Introduction
Despite the apparent decline in the incidence of GC
in the last few decades, GC currently remains one of the most
common cancers worldwide (1). It
was reported that the GC mortality rate in East Asia countries is
~28.1% for men and 13.0% for women (2). At present, the major therapeutic
strategy of GC includes surgery combined with adjuvant
chemotherapy, and evidence suggests that this approach is effective
for early GC (3), However, the
efficacy of these measures on patients with advanced GC is still
unsatisfactory. Due to the lack of effective early diagnostic
biomarkers, most patients are diagnosed at a late clinical stage
with extremely poor prognosis (2,4). As
known, diet and family inheritance are two risk factors that
contribute to the occurrence of GC, but chronic gastritis caused by
Helicobacter pylori is the primary cause (5–7). In
spite of the extraordinary progress achieved in the prevention and
treatment of GC, issues remain in terms of the exact underlying
mechanism. Thus, elucidating the molecular mechanism of GC is of
significant importance for exploring new effective therapies.
It was demonstrated the angiopoietin (ANGPTL) family
is associated with many physiological processes, including lipid
metabolism (8), cell activity
regulation (9) and tumor growth
(10). The angiopoietin family
consists of 7 ANGPTL proteins, named ANGPTL 1–7. As identified in
systemic circulation, their amino acid sequences are slightly
conserved. Angiopoietin-like 4 (ANGPTL4), widely expressed in
different human organs, contains a C-terminal fibrinogen-like
domain and an N-terminal coiled-coil fragment (11–13).
Previous reports have revealed the ANGPTL4 is involved in the
progression of several human tumors, such as colorectal cancer
(14,15), prostate cancer (16), and breast cancer (13). However, it remains unclear whether
ANGPTL4 is associated with the progression of GC, and there is an
urgent need to explore the basic mechanisms by which they
participate and discover new therapies for GC patients.
In the present study, we firstly analyzed the
expression of ANGPTL4 in GC tissues. In addition, we generated two
effective siRNAs of ANGPTL4, named siRNA1 and siRNA2. We used
siRNA1 or siRNA2 to transfect human GC cell lines to block the
expression of ANGPTL4. We found that knockdown of ANGPTL4 by siRNAs
inhibited cell proliferation, migration and invasion, and promoted
apoptosis in both SNU-1 and BGC823 cell lines. In addition, we also
found that knockdown of ANGPTL4 effectively increased the
cisplatin-induced apoptosis in both SNU-1 and BGC823 cells in a
dose-dependent manner. These results demonstrated that ANGPTL4
plays a critical role in the progression of GC, and it may provide
a new potential target for the treatment of GC.
Materials and methods
Patients and tissue samples
A total of 40 GC patients were recruited at the Sun
Yat-sen Memorial Hospital of Sun Yat-sen University (Guangzhou,
Guangdong, China), and tumor and corresponding non-tumor control
tissues were collected. After washing with sterile
phosphate-buffered saline (PBS), all tissue samples were
immediately stored at −80°C until further study. This study was
approved by the Ethics Committee of Sun Yat-sen Memorial Hospital,
and written informed consent was obtained from all patients.
Cell lines and culture
The GC cell line SNU-1 was obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA), and GC
cell line BGC823 was purchased from Shanghai Institutes for
Biological Sciences, Chinese Academy of Sciences. Both SNU-1 and
BGC823 cells were maintained in RPMI-1640 medium with 100 U/ml
penicillin, streptomycin and 10% fetal bovine serum (FBS, cat. no.
100–106; Gemini Bio Products, West Sacramento, CA, USA) at 37°C and
5% CO2.
Knockdown of human ANGPTL4 in GC cell
lines
Negative control siRNA (NC) and two siRNAs against
human ANGPTL4 (siRNA1, siRNA2) were designed and obtained from
GenePharma (Shanghai, China). The sequence of siRNA1 was
5′-GGUGACUCUUGGCUCUGCC-3′ (sense) and 5′-GGUGACUCUUGGCUCUGCC-3′
(antisense). The sequence of siRNA2 was 5′-AGGGAAUCUUCUGGAAGAC-3′
(sense) and 5′-GUCUUCCAGAAGAUUCCCU-3′ (antisense). According to the
manufacturer's instructions, SNU-1 or BGC823 cells were transfected
with 40 nmol/l of ANGPTL4 siRNA1, siRNA2 or NC siRNA using
Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA).
qRT-PCR and western blot analysis were used to detect the knockdown
efficacy of the ANGPTL4 siRNAs.
RNA extraction and quantitative
real-time PCR (qRT-PCR)
Total RNA was extracted from the SNU-1 and BGC823
cells using TRIzol reagent (Invitrogen), and 5 µg RNA was used for
synthesizing cDNA using the Revert Aid First Strand cDNA Synthesis
kit (Invitrogen, UK) according to the manufacturer's instructions.
Quantitative real-time PCR was performed using a 7900 HT Fast
system (Applied Biosystems, Life Technologies, Foster City, CA,
USA). During the course of the reaction, the Cq value was obtained,
and the results were analyzed using 2−∆∆Cq calculation.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
endogenous housekeeping gene. The sequence of human ANGPTL4 PCR
primers was 5′-GGCGAGTTCTGGCTGGGTCT-3′ (sense) and
5′-TGGCCGTTGAGGTTGGAATG-3′ (antisense). The sequences of the GAPDH
primers were: 5′-ATGTCGTGGAGTCTACTGGC-3′ (forward) and
5′-TGACCTTGCCCACAGCCTTG-3′ (reverse).
