Introduction
The precise regulation of cell proliferation and
apoptosis plays an important role in maintaining an appropriate
organ size during organ development and tissue homeostasis during
postnatal life (1). The Hippo
pathway was initially identified in Drosophila through
genetic mosaic screening. As an evolutionarily and functionally
conserved signaling network, the Hippo pathway has been discovered
to participate in controlling organ size by regulating cell
proliferation and apoptosis (2–5).
Following these pioneering discoveries, research
concerning the Hippo pathway in mammalian tissues has currently
become a burgeoning field (2,3).
Generally, in Drosophila, the terminal effector component of
the Hippo pathway is a transcription co-activator, named Yorkie
(4). However in mammalians,
Yes-associated protein (YAP) and its homolog transcriptional
co-activator TAZ (also called WWTR1) with PDZ-binding motif are key
downstream terminal effectors of the Hippo pathway (5). In normal tissue, YAP/TAZ proteins are
phosphorylated at specific serine residues in order to confine
their subsequent degradation in the cytoplasm, which consists of
the final effect of the Hippo pathway on YAP/TAZ proteins. However
in tumors, YAP/TAZ proteins are translocated into the nucleus where
they bind to TEA domain (TEAD) proteins. This eventually results in
cell proliferation, evasion of apoptosis and amplification of
progenitor/stem cells for the promotion of organ size (6–8).
Increasing evidence shows that alterations to the
Hippo pathway are significantly associated with cancer development
(9,10). A high percentage of patients
suffering from liver cancer, breast cancer or cancer of the pharynx
are reported to harbor causative overexpression of YAP/TAZ genes
(11). Moreover, aberrant
expression of YAP/TAZ has been demonstrated to be an independent
prognostic predictor and indicator of rapid proliferation,
metastasis and poor survival outcome of patients with colorectal
cancer (CRC) (12).
Herein, we review the functions of YAP/TAZ on
tumorigenesis, cell proliferation, metastasis and apoptosis.
Furthermore, the importance of YAP/TAZ in cancer treatment is also
highlighted in light of the interactive molecular pathways noted
among Hippo, Wnt and G-protein-coupled receptor (GPCR) in the
regulation of tumor progression and drug resistance. Briefly, we
focused on the considerable role of YAP/TAZ in oncotherapy,
illuminating their promising application potential as new drug
targets for tumor therapeutic intervention.
Hippo pathway in mammals
The Hippo pathway, conserved in mammals, has
received immense research attention in recent years (4). The study from fruit flies to humans
shows that the Hippo pathway is highly conserved under normal
conditions and functions as a means of inhibiting cancer
development (13). Basically, the
Hippo pathway consists of three parts: upstream regulatory elements
(NF2, Mel), core components (mainly Mst1/2, Lats1/2) and downstream
effector molecules (YAP/TAZ). Meanwhile, the central components of
the Hippo pathway comprise a regulatory serine-threonine kinase
module and a transcriptional module (14) (Table
I). In detail, the kinase module includes mammalian STE20-like
protein kinase 1 (Mst1, also known as Stk4) and Mst2 (also known as
Stk3), as well as large tumor suppressor 1 (Lats1) and Lats2
(11,15). The transcriptional module includes
YAP and TAZ. They are two closely related paralogues which
primarily mediate the downstream effects of the Hippo pathway via a
feedback mechanism. It is now widely acknowledged that the
components of the kinase module are tumor suppressors and those of
the transcriptional module are oncogenes (11,16).
 | Table I.Elements of the Hippo pathway. |
Table I.
Elements of the Hippo pathway.
| Location | Elements |
|---|
| Upstream | NF2 and Mel |
| Core
components | Mainly Mst1/2,
Lats1/2 |
| Downstream | YAP/TAZ |
| Central
components | Serine-threonine
kinase module (Mst1/2, Lats1/2), a transcriptional module
(YAP/TAZ) |
In normal tissue, Mst1/2, activated by certain
upstream signals, can phosphorylate and activate the substrate
Lats1/2 (17). Subsequently, the
activated Lats1/2 can directly phosphorylate downstream effector
molecules YAP/TAZ. In addition, the phosphorylated YAP/TAZ
interacts with the 14-3-3 protein, resulting in the cytoplasm
retention of YAP/TAZ. Consequently, phosphorylated YAP/TAZ may lose
their function as transcription cofactors, which leads to
ubiquitin-mediated proteasomal degradation (18,19).
On the contrary, YAP/TAZ can function as transcriptional
co-activators that shuttle between the cytoplasm and nucleus. In
the nucleus, they induce expression of cell-proliferative and
anti-apoptotic genes via interacting with transcriptional factors,
particularly the TEA domain (TEAD) (6,20,21).
In short, the Hippo pathway is an important pathway to maintain the
homeostasis of cell proliferation and apoptosis (22). Noteworthy, deactivation of the Hippo
pathway and the upregulation of YAP/TAZ have been frequently
observed in many human cancers (10,23).
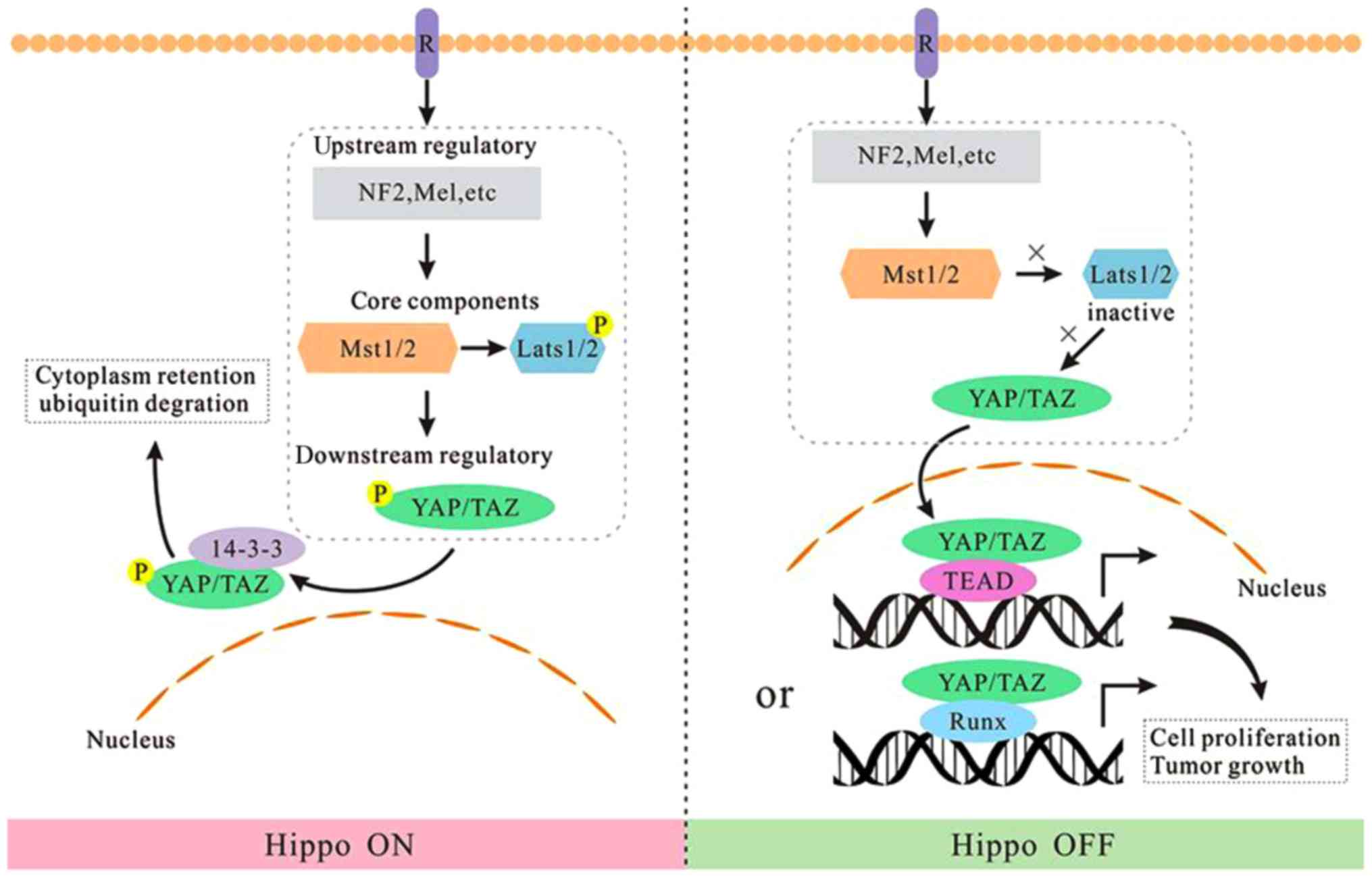
Based on the aforementioned analysis, we regard the
Hippo kinase module as a switch button. When Hippo kinase module is
‘on’, LATS1/LATS2 phosphorylates and inactivates YAP/TAZ, thus the
output gene production is turned off. Oppositely, when the kinase
module is ‘off’, hypophosphorylated YAP/TAZ are translocated into
the nucleus and induce the expression of target genes (Fig. 1).
Structural and functional characteristics of
YAP/TAZ
The YAP1 (Yes-associated protein 1) gene, located on
chromosome 11q22 in humans and having a 65-kDa molecular mass
(known as YAP or YAP65), is ubiquitously expressed in human tissues
throughout the developmental process (24–26).
To date, eight splicing isoforms of the YAP1 gene product (YAP1-1
α, β, γ, δ and YAP1-2 α, β, γ and δ) have been initially identified
in humans and are regarded as YAP1 and YAP2, which differ by the
presence of an extra 38 amino acids that encode the WW domain
(27). Although the existence of
YAP1 (with one WW domain) and YAP2 (with two WW domains) isoforms
has been previously unveiled, there are few studies that separate
these two types of isoforms in the research of the Hippo pathway
(24). As an oncogene, YAP is
amplified in various human cancers, which leads to abnormalities of
the Hippo-YAP pathway and induces an imbalance between cell
proliferation and apoptosis (28).
Research in vitro has demonstrated that YAP2 is the
predominantly expressed YAP isoform in both ovarian surface
epithelium and epithelial ovarian cancers (29). Notably, whether YAP has biological
activity or not depends on the location of YAP in cells.
Phosphorylated YAP binds to the 14-3-3 protein in the cytoplasm,
leading to its cytoplasmic retention and inactivation (30). As for the TAZ gene, it is located on
3q23-q24 and encodes a 43-kDa protein (31) with abundant expression in various
tissues except for thymus and peripheral blood leukocytes and
amplification in many human cancers (32). However, it is also known as WWTR1
(WW-domain containing transcriptional regulator 1) and is similarly
identified as a 14-3-3 binding protein as well.
Structurally, YAP and TAZ share nearly half of the
amino acid sequence and have very similar topology features. YAP
protein, consisting of 488 amino acids, has a TEAD-binding region
(TB), two WW domains (two conserved tryptophan/W residues separated
by 20-23 amino acids), an SH3-binding motif, a coiled-coil domain,
a transcription activation domain, an N-terminal proline-rich
domain, and a C-terminal PDZ-binding motif. Whereas TAZ protein,
consisting of 400 amino acids, has a similar domain organization
with YAP, although it lacks the second WW domain, the SH3-binding
motif and the proline-rich domain (33,34)
(Fig. 2).
