Introduction
Lung cancer is the most common cause of
cancer-related death, accounting for ~1.6 million premature deaths.
The majority (80%) of lung cancer cases are non-small cell lung
cancer (NSCLC), which is associated with a poor 5-year patient
survival (1,2). NSCLC therapy has traditionally relied
on chemotherapeutics but poor response results in rare remissions
and the side-effects are significant (3,4). Thus,
novel therapeutic strategies to improve treatment responses are a
focus of anticancer research.
Cellular senescence is an important defense
mechanism that prevents cancer development. Multiple stimuli can
trigger senescence, including telomere attrition, epigenetic
alterations, activation of oncogenes, DNA damage and oxidative
stress, which cause functional and morphological changes in cells
(5,6). Senescent cells have distinctive
phenotypes and biomarkers, such as an enlarged flat morphology,
enhanced senescence-associated β-galactosidase (SA-β-gal) activity,
and a senescence-associated secretory phenotype (SASP).
Additionally, increased p53 and p21 proteins and activation of DNA
damage response (DDR) are universal features of senescent cells
(7).
The ligand of CD40 (CD40L) is a type II
membrane-associated glycoprotein and a member of the TNF gene
family (8). CD40L helps regulate
the immune response and suppresses tumor growth. CD40L transgene
expression in CD40-positive malignant cells, such as breast or
bladder cancer cells, was found to produce a direct
growth-inhibitory effect via apoptosis (9,10).
However, membrane-stable CD40L can be cleaved by matrix
metalloproteases (MMPs), releasing soluble fragment sCD40L which
promotes various systemic inflammatory responses (11). To diminish the adverse effects of
CD40L immunotherapy, we used a membrane-stable mutant form CD40L
(CD40L-M) resistant to cleavage. Widespread expression of CD40 by
carcinomas indicates that more research is required to explore the
therapeutic benefits of CD40L-M.
In the present study, we evaluated the effect of
CD40L-M expression on senescence in CD40-positive NSCLC cells as
well as the molecular mechanisms underlying CD40L-M-induced cancer
cell senescence regulation. Our data offer a better understanding
of the multiple antitumor effects of CD40L-M in NSCLC.
Materials and methods
Cell culture and reagents
Human CD40-positive lung adenocarcinoma cell lines
A549 and H460 and human bronchial 16HBE epithelial cell line were
purchased from the Cell Resource Center (Shanghai Institutes for
Biological Sciences, Shanghai, China). The cisplatin-resistant A549
(A549/DDP) and paclitaxel-resistant A549 (A549/TR) cell lines were
kindly provided by Professor Zhou at the Shanghai Pulmonary
Hospital. All cell lines were maintained at 37°C in a 5%
CO2 atmosphere in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal bovine serum (FBS; ScienCell Research
Laboratories, San Diego, CA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin. To maintain drug resistance, A549/DDP and
A549/TR cells were grown in DMEM containing 6.67 µM cisplatin or
0.23 µM paclitaxel (Taxol®) (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) respectively, and then in drug-free DMEM two
days before the experiments. We previously constructed a plasmid
expressing CD40L-M, which contained 6 substitutions
(Gln114 to Pro114, Lys115 to
Arg115, Asp117 to Glu117,
Gln118 to Glu118, Asn119 to
Asp119 and Pro120 to Ser120) by
Invitrogen Biotechnology (Shanghai, China). A549/TR, A549/DDP, H460
and A549 cells were transfected with an entry vector pcDNA3.1+,
pcDNA3.1+-CD40L-WT or pcDNA3.1+-CD40L-M according to a previously
described method (12). The ATM
inhibitor KU-55933 was obtained from Selleck Chemicals (Houston,
TX, USA).
siRNA transfection
To knockdown GATA4, cells were transfected with
GATA4 siRNAs using Invitrogen Lipofectamine 2000 transfection
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. The siRNAs targeting
sequences are as follows: CGAUAUGUUUGACGACUUC.
β-galactosidase senescence assay
SA-β-gal activity was measured with a
β-galactosidase staining kit (Beyotime Institute of Biotechnology,
Shanghai, China) according to the manufacturer's protocol. Briefly,
the cells were cultured after transfection for 48 h. Then, the
cells were washed twice with PBS and fixed with a 3.5%
paraformaldehyde solution for 15 min at room temperature. Cells
were washed every 5 min 3 times in PBS, and the SA-β-gal staining
solution was added and incubated in a 37°C water bath for 16 h.
Images of the representative fields observed under a light
microscope (Olympus IX-71; Olympus, Tokyo, Japan) were captured
under 20× magnification.
Cell proliferation assay
Cell viability was measured using a Cell Counting
Kit-8 (Bimake, Houston, TX, USA). Briefly, 5×103 cells
were plated into each well of 96-well flat-bottomed plates. After
24 h, the cells were transfected with an empty vector pcDNA3.1+,
pcDNA3.1+-CD40L-WT or pcDNA3.1+-CD40L-M. Then cells were cultured
for an additional 48 h. A colorimetric assay was performed after
addition of 10 µl Cell Counting Kit-8 reagent to each well, and
plates were incubated at 37°C for 2–4 h. Absorbance at 450 nm was
read using a multiplate reader (Tecan Group Ltd., Männedorf,
Switzerland).
Cell cycle analysis
For cell cycle analysis, 2×106 cells were
harvested, fixed with 3 ml of cold 75% ethanol at −20°C overnight,
and washed twice with PBS. The cells were then resuspended in 500
µl of PBS and simultaneously stained with 200 µl of DNA staining
solution (MultiSciences, Hangzhou, China) at 25°C for 30 min. The
percentage of cells in each cell cycle phase was determined using a
FACStation (FV500; Beckman Coulter, La Brea, CA, USA) and analyzed
using Kaluza Flow Analysis software (Beckman Coulter, Inc.).
Immunofluorescence
Transfected cells were seeded on a micro-cover glass
for 48 h. Cells were fixed with 4% paraformaldehyde for 15 min and
permeabilized with 0.3% Triton X-100 in PBS for 1 h at room
temperature. After treatment, the slides were incubated with
anti-GATA4 rabbit polyclonal antibody (1:100; cat. no. ab84593;
Abcam, Cambridge, MA, USA) at 4°C. Cells were washed and then
incubated with goat anti-rabbit IgG (H+L) highly cross-adsorbed
secondary antibody Alexa Fluor® 488 conjugate (1:500;
cat. no. A-11034; Thermo Fisher Scientific) for 1 h at room
temperature. All slides were counterstained with
4′-6-diamidino-2-phenylindole (DAPI). Photomicrographs were
captured using a fluorescence microscope (Olympus IX-71;
Olympus).
