Introduction
The incidence and mortality rate of esophageal
cancer (EC) are ranked as the eighth and sixth highest among all
cancers worldwide (1). China is one
of the countries with the highest incidence and mortality of EC in
the world. Newly diagnosed EC cases are estimated at 4,292,000
annually, ranking as the third among all cancer cases, and
accounting for 2,814,000 cancer-related deaths, which ranks fourth
among all cancer cases (2). It is
widely known that the Taihang Mountain region is an area with a
high incidence of EC cases located in the junction of Shanxi, Hebei
and Henan Province (3). Esophageal
squamous cell carcinoma (ESCC) is the main type of EC and ~70% of
worldwide ESCC cases occur in China. The 5-year survival rate of
ESCC remains between 10 and 25% due to the absence of clinical
approaches for early diagnosis and treatment (4,5).
Therefore, identification of the critical driving genes of ESCC is
urgent in order to improve the management of ESCC patients.
In our preliminary experiment, we performed
whole-genome sequencing (WGS) of 14 and whole-exome sequencing
(WES) of 90 ESCC tumor and adjacent normal tissues from patients
recruited from the Taihang Mountain region in China. We identified
eight significantly mutated genes (SMGs) including FAT atypical
cadherin 1 (FAT1). Among these SMGs, FAT1 was mutated in 15% of
ESCC tumors (6,7). Furthermore, our studies identified a
novel role for FAT1 in inhibiting tumor growth and
epithelial-mesenchymal transition (EMT) in ESCC by disrupting the
MAPK/ERK pathway (8).
Fat cadherins are extremely large cell adhesion
molecules, with >30 cadherin repeats, including FAT1, FAT2,
FAT3, FAT4. FAT1 is an important trans-membrane protein
involved in the regulation of cell adhesion and growth, migration,
actin dynamics and orientation, playing critical roles in tumor
development. It is often regarded as a tumor-suppressor gene or
oncogene in different types of human cancer (9–11).
Morris et al reported that recurrent somatic mutation of
FAT1 was found to lead to aberrant activation of the Wnt/β-catenin
signaling pathway in human glioblastoma multiforme (12). Moreover, depression of FAT1 was
found to accelerate cell migration in cholangiocarcinoma and breast
cancer (13). However, it was
reported that FAT1 acts as an oncogene in hepatic cancer (11). Noteworthy, our previous study showed
that FAT1 acts as a tumor-suppressor gene in ESCC (8).
Atomic force microscopy (AFM) has provided a new
screening test to observe the morphological and mechanical
properties of a single cell (14).
AFM is a type of scanning probe microscopy with high resolution,
that can be used to detect changes in cellular biophysical
properties, such as roughness, adhesion and elasticity (15,16).
With the development of AFM technology, AFM is used more and more
extensively in the tumor field. Kaul-Ghanekar et al observed
and analyzed breast cancer cell lines by AFM and found that SMAR1
acts as tumor suppressor by regulating expression of cell surface
proteins (17). Cross et al
reported the stiffness of live metastatic cancer cells taken from
the pleural fluids of patients with suspected lung, breast and
pancreas cancer. The results showed that mechanical analysis can
distinguish cancer cells from normal cells using AFM (18).
The aim of our present study was to confirm the
effect of FAT1 on the migration and invasion of ESCC cell lines
YSE2 and Colo680N. Moreover, the cell adhesive force and cell
elasticity force after FAT1 knockdown were detected by AFM. The
present study will contribute to the understanding of the
mechanisms that drive the development and progression of ESCC and
may provide a new therapeutic target for ESCC treatment.
Materials and methods
Cell culture
All ESCC cell lines used in the study were obtained
from the Translational Medicine Research Center, Shanxi Medical
University (Taiyuan, China) and cultured in HyClone™ RPMI-1640
medium (GE Healthcare Life Sciences, HyClone Laboratories, Logan,
UT, USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a 5% CO2
incubator. Culture medium was replaced every two to three days.
Subculture was carried out when the cells were fused to 80–90%
confluency and logarithmic phase cells were used in the following
experiments.
Ethics statement
All experimental protocols were approved by the
Ethics Committee of Shanxi Medical University. All samples were
obtained before treatment according to the guidelines of the local
ethics committees and written informed consent was received from
all participants.
TMAs and immunohistochemistry
(IHC)
Tissue microarrays (TMAs) consisting of 125 primary
ESCC tumor tissues and 125 matched non-tumor tissues were obtained
from Shanxi Cancer Hospital from 2011 to 2014. IHC was performed to
detect the protein expression of the corresponding genes. Briefly,
the TMA sections (4 µm) were deparaffinized and rehydrated with
xylene and a series of grades of alcohol and then soaked in 3%
H2O2 for 15 min. Antigen retrieval was
implemented in sodium citrate buffer (pH 6.0) for 2 min in a
pressure cooker, followed by incubation with the anti-FAT1 antibody
(1:300 dilution; rabbit polyclonal antibody; cat. no. HPA023882;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C overnight.
After washing with PBS, the TMA sections were incubated with the
secondary antibody (HRP-polymer anti-mouse/rabbit IHC kit, goat;
cat. no. KIT-5920; Maixin Biotechnology, Co., Ltd., Fuzhou, China)
at 37°C for 20 min. Slides were stained with DAB and counterstained
with hematoxylin. The levels and location of FAT1 were assessed
using IHC and analyzed with Aperio Cytoplasma 2.0 software (Leica
Microsystems GmbH, Wetzlar, Germany). The protein expression of
FAT1 was calculated by a semi-quantitative assessment of both the
staining intensity and the percentage of positive cells.
RNA extraction and reverse
transcription PCR (RT-PCR) and quantitative real-time PCR
(qPCR)
Total RNA was isolated from cells by TRIzol reagent
(Takara Biotechnology Co., Ltd., Dalian, China). RNA (2 µg) was
reverse-transcribed in 20 µl using the PrimeScript® RT
Master Mix (Perfect Real-Time) for RT-PCR (Takara Biotechnology).
The reaction conditions of reverse-transcription PCR were set as
follows: 94°C for 5 min, 30 cycles at 94°C for 50 sec, 55°C for 30
sec, and 72°C for 50 sec; followed by extension at 72°C for 10 min.
Quantitative real-time PCR (qPCR) experiment was implemented with
SYBR® Premix Ex Taq™ (Takara Biotechnology).