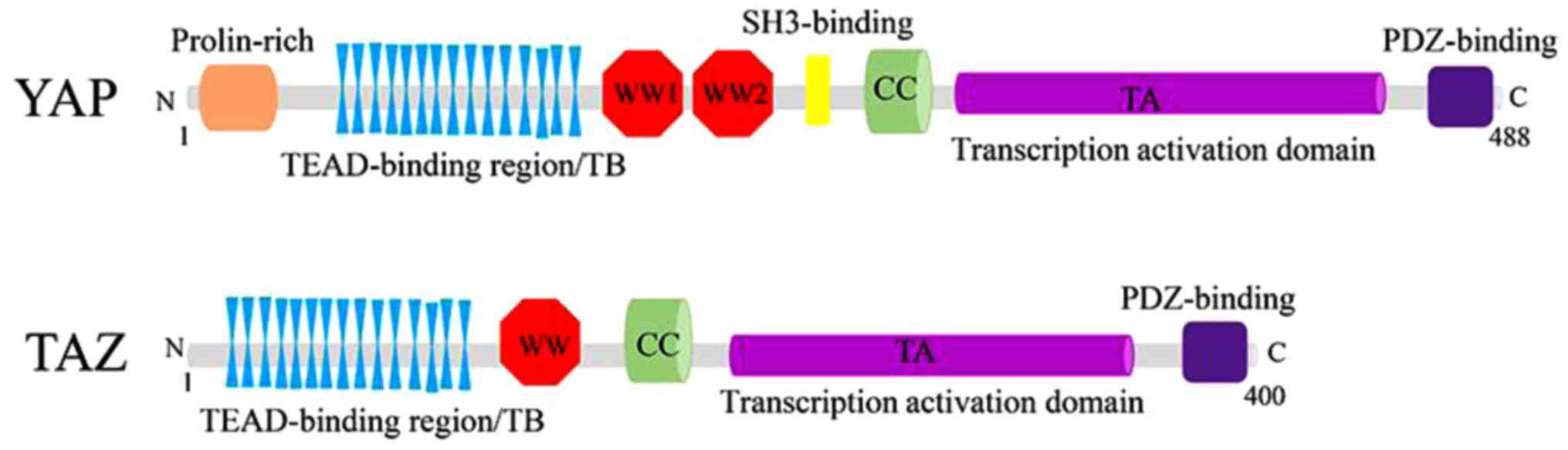 | Figure 2.Structures of YAP/TAZ. YAP protein
consists of a TEAD-binding region, two WW domains, an SH3-binding
motif, a coiled-coil domain, a transcription activation domain, an
N-terminal proline-rich domain and a C-terminal PDZ-binding motif
(above). Similarly, TAZ protein consists of a TEAD-binding region,
only one WW domain, a coiled-coil domain, a transcription
activation domain and a C-terminal PDZ-binding motif, without
SH3-binding motif and proline-rich domain compared with YAP
(below). TEAD, YAP/TAZ binds to TEA domain. |
Functionally, the WW domains of YAP and TAZ are
shown to interact with PPXY motifs of various transcriptional
factors (35). The TB domains
recognize transcriptional factors, the TEAD family, and activate
the expression of target genes. In addition, the 14-3-3 binding
motif is also crucial for the regulation of YAP and TAZ (36). In other words, both YAP and TAZ
serve as transcriptional co-activators and share various
transcriptional factor partners (37). However, there are still various
studies indicating that YAP/TAZ have their own unique target
transcriptional factors, such as ErbB4 and p73 for YAP and PPARγ,
Pax3, TBX5 and TTF-1 for TAZ. These different transcriptional
factors may contribute to the distinct functions of YAP and TAZ
(38–42).
YAP/TAZ in cancer
YAP/TAZ as biomechanic mediators
It is widely recognized that biomechanics is an
important regulator of cell physiology and a pivot in cell
development and pathological abnormalities (43). YAP/TAZ, two proto-oncogene proteins,
are able to respond to complex physical milieu characterized by the
rigidity of extracellular matrix (ECM), mechanical stretching, cell
geometry and status of the actin cytoskeleton (44).
It was revealed that breast cancer has elevated
tissue stiffness due to the alteration of the ECM (45). Nevertheless, the softening of the
tumor microenvironment may contribute to the alleviation of tumor
growth and progression. Intriguingly, remodeling of the ECM is
partly dependent on YAP activity. In cancer-associated fibroblasts
(CAFs), the activation of YAP promotes matrix stiffening through
extensive deposition of collagen. Subsequently, the YAP-induced
matrix stiffening creates tension within CAFs, leading to the
activation of Src kinase and the nuclear translocation of YAP. This
is conducive to further matrix stiffening (46), thereby establishing a self-enhancing
loop during tumorigenesis. Beyond that, mechanical stretching can
indeed induce the entry of YAP/TAZ into the nucleus, stimulating
the proliferation of contact-inhibited mammary epithelial cells
(47). In detail, mechanical
stretching is able to override CIP (contact inhibition of
proliferation) via YAP/TAZ, although CIP is deregulated in cancer
(48). Namely, this is one of the
major hallmarks of cell neoplastic transformation.
Crucially, in tumors, the extensive diversity of
cell geometry regulates cell proliferation, which is identified by
YAP/TAZ (49,50). However, it is not clear how YAP/TAZ
respond to the diversity of cell geometry in cancer. It has been
shown that the adhesion sites of YAP/TAZ and their associated
F-actin cytoskeleton are affected differently in rounded cells and
spread cells (51,52). Moreover, the regulation of YAP/TAZ
localization by mechanical stress also depends on F-actin and Rho
family GTPases (53,54). Induced actin polymerization by the
overexpression of F-actin nucleator precisely correlates with
activation of YAP/TAZ (55). Along
with these clues, we may explain how YAP/TAZ interact with the
F-actin cytoskeleton and sense cell geometry, which eventually
leads to the acceleration of tumor cell proliferation. Furthermore,
the pro-fibrotic microenvironment of tumors characterized by
enhanced stiffness stimulates mesenchymal stromal cells (MSCs) to
express α-smooth muscle actin (α-SMA). And α-SMA incorporates into
hMSC stress fibers and promotes downstream translocation of YAP/TAZ
transcription factors into the nucleus. Moreover, the nuclear
localization of YAP/TAZ is positively correlated with
α-SMA-expressing stromal cells of adiposarcoma and osteosarcoma
(56), further suggesting the
significant interaction between YAP/TAZ and biomechanic
indicators.
YAP/TAZ as modulators of the Wnt
pathway
The canonical Wnt pathway is initially stimulated by
Wnt receptors on the cell surface. In the activated Wnt pathway,
β-catenin destruction complex is decomposed and the accumulation of
β-catenin in the nucleus is also promoted. Subsequently, these
effects can facilitate the expression of Wnt-targeted genes and
tumorigenesis (57,58). It was mentioned above that the
dysregulation of YAP/TAZ may contribute to human cancer (59). Notably, previous research has
discovered an unexpected role of Wnt/β-catenin on promoting YAP
protein level by activating YAP transcription and interacting with
Hippo/YAP. Their interaction considerably contributes to
homeostasis, organ repair and tumorigenesis (60). As for colorectal cancer (CRC),
β-catenin was found to associate with TCF/LEF sequence-specific
transcriptional factors for the activation of target gene
expression. In experiments using human CRC cell lines HCT116,
SW620, SW480, RKO, LS174 and HT29, the β-catenin/TCF4 complex binds
to a DNA enhancer element within the first intron of YAP gene to
trigger YAP expression (61). It is
also noteworthy that as a modulator, the Wnt pathway can stimulate
the stabilization of β-catenin and TAZ (43). Therefore, TAZ activation is
speculated as a general feature of the Wnt pathway and is
functionally relevant to mediate Wnt biological effects.
Furthermore, the stability of TAZ is regulated by the
phosphorylation of a C-terminal phospho-degron via the Hippo
pathway and the phosphorylation of an N-terminal degron via GSK3 in
the Wnt pathway (62), indicating
the substantial connection of the Wnt pathway and Hippo pathway.
However, the Wnt pathway has other branches including Wnt/PCP
(regulating cell surface polarity), Wnt/Ca2+ (regulating
intracellular calcium signal) and Wnt/ROR1/2. Collectively, these
branches are known as the non-canonical Wnt pathway (63). In vertebrates, the non-canonical Wnt
pathway can induce osteogenic differentiation and the migration of
tumor cells, and inhibit canonical Wnt/β-catenin signals (64). Surprisingly, YAP/TAZ are also found
to be the downstream effector of the non-canonical Wnt axis,
Wnt-FZD/ROR-Gα12/13-Rho GTPases-Lats1/2. In this axis, Wnt5a/b and
Wnt3a induce YAP/TAZ activation and nuclear localization, which is
independent of canonical Wnt/β-catenin signaling. However,
upregulation of the expression of YAP/TAZ-TEAD target genes
including DKK1, BMP4 and IGFBP4, may lead to Wnt/β-catenin
inhibition (65).