Western blot analysis
Forty-eight hours after transfection, the cells were
lysed using ice-cold RIPA buffer containing a mixture of
phosphatase and protease inhibitors. Lysates were then centrifuged
at 4°C, and the supernatant was collected. Protein was measured in
supernatants using the BCA method. Equal amounts of proteins (30
µg) were separated on 10–12% SDS-polyacrylamide gels and then
blocked with 5% non-fat dry milk diluted in Tris-buffered saline
0.1 M added to 0.1% Tween-20 (TBST). Membranes were incubated
overnight at 4°C with one of the specific antibodies. Phospho-NF-κB
p65 (Ser536) (93H1) rabbit monoclonal antibody (mAb; 1:1,000; cat.
no. 3033), NF-κB p65 (D14E12) rabbit mAb (1:1,000; cat. no. 8245),
IκBα (44D4) rabbit mAb (1:1,000; cat. no. 4812), p53 (DO-7) mouse
mAb (1:1,000; cat. no. 48818), p21 Waf1/Cip1 (12D1) rabbit mAb
(1:1,000; cat. no. 2947), phospho-Chk2 (Thr68) (C13C1) rabbit mAb
(1:1,000; cat. no. 2197), Chk2 (D9C6) rabbit mAb (1:1,000; cat. no.
6334), phospho-Chk1 (Ser317) (D12H3) rabbit mAb (1:1,000; cat. no.
12302), Chk1 (2G1D5) mouse mAb (1:1,000; cat. no. 2360), GAPDH
(14C10) rabbit mAb (1:1,000; cat. no. 2118), β-Tubulin (9F3) rabbit
mAb (1:1,000; cat. no. 2128,) and a HRP-conjugated secondary
antibody (1:2,000; cat. no. 7075) were obtained from Cell Signaling
Technology (Beverly, MA, USA). CD40L (1:1,000; cat. no. ab65854)
and GATA4 (1:1,000; cat. no. ab84593) were obtained from Abcam
(Cambridge, MA, USA). After washing three times in TBST, a
horseradish peroxidase-conjugated goat anti-rabbit antibody was
applied. Proteins were visualized using Pierce ECL reagent (Thermo
Fisher Scientific).
Methylation-specific PCR
DNA from A549, A549/TR and 16HBE cells was treated
with sodium bisulfite and purified using EZ DNA methylation kit
(Zymo Research, Irvine, CA, USA). Methylation-specific PCR (MSP)
was used to determine bisulfite-induced changes affecting
unmethylated (U) and methylated (M) alleles. Each MSP reaction
incorporated 100 ng of bisulfite-treated DNA, 25 pM of each primer,
100 pM dNTPs, 10X PCR buffer, and 1 U/ml JumpStart Red Taq
Polymerase (Sigma-Aldrich; Merck KGaA) in a final reaction volume
of 25 µl. Cycle conditions were as previously described (13). MSP products were separated on a 2%
agarose gel and stained with ethidium bromide. MSP primer sequences
for GATA4 were as follows: GATA-4-M-sense,
5-GTATAGTTTCGTAGTTTGCGTTTAGC-3 and GATA-4-M-antisense,
5-AACTCGCGACTCGAATCCCCG-3; GATA-4-U-sense,
5-TTTGTATAGTTTTGTAGTTTGTGTTTAGT-3 and GATA-4-U-antisense,
5-CCCAACTCACAACTCAAATCCCCA-3.
Statistical analyses
Results are expressed as means ± SD. All statistical
analyses were performed using SPSS for Windows v.16.0 (SPSS, Inc.,
Chicago, IL, USA). Continuous data were analyzed using an
independent Student's t-test between two groups. P<0.05 was
considered to indicate a statistically significant result.
Results
Expression of CD40L-M induces cellular
senescence
To investigate the multiple effects of CD40L-M, we
used pcDNA3.1+, pcDNA3.1+-CD40L-WT and pcDNA3.1+-CD40L-M to
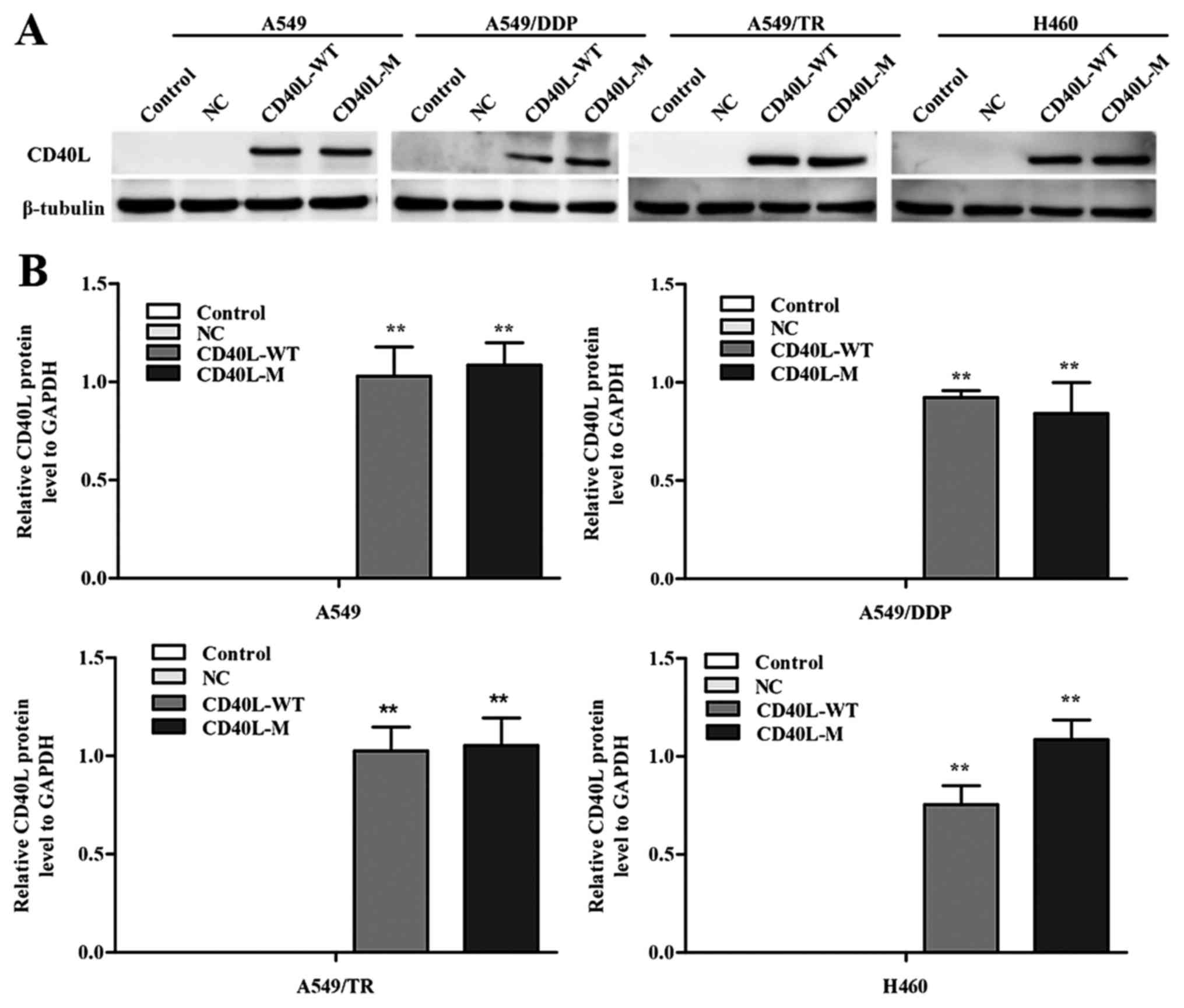
transfect CD40-positive NSCLC cell lines. CD40L expression was
increased in the A549, A549/TR, A549/DDP and H460 cells 48 h after
transfection (Fig. 1A and B). In
addition, the culture cell size was enlarged in the A549/TR,
A549/DDP and H460 cells, a trait associated with senescence. These
data were confirmed with SA-β-gal staining (Fig. 1C and D), yet SA-β-gal staining was
not observed in the A549 cells. Thus, CD40L-M expression is
implicated in cellular senescence in various CD40-positive NSCLC
cells.
