Introduction
Gastric cancer (GC) is currently the fifth most
common cancer worldwide (1). As
reported in the World Health Organization's World Cancer Report,
there were an estimated 22,220 new cases of GC (7% of all new
cancer diagnoses) and 10,990 deaths from GC (9% of all
cancer-related deaths) globally in 2014. In China, gastric and
liver cancers have the highest mortality rates; the incidence of
new cases of GC is second only to that of liver cancer (2).
Melatonin (N-acetyl-5-methoxytryptamine)
biosynthesis is initiated by the uptake of the essential amino acid
tryptophan by the pineal gland. Melatonin possesses diverse
physiological functions, including regulation of circadian rhythms,
controlling the maturation of the reproductive system and promoting
skeletal growth; melatonin also has antitumor, immunomodulatory,
antioxidant and free-radical scavenging activities (3,4). Many
tissues and organs have the ability to synthesize melatonin in
addition to the pineal gland, including the retina, striatum,
spleen, liver and gastrointestinal tract (5). In the gastrointestinal tract, for
example, the amount of melatonin produced far exceeds that produced
by other organs: Approximately 400 times higher than that in the
pineal gland, with concentrations 10–100 times higher than those
found in plasma (6). Cells of
gastrointestinal tissues not only have the ability to secrete
melatonin but also express melatonin receptors. Melatonin exerts
important protective and regulatory effects via autocrine and
paracrine activities in the gastrointestinal tract. A previous
study found that melatonin enhances the immune system of the gut
(7), regulates fecal water content,
reduces peristalsis, and prevents gut damage due to digestive
enzymes, hydrochloric acid (6) and
exogenously administered drugs (8).
In previous studies, melatonin was found to inhibit
various carcinomas, such as GC (9–13),
liver (14–17), breast (18–21),
oral (22,23) and prostate cancer (24–26).
Melatonin exerts anticancer effects by promoting cellular
apoptosis, inhibiting cell growth, regulating anticancer immunity,
scavenging free radicals and competitively inhibiting estrogen.
However, the mechanism by which melatonin exerts these effects is
unclear. Our previous studies found that melatonin exhibited
effective anticancer effects that were mediated by the stimulation
of apoptosis, inhibition of cell growth, and reduction in the
number of CD4+CD25+ regulatory T cells in
mouse GC cells and in vivo (27,28).
We also found that the nuclear receptor RORγ is involved in the
effects of melatonin on human GC cells (12,13). A
recent study demonstrated that melatonin induces AGS cell apoptosis
via the activation of JNK and p38, and the suppression of NF-κB
(13). To further elucidate the
effects of melatonin on human GC cells and the molecular mechanism
involved, we selected the human GC cell line SGC-7901 and analyzed
the resulting changes in proteins using protein chip technology.
Several proteins related to apoptosis and cell proliferation were
identified and further tested in SGC-7901 cells, including AKT,
MDM2, CDC25A, p53, p21, Bcl-xL and Bax. Both CDC25A and p21 are
known to regulate cell cycle progression via their interactions
with cyclin/CDK complexes. Bcl-xL and Bax are both mitochondrial
proteins that control the release of cytochrome c and are
involved in mitochondrial apoptosis. AKT, MDM2 and p53 are upstream
regulators of the mitochondrial apoptosis pathway. The aim of the
present study was to elucidate the mechanism by which melatonin
elicits its anticancer effects in GC cells.
Materials and methods
Cell culture and reagents
Human GC cell line SGC-7901 was purchased from the
Chinese Academy of Sciences, Shanghai Institute for Biological
Science (Shanghai, China). SGC-7901 cells were cultured in Roswell
Park Memorial Institute (RPMI)-1640 medium supplemented with 10%
fetal bovine serum (both from GE Healthcare Life Sciences, HyClone
Laboratories, Logan, UT, USA). The cultures were maintained at 37°C
in 5% CO2. Melatonin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was dissolved in ethanol prior to use. The
final concentration of ethanol in the culture medium never exceeded
1%.
Cell viability assays
After exposure of GC cells to various concentrations
of melatonin for various times, we assessed cell viability using
the MTS assay (CellTiter 96® AQueous One
Solution Cell Proliferation Assay; Promega, Madison, WI, USA),
which is based on the mitochondrial conversion of MTS (a
tetrazolium salt) into a water-soluble, colored, formazan
precipitate that can be quantified by spectrophotometry. SGC-7901
cells were seeded at a density of 1×104 cells/ml in
96-well plates. After 24 h of culture, the cells were treated with
0 (1% ethanol as control was added), 1, 2, 3, 4 or 5 mM melatonin
for 24, 48 or 72 h. Absorbance of cells at 490 nm was measured
using a microplate reader (Synergy HT; BioTek Instruments Inc.,
Winooski, VT, USA) when MTS was added 2 h later. Effects on
SGC-7901 cell viability was measured by determining the percentage
of viable cells relative to the control: % cell inhibition = [1 -
(ODmt - ODblank)]/ (ODc
-ODblank) × 100%, where ODmt is the average
OD value of the melatonin-treated samples, ODc is the
average OD value of the control samples, and ODblank is
the average OD value of the blank samples without cells.
Cell morphology at the microscopic and
ultramicroscopic levels
SGC-7901 cells were seeded at a density of
2×105 cells/ml in 6-well plates. After 24 h of culture,
the cells were treated with 3 mM melatonin or 1% ethanol (control)
for 24 h. Cell morphology was observed with an inverted microscope
(Primo Vert; Carl Zeiss Microscopy GmbH, Jena, Germany) and an
electron microscope (EM208; FEI, Hillsboro, OR, USA).
Cell cycle analysis
After treatment with melatonin, cells were collected
and stained with propidium iodide (PI; BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA). Single-cell suspensions were
sorted by flow cytometry, which revealed the distribution of cells
in the three major phases of the cycle (G0/G1
vs. S vs. G2/M).
Analysis of apoptosis
We use the TUNEL assay (DeadEnd™ Fluorometric TUNEL
System; Promega) to analyze cell apoptosis in situ. This
assay measures nuclear DNA fragmentation of apoptotic cells tagged
with fluorescein-12-dUTP, which can be visualized by fluorescence
microscopy. SGC-7901 cells were cultured on coverslips and treated
with either 3 mM melatonin or 1% ethanol for 24 h. After three
washes with phosphate-buffered saline, the cells were fixed in 4%
paraformaldehyde and incubated at 4°C for 25 min. Fixed cells were
permeabilized with 0.2% Triton X-100 solution for 5 min. The cells
were then incubated with a nucleotide mixture and rTdT buffer
solution, and incubated at 37°C for 60 min to allow the tailing
reaction to occur in the dark. After terminating the reaction in 2X
SSC, the cells were counterstained with PI for 15 min at room
temperature. Positive apoptotic cells were identified under 5
random fields of view by fluorescence microscopy (Axio Observer A1;
Carl Zeiss Microscopy GmbH, Jena, Germany).
To analyze the rate of cellular apoptosis, we used
the FITC Annexin V apoptosis detection kit I (BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA), following the manufacturer's
instructions. Briefly, SGC-7901 cells were treated with 3 mM
melatonin for 24 h and collected. In cells that have undergone
apoptosis, phosphatidylserine (PS), which is usually located in the
inner leaflet of the plasma membrane, is translocated to the outer
leaflet of the plasma membrane. Once on the outer surface of the
membrane, PS is bound by FITC-labeled Annexin V and detected by
flow cytometry.