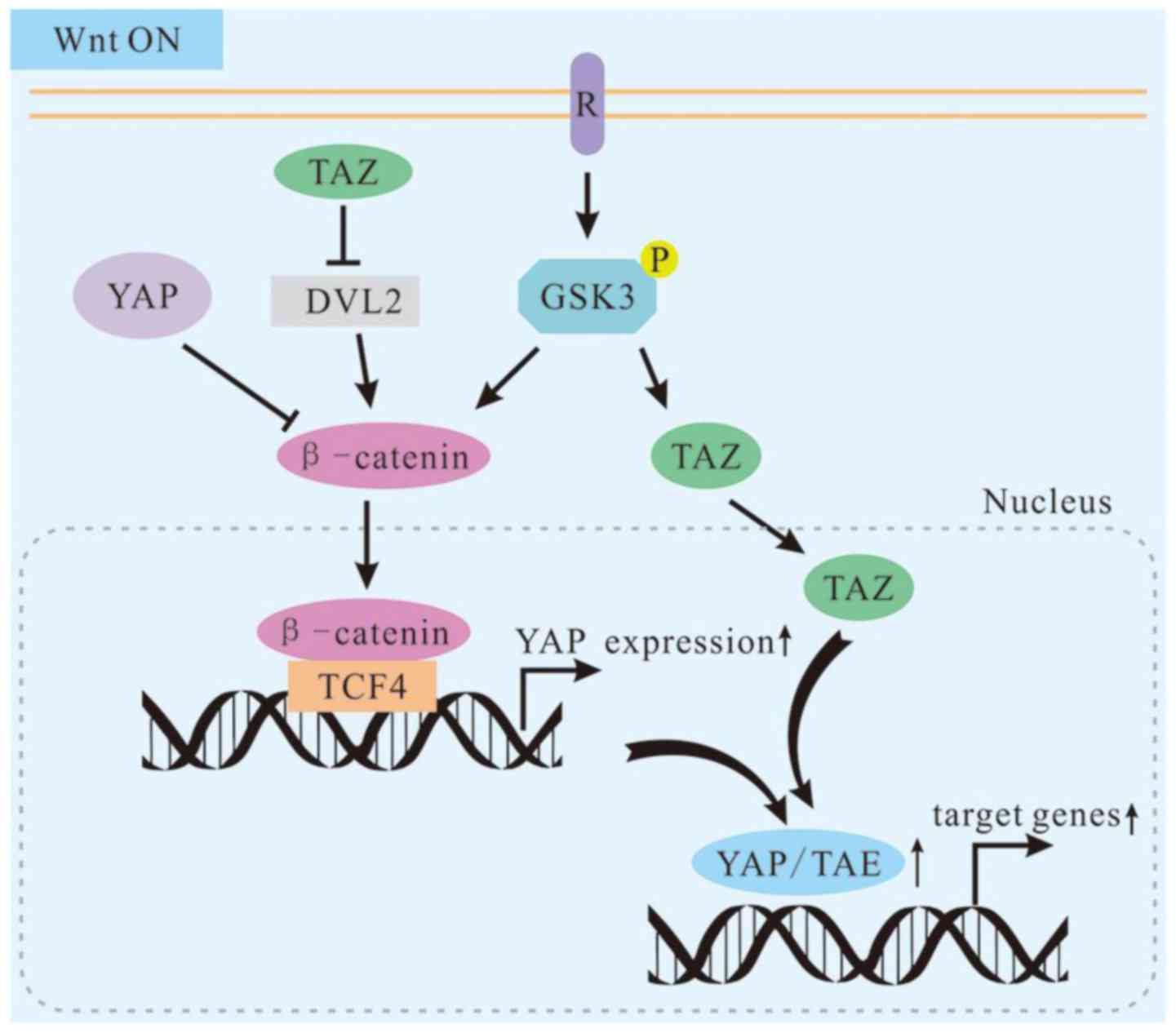
In terms of biochemical, functional and genetic
aspects, YAP/TAZ are integral components of the β-catenin
destruction complex that serves as a cytoplasmic sink for YAP/TAZ
(66). Cytoplasmic YAP may directly
sequester β-catenin into the cytoplasm. Cytoplasmic TAZ may
sequester DVL2 to impede its activity in promoting β-catenin
accumulation in response to Wnt stimulation (67,68).
In other words, the cytoplasmic YAP/TAZ may have an opposite role
here in regulating β-catenin activity. Hence, the bHippo pathway,
known to be involved in tumor suppression, also promotes
cytoplasmic localization of YAP/TAZ and inhibits the Wnt pathway.
This indicates that the Hippo pathway promotes cytoplasmic
retention and degradation of β-catenin via YAP/TAZ and the Hippo
pathway serves as a tumor inhibitor in a novel manner (Fig. 3).
Regulation of YAP/TAZ via GPCR
signaling
G protein-coupled receptors (GPCRs) are the largest
family of cell surface receptors mediating the responses to a wide
range of physiological signals (69). However, abnormal GPCR signaling is
involved in tumor development as well. For example, elevated
expression of GCPRs such as PAR1 has been demonstrated in
high-grade breast cancers, while activated mutations of GPCRs have
been found in melanomas and thyroid carcinomas (70–72).
As mentioned above, the Hippo pathway is regarded as a tumor
suppressor that regulates organ size and tumorigenesis. Based on
that, the relationship between activation of YAP/TAZ in cancer and
aberrant GPCR signaling has attracted increasing attention
(73).
Recently, groundbreaking research has found that
serum-derived sphingosine-1-phosphate (S1P) and lysophosphatidic
acid (LPA) are small-molecule activators of YAP (74). However, S1P is independent of the
core Hippo pathway kinase. It induces YAP nuclear localization
through the S1P2 receptor, Rho GTPase activation and F-actin
polymerization. Crucially, LPA and S1P activate YAP by binding to
their respective GPCRs on the cell surface and by activation of
downstream G proteins (75).
Namely, the various GPCRs and their agonists importantly serve as
Hippo pathway regulators. But notably, activation of GPCRs by
epinephrine or glucagon stimulation increases the activity of
Lats1/2 kinase, thus resulting in the inhibition of YAP function.
In contrast, G12/13- or Gq/11-coupled receptors are activated by
LPA or S1P, which can inhibit Lats1/2 kinases and result in YAP
activation.
However, protein kinase D (PKD) is activated within
cells by the stimulation of multiple GPCRs (76). Moreover, YAP/TAZ are necessary for
the stimulation of the proliferative response to GPCR agonists that
act via PKD (77,78). It was shown that stimulation of
intestinal epithelial IEC-18 cells with angiotensin II (GPCR
agonist) induces rapid cytoplasmic accumulation of YAP and
concomitant increase of YAP phosphorylation at Ser 127 and Ser 397
(77). However, addiitonal research
using human pancreatic cancer cell lines PANC-1 and MiaPaCa-2
revealed that the stimulation of tumor cells with insulin and
neurotensin promoted YAP nuclear localization and decreased YAP
phosphorylation at Ser 127 and Ser 397, which was mediated by PI3K
and PKD. In addition, this would subsequently induce the expression
of YAP/TEAD-regulated genes including connective tissue growth
factor (CTGF), cysteine-rich angiogenic inducer 61 (CYR61) and
CXCL5. siRNA-mediated knockdown of YAP/TAZ, PI3K inhibitor A66 and
PKD family inhibitors/siRNAs, would prevent the increase in the
mRNA levels of CTGF, CYR61 and CXCL5 in response to insulin and
neurotensin stimulation (78). This
indicates that PI3K or PKD can promote the crosstalk between
insulin receptor and GPCR signaling systems by inducing
YAP/TEAD-regulated gene expression in pancreatic cancer cells
(Fig. 4).
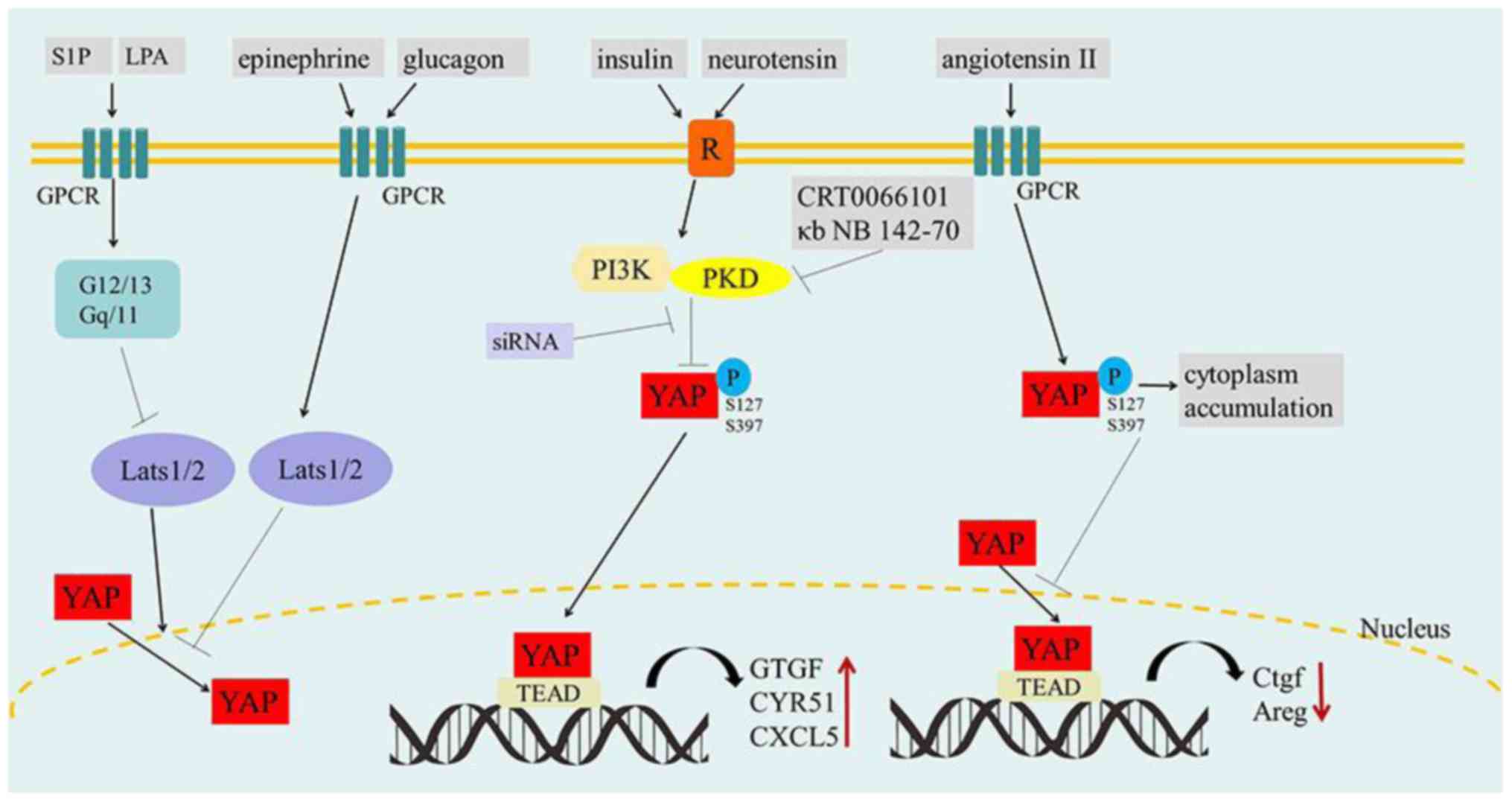 | Figure 4.The effect of GPCR signaling on
YAP/TAZ. S1P and LPA inhibit Lats1/2 kinases by activating G12/13-
or Gq/11-coupled receptors, resulting in YAP activation and nuclear
localization. Conversely, epinephrine and glucagon stimulate GPCRs
and increase the activity of Lats1/2 kinase to inactivate YAP
(left). Insulin and neurotensin promote striking YAP nuclear
localization and decreased YAP phosphorylation by PI3K/PKD in GPCR
signaling, which accelerates tumorigenesis. In contrast, the PKD
family inhibitors (CRT0066101 and κb NB 142-70) and PDK siRNA can
prohibit this process (medium). Angiotensin II increases the
phosphorylation of YAP by stimulating GPCRs, which may induce the
cytoplasmic accumulation of YAP and reduce the expression of Ctgf
and Areg. GPCRs, G-protein-coupled receptors; S1P,
sphingosine-1-phosphate; LPA, lysophosphatidic acid. P,
phosphorylation; R, insulin/neurotensin receptor; ↓, activation; ⊥,
inhibition. |
Nuclear YAP/TAZ in tumorigenesis
The mechanisms of YAP/TAZ translocation are
complicated. Apart from the aforementioned pathway, studies on
signaling molecules involved in YAP/TAZ nuclear translocation are
still underway. For instance, apoptosis-stimulating protein of p53
1 (ASPP1) is able to inhibit the interaction between YAP and Lats1
(79), therefore enhancing nuclear
accumulation of YAP/TAZ and YAP/TAZ-dependent transcriptional
regulation. Eventually, the nuclear YAP/TAZ could inhibit apoptosis
and enhance cell migration.