CD40L-M contributes to the inhibition
of cell proliferation and cell cycle arrest
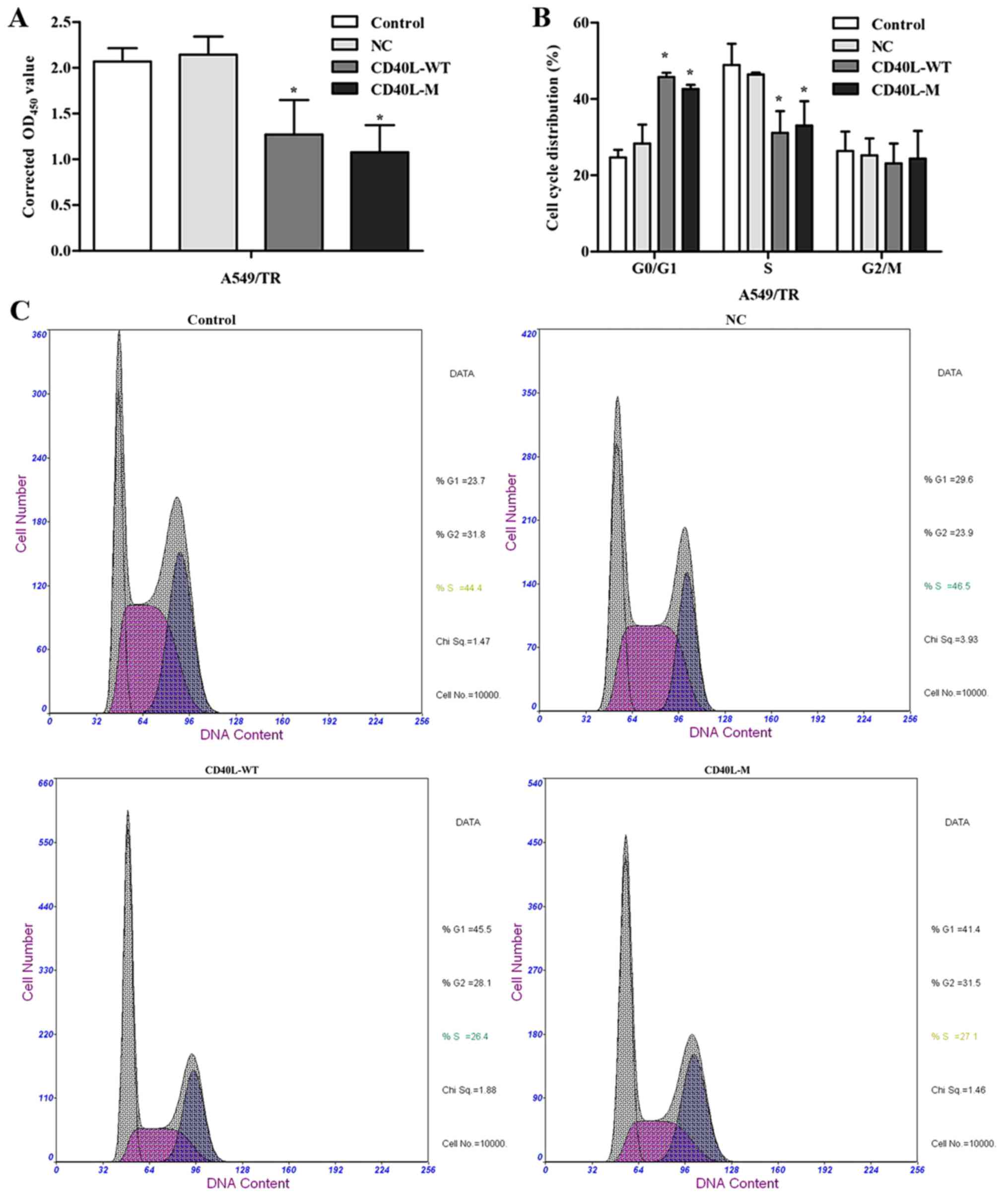
There may be several features and cell cycle
regulators in senescence networks. After 48 h of culture, total
CD40L-M/A549/TR cells were not significantly increased compared
with the controls or negative groups. CCK-8 assay data showed that
CD40L-M expression significantly reduced cell proliferation
(Fig. 2A).
To explore growth inhibition after CD40L-M
upregulation, we evaluated the effect of CD40L-M on cell cycle
progression. Flow cytometry data showed that the percentage of S
phase cells was decreased and the percentage of G0/G1 phase cells
was increased in the CD40L-M-expressed cell group compared with
these percentages noted in the controls or negative cells (Fig. 2B and C), indicating that CD40L-M
expression blocked cell cycle progression. Expression of cell cycle
regulators p53 and p21 were determined using western blot analysis
and data showed that p53 and p21 expression was significantly
increased in the CD40L-M-upregulated cells (Fig. 2D).