Protein extraction and western blot
analysis
Melatonin-and vehicle-treated SGC-7901 cells were
lysed in cell lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China) supplemented with a protease inhibitor cocktail
and phosphatase inhibitors (Roche, Basel, Switzerland). Protein
concentrations were measured using the enhanced BCA protein assay
kit (Beyotime Institute of Biotechnology). Protein extracts (40 µg)
were subjected to 12 or 15% polyacrylamide gel electrophoresis. The
proteins in the gels were transferred to polyvinylidene difluoride
membranes, which were then blocked in Tris-buffered saline
containing 0.5% bovine serum albumin. Blocked membranes were
incubated with primary antibodies: anti-MDM2 (cat. no. ab137413;
dilution, 1:1,000), anti-phospho-MDM2 (at Ser166; cat. no.
ab170880; dilution, 1:50,000), anti-CDC25A (cat. no. ab140247;
dilution, 1:200), anti-phospho-CDC25A (at Ser75; cat. no. ab47279;
dilution, 1:1,000), anti-p21 (cat. no. ab109199; dilution,
1:5,000), and anti-phospho-p21 (at Thr145; cat. no. ab47300;
dilution, 1:1,000) were purchased from Abcam (Cambridge, UK);
anti-AKT (cat. no. 4685S; dilution, 1:1,000), anti-phospho-AKT (at
Thr308; cat. no. 4056S; dilution, 1:1,000), anti-Bcl-xL (cat. no.
2764S; dilution, 1:1,000), anti-Bax (cat. no. 5023P; dilution,
1:1,000), anti-caspase-9 (cat. no. 9508S; dilution, 1:1,000) and
anti-GAPDH (cat. no. 2118S; dilution, 1:1,000) were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA) and anti-p53 was
purchased from Medical and Biological Laboratories Co., Ltd.,
Nagoya, Japan (cat. no. K0181-3; dilution, 1:5,000). Proteins were
detected by the addition of alkaline phosphatase-conjugated
secondary antibody, goat anti-rabbit IgG (cat. no. ab98505;
dilution, 1:5,000; Abcam) or goat anti-mouse IgG (cat. no. sc-2008;
dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Target proteins were visualized by the addition of CDP-Star
reagents (Roche Diagnostics, Mannheim, Germany). The bands were
detected using an ImageQuant LAS 4000 mini (GE Healthcare, Chicago,
IL, USA). Band intensities were quantified using ImageJ2× software
(National Institutes of Health, Bethesda, MD, USA) and the relative
intensities to the internal GAPDH control were calculated.
Analysis of caspase-3 activity
SGC-7901 cells were seeded at a density of
1×104 cells/ml in 96-well plates. After 24 h of culture,
the cells were treated with 0 (1% ethanol as control) or 3 mM
melatonin for 24 h. Melatonin- and vehicle-treated SGC-7901 cells
were added together with 100 µl of Caspase-Glo® 3/7
Reagent according to the caspase-Glo® 3/7 assay kit
(Promega Corp., Madison, WI, USA) manufacturer's instructions. This
assay provides a luminogenic caspase-3/7 substrate that is released
following caspase cleavage, and the subsequent production of light
can be detected by a microplate luminometer (Orion microplate
luminometer; Berthold Detection Systems GmbH, Pforzheim,
Germany).
Data analysis
The data represent the means ± standard deviations
(SD) from at least three independent experiments. One-way ANOVA and
the Student's paired t-test were used to determine statistical
significance. P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA).
Results
Melatonin inhibits the proliferation
of SGC-7901 cells
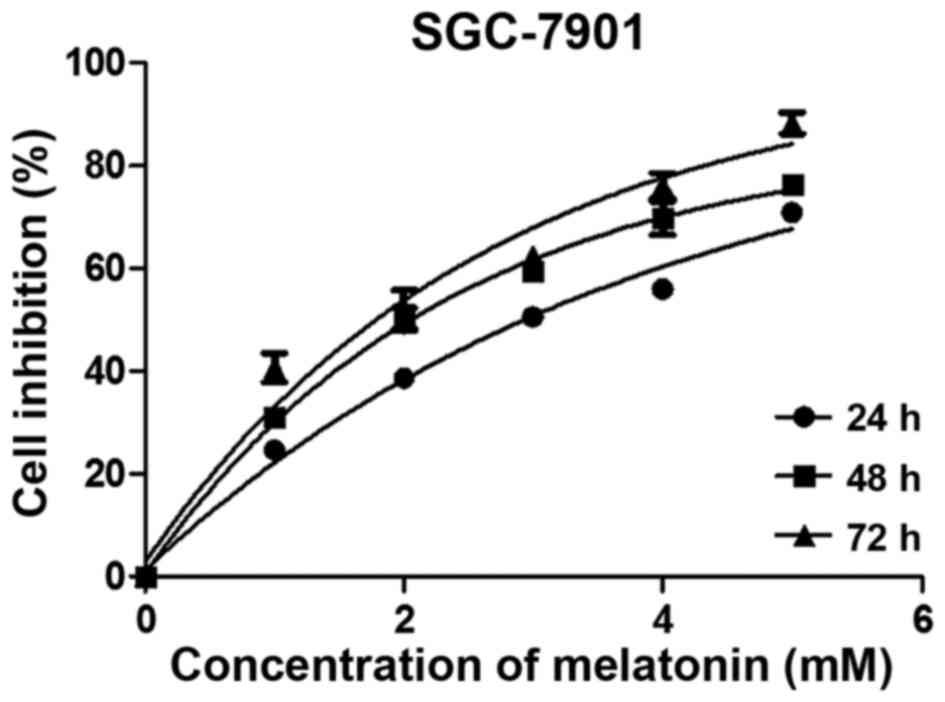
We assessed the cell viability using the MTS assay.