The detailed protocol was as follows: 95°C for 10 min, 40 cycles of
95°C for 15 sec, and 60°C for 1 min. The relative expression of
target genes was determined by normalization to GAPDH expression
and was determined as ΔΔCt. The primers used to measure the
expression level of FAT1 were: 5′-TGCTGGAGGAAAAGTTGCTT-3′ (sense),
and 5′-GATTACGCCGGACAGTTTGT-3′ (antisense). The primers of GAPDH
were: 5′-AGTCAACGGATTTGGTCGTA-3′ (sense), and
5′-AGTCAACGGATTTGGTCGTA-3′ (antisense). The experiment was
completed in triplicate independently.
In-cell western assay
The FAT1 protein in the ESCC cell lines was
determined using in-cell western assay. Briefly, 4×104
cells were seeded in 96-well plates and incubated under normal
conditions to culture to a final volume of 200 µl/well overnight.
The cells were fixed with 4% formaldehyde and permeabilized using
0.2% Triton X-100. LI-COR Odyssey Blocking Buffer (150 µl) (LI-COR
Biosciences, Lincoln, NE, USA) was added to each well. The cells
were then incubated with the FAT1 primary antibody (1:1,000; rabbit
polyclonal antibody; cat. no. ab190242; Abcam, Cambridge, UK).
Then, the wells were incubated with corresponding fluorescence
stain CellTag™ 700 Stain (red fluorescence; LI-COR
Biosciences, Lincoln, NE, USA) or fluorescence antibody
IRDye™ 800CW (green fluorescence; LI-COR Biosciences).
Images of FAT1 were obtained using the Odyssey Infrared Imaging
System (LI-COR Biosciences GmbH, Bad Homburg, Germany). The protein
level of FAT1 was calculated as the ratio of the intensity of FAT1
to that of the cell number. At least three independent experiments
were carried out; for each independent experiment, three duplicates
were performed for each group.
Plasmid constructs and
transfection
For knockdown of endogenous FAT1, we used vectors
containing the sequence: 5′-GCCTGTGGGTTCCAGTGTAAT-3′ (FAT1shRNA1)
and 5′-GCTGGAAATGAACTGGATTTC-3′ (FAT1shRNA1) and scramble control
sequence: 5′-TTCTCCGAACGTGTCACGTTTC-3′ (NC). These shRNAs were
cloned into the vector pGLV-H1-GFP+Puro and co-transfected into
293T cells with packaging plasmids (Shanghai GenePharma Co., Ltd.,
Shanghai, China). The lentivirus supernatant was used to infect the
YSE2 and Colo680N cell lines. The corresponding empty vectors were
used as the negative control. Stable cell lines were screened out
for 2 weeks with 2 µg/ml puromycin (Invitrogen; Thermo Fisher
Scientific, Inc.). The efficiency of silence was determined by
means of RT-PCR, qPCR and in-cell western assay.
Transwell migration and invasion
assays
Migration and invasion assays were performed using
Transwell plates (pore size is 8-µm; Corning, Inc., Corning, NY,
USA). In brief, 50,000 cells were seeded into each well with
serum-free medium in the upper compartment of the Transwell plates
coated with or without BD Matrigel Basement Membrane Matrix (BD
Biosciences, Franklin Lakes, NJ, USA), and the ratio of the
Matrigel Basement Membrane Matrix and serum-free medium was 1:6.
The lower compartment of the chamber was filled with medium with
10% FBS. Following a 24-h culture, the cell numbers that passed
through the membrane were fixed with 4% formaldehyde and stained
using 0.1% crystal violet and counted under a microscope
(IX71-A12FL/PH; Olympus Corp., Tokyo, Japan).
Wound healing assay
The stably transfected YSE2 and Colo680N cells were
seeded in 6-well plates. When cells grew to 95–100% density, the
cell monolayer was scratched using a pipette tip, followed by being
washed with culture medium to remove any loosely held cells. The
wound closure was monitored at 0 and 24 h after scratching, and the
wound area was calculated using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). At least three
independent experiments were carried out; for each independent
experiment, three duplicates were performed for each group.
Atomic force microscopy (AFM)
analysis
The morphology and biomechanical properties of the
YSE2-FAT1shRNA and Colo680N-FAT1shRNA cells and the corresponding
controls were detected by AFM, as previously described (19). Briefly, AFM images were acquired in
the tapping mode and in contact mode using Si3N4 tips (NSC19, 0.68
N/m normal spring constant; Schaefer Technologie GmbH, Langen,
Germany) and gold-coated tips (CSC 38; Schaefer Technologie GmbH),
respectively. AFM measurements were performed in aqueous solution
at room temperature, using a 5500 atomic force microscope (Agilent
Technologies, Inc., Santa Clara, CA, USA). The spring constants of
the cantilevers used for AFM force spectroscopy were ~0.1 N/m.
Adhesion and elasticity maps were obtained by recording 16×16
force-distance curves on areas of a given size (2×2 µm),
calculating the adhesion force and elasticity modulus for each
force curve and displaying these values as gray and colorized scale
pixels, respectively. These maps qualitatively and quantitatively
demonstrated the viscoelasticity of individual cells at the
nanoscale level.
Statistical analyses
Statistical analyses were performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Experiments were performed
in triplicate, and data are presented as the mean ± SEM. Data from
two groups were analyzed by unpaired t-test and >2 groups were
analyzed by one-way ANOVA with post hoc contrasts by the S-N-K
method. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
FAT1 exhibits a high frequency of
mutations and shows downregulated expression in ESCC
In our previous study, we analyzed the genomic
sequencing data from 4 ESCC cohorts, including 424 tumors and
matched normal DNA samples, from patients recruited in the Taihang
Mountain region of north-central China and Chaoshan District of
Guangdong Province, the areas of high ESCC incidence in China. We
found that FAT1 was mutated frequently in ESCC. The total mutation
rate was ~13.4% (57/424), including nonsense in 21/57 (36.8%),
missense in 22/57 (38.6%), insertion or deletion in 14/57 (24.6%)
in these 4 cohorts (6,8,20–22).
