
supplemented with 10% fetal bovine serum (FBS), 100 µg/ml
penicillin and 100 µg/ml streptomycin (all obtained from GE
Healthcare Life Sciences, Logan, UT, USA) in humidified 5%
CO2 at 37°C. Trypsin solution (0.25%; GE Healthcare Life
Sciences) was used to detach cells from the culture flask. Culture
medium was changed every 2 days.
Animals
Male SD mice were purchased from the Experimental
Center of Yangzhou University (Jiangsu, China) and housed in the
Central Animal Facility at Liaoning Medical College. All the mice
were aged 6-8 weeks at the start of the investigation and their
body weight was in the range of 160–180 g. The animals were
acclimated for at least 1 week before any of the experiments were
undertaken. All studies involving mice were approved by the
Institute's Animal Care and Use Committee (Liaoning Provincial
Science and Technology Department).
Construction of the expression vector
encoding the extracellular domain (ECD) of EGFR
The EGFR-wt plasmid was obtained from Addgene
(Cambridge, CA, USA). The plasmid corresponding to the ECD of EGFR
was amplified by reverse transcription polymerase chain reaction
(RT-PCR). The upstream primer, 3′-gCTGGAGGAAAAGAAAGTTTGCCA AGG-5′
includes a SmaI excision site. The downstream primer,
3′-GGGGACGGGATCTTAGGCCC-5′ contains a SmaI excision site and
a stop codon. Total RNA was reverse-transcribed using the specific
downstream primer. PCR cycle parameters were 95°C for 30 sec, 66°C
for 30 sec, and 72°C for 90 sec, for a total 30 cycles, followed by
a 10 min final extension at 72°C. The RT-PCR product, a 2-kb
fragment, was cloned into the pGEM-T easy vector for sequence
analysis. The fragment encoding for the ECD of EGFR was recovered
using SmaI enzymes and cloned into SmaI sites of the
pGEX-4T-2 expression vector, generating the pGEX-4T-2-EGFR
plasmid.
Expression and purification of
GST-EGFR fusion and GST proteins
The pGEX-4T-2-EGFR and pGEX-4T-2 plasmids were
transformed into Escherichia coli (E. coli) XL1-Blue,
respectively, and the expression of GST-EGFR fusion or GST proteins
was induced with 0.1 mM isopropyl β-D-thiogalactoside (IPTG) at
23°C overnight. The GST fusion protein used in the present study
was to facilitate the expression of E. coli and purification
of the recombinant protein. The expression of the proteins was
identified by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and protein immunoblots (western
blotting) with an anti-GST antibody. The MagneGST Protein
Purification System was used to purify the soluble GST-EGFR fusion
and GST proteins. The proteins were eluted with buffer (50 mM
glutathione, pH 7.0–8.0; 50 mM Tris-HCl, pH 8.1) and the purified
proteins were examined by SDS-PAGE.
Generating DCs
Bone marrow-derived DCs were isolated and maintained
as previously described with minor modifications (18). Briefly, bone marrow cells were
obtained from femurs and tibias of SD mice and filtered through a
nylon mesh. Red blood cells (RBC) were depleted with lysis buffer
and washed with phosphate buffered-saline (PBS) twice. Then, the
cells were seeded at 2×106 cells/100-mm dish in
RPMI-1640 medium supplemented with 10% FBS and 500 U/ml rmGM-CSF,
500 U/ml rmIL-4 and 500 U/ml TNF-α at 37°C and 5% CO2.
On days 4, 6 and 7, half of the culture supernatants were collected
and centrifuged, respectively. Each cell pellet was re-suspended in
5 ml of a fresh RPMI-1640 medium containing 500 U/ml rmGM-CSF,
rmIL-4 and TNF-α and returned to the original plate. On day 7, the
DCs were harvested for subsequent experiments.
Synthesis of the siSOCS1 gene and gene
transfection
The siRNA molecules used for suppression of the
murine SOCS1 gene and the negative control siRNA (which does not
target any sequence present in the murine genome) were all obtained
from Biosci Co., Ltd. (Hangzhou, China). Recombinant adenovirus
expression vector GV248 was used as the vector for SOCS1-siRNA.
Transient transfection of siRNA was carried out with Lipofectamine
2000 reagent (Invitrogen, Waltham, MA, USA) according to the
manufacturer's instructions.
Quantitative RT-PCR
The relative expression of SOCS1 mRNA in transfected
DCs was evaluated by quantitative real-time PCR (qRT-PCR). Total
RNA was prepared using TRIzol reagent (Invitrogen) according to the
manufacturer's instructions. cDNA was synthesized with 1 µg of
total RNA by reverse transcriptase (Takara, Shiga, Japan). For
quantitative determination of SOCS1 expression, qRT-PCR analysis
was performed with LightCycler (Roche Diagnostics, Basel,
Switzerland).
Western blot analysis
In western blot analysis, cell lysates were
separated by SDS-polyacrylamide gel electrophoresis, transferred
onto polyvinylidene fluoride (PVDF) membranes, and probed with a
rabbit monoclonal anti-SOCS1 antibody (cat. no. 10-P1074; ARP) was
reconstitutioned with distilled water. Bound antibodies were
detected using a horseradish peroxidase (HRP)-labeled goat
anti-rabbit IgG (cat. no. BG08T-1; Genemark Technology Co., Ltd.,
Tainan, Taiwan), and then were developed using
3,3,5,5-tetramethylbenzidine (TMB; cat. no. 1215-100; BioVision,
Inc., Milpitas, CA, USA).