Results demonstrated that cell growth was inhibited after melatonin
exposure in a dose- and time-dependent manner (Fig. 1). Based on these results, we
selected 3 mM melatonin and 24 h of exposure for the follow-up
experiments; these parameters reflect a 50% inhibition of cell
viability. Cells treated with higher concentrations of melatonin
for longer times were not suitable for use in subsequent
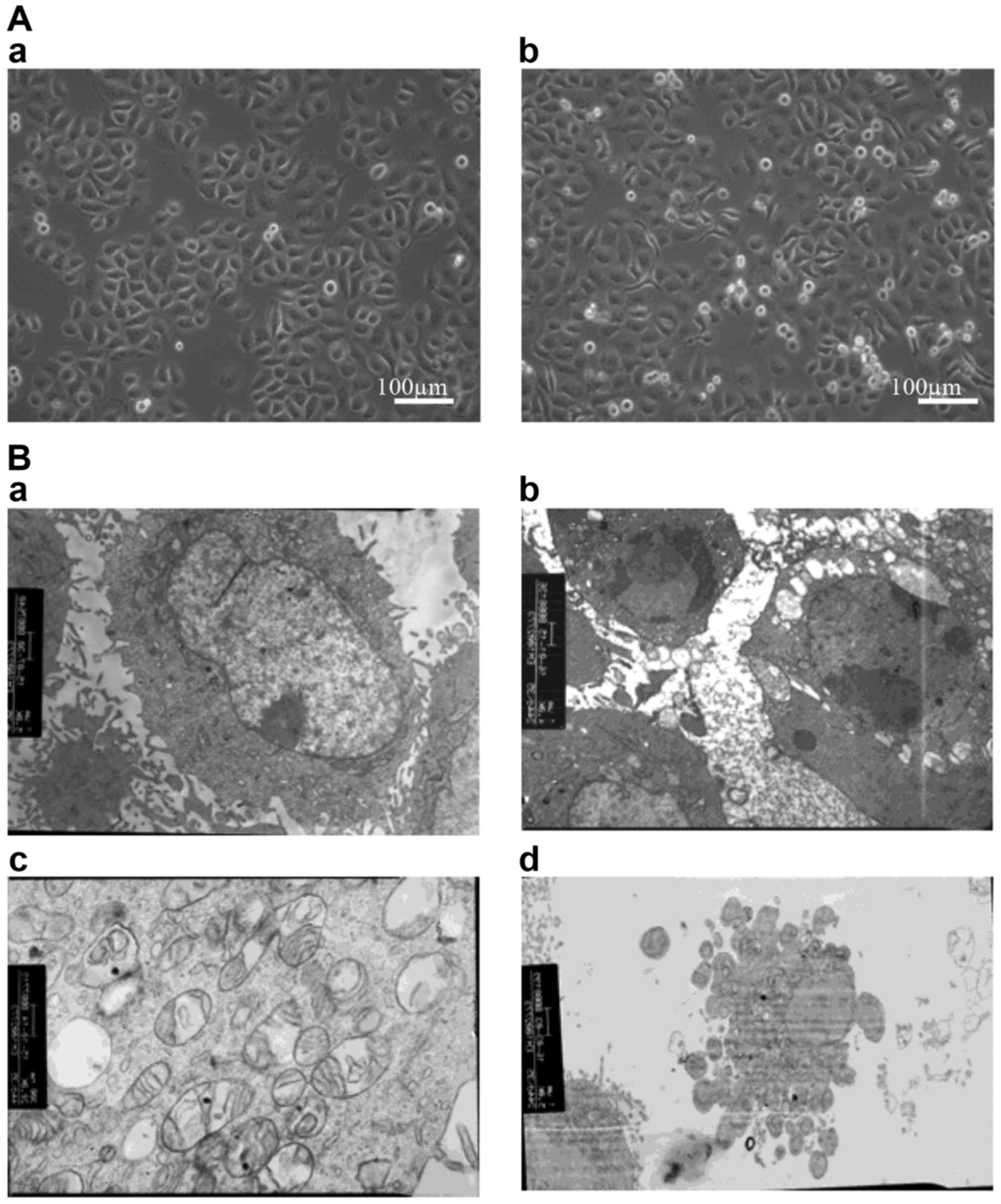
experiments. The morphology of treated SGC-7901 cells was assessed
microscopically (Fig. 2). At the
microstructural level, melatonin-treated cells appeared thinner and
more elongated than control cells, with twisted cytoplasmic
extensions and a greater number of floating dead cells. At the
ultrastructural level, apoptotic cells were evident, surface
microvilli were absent, plasmolysis and nuclear pyknosis were
present, mitochondria exhibited vacuolation, and apoptotic bodies
appeared in the cultures of melatonin-treated cells.
Melatonin induces cell cycle arrest and
apoptosis in SGC-7901 cells
Melatonin arrests SGC-7901 cells in
the G1/S phase of the cell cycle
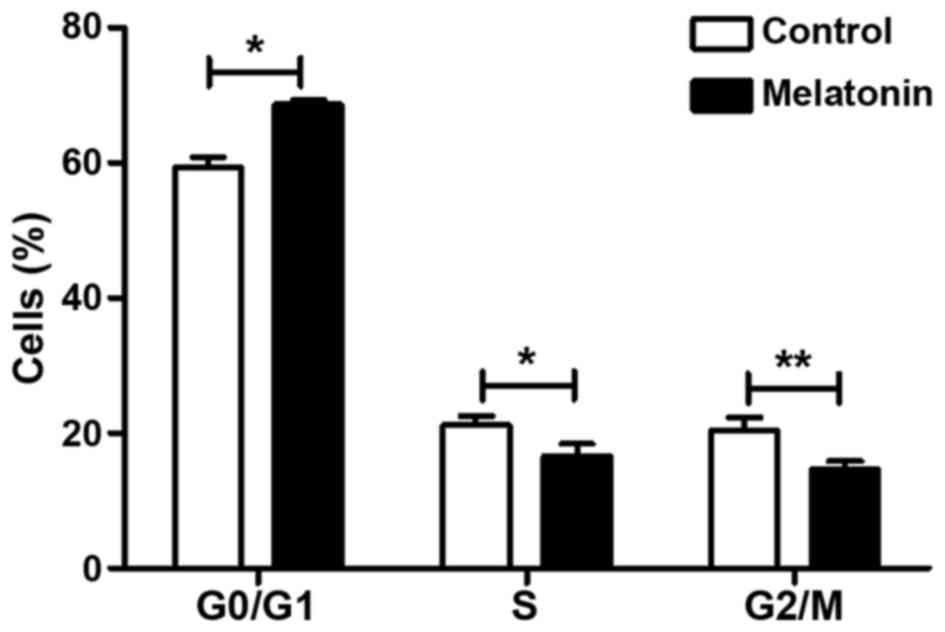
We assessed the distribution of SGC-7901 cells in
various phases of the cell cycle by flow cytometry. The analysis
showed that the proportion of cells in the
G0/G1 phase increased from 59.357±1.518 to
68.583±0.649% (P<0.05) in the melatonin-treated SGC-7901 cells
compared to the controls (Fig. 3).
Consistent with this finding, the proportion of cells in the S and
G2/M phases decreased relative to the controls: As shown
in Fig. 3, the proportion of
S-phase cells decreased from 21.177±1.322 to 16.590±1.874%
(P<0.05), and the proportion of G2/M-phase cells
decreased from 20.450±1.868 to 14.727±1.194% (P<0.01). All
differences were statistically significant. These results indicate
that melatonin effectively arrested SGC-7901 cells in the
G1/S phase of the cell cycle.