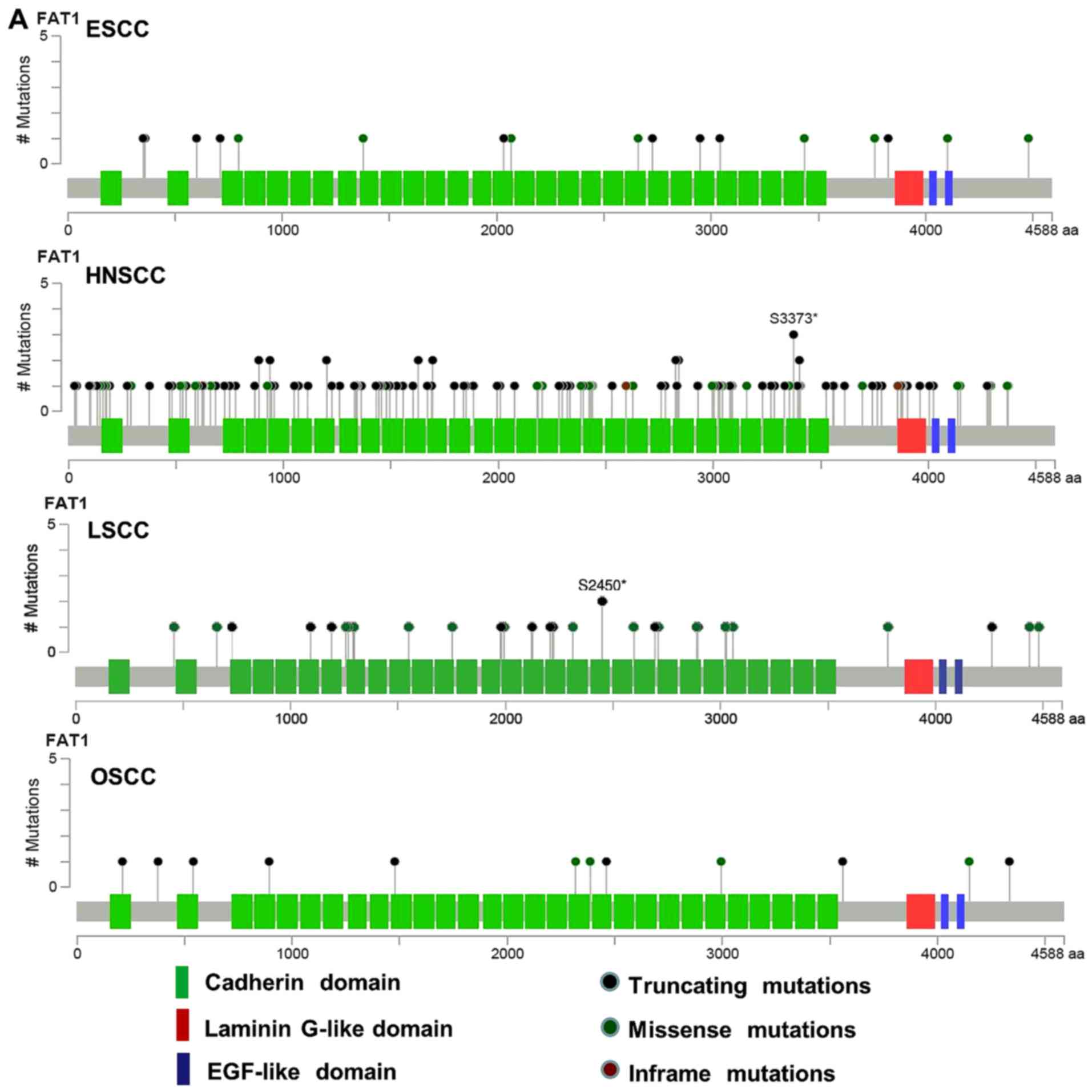
In addition, we analyzed several types of squamous cell carcinomas
in a TCGA database using cBioPortal (23,24),
and found that FAT1 was mutated frequently in squamous cell
carcinomas (Fig. 1A). In ESCC, the
frequency of FAT1 mutations was 11.7% (20), in head and neck squamous cell
carcinoma (HNSCC), the frequency of FAT1 mutations was 21.7%
(23), in lung squamous cell
carcinoma (LSCC), the frequency of FAT1 mutations was 14.6%
(25), and in oral squamous cell
carcinoma (OSCC), the frequency of FAT1 mutations was 30% (26). Then, we detected FAT1 protein
expression via TMA that included 76 cases of primary esophageal
tumor tissues and matched adjacent non-tumor tissues, since the
other 49 cases of tissues were incomplete for further statistics.
We observed that FAT1 expression was strongly lower in the tumor
tissues than that noted in the stained non-tumor tissues (Fig. 1B). Data on the 125 patients from
which the tumor and normal samples were derived for the TMA study
are provided in Table I.
 | Table I.Information concerning the 125
patients in the tissue microarray study. |
Table I.
Information concerning the 125
patients in the tissue microarray study.