Pulsing DCs and flow cytometric
analysis
After adenovirus SOCS1 transfection for 4 h, DCs
were incubated with GST-EGFR fusion protein (20, 50 and 100 µg/ml)
for 12 h. As a control, unpulsed DCs were also cultured for 12 h.
Then, DCs were collected, and the expression of surface molecules
on DCs was quantified by flow cytometry using FITC- or
PE-conjugated Ab (anti-CD83, anti-HLA-DR and anti-CD86). The
samples were analyzed using a FACSCalibur flow cytometer and
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
T-cell proliferation assays
T lymphocytes were isolated from the spleens of SD
mice by negative immunomagnetic selection using a Ficoll-Hypaque
kit (GE Healthcare Life Sciences, Buckinghamshire, UK). For the
proliferation assays, T cells were seeded into 96-well plates at a
density of 2×106/ml. The cells were divided into six
groups: blank (no cells), control (T cells), DCs (T cells + DCs),
EGFR (T cells + EGFR-DCs), SOCS1-silenced (T cells +
SOCS1-siRNA-DCs), EGFR + SOCS1-silenced (T cells +
EGFR-SOCS1-siRNA-DCs). T cells were co-cultured with DCs at
different DC/T cell ratios (1:5, 1:10 and 1:20) in RPMI-1640 for 3
days at 37°C in triplicate. After 3 days of incubation, T-cell
proliferation status was examined by MTT assay. Cells were
incubated at 37°C for 4 h following the addition of 20 µl MTT to
each well and the absorbance at 490 nm was detected using a
microplate reader.
Cytotoxic T lymphocyte assay
For the cytotoxic T lymphocyte (CTL) assay,
1×106 Hep-2 cells were subcutaneously injected into the
left flanks of 6-week-old SD mice. After 10 days, the mice that had
a mean tumor size of 10 mm were chosen for the following
experiments. The mice were divided into 3 groups, and were
