Introduction
Lung cancer is the leading cause of cancer-related
deaths in both men and women and its incidence has been increasing
over the past few decades (1,2). Lung
cancer is clinically classified into small-cell lung cancer (SCLC)
and non-small cell lung cancer (NSCLC). It is estimated that over
80% of lung cancer cases are NSCLC (3). NSCLC is further divided into squamous
carcinoma, adenocarcinoma and large cell carcinoma based on
histological classification. In addition, the classification of
NSCLC into subtypes using genetic markers has great importance in
relation to the selection of the therapeutic procedure (4). Squamous cell carcinoma is mainly
located in the large airways such as the bronchial tree. Large cell
carcinoma may occur in any part of the lung and its growth and
metastasis are aggressive (5,6).
Chemotherapy is the primary treatment option in most cases of lung
cancer (7,8). However, treatment with traditional
drugs such as cisplatinum is not considered ideal due to its
adverse effects and drug resistance (9). Therefore, research has focused on
discovering new chemical drugs. Conversely, natural medicine has
certain advantages, such as a wide variety of compounds with low
toxicity, that could serve as a source of new drugs.
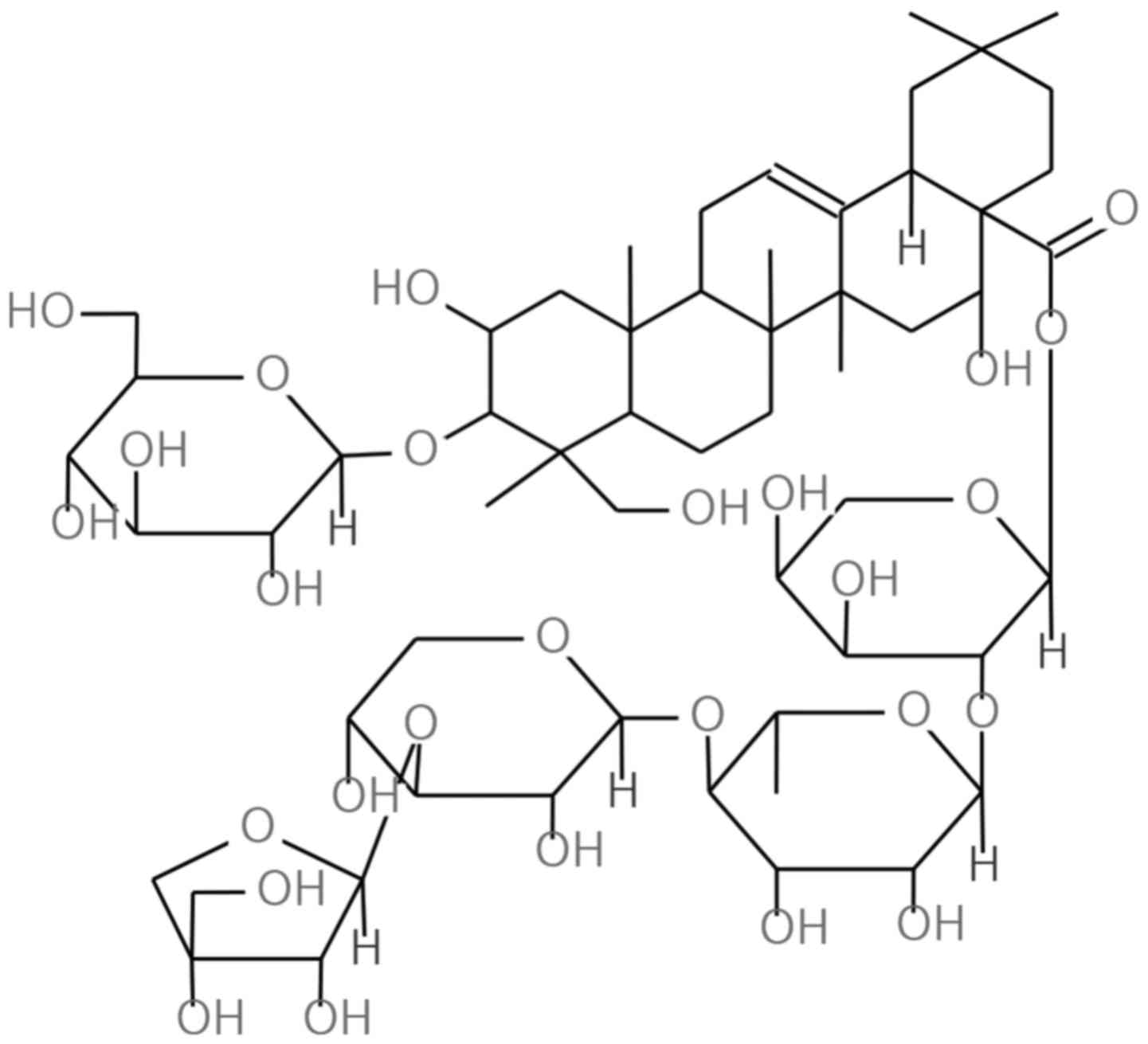
Platycodon grandiflorum (Jacq.) A. DC. (P.
grandiflorum) is a well known medicinal plant in East Asian
countries. P. grandiflorum has been reported to be a plant
that contains rich triterpenoid saponins such as platycodin D, D2,
D3, deapioplatycodin D, D2, polygalacin D (PGD) and platyconic acid
A (10). PGD is classified as a
triterpenoid saponin and is one of the main chemical components of
P. grandiflorum. PGD has a very similar structure to
Platycodin D, a well known anticancer agent (11–14).
Based on this relationship, we hypothesized that PGD would also
have anticancer effects. However, there are only a few studies
concerning the anticancer effects of PGD. In the present study, we
investigated the effects of PGD on NSCLC using the adenocarcinoma
cell line A549 and the large cell carcinoma NCI-H460 cell line.
Materials and methods
Reagents
RPMI-1640, fetal bovine serum (FBS), penicillin and
streptomycin were purchased from HyClone Laboratories Inc., (Logan,
UT, USA). Bovine serum albumin (BSA),
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS), 4,6-diamidino −2-phenylindole (DAPI) and dimethyl sulfoxide
(DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
TIMP-1 (mouse, monoclonal, sc-365905), Cdk2 (mouse, monoclonal,
sc-6248), cyclin A (rabbit, polyclonal, sc-751), cyclin E (rabbit,
polyclonal, sc-198), P-GSK3β (mouse, monoclonal, sc-81495), GSK3β
(mouse, monoclonal, sc-81462), PI3K (rabbit, polyclonal, sc-602)
and β-actin (mouse, monoclonal, sc-47778) were all purchased from
Santa Cruz Biotechnology Inc., (Santa Cruz, CA, USA). Cleaved PARP
(rabbit, polyclonal, #9541s), survivin (rabbit, monoclonal,
#2808s), Cdk4 (rabbit, monoclonal, #12790s), cyclin D (rabbit,
monoclonal, #2978s), p-Akt (rabbit, monoclonal, #4058s) and Akt
(rabbit, polyclonal, #9272s) were purchased from Cell Signaling
Technology (Beverly, MA, USA). Caspase-3 (active, rabbit,
polyclonal, ALX-210-807-c100), caspase-9 (active, rabbit,
polyclonal, ALX-210-816-c100), cIAP-1 (rat, monoclonal,
ALX-803-335-c100) and cIAP-2 (rat, monoclonal, ALX-803-341-c100)
were purchased from Enzo Life Sciences (Farmingdale, NY, USA) and
diluted 1:1,000. Primary antibodies were diluted 1:1,000.