|
Characteristics | No. of cases
(N=125) |
|---|
| Age (years) |
|
|
<60 | 69 |
|
≥60 | 56 |
| Sex |
|
|
Male | 86 |
|
Female | 39 |
| Smoking |
|
| No | 46 |
|
Yes | 79 |
| Drinking |
|
| No | 81 |
|
Yes | 44 |
| Family history |
|
| No | 97 |
|
Yes | 28 |
|
Differentiation |
|
|
High | 4 |
|
Middle | 89 |
|
Low | 32 |
| T
Classification |
|
| T1 | 10 |
| T2 | 27 |
| T3 | 88 |
| T4 | 0 |
| N
classification |
|
| N
<1 | 47 |
| N
≥1 | 78 |
| TNM stage |
|
| I | 6 |
| II | 50 |
|
III | 68 |
| IV | 1 |
Knockdown of FAT1 in ESCC cells
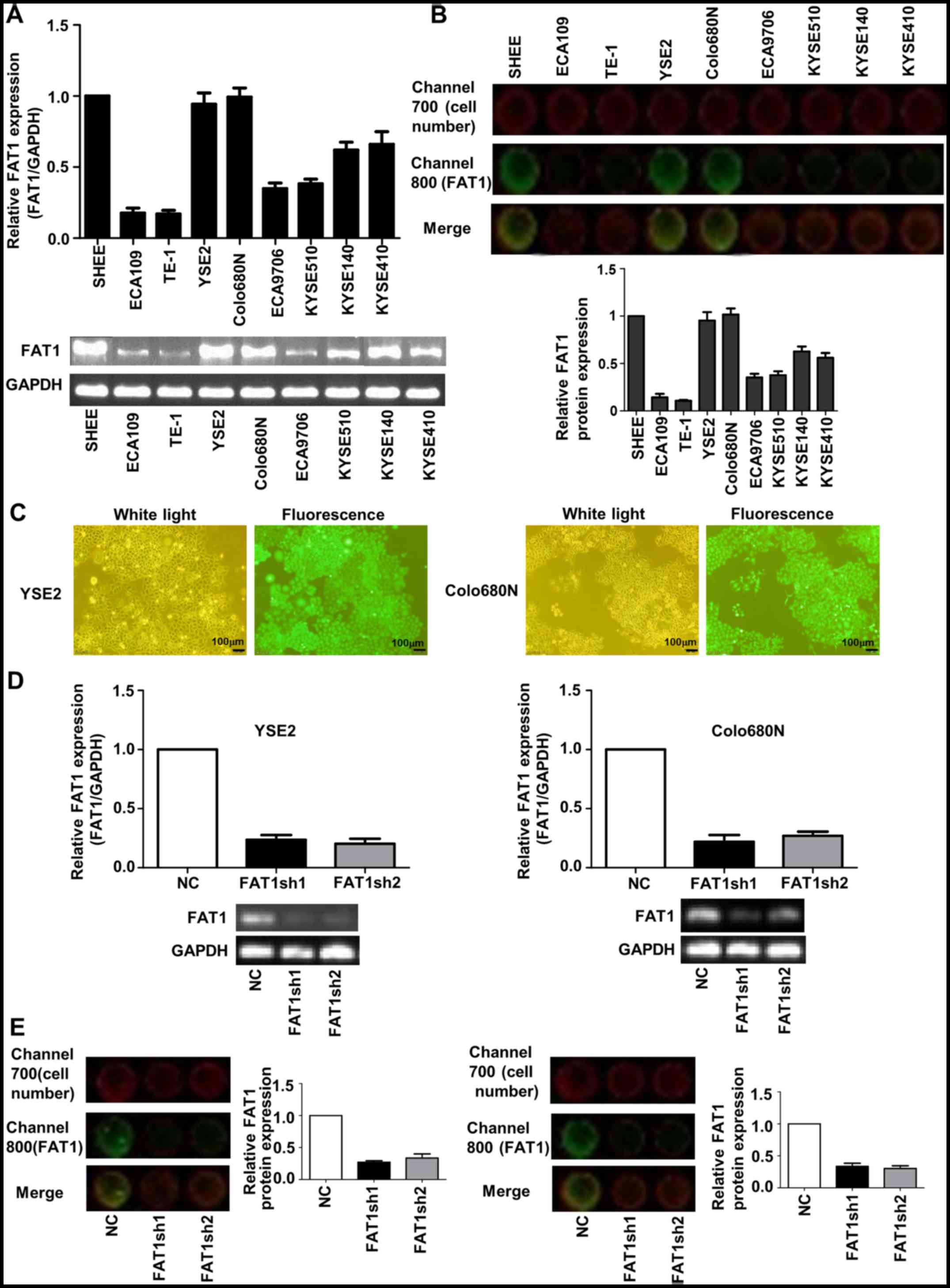
First, we measured FAT1 mRNA levels in
non-transformed esophageal epithelial SHEE cells and 8 ESCC cell
lines by RT-PCR and in-cell western assay and found different
expression levels in the various of ESCC cell lines (Fig. 2A and B). Of these cell lines, YSE2
and Colo680N cell lines with a relative high endogenous FAT1 level
were used for the knockdown experiment. The transfection efficiency
of YSE2 and Colo680N cells was >90% (Fig. 2C). RT-PCR and qPCR were used to
assess the mRNA expression of FAT1 in the FAT1-knockdown ESCC cells
(Fig. 2D). In-cell western assay
was used to assess the protein of FAT1 in FAT1-knockdown ESCC cells
(Fig. 2E). The data showed that the
efficiency of knockdown was >70%.
FAT1 knockdown promotes cell migration
and invasion in ESCC
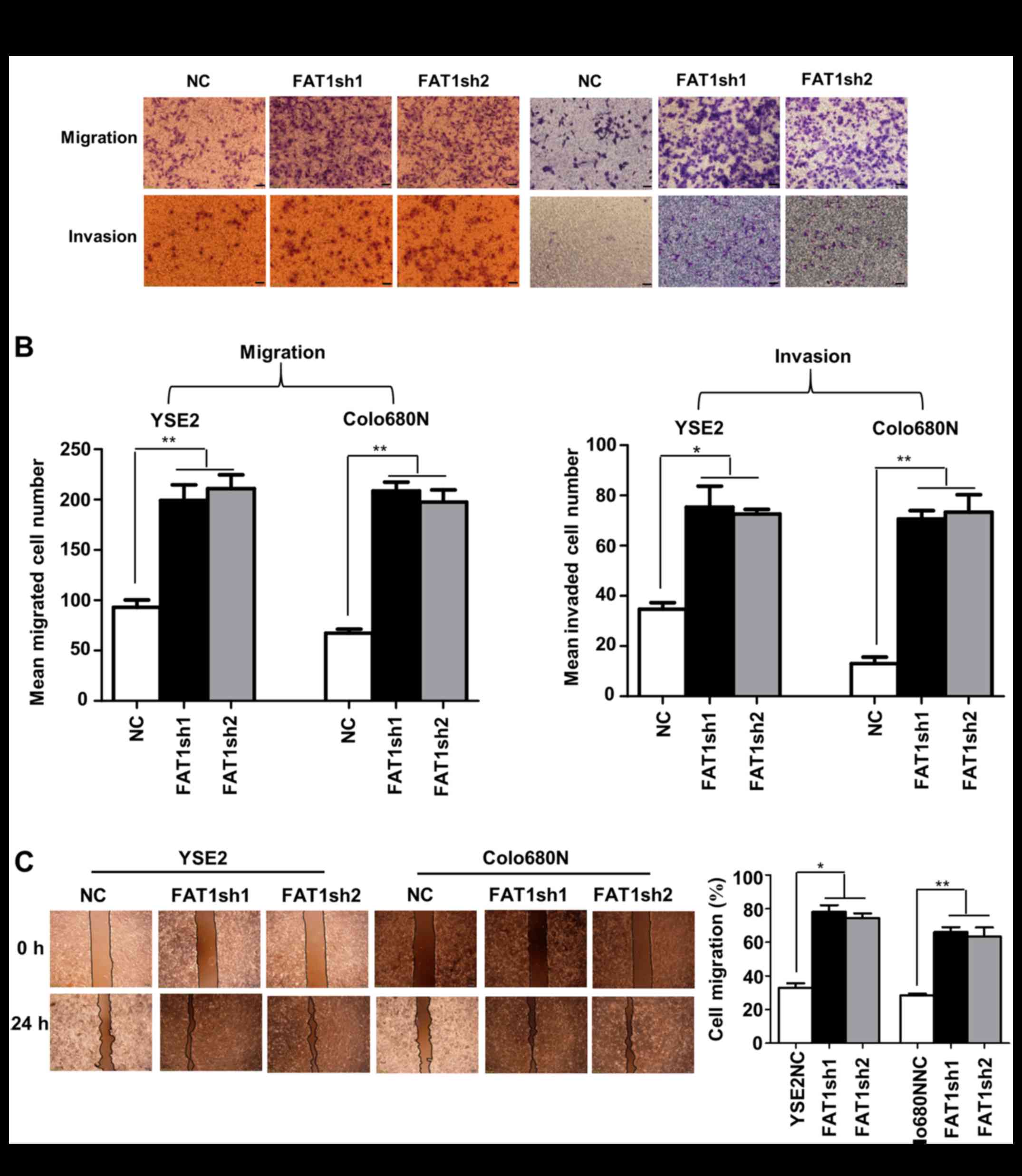
Transwell assays were performed to examine the
effect of FAT1 on the migration and invasion of ESCC cells. The
results showed that FAT1 depletion led to a significant increase in
cell migration and invasion abilities in the YSE2 and Colo680N cell
lines (Fig. 3A and B), which were
also confirmed by the wound healing migration assay (Fig. 3C).