subcutaneously injected into the right flanks with untreated DCs,
EGFR-pulsed DCs and EGFR-SOCS1 silenced pulsed DCs, respectively.
After 7 days, T cells were separated and enriched from each group
of mice as previously described. Hep-2 cells were seeded in 96-well
plates at a density of 2×106/ml. The Hep-2 cells were
divided into 6 groups: experimental (Hep-2 cells plus T cells from
DCs, EGFR-DCs and EGFR plus SOCS1-silenced DC mice), blank (no
cells), spontaneous (Hep-2 cells only) and the maximum group (Hep-2
cells plus cell lysis buffer). Then, Hep-2 cells were co-cultured
with T cells at different T/Hep-2 cell ratios (1:25, 1:50 and
1:100) in RPMI-1640 for 30 min at 37°C in triplicate. After 30 min
of incubation, samples of the cultured wells were then harvested
and the cytotoxicity of the T cells against Hep-2 cells was
determined using an LDH activity assay kit. Wavelength (450 nm)
absorbance data were collected using a standard 96-well plate
reader. The percentage of specific lysis was calculated as:
(experimental - spontaneous)/(maximum - spontaneous) × 100%.
Statistical analysis
Data are presented as the means ± SD. The
significance of differences between the values of different groups
was evaluated by Student's t-test or ANOVA test, and p<0.05 was
considered to indicate a statistically significant result. SPSS
21.0 software was used for these analyses.
Results
Construction of the expression vector
encoding the ECD of EGFR and GST-EGFR fusion protein expression and
purification
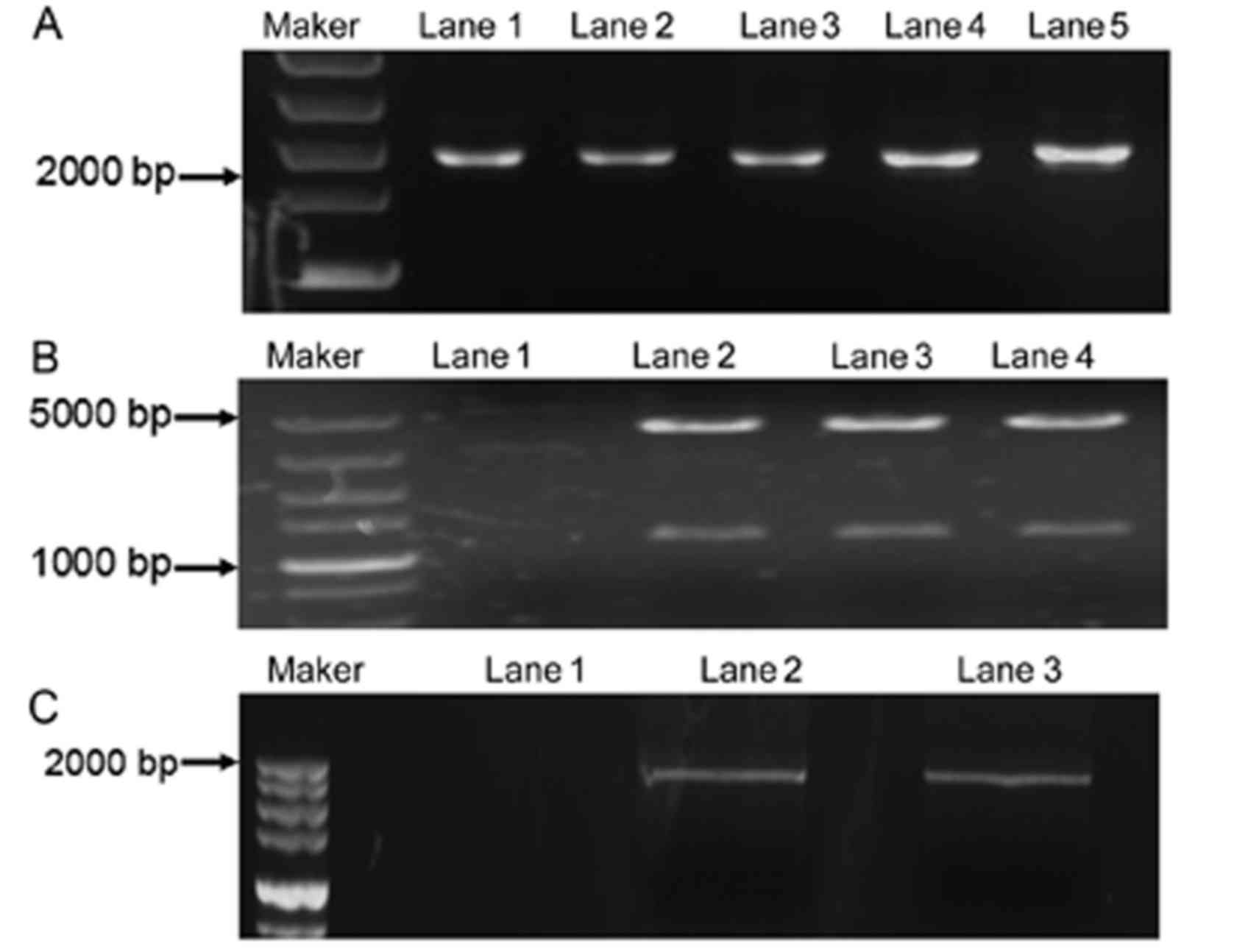
The EGFR-wt plasmid was used as a template for
EGFR-ECD cDNA expression. The RT-PCR product was separated by 1%
agarose gel electrophoresis and stained with ethidium bromide. The
expected length was 1.6 kbp (Fig.