Accumulation of YAP/TAZ broadly participates in the
expression of cancer-related genes. It was reported that YAP/TAZ
promote cell growth by modulating amino acid signaling to mTORC1
(mammalian target of rapamycin 1) (80,81).
In the progress of amino acid-induced mTORC1 activation,
particularly under nutrient-limiting conditions, YAP/TAZ potentiate
mTORC1 activity by increasing the expression of high-affinity LAT1
(L-type amino acid transporter), a heterodimer of SLC7A5 and
SLC3A2. In addition, the YAP/TAZ-TEAD complex directly induces the
transcription of SLC7A5, which forms a dimer with SLC3A2 in order
to increase LAT1 expression and provide a competitive growth
advantage for tumor cells (80). At
the same time, YAP/TAZ also inhibit tumor-suppressor genes
including DNA-damage-inducible transcript 4 (DDIT4) and TNF-related
apoptosis-inducing ligand (TRAIL) that are inhibitors of mTORC1
(81). Herein, we directly address
that YAP/TAZ can function as oncogenes by repressing
antiproliferative and cell-death genes (Fig. 5A).
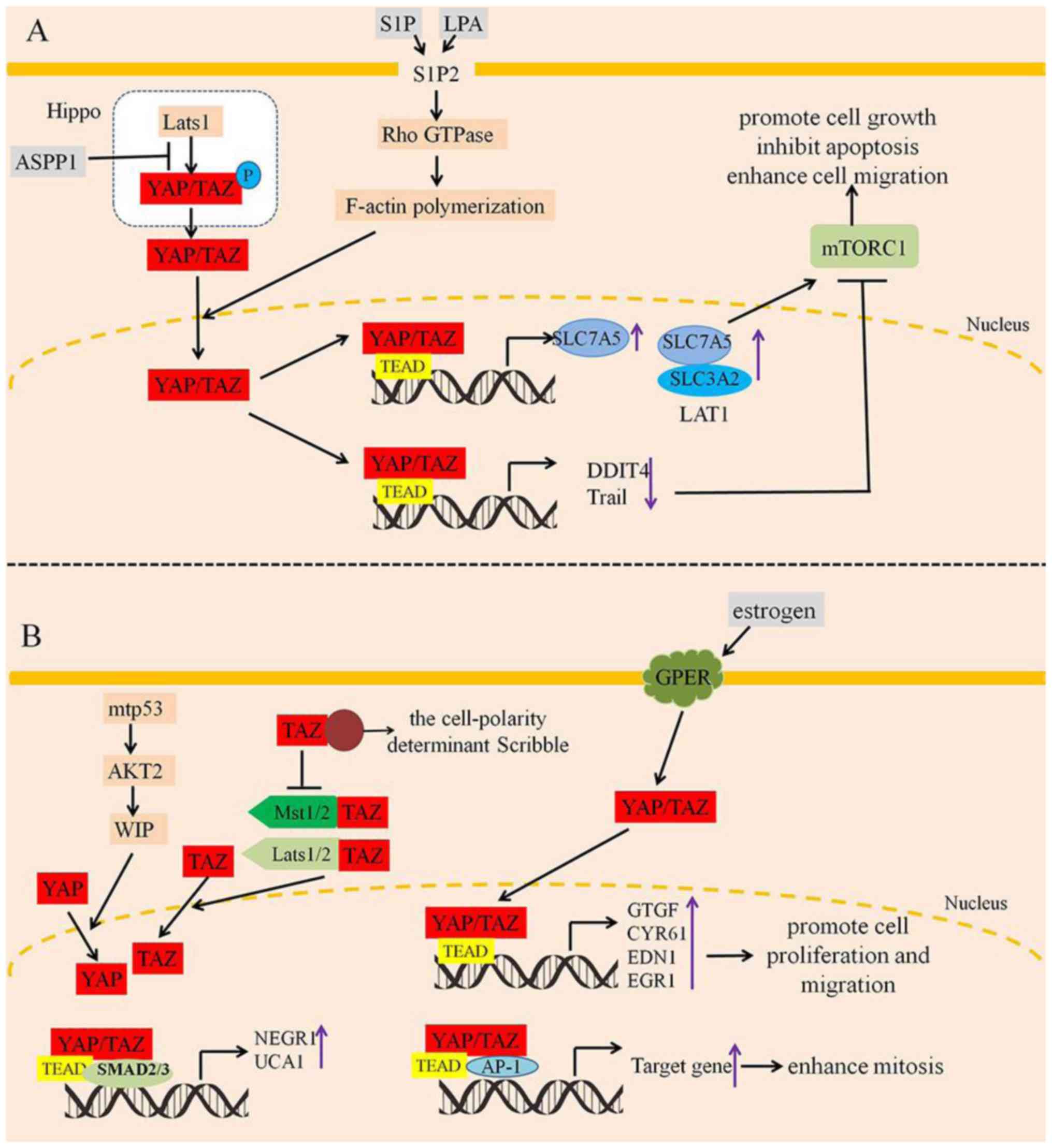 | Figure 5.Nuclear YAP/TAZ promote tumor cell
proliferation, inhibit apoptosis and enhance cell migration. (A)
S1P and LPA promote YAP/TAZ nuclear translocation by S1P2/Rho
GTPase/F-actin polymerization. Moreover, ASPP1 inhibits the
interaction between YAP and Lats1, which promotes YAP/TAZ nuclear
translocation. As a result, YAP/TAZ can enhance cell proliferation,
inhibit apoptosis and increase the activation of mTORC1 by
regulating the expression of SLC7A5, DDIT4 and Trail. (B) WIP
induces YAP activation and translocation into the nucleus with
consequent activation of YAP/TAZ oncogenic targets. In the nucleus,
YAP/TAZ both promote the gene expression of NEGR1 and UCA1 that are
necessary for maintaining tumorigenic activity. On the contrary,
the cell-polarity determinant scribble could disrupt the inhibitory
association of TAZ with Mst1/2 and Lats1/2 (left). However,
estrogen stimulation activates YAP/TAZ nuclear translocation, and
then promotes YAP/TAZ-targeted genes, which enhances cell
proliferation, migration, and mitosis (right). S1P,
sphingosine-1-phosphate; LPA, lysophosphatidic acid; ASPP1,
apoptosis-stimulating protein of p53 1. P, phosphorylation; ↓,
activation; ⊥, inhibition. |
In breast cancer, estrogen stimulation activates
YAP/TAZ via the G protein-coupled estrogen receptor (GPER)
(82). Moreover, GPER mediates the
expression of CTGF, CYR61, EDN1 and EGR1 that are well-established
YAP/TAZ target genes (83). In
addition, TEAD factors of YAP/TAZ and activator protein-1 (AP-1)
form a complex that synergistically activates target genes involved
in the control of S phase entry and mitosis (84). Moreover, wild-type p53 (wtp53) is
described as a tumor-suppressor gene, while mutations in this gene
(mtp53) occur in many human cancers and promote oncogenic capacity.
WIP, phosphorylated by AKT2 in downstream of mtp53, induces YAP/TAZ
activation and translocation into the nucleus with consequent
activation of YAP/TAZ oncogenic targets (85). From another perspective, TAZ forms a
complex with the cell-polarity determinant Scribble. This complex
disrupts the inhibitory association of TAZ with the Hippo kinases
Mst1/2 and Lats1/2 (86), which
promote tumorigenesis. In addition, YAP/TAZ also promote and
maintain transforming growth factor-β (TGFβ)-induced tumorigenic
phenotypes in late-stage breast cancer cells. The TEAD
transcription factors of YAP/TAZ interact with TGFβ-induced SMAD2/3
in the nucleus, eventually promoting the gene expression of NEGR1
and UCA1 that are necessary for maintaining tumorigenic activity
(87). Therefore, YAP/TAZ are
involved in the tumor phenotype and convergence of
TAZ/YAP-TEAD-TGFβ signals are critical for late-stage breast cancer
phenotypes (Fig. 5B).
Nuclear YAP/TAZ also function as transcriptional
regulators of the Hippo pathway controlling tumorigenesis and tumor
progression in other cancers including lung cancer, oral squamous
cell carcinoma (OSCC), and glioblastoma (GBM) (37,88,89).
In murine tumor propagating cells (TPCs) marked with Sca1 and CD24,
the knockdown of YAP/TAZ was found to decrease the migration and
metastatic potential of lung cancer cells (89). However, the concrete mechanism of
YAP/TAZ in TPCs is still under investigation. Moreover, nuclear
YAP/TAZ activity drives OSCC cell proliferation, survival and
migration. In detail, the transcriptional signature regulated by
YAP/TAZ significantly correlates with gene expression changes
occurring in human OSCCs including CCNE2, CDK2, CDC6, PCNA, AURKA
and PLK4, which has been identified by The Cancer Genome Atlas
(TCGA) (37). Additionally,
patients with a mesenchymal (MES) gene expression signature exhibit
poor overall survival and treatment resistance in glioblastoma. TAZ
is directly recruited to a majority of MES gene promoters as a
complex with TEAD2, resulting in high-grade tumors with MES
features (88). The usual
perception is that nuclear YAP/TAZ function as regulators of the
Hippo pathway in tumorigenesis; however, when the Hippo pathway is
‘on’, phosphorylated YAP/TAZ in the cytoplasm act as a retardant
for SHP2 nuclear translocator. Namely, only the non-phosphorylated
YAP/TAZ promote nuclear translocalization of SHP2. This effect
stimulates TCF/LEF- and TEAD-regulated genes via parafibromin (a
tumor-suppressor factor) dephosphorylation leading to malignant
neoplasms and developmental disorders (90).
YAP/TAZ in oncotherapy
YAP/TAZ, two transcriptional co-activators of the
Hippo pathway, are receiving increased attention owing to their
fundamental roles in organ growth and tumor cell proliferation
(7). In particular, the
participation of YAP/TAZ in cancer indicates the potential of
YAP/TAZ as targets for designing anticancer drugs. Fortunately,
some inhibitors, directly and indirectly suppressing the activation
of YAP/TAZ, have been demonstrated to significantly contribute to
effective cancer treatment (91–93).
Herein, we highlighted some possible routes for therapeutic
intervention.
Regulation of tumor metabolism by
targeting YAP/TAZ
The Warburg effect, reprogramming the metabolism of
cancer cells towards aerobic glycolysis, supports oncogenic
signaling to promote tumor malignancy. Herein, phosphofructokinase
1 (PFK1), regulating the first committed step of glycolysis, binds
to the YAP/TAZ transcriptional co-factors TEADs and increases their
transcriptional activity (94),
whereas 2-deoxy-D-glucose (2-DG), as an inhibitor of glycolysis,
inhibits YAP/TAZ transcriptional activity and transforming capacity
in multiple ways. On the one hand, 2-DG increases the activity of
Lats1, the kinase that phosphorylates YAP at Ser 127. On the other
hand, 2-DG promotes the interaction between AMP-activated protein
kinase (AMPK) and phosphorylated YAP (95). However, tumor cellular energy stress
induces YAP phosphorylation due to the AMPK-dependent activation of
Lats, and AMPK can also directly phosphorylate YAP at Ser 94, thus
disrupting the YAP-TEAD interaction (96). In other words, energy stress induces
YAP cytoplasmic retention and its phosphorylation at Ser 127,
ultimately inhibiting YAP transcriptional activity. In addition,
AMPK phosphorylates angiomotin-like 1 (AMOTL1) at Ser 793 and
increases AMOTL1 steady-state protein levels, which contributes to
YAP inhibition (97). Therefore,
tumor growth can be suppressed by knockdown of YAP/TAZ or inhibited
through the treatment with metformin or AMP mimic aminoimidazole
carboxamide ribonucleotide (AICAR) that activates AMPK (95). Metformin, as a widely used oral
antidiabetic agent, has been reported to increase AMPK activity and
YAP/TAZ phosphorylation in primary mouse hepatocytes after
treatment with different doses of metformin for 4 or 8 h.