Western blot analysis
Total proteins of the human GC cells were prepared
using RIPA buffer (cat. no. R3792). Cell lysis was preformed on ice
using ultrasonication. Cell debris was centrifuged (13,000 rmp, 20
min, 4°C) and the supernatant was collected. The concentrations of
proteins in each sample were detected using a protein assay reagent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). SDS-PAGE (10%) was
used to separate total proteins (40 µg). The separated proteins
were then transferred onto Immun-Blot® PVDF membranes
(cat. no. 162-0177; Bio-Rad Laboratories). The membranes were
blocked with 5% skim milk in TBS for 2 h at room temperature, and
incubated with the primary antibody overnight at 4°C. Then the
membranes were incubated with secondary horseradish peroxidase
antibody (HRP, cat. no. 074-1806; KPL Co., USA). The results were
analyzed using a chemiluminescence substrate kit and an ECL system
(both from Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA).
The primary antibodies were ANGPTL4 (1:1,000; AF3485; R&D
Systems Inc., Minneapolis, MA, USA) and GAPDH (1:200; sc-51631;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Cell migration and invasion
assays
Cell migration and cell invasion assays were
performed using Transwell chambers (8-µm pore size; BD Biosciences,
San Jose, CA, USA) with or without Matrigel coating (BD
Biosciences). Briefly, SNU-1 or BGC823 cells (0.2 ml,
2×105 for the invasion assay; 1×105 for the
migration assay) were seeded in the upper chamber, and the lower
chamber was filled with 10% FBS containing medium (0.6 ml). After
24 h, cells on the upper chamber were removed, and migrated cells
were fixed and stained with 5% crystal violet. The cells were
captured and counted under a microscope.
Cell viability assay (MTT assay)
After transfection, SNU-1 or BGC823 cells were
seeded into a 96-well plate at a concentration of 2×104
cells per well. Incubated for 24 h, cells were then washed with PBS
and 20 µl of MTT solution was added to each well. After at least 30
min of incubation at 37°C, 150 µl dimethyl sulfoxide (DMSO) was
added to each well. The plate was then shaken at room temperature
for ≤10 min, and absorbance was determined at 490 nm by a
microplate reader (Sunrise™; Tecan Group Ltd., Switzerland).
Apoptosis analysis
Apoptosis rate of GC cells after the transfection of
ANGPTL4 siRNA was measured by flow cytometry using Annexin V/PI
double staining (BD Biosciences, Franklin Lakes, NJ, USA). The
cells were seeded in a 24-well plate at a concentration of
2×104 per well. Following overnight incubation, cells
were washed at least twice with cold phosphate-buffered saline
(PBS). Cells were re-suspended with 0.5 ml binding buffer
supplemented with 5 µl PI and 5 µl FITC-labeled Annexin V and
incubated at room temperature for 10 min (protected from light).
Apoptotic SNU-1 or BGC823 cells were analyzed in triplicates and
the analysis was repeated three times independently by flow
cytometry on a FACScan (Beckman Instruments, Fullerton, CA,
USA).
Immunohistochemical (IHC)
staining
Paraffin-embedded tissues were sliced into 4-µm
sections, and then the sections were treated with xylene and
ethanol to remove paraffin, and were rehydrated in PBS. All
sections were immersed in 0.01 M sodium citrate buffer (pH 6.0) at
95°C for ≤20 min for antigen retrieval. The sections were then
treated with 3% H2O2 for 10 min to block the
endogenous peroxidase activity, and incubated with 5% normal goat
serum to block the non-specific binding. Samples were then
incubated overnight at 4°C with primary rabbit antibody against
human ANGPTL4 (R&D Systems). After washing with PBS, the
sections were sequentially incubated with goat anti-rabbit HRP, and
the reaction signals were visualized by the diaminobenzidine
reaction (DAB; Dako Ltd.) and sections were counterstained with
hematoxylin.
Cisplatin cytotoxicity assay
After transfection with ANGPTL4 siRNA, SNU-1 or
BGC823 cells (2×104) were seeded in a 96-well plate and
incubated overnight at 37°C. Then cells were treated with various
concentrations (0, 2.5, 5.0, 10.0, 20.0 and 40.0 µg/ml) of
cisplatin (Sigma, Santa Clara, CA, USA). MTT assay was applied to
examine cell viability.
Statistical analysis
Statistical analyses were conducted using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). All data are expressed as
the mean ± standard deviation (SD). The Student's t-test was
carried out to compare the level of cell proliferation, apoptosis,
migration, invasion and cisplatin resistance among groups, and
P<0.05 was considered to be indicative of statistical
significance.