1A). The sequence of the ECD of EGFR was confirmed, by
dideoxynucleotide sequencing analysis, to be identical to those
previously reported (data not shown) (19). Then, the EGFR-ECD cDNA was
successfully cloned into the pGEX-4T-2 expression vector as
previously described. The EGFR-ECD expression was reconfirmed by
the RT-PCR (Fig. 1B). The
pGEX-4T-2-EGFR plasmid was established by SamI digestion.
The product was separated by 1% agarose, and 2 expected fragments
appeared (Fig. 1C). The expression
of the soluble GST-EGFR or GST was analyzed by SDS-PAGE and western
blotting. The proteins were purified by the MagneGST Protein
Purification System and confirmed by SDS-PAGE, revealing the
protein bands again (data not shown). The purity of the acquired
proteins was >90%.
Preparation, sensitization and
characterization of DCs
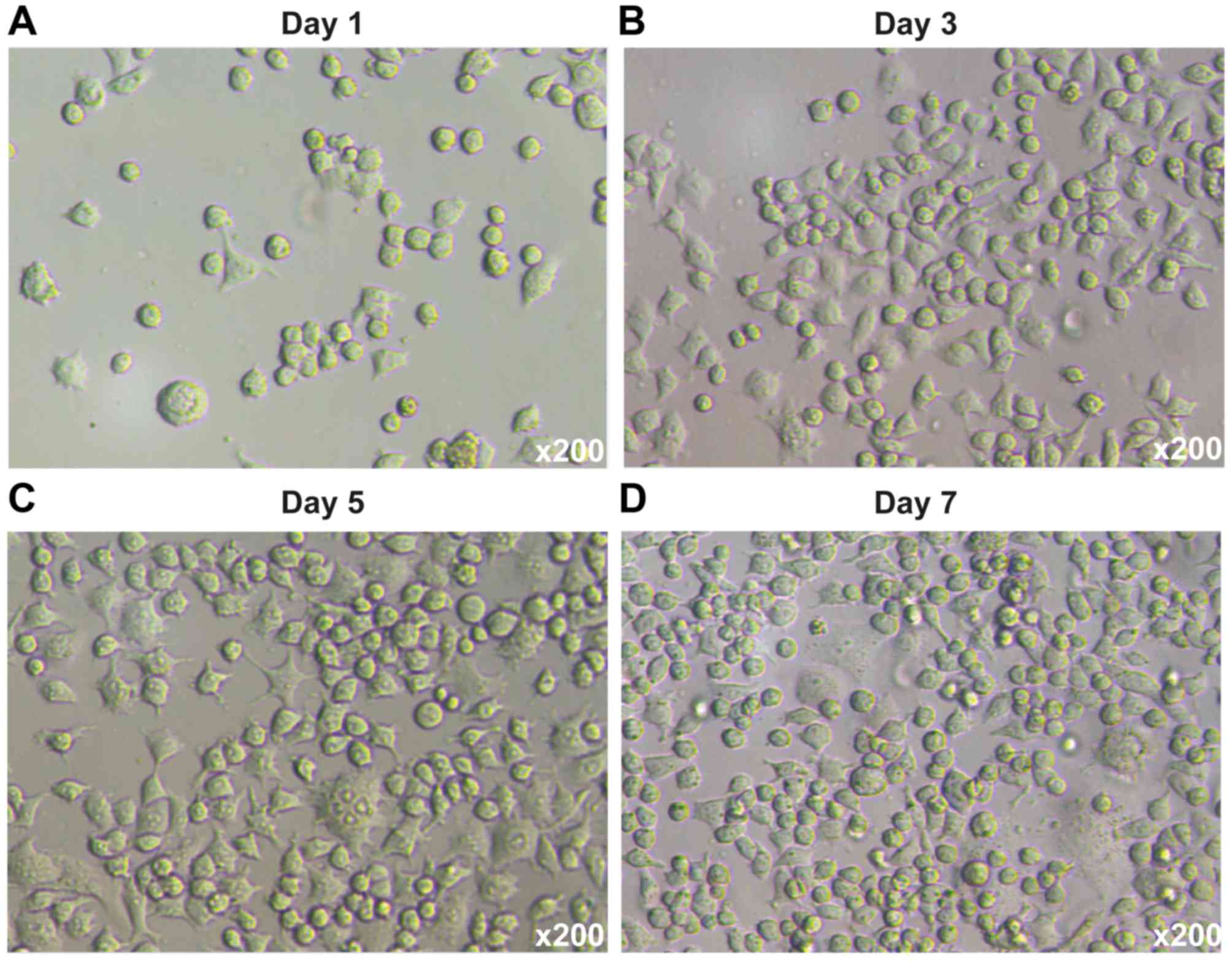
Bone marrow-derived DCs were isolated from SD mice
and maintained as previously described (20). The DCs were cultured in media
supplemented with GM-CSF, rmIL-4 and TNF-α for 7 days. On day 7,
the DCs were harvested for subsequent experiments. The phase
contrast micrographs illustrating the development and isolation of
DCs are shown in Fig. 2. For DC
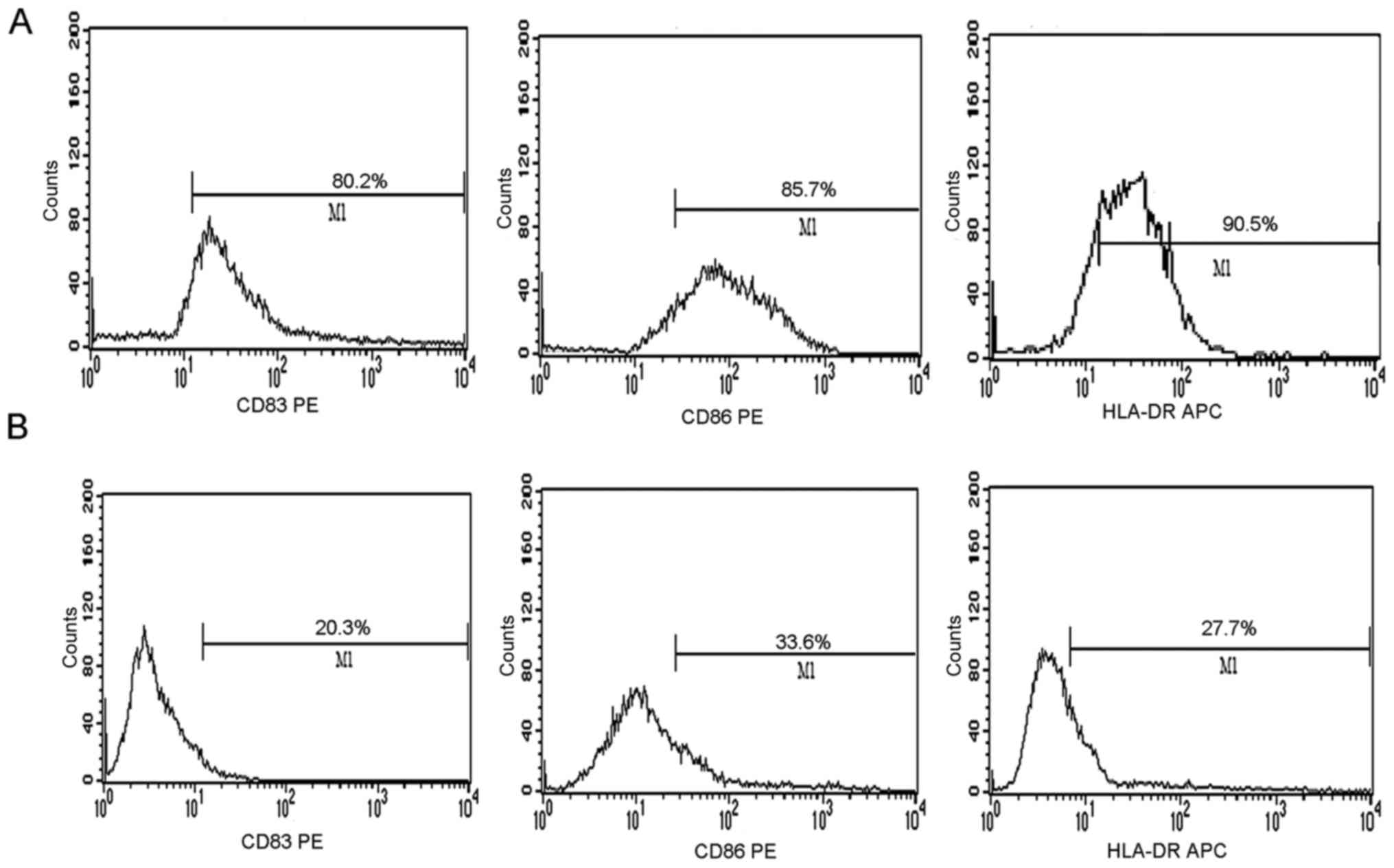
sensitization, the DCs were incubated with GST-EGFR fusion protein
at different concentrations. The degree of expression of CD83, CD86
and HLA-DR on the DC surfaces was evaluated by flow cytometry after
a 48-h stimulation with proteins. The positive cells among the DCs
pulsed with GST-EGFR were 80.2, 85.7 and 90.5%, respectively. These
results were greater than those of the DCs unpulsed with protein,
in which the positive cells were 20.3, 33.6 and 27.7%, respectively
(Fig. 3A and B).