ATM/Chk2 is necessary for
CD40L-M-induced senescence
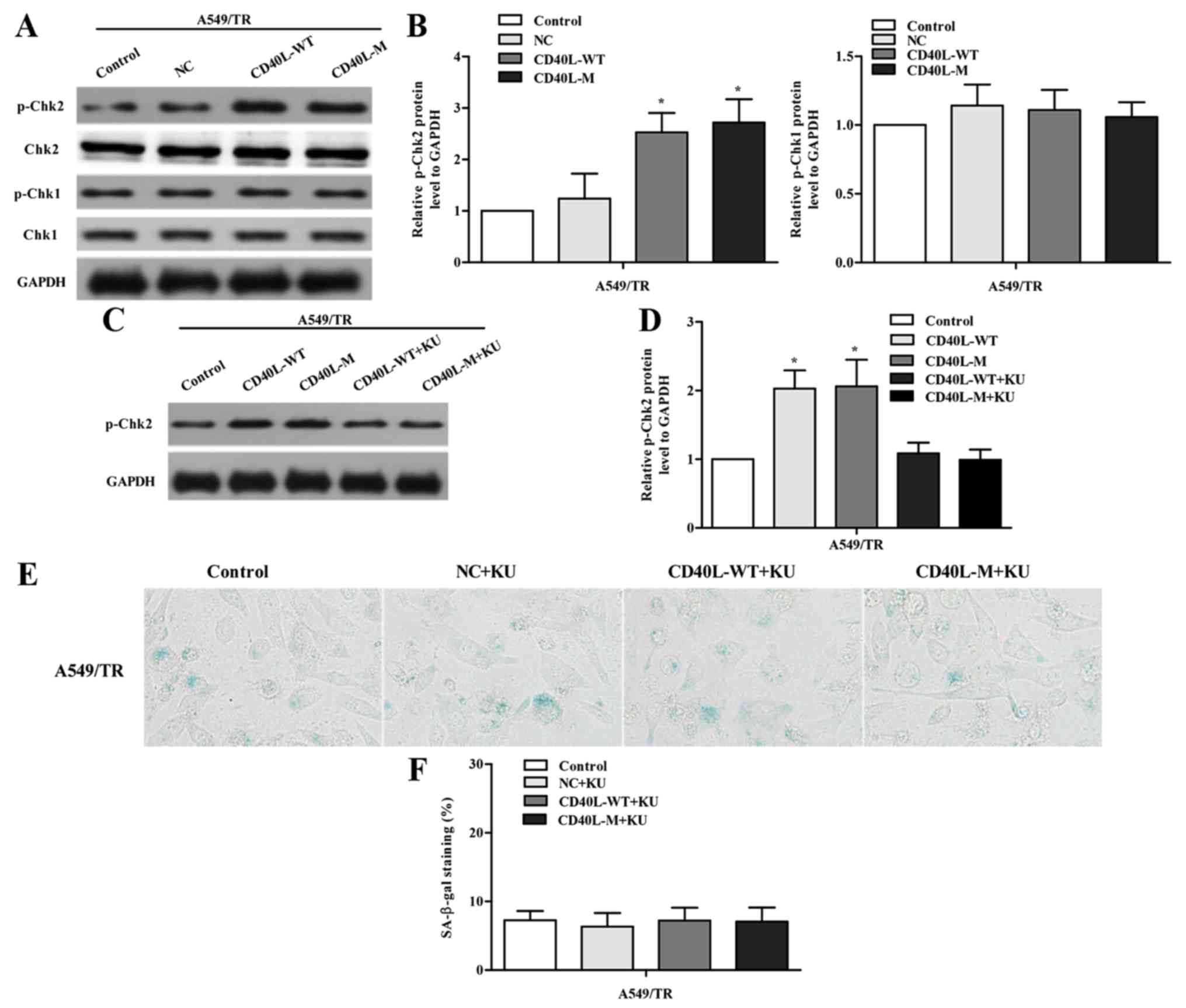
DNA damage response (DDR) is a trait of
multi-faceted senescent phenotypes. To identify whether DDR is
activated, we assessed p-Chk2 and p-Chk1 in CD40L-induced senescent
A549/TR cells. Data showed that only p-Chk2 was significantly
induced 48 h after transfection (Fig.
3A and B), indicating that the ATM/Chk2 pathway was activated
in response to CD40L-M-induced senescence. To address the
functional role of the ATM/Chk2 pathway, we treated
CD40L-M-transfected A549/TR cells with an inhibitor of ATM kinase,
KU-55933. KU-55933 inhibited phosphorylation of the ATM target
protein Chk2 (Fig. 3C and D) and
decreased SA-β-gal activity (Fig. 3E
and F; P>0.05, compared to controls), suggesting that
ATM/Chk2 mediated CD40L-M-induced senescence.
GATA4 is restored in CD40L-M-induced
senescent cells
GATA4 is a zinc-finger transcription factor,
critical for the development of organogenesis, proliferation,
differentiation and apoptosis. Numerous studies have shown that
silencing of the GATA4 gene by promoter methylation has been
implicated in carcinogenesis of the lung (13,14).