Results
ANGPTL4 expression is upregulated in
GC tissues
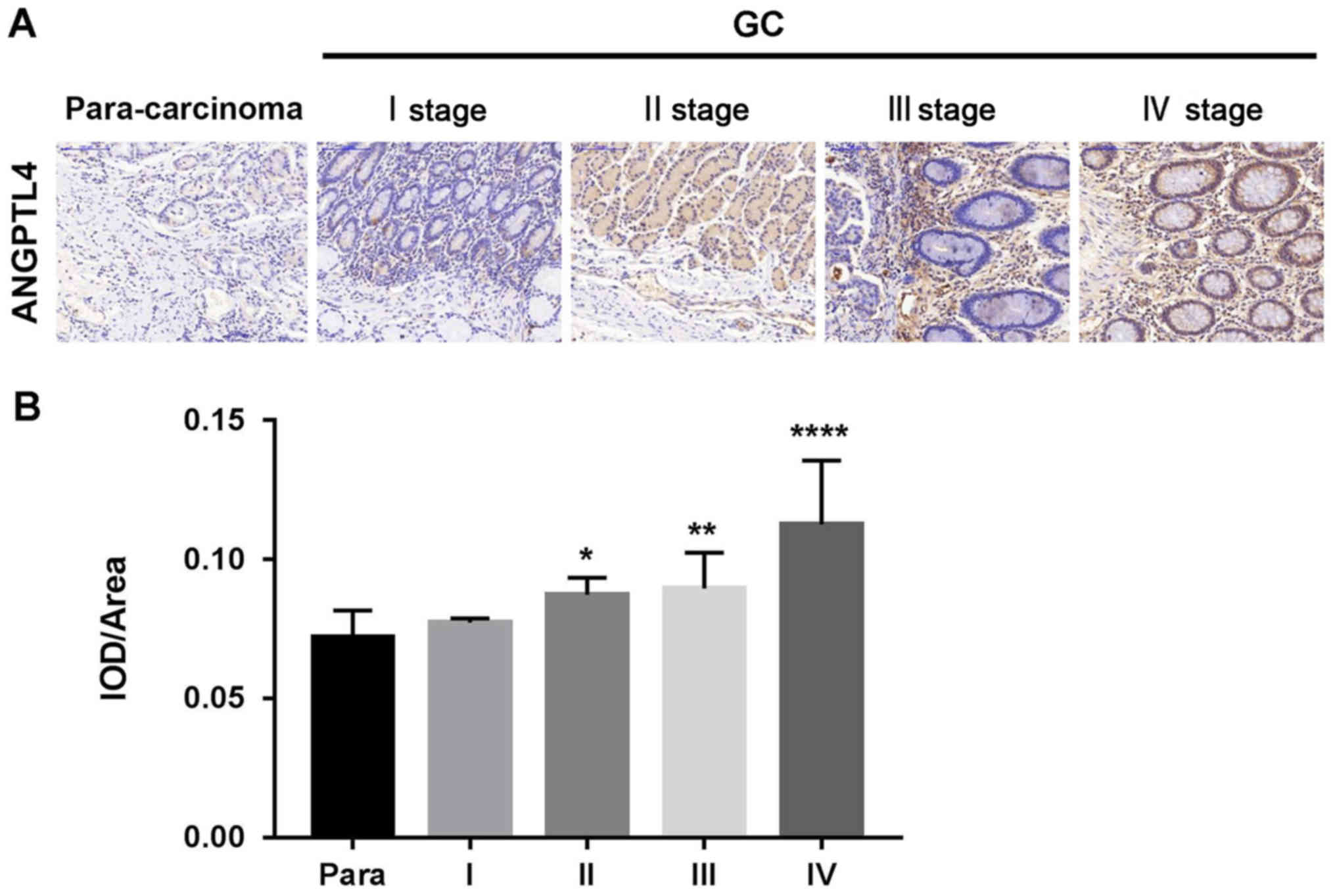
To study the roles of ANGPTL4 in GC pathogenesis,
protein expression of ANGPLT4 was analyzed by IHC in tumor samples
and adjacent normal specimens of 40 patients with GC (I, II, III
and IV stage). The results indicated that the expression of ANGPTL4
was significantly increased in the GC tissues compared with that
noted in the normal tissues. In addition, ANGPTL4 expression levels
were associated with the clinical phase of GC (Fig. 1).
The expression level of ANGPTL4 is
downregulated by siRNA in human GC cell lines
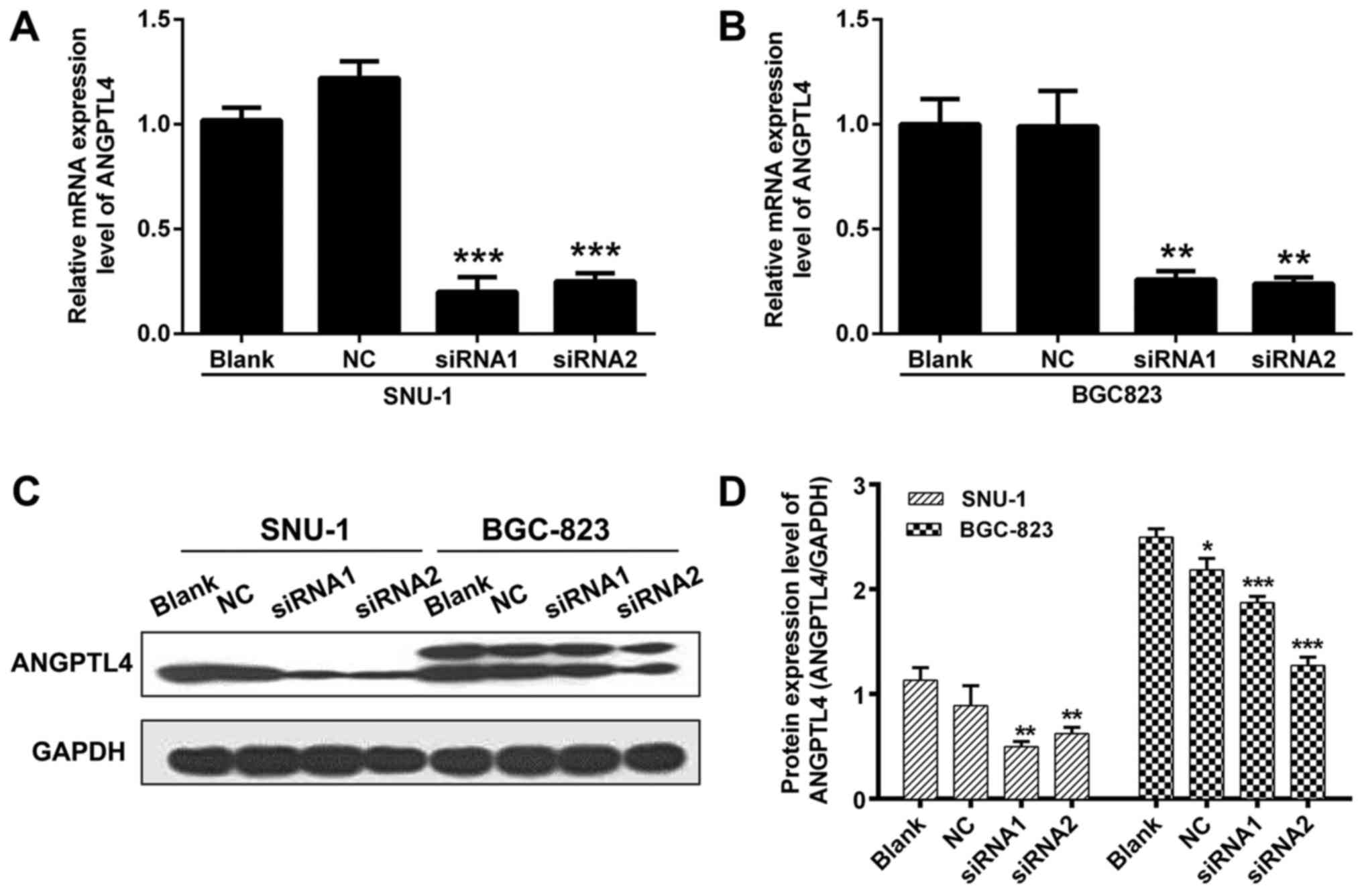
To investigate the role of ANGPTL4 in human GC, two
siRNAs targeting ANGPTL4 were designed, and SNU-1 and BGC823 cells
were transfected. The knockdown efficiency of those two siRNAs was
validated by detecting the mRNA expression level using qRT-PCR
assay. Results from qRT-PCR showed that, compared to NC, the mRNA
expression of ANGPTL4 in SNU-1 and BGC823 cells was extremely
decreased after siRNA1 and siRNA2 transfection (P<0.01 and
P<0.001, respectively; Fig. 2A and
B). We further assessed the ANGPTL4 protein level in the
transfected SNU-1 and BGC823 cells, and the results indicated that
ANGPTL4 protein was also downregulated (Fig. 2C and D). These results demonstrated
that the siRNAs were effective in silencing ANGPTL4 expression.