SOCS1 silencing effectively enhances
T-lymphocyte proliferation
The SOCS1 gene is an important regulator that
suppresses DC maturation and cytokine production during
inflammatory response. To clarify the ability of T cell
differentiation under SOCS1 gene silencing, we compared the
proliferation of T cells after incubation with SOSC1-siRNA DCs or
negative control DCs after being pulsed with EGFR fusion protein by
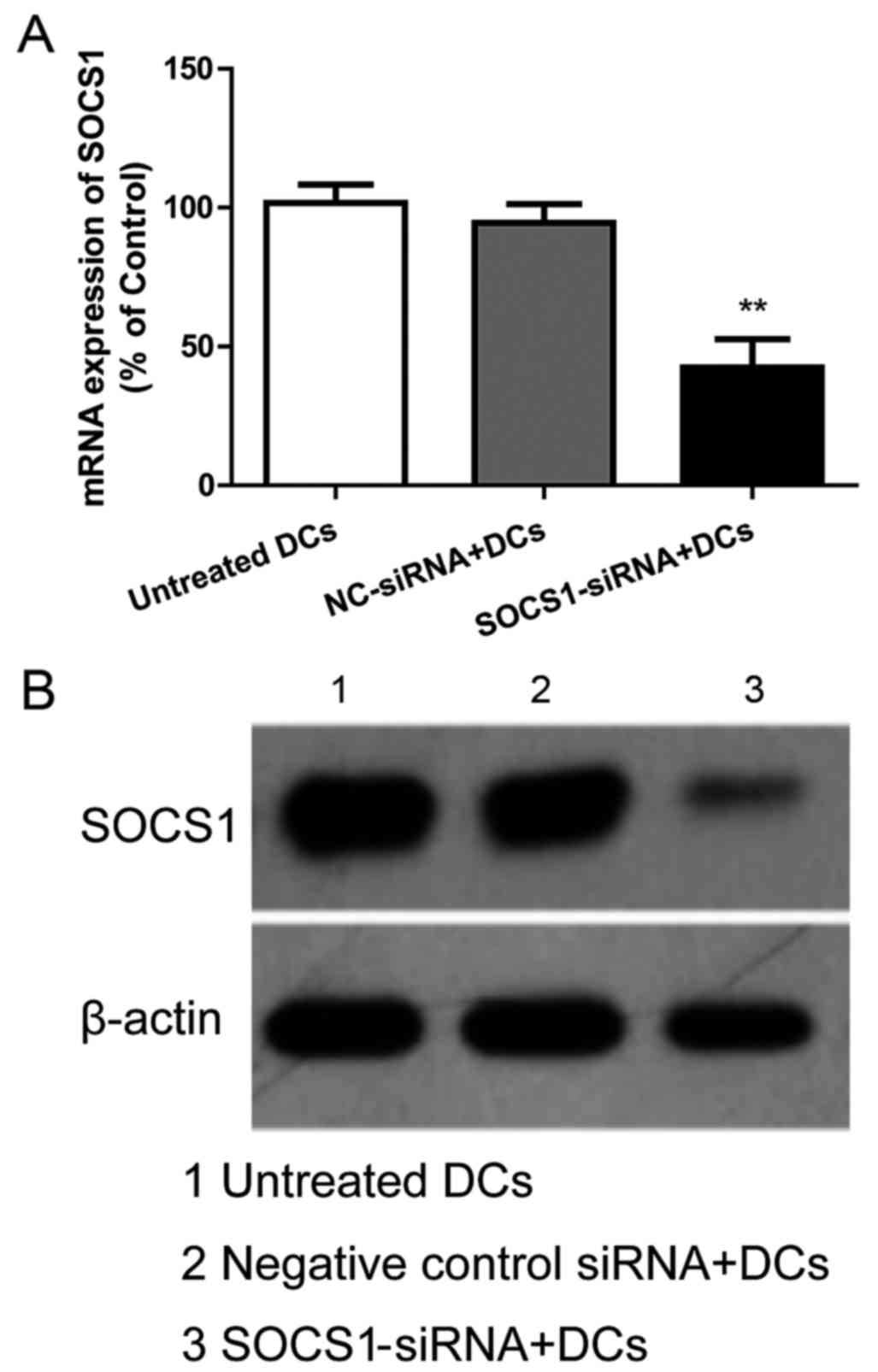
MTT assay. The results in Fig. 4
indicated that the mRNA and protein expression of SOCS1 in DCs were
knocked down by 50–70%. As shown in Fig. 5, all of the EGFR-pulsed DCs,
SOCS1-silenced DCs and EGFR plus SOCS1-silenced DCs had an enhanced
capacity to stimulate the proliferation of T lymphocytes at DC/T
cell ratios of 1:5 and 1:10. As expected, EGFR plus SOCS1-silenced
DCs had the strongest effects on T cell proliferation. At the DC/T
cell ratio of 1:20, only EGFR plus SOCS1-silenced DCs enhanced
T-cell proliferation while EGFR-pulsed and SOCS1-silenced DCs had
no effect on T-cell proliferation.