Furthermore, after the treatment of AICAR for 4 h, interaction
disruption between YAP and TEAD has also been found in primary
mouse hepatocytes with induced YAP/TAZ phosphorylation (96). These findings suggest that aerobic
glycolysis endows cancer cells with particular metabolic
properties, while energy stress and inhibition of glucose
metabolism could inhibit YAP/TAZ. Additionally, these results
provide molecular mechanisms for the correlation between cellular
metabolism and tumorigenesis in oncotherapy (Fig. 6A).
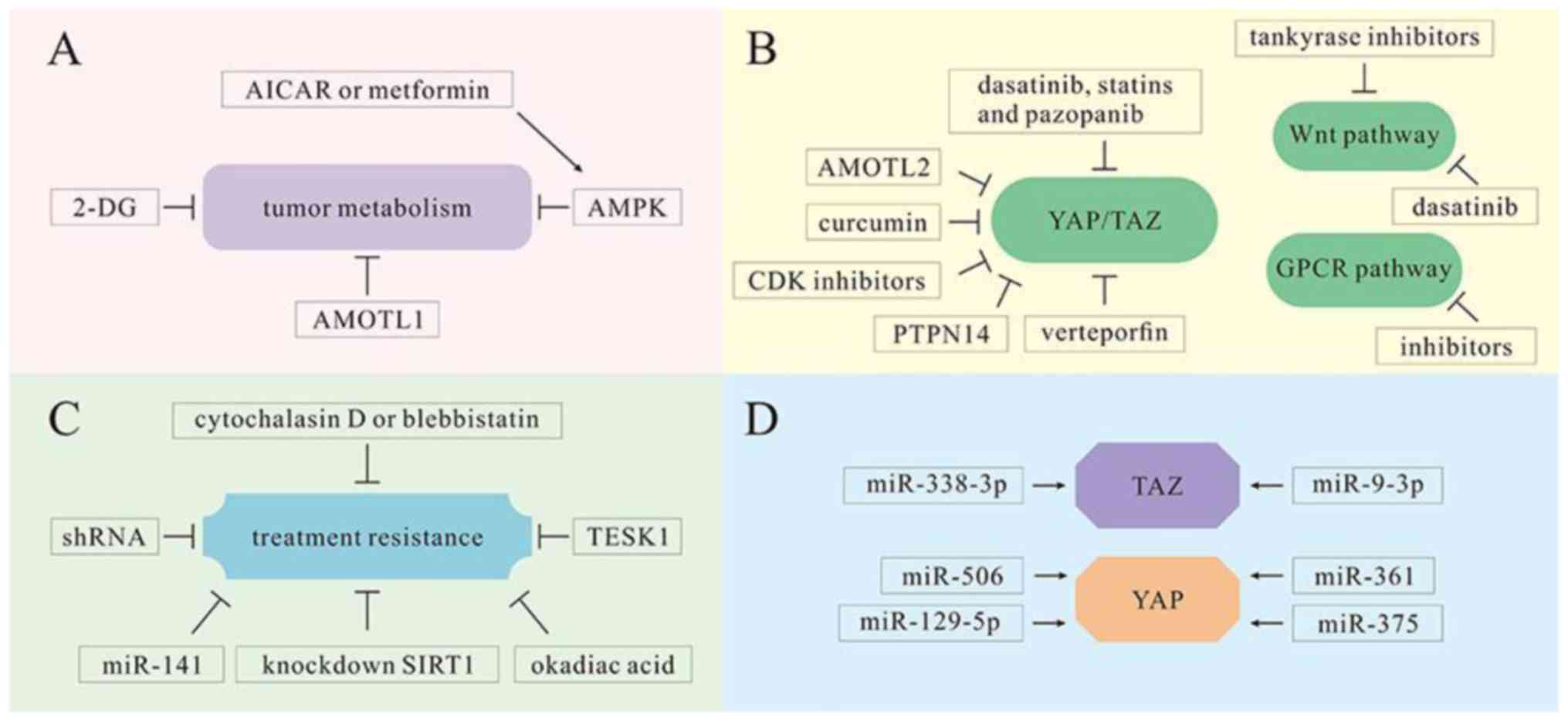 | Figure 6.YAP/TAZ as therapeutic targets in
multiple mechanisms. (A) Tumor cell metabolism is inhibited by
various molecules, including 2-DG, AICAR, metformin and AMOTL1. (B)
Various molecules and medicines, including AMOTL2, CDK inhibitors,
PTPN14, curcumin, dasatinib, statins, pazopanib and verteporfin,
serve as inhibitors of YAP/TAZ in the Hippo pathway and are
conducive to cancer treatment. However, tankyrase inhibitors and
dasatinib can inhibit the proliferation of tumor cells in the Wnt
pathway. There are also inhibitors that can reduce tumor cell
proliferation by inhibiting GPCRs. (C) Various molecules and
technologies, including cytochalasin D, vlebbistatin, okadiac acid,
TESK1, miRNA-141 and shRNAs, can reduce treatment resistance and
promote tumor cell sensitivity to oncotherapy. (D) miRNA-338-3p and
miR-9-3p can target TAZ in HCC treatment. Other miRNAs, including
miR-375, miR-129-5p, miR-506 and miR-361, can target YAP and serve
as tumor suppressors. 2-DG, 2-deoxy-D-glucose; AICAR,
5-aminoimidazole-4-carboxamide ribonucleotide; AMOTL1, angiomotin
like 1; GPCRs, G-protein-coupled receptors; HCC, hepatocellular
carcinoma. ↓, activation; ⊥, inhibition. |
In addition to aerobic glycolysis mentioned above,
the activity of YAP/TAZ was recently found to be regulated by other
metabolic pathways such as mevalonate synthesis, nutrient-sensing
LKB1-AMPK and TSC-mTOR pathways (98). Among them, the mevalonate-YAP/TAZ
axis plays a vital role in proliferation and self-renewal of breast
cancer cells, due to the activation of YAP/TAZ promoted by
increased levels of mevalonic acid (99). Hence, YAP/TAZ regulation is greatly
beyond the regulation by core Hippo kinases, and these new
regulatory mechanisms significantly open unexplored routes for
therapeutic intervention.
Agents of Hippo, Wnt and GPCR
signaling pathways via the regulation of YAP/TAZ
YAP and its paralog protein TAZ are downstream
effectors of the Hippo tumor-suppressor pathway and they can be
inhibited through phosphorylation-induced cytoplasmic retention and
degradation (100). Recently, in
polarized Madin-Darby canine kidney (MDCK) cells, some scholars
found that knockdown of the AMOT family protein AMOTL2 decreased
YAP tight junction localization, reduced the accumulation of
nuclear YAP, attenuated YAP phosphorylation, and induced the
expression of YAP target gene (101). It was implied that AMOTL2 has an
inhibitory effect on YAP. Quickly, the conjecture was confirmed in
low metastatic CL1-0 lung cancer cells and in a nude mouse model.
AMOT upregulation was transduced from nuclear translocation to
cytoplasmic sequestration of YAP/TAZ, leading to decreased
expression of Cyr61 (a growth factor) (102). These findings suggest that AMOT is
a crucial tumor suppressor and it has bne potential to be a
prognostic biomarker and therapeutic target for cancer. Moreover,
high mobility group A1 (HMGA1) and its downstream molecule cyclin
E2 (CCNE2) exert their stimulatory effect on cell migration by
regulating YAP cellular localization and activity, which is called
the HMGA1/CCNE2/YAP axis. However, CDK inhibitors induce the
translocation of YAP from the nucleus to the cytoplasm, which can
interdict the axis (103).
The Hippo pathway regulates cellular proliferation
and tumorigenesis, which is also controlled by non-receptor
tyrosine phosphatase 14 (PTPN14). It was demonstrated that the PPXY
domain of PTPN14 binds to WW domain of Kibra, independently and
cooperatively promoting the activation and stability of the Lats1
protein (104). Similarly, PPXY
motifs of PTPN14 and WW domains of YAP can also form a protein
complex, confirming the function of PTPN14 as a negative regulator
of YAP. Meanwhile, PTPN14 inhibits YAP-mediated transcriptional
activities and sensitizes cancer cells to various anticancer agents
such as cisplatin, erlotinib and S12 (105). In addition, in bladder cancer,
Kruppel-like factor 5 (KLF5) serves as a modulator to promote cell
cycle progression by inducing cyclin D1 expression. Notably,
curcumin was found to downregulate the expression of YAP/TAZ and to
protect KLF5 protein from proteasome degradation both in human
bladder cancer 5637 cells and in xenografted nude mouse models
(106). On the basis of these
studies, some drug molecules have been verified to play a role in
inhibiting YAP in cancer treatment. In uveal melanoma (UM), the
activity of cancer-associated Gq/11 mutants was found to be
mediated by YAP. Inversely, the YAP inhibitor verteporfin blocked
the growth of UM cells containing Gq/11 mutations (28). Moreover, the results have been also
validated by animal experiments. Experiments using breast cancer
cell lines (MDA-MB-231, MDA-MB-453, HBC-4, HBC-5, MCF-7, BSY-1,
ZR-75-1 and SKBR-3) indicates that dasatinib, statins and pazopanib
can inhibit the nuclear localization and target gene expression of
YAP/TAZ by promoting phosphorylation and proteasomal degradation of
YAP/TAZ (107).
As discussed above, cytoplasmic YAP/TAZ, as
downstream elements of the Wnt/β-catenin cascade, are involved in
the degradation and sequestration of β-catenin in the Wnt pathway
(66). YAP/TAZ activation by the
Wnt pathway can be prevented by the reactivation of β-catenin
destruction complex with the treatment of tankyrase inhibitors
(43). Furthermore, it has been
revealed that β-catenin-active cancers are dependent on a signaling
pathway involving YAP1, one of YAP isoforms. The tyrosine kinase
YES1 phosphorylates YAP1. Consequently, YAP1 and the transcription
factor TBX5 form a complex with β-catenin. In addition, this
complex is essential to the transformation and survival of tumor
cells. Moreover, the phosphorylation of YAP1 promotes the
localization of this complex and the expression of antiapoptotic
genes including BCL2L1 and BIRC5. Importantly, the YES1 inhibitor
dasatinib can successfully inhibit the proliferation of
β-catenin-active tumor cells (108).
Furthermore, GPCR is able to upregulate YAP/TAZ
activity depending on the specific subset of GPCRs including
Gα12/13-, Gα11- and Gαi/o-coupled receptors (28). GPCRs are also prominent
pharmacological targets, suggesting that their selective inhibition
may blunt YAP/TAZ activity. However, the 99 mechanisms of targeting
YAP/TAZ in these two pathways warrant further research (Fig. 6B).