Peroxidase-conjugated secondary antibodies (rabbit, monoclonal,
sc-2357; mouse, polyclonal, sc-2005; rat, polyclonal sc-2006;
dilution 1:2,000) were purchased from Santa Cruz Biotechnology. An
enhanced chemiluminescent (ECL) kit was obtained from Amersham
Pharmacia Biotech (Buckinghamshire, UK).
Preparation of PGD
For the preparation of PGD dried roots, P.
grandiflorum (5 kg) underwent extraction three times with
methanol at room temperature for seven days. Concentration of the
solvent gave a brown syrupy extract (1.4 kg) which was suspended in
water and then partitioned successively with ethyl acetate (63 g)
and n-butanol (130 g). The n-butanol layer was suspended in
H2O (2 liters) and poured into a Diaion HP-20 column (Φ
= 5.0×100 cm; Mitsubishi Chemicals Corp., Tokyo, Japan), which was
stabilized with H2O. The column was washed with
H2O (2 liters) and then eluted with MeOH (5 liters).
Polygalacin D2 (PD2) was prepared from the extract of P.
grandiflorum for experimental procedures using our method
(15).
Cell culture and proliferation
assay
Human lung cancer cells NCI-A549 and H460 were
obtained from the Korean Cell Line Bank (Seoul, Korea) and grown in
RPMI-1640 containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. BEAS-2B, a human bronchial epithelial cell line, was
purchased from the American Type Culture Collection (CRL-9609;
ATCC, Manassas, VA, USA) and maintained in bronchial epithelial
cell basal medium (BEBM) containing 1% penicillin/streptomycin. The
cell lines were maintained in a humidified incubator, with an
atmosphere of 5% CO2 at 37°C. The effect of PGD on cell
proliferation was determined using an MTS assay. The cells
(2×103/well) were seeded in 96-well culture plates and
incubated for 24 h. Various concentrations (0–40 µM) of PGD were
added and the cells were incubated for 48 h. The optical density
(OD) of each culture well was assessed at 490 nm using a microplate
reader (Titertek Multiskan; Flow Laboratories, North Ryde, NSW,
Australia). Cell proliferation was calculated using the following
formula: (mean absorbance value of treated cells/mean absorbance
value of untreated cells) × 100.
DAPI staining
Cell nuclear morphology was assessed by fluorescence
microscopy following DAPI staining. A549 and H460 cells were
treated with PGD for 48 h. The cells were washed with
phosphate-buffered saline (PBS), fixed with ice cold 70% ethanol,
stained with DAPI and incubated for 5 min at 37°C, in the dark. The
cells were then washed with PBS and visualized using a fluorescence
microscope (Carl Zeiss AG, Oberkochen, Germany).
Detection of apoptosis
The degree of apoptosis was assessed using Muse cell
analyzer (EMD Millipore, Billerica, MA, USA) according to the
manufacturers instructions. A549 and H460 cells were seeded in
6-well plates (105 cells/well) and treated with PGD (0,
10, 20 and 40 µM) for 48 h. The harvested cells were washed with 1
ml of PBS, resuspended in 150 µl FBS free media and 150 µl of the
Muse™ Annexin V Dead Cell kit (EMD Millipore) was added. Then, the
cells were incubated at room temperature (RT) for 20 min in the
dark. The samples were assessed with the Muse cell analyzer (EMD
Millipore).
Cell cycle analysis
A549 and H460 cells were seeded in 6-well plates
(105 cells/well) and treated with PGD (0, 10, 20 and 40
µM) for 48 h. The harvested cells were washed with 1 ml PBS and
fixed with ice-cold 70% EtOH over 3 h. After fixation, the cells
were washed with PBS and suspended in 200 µl PBS. Muse™ Cell cycle
reagent (200 µl; EMD Millipore) was then added and the cells were
incubated at RT for 30 min in the dark. The samples were assessed
with the Muse cell analyzer (Merck Millipore).
Western blotting
Cell extracts were subjected to SDS gel
electrophoresis and then transferred onto a polyvinylidene fluoride
membrane (PVDF; EMD Millipore). The membrane was blocked with 5%
skim milk for 1 h and probed with primary antibodies. After a
series of washes, the membrane was further incubated with a
secondary antibody conjugated with horseradish peroxidase (HRP).
The proteins were then supplemented with ECL prime western blotting
detection reagents (GE Healthcare Life Sciences, Little Chalfont,
UK). The bands were evaluated using the ImageQuant LAS 4000 mini
biomolecular imager (GE Healthcare Life Sciences).
Statistical analysis
The statistical analysis was performed using one-way
analysis of variance (ANOVA), followed by Scheffes test for
multiple comparisons. Data are presented as the means ± standard
deviations (n=5). All calculations were performed using SPSS
statistics 23 software (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
PGD inhibits the proliferation of
NSCLC cell lines
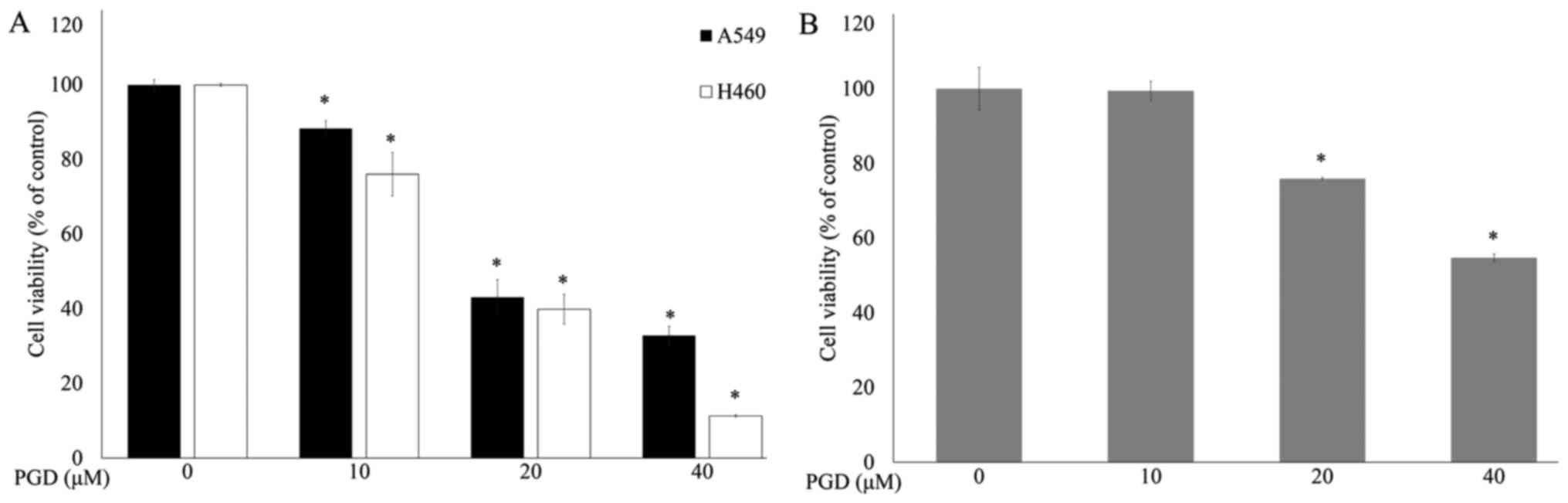
MTS assays were performed to determine the effects
of PGD (Fig. 1) on the
proliferation of lung cancer cell lines. Cancer cells were treated
with increasing concentrations of PGD. As shown in Fig. 2A, PGD inhibited the growth of the
treated cell lines in a dose-dependent manner. The IC50
concentrations of PGD were 26.49±1.45 µM in the A549 cells and
20.52±0.30 µM in the H460 cells. Treatment with PGD at 40 µM
reduced cell viability below 70% in normal airway bronchus cell
line (Fig. 2B). Additional
experiments were performed using PGD under 20 µM.