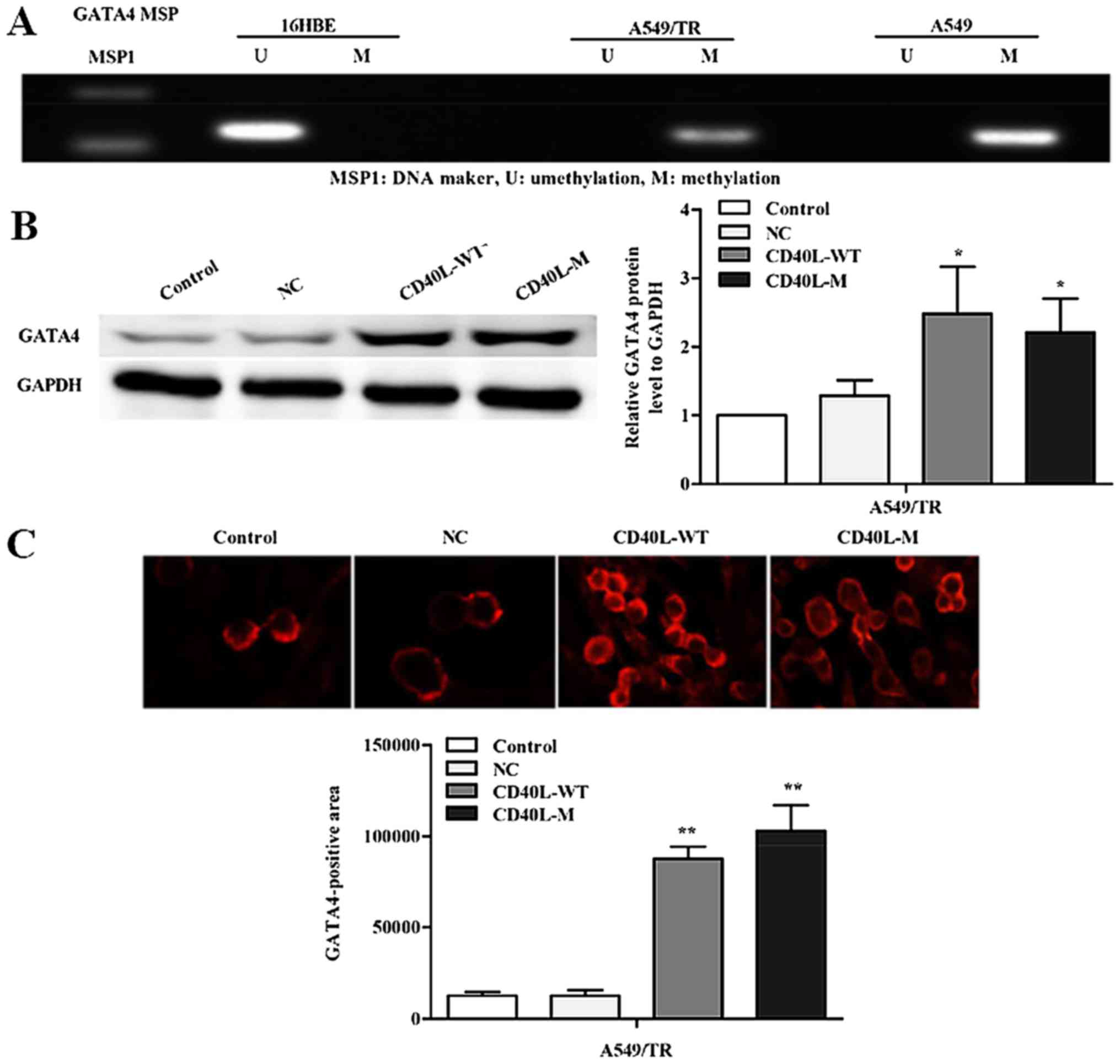
To investigate whether silencing of GATA4 expression is related to
promoter region methylation, we used methylation-specific PCR to
determine the methylation status of A549, A549/TR and 16HBE cells
(Fig. 4A). Data showed that GATA4
methylation occurred in the A549 and A549/TR cells. In contrast, we
observed no aberrant promoter hypermethylation for the GATA4 gene
in 16HBE cells.
Cellular senescence causes widespread changes in
chromatin organization (15). Prior
studies have shown that DNA methylation is reduced in response to
DNA damage or cell senescence (16). Thus, we hypothesized that
CD40L-M-induced senescence might be accompanied by GATA4
demethylation. Thus, we assessed the expression of GATA4 in
CD40L-M-induced senescent A549/TR cells by western blot analysis.
GATA4 protein was significantly increased in the CD40L-M-expressed
A549/TR cells compared with that noted in the controls or negative
groups (Fig. 4B). These data were
confirmed by immunofluorescence (Fig.
4C).
GATA4 regulates senescence dependent
on DDR
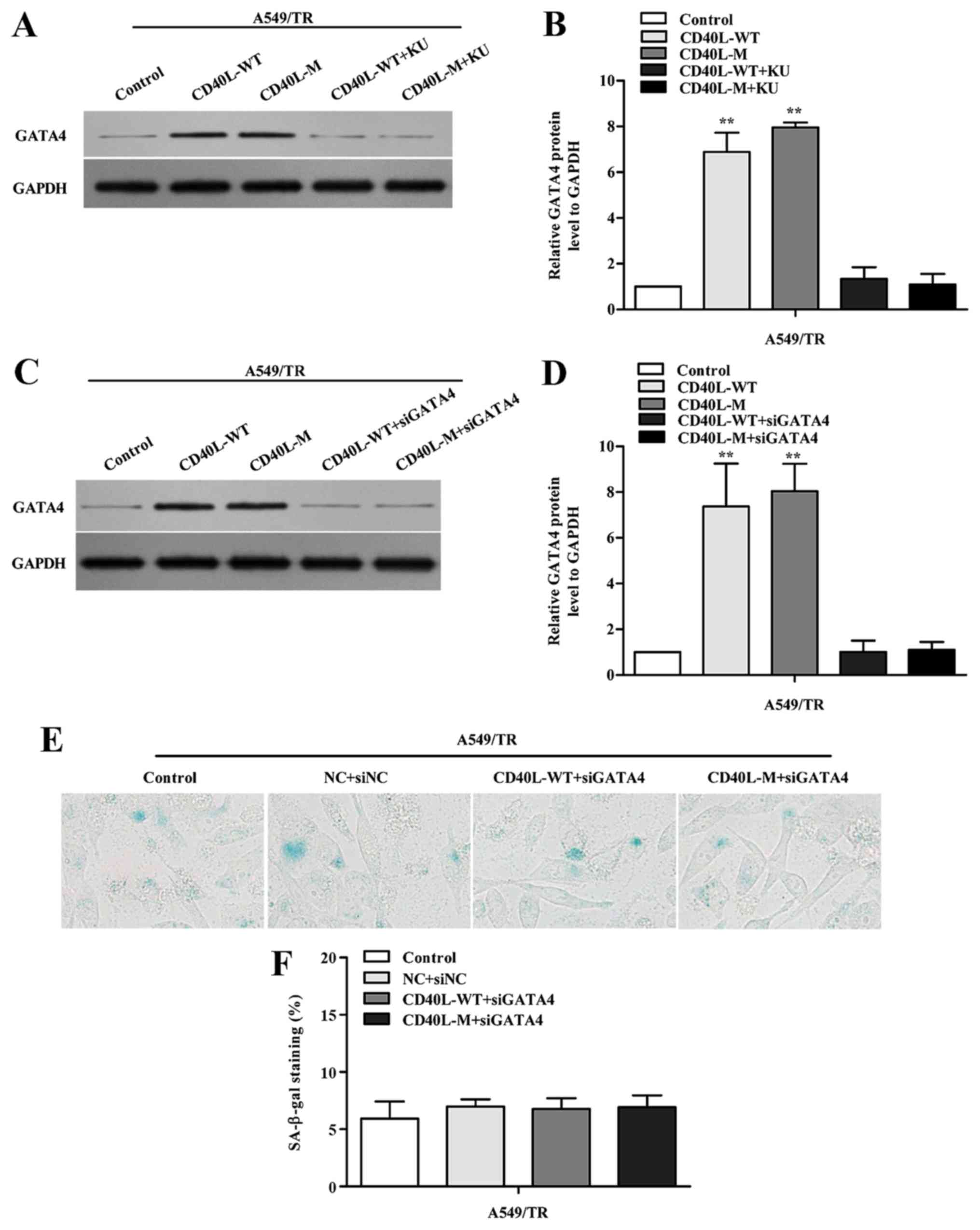
To determine whether GATA4 demethylation responds to
DDR activation, KU-55933 was used to block ATM/Chk2 DDR. When the
ATM/Chk2 pathway was suppressed, GATA4 expression was reduced in
the CD40L-M-induced senescent cells (Fig. 5A and B).
