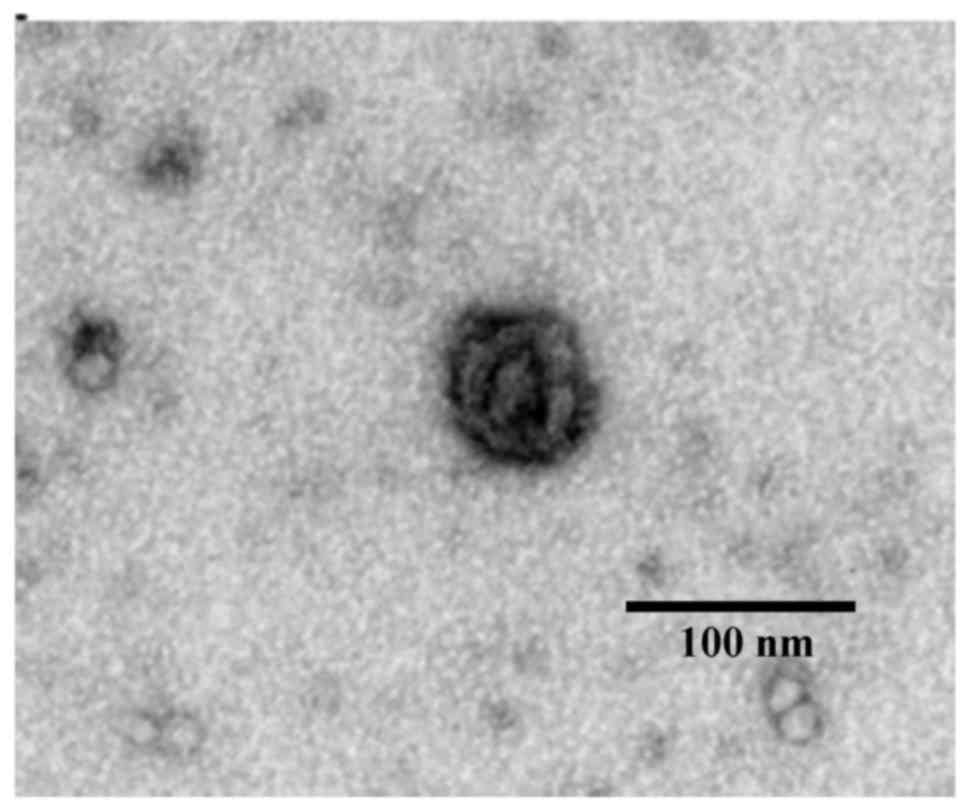
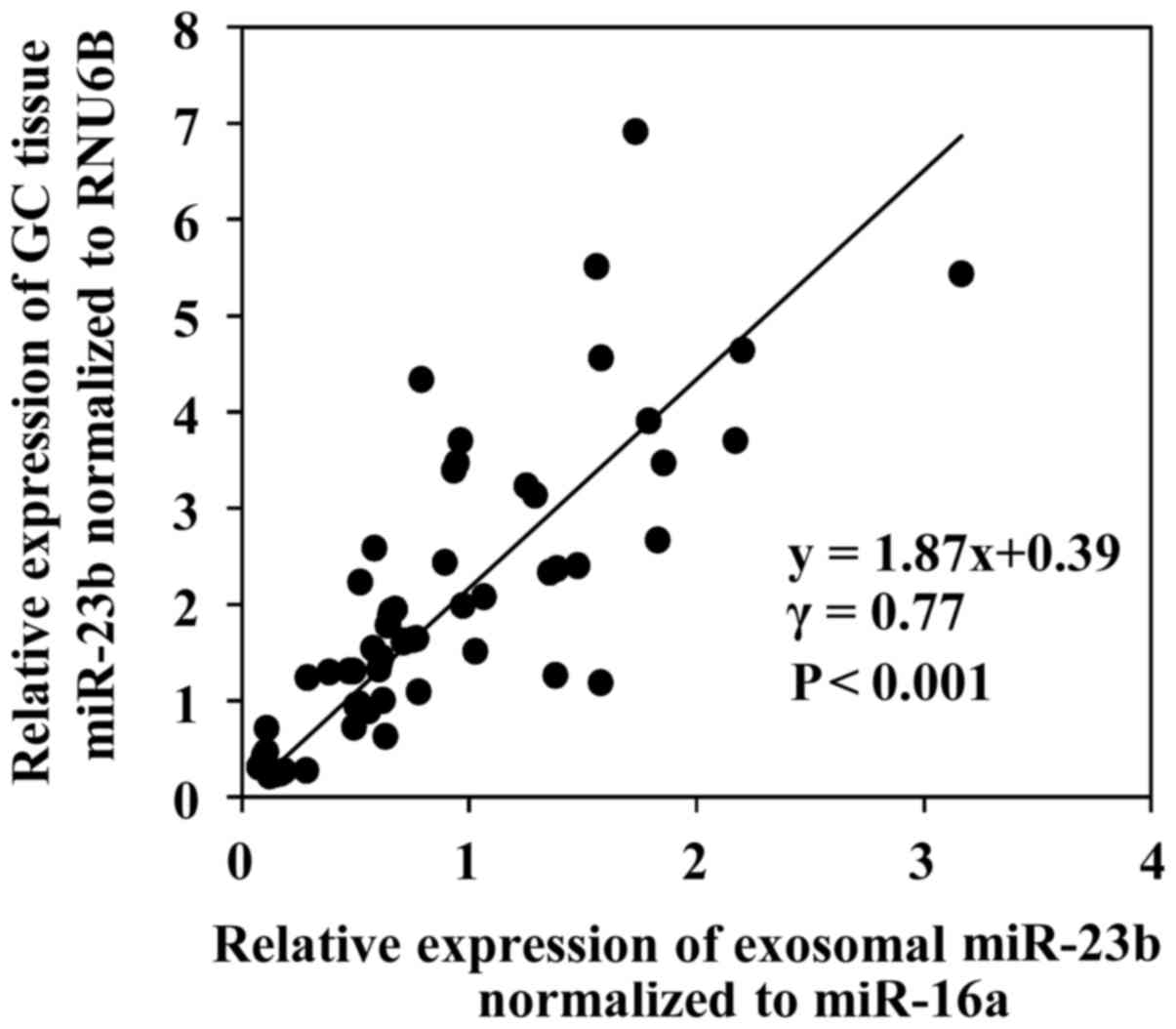
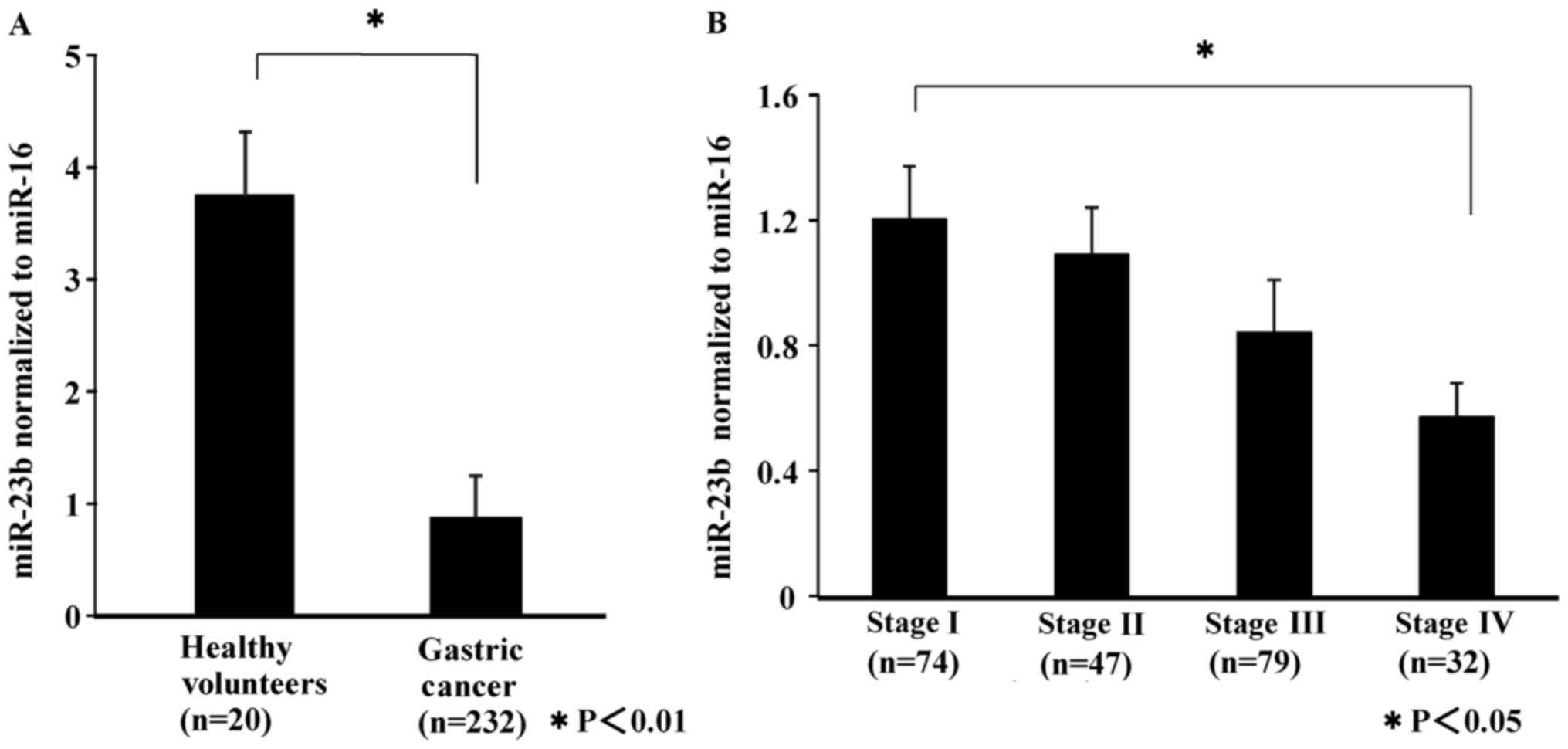
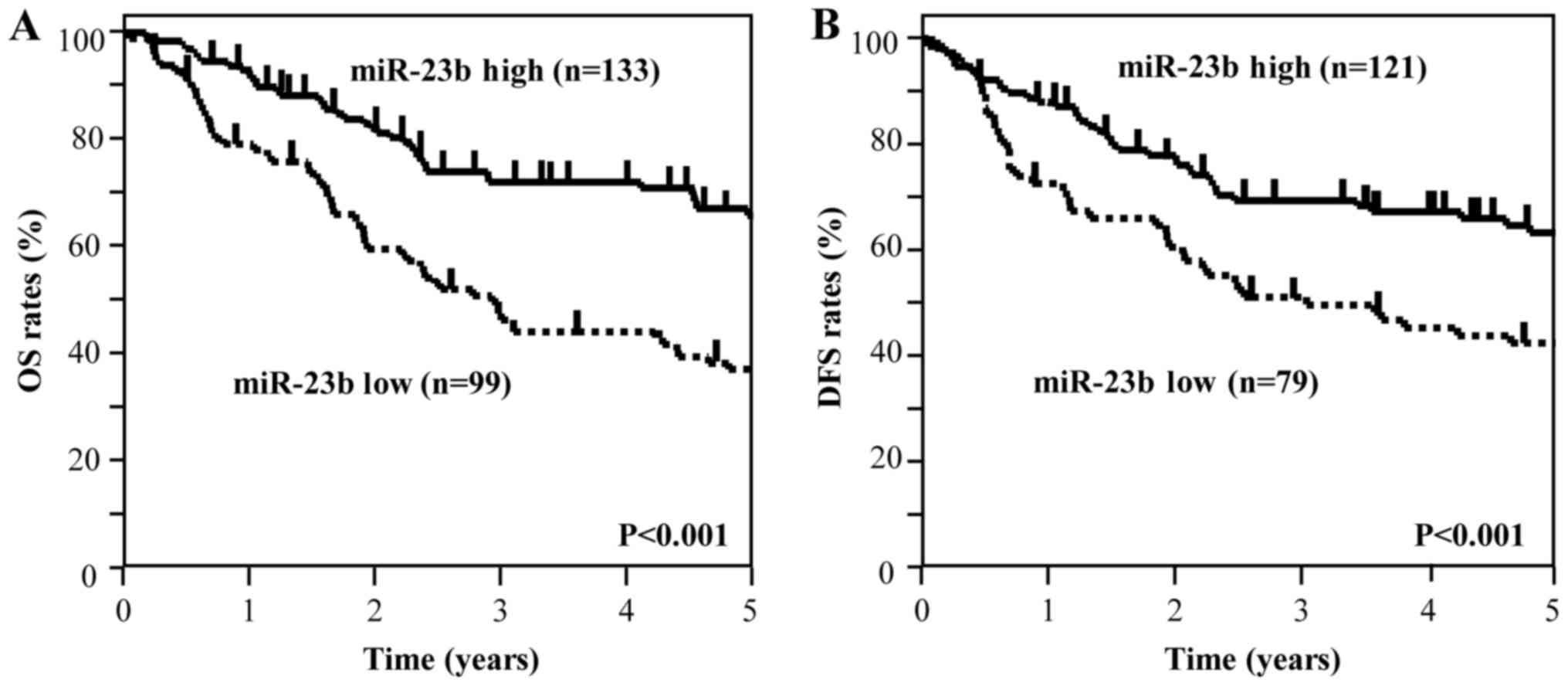
|
1
|
Uemura N, Okamoto S, Yamamoto S, Matsumura
N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N and Schlemper RJ:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grady WM and Tewai M: The next thing in
prognostic molecular markers: microRNA signatures of cancer. Gut.