Changes in the morphologic and
biomechanical properties of the ESCC cells after FAT1 knockdown as
determined by AFM
In the present study, AFM was used to visualize the
morphology of YSE2 and Colo680N FAT1-knockdown cells in comparison
to the corresponding control groups, respectively. The results
demonstrated that the cells became relatively thinner following
FAT1 knockdown. In addition, we detected the roughness of the cell
membrane surface. The results showed that, although the cell
surface roughness in the FAT1-knockdown cells had no statistical
difference when compared with the NC group, there was a downward
trend. The results indicate that the cell surface may be smoother
after knockdown of FAT1 (Fig.
4).
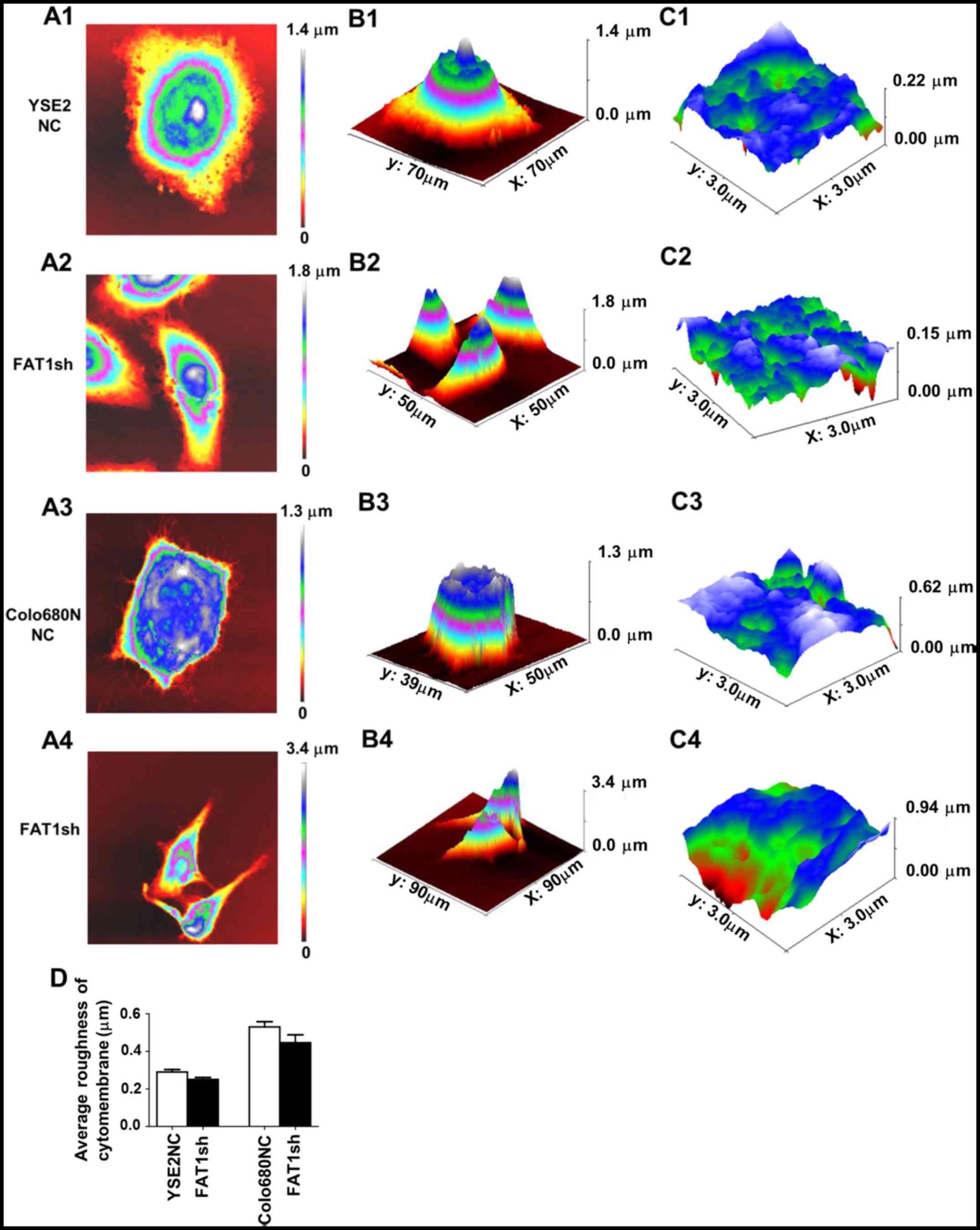 | Figure 4.The morphological images of YSE2 and
Colo680N cells following FAT1 knockdown and the matched negative
control groups by atomic force microscopy (AFM). (A) The 2D cell
morphology of YSE2 and Colo680N cells following FAT1 knockdown and
the matched negative control (NC) groups by AFM. A1, NC group (YSE2
cells); A2, FAT1-knockdown group (YSE2 cells); A3, NC group
(Colo680N cells); A4, FAT1-knockdown group (Colo680N cells). (B) 3D
cell morphology. B1, NC group (YSE2 cells); B2, FAT1-knockdown
group (YSE2 cells); B3, NC group (Colo680N cells); B4,
FAT1-knockdown group (Colo680N cells). (C) The cell ultra-structure
of YSE2 and Colo680N cells of the FAT1-knockdown and matched
negative groups by AFM. C1, NC group (YSE2 cells); C2,
FAT1-knockdown group (YSE2 cells); C3, NC group (Colo680N cells);
C4, FAT1-knockdown group (Colo680N cells). (D) The statistical
histogram of average roughness of the cytomembrane of ESCC cells
with or without FAT1 knockdown. All experiments were repeated three
times independently. *P<0.05, **P<0.01. FAT1, FAT atypical
cadherin 1. |
Furthermore, compared to the NC groups, the adhesive
force was significantly reduced (YSE2NC, 1200±300 pN vs. FAT1shRNA,
480±200 pN; Colo680NNC, 1400±400 pN vs. FAT1shRNA, 560±230 pN),
while the elasticity force was markedly increased (YSE2NC, 3.9±0.7
MPa vs. FAT1shRNA, 19±6.3 MPa; Colo680NNC, 4.2±1.3 MPa vs.