ANGPTL4 knockdown inhibits cell
proliferation and promotes cell apoptosis in GC cell lines
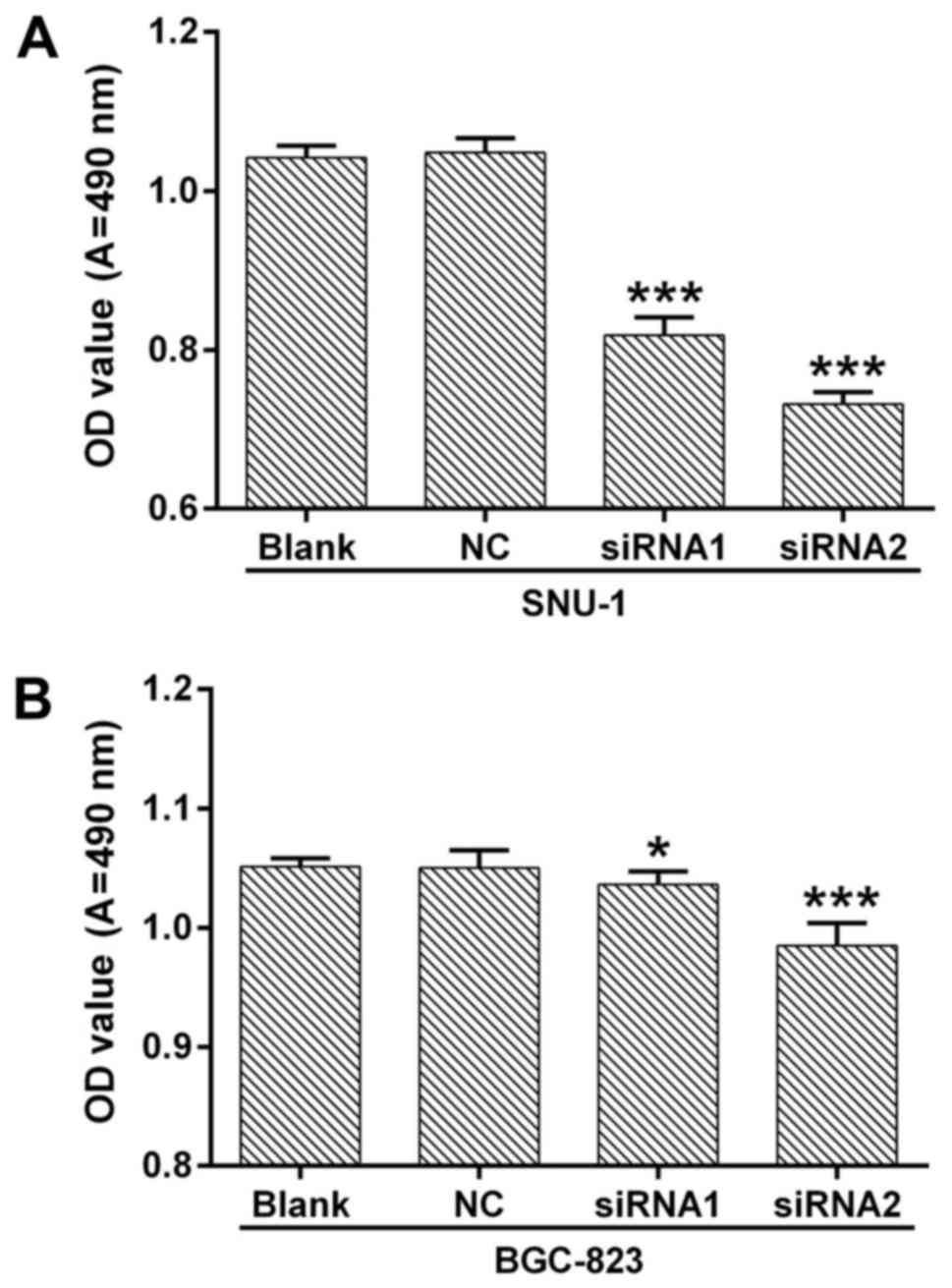
In order to explore the biological function of
ANGPTL4 in human GC, we silenced the ANGPTL4 expression by siRNA in
SNU-1 and BGC823 cells. Cell proliferation and apoptosis were then
detected. MTT assay indicated that knockdown of ANGPTL4
significantly inhibited the cell proliferation in both SNU-1 and
BGC823 cells, compared with NC (P<0.05, P<0.01 and
P<0.001, respecively; Fig. 3).