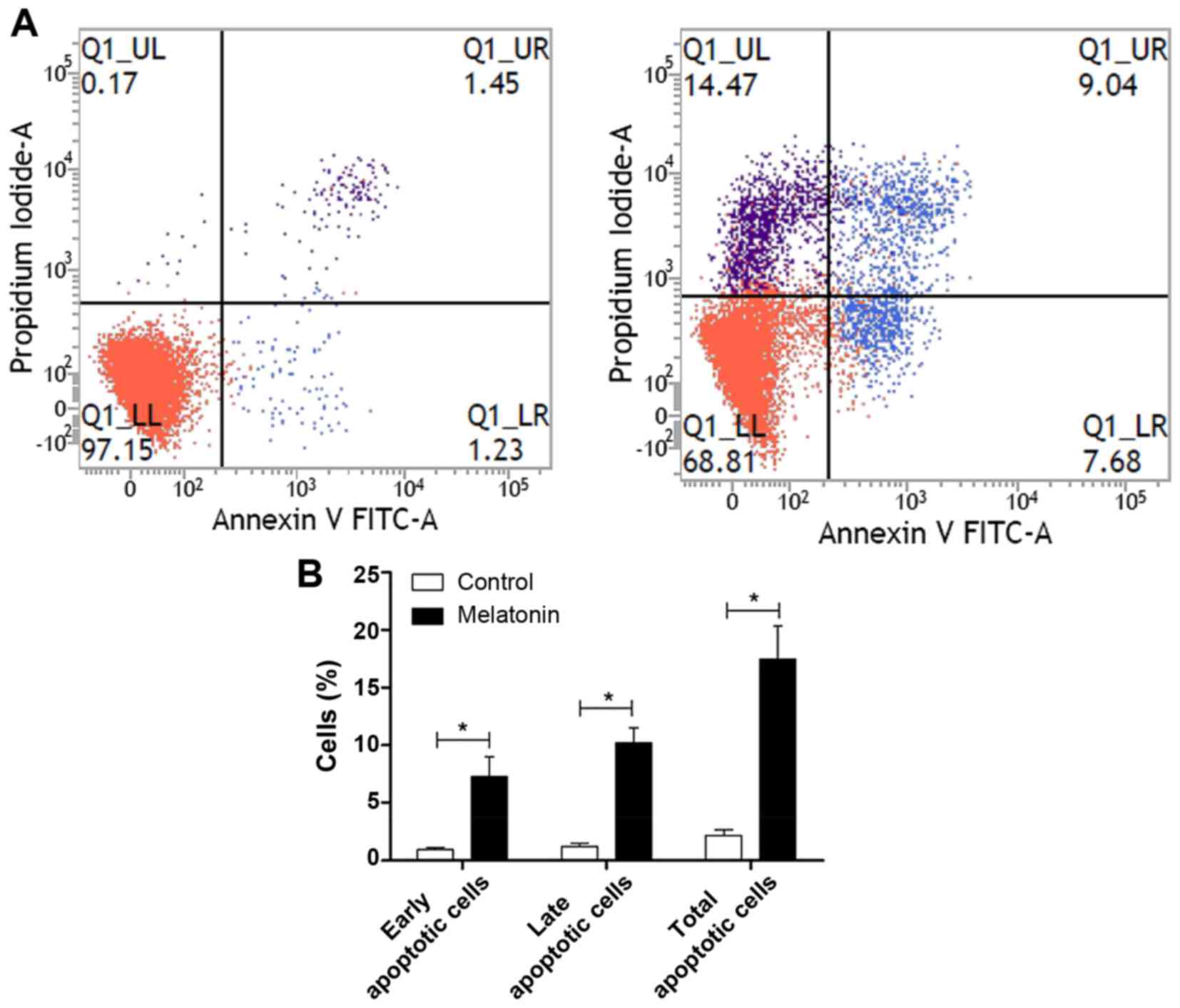
Melatonin induces apoptosis in
SGC-7901 cells
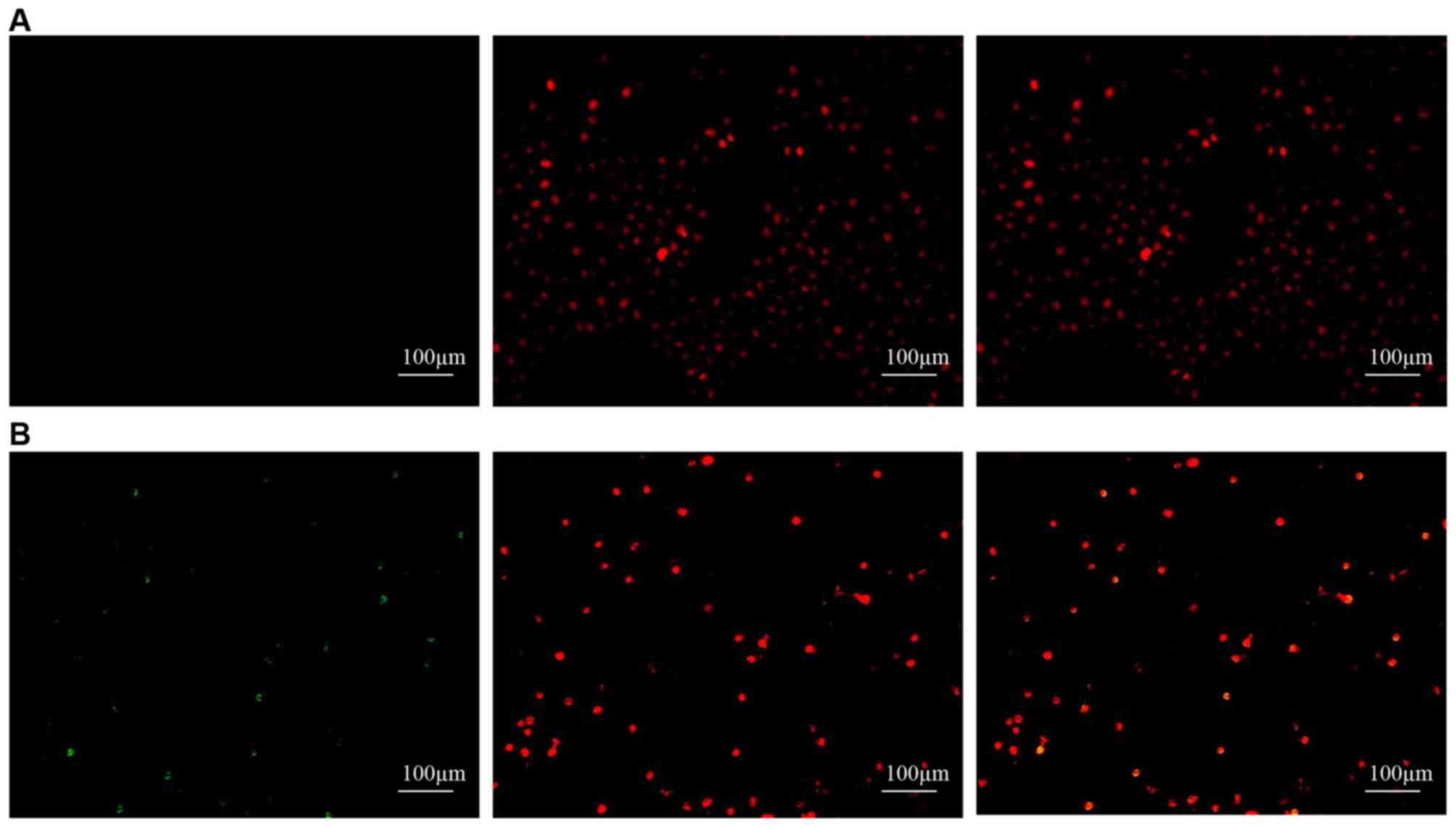
We used two methods to determine whether melatonin
stimulates the apoptosis of SGC-7901 cells. First, we used the
TUNEL assay to measure apoptotic SGC-7901 cells in situ. As
shown in Fig. 4, apoptosis could be
observed in the SGC-7901 cells treated with melatonin but not in
the controls. We then used flow cytometry to determine the
percentage of apoptotic cells using the FITC Annexin V apoptosis
detection kit I. The percentages of melatonin-treated cells in the
early and late stages of apoptosis were found to increase compared
with the controls (Fig. 5). Early
apoptotic cells increased from 0.97±0.31 to 7.25±3.00 (P<0.05),
late apoptotic cells increased from 1.23±0.53 to 10.22±2.22
(P<0.05), and the total number of apoptotic cells increased from
2.20±0.81 to 17.48±4.98 (P<0.05). All differences were
statistically significant.