FAT1shRNA, 26±5.5 MPa) in the YSE2-FAT1shRNA and Colo680N-FAT1shRNA
groups, and the difference was statistically significant (Fig. 5). Taken together, these results
suggest that FAT1 may affect cell microscopic morphology and
mechanical properties consequently impacting ESCC cell migration
and invasion ability.
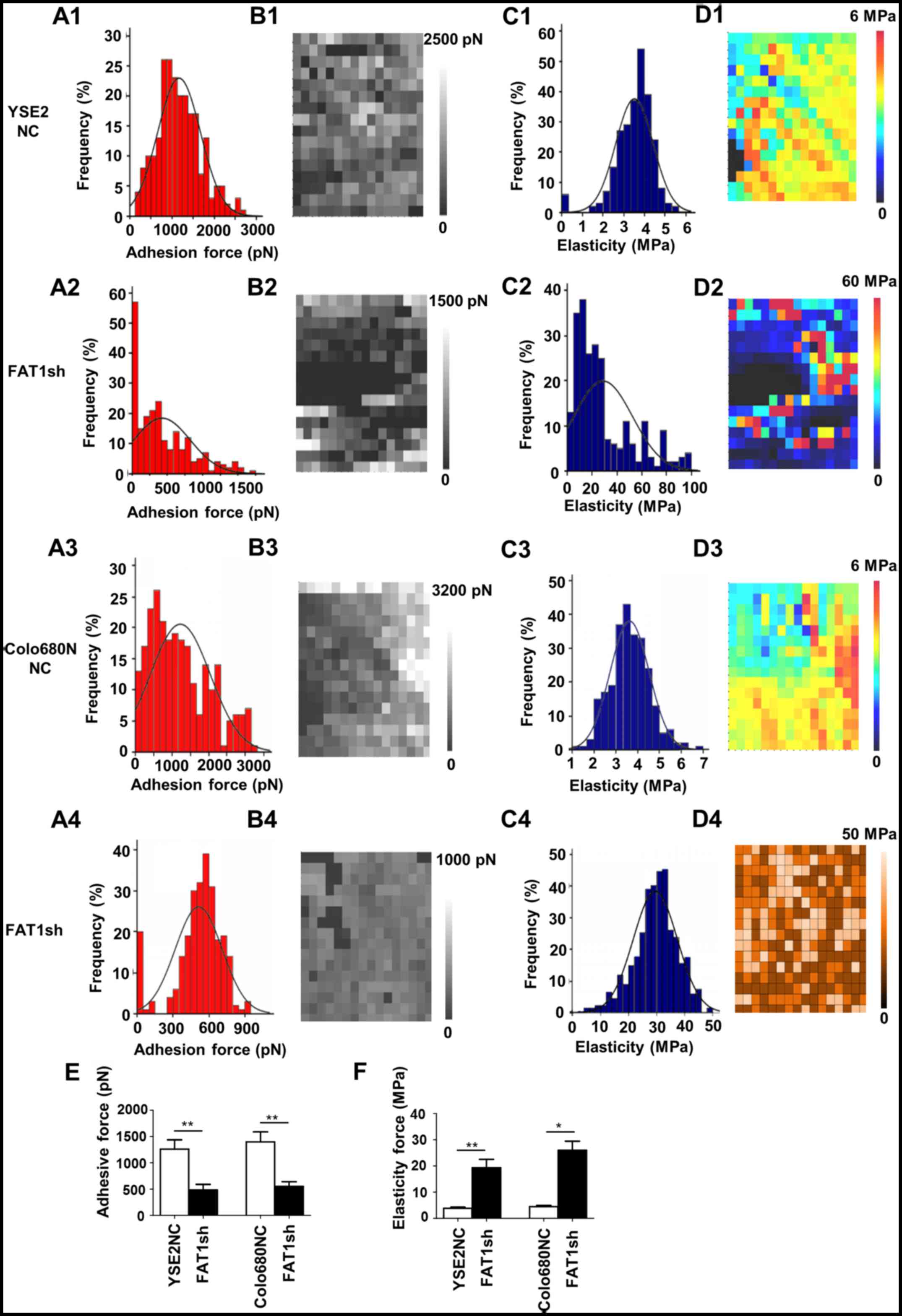 | Figure 5.AFM force-distance curve analyses to
detect the adhesive force and elasticity of YSE2 and Colo680N cells
in the FAT1-knockdown and matched negative groups. (A) Adhesion
force histograms showing the adhesive force and elasticity of YSE2
and Colo680N cells in the FAT1-knockdown and matched negative
control (NC) groups by atomic force microscopy (AFM). A1, NC group
(YSE2 cells); A2, FAT1-knockdown group (YSE2 cells); A3, NC group
(Colo680N cells); A4, FAT1-knockdown group (Colo680N cells). (B)
Adhesion force map of the same cell-surface area. B1, NC group
(YSE2 cells); B2, FAT1-knockdown group (YSE2 cells); B3, NC group
(Colo680N cells); B4, FAT1-knockdown group (Colo680N cells). (C)
Elasticity histogram. C1, NC group (YSE2 cells); C2, FAT1-knockdown
group (YSE2 cells); C3, NC group (Colo680N cells); C4,
FAT1-knockdown group (Colo680N cells). (D) Elasticity map of the
same cell-surface area. D1, NC group (YSE2 cells); D2,
FAT1-knockdown group (YSE2 cells); D3, NC group (Colo680N cells);
D4, FAT1-knockdown group (Colo680N cells). (E) Statistical
histogram showing the adhesive force of ESCC cells with or without
FAT1 knockdown. (F) Statistical histogram of the elasticity force
of ESCC cells with or without FAT1 knockdown. All experiments were
repeated three times independently. *P<0.05, **P<0.01. FAT1,
FAT atypical cadherin 1. pN, picoNewton (10−12 N); MPa,
mega Pascal (106 Pa). |
Discussion
As one of the members of the cadherin family, FAT
atypical cadherin 1 (FAT1) is regarded as a key molecule that
regulates cellular growth, migration and orientation. The effect of
FAT1 on tumor occurrence and development is still in discussion
(27). The dual role of FAT1 in
human cancer development has been reported as it functions as both
a tumor-suppressor gene as well as an oncogene (9–11,28).