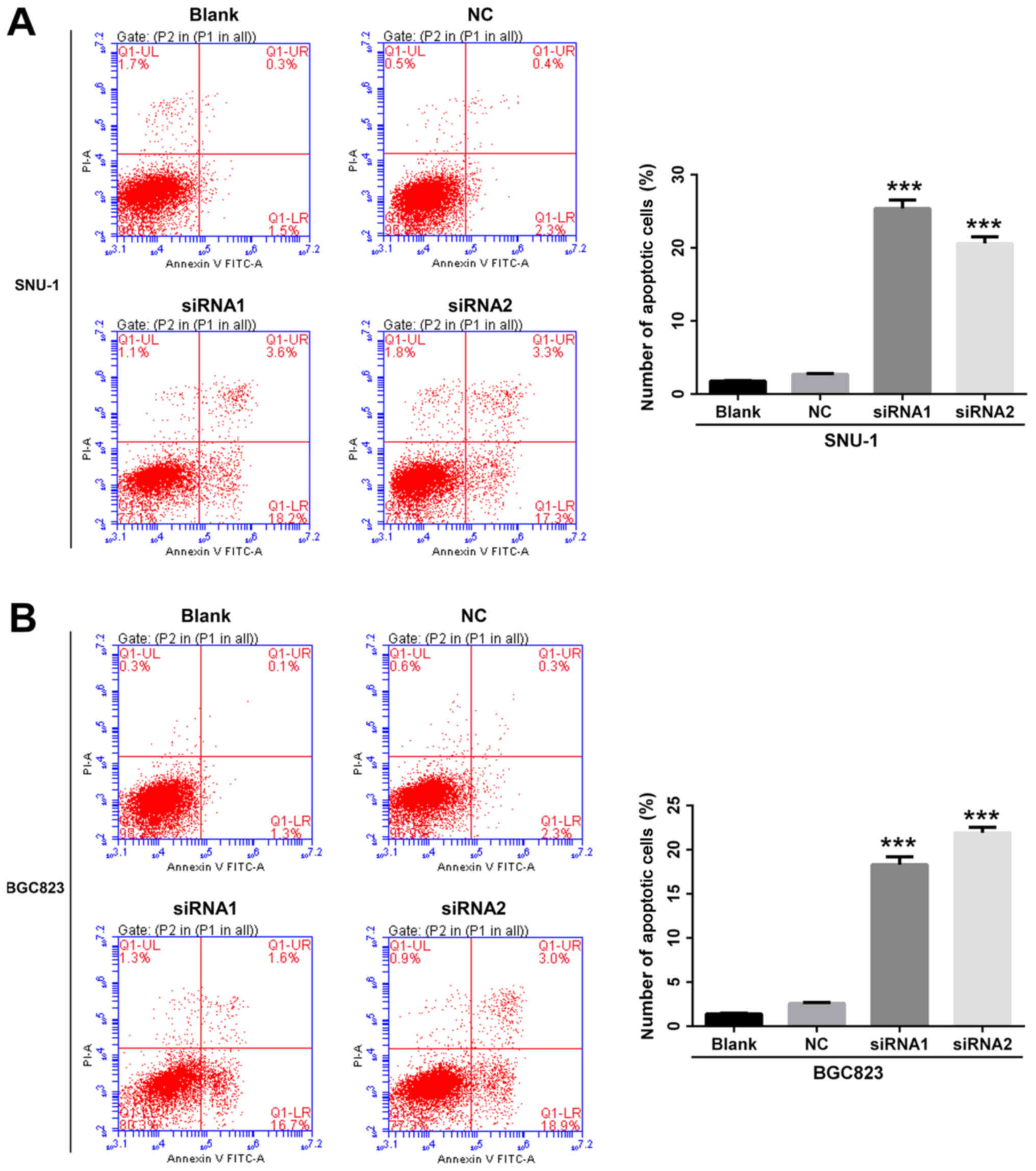
Annexin V/PI staining and flow cytometry assay were employed to
further determine whether cell apoptosis could be affected by
ANGPTL4. We found that the percentage of apoptosis was
significantly upregulated after the silencing of ANGPTL4 in both
the SNU-1 (P<0.001, Fig. 4A) and
BGC823 cells (P<0.001, Fig. 4B).
This indicated that knockdown of ANGPTL4 inhibited cell
proliferation and promoted cell apoptosis in human GC cell
lines.