PGD induces apoptosis of NSCLC cell
lines
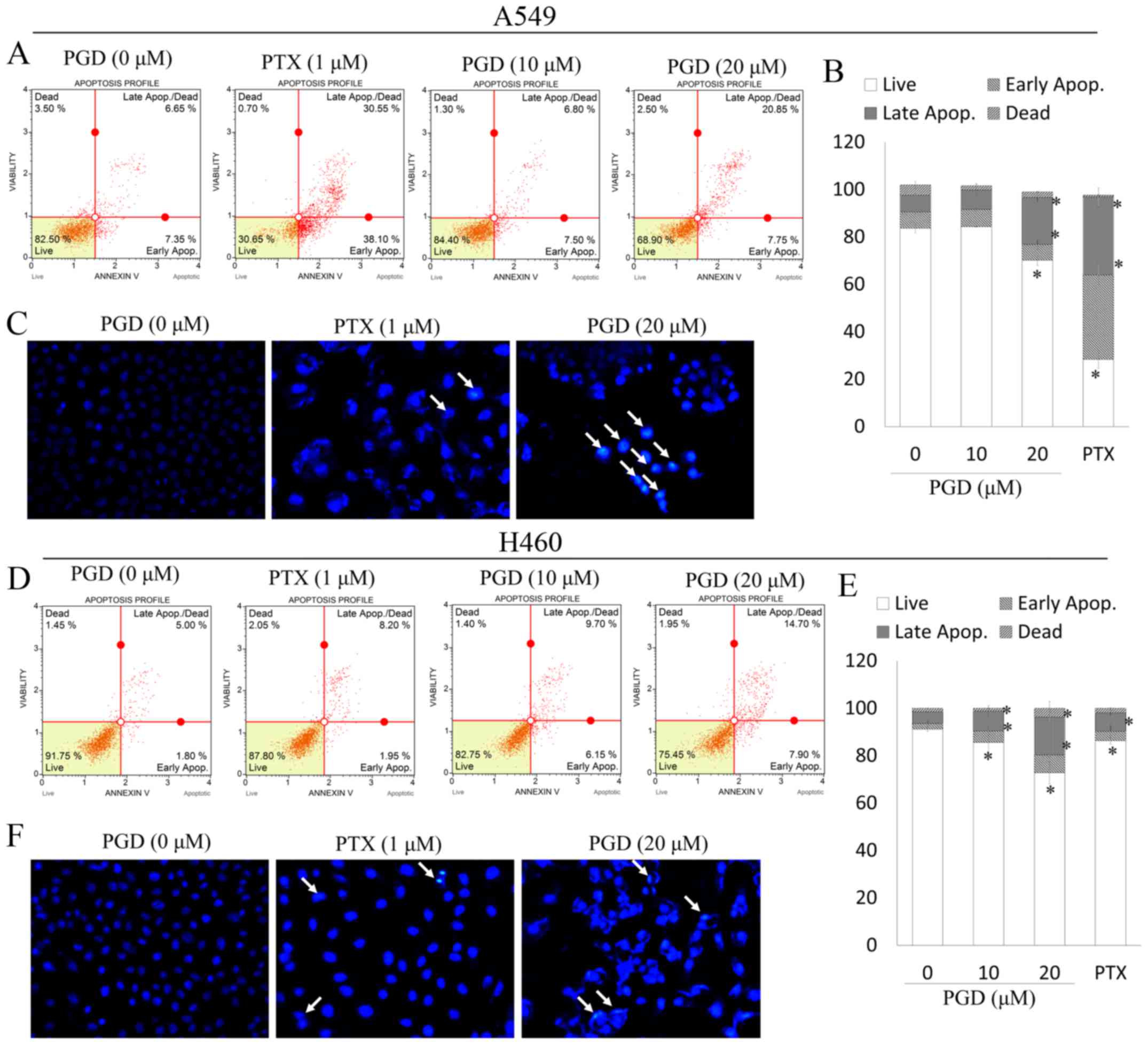
To determine whether the decreased viability of A549
and H460 cells occurs as a result of apoptosis, we investigated the
apoptotic effects of PGD in NSCLC cell lines. In the present study,
pemetrexed (PTX) was used as a control reagent to assess the
apoptotic effects of PGD. PTX has been widely used in the clinical
treatment of non-small cell lung cancer. As displayed in Fig. 3C and F, after treatment with PGD,
nuclear condensation (white arrows) was observed in A549 and H460
cells. In addition, treatment with PGD increased the proportion of
early and late apoptotic cells (Fig.
3A, B, D and E). Collectively, PGD-inhibited proliferation of
NSCLC cells was due to cell apoptosis.
