Introduction
Pancreatic cancer is one of the major human cancers
with extremely poor prognosis compared with other leading cancers
(1). In the USA, the mortality rate
of pancreatic cancer is ranked fourth among all malignancies, and
the overall 5-year survival rate of patients with pancreatic cancer
is less than 5% (2,3). It is estimated that by the year 2030,
pancreatic cancer may become the second leading cause of
cancer-related deaths in the USA (4). The primary reason for this poor
prognosis is a failure to diagnose the disease at an early stage
(5). Furthermore, apart from
surgery, chemotherapy and radiation therapy, there are no effective
therapies for the treatment of pancreatic cancer (6). Hence, novel biomarkers and therapeutic
strategies are urgently required.
Previous studies have suggested that
epithelial-mesenchymal transition (EMT) contributes to early-stage
dissemination of cancer cells and is pivotal for invasion and
metastasis of pancreatic cancer (7). EMT results in the loss of E-cadherin
expression and the acquisition of mesenchymal markers including
fibronectin or vimentin (8). Long
non-coding RNAs (lncRNAs) are RNA molecules (>200 nucleotides in
length) lacking coding capacity (9). Increasing evidence suggests that
lncRNAs play important role in diverse cellular biological
processes. Plasmacytoma variant translocation 1 (PVT1) is an
oncogenic lncRNA (10) and was
initially found in the translocations occurring in variant
Burkitt's lymphoma and murine plasmacytoma (11). PVT1 is a downstream target of the
well-known Myc oncogene (12).
Recent studies have indicated that PVT1 can regulate a series of
human tumors, such as breast and ovarian (13,14),
prostate (15), lung (16) and gastric cancer (17). In ovarian and breast cancer, the
PVT1 gene was revealed to be overexpressed and to contribute
independently to ovarian and breast cancer pathogenesis and inhibit
apoptosis (13). Yang et al
revealed that the expression level of PVT1 was significantly
upregulated in non-small cell lung carcinoma (NSCLC) tissues and
cell lines (16). Using a
genome-wide screening strategy, PVT1 was identified as a regulator
of gemcitabine sensitivity in pancreatic cancer cells, and
upregulation of PVT1 was negatively associated with gemcitabine
sensitivity (18). Finally, PVT1
may serve as an independent prognostic factor for poor overall
survival rate in patients with pancreatic ductal adenocarcinoma
(PDAC) (19). However, the role of
PVT1 in pancreatic cancer cells remains to be clarified.
In the present study, we found that PVT1 had a
significantly higher expression in tumor tissues than that in
adjacent normal tissues, and was critical for cell viability, the
ability of adhesion, migration and invasion in pancreatic cancer
cells. Moreover, it was demonstrated that PVT1 promoted EMT in
pancreatic cancer cells possibly via TGF-β/Smad signaling. All
these findings demonstrated for the first time that PVT1 may be a
facilitator in pancreatic cancer progression.
Materials and methods
Tissue samples
Human tumor tissue samples and adjacent normal
controls were obtained by surgical resection from nine patients
with pancreatic cancer, at the Department of General Surgery,
Changhai Hospital of Shanghai, Second Military Medical University.
All samples were snap-frozen and stored in liquid nitrogen
(−176°C). Signed informed consent was obtained from all patients,
and the study was approved by the Ethics Committee of the
hospital.
Cell cultures
The human pancreatic cancer cell lines (PANC1,
PaTu8988 and SW1990) were kindly provided by the Second Military
Medical University of Shanghai. The cell lines were cultured with
DMEM (Hyclone Laboratories; GE Healthcare Life Sciences, Shanghai,
China) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in a humidified 5%
CO2 incubator at 37°C.
Quantitative RT-PCR
Total RNA was extracted from tissues and cultured
cells with Trizol Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. RNA concentration and
integrity were determined by spectrophotometry. Reverse
transcription was performed using the PrimeScrip™ RT reagent kit
(Takara Bio, Inc., Otsu, Japan). Quantitative RT-PCR was carried
out using the SYBR® Premix Dimmer Eraser kit (Takara
Bio, Inc.). GAPDH was used as an internal control. The primer pair
used for the amplification was as follows: PVT1 forward,
5′-TGAGAACTGTCCTTACGTGACC-3′ and reverse,
5′-AGAGCACCAAGACTGGCTCT-3′; GAPDH forward,
5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTTGGCAGGTT-3′. PCR reactions were performed on the
ABI7300 system (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Sunnyvale, CA, USA). The relative expression fold change of mRNAs
was calculated using the 2−ΔΔCt method.