59:706–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi R, Li Y, Wang FL, Miao G, Qi RM and
Zhao YY: MicroRNAs as diagnostic and prognostic biomarkers in
colorectal cancer. World J Gastrointest Oncol. 8:330–340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai MM, Wand CS, Tsai CY, Huang HW, Chi
HC, Lin YH, Lu PH and Lin KH: Potential diagnostic, prognostic and
therapeutic target microRNAs in human gastric cancer. Int J Mol
Sci. 17:E9452016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Wang L, Wu Z, Sun R, Jin H, Ma J,
Liu L, Ling R, Yi J, Wang L, et al: Three dysregulated microRNAs in
serum as novel biomarkers for gastric cancer screening. Med Oncol.
31:2982014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsujiura M, Komatsu S, Ichikawa D,
Shiozaki A, Konishi H, Takeshita H, Moriumura R, Nagata H,
Kawaguchi T, Hirajima S, et al: Circulating miR-18a in plasma
contributes to cancer detection and monitoring in patients with
gastric cancer. Gastric Cancer. 18:271–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma GJ, Gu RM, Zhu M, Wen X, Li JT, Zhang
YY, Zhang XM and Chen SQ: Plasma post-operative miR-21 expression
in the prognosis of gastric cancers. Asian Pac J Cancer Prev.