PGD regulates the expression of
apoptosis-related proteins in NSCLC cells
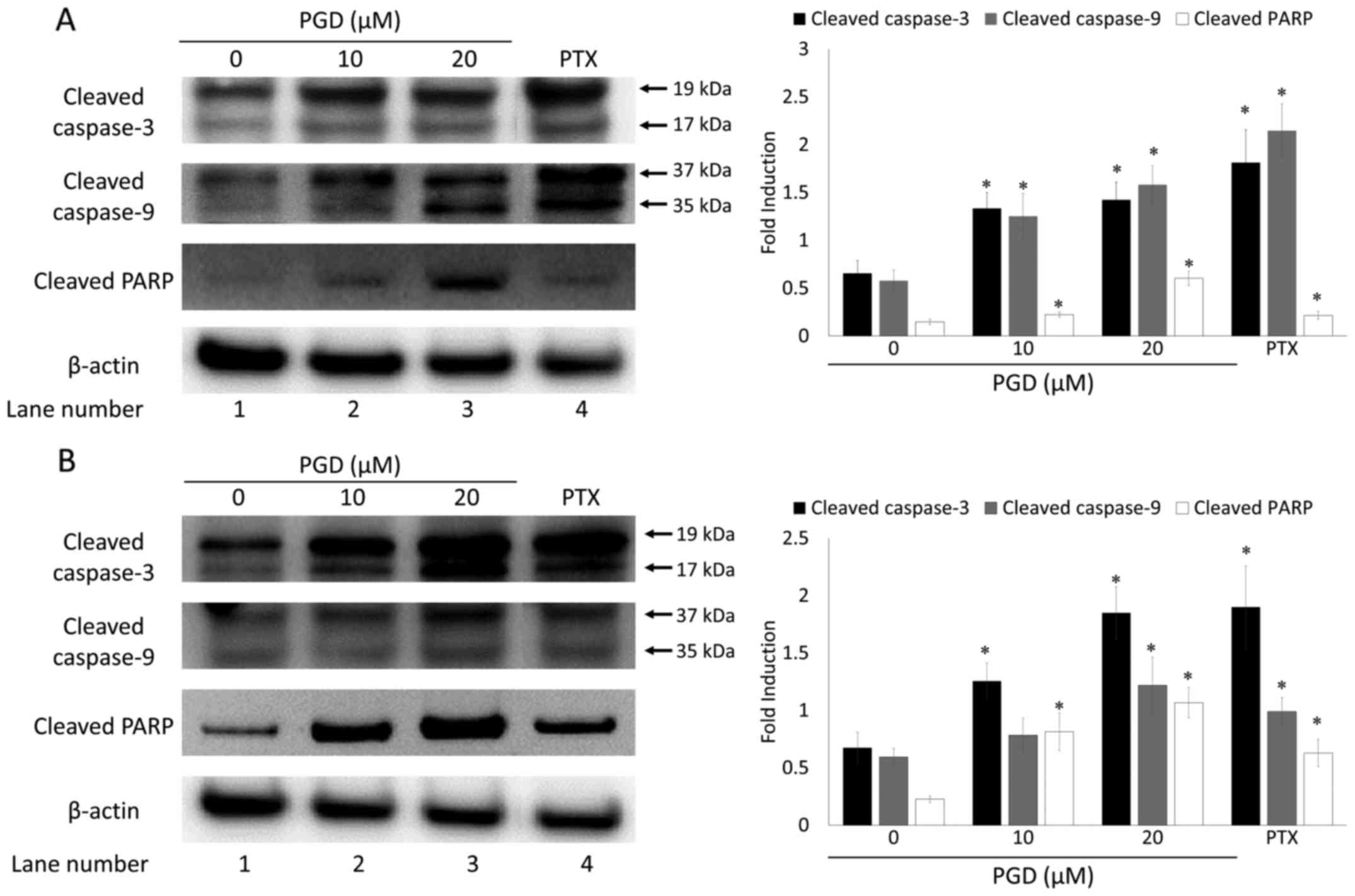
To elucidate the mechanism by which PGD induced
apoptosis, the expression levels of proteins involved in the
apoptosis pathway were examined using western blot analysis. The
results indicated that treatment with PGD induced the cleavage of
caspase-3 and −9, as well as PARP in both A549 and H460 cells (in
lanes 2 and 3 compared with lane 1; Fig. 4). The expression levels of cleaved
caspase-3 and −9, as well as PARP were markedly increased when
compared with those in non-treated cells.
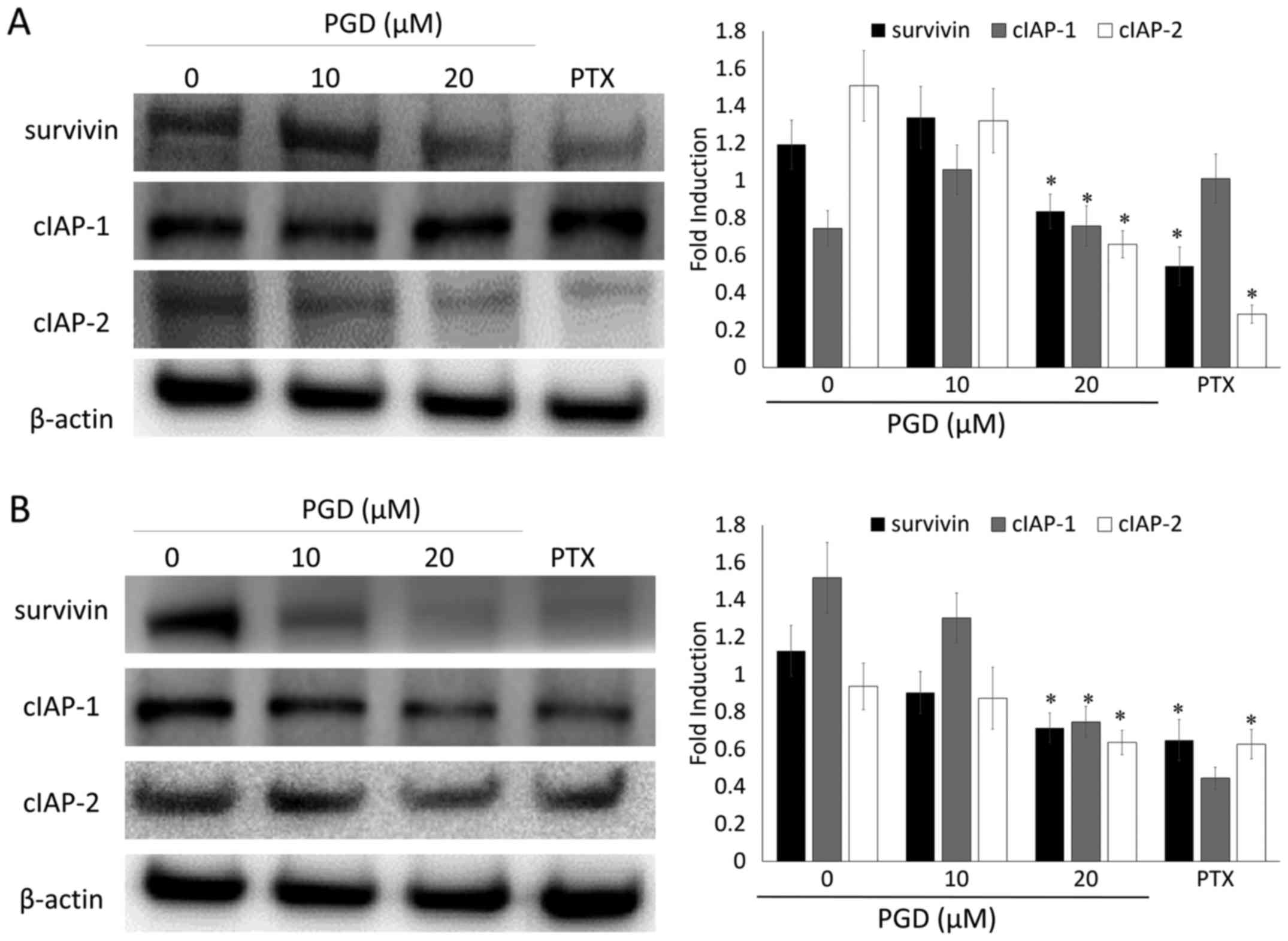
PGD inhibits the IAP pathway in NSCLC
cells
To further investigate the effects of PGD on the
expression of the IAP family of proteins which play a significant
role in intrinsic programmed cell death, NSCLC cells were treated
with various concentrations of PGD and then, the expression levels
of the IAP family of proteins including survivin, c-IAP-1 and
c-IAP-2 were determined using a western blot assay. The expression
of survivin, c-IAP-1 and c-IAP-2 was decreased, notably at the
highest concentration of PGD, compared with that in non-treated
cells. The expression of c-IAP-2 in A549 cells and the expression
of survivin and c-IAP-2 in H460 cells was decreased to undetectable
levels (Fig. 5). As a result, PGD
may exert its apoptotic effects by regulating the apoptotic
proteins and the IAP family of proteins.