Agents for treatment resistance
through the downregulation of YAP/TAZ
Actin remodeling is the result of oncogenic actin
signaling pathway activation or inactivation of several important
actin-binding proteins that have tumor-suppressor functions
(109). Although the influence of
actin remodeling on cancer drug resistance remains unclear, there
is research verifying that YAP/TAZ become activated in response to
changes in the cytoskeleton. The activation of YAP/TAZ stimulates
the actin remodeling of the extracellular matrix (110). BRAF V600E mutant melanoma cells,
resistant to the BRAF inhibitor PLX4032 (vemurafenib), exhibit an
increase in actin stress fiber formation, which promotes the
nuclear accumulation of YAP/TAZ. Actin dynamics regulator TESK1,
identified by siRNA library screening, serves as a potential target
of PLX4032-resistance in melanoma cells via promoting YAP/TAZ
localization (111). Similarly,
cytochalasin D or blebbistatin inhibit actomyosin and actin
polymerization, respectively, causing YAP/TAZ localization in the
cytoplasm and a decrease in the viability of vemurafenib-resistant
cells (112). Although
post-translational modification, particularly Mst/Lats-mediated
phosphorylation at serine 127, has been confirmed to regulate the
activity of YAP, its dephosphorylation and acetylation are still
unclear. However, researchers have demonstrated that via cisplatin
treatment, the catalytic subunit of protein phosphatase-1 (PP1A)
interacts with YAP2 and dephosphorylates YAP2, which consequently
induces the nuclear accumulation and transcriptional activation of
YAP2; whereas, the inhibition of PP1 by okadiac acid (OA) increased
the phosphorylation of YAP2 and sensitized ovarian cancer cells to
cisplatin treatment both in vivo and in vitro
(113). However, YAP2 is
deacetylated by Sirtuin 1 (SIRT1), and the deacetylation of YAP2
upregulates YAP2/TEAD binding, leading to YAP2 transcriptional
activation and cell growth in hepatocellular carcinoma (HCC) cells;
whereas, the knockdown of SIRT1 was found to block the
cisplatin-induced nuclear translocation of YAP2 and enhance the
chemosensitivity of HCC cells to cisplatin treatment (114). Similarly, it was revealed that
miRNA-141 reduced the cisplatin resistance in esophageal squamous
cell carcinoma (ESCC) cell lines (KYSE series) via directly
targeting the 3′-untranslated region of YAP1 and downregulating
YAP1 expression, which has a crucial role in apoptosis induced by
DNA-damaging agents (115).
Taxol (paclitaxel) resistance represents a major
challenge in breast cancer treatment, but a recent study reports
its close relation to TAZ overexpression (85). Short hairpin RNA (shRNA)-mediated
knockdown of both Cyr61 and CTGF (downstream transcriptional
targets of TAZ) was found to reverse TAZ-induced Taxol resistance
in breast cancer cells (85).
Likewise, TAZ was found to function as an oncogene in non-small
cell lung cancer (NSCLC), and it was knocked down by shRNA in NSCLC
cell lines, suppressing tumor cell proliferation and
anchorage-independent growth in vitro (116) (Fig.
6C).
MicroRNA (miRNA-associated treatment
by modulating YAP/TAZ (Table
II)
miRNAs, non-coding small RNAs (117–121), are implicated in cell development,
cell proliferation, differentiation and apoptosis of cancers
(121–128). Recent studies have identified that
the hyperexpression of miRNAs are essential for abnormal
proliferation and survival of cancer cells though their effect on
the Hippo pathway (129). This
gives us a hint that miRNAs may provide novel potential for cancer
treatment.
In HCC, YAP is involved in HBx-mediated
hepatocarcinogenesis. And TAZ is upregulated by preS2. HBx and
preS2, transactivators encoded by HBV, can promote YAP/TAZ at the
protein level but not at the mRNA level. Surprisingly, miRNA-338-3p
downregulates TAZ and interdicts preS2, while miRNA-338-3p
inhibitor restores the expression of TAZ, suggesting that
miRNA-338-3p directly targets TAZ (130). In addition, in experiments using
HCC cell lines (HepG2, HuH1, HuH7, HLE, HLF, PLC and SKHep1) and
HCC clinical samples (frozen HCC tissue), miR-9-3p, as a
tumor-suppressor miRNA, was found to target TAZ and reduce TAZ
expression. Treatment with miR-9-3p mimic inhibited the cell
proliferative ability and downregulated the phosphorylation of
Erk1/2, AKT and β-catenin (cancer promotion factors) (131). Notably, YAP, as a potent oncogenic
trigger and independent prognostic risk factor of HCC, was found to
be targeted by miR-375. miR-375 was found to be able to inhibit the
proliferation and invasion of HCC cells (PLC/PRF/5 and MHCC-97L),
and it plays a potential therapeutic role in HCC treatment
(132). Coincidentally, miRNAs are
also identified to participate in the treatment of ovarian, breast
and lung cancer. In experiments using ovarian cancer cell lines
(OV56, OVCAR4, TOV-21G, COV362, TOV-112D, SKOV3, COV644, CaOV3,
A2780, OV90, COV434 and COV504), miR-129-5p directly repressed YAP
and TAZ expression. This repression led to inactivation of TEA
domain (TEAD) transcription and the downregulation of Hippo
downstream genes including connective tissue growth factor (CTGF)
and cyclin A. Thus, miR-129-5p contributes to the subsequent
inhibition of cell proliferation, survival and tumorigenicity in
ovarian cancer cells (133), but
it is not known whether YAP or TAZ is targeted by this miRNA.
Recently, the negative correlation of miR-506 with YAP was
demonstrated in clinical human breast cancer tissues. A study using
MDA-MB-231 breast cancer cell line, MCF-7 breast cancer cell line
and MCF-10A cell line demonstrated that ectopic miR-506 expression
significantly suppressed cell proliferation and affected the cell
cycle via targeting YAP (134).
Similarly, both in human lung cancer cell line A549 and lung cancer
tissues, miR-361 was found to be a tumor suppressor in the same way
(135). On the other hand, there
is increased evidence supporting the function of miRNAs as a target
of YAP/TAZ in NSCLC cell lines. In detail, YAP/TAZ inducd
transcription of the MCM7 gene and its hosted microRNAs including
miR-25, miR-93 and miR-106b, thereby promoting cell proliferation
in human H1299 and H1975 cells (136). In summary, these studies provide
evidence for a novel modulation mechanism of inhibiting the
pro-tumorigenic functions of YAP/TAZ through miRNAs. It goes
without saying that, miRNAs function as a novel mechanism for
YAP/TAZ regulation. However, their potential in the clinical
intervention of human cancers warrants further research (Fig. 6D).
Conclusion
It is well-known that the Hippo pathway, an
evolutionarily conserved signaling pathway, is involved in
regulating organ size through controlling the balance of cell
proliferation and apoptosis. YAP/TAZ, two key downstream terminal
effectors in this pathway, are negatively regulated by its
phosphorylation. Accompanied by deactivated Hippo pathway, YAP/TAZ
are translocated into the nucleus and promote the transcription of
downstream genes through their binding with TEAD. As a result, they
facilitate cell proliferation and the amplification of stem cells.
Meanwhile, cell apoptosis is also inhibited for the promotion of
organ size. However, co-overexpression of YAP/TAZ and deactivation
of the Hippo pathway are significantly observed in many malignant
cancers, including liver and breast cancer. Moreover, YAP/TAZ also
function as modulators of the Wnt pathway and participate in GPCR
signaling, playing a vital role in tumorigenesis and tumor
progression.
Due to the pivotal effects of YAP/TAZ on organ
growth and tumor cell proliferation, it cannot be ignored that
targeting YAP/TAZ may be a potential therapeutic strategy for many
human cancers. Based on this, direct or indirect inhibition of
YAP/TAZ may effectively restrain tumor cell growth, suggesting a
novel method for oncotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Sichuan
Health and Family Planning Commission Funding (no. 16ZD0253), the
Sichuan National Science Research Funding (no. 2015JY0183), funding
from the Sichuan Provincial People's Hospital, and a Sichuan
Scientific Research Grant for Returned Overseas Chinese Scholars
for YW. This study was also funded by the Science and Technology
Department of Sichuan Province, China (nos. 16FZ0126, 2011FZ0068
and 14010159) for RT. The study was also supported by the National
Key Specialty Construction Project of Clinical Pharmacy (no.
30305030698).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RT and YW designed the study. SD and HL wrote the
manuscript. TL prepared the figures. HW and XH reviewed and edited
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This review does not contain any studies with human
participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interests.
References
|
1
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The Hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai J, Zhang N, Zheng Y, de Wilde RF,
Maitra A and Pan D: The Hippo signaling pathway restricts the
oncogenic potential of an intestinal regeneration program. Genes
Dev. 24:2383–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong W and Guan KL: The YAP and TAZ
transcription co-activators: Key downstream effectors of the
mammalian Hippo pathway. Semin Cell Dev Biol. 23:785–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barry ER, Morikawa T, Butler BL, Shrestha
K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et
al: Restriction of intestinal stem cell expansion and the
regenerative response by YAP. Nature. 493:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma Y, Yang Y, Wang F, Wei Q and Qin H:
Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int
J Cancer. 137:2275–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
11
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang
A, Wen W and Zhu Q: Overexpression of YAP and TAZ is an independent
predictor of prognosis in colorectal cancer and related to the
proliferation and metastasis of colon cancer cells. PLoS One.
8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halder G and Johnson RL: Hippo signaling:
Growth control and beyond. Development. 138:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moroishi T, Park HW, Qin B, Chen Q, Meng
Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al: A
YAP/TAZ-induced feedback mechanism regulates Hippo pathway
homeostasis. Genes Dev. 29:1271–1284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Chen Z, Wang Y, Chang D, Su L, Guo
Y and Liu C: TR1 promotes cell proliferation and inhibits apoptosis
through cyclin A and CTGF regulation in non-small cell lung cancer.
Tumour Biol. 35:463–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishioka N, Inoue K, Adachi K, Kiyonari H,
Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N,
et al: The Hippo signaling pathway components Lats and Yap pattern
Tead4 activity to distinguish mouse trophectoderm from inner cell
mass. Dev Cell. 16:398–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xin M, Kim Y, Sutherland LB, Murakami M,
Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et
al: Hippo pathway effector Yap promotes cardiac regeneration. Proc
Natl Acad Sci USA. 110:13839–13844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaidi SK, Sullivan AJ, Medina R, Ito Y,
van Wijnen AJ, Stein JL, Lian JB and Stein GS: Tyrosine
phosphorylation controls Runx2-mediated subnuclear targeting of YAP
to repress transcription. EMBO J. 23:790–799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Z, Hu T, Xu Z, Lin Z, Zhang Z, Feng
T, Zhu L, Rong Y, Shen H, Luk JM, et al: Targeting Hippo pathway by
specific interruption of YAP-TEAD interaction using cyclic YAP-like
peptides. FASEB J. 29:724–732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varelas X: The Hippo pathway effectors TAZ
and YAP in development, homeostasis and disease. Development.