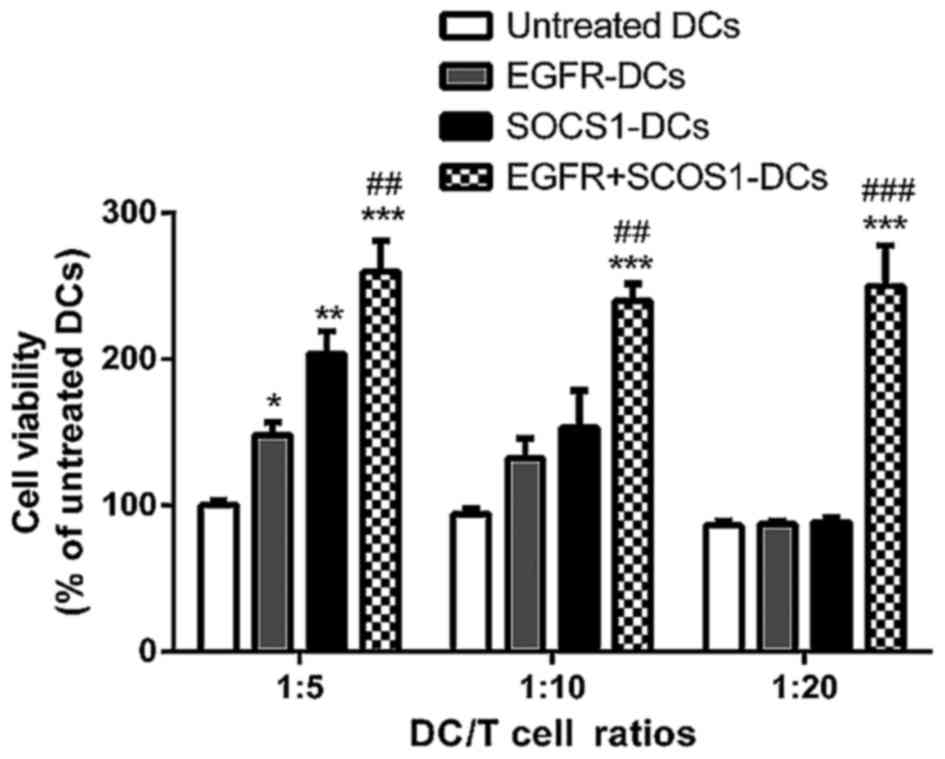 | Figure 5.SOCS1-siRNA effectively enhances
T-lymphocyte proliferation. MTT assays indicated that DCs treated
with EGFR, SOCS1-siRNA and EGFR plus SOCS1-siRNA have enhanced
capacity to activate T-cell proliferation at different DC/T cell
ratios, including 1:5, 1:10 and 1:20. The mean ± SD of data from 3
experiments is shown. (*p<0.05, **p<0.01, ***p<0.001,
compared with the untreated DC groups; ##p<0.01,
###p<0.001, compared with the EGFR-DC groups). SOCS1,
cytokine signaling suppressor 1; DCs, dendritic cells; EGFR,
epidermal growth factor receptor. |
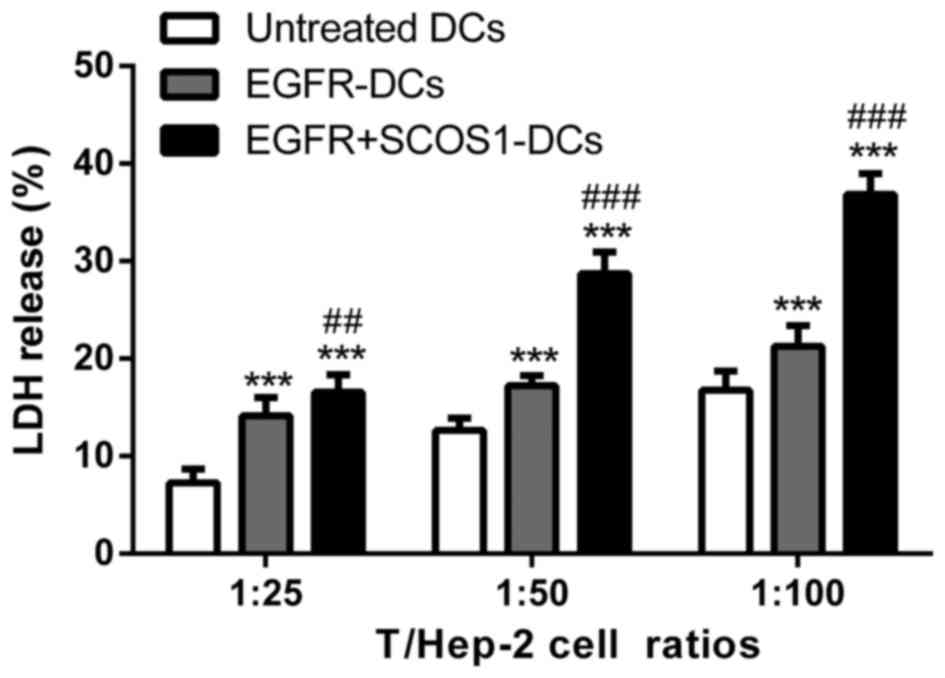
EGFR plus SOCS1-silenced DC
immunization effectively enhances cytotoxic T-lymphocyte activity
against Hep-2 cells
A CTL assay was performed at different T/Hep-2 cell
ratios of 1:25, 1:50 and 1:100. The splenic T cells isolated from
both EGFR-pulsed DC-immunized mice and EGFR plus SOCS1-silenced
DC-immunized mice enhanced the cytotoxicity against Hep-2 cells,
while T cells from EGFR plus SOCS1-silenced DC-immunized mice
exhibited significantly higher cytotoxicity than those from
EGFR-DC-immunized mice (Fig.
6).
Discussion
The development of cancer immunotherapy has led to
great clinical advances and provided a new weapon against cancer
(21). The prerequisite of
immunotherapy is to identify an efficient tumor-specific antigen.