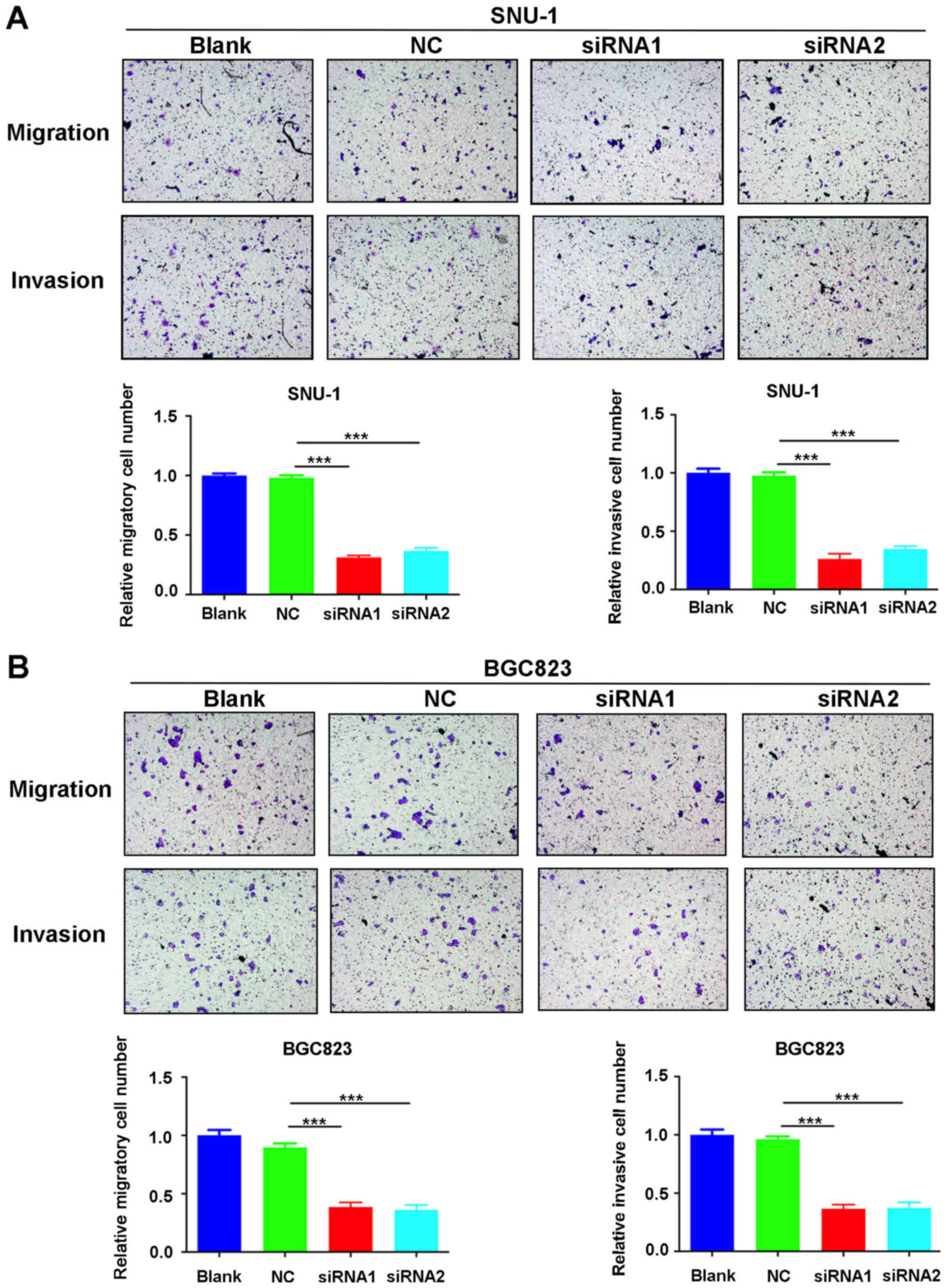
ANGPTL4 knockdown suppresses the
ability of cell migration and invasion in human GC cell lines
Migration and invasion are two important factors
involved in the progression of cancer cells. To determine whether
ANGPTL4 is involved in the migration and invasion of human GC
cells, we performed Transwell assays. The results showed that cell
migration and invasion were significantly reduced after
transfection of SNU-1 and BGC823 cells by siRNA1 or siRNA2 compared
to the blank control (P<0.001, Fig.
5). These results confirm that knockdown of ANGPTL4 inhibited
human GC cell migration and invasion.
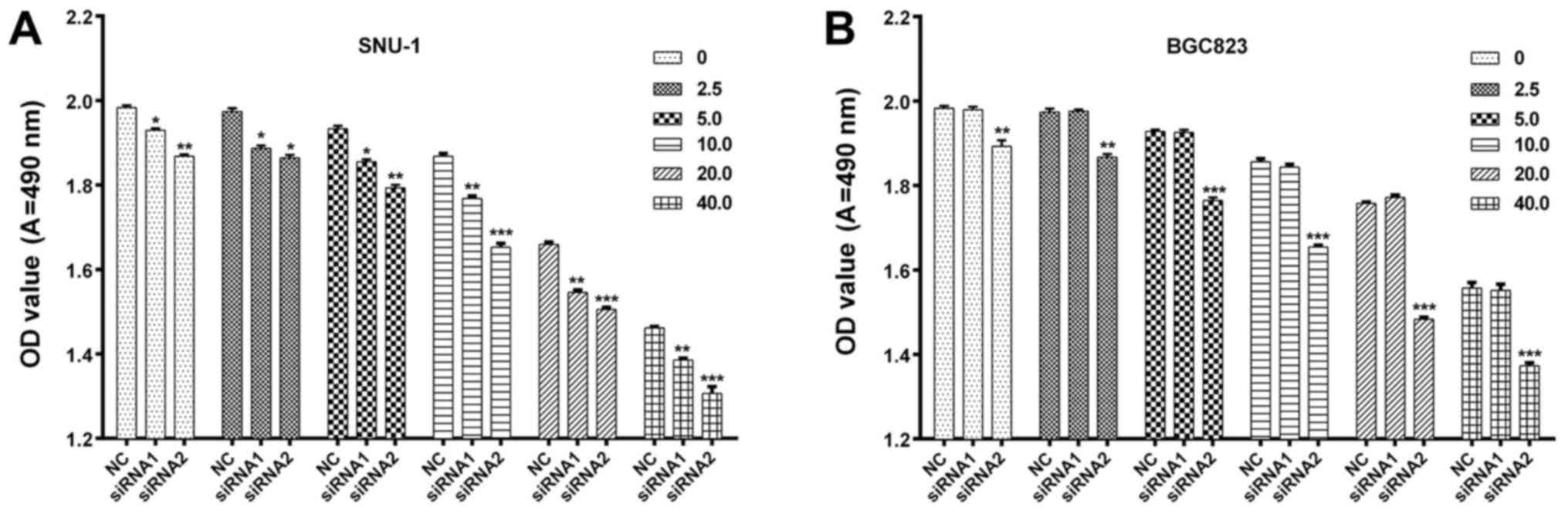
Knockdown of ANGPTL4 enhances the in
vitro sensitivity of human GC cells to cisplatin
Whether the expression of ANGPTL4 affects the
sensitivity of human GC cells to cisplatin remains unexplored. To
ascertain the effects, after transfection with ANGPTL4 siRNA and
stabilization, human GC cells were treated with various
concentrations (0, 2.5, 5.0, 10.0, 20.0 and 40.0 µg/ml) of
cisplatin for 24 h. The results of MTT assay revealed that
knockdown of ANGPTL4 significantly decreased the viability of the
SNU-1 (P<0.05, P<0.01, P<0.001, Fig. 6A) and BGC823 cells (P<0.05,
P<0.01, P<0.001, Fig. 6B) in
response to cisplatin in a dose-dependent manner. These results
obviously indicated that ANGPTL4 knockdown enhanced the sensitivity
of human GC cells to cisplatin.