To investigate whether GATA4 is associated with
cellular senescence in CD40L-M-expressed A549/TR cells, we knocked
down GATA4 using GATA4 siRNAs. Data showed that GATA4 protein was
significantly downregulated (Fig. 5C
and D) and suppression of GATA4 in CD40L-M-expressed A549/TR
cells decreased SA-β-gal activity (Fig.
5E and F; P>0.05, compared to controls). Thus, GATA4 was
responsible for CD40L-M-induced senescence which was dependent on
DDR activation.
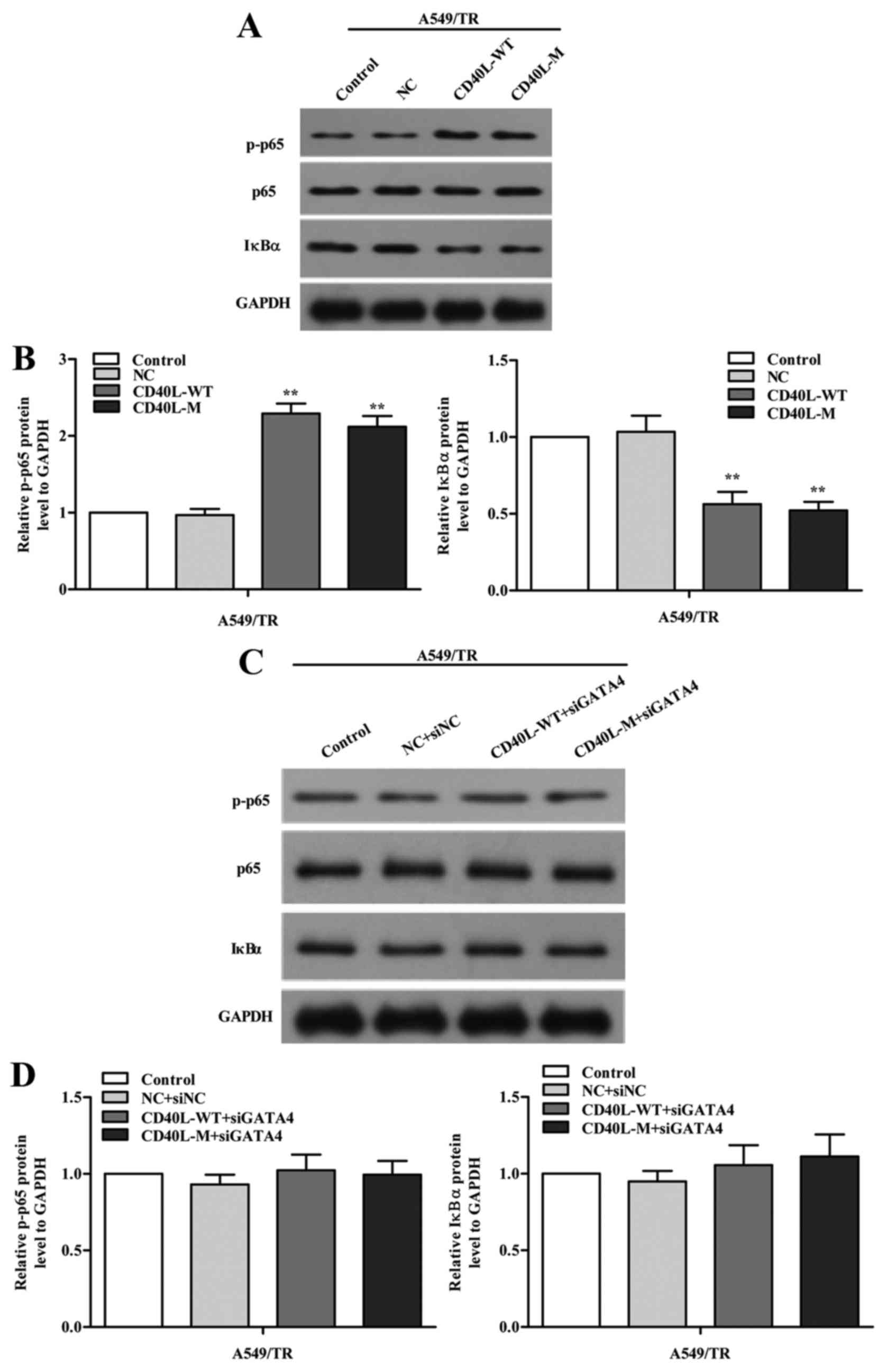
GATA4 regulates the NF-κB signaling
pathway
To investigate how GATA4 regulates cell senescence,
we explored the signaling pathways implicated in senescence. We
measured phosphorylated NF-κB p65 (p-p65) and its negative
regulator IκBα using western blot analysis in the CD40L-M-induced
senescent A549/TR cells and we found that expression of p-p65 was
increased and IκBα was reduced in the CD40L-M-expressing A549/TR
cells (Fig. 6A and B). To confirm
our results, we knocked down GATA4 with GATA4 siRNAs and found that
p-p65 was partially decreased (Fig. 6C
and D). Thus, the NF-κB signaling pathway was regulated by
GATA4 in our cell systems.