EGFR is a tumor-associated antigen of HNSCC. More than 80% of head
and neck tumors overexpress EGFR (22). The knowledge that EGFR is
overexpressed in the majority of HNSCC cells provides a rationale
for the use of anti-EGFR therapies (23,24).
Cetuximab, a chimeric monoclonal antibody (mAb) targeting the
extracellular portion of EGFR, was found to enhance survival when
combined with radiotherapy in patients with advanced HNSCC
(25). Therefore, EGFR is an ideal
target in antitumor immunotherapy of HNSCC. Dendritic cells (DCs)
are the most potent antigen-presenting cells in the immune system
and have the unique ability to take up and efficiently present
antigens to naive T lymphocytes. DCs can also interact with B cells
and natural killer (NK) cells, thus bridging the gap between innate
and adaptive immunities. DC vaccinations have been demonstrated to
be safe and efficient in inducing the expansion of circulating CTLs
that are specific for tumor antigens (26). Thus, in the present study, we pulsed
DCs with a GST-EGFR fusion protein in order to provoke the specific
immune response targeted against EGFR and HNSCC.
Several cancer vaccine studies have suggested that
the therapeutic vaccination outcome (success or failure) is
correlated with the vaccine-induced expansion of antigen-specific
effector T cells (27,28). In the present study, we compared
EGFR-pulsed DCs with control DCs and found that the former
displayed even higher expression of cell surface molecules, such as
CD83, CD860 and HLA-DR (which are common indicators of the
maturation of DCs). Effective therapeutic antitumor activities of
the EGFR-pulsed DC vaccine against SCC tumor cells were confirmed
in a previous study (29). Similar
to the results of previous studies, EGFR fusion protein-immunized
DCs had the strongest effects on activated T-cell differentiation.
Meanwhile, the strongest CTL response against Hep-2 cells was found
in the group immunized with EGFR-pulsed DCs compared with the
control groups. CTLs are believed to be critical effectors of
antitumor immune responses (30),
whereas the CD4+ T cells, characterized by the secretion
of IFN-γ, are primarily responsible for activating and regulating
the development and persistence of CTLs (31). Previous studies in mice revealed
that the in vivo induction of CTL responses, particularly
those induced through cross-priming of exogenous antigens by DCs,
is dependent on a CD4+ T-cell response (32,33).
Moreover, CD4+ T cells are also essential for the
activation of memory CTLs into tumor killer cells (34). Therefore, our in vitro
observations indicated that GST-EGFR-pulsed DCs induced effective
therapeutic and preventive antitumor immunity against Hep-2
cells.
In addition to the expression of co-stimulatory
molecules that facilitate an immune response, DCs are also equipped
with negative feedback mechanisms that control their cytokine
function. According to previous literature, the SOCS1 gene is an
important regulator that suppresses DC maturation and cytokine
production during inflammatory response. In the present study, we
demonstrated that a SOCS1-siRNA approach can be used to silence
negative regulatory molecules in DCs and thereafter to modulate
immune response. Although, the abolition of SOCS1 in DCs could by
itself induce the expression of co-stimulatory molecules as
suggested by other studies (35,36),
we found that there was no significant difference in surface
molecule expression between EGFR plus SOCS1-silenced DCs and only
EGFR-pulsed DCs (data not shown). Ablation of SOCS1 in
antigen-presenting cells has been reported to enhance cellular
immune and subsequent cytokine responses in mice when they are
challenged by a pathogen (37). Our
data revealed that reversing the immunity-attenuating mechanism of
SOCS1 activated the function of DCs, including the promotion of DC
maturation and activation of T-cell differentiation. Concurrently,
SOCS1-siRNA-treated DCs also mediated innate immunity by possessing
a stronger CTL activity against Hep-2 cells. Compared to the
EGFR-pulsed only DCs, EGFR plus SOCS1-silenced DCs had a stronger
effect on T-cell proliferation and CTL against Hep-2 cells. These
results revealed that the targeted modulation of SOCS1 expression
in DCs could be exploited as a novel molecular adjunct to improve
the potency of vaccine-induced T cells against HNSCC.
In summary, the present study revealed that SOCS1
silencing can be used to silence immunosuppressive molecules in
GST-EGFR pulsed DCs, enhance the T-cell proliferation and the CTL
activity against Hep-2 cells in vitro. This provides a
promising approach for increasing antitumor activity of
EGFR-targeted therapy of HNSCC.
Acknowledgements
The present study was funded by the Youth Foundation
of the First Affiliated Hospital of Liaoning Medical University and
the Liaoning Science and Technology Project (2012225019).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao L, Hong WK and Papadimitrakopoulou VA:
Focus on head and neck cancer. Cancer Cell. 5:311–316. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37 Suppl 4:S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woodburn JR: The epidermal growth factor
receptor and its inhibition in cancer therapy. Pharmacol Ther.
82:241–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiley HS: Trafficking of the ErbB
receptors and its influence on signaling. Exp Cell Res. 284:78–88.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 Suppl 4:S9–S15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS: Targeted therapy in
non-small-cell lung cancer. Oncology. 16 Suppl 9:19–24.
2002.PubMed/NCBI
|
|
9
|
Galanis E, Buckner J, Kimmel D, Jenkins R,
Alderete B, O'Fallon J, Wang CH, Scheithauer BW and James CD: Gene
amplification as a prognostic factor in primary and secondary
high-grade malignant gliomas. Int J Oncol. 13:717–724.