Discussion
The poor prognosis of gastric cancer (GC) is mainly
due to the lack of early diagnostic biomarkers and effective
treatments. Since the prognosis of GC is closely associated with
the clinical stage of disease at diagnosis, there is an urgent need
to explore novel early diagnostic markers and new effective GC
therapies (17).
The communication between tumor cells and their
microenvironment is extremely important, as it can significantly
affect the efficacy of antitumor treatments (13,18).
Matricellular proteins are a type of extracellular matrix
(ECM)-associated glycoproteins which are secreted by various tumor
cells into the extracellular matrix (19,20).
Increasing evidence has shown that several matricellular proteins
are involved in the development of various tumor cells, including
breast, colorectal and prostate cancer (15,16,21–24).
The ANGPTL family is an important novel member of the matricellular
proteins and was reported to be involved in many metastatic
cancers, indicating that it may play a critical role in the
processes of tumor cell metastasis. Among the ANGPTL family,
ANGPTL2 has been reported to act as an agent in tumor progression
and metastasis in various cancers, such as lung cancer, ovarian
cancer and GC (25–27), and may be a key factor in cancer
progression. In addition, emerging evidence has shown that ANGPTL4
is upregulated in many human epithelial tumors and is involved in
cell migration and prolifration. Zhu et al found that
ANGPTL4 overexpression is widespread in tumors, and promoted tumor
growth by elevating the
O2−:H2O2 ratio
(22). Nakayama et al
examined the mRNA and protein expression level of ANGPTL4 in 144
human colorectal cancer cases. Their findings suggested that
ANGPTL4 is involved in the process of invasion and metastasis
(15). Huang et al reported
that ANGPTL4 promotes lung cancer metastasis by modulating vascular
junction integtity (28). However,
the involvement of ANGPTL4 in human GC is unclear and needs further
investigation.
In the present study, we found that ANGPTL4
expression was upregulated in GC tissues. In order to further
determine the biological functions of ANGPTL4 in human GC, we used
siRNA to block the expression of ANGPTL4 in two human GC cell
lines, SNU-1 and BGC823, and validated the knockdown efficiency at
the mRNA and protein levels by qRT-PCR, and western blot analysis,
respectively. MTT and Transwell assays, Annexin V/PI staining and
flow cytometry were used to assess the effects of ANGPTL4 knockdown
on the GC cells, and results from these assays indicated that
knockdown of ANGPTL4 inhibited proliferation, metastasis, invasion
and promoted apoptosis in both SNU-1 and BGC823 cell lines.
Surgery and chemotherapy are two main curative
treatments for patients with human GC. However, surgery is only
effective for patients with early or locally advanced GC (29). Since most patients have advanced or
metastatic GC at the time of diagnosis, the efficacy of
chemotherapy is limited (30,31).
To date, cisplatin remains the most widely used chemotherapeutic
agent for advanced GC patients worldwide, although it has several
severe side effects, such as asthenia, gastrointestinal disorders
and long-term neurological consequences (32). One study demonstrated that ANGPTL4
increased the survival of melanoma cells against cisplatin-induced
apoptosis (33). Whether ANGPTL4
affects the efficacy of cisplatin in human GC remains unknown. To
explore this, we knocked down ANGPTL4 in SNU-1 and BGC823 cells
with siRNA1 or siRNA2 and treated the cells with different
concentrations of cisplatin. The results showed that the silencing
of ANGPTL4 obviously enhanced the sensitivity of the SNU-1 and
BGC823 cells to cisplatin in a dose-dependent manner.
In conclusion, we found that ANGPTL4 participated in
GC cell proliferation, apoptosis, and metastasis in vitro,
suggesting that it might act as a new potential therapeutic target
for GC patients. However, the roles of ANGPTL4 in vivo
warrant further investigation.
Acknowledgements
The present study was supported by a research grant
from a project by the Leading Talents in Pearl River Talent Plan of
Guangdong Province (no. 81000-42020004) and The Natural Science
Foundation of Guangdong (2017A030313652).
References
|
1
|
Zhang XY and Zhang PY: Gastric cancer:
Somatic genetics as a guide to therapy. J Med Genet. 54:305–312.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li H, Yu B, Li J, Su L, Yan M, Zhu Z and
Liu B: Overexpression of lncRNA H19 enhances carcinogenesis and
metastasis of gastric cancer. Oncotarget. 5:2318–2329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y,
Liu C, Song W, Wang F, Zhang J, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng EK, Chong WW, Jin H, Lam EK, Shin VY,
Yu J, Poon TC, Ng SS and Sung JJ: Differential expression of
microRNAs in plasma of patients with colorectal cancer: A potential
marker for colorectal cancer screening. Gut. 58:1375–1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loffeld RJ, Willems I, Flendrig JA and
Arends JW: Helicobacter pylori and gastric carcinoma.