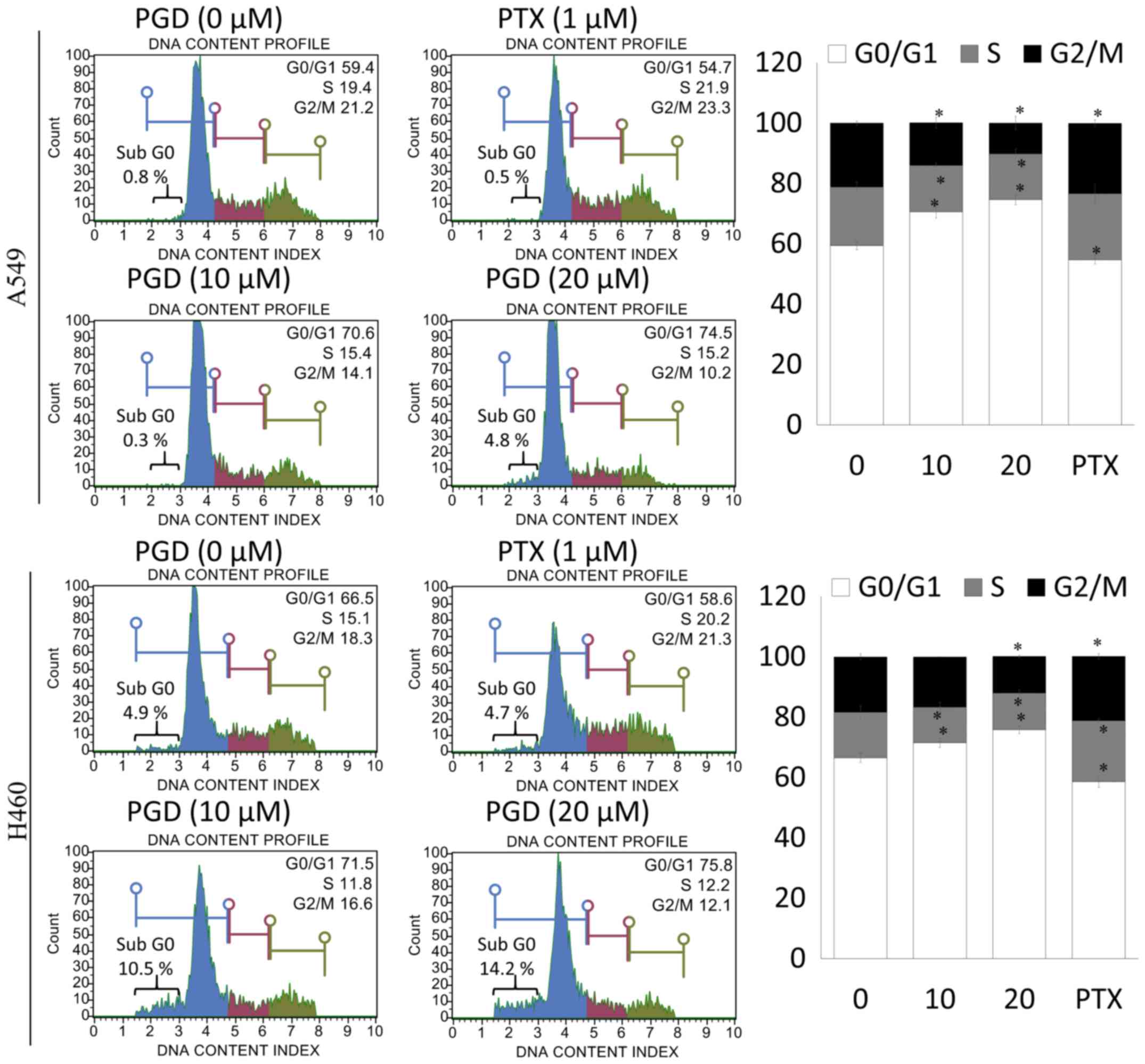
PGD induces cell cycle arrest in NSCLC
cells
To determine whether the effects of PGD on the
inhibition of NSCLC cell-proliferation were related to cell-cycle
arrest, the number of cells in the G0/G1, S and G2/M phases were
assessed by flow cytometry. Treatment with PGD inhibited the
progression of cell cycle in A549 and H460 cells. As displayed in
Fig. 6, the percentage of cells in
the G0/G1 phase was significantly increased in PGD-treated A549 and
H460 cells compared with non-treated cells. Furthermore, treatment
with PGD significantly increased the percentages of the sub G0
phase in both cell lines. In addition, PGD decreased the percentage
of cells in the S and G2/M phases.
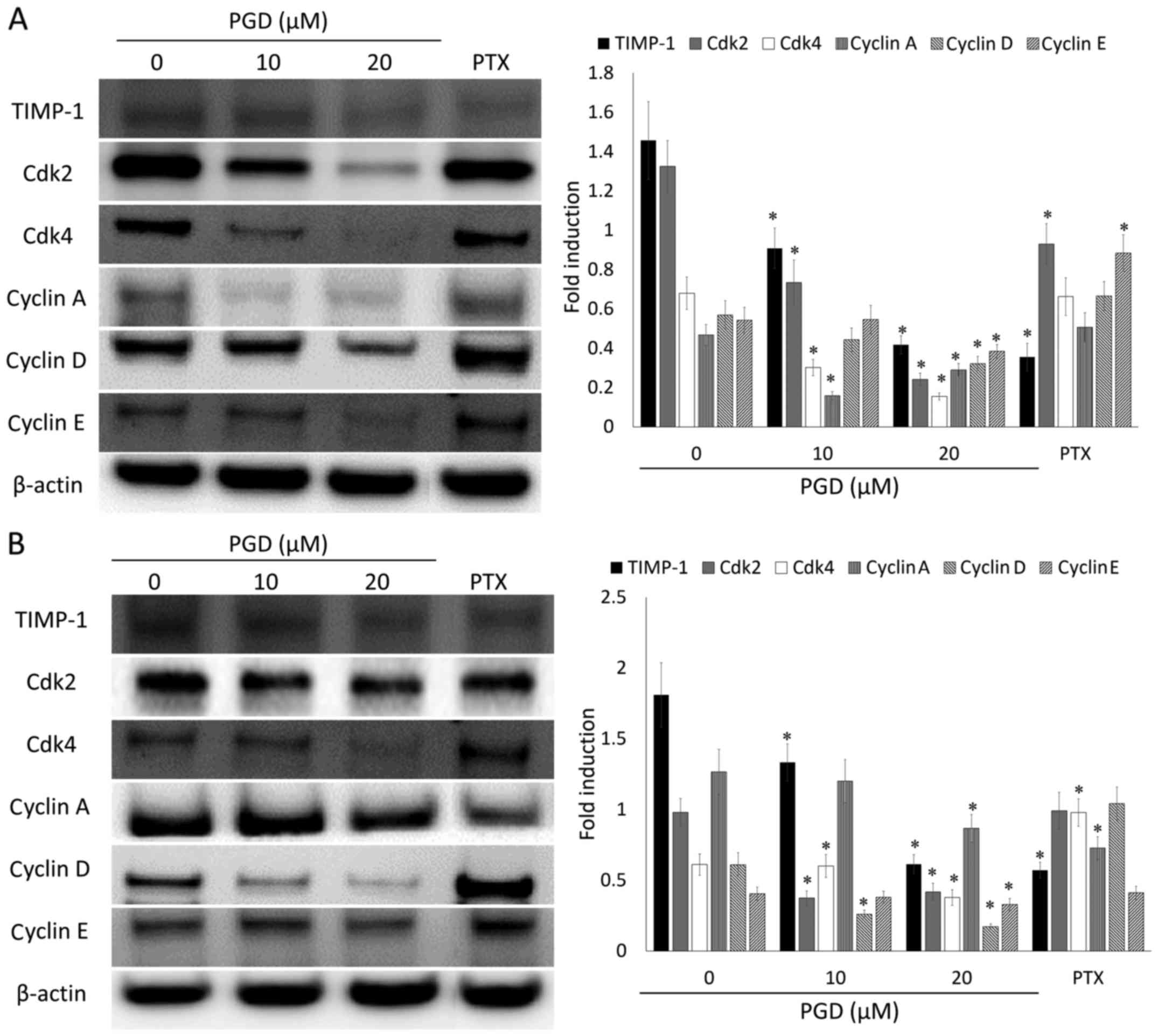
PGD regulates the expression levels of
proteins related to cell cycle progression in NSCLC cells
To evaluate the underlying mechanism of the effects
of the cell cycle-arrest of PGD, the expression levels of proteins
involved in cell cycle progression were determined using western
blot analysis. As displayed in Fig.