FAT1 was first deemed to posses a tumor suppressive function in
Drosophila melanogaster by regulating the
Salvador/Warts/Hippo signaling pathway (29,30).
In glioblastoma multiforme, colorectal cancer and head and neck
cancer, FAT1 was identified as a tumor suppressor, for which
somatic mutations lead to aberrant Wnt signaling pathway activation
(12). In breast carcinoma,
suppression of FAT1 expression was found to promote breast cancer
cell invasion (31). However, in
hepatocellular carcinoma, FAT1 acts as a tumor promoter and
promotes the proliferation and migration as well as inhibits
apoptosis (11). The possible
switch of FAT1 from an oncogene to an oncogenesis-preventive gene
may be due to the specificity of the tissues and organs and the
complexity of the signaling pathways. In our previous study, we
identified a novel role of FAT1 in inhibiting tumor growth and EMT
occurrence in ESCC by means of disruption of the MAPK/ERK pathway
(8). In the present study, we
further identified the tumor-suppressor function of FAT1 in light
of the changes in the morphologic and biomechanical properties of
the cells using AFM.
A change in cell mechanical properties can affect
the body's physiological function and cause disease (32); in particular, cell mechanical
properties play an important role in the study of human cancer
(33,34). In recent years, more and more
research has shown that AFM can be used as a tool for the detection
of the biological behaviors of tumor cells and for assessment of
antitumor efficacy (35). Cell
adhesion and elasticity force may reflect the cell membrane and
cytoskeleton mechanics state and are associated with cellular
deformability (36). Increased cell
adhesion strength and decreased elasticity lead to deformation
capacity reduction. Tumor cells need deformation to complete
migration, invasion and a series of biological behaviors (37). Propofol was found to decrease the
migratory ability by influencing the cell cytoskeleton in cervical
cancer cell lines (38). Lian et
al reported that artesunate attenuates cellular migration and
invasion by affecting cellular mechanical properties in glioma
(39). In addition, studies have
shown that the mechanical properties of cells and tissues as
determined by AFM can be used as markers for the diagnosis of
various pathological conditions such as cancer, arthritis,
osteoporosis (40), pulmonary
fibrosis (41), blood and
cardiovascular pathologies (40).
Therefore, we speculate that AFM may serve as a means of clinical
laboratory examination in the future. In the present study, we
detected cell adhesive force and cell elasticity force by AFM and
found that suppression of endogenous expression of FAT1 led to a
decrease in cell adhesive force and an increase in cell elasticity
force compared with the control groups.
Although FAT1 was recently implicated as a tumor
suppressor or an oncogene in other cancers, its roles in ESCC are
limited. In our previous research, we found that FAT1 was one of
the significantly mutated genes in ESCC and FAT1 expression was
decreased in ESCC tissues compared with that noted in matched
normal adjacent tissues (8). In the
present study, we verified that FAT1 knockdown effectively
accelerated cell migration and invasion. Moreover, the impact of
FAT1 disruption on the cellular mechanical properties was also
evaluated by AFM. The cytoskeleton consists of microtubules,
microfilaments and microvilli, which determines the cell morphology
and biomechanical characteristics under various physiological and
pathological statuses (42). Yao
et al showed that MARVELD1, a potential tumor suppressor,
increased the length of microvilli and suppressed EMT in non-small
cell lung cancer (43). In breast
cancer MCF-7 cell lines, rhBMP-2 was found to promote EMT
progression and AFM observation showed that the MCF-7 cells became
narrower and flatter, with lamellipodia formation following rhBMP-2
treatment (44). Our previous study
showed that FAT1 prevented EMT and inhibited the migration and
invasion of ESCC cells. Our present study showed that FAT1 may
affect cytoskeleton proteins, and therefore alter the ability of
cell migration and invasion. The specific mechanism will be
explored in subsequent research.
In conclusion, the present study showed that FAT1
inhibited cell migration and invasion by affecting the cellular
mechanical properties of ESCC cells. The present findings aid in
our understanding of the potential mechanisms that drive the
development of ESCC and may provide new insight concerning the
association between FAT1 and ESCC.
Acknowledgements
We would like to thank the patients with ESCC and
their families for participation. We would also like to thank Dr
Xun Huang (Department of Materials Science and Engineering, Jinan
University) for his contributions to the determination of cell
mechanics properties.
References
|
1
|
Wheeler JB and Reed CE: Epidemiology of
esophageal cancer. Surg Clin North Am. 92:1077–1087. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Zhou Y, Cheng C, Cui H, Cheng L,
Kong P, Wang J, Li Y, Chen W, Song B, et al: Genomic analyses
reveal mutational signatures and frequently altered genes in
esophageal squamous cell carcinoma. Am J Hum Genet. 96:597–611.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng C, Zhou Y, Li H, Xiong T, Li S, Bi
Y, Kong P, Wang F, Cui H, Li Y, et al: Whole-genome sequencing
reveals diverse models of structural variations in esophageal
squamous cell carcinoma. Am J Hum Genet. 98:256–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Zhai Y, Kong P, Cui H, Yan T, Yang
J, Qian Y, Ma Y, Wang F, Li H, et al: FAT1 prevents epithelial
mesenchymal transition (EMT) via MAPK/ERK signaling pathway in
esophageal squamous cell cancer. Cancer Lett. 397:83–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadeqzadeh E, de Bock CE, Zhang XD,
Shipman KL, Scott NM, Song C, Yeadon T, Oliveira CS, Jin B, Hersey
P, et al: Dual processing of FAT1 cadherin protein by human
melanoma cells generates distinct protein products. J Biol Chem.