Histopathology. 17:537–541. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Recavarren-Arce S, León-Barúa R, Cok J,
Berendson R, Gilman RH, Ramírez-Ramos A, Rodríguez C and Spira WM:
Helicobacter pylori and progressive gastric pathology that
predisposes to gastric cancer. Scand J Gastroenterol Suppl.
181:51–57. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang B, Yong H, Zhu H, Ni D, Tang S, Zhang
S, Wang W, Zhou Y, Zhao W, Ding G, et al: Abnormal amphiregulin
expression correlates with gastric cancer prognosis. Oncotarget.
7:76684–76692. 2016.PubMed/NCBI
|
|
8
|
Abu-Farha M, Al-Khairi I, Cherian P,
Chandy B, Sriraman D, Alhubail A, Al-Refaei F, AlTerki A and
Abubaker J: Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression
levels in obesity and T2D. Lipids Health Dis. 15:1812016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masuko K: Angiopoietin-like 4: A molecular
link between insulin resistance and rheumatoid arthritis. J Orthop
Res. 35:939–943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parri M, Pietrovito L, Grandi A,
Campagnoli S, De Camilli E, Bianchini F, Marchiò S, Bussolino F,
Jin B, Sarmientos P, et al: Angiopoietin-like 7, a novel
pro-angiogenetic factor over-expressed in cancer. Angiogenesis.
17:881–896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv
XJ, Wu XL and Qian GS: Role of Angptl4 in vascular permeability and
inflammation. Inflamm Res. 63:13–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu P, Goh YY, Chin HFA, Kersten S and Tan
NS: Angiopoietin-like 4: A decade of research. Biosci Rep.
32:211–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan MJ, Teo Z, Sng MK, Zhu P and Tan NS:
Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer
Res. 10:677–688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SH, Park YY, Kim SW, Lee JS, Wang D
and DuBois RN: ANGPTL4 induction by prostaglandin E2 under hypoxic
conditions promotes colorectal cancer progression. Cancer Res.
71:7010–7020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama T, Hirakawa H, Shibata K, Nazneen
A, Abe K, Nagayasu T and Taguchi T: Expression of angiopoietin-like
4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous
invasion and distant metastasis. Oncol Rep. 25:929–935. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ifon ET, Pang AL, Johnson W, Cashman K,
Zimmerman S, Muralidhar S, Chan WY, Casey J and Rosenthal LJ: U94
alters FN1 and ANGPTL4 gene expression and inhibits tumorigenesis
of prostate cancer cell line PC3. Cancer Cell Int. 5:192005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ayaz L, Görür A, Yaroğlu HY, Özcan C and
Tamer L: Differential expression of microRNAs in plasma of patients
with laryngeal squamous cell carcinoma: Potential early-detection
markers for laryngeal squamous cell carcinoma. J Cancer Res Clin
Oncol. 139:1499–1506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiodoni C, Colombo MP and Sangaletti S:
Matricellular proteins: From homeostasis to inflammation, cancer,
and metastasis. Cancer Metastasis Rev. 29:295–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bornstein P: Matricellular proteins: An
overview. J Cell Commun Signal. 3:163–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnold SA and Brekken RA: SPARC: A
matricellular regulator of tumorigenesis. J Cell Commun Signal.
3:255–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular
O2(−):H2O2 ratio and confers
anoikis resistance to tumors. Cancer Cell. 19:401–415. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Wong CC, Wei H, Gilkes DM,
Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B,
Winnard PT Jr, et al: HIF-1-dependent expression of
angiopoietin-like 4 and L1CAM mediates vascular metastasis of
hypoxic breast cancer cells to the lungs. Oncogene. 31:1757–1770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakayama T, Hirakawa H, Shibata K, Abe K,
Nagayasu T and Taguchi T: Expression of angiopoietin-like 4 in
human gastric cancer: ANGPTL4 promotes venous invasion. Oncol Rep.
24:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Yu X, Xie J, Zhan M, Yu Z, Xie L,
Zeng H, Zhang F, Chen G, Yi X, et al: ANGPTL2/LILRB2 signaling
promotes the propagation of lung cancer cells. Oncotarget.
6:21004–21015. 2015.PubMed/NCBI
|
|
26
|
Kikuchi R, Tsuda H, Kozaki K, Kanai Y,
Kasamatsu T, Sengoku K, Hirohashi S, Inazawa J and Imoto I:
Frequent inactivation of a putative tumor suppressor,
angiopoietin-like protein 2, in ovarian cancer. Cancer Res.
68:5067–5075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshinaga T, Shigemitsu T, Nishimata H,
Takei T and Yoshida M: Angiopoietin-like protein 2 is a potential
biomarker for gastric cancer. Mol Med Rep. 11:2653–2658. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ,
Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, et al: ANGPTL4 modulates
vascular junction integrity by integrin signaling and disruption of
intercellular VE-cadherin and claudin-5 clusters. Blood.
118:3990–4002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Young K and Chau I: Targeted therapies for
advanced oesophagogastric cancer: Recent progress and future
directions. Drugs. 76:13–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10 Suppl 3:49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Van Cutsem E, Dicato M, Geva R, Arber N,
Bang Y, Benson A, Cervantes A, Diaz-Rubio E, Ducreux M,
Glynne-Jones R, et al: The diagnosis and management of gastric
cancer: Expert discussion and recommendations from the 12th
ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010.
Ann Oncol. 22 Suppl 5:v1–v9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kostova I: Platinum complexes as
anticancer agents. Recent Patents Anticancer Drug Discov. 1:1–22.
2006. View Article : Google Scholar
|
|
33
|
Sun Y, Long J and Zhou Y:
Angiopoietin-like 4 promotes melanoma cell invasion and survival
through aldolase A. Oncol Lett. 8:211–217. 2014. View Article : Google Scholar : PubMed/NCBI
|