7, PGD reduced the expression levels of proteins related to the
progression of the cell cycle such as TIMP-1, Cdk2, Cdk4, cyclin A,
D and E in both cell lines. In addition, PGD reduced the
phosphorylation of GSK3β (Fig. 8),
which is also involved in cell cycle progression. These results
indicated that the inhibitory effects of PGD on cell growth via
cell cycle arrest were induced as a result of a decrease in the
expression of proteins related to cell cycle progression.
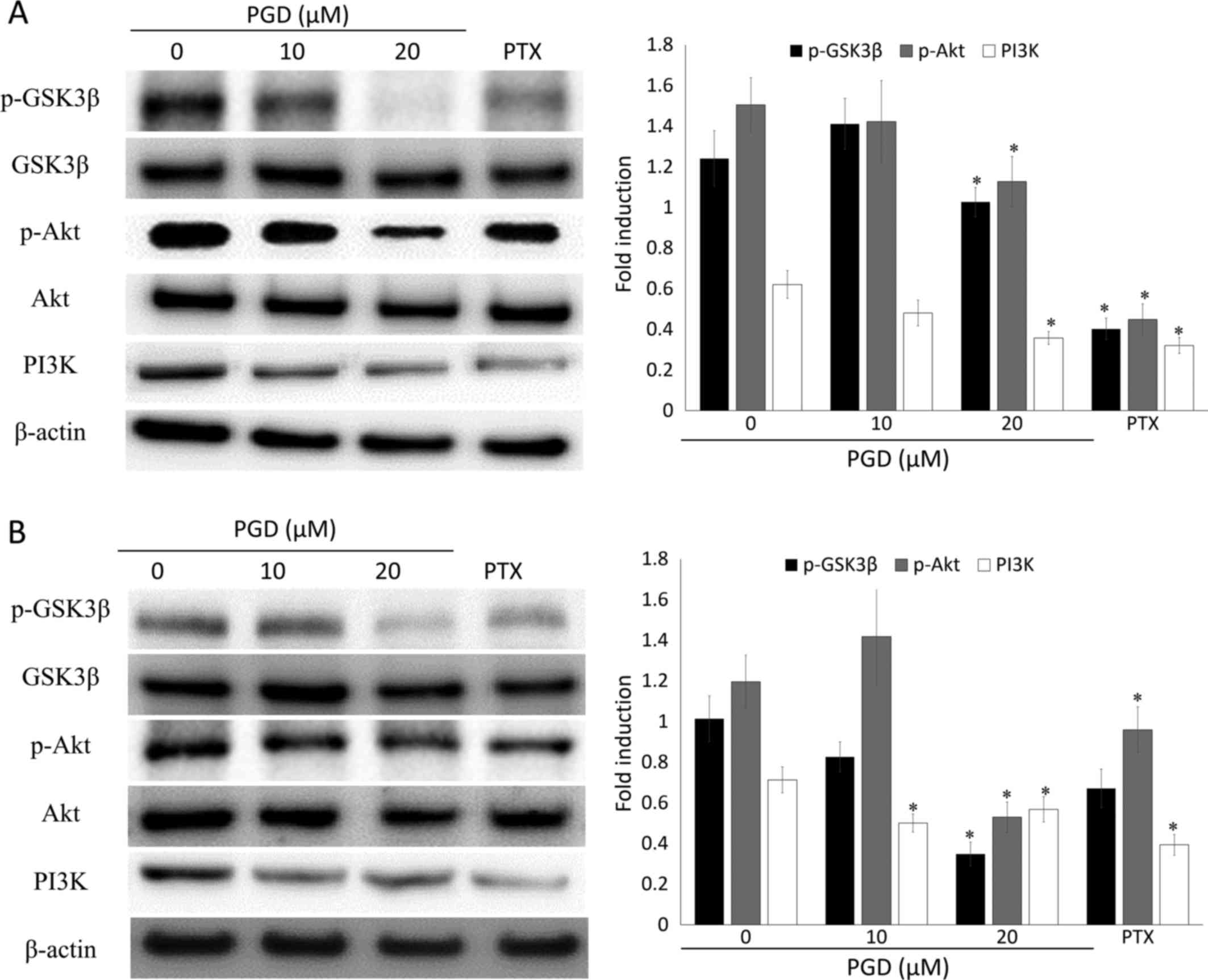
PGD inhibits the PI3K/Akt signaling
pathway
Previous research reported that the PI3K/Akt
signaling pathway plays a critical role in cell survival. To
confirm whether the inhibitory activity of PGD on cell growth was
related to the PI3K/Akt signaling pathway, western blot analysis
was performed. Treatment with PGD reduced the phosphorylation of
Akt, while total Akt remained the same (Fig. 8). In addition, the expression of
PI3K was reduced at the highest concentration of PGD compared with
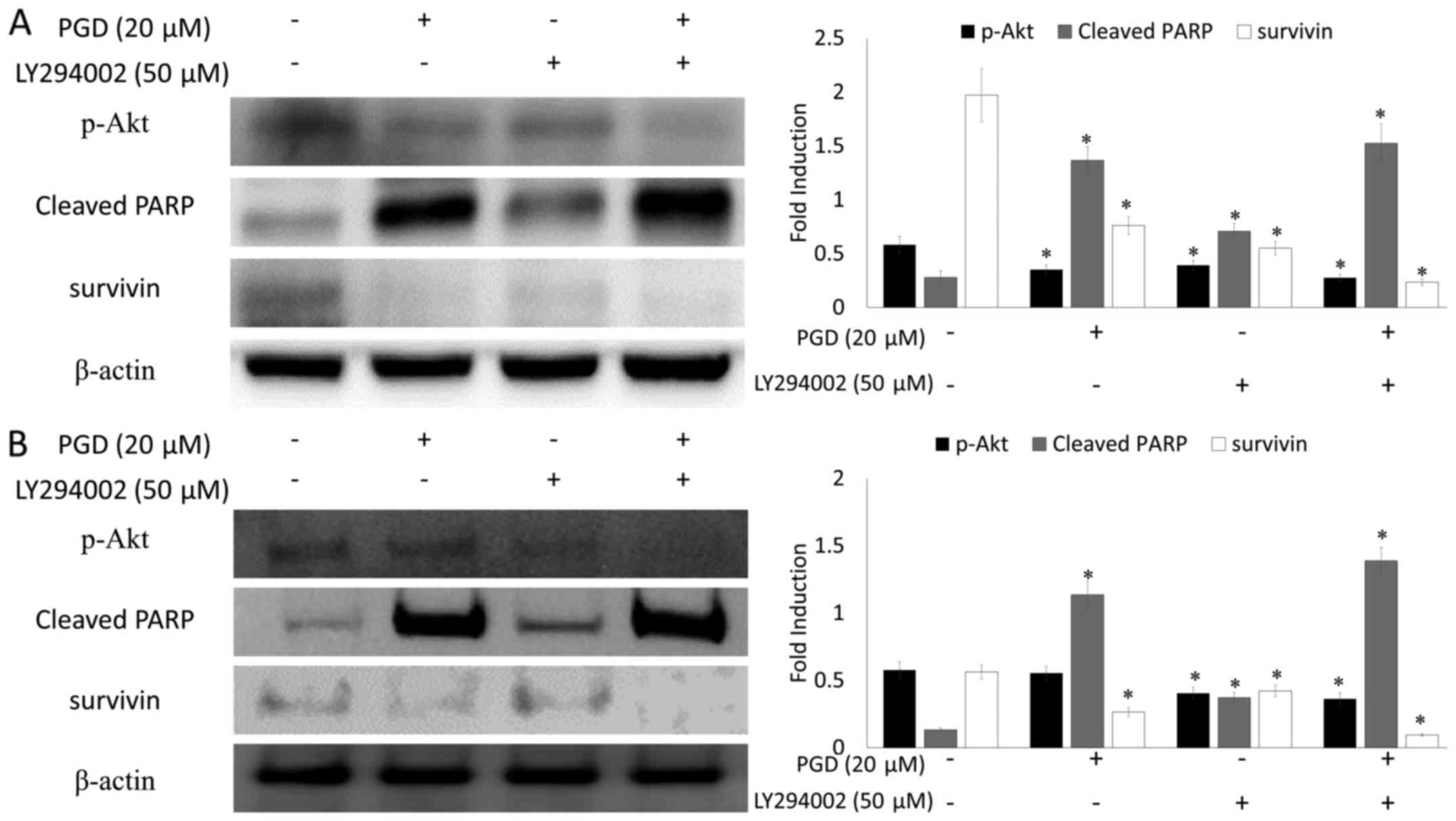
the non-treated group. In addition, as displayed in Fig. 9, co-treatment with LY294002 (the
inhibitor of PI3K) decreased the expression of survivin and
enhanced cleavage of PARP.