1998.PubMed/NCBI
|
|
10
|
Kabolizadeh P, Kubicek GJ, Heron DE,
Ferris RL and Gibson MK: The role of cetuximab in the management of
head and neck cancers. Expert Opin Biol Ther. 12:517–528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Celluzzi CM, Mayordomo JI, Storkus WJ,
Lotze MT and Falo LD Jr: Peptide-pulsed dendritic cells induce
antigen-specific CTL-mediated protective tumor immunity. J Exp Med.
183:283–287. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mayordomo JI, Zorina T, Storkus WJ,
Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM,
Deleo AB, et al: Bone marrow-derived dendritic cells pulsed with
synthetic tumour peptides elicit protective and therapeutic
antitumour immunity. Nat Med. 1:1297–1302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubo M, Hanada T and Yoshimura A:
Suppressors of cytokine signaling and immunity. Nat Immunol.
4:1169–1176. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanada T, Yoshida H, Kato S, Tanaka K,
Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M and Yoshimura A:
Suppressor of cytokine signaling-1 is essential for suppressing
dendritic cell activation and systemic autoimmunity. Immunity.
19:437–450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanada T, Tanaka K, Matsumura Y, Yamauchi
M, Nishinakamura H, Aburatani H, Mashima R, Kubo M, Kobayashi T and
Yoshimura A: Induction of hyper Th1 cell-type immune responses by
dendritic cells lacking the suppressor of cytokine signaling-1
gene. J Immunol. 174:4325–4332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi D, Li D, Yin Q, Qiu Y, Yan H, Shen Y,
Lu G and Liu W: Silenced suppressor of cytokine signaling 1 (SOCS1)
enhances the maturation and antifungal immunity of dendritic cells
in response to Candida albicans in vitro. Immunol Res. 61:206–218.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reiter JL, Threadgill DW, Eley GD, Strunk
KE, Danielsen AJ, Sinclair CS, Pearsall RS, Green PJ, Yee D,
Lampland AL, et al: Comparative genomic sequence analysis and
isolation of human and mouse alternative EGFR transcripts encoding
truncated receptor isoforms. Genomics. 71:1–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabrilovich DI, Nadaf S, Corak J,
Berzofsky JA and Carbone DP: Dendritic cells in antitumor immune
responses. II. Dendritic cells grown from bone marrow precursors,
but not mature DC from tumor-bearing mice, are effective antigen
carriers in the therapy of established tumors. Cell Immunol.
170:111–119. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herbst RS and Shin DM: Monoclonal
antibodies to target epidermal growth factor receptor-positive
tumors: A new paradigm for cancer therapy. Cancer. 94:1593–1611.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharafinski ME, Ferris RL, Ferrone S and
Grandis JR: Epidermal growth factor receptor targeted therapy of
squamous cell carcinoma of the head and neck. Head Neck.
32:1412–1421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferris RL, Jaffee EM and Ferrone S: Tumor
antigen-targeted, monoclonal antibody-based immunotherapy: Clinical
response, cellular immunity, and immunoescape. J Clin Oncol.
28:4390–4399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paczesny S, Banchereau J, Wittkowski KM,
Saracino G, Fay J and Palucka AK: Expansion of melanoma-specific
cytolytic CD8+ T cell precursors in patients with
metastatic melanoma vaccinated with CD34+
progenitor-derived dendritic cells. J Exp Med. 199:1503–1511. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Welters MJ, Kenter GG, de Vos van
Steenwijk PJ, Löwik MJ, Berends-van der Meer DM, Essahsah F,
Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, et al:
Success or failure of vaccination for HPV16-positive vulvar lesions
correlates with kinetics and phenotype of induced T-cell responses.
Proc Natl Acad Sci USA. 107:pp. 11895–11899. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang BB, Jiang H, Chen J, Zhang X, Ye JJ
and Cao J: Dendritic cells pulsed with GST-EGFR fusion protein:
Effect in antitumor immunity against head and neck squamous cell
carcinoma. Head Neck. 32:626–635. 2010.PubMed/NCBI
|
|
30
|
Melief CJ: Tumor eradication by adoptive
transfer of cytotoxic T lymphocytes. Adv Cancer Res. 58:143–175.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knutson KL and Disis ML: Tumor
antigen-specific T helper cells in cancer immunity and
immunotherapy. Cancer Immunol Immunother. 54:721–728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bennett SR, Carbone FR, Karamalis F,
Miller JF and Heath WR: Induction of a CD8+ cytotoxic T
lymphocyte response by cross-priming requires cognate
CD4+ T cell help. J Exp Med. 186:65–70. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schoenberger SP, Toes RE, van der Voort
EI, Offringa R and Melief CJ: T-cell help for cytotoxic T
lymphocytes is mediated by CD40-CD40L interactions. Nature.
393:480–483. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao FG, Khammanivong V, Liu WJ, Leggatt
GR, Frazer IH and Fernando GJ: Antigen-specific CD4+
T-cell help is required to activate a memory CD8+ T cell
to a fully functional tumor killer cell. Cancer Res. 62:6438–6441.
2002.PubMed/NCBI
|
|
35
|
Kobayashi T and Yoshimura A: Keeping DCs
awake by putting SOCS1 to sleep. Trends Immunol. 26:177–179. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu Q, Qin X, Qian G, Jiang S, Li H, Jiang
M, Li X, Chen SY and Zang YQ: SOCS1 silencing can break high-dose
dendritic cell immunotherapy-induced immune tolerance. Mol Med Rep.
1:61–70. 2008.PubMed/NCBI
|
|
37
|
Wurtz O, Bajénoff M and Guerder S:
IL-4-mediated inhibition of IFN-gamma production by CD4+
T cells proceeds by several developmentally regulated mechanisms.
Int Immunol. 16:501–508. 2004. View Article : Google Scholar : PubMed/NCBI
|