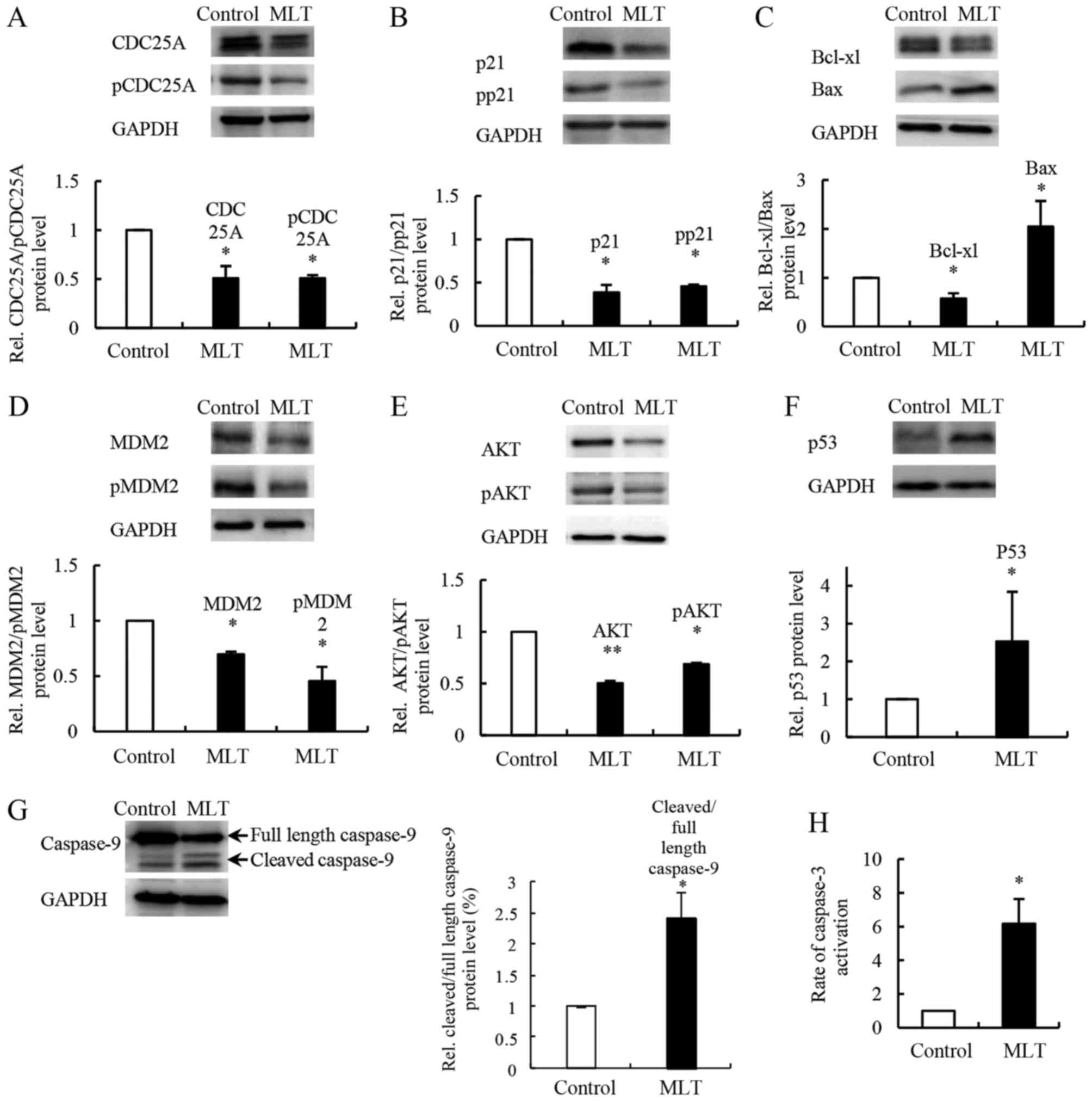
Melatonin affects proteins associated
with the cell cycle and apoptosis
CDC25A and p21 are known regulators of cell cycle
progression that interact with cyclin/CDK complexes. Melatonin
reduced the expression of CDC25A and the level of CDC25A
phosphorylation at Ser75 in SGC-7901 cells compared to the controls
(Fig. 6A). Moreover, we found that
both the level of p21 and its phosphorylation at Thr145 were
decreased (Fig. 6B). We examined
changes in apoptosis-associated proteins Bcl-xL, Bax, caspase-9 and
caspase-3. In SGC-7901 cells treated with melatonin, western blot
analysis showed evidence of a reduced expression level of Bcl-xL
and a significantly increased expression level of Bax compared with
controls (Fig. 6C). Melatonin
treatment also increased cleaved caspase-9 levels and caspase-3
activity (Fig. 6G and H).
Melatonin affects the expression of
upstream regulators MDM2, p53 and AKT
MDM2, p53 and AKT are upstream regulators of the
apoptosis- and cell cycle-related proteins mentioned above. To
further understand the molecular mechanism underlying
melatonin-induced cell apoptosis and inhibition of cell
proliferation, we evaluated the expression levels of MDM2, p53 and
AKT in SGC-7901 cells after melatonin exposure. Western blot
analysis showed that the expression levels of MDM2 and phospho-MDM2
(at Ser166) were decreased compared with the controls (Fig. 6D), whereas the expression level of
p53 was increased compared with the controls (Fig. 6F). Levels of both AKT and
phospho-AKT (at Thr308) were decreased after melatonin treatment of
SGC-7901 cells compared to the controls (Fig. 6E).
Discussion
The results of the present study confirmed that
melatonin arrests SGC-7901 GC cells in the G1/S phase of the cell
cycle and promotes apoptosis via a mitochondrial apoptosis pathway,
involving a key factor, MDM2. We detected increased expression
levels of MDM2, phospho-MDM2 (at Ser166), and the upstream
regulator AKT in response to melatonin. Murine double 2 min, also
HDM) (MDM2) was originally identified as an amplified oncogene on
double-minute chromosomes in transformed mouse fibroblasts
(13). While it is involved in the
regulation of gene expression as E3 ubiquitin-protein ligase, MDM2
itself is overexpressed in various human cancers (30,31),
including GC (32–36). MDM2 is an important negative
regulator of the p53 tumor suppressor. Overexpression of MDM2
promotes tumor development by suppressing the function of p53
(37). At the protein level, MDM2
increases both the polyubiquitination of p53, which drives its
proteasomal degradation, and the monoubiquitination of p53, which
exposes a nuclear export signal, leading to the cytoplasmic
translocation of p53 (38,39) and inhibition of its interaction with
DNA (40–43). MDM2 also directly inhibits p53
transcription by binding to the transactivation domain of the p53
gene at the transcriptional level (44,45).
Furthermore, MDM2 inhibits p53 mRNA translation by binding the
5′-UTR of p53 mRNA, likely through interactions with ribosomal
protein L26, a positive regulator of p53 expression (46). The phosphorylated form of MDM2 at
Ser166 is a substrate for AKT that is activated when its Thr308 is
phosphorylated by PDPK1, which increases the nuclear localization
of MDM2 and the subsequent degradation of p53 (47–49).
In our experiments, melatonin exposure caused the downregulation of
AKT, phospho-AKT (at Thr308), MDM2, and phospho-MDM2 (at Ser166),
and the upregulation of p53, suggesting that melatonin inhibits
cancer cell growth by attenuating AKT activity, which leads to the
inactivation of MDM2.
However, the presence of p53 mutations in cancer
diminishes the ability of p53 to inhibit tumor growth. Many studies
have argued that there are p53-independent effects of MDM2
(50,51). MDM2 could influence the activities
of other transcription factors, such as p73 (52–55),
p65 (56), Smad proteins (57,58),
cyclin D1, c-Jun, c-Myc and pRb (E2F1) (59,60).
Moreover, MDM2 has been shown to influence chromatin modifications
by interacting directly with chromatin (61–63). A
p53 mutation was reported in SGC-7901 cells, but its oncostatic
function was not blocked completely (64). However, this infers that other
components may be involved in the melatonin-induced inhibition of
GC cell growth.