Discussion
In the present study, we examined the potential
inhibitory effects of PGD, a triterpenoid saponin contained in
P. grandiflorum, which has a similar structure to platycodin
D, on cell proliferation in NSCLC cells such as A549 and H460 cell
lines. Structure-activity relationships of Platycodon saponins
provide important information regarding their bioactive roles
(16) and it is expected that they
have similar biological activities. However, the anticancer
activity of PGD and its mechanisms still remain unclear. As shown
in Results, PGD significantly reduced cell viability via the
induction of apoptosis and cell cycle arrest. In addition, our
finding indicated that PGD inhibited cell survival via regulation
of the PI3K/Akt signaling pathway.
Apoptosis is one of the major goals of
chemopreventive agents which inhibit the overall growth of cancer
cells. Caspases are well-defined proteases related to apoptosis
(17). Activation of caspase-3 and
−9 induces the cleavage of PARP, eventually resulting in cell
apoptosis. IAPs are a group of structurally-related proteins that
block apoptosis either by binding and inhibiting caspases or
through caspase-independent mechanisms (18). Notably, cIAPs are positive
regulators of cell proliferation (19) and expression of cIAPs is associated
with advanced disease stage, poor patient prognosis and squamous
cell carcinoma (20–22). Survivin is one of the members of the
IAP family and is known to play a critical role in cancer cell
progression (23). As shown in
Fig. 2, PGD significantly reduced
cell viability in a dose-dependent manner and revealed
IC50 values of 26.49±1.45 µM and 20.52±0.30 µM in A549
and H460 cells, respectively. Furthermore, PGD significantly
induced apoptosis, which was accompanied by DNA damage, cleavage of
caspase-3 and −9, as well as PARP and a decrease in the expression
levels of the IAP proteins such as survivin, cIAP-1 and cIAP-2
(Figs. 3–5). These results indicated that the
antiproliferative effects of PGD on the NSCLC cell lines were
induced by activation of apoptosis-related proteins such as
caspase-3 and −9 as well as PARP and inhibition of the IAP family
of proteins such as survivin, cIAP-1 and cIAP-2.
In addition, the inhibitory effects of PGD on cell
growth were related to the arrest of cell cycle progression. Our
data revealed that treatment with PGD significantly reduced the
proportion of cells in the S and G2/M phases, followed by an
increase in the proportion of cells in the G0/G1 phase. These
results were in agreement with the findings of the proliferation
assay. Tissue inhibitor of metalloproteinases-1 (TIMP-1) was
initially characterized as an endogenous inhibitor of matrix
metalloproteinases (MMPs) (24).
TIMP-1 is frequently overexpressed in several types of human
cancers and promotes cell proliferation (25–27).
Cell cycle progression is regulated by cyclins and cyclin-dependent
kinases (CDKs). Specifically, cyclin D1 and CDK4 play pivotal roles
in the cell cycle transition from the G1 to the S phase (28,29).
Overexpression of cyclin D1 and CDK4 has been observed in various
cancer cells and was strongly associated with the risk of tumor
progression and metastasis (30,31).
CDK2 is a protein kinase essential for the G1/S transition and its
activity is limited in the G1/S phase (32). CDK2 forms a complex with cyclin E
and this complex leads to the progression of the G1 phase to the S
phase (33). The CDK2/cyclin E
complex also induces an increase in cyclin A expression, which
facilitates G1 to S-phase transition. Cyclin A binds to CDK2
instead of cyclin E and this CDK2/cyclin A complex initiates DNA
replication, which is required for the progression to the S phase
(34). PGD reduced the expression
of TIMP-1, CDK2, cyclin A and E and these results were related to
the effects of cell cycle arrest of PGD at the G0/G1 phase.
Finally, we investigated the anticipated mechanism
that is involved in the aforementioned effects of PGD. The PI3K/Akt
pathway is a well-known essential pathway involved in cell
proliferation, cycle progression, metastasis, survival and
apoptosis and it is one of the most important oncogenic targets in
almost all kinds of cancers (35,36).
Furthermore, this pathway increases the resistance to DNA
damage-induced apoptosis (37). In
addition, increased phosphorylation levels of GSK-3β, which is a
known downstream target, lead to the expression of cyclin D and
cMyc, which play a role in the G1/S-phase check point of cell cycle
progression and migration (38,39).
Therefore, PGD-induced G1 arrest may be attributed to its influence
on GSK-3β and upstream Akt and PGD-induced apoptosis may be
attributed to the inhibition of Akt phosphorylation.
In conclusion, the anti-proliferative effects of PGD
on NSCLC cells may be due to modulation of the PI3K/Akt pathway,
which subsequently leads to apoptosis induced by the activation of
apoptotic proteins, a decrease in the expression of the IAP family
of proteins and growth inhibition induced by cell cycle arrest
regulated by CDK2/4, cyclin A, D and E. Based on our findings, PGD
exhibits a potential role as a new therapeutic agent for the
treatment of NSCLC.