PVT1-siRNA and plasmid
construction
The specific sequences of small interfering RNAs for
PVT1 were: siPVT1-1 sense, GCUUGGAGGCUGAGGAGUUTT and antisense,
AACUCCUCAGCCUCCAAGCTT; siPVT1-2 sense, CCCAACAGGAGGACAGCUUTT and
antisense, AAGCUGUCCUCCUGUUGGGTT. They were synthesized by GE
Healthcare Dharmacon (Lafayette, CO, USA). A non-specific siRNA
sequence was used as a negative control. For ectopic expression, we
amplified the entire PVT1 sequence with RT-PCR using the following
primers: Forward, TGCTCTAGACTCAAGATGGCTGTGCCTGTCAGCT and reverse,
CCGGAATTCAGTAGAAAAAGAATTTAATAGACACG, and then cloned it into
pCD513B-1 (SBI Pharmaceuticals, Co., Ltd., Tikyo, Japan) at
XbaI and EcoRI sites. All PCR products were confirmed
by DNA sequencing.
Cell transfection
Cells in logarithmic growth phase were diluted and
seeded at 2.5×105 cells/well into 6-well plates. The
siRNAs or plasmid were transfected into pancreatic cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The cells were incubated
in a humidified chamber for 48 h before use in the following
assays.
Cell viability assay
A cell viability assay was performed using a CCK-8
kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
following the manufacturer's protocol. Cells were seeded in 96-well
plates at a density of 5,000 cells/well and cultured with medium
containing 10% FBS for 10, 24, 48 and 72 h. After the cells were
washed twice with cold PBS, 10 µl CCK-8 and 100 µl serum-free
medium were added into each well and the plates were incubated for
a further 45 min at 37°C. The absorbance was assessed at a
wavelength of 450 nm using Wellscan MK-3 ELISA (Labsystems Dragon,
Helsinki, Finland).
Western blotting
Protein was extracted from transfected cells with
RIPA lysis buffer at 4°C for 10 min. Equal amounts of protein were
loaded and separated by 10% SDS-PAGE and transferred to PVDF
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat dried milk in TBST for 1 h at room
temperature and then incubated with primary antibodies at 4°C
overnight. After washing with TBST, the membranes were further
incubated for 1 h at room temperature with corresponding
horseradish peroxidase-conjugated secondary antibody in an
appropriate dilution (goat anti-mouse; 1:5,000; cat. no. A0216; and
goat anti-rabbit; 1:10,000; cat. no. A0208) and then washed three
times with the same buffer. The immunoreactive protein bands were
visualized using an ECL kit (Pierce; Thermo Fisher Scientific,
Inc.). The antibodies used were: rabbit anti-TGF-β (cat. no. 3712;
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
anti-MMP2 (cat. no. YT2798) and rabbit anti-MMP9 (cat. no. YT1892;
both from ImmunoWay Biotechnology Co., Plano, TX, USA), an EMT
antibody sampler kit (cat. no. 9782), a Smad2/3 antibody sampler
kit (cat. no. 12747), mouse anti-β-Tubulin (cat. no. 4466), and
rabbit anti-β-actin (cat. no. 4970; all from Cell Signaling
Technology, Inc.) and all above antibodies diluted with 1:1,000.
β-actin and β-tubulin were used as internal controls. Signals were
visualized using an enhanced chemiluminescence system.
Transwell migration and invasion
assay
Briefly, migration assays were conducted using
8.0-µm culture insert chambers (EMD Millipore). Invasion assays
were performed using the Corning® Matrigel®
Invasion Chamber (Corning Inc., Corning, NY, USA). FBS-containing
medium (10%) was placed in the bottom chamber to act as a
chemoattractant. Then, 5×104 cells in a 100-µl volume of
serum-free medium were placed in the upper chamber. Invasive cells
on the lower surface of the membrane were those that had invaded
the Matrigel or had migrated through the polycarbonate membrane.
After incubation at 37°C for 24 h, the cells were fixed in
paraformaldehyde solution and stained with crystal violet. The top
surface was gently scrubbed with a cotton bud and the remaining
cells at the bottom surface were counted under a microscope
(Olympus GX41; Olympus Corp. Tokyo, Japan) in five randomly
selected fields at a magnification of ×20.
Wound healing assay
Transfected cells were grown to 95% confluence. A
wound was scratched in the monolayer with a 200-µl pipette tip.
Suspended cells and debris were washed with PBS three times, then
the cells were incubated in medium containing 2% FBS at 37°C. At 0
and 24 h the wound image was captured using an OLYMPUS inverted
microscope at a magnification of ×100, respectively, to evaluate
the cell migratory potential.