In the present study, melatonin was found to affect
other components of the mitochondrial apoptosis pathway downstream
of MDM2 and AKT. Melatonin treatment caused a decrease in Bcl-xL
levels and an increase in Bax levels, which were consistent with
the results of our previous studies concerning the inhibitory
effect of melatonin on the proliferation of mouse precancerous
cells (28). The changes in Bcl-xL
and Bax as well as the upregulation of cleaved caspase-9 and
activated caspase-3 indicated that melatonin induced apoptosis via
a mitochondrial apoptosis pathway. Cell cycle-related proteins
CDC25A and p21 were also affected by melatonin treatment. CDC25A is
a dual-specificity phosphatase that activates the G1/S-phase
cyclin-dependent kinases CDK4 and CDK2, which are required for cell
cycle progression (65). CDC25A was
also found to suppress apoptosis by inhibiting ASK1 activity
(66). The degradation of CDC25A by
ubiquitination is blocked by the phosphorylation of CDC25A at Ser75
(67). Furthermore, CDC25A is
overexpressed in a variety of tumor types, such as lung, breast,
prostate and GC and is correlated with poor prognosis (68). Melatonin induces the downregulation
of CDC25A and phospho-CDC25A (Ser75), causing cell cycle arrest in
the G1/S phase. Surprisingly, levels of p21, a cyclin/CDK complex
inhibitor, were decreased in SGC-7901 cells after melatonin
treatment which was different from previous studies in mouse
precancerous cells (28). However,
some researchers have shown increases in p21 levels in response to
mitogenic signals. The binding of p21 to cyclin/CDK forms a complex
that stimulates cell cycle progression (69). Martin et al (70) also found increased p21 expression
levels in C6 glioma cells treated with melatonin. Their results are
consistent with those of our experiments. Moreover, Rother et
al (71) proposed that p53
suppresses CDC25A expression, independent of p21, therefore, the
role of p21 is complex and may vary in different conditions.
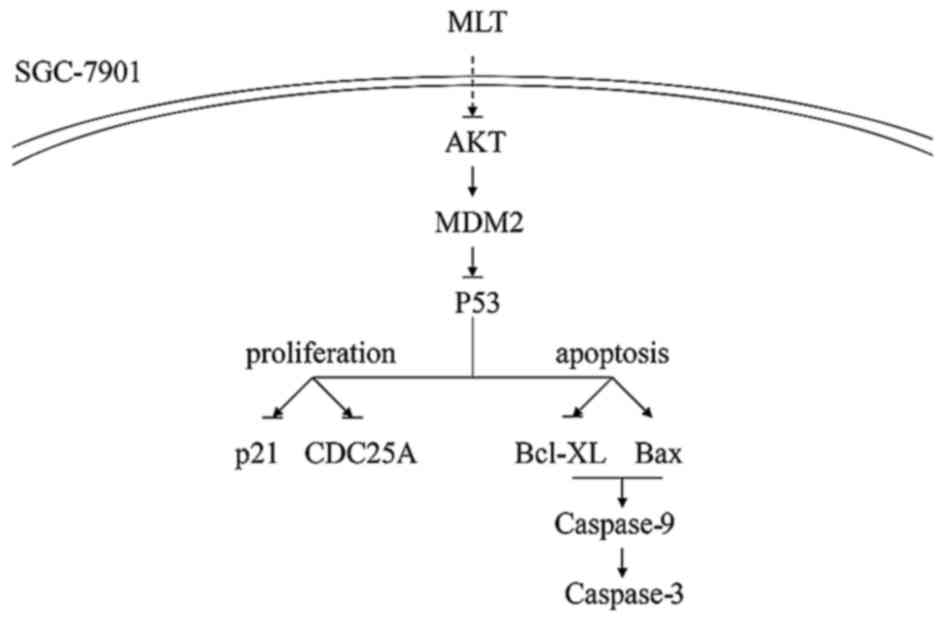
In conclusion, the downregulation of AKT, MDM2, and
changes in the above-mentioned factors suggest that the AKT/MDM2
pathway is involved in the mechanism by which melatonin inhibits
the growth of SGC-7901 GC cells (Fig.
7). Melatonin induces apoptosis and inhibits the proliferation
of SGC-7901 via downregulation of AKT and MDM2, inducing an
increase in p53. Previous studies suggest that p53 activates the
expression of the Cdk inhibitor p21, resulting in CDC25A decrease,
and cell cycle arrest in the G1/S phase. However, we found a
decrease in p21 indicating that the CDC25A decrease is independent
of p21. Further research should be conducted concerning the role of
p21 in the effect of melatonin against GC SGC-7901 cell growth.
There are other points needed to be tested in future studies.
Firstly, the flow cytometric analysis showed that the proportion of
cells in the G0/G1 phase was increased and in
the S, G2/M phases was decreased relative to the
controls. It would be better if the G0 and G1
phase of SGC-7901 cells are differentiated further, since there
would be a few early apoptotic cells induced by the melatonin
mixing with the cells of G0 phase. The conclusion that
melatonin induces cell cycle arrest in G1/S would be
confirmed to a higher degree removing these potential apoptotic
cells. Secondly, this study analyzes only one type of human GC cell
line. We are currently in the selection of more cell lines and a
PDX model to confirm the anticancer effect of melatonin. The
experiments are still underway and the results need further
verification.
Acknowledgements
The authors would like to thank JJL and ZHH from the
Public Technology Service Center (Fujian Medical University,
Fuzhou, China) for their technical assistance.
Funding
The present study was sponsored by the Project of
the Education Department of Fujian Province of China (nos. JA12153
and JAT160202), the Natural Science Foundation of Fujian Provincial
Department of Science & Technology (nos. 2016J01535 and
2017J01530), the Young and Middle-aged Key Personnel Training
Program of Fujian Provincial Health and family planning commission
(no. 2016-ZQN-51) and the Nursery Research Fund Project of Fujian
Medical University (2015MP006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RXZ was involved in the study concept and design,
supervision and provided final approval of the version to be
published. JS was involved in the drafting of the manuscript, the
analysis and interpretation of the data, performed experiments and
obtained funding. SJM, JHL and HZ were involved in performing
experiments, analysis and interpretation of the data. RXW, HL, LL
and ZGZ assisted with the experimental design, data interpretation,
acquisition of funding. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
MLT
|
melatonin
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization, . World Cancer
Report 2014. World Health Organization; Geneva: 2014
|
|
3
|
Voiculescu SE, Zygouropoulos N, Zahiu CD
and Zagrean AM: Role of melatonin in embryo fetal development. J
Med Life. 7:488–492. 2014.PubMed/NCBI
|
|
4
|
Karaaslan C and Suzen S: Antioxidant
properties of melatonin and its potential action in diseases. Curr
Top Med Chem. 15:894–903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Acuña-Castroviejo D, Escames G, Venegas C,
Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX
and Reiter RJ: Extrapineal melatonin: Sources, regulation, and
potential functions. Cell Mol Life Sci. 71:2997–3025. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bubenik GA: Thirty four years since the
discovery of gastrointestinal melatonin. J Physiol Pharmacol. 59
Suppl 2:33–51. 2008.PubMed/NCBI
|
|
7
|
Bubenik GA: Gastrointestinal melatonin:
Localization, function, and clinical relevance. Dig Dis Sci.