141:1614–1626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang
LJ, Li M, Ying X and Zhu Q: Expression and clinical significance of
YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res.
2014:2613652014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sudol M, Bork P, Einbond A, Kastury K,
Druck T, Negrini M, Huebner K and Lehman D: Characterization of the
mammalian YAP (Yes-associated protein) gene and its role in
defining a novel protein module, the WW domain. J Biol Chem.
270:14733–14741. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morin-Kensicki EM, Boone BN, Howell M,
Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W and Milgram
SL: Defects in yolk sac vasculogenesis, chorioallantoic fusion, and
embryonic axis elongation in mice with targeted disruption of
Yap65. Mol Cell Biol. 26:77–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muramatsu T, Imoto I, Matsui T, Kozaki K,
Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T and Inazawa J: YAP
is a candidate oncogene for esophageal squamous cell carcinoma.
Carcinogenesis. 32:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaffney CJ, Oka T, Mazack V, Hilman D, Gat
U, Muramatsu T, Inazawa J, Golden A, Carey DJ, Farooq A, et al:
Identification, basic characterization and evolutionary analysis of
differentially spliced mRNA isoforms of human YAP1 gene. Gene.
509:215–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng
Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al: Mutant Gq/11 promote
uveal melanoma tumorigenesis by activating YAP. Cancer Cell.
25:822–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sudol M: Yes-associated protein (YAP65) is
a proline-rich phosphoprotein that binds to the SH3 domain of the
Yes proto-oncogene product. Oncogene. 9:2145–2152. 1994.PubMed/NCBI
|
|
31
|
Kanai F, Marignani PA, Sarbassova D, Yagi
R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley
LC, et al: TAZ: A novel transcriptional co-activator regulated by
interactions with 14-3-3 and PDZ domain proteins. EMBO J.
19:6778–6791. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li PD, Wang XJ, Shan Q, Wu YH and Wang Z:
Evaluation of TAZ expression and its effect on tumor invasion and
metastasis in human glioma. Asian Pac J Trop Med. 7:757–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagaraj R, Gururaja-Rao S, Jones KT,
Slattery M, Negre N, Braas D, Christofk H, White KP, Mann R and
Banerjee U: Control of mitochondrial structure and function by the
Yorkie/YAP oncogenic pathway. Genes Dev. 26:2027–2037. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo L and Teng L: YAP/TAZ for cancer
therapy: Opportunities and challenges (Review). Int J Oncol.
46:1444–1452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oka T and Sudol M: Nuclear localization
and pro-apoptotic signaling of YAP2 require intact PDZ-binding
motif. Genes Cells. 14:607–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sawada A, Kiyonari H, Ukita K, Nishioka N,
Imuta Y and Sasaki H: Redundant roles of Tead1 and Tead2 in
notochord development and the regulation of cell proliferation and
survival. Mol Cell Biol. 28:3177–3189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hiemer SE, Zhang L, Kartha VK, Packer TS,
Almershed M, Noonan V, Kukuruzinska M, Bais MV, Monti S and Varelas
X: A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral
Squamous Cell Carcinoma. Mol Cancer Res. 13:957–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chan SW, Lim CJ, Chong YF, Pobbati AV,
Huang C and Hong W: Hippo pathway-independent restriction of TAZ
and YAP by angiomotin. J Biol Chem. 286:7018–7026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu C, Huang W and Lei Q: Regulation and
function of the TAZ transcription co-activator. Int J Biochem Mol
Biol. 2:247–256. 2011.PubMed/NCBI
|
|
40
|
Yue G, Sun X, Gimenez-Capitan A, Shen J,
Yu L, Teixido C, Guan W, Rosell R, Liu B and Wei J: TAZ is highly
expressed in gastric signet ring cell carcinoma. BioMed Res Int.
2014:3930642014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aqeilan RI, Donati V, Palamarchuk A,
Trapasso F, Kaou M, Pekarsky Y, Sudol M and Croce CM: WW
domain-containing proteins, WWOX and YAP, compete for interaction
with ErbB-4 and modulate its transcriptional function. Cancer Res.
65:6764–6772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Donzelli S, Strano S and Blandino G: YAP
and p73: A matter of mutual specificity in tumor suppressionThe
Hippo Signaling Pathway and Cancer. Springer; New York: pp.
147–172. 2013, View Article : Google Scholar
|
|
43
|
Azzolin L, Zanconato F, Bresolin S,
Forcato M, Basso G, Bicciato S, Cordenonsi M and Piccolo S: Role of
TAZ as mediator of Wnt signaling. Cell. 151:1443–1456. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Low BC, Pan CQ, Shivashankar GV,
Bershadsky A, Sudol M and Sheetz M: YAP/TAZ as mechanosensors and
mechanotransducers in regulating organ size and tumor growth. FEBS
Lett. 588:2663–2670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Acerbi I, Cassereau L, Dean I, Shi Q, Au
A, Park C, Chen YY, Liphardt J, Hwang ES and Weaver VM: Human
breast cancer invasion and aggression correlates with ECM
stiffening and immune cell infiltration. Integr Biol. 7:1120–1134.
2015. View Article : Google Scholar
|
|
46
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G, et al: Mechanotransduction and YAP-dependent matrix
remodelling is required for the generation and maintenance of
cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Halder G, Dupont S and Piccolo S:
Transduction of mechanical and cytoskeletal cues by YAP and TAZ.
Nat Rev Mol Cell Biol. 13:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aragona M, Panciera T, Manfrin A, Giulitti
S, Michielin F, Elvassore N, Dupont S and Piccolo S: A mechanical
checkpoint controls multicellular growth through YAP/TAZ regulation
by actin-processing factors. Cell. 154:1047–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gibson WT and Gibson MC: Cell topology,
geometry, and morphogenesis in proliferating epithelia. Curr Top
Dev Biol. 89:87–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vogel V and Sheetz M: Local force and
geometry sensing regulate cell functions. Nat Rev Mol Cell Biol.
7:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Furukawa KT, Yamashita K, Sakurai N and
Ohno S: The epithelial circumferential actin belt regulates YAP/TAZ
through nucleocytoplasmic shuttling of merlin. Cell Reports.
20:1435–1447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Matsui Y and Lai ZC: Mutual regulation
between Hippo signaling and actin cytoskeleton. Protein Cell.
4:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao B, Li L, Wang L, Wang CY, Yu J and
Guan KL: Cell detachment activates the Hippo pathway via
cytoskeleton reorganization to induce anoikis. Genes Dev. 26:54–68.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wada K, Itoga K, Okano T, Yonemura S and
Sasaki H: Hippo pathway regulation by cell morphology and stress
fibers. Development. 138:3907–3914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sansores-Garcia L, Bossuyt W, Wada K,
Yonemura S, Tao C, Sasaki H and Halder G: Modulating F-actin
organization induces organ growth by affecting the Hippo pathway.
EMBO J. 30:2325–2335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Talele NP, Fradette J, Davies JE, Kapus A
and Hinz B: Expression of α-smooth muscle actin determines the fate
of mesenchymal stromal cells. Stem Cell Reports. 4:1016–1030. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miyabara S, Yuda Y, Kasashima Y, Kuwano A
and Arai K: Regulation of Tenomodulin Expression Via Wnt/β-catenin
Signaling in Equine Bone Marrow-derived Mesenchymal Stem Cells. J
Equine Sci. 25:7–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
59
|
Yu FX, Zhao B and Guan KL: Hippo pathway
in organ size control, tissue homeostasis, and cancer. Cell.
163:811–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Konsavage WM Jr and Yochum GS:
Intersection of Hippo/YAP and Wnt/β-catenin signaling pathways.
Acta Biochim Biophys Sin (Shanghai). 45:71–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Konsavage WM Jr, Kyler SL, Rennoll SA, Jin
G and Yochum GS: Wnt/β-catenin signaling regulates Yes-associated
protein (YAP) gene expression in colorectal carcinoma cells. J Biol
Chem. 287:11730–11739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang W, Lv X, Liu C, Zha Z, Zhang H,
Jiang Y, Xiong Y, Lei QY and Guan KL: The N-terminal phosphodegron
targets TAZ/WWTR1 protein for SCFβ-TrCP-dependent degradation in
response to phosphatidylinositol 3-kinase inhibition. J Biol Chem.
287:26245–26253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nishita M, Endo M and Minami Y: Regulation
of cellular responses by non-canonical Wnt signaling. Clin Calcium.
23:809–815. 2013.(In Japanese). PubMed/NCBI
|
|
64
|
Korswagen HC: Canonical and non-canonical
Wnt signaling pathways in Caenorhabditis elegans: Variations on a
common signaling theme. BioEssays. 24:801–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Park HW, Kim YC, Yu B, Moroishi T, Mo JS,
Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al: Alternative
Wnt Signaling Activates YAP/TAZ. Cell. 162:780–794. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Azzolin L, Panciera T, Soligo S, Enzo E,
Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V,
et al: YAP/TAZ incorporation in the β-catenin destruction complex
orchestrates the Wnt response. Cell. 158:157–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Varelas X, Miller BW, Sopko R, Song S,
Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill
H, et al: The Hippo pathway regulates Wnt/beta-catenin signaling.
Dev Cell. 18:579–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Imajo M, Miyatake K, Iimura A, Miyamoto A
and Nishida E: A molecular mechanism that links Hippo signalling to
the inhibition of Wnt/β-catenin signalling. EMBO J. 31:1109–1122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lappano R and Maggiolini M: G
protein-coupled receptors: Novel targets for drug discovery in
cancer. Nat Rev Drug Discov. 10:47–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Prickett TD, Wei X, Cardenas-Navia I, Teer
JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A,
et al: Exon capture analysis of G protein-coupled receptors
identifies activating mutations in GRM3 in melanoma. Nat Genet.
43:1119–1126. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Paschke R and Ludgate M: The thyrotropin
receptor in thyroid diseases. N Engl J Med. 337:1675–1681. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hernández NA, Correa E, Avila EP, Vela TA
and Pérez VM: PAR1 is selectively over expressed in high grade
breast cancer patients: A cohort study. J Transl Med. 7:472009.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Guo X and Zhao B: Integration of
mechanical and chemical signals by YAP and TAZ transcription
coactivators. Cell Biosci. 3:332013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Miller E, Yang J, DeRan M, Wu C, Su AI,
Bonamy GMC, Liu J, Peters EC and Wu X: Identification of
serum-derived sphingosine-1-phosphate as a small molecule regulator
of YAP. Chem Biol. 19:955–962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yu FX, Zhao B, Panupinthu N, Jewell JL,
Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al:
Regulation of the Hippo-YAP pathway by G-protein-coupled receptor
signaling. Cell. 150:780–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Waldron RT, Innamorati G, Torres-Marquez
ME, Sinnett-Smith J, Rozengurt E and RT W: Differential
PKC-dependent and -independent PKD activation by G protein α
subunits of the Gq family: Selective stimulation of PKD
Ser748 autophosphorylation by Gαq. Cell Signal.
24:914–921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang J, Sinnett-Smith J, Stevens JV, Young
SH and Rozengurt E: Biphasic regulation of Yes-associated protein
(YAP) cellular localization, phosphorylation, and activity by G
protein-coupled receptor agonists in intestinal epithelial cells: A
novel role for protein kinase D (PKD). J Biol Chem.