Acknowledgements
The present study was supported by Wonkwang
University in 2017.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Couraud S, Zalcman G, Milleron B, Morin F
and Souquet PJ: Lung cancer in never smokers: a review. Eur J
Cancer. 48:1299–1311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Travis WD, Travis LB and Devesa SS: Lung
cancer. Cancer. 75 Suppl:191–202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al WHO Panel, : The 2015 World Health Organization
Classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soria JC, Jang SJ, Khuri FR, Hassan K, Liu
D, Hong WK and Mao L: Overexpression of cyclin B1 in early-stage
non-small cell lung cancer and its clinical implication. Cancer
Res. 60:4000–4004. 2000.PubMed/NCBI
|
|
6
|
Yoshida T, Tanaka S, Mogi A, Shitara Y and
Kuwano H: The clinical significance of Cyclin B1 and Wee1
expression in non-small-cell lung cancer. Ann Oncol. 15:252–256.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniel C: Lung cancer, a worrying
epidemiological evolution. Rev Infirm. 184:14–16. 2012.(In
French).
|
|
8
|
Ball D, Mitchell A, Giroux D and
Rami-Porta R; IASLC Staging Committee and Participating
Institutions, : Effect of tumor size on prognosis in patients
treated with radical radiotherapy or chemoradiotherapy for
non-small cell lung cancer. An analysis of the staging project
database of the International Association for the Study of Lung
Cancer. J Thorac Oncol. 8:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JW, Park SJ, Lim JH, Yang JW, Shin JC,
Lee SW, Suh JW and Hwang SB: Triterpenoid saponins isolated from
Platycodon grandiflorum inhibit hepatitis C virus replication. Evid
Based Complement Alternat Med. 2013:5604172013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan M, Maryam A, Zhang H, Mehmood T and
Ma T: Killing cancer with platycodin D through multiple mechanisms.
J Cell Mol Med. 20:389–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim MO, Moon DO, Choi YH, Lee JD, Kim ND
and Kim GY: Platycodin D induces mitotic arrest in vitro, leading
to endoreduplication, inhibition of proliferation and apoptosis in
leukemia cells. Int J Cancer. 122:2674–2681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou R, Lu Z, Liu K, Guo J, Liu J, Zhou Y,
Yang J, Mi M and Xu H: Platycodin D induces tumor growth arrest by
activating FOXO3a expression in prostate cancer in vitro and in
vivo. Curr Cancer Drug Targets. 14:860–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu C, Sun G, Yuan G, Wang R and Sun X:
Effects of platycodin D on proliferation, apoptosis and PI3K/Akt
signal pathway of human glioma U251 cells. Molecules.
19:21411–21423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YH, Yoo DS, Choi CW, Cha MR, Kim YS,
Lee HS, Lee KR and Ryu SY: Platyconic acid A, a genuine
triterpenoid saponin from the roots of Platycodon grandiflorum.
Molecules. 13:2871–2879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chun J, Ha IJ and Kim YS:
Antiproliferative and apoptotic activities of triterpenoid saponins
from the roots of Platycodon grandiflorum and their
structure-activity relationships. Planta Med. 79:639–645. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie CQ, Zhou P, Zuo J, Li X, Chen Y and
Chen JW: Triptolide exerts pro-apoptotic and cell cycle arrest
activity on drug-resistant human lung cancer A549/Taxol cells via
modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett.
12:3586–3590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nachmias B, Ashhab Y and Ben-Yehuda D: The
inhibitor of apoptosis protein family (IAPs): An emerging
therapeutic target in cancer. Semin Cancer Biol. 14:231–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samuel T, Okada K, Hyer M, Welsh K, Zapata
JM and Reed JC: cIAP1 localizes to the nuclear compartment and
modulates the cell cycle. Cancer Res. 65:210–218. 2005.PubMed/NCBI
|
|
20
|
Imoto I, Tsuda H, Hirasawa A, Miura M,
Sakamoto M, Hirohashi S and Inazawa J: Expression of cIAP1, a
target for 11q22 amplification, correlates with resistance of
cervical cancers to radiotherapy. Cancer Res. 62:4860–4866.
2002.PubMed/NCBI
|
|
21
|
Tanimoto T, Tsuda H, Imazeki N, Ohno Y,
Imoto I, Inazawa J and Matsubara O: Nuclear expression of cIAP-1,
an apoptosis inhibiting protein, predicts lymph node metastasis and
poor patient prognosis in head and neck squamous cell carcinomas.
Cancer Lett. 224:141–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Che X, Yang D, Zong H, Wang J, Li X, Chen
F, Chen X and Song X: Nuclear cIAP1 overexpression is a tumor
stage- and grade-independent predictor of poor prognosis in human
bladder cancer patients. Urol Oncol. 30:450–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kishi H, Igawa M, Kikuno N, Yoshino T,
Urakami S and Shiina H: Expression of the survivin gene in prostate
cancer: Correlation with clinicopathological characteristics,
proliferative activity and apoptosis. J Urol. 171:1855–1860. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Docherty AJ, Lyons A, Smith BJ, Wright EM,
Stephens PE, Harris TJ, Murphy G and Reynolds JJ: Sequence of human
tissue inhibitor of metalloproteinases and its identity to
erythroid-potentiating activity. Nature. 318:66–69. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gouyer V, Conti M, Devos P, Zerimech F,
Copin MC, Créme E, Wurtz A, Porte H and Huet G: Tissue inhibitor of
metalloproteinase 1 is an independent predictor of prognosis in
patients with non small cell lung carcinoma who undergo resection
with curative intent. Cancer. 103:1676–1684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aaberg-Jessen C, Christensen K, Offenberg
H, Bartels A, Dreehsen T, Hansen S, Schrøder HD, Brünner N and
Kristensen BW: Low expression of tissue inhibitor of
metalloproteinases-1 (TIMP-1) in glioblastoma predicts longer
patient survival. J Neurooncol. 95:117–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayakawa T, Yamashita K, Tanzawa K,
Uchijima E and Iwata K: Growth-promoting activity of tissue
inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of
cells. A possible new growth factor in serum. FEBS Lett. 298:29–32.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dworakowska D: Clinical significance of
cyclin Dl expression in non-small cell lung cancer. Pneumonol
Alergol Pol. 73:297–300. 2005.(In Polish). PubMed/NCBI
|
|
31
|
Ishii Y, Pirkmaier A, Alvarez JV, Frank
DA, Keselman I, Logothetis D, Mandeli J, O'Connell MJ, Waxman S and
Germain D: Cyclin D1 overexpression and response to bortezomib
treatment in a breast cancer model. J Natl Cancer Inst.
98:1238–1247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hinds PW, Mittnacht S, Dulic V, Arnold A,
Reed SI and Weinberg RA: Regulation of retinoblastoma protein
functions by ectopic expression of human cyclins. Cell.
70:993–1006. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yam CH, Fung TK and Poon RY: Cyclin A in
cell cycle control and cancer. Cell Mol Life Sci. 59:1317–1326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong LW, Yang GZ, Pan YF, Chen Y, Tan YX,
Dai RY, Ren YB, Fu J and Wang HY: The oncoprotein p28GANK
establishes a positive feedback loop in β-catenin signaling. Cell
Res. 21:1248–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neumeister P, Pixley FJ, Xiong Y, Xie H,
Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER, et al:
Cyclin D1 governs adhesion and motility of macrophages. Mol Biol
Cell. 14:2005–2015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arber N, Doki Y, Han EK, Sgambato A, Zhou
P, Kim NH, Delohery T, Klein MG, Holt PR and Weinstein IB:
Antisense to cyclin D1 inhibits the growth and tumorigenicity of
human colon cancer cells. Cancer Res. 57:1569–1574. 1997.PubMed/NCBI
|