286:28181–28191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KT, Kim BS and Kim JH: Association
between FAT1 mutation and overall survival in patients with human
papillomavirus-negative head and neck squamous cell carcinoma. Head
Neck. 38 Suppl 1:E2021–E2029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valletta D, Czech B, Spruss T, Ikenberg K,
Wild P, Hartmann A, Weiss TS, Oefner PJ, Müller M, Bosserhoff AK
and Hellerbrand C: Regulation and function of the atypical cadherin
FAT1 in hepatocellular carcinoma. Carcinogenesis. 35:1407–1415.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris LG, Kaufman AM, Gong Y, Ramaswami
D, Walsh LA, Turcan Ş, Eng S, Kannan K, Zou Y, Peng L, et al:
Recurrent somatic mutation of FAT1 in multiple human cancers leads
to aberrant Wnt activation. Nat Genet. 45:253–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Settakorn J, Kaewpila N, Burns GF and
Leong AS: FAT, E-cadherin, beta catenin, HER 2/neu, Ki67
immuno-expression, and histological grade in intrahepatic
cholangiocarcinoma. J Clin Pathol. 58:1249–1254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi X, Zhang X, Xia T and Fang X: Living
cell study at the single-molecule and single-cell levels by atomic
force microscopy. Nanomedicine. 7:1625–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Yang F, Cai JY, Yang PH and Liang
ZH: In-situ detection of resveratrol inhibition effect on epidermal
growth factor receptor of living MCF-7 cells by atomic force
microscopy. Biosens Bioelectron. 56:271–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang J, Jiang C, Xiao X, Fang Z, Li L, Han
L, Mei A, Feng Y, Guo Y, Li H and Jiang W: Changes in red blood
cell membrane structure in G6PD deficiency: An atomic force
microscopy study. Clin Chim Acta. 444:264–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kaul-Ghanekar R, Singh S, Mamgain H,
Jalota-Badhwar A, Paknikar KM and Chattopadhyay S: Tumor suppressor
protein SMAR1 modulates the roughness of cell surface: Combined AFM
and SEM study. BMC Cancer. 9:3502009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cross SE, Jin YS, Rao J and Gimzewski JK:
Nanomechanical analysis of cells from cancer patients. Nat
Nanotechnol. 2:780–783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi R, Cui H, Bi Y, Huang X, Song B, Cheng
C, Zhang L, Liu J, He C, Wang F, et al: Artesunate altered cellular
mechanical properties leading to deregulation of cell proliferation
and migration in esophageal squamous cell carcinoma. Oncol Lett.
9:2249–2255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun
ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, et al: Genetic landscape of
esophageal squamous cell carcinoma. Nat Genet. 46:1097–1102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cancer Genome Atlas Research Network:
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pickering CR, Zhang J, Yoo SY, Bengtsson
L, Moorthy S, Neskey DM, Zhao M, Alves Ortega MV, Chang K, Drummond
J, et al: Integrative genomic characterization of oral squamous
cell carcinoma identifies frequent somatic drivers. Cancer Discov.
3:770–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh M: Function and cancer genomics of
FAT family genes (Review). Int J Oncol. 41:1913–1918. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanoue T and Takeichi M: New insights into
fat cadherins. J Cell Sci. 118:2347–2353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bennett FC and Harvey KF: Fat cadherin
modulates organ size in Drosophila via the Salvador/Warts/Hippo
signaling pathway. Curr Biol. 16:2101–2110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reddy BV and Irvine KD: The fat and warts
signaling pathways: New insights into their regulation, mechanism
and conservation. Development. 135:2827–2838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee S, Stewart S, Nagtegaal I, Luo J, Wu
Y, Colditz G, Medina D and Allred DC: Differentially expressed
genes regulating the progression of ductal carcinoma in situ to
invasive breast cancer. Cancer Res. 72:4574–4586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee GY and Lim CT: Biomechanics approaches
to studying human diseases. Trends Biotechnol. 25:111–118. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guck J, Schinkinger S, Lincoln B, Wottawah
F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R,
Mitchell D, et al: Optical deformability as an inherent cell marker
for testing malignant transformation and metastatic competence.
Biophys J. 88:3689–3698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suresh S: Biomechanics and biophysics of
cancer cells. Acta Biomater. 3:413–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai X, Gao S, Cai J, Wu Y and Deng H:
Artesunate induced morphological and mechanical changes of Jurkat
cell studied by AFM. Scanning. 31:83–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fletcher DA and Mullins RD: Cell mechanics
and the cytoskeleton. Nature. 463:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar S and Weaver VM: Mechanics,
malignancy, and metastasis: The force journey of a tumor cell.
Cancer Metastasis Rev. 28:113–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang F, Wang C, Cui Y, Li S, Yao Y, Ci Y,
Wang J, Hou W, Wu A and Li E: Effects of propofol on several
membrane characteristics of cervical cancer cell lines. Cell
Physiol Biochem. 40:172–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lian S, Shi R, Huang X, Hu X, Song B, Bai
Y, Yang B, Dong J, Du Z, Zhang Y, et al: Artesunate attenuates
glioma proliferation, migration and invasion by affecting cellular
mechanical properties. Oncol Rep. 36:984–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Khalili AA and Ahmad MR: A review of cell
adhesion studies for biomedical and biological applications. Int J
Mol Sci. 16:18149–18184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Siamantouras E, Hills CE, Squires PE and
Liu KK: Quantifying cellular mechanics and adhesion in renal
tubular injury using single cell force spectroscopy. Nanomedicine.
12:1013–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuchs E and Cleveland DW: A structural
scaffolding of intermediate filaments in health and disease.
Science. 279:514–519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yao Y, Shi M, Liu S and Li Y, Guo K, Ci Y,
Liu W and Li Y: MARVELD1 modulates cell surface morphology and
suppresses epithelial-mesenchymal transition in non-small cell lung
cancer. Mol Carcinog. 55:1714–1727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang D, Huang P, Zhu B, Sun L, Huang Q and
Wang J: Induction of estrogen receptor α-36 expression by bone
morphogenetic protein 2 in breast cancer cell lines. Mol Med Rep.
6:591–596. 2012. View Article : Google Scholar : PubMed/NCBI
|