Discussion
We reported that CD40L-M overexpression is
associated with induction of senescence in CD40-positive NSCLC
A549/TR, H460 and A549/DDP cell lines, whereas A549 cells displayed
no senescent phenotype after CD40L-M upregulation. In accordance
with our previous studies, treatment with scAAV5 CD40L-M resulted
in the significant reduction in cell number in the CD40 positive
A549 cells by inducing apoptosis (17). In contrast to H460 and A549/DDP
cells, more CD40L-M-expressed A549/TR cells exhibited an enlarged,
flattened morphology accompanied by an increase in SA-β-gal
staining activity. This might be attributed to the possibilities
that A549/TR cells are inclined to senesce due to the DNA damage by
Taxol previously, or A549/TR cells are more vulnerable to
CD40L-induced senescence. Additionally, CD40L-M upregulation
decreased cell proliferation and induced cell cycle arrest. p53 and
its target gene p21 are essential regulators for cell cycle arrest
after induction of senescence in response to DNA damage signals
(18). We also showed that p53 and
p21 protein increased in the CD40L-M-induced senescent A549/TR
cells. Thus, CD40L-M is involved in induction of cellular
senescence in CD40-positive NSCLC cells with cell
heterogeneity.
CD40L a key costimulatory molecule for
antigen-presenting cells (APCs), which is preferentially expressed
on activated CD4+ T cells and activated platelets
(19,20). Signaling from CD40L binding to its
receptor CD40 induces antigen-presenting cells to express various
immune accessory molecules and activates transcription factors,
such as AP-1 and NF-κB, which are crucial for the development of
humoral and cellular immunity (21,22).
In addition, various forms of CD40L have been used to directly
promote pro-apoptotic induction in CD40-positive malignant cells
(23,24). However, membrane-stable CD40L can be
cleaved by various MMPs into a soluble form that induces survival
signals in CD40-positive carcinomas causing inflammatory diseases
(11,25). To optimize CD40L gene therapy, we
generated a membrane-stable mutant form CD40L resistant to MMPs.
Our previous research showed that CD40L-M conferred a direct
antitumor effect in vitro and in vivo with few
side-effects (12,17).
DNA damage response (DDR) is a senescent biomarker
(26) and senescence-inducing
stimuli can cause genomic damage, subsequently activating DDR
(27). Our results showed that the
ATM/Chk2 pathway was activated in CD40L-M-induced senescent NSCLC
cells. Previous reports have shown that ATM or ATR activation is
sufficient to induce cellular senescence (28,29).
Chk2 can promote cellular senescence through either p53/p21 or
other pathways (30). Therefore, we
investigated whether Chk2 upregulation influences the regulation of
cellular senescence in this context. Our data agree with these
previous studies. We showed that p-Chk2 suppression impaired cell
senescence when we used an ATM inhibitor to block the ATM/Chk2
pathway. Thus, CD40L-M-induced senescence may be mediated by
ATM/Chk2.
GATA4 is a transcription protein family member and
common to other GATA factors, GATA4 contains two highly conserved
zinc fingers that mediate DNA binding, and many protein
interactions. GATA4 is frequently silenced by promoter methylation
in lung, colorectal, prostate, ovarian, and breast cancers
(13,14). In contrast to tumor and surrounding
normal tissue, the GATA4 promoter is either non-methylated or
hypomethylated in healthy lung tissue (31). Consistent with these studies, our
results showed that hypermethylation of GATA4 was determined in
NSCLC A549/TR and A549 cell lines but not in 16HBE cells.
Epigenomic perturbations are an inducer of cell senescence in
response to various stimuli (32).
Previous research has shown that epigenomic perturbations can
activate DDR signaling (27). In
contrast, our results showed that DDR contributed to GATA4
demethylation in senescent A549/TR cells expressing CD40L-M. A
recent study showed that GATA4 is a key regulator of senescent
phenotypes (33) and our data
showed that GATA4 knockdown decreased SA-β-gal activity. Therefore,
GATA4 expression was induced and positively regulated senescence in
CD40L-M-upregulated A549/TR cells.
NF-κB can be activated by diverse external and
internal stimuli associated with senescence, such as DNA damage and
genotoxic stresses (34). Because
the NF-κB signaling pathway can promote cellular senescence
(35), we investigated the
relationship between GATA4 and the NF-κB pathway during
CD40L-M-induced senescence in NSCLC cells. Data showed that the
NF-κB pathway was activated in the CD40L-M-overexpressed A549/TR
cells. In addition, knockdown of GATA4 resulted in markedly reduced
NF-κB activity. In fact, it has been clearly established that NF-κB
positively regulates the senescence-associated secretory phenotype
(SASP) that is a prominent property of senescent cells. Some SASP
factors can reinforce senescent growth arrest in an autocrine
manner (36). Others can stimulate
the immune system to clear senescent cells, suppress tumorigenesis,
and promote optimal repair of damaged tissues (15,37).
In summary, CD40L-M induces senescence, activates
DDR, and inhibits cell proliferation in CD40-positive NSCLC cells.
We demonstrated that GATA4 expression is restored by demethylation
and triggers NF-κB pathway activation to promote senescence in
CD40L-M-overexpressing A549/TR cells. This is positively correlated
with DDR. Thus, we predict that CD40L-M transgenes may offer an
approach to therapeutic intervention via senescence for lung
cancer.
Acknowledgements
The present study was supported by Jiangsu
Provincial Key Discipline of Medicine (ZDXKA2016003).
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
CD40L-M
|
CD40 ligand mutant
|
|
SA-β-gal
|
senescence-associated
β-galactosidase
|
|
ATM
|
ataxia telangiectasia mutated
|
|
ATR
|
ATM-related kinase
|
References
|
1
|
Cao X, Lai S, Hu F, Li G, Wang G, Luo X,
Fu X and Hu J: miR-19a contributes to gefitinib resistance and
epithelial mesenchymal transition in non-small cell lung cancer
cells by targeting c-Met. Sci Rep. 7:29392017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang W, Chen P, Tang M, Li J, Pei Y, Cai
S, Zhou X and Chen S: Tumstatin 185–191 increases the sensitivity
of non-small cell lung carcinoma cells to cisplatin by blocking
proliferation, promoting apoptosis and inhibiting Akt activation.
Am J Transl Res. 7:1332–1344. 2015.PubMed/NCBI
|
|
4
|
Ge H, Ni S, Wang X, Xu N, Liu Y, Wang X,
Wang L, Song D, Song Y and Bai C: Dexamethasone reduces sensitivity
to cisplatin by blunting p53-dependent cellular senescence in
non-small cell lung cancer. PLoS One. 7:e518212012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: The hallmarks of aging. Cell.