Adhesion assay
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was diluted in serum-free media to a concentration of 0.04 µg/µl,
and then 50 µl of this Matrigel was transferred into 96-well
plates. Transfected cells (4×104) in a 100-µl volume of
serum-free medium were plated onto the Matrigel-precoated 96-well
plates in triplicate. After incubation at 37°C for 4 h, the medium
was then carefully removed, and the plate was washed with PBS to
remove the non-adherent cells. The Matrigel-precoated plate without
cells served as the negative control. After washing, adherent cells
were assessed with a CCK-8 kit (Dojindo Molecular Technologies,
Inc.) following the manufacturer's protocol. The relative optical
density (OD) value was determined at 490 nm using Wellscan MK-3
ELISA. The OD values reflected the proportion of cells in the
Matrigel-coated 96-well plate.
Statistical analysis
All the presented data and results were confirmed in
at least three independent experiments and were expressed as the
mean ± SD. Statistical comparisons were performed using Student's
t-test and one-way ANOVA. The data was analyzed using GraphPad
Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of PVT1 is profiled in
human pancreatic cancer tissues and cell lines
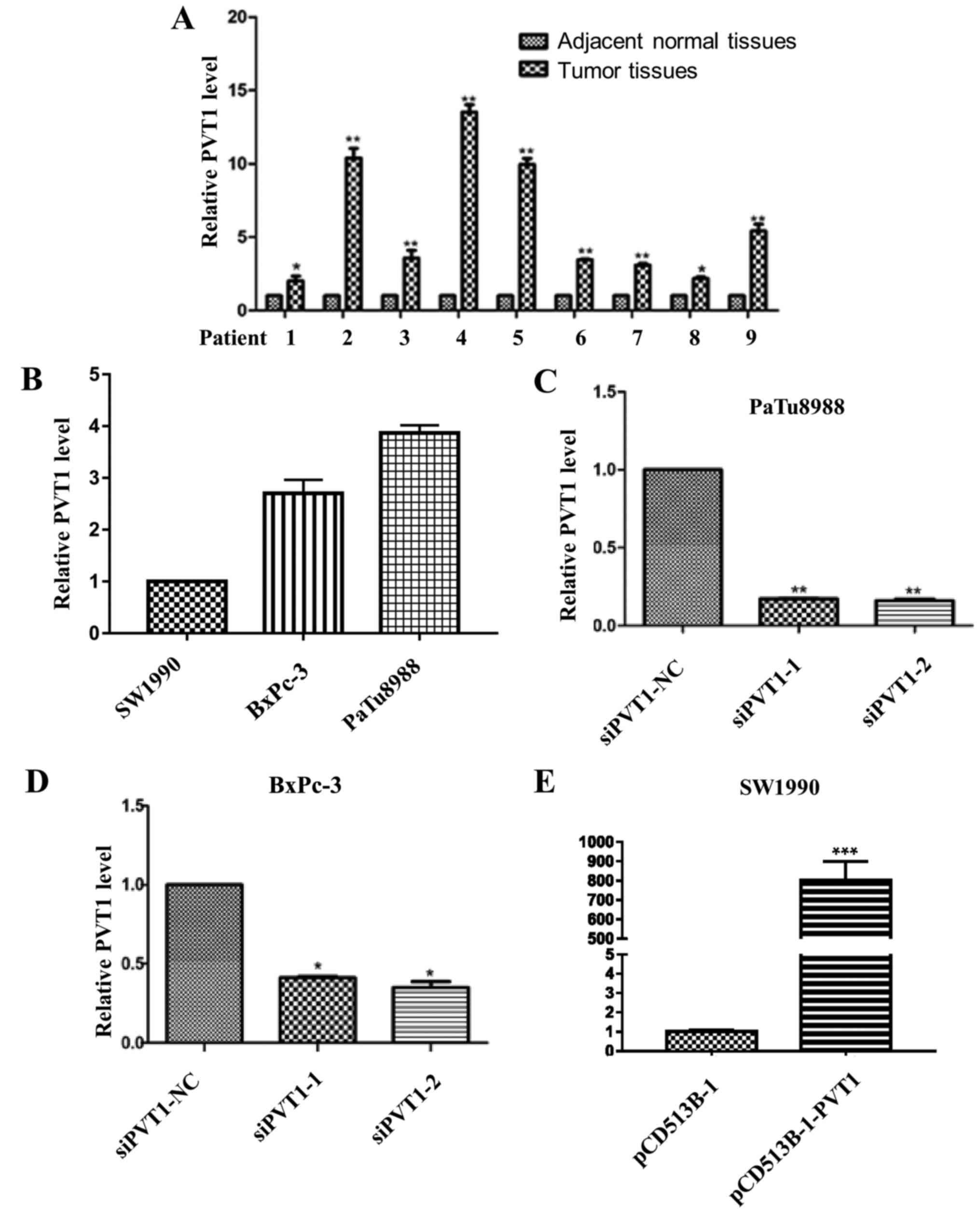
To confirm the role of PVT1 in pancreatic cancer, we
first examined its expression profile in 9 pairs of matched human
pancreatic tumors and adjacent normal tissues by qRT-PCR. The
results indicated that the expression level of PVT1 in tumor
tissues was significantly higher than that in adjacent normal
tissues (Fig. 1A). In addition, it
was revealed that PaTu8988 cells expressed the highest level of
PVT1, whereas SW1990 cells expressed the lowest level of PVT1 among
the 3 cell lines (Fig. 1B). Since
PaTu8988 and BxPc-3 cells expressed a high level of PVT1, these two
cell lines were selected to investigate the effect of specific
depletion of PVT1 (Fig. 1C and D),
and SW1990 was selected to study the effect of overexpression of
PVT1 assays (Fig. 1E).