47:2336–2348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolli VK, Kanakasabapathy I, Faith M,
Ramamoorthy H, Isaac B, Natarajan K and Abraham P: A preclinical
study on the protective effect of melatonin against
methotrexate-induced small intestinal damage: Effect mediated by
attenuation of nitrosative stress, protein tyrosine nitration, and
PARP activation. Cancer Chemother Pharmacol. 71:1209–1218. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lissoni P, Barni S, Crispino S, Tancini G
and Fraschini F: Endocrine and immune effects of melatonin therapy
in metastatic cancer patients. Eur J Cancer Clin Oncol. 25:789–795.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Qi Y, Zhang H, He W, Zhou Q, Gui
S and Wang Y: Melatonin inhibits cell growth and migration, but
promotes apoptosis in gastric cancer cell line, SGC7901. Biotech
Histochem. 88:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RX, Liu H, Xu L, Zhang H and Zhou RX:
Involvement of nuclear receptor RZR/RORγ in melatonin-induced
HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol
Rep. 34:2541–2546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang RX, Liu H, Xu L, Zhang H and Zhou RX:
Melatonin downregulates nuclear receptor RZR/RORγ expression
causing growth-inhibitory and anti-angiogenesis activity in human
gastric cancer cells in vitro and in vivo. Oncol Lett. 12:897–903.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Fan M, Chen Y, Zhao Q, Song C, Yan
Y, Jin Y, Huang Z, Lin C and Wu J: Melatonin induces cell apoptosis
in AGS cells through the activation of JNK and P38 MAPK and the
suppression of nuclear factor-kappa B: A novel therapeutic
implication for gastric cancer. Cell Physiol Biochem. 37:2323–2338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, García-Palomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan L, Sun G, Ma T, Zhong F and Wei W:
Melatonin overcomes apoptosis resistance in human hepatocellular
carcinoma by targeting survivin and XIAP. J Pineal Res. 55:174–183.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan L, Sun G, Ma T, Zhong F, Lei Y, Li X
and Wei W: Melatonin reverses tunicamycin-induced endoplasmic
reticulum stress in human hepatocellular carcinoma cells and
improves cytotoxic response to doxorubicin by increasing CHOP and
decreasing survivin. J Pineal Res. 55:184–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ordoñez R, Carbajo-Pescador S,
Prieto-Dominguez N, García-Palomo A, González-Gallego J and Mauriz
JL: Inhibition of matrix metalloproteinase-9 and nuclear factor
kappa B contribute to melatonin prevention of motility and
invasiveness in HepG2 liver cancer cells. J Pineal Res. 56:20–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alvarez-García V, González A,
Alonso-González C, Martínez-Campa C and Cos S: Regulation of
vascular endothelial growth factor by melatonin in human breast
cancer cells. J Pineal Res. 54:373–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blask DE, Dauchy RT, Dauchy EM, Mao L,
Hill SM, Greene MW, Belancio VP, Sauer LA and Davidson L: Light
exposure at night disrupts host/cancer circadian regulatory
dynamics: Impact on the Warburg effect, lipid signaling and tumor
growth prevention. PLoS One. 9:e1027762014.doi:
10.1371/journal.pone.0102776. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cos S, Alvarez-García V, González A,
Alonso-González C and Martínez-Campa C: Melatonin modulation of
crosstalk among malignant epithelial, endothelial and adipose cells
in breast cancer (Review). Oncol Lett. 8:487–492. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Proietti S, Cucina A, Dobrowolny G,
D'Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A,
Morini V, Reiter RJ, et al: Melatonin down-regulates MDM2 gene
expression and enhances p53 acetylation in MCF-7 cells. J Pineal
Res. 57:120–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cutando A, López-Valverde A, DE Vicente J,
Gimenez JL, Carcía IA and DE Diego RG: Action of melatonin on
squamous cell carcinoma and other tumors of the oral cavity
(Review). Oncol Lett. 7:923–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goncalves NN, Rodrigues RV, Jardim-Perassi
BV, Moschetta MG, Lopes JR, Colombo J and Zuccari DA: Molecular
markers of angiogenesis and metastasis in lines of oral carcinoma
after treatment with melatonin. Anticancer Agents Med Chem.
14:1302–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez-Garcia A, Mayo JC, Hevia D,
Quiros-Gonzalez I, Navarro M and Sainz RM: Phenotypic changes
caused by melatonin increased sensitivity of prostate cancer cells
to cytokine-induced apoptosis. J Pineal Res. 54:33–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiu SY, Leung WY, Tam CW, Liu VW and Yao
KM: Melatonin MT1 receptor-induced transcriptional up-regulation of
p27(Kip1) in prostate cancer antiproliferation is mediated via
inhibition of constitutively active nuclear factor kappa B (NF-κB):
Potential implications on prostate cancer chemoprevention and
therapy. J Pineal Res. 54:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paroni R, Terraneo L, Bonomini F, Finati
E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ,
Rezzani R, et al: Antitumour activity of melatonin in a mouse model
of human prostate cancer: Relationship with hypoxia signalling. J
Pineal Res. 57:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Xu L, Wei JE, Xie MR, Wang SE and
Zhou RX: Role of CD4+ CD25+ regulatory T
cells in melatonin-mediated inhibition of murine gastric cancer
cell growth in vivo and in vitro. Anat Rec (Hoboken). 294:781–788.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu L, Jin QD, Gong X, Liu H and Zhou RX:
Anti-gastric cancer effect of melatonin and Bcl-2, Bax, p21 and p53
expression changes. Sheng Li Xue Bao. 66:723–729. 2014.(In
Chinese). PubMed/NCBI
|
|
29
|
Fakharzadeh SS, Trusko SP and George DL:
Tumorigenic potential associated with enhanced expression of a gene
that is amplified in a mouse tumor cell line. EMBO J. 10:1565–1569.
1991.PubMed/NCBI
|
|
30
|
Zak K, Pecak A, Rys B, Wladyka B, Dömling
A, Weber L, Holak TA and Dubin G: Mdm2 and MdmX inhibitors for the
treatment of cancer: A patent review (2011-present). Expert Opin
Ther Pat. 23:425–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shaikh MF, Morano WF, Lee J, Gleeson E,
Babcock BD, Michl J, Sarafraz-Yazdi E, Pincus MR and Bowne WB:
Emerging role of MDM2 as target for anti-cancer therapy: A review.
Ann Clin Lab Sci. 46:627–634. 2016.PubMed/NCBI
|
|
32
|
He J, Zhu G, Gao L, Chen P, Long Y, Liao
S, Yi H, Yi W, Pei Z, Wu M, et al: Fra-1 is upregulated in gastric
cancer tissues and affects the PI3K/Akt and p53 signaling pathway
in gastric cancer. Int J Oncol. 47:1725–1734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eischen CM and Lozano G: p53 and MDM2:
Antagonists or partners in crime? Cancer Cell. 15:161–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakajima N, Ito Y, Yokoyama K, Uno A,
Kinukawa N, Nemoto N and Moriyama M: The expression of murine
double minute 2 (MDM2) on Helicobacter pylori-infected intestinal
metaplasia and gastric cancer. J Clin Biochem Nutr. 44:196–202.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Günther T, Schneider-Stock R, Häckel C,
Kasper HU, Pross M, Hackelsberger A, Lippert H and Roessner A: Mdm2
gene amplification in gastric cancer correlation with expression of
Mdm2 protein and p53 alterations. Mod Pathol. 13:621–626. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun LP, Jiang NJ, Fu W, Xue YX and Zhao
YS: Relationship between gastric cancer and gene amplification of
p14 and mdm2. Ai Zheng. 23:36–39. 2004.(In Chinese). PubMed/NCBI
|
|
37
|
Manfredi JJ: The Mdm2-p53 relationship
evolves: Mdm2 swings both ways as an oncogene and a tumor
suppressor. Genes Dev. 24:1580–1589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carter S, Bischof O, Dejean A and Vousden
KH: C-terminal modifications regulate MDM2 dissociation and nuclear
export of p53. Nat Cell Biol. 9:428–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lohrum MA, Woods DB, Ludwig RL, Bálint E
and Vousden KH: C-terminal ubiquitination of p53 contributes to
nuclear export. Mol Cell Biol. 21:8521–8532. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zauberman A, Barak Y, Ragimov N, Levy N
and Oren M: Sequence-specific DNA binding by p53: Identification of
target sites and lack of binding to p53 - MDM2 complexes. EMBO J.