153:1194–1217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schönbeck U, Mach F and Libby P: CD154
(CD40 ligand). Int J Biochem Cell Biol. 32:687–693. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomes EM, Rodrigues MS, Phadke AP, Butcher
LD, Starling C, Chen S, Chang D, Hernandez-Alcoceba R, Newman JT,
Stone MJ and Tong AW: Antitumor activity of an oncolytic
adenoviral-CD40 ligand (CD154) transgene construct in human breast
cancer cells. Clin Cancer Res. 15:1317–1325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vardouli L, Lindqvist C, Vlahou K, Loskog
AS and Eliopoulos AG: Adenovirus delivery of human CD40 ligand gene
confers direct therapeutic effects on carcinomas. Cancer Gene Ther.
16:848–860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuda H, Mori M, Uchida T, Uzawa A,
Ohtani R and Kuwabara S: Soluble CD40 ligand contributes to
blood-brain barrier breakdown and central nervous system
inflammation in multiple sclerosis and neuromyelitis optica
spectrum disorder. J Neuroimmunol. 305:102–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu W, Li Y, Wang X, Wang C, Zhao W and Wu
J: Anti-tumor activity of gene transfer of the membrane-stable
CD40L mutant into lung cancer cells. Int J Oncol. 37:935–941.
2010.PubMed/NCBI
|
|
13
|
Agnihotri S, Wolf A, Munoz DM, Smith CJ,
Gajadhar A, Restrepo A, Clarke ID, Fuller GN, Kesari S, Dirks PB,
et al: A GATA4-regulated tumor suppressor network represses
formation of malignant human astrocytomas. J Exp Med. 208:689–702.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng R and Blobel GA: GATA transcription
factors and cancer. Genes Cancer. 1:1178–1188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams PD: Healing and hurting: Molecular
mechanisms, functions, and pathologies of cellular senescence. Mol
Cell. 36:2–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Sullivan RJ, Kubicek S, Schreiber SL and
Karlseder J: Reduced histone biosynthesis and chromatin changes
arising from a damage signal at telomeres. Nat Struct Mol Biol.
17:1218–1225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu W, Xu Y, Wei Y, Tan Y, Zhao H, Zhao W
and Wu J: Self-complementary adeno-associated virus 5-mediated gene
transduction of a novel CD40L mutant confers direct antitumor
effects in lung carcinoma. Mol Med Rep. 11:482–488. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heo JI, Kim W, Choi KJ, Bae S, Jeong JH
and Kim KS: XIAP-associating factor 1, a transcriptional target of
BRD7, contributes to endothelial cell senescence. Oncotarget.
7:5118–5130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fiumara P and Younes A: CD40 ligand
(CD154) and tumour necrosis factor-related apoptosis inducing
ligand (Apo-2L) in haematological malignancies. Br J Haematol.
113:265–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner AH, Güldenzoph B, Lienenlüke B and
Hecker M: CD154/CD40-mediated expression of CD154 in endothelial
cells: Consequences for endothelial cell-monocyte interaction.
Arterioscler Thromb Vasc Biol. 24:715–720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Srahna M, Remacle JE, Annamalai K, Pype S,
Huylebroeck D, Boogaerts MA and Vandenberghe P: NF-kappaB is
involved in the regulation of CD154 (CD40 ligand) expression in
primary human T cells. Clin Exp Immunol. 125:229–236. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Durie FH, Foy TM, Masters SR, Laman JD and
Noelle RJ: The role of CD40 in the regulation of humoral and
cell-mediated immunity. Immunol Today. 15:406–411. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loskog A, Maleka A, Mangsbo S, Svensson E,
Lundberg C, Nilsson A, Krause J, Agnarsdóttir M, Sundin A, Ahlström
H, et al: Immunostimulatory AdCD40L gene therapy combined with
low-dose cyclophosphamide in metastatic melanoma patients. Br J
Cancer. 114:872–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beatty GL, Chiorean EG, Fishman MP,
Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL,
et al: CD40 agonists alter tumor stroma and show efficacy against
pancreatic carcinoma in mice and humans. Science. 331:1612–1616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elmetwali T, Young LS and Palmer DH: CD40
ligand-induced carcinoma cell death: A balance between activation
of TNFR-associated factor (TRAF) 3-dependent death signals and
suppression of TRAF6-dependent survival signals. J Immunol.
184:1111–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campisi J: Cellular senescence: Putting
the paradoxes in perspective. Curr Opin Genet Dev. 21:107–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pazolli E, Alspach E, Milczarek A, Prior
J, Piwnica-Worms D and Stewart SA: Chromatin remodeling underlies
the senescence-associated secretory phenotype of tumor stromal
fibroblasts that supports cancer progression. Cancer Res.
72:2251–2261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Hawkins OE, Su Y, Vilgelm AE,
Sobolik T, Thu YM, Kantrow S, Splittgerber RC, Short S, Amiri KI,
et al: Targeting aurora kinases limits tumour growth through DNA
damage-mediated senescence and blockade of NF-κB impairs this
drug-induced senescence. EMBO Mol Med. 5:149–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toledo LI, Murga M, Gutierrez-Martinez P,
Soria R and Fernandez-Capetillo O: ATR signaling can drive cells
into senescence in the absence of DNA breaks. Genes Dev.
22:297–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gire V, Roux P, Wynford-Thomas D,
Brondello JM and Dulic V: DNA damage checkpoint kinase Chk2
triggers replicative senescence. EMBO J. 23:2554–2563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azhikina T, Kozlova A, Skvortsov T and
Sverdlov E: Heterogeneity and degree of TIMP4, GATA4, SOX18, and
EGFL7 gene promoter methylation in non-small cell lung cancer and
surrounding tissues. Cancer Genet. 204:492–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang C, Xu Q, Martin TD, Li MZ, Demaria M,
Aron L, Lu T, Yankner BA, Campisi J and Elledge SJ: The DNA damage
response induces inflammation and senescence by inhibiting
autophagy of GATA4. Science. 349:aaa56122015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freund A, Patil CK and Campisi J: p38MAPK
is a novel DNA damage response-independent regulator of the
senescence-associated secretory phenotype. EMBO J. 30:1536–1548.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rovillain E, Mansfield L, Caetano C,
Alvarez-Fernandez M, Caballero OL, Medema RH, Hummerich H and Jat
PS: Activation of nuclear factor-kappa B signalling promotes
cellular senescence. Oncogene. 30:2356–2366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Acosta JC, Banito A, Wuestefeld T,
Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka
F, Andrulis M, et al: A complex secretory program orchestrated by
the inflammasome controls paracrine senescence. Nat Cell Biol.
15:978–990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang TW, Yevsa T, Woller N, Hoenicke L,
Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova
A, et al: Senescence surveillance of pre-malignant hepatocytes
limits liver cancer development. Nature. 479:547–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|