14:7551–7554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorur A, Fidanci Balci S, Unal Dogruer N,
Ayaz L, Akbayir S, Yaroglu Yildirim H, Dirlik M, Serin MS and Tamer
L: Determination of plasma microRNA for early detection of gastric
cancer. Mol Biol Rep. 40:2091–2096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kowal J, Tkach M and Théry C: Biogenesis
and secretion of exosome. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge Q, Zhou Y, Lu J, Bai Y, Xie X and Lu Z:
miRNA in plasma exosome is stable under different storage
conditions. Molecules. 19:1567–1575. 2014. View Article : Google Scholar
|
|
11
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion and intercellular interactions of exosomes
and other extracellular vesicles. Ann Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar
|
|
12
|
Kadota T, Yoshioka Y, Fujita Y, Kuwano K
and Ochiya T: Extracellular vesicles in lung cancer-From bench to
bedside. Semin Cell Dev Biol. 67:39–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogata-Kawata H, Izumiya M, Kurioka D,
Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H,
et al: Circulating exosomal microRNAsas biomarkers of colon cancer.
PLoS One. 9:e929212014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hannafon BN and Ding WQ: Intercelluar
communication by exosome-derived microRNAs in cancer. Int J Mol
Sci. 14:14240–14269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tovar-Camargo OA, Toden S and Goel A:
Exosomal microRNA biomarkers: Emerging frontiers in colorectal and
other human cancers. Expert Rev Mol Diagn. 16:553–567. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dejima H, Iinuma H, Kanaoka R, Matsutani N
and Kawamura K: Exosomal microRNA in plasma as a non-invasive
biomarker for the recurrence of non-small cell lung cancer. Oncol
Lett. 13:1256–1263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsukamoto M, Iinuma H, Yagi T, Matsuda K
and Hashiguchi Y: Circulating exosomal MicroRNA-21 as a biomarker
in each tumor stage of colorectal cancer. Oncology. 92:360–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japanese Gastric Cancer Association:
Japanese gastric cancer treatment guidelines 2014 (ver.4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
19
|
Salvi A, Sabelli C, Moncini S, Venturin M,
Arici B, Riva P, Portolani N, Giulini SM, De Petro G and Barlati S:
MicroRNA-23b mediates urokinase and c-met downmodulation and a
decreased migration of human hepatocellular carcinoma cells. FEBS
J. 276:2966–2982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Liu Z, Chen L, Zhou L and Yao Y:
MicroRNA-23b is an independent prognostic marker and suppresses
ovarian cancer progression by targeting runt-related transcription
factor-2. FEBS Lett. 588:1608–1615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loftus JC, Ross JT, Paquette KM, Paulino
VM, Nasser S, Yang Z, Kloss J, Kim S, Berens ME and Tran NJ: miRNA
expression profiling in migration glioblastoma cells: Regulation of
cell migration and invasion by miR-23b via targeting of Pyk2. PLoS
One. 7:e398182012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin
S, Sun C, Ma M, Huang Y and Xi JJ: Genome-wide functional screening
of miR-23b as a pleiotropic modulator suppressing cancer
metastasis. Nat Commun. 2:5542011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pellegrino L, Stebbing J, Braga VM,
Frampton AE, Jacob J, Buluwela L, Jiao LR, Periyasamy M, Madsen CD,
Caley MP, et al: miR-23b regulates cytoskeletal remodeling,
motility and metastasis by directly targeting multiple transcripts.
Nucleic Acids Res. 41:5400–5412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Majid S, Dar AA, Saini S, Arora S,
Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G and
Dahiya R: miR-23b represses proto-oncogene Src kinase and functions
as methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res.
72:6435–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kou CH, Zhou T, Han XL, Zhuang HJ and Qian
HX: Downregulation of miR-23b in plasma is associated with poor
prognosis in patients with colorectal cancer. Oncol Lett.
12:4838–4844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma G, Dai W, Sang A, Yang X and Gao C:
Upregulation of microRNA-23a/b promotes tumor progression and
confers poor prognosis in patients with gastric cancer. Int J Clin
Exp Pathol. 7:8833–8840. 2014.PubMed/NCBI
|
|
28
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Y, Zhang Z, Shang Y, Jiang X, Dong J,
Yu P, Nie Y and Zhao Q: miR-23b-3p regulates the chemoresistance of
gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis.
6:e17662015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|