291:17988–18005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hao F, Xu Q, Zhao Y, Stevens JV, Young SH,
Sinnett-Smith J and Rozengurt E: Insulin receptor and GPCR
crosstalk stimulates YAP via PI3K and PKD in pancreatic cancer
cells. Mol Cancer Res. 15:929–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vigneron AM, Ludwig RL and Vousden KH:
Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP.
Genes Dev. 24:2430–2439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hansen CG, Ng YL, Lam WL, Plouffe SW and
Guan KL: The Hippo pathway effectors YAP and TAZ promote cell
growth by modulating amino acid signaling to mTORC1. Cell Res.
25:1299–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kim M, Kim T, Johnson RL and Lim DS:
Transcriptional co-repressor function of the hippo pathway
transducers YAP and TAZ. Cell Reports. 11:270–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv
XB, Li F, Yu FX, Sun Y, Yuan H, et al: Estrogen regulates Hippo
signaling via GPER in breast cancer. J Clin Invest. 125:2123–2135.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zanconato F, Forcato M, Battilana G,
Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M
and Piccolo S: Genome-wide association between YAP/TAZ/TEAD and
AP-1 at enhancers drives oncogenic growth. Nat Cell Biol.
17:1218–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Escoll M, Gargini R, Cuadrado A, Anton IM
and Wandosell F: Mutant p53 oncogenic functions in cancer stem
cells are regulated by WIP through YAP/TAZ. Oncogene. 36:3515–3527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hiemer SE, Szymaniak AD and Varelas X: The
transcriptional regulators TAZ and YAP direct transforming growth
factor β-induced tumorigenic phenotypes in breast cancer cells. J
Biol Chem. 289:13461–13474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Bhat KP, Salazar KL, Balasubramaniyan V,
Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL,
Kim SH, et al: The transcriptional coactivator TAZ regulates
mesenchymal differentiation in malignant glioma. Genes Dev.
25:2594–2609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lau AN, Curtis SJ, Fillmore CM, Rowbotham
SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW,
Walton ZE, et al: Tumor-propagating cells and Yap/Taz activity
contribute to lung tumor progression and metastasis. EMBO J.
33:468–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tsutsumi R, Masoudi M, Takahashi A, Fujii
Y, Hayashi T, Kikuchi I, Satou Y, Taira M and Hatakeyama M: YAP and
TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2
function. Dev Cell. 26:658–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bae JS, Kim SM and Lee H: The Hippo
signaling pathway provides novel anti-cancer drug targets.
Oncotarget. 8:16084–16098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Tao Y, Cai F, Shan L, Jiang H, Ma L and Yu
Y: The Hippo signaling pathway: An emerging anti-cancer drug
target. Discov Med. 24:7–18. 2017.PubMed/NCBI
|
|
93
|
Zanconato F, Battilana G, Cordenonsi M and
Piccolo S: YAP/TAZ as therapeutic targets in cancer. Curr Opin
Pharmacol. 29:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Enzo E, Santinon G, Pocaterra A, Aragona
M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo
G, et al: Aerobic glycolysis tunes YAP/TAZ transcriptional
activity. EMBO J. 34:1349–1370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wong W: Keeping cells from getting
Hippo-sized under energy stress. Sci Signal. 8:ec1082015.
View Article : Google Scholar
|
|
96
|
Mo JS, Meng Z, Kim YC, Park HW, Hansen CG,
Kim S, Lim DS and Guan KL: Cellular energy stress induces
AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell
Biol. 17:500–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
DeRan M, Yang J, Shen CH, Peters EC,
Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al:
Energy stress regulates hippo-YAP signaling involving AMPK-mediated
regulation of angiomotin-like 1 protein. Cell Reports. 9:495–503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Santinon G, Pocaterra A and Dupont S:
Control of YAP/TAZ activity by metabolic and nutrient-sensing
pathways. Trends Cell Biol. 26:289–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Sorrentino G, Ruggeri N, Specchia V,
Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio
R, Piazza S, et al: Metabolic control of YAP and TAZ by the
mevalonate pathway. Nat Cell Biol. 16:357–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nallet-Staub F, Marsaud V, Li L, Gilbert
C, Dodier S, Bataille V, Sudol M, Herlyn M and Mauviel A:
Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in
cutaneous melanoma. J Invest Dermatol. 134:123–132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q
and Guan KL: Angiomotin is a novel Hippo pathway component that
inhibits YAP oncoprotein. Genes Dev. 25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hsu YL, Hung JY, Chou SH, Huang MS, Tsai
MJ, Lin YS, Chiang SY, Ho YW, Wu CY and Kuo PL: Angiomotin
decreases lung cancer progression by sequestering oncogenic YAP/TAZ
and decreasing Cyr61 expression. Oncogene. 34:4056–4068. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Pegoraro S, Ros G, Ciani Y, Sgarra R,
Piazza S and Manfioletti G: A novel HMGA1-CCNE2-YAP axis regulates
breast cancer aggressiveness. Oncotarget. 6:19087–19101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wilson KE, Li YW, Yang N, Shen H, Orillion
AR and Zhang J: PTPN14 forms a complex with Kibra and LATS1
proteins and negatively regulates the YAP oncogenic function. J
Biol Chem. 289:23693–23700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Huang JM, Nagatomo I, Suzuki E, Mizuno T,
Kumagai T, Berezov A, Zhang H, Karlan B, Greene MI and Wang Q: YAP
modifies cancer cell sensitivity to EGFR and survivin inhibitors
and is negatively regulated by the non-receptor type protein
tyrosine phosphatase 14. Oncogene. 32:2220–2229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D, et al: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Oku Y, Nishiya N, Shito T, Yamamoto R,
Yamamoto Y, Oyama C and Uehara Y: Small molecules inhibiting the
nuclear localization of YAP/TAZ for chemotherapeutics and
chemosensitizers against breast cancers. FEBS Open Bio. 5:542–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Rosenbluh J, Nijhawan D, Cox AG, Li X,
Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et
al: β-Catenin-driven cancers require a YAP1 transcriptional complex
for survival and tumorigenesis. Cell. 151:1457–1473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rao J and Li N: Microfilament actin
remodeling as a potential target for cancer drug development. Curr
Cancer Drug Targets. 4:345–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Morikawa Y, Zhang M, Heallen T, Leach J,
Tao G, Xiao Y, Bai Y, Li W, Willerson JT and Martin JF: Actin
cytoskeletal remodeling with protrusion formation is essential for
heart regeneration in Hippo-deficient mice. Sci Signal. 8:ra412015.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kim MH and Kim J, Hong H, Lee SH, Lee JK,
Jung E and Kim J: Actin remodeling confers BRAF inhibitor
resistance to melanoma cells through YAP/TAZ activation. EMBO J.
35:462–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ferrarelli LK: Actin against BRAF
inhibitors. Sci Signal. 9:ec512016. View Article : Google Scholar
|
|
113
|
Wang P, Bai Y, Song B, Wang Y, Liu D, Lai
Y, Bi X and Yuan Z: PP1A-mediated dephosphorylation positively
regulates YAP2 activity. PLoS One. 6:e242882011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Imanaka Y, Tsuchiya S, Sato F, Shimada Y,
Shimizu K and Tsujimoto G: MicroRNA-141 confers resistance to
cisplatin-induced apoptosis by targeting YAP1 in human esophageal
squamous cell carcinoma. J Hum Genet. 56:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS
and Yang X: TAZ is a novel oncogene in non-small cell lung cancer.
Oncogene. 30:2181–2186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Anastasiadou E, Jacob LS and Slack FJ:
Non-coding RNA networks in cancer. Nat Rev Cancer. 18:5–18. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Sassen S, Miska EA and Caldas C: MicroRNA:
Implications for cancer. Virchows Arch. 452:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kim HS, Lee KS, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Yang HD, Park M, Park WS, et al: MicroRNA-31
functions as a tumor suppressor by regulating cell cycle and
epithelial-mesenchymal transition regulatory proteins in liver
cancer. Oncotarget. 6:8089–8102. 2015.PubMed/NCBI
|
|
124
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Nishikawa R, Goto Y, Kojima S, Enokida H,
Chiyomaru T, Kinoshita T, Sakamoto S, Fuse M, Nakagawa M, Naya Y,
et al: Tumor-suppressive microRNA-29s inhibit cancer cell migration
and invasion via targeting LAMC1 in prostate cancer. Int J Oncol.
45:401–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Nishikawa R, Goto Y, Sakamoto S, Chiyomaru
T, Enokida H, Kojima S, Kinoshita T, Yamamoto N, Nakagawa M, Naya
Y, et al: Tumor-suppressive microRNA-218 inhibits cancer cell
migration and invasion via targeting of LASP1 in prostate cancer.
Cancer Sci. 105:802–811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cirilo PDR, de Sousa Andrade LN, Corrêa
BRS, Qiao M, Furuya TK, Chammas R and Penalva LOF: MicroRNA-195
acts as an anti-proliferative miRNA in human melanoma cells by
targeting Prohibitin 1. BMC Cancer. 17:7502017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Halytskiy V: Shifts in miRNA expression
pattern can lead to the loss of contact inhibition in breast cancer
cells. Eur J Cancer. 57 Suppl 2:S1162016.
|
|
130
|
Liu P, Zhang H, Liang X, Ma H, Luan F,
Wang B, Bai F, Gao L and Ma C: HBV preS2 promotes the expression of
TAZ via miRNA-338-3p to enhance the tumorigenesis of hepatocellular
carcinoma. Oncotarget. 6:29048–29059. 2015.PubMed/NCBI
|
|
131
|
Higashi T, Hayashi H, Ishimoto T, Takeyama
H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, et al:
miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in
hepatocellular carcinoma cells. Br J Cancer. 113:252–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liu AM, Poon RT and Luk JM: MicroRNA-375
targets Hippo-signaling effector YAP in liver cancer and inhibits
tumor properties. Biochem Biophys Res Commun. 394:623–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Tan G, Cao X, Dai Q, Zhang B, Huang J,
Xiong S, Zhang Y, Chen W, Yang J and Li H: A novel role for
microRNA-129-5p in inhibiting ovarian cancer cell proliferation and
survival via direct suppression of transcriptional co-activators
YAP and TAZ. Oncotarget. 6:8676–8686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hua K, Yang W, Song H, Song J, Wei C, Li D
and Fang L: Up-regulation of miR-506 inhibits cell growth and
disrupt the cell cycle by targeting YAP in breast cancer cells. Int
J Clin Exp Med. 8:12018–12027. 2015.PubMed/NCBI
|
|
135
|
Zhang S, Liu Z, Wu L and Wang Y: MiR-361
targets Yes-associated protein (YAP) mRNA to suppress cell
proliferation in lung cancer. Biochem Biophys Res Commun.
492:468–473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Lo Sardo F, Forcato M, Sacconi A, Capaci
V, Zanconato F, Di Agostino S, Del Sal G, Pandolfi PP, Strano S,
Bicciato S, et al: MCM7 and its hosted miR-25, 93 and 106b cluster
elicit YAP/TAZ oncogenic activity in lung cancer. Carcinogenesis.
38:64–75. 2017. View Article : Google Scholar : PubMed/NCBI
|