12:2799–2808. 1993.PubMed/NCBI
|
|
41
|
Poyurovsky MV, Katz C, Laptenko O,
Beckerman R, Lokshin M, Ahn J, Byeon IJ, Gabizon R, Mattia M,
Zupnick A, et al: The C terminus of p53 binds the N-terminal domain
of MDM2. Nat Struct Mol Biol. 17:982–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cross B, Chen L, Cheng Q, Li B, Yuan ZM
and Chen J: Inhibition of p53 DNA binding function by the MDM2
protein acidic domain. J Biol Chem. 286:16018–16029. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Biderman L, Poyurovsky MV, Assia Y, Manley
JL and Prives C: MdmX is required for p53 interaction with and full
induction of the Mdm2 promoter after cellular stress. Mol Cell
Biol. 32:1214–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bond GL, Hu W, Bond EE, Robins H, Lutzker
SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al: A
single nucleotide polymorphism in the MDM2 promoter attenuates the
p53 tumor suppressor pathway and accelerates tumor formation in
humans. Cell. 119:591–602. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bond GL, Hu W and Levine A: A single
nucleotide polymorphism in the MDM2 gene: From a molecular and
cellular explanation to clinical effect. Cancer Res. 65:5481–5484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ofir-Rosenfeld Y, Boggs K, Michael D,
Kastan MB and Oren M: Mdm2 regulates p53 mRNA translation through
inhibitory interactions with ribosomal protein L26. Mol Cell.
32:180–189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:pp. 11598–11603. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B and
Hung MC: HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2
phosphorylation. Nat Cell Biol. 3:973–982, 2001. Nat Cell Biol 3:
973–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gottlieb TM, Leal JF, Seger R, Taya Y and
Oren M: Cross-talk between Akt, p53 and Mdm2: Possible implications
for the regulation of apoptosis. Oncogene. 21:1299–1303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jones SN, Hancock AR, Vogel H, Donehower
LA and Bradley A: Overexpression of Mdm2 in mice reveals a
p53-independent role for Mdm2 in tumorigenesis. Proc Natl Acad Sci
USA. 95:pp. 15608–15612. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McDonnell TJ, Montes de Oca Luna R, Cho S,
Amelse LL, Chavez-Reyes A and Lozano G: Loss of one but not two
mdm2 null alleles alters the tumour spectrum in p53 null mice. J
Pathol. 188:322–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dobbelstein M, Wienzek S, König C and Roth
J: Inactivation of the p53-homologue p73 by the mdm2-oncoprotein.
Oncogene. 18:2101–2106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zeng X, Chen L, Jost CA, Maya R, Keller D,
Wang X, Kaelin WG Jr, Oren M, Chen J and Lu H: MDM2 suppresses p73
function without promoting p73 degradation. Mol Cell Biol.
19:3257–3266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gu J, Nie L, Kawai H and Yuan ZM:
Subcellular distribution of p53 and p73 are differentially
regulated by MDM2. Cancer Res. 61:6703–6707. 2001.PubMed/NCBI
|
|
55
|
Watson IR, Blanch A, Lin DC, Ohh M and
Irwin MS: Mdm2-mediated NEDD8 modification of TAp73 regulates its
transactivation function. J Biol Chem. 281:34096–34103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cheney MD, McKenzie PP, Volk EL, Fan L and
Harris LC: MDM2 displays differential activities dependent upon the
activation status of NFkappaB. Cancer Biol Ther. 7:38–44. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun P, Dong P, Dai K, Hannon GJ and Beach
D: p53-independent role of MDM2 in TGF-beta1 resistance. Science.
282:2270–2272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yam CH, Siu WY, Arooz T, Chiu CH, Lau A,
Wang XQ and Poon RY: MDM2 and MDMX inhibit the transcriptional
activity of ectopically expressed SMAD proteins. Cancer Res.
59:5075–5078. 1999.PubMed/NCBI
|
|
59
|
Xiao ZX, Chen J, Levine AJ, Modjtahedi N,
Xing J, Sellers WR and Livingston DM: Interaction between the
retinoblastoma protein and the oncoprotein MDM2. Nature.
375:694–698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sdek P, Ying H, Zheng H, Margulis A, Tang
X, Tian K and Xiao ZX: The central acidic domain of MDM2 is
critical in inhibition of retinoblastoma-mediated suppression of
E2F and cell growth. J Biol Chem. 279:53317–53322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou S, Gu L, He J, Zhang H and Zhou M:
MDM2 regulates vascular endothelial growth factor mRNA
stabilization in hypoxia. Mol Cell Biol. 31:4928–4937. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Thut CJ, Goodrich JA and Tjian R:
Repression of p53-mediated transcription by MDM2: A dual mechanism.
Genes Dev. 11:1974–1986. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Minsky N and Oren M: The RING domain of
Mdm2 mediates histone ubiquitylation and transcriptional
repression. Mol Cell. 16:631–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ji W, Ma J, Zhang H, Zhong H, Li L, Ding
N, Jiao J and Gao Z: Role of p53β in the inhibition of
proliferation of gastric cancer cells expressing wild-type or
mutated p53. Mol Med Rep. 12:691–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hoffmann I, Draetta G and Karsenti E:
Activation of the phosphatase activity of human cdc25A by a
cdk2-cyclin E dependent phosphorylation at the G1/S transition.
EMBO J. 13:4302–4310. 1994.PubMed/NCBI
|
|
66
|
Zou X, Tsutsui T, Ray D, Blomquist JF,
Ichijo H, Ucker DS and Kiyokawa H: The cell cycle-regulatory CDC25A
phosphatase inhibits apoptosis signal-regulating kinase 1. Mol Cell
Biol. 21:4818–4828. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Goloudina A, Yamaguchi H, Chervyakova DB,
Appella E, Fornace AJ Jr and Bulavin DV: Regulation of human Cdc25A
stability by Serine 75 phosphorylation is not sufficient to
activate a S phase checkpoint. Cell Cycle. 2:473–478. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Boutros R, Lobjois V and Ducommun B: CDC25
phosphatases in cancer cells: Key players? Good targets? Nat Rev
Cancer. 7:495–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cheng M, Olivier P, Diehl JA, Fero M,
Roussel MF, Roberts JM and Sherr CJ: The p21(Cip1) and p27(Kip1)
CDK ‘inhibitors’ are essential activators of cyclin D-dependent
kinases in murine fibroblasts. EMBO J. 18:1571–1583. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Martín V, Herrera F, García-Santos G,
Antolín I, Rodriguez-Blanco J, Medina M and Rodriguez C:
Involvement of protein kinase C in melatonin's oncostatic effect in
C6 glioma cells. J Pineal Res. 43:
|
|
71
|
Rother K, Kirschner R, Sänger K, Böhlig L,
Mössner J and Engeland K: p53 downregulates expression of the
G1/S cell cycle phosphatase Cdc25A. Oncogene.
26:1949–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|