PVT1 promotes cell viability and the
ability of adhesion in pancreatic cancer cells
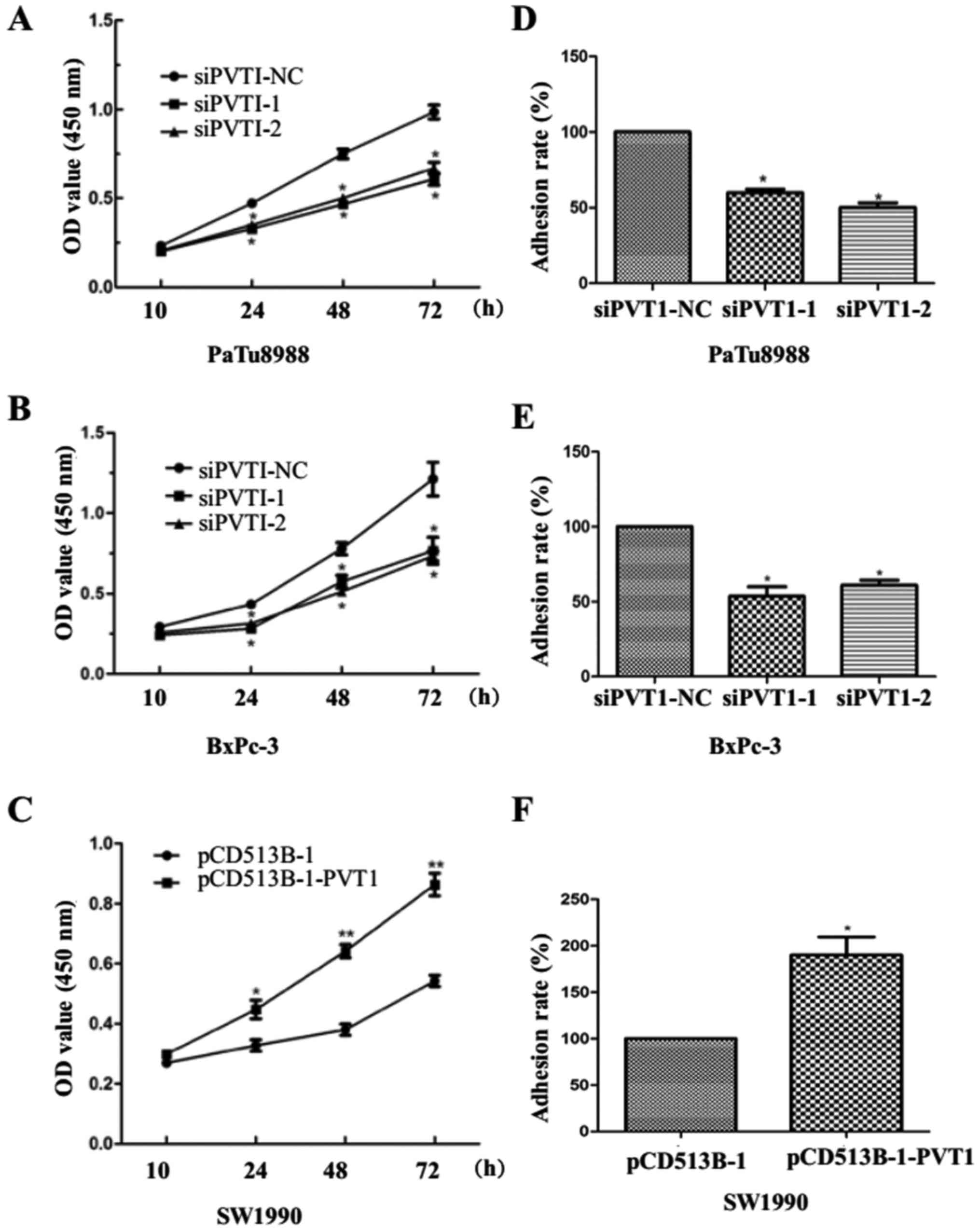
To determine the role of PVT1 in pancreatic cancer
cells, we utilized a CCK-8 assay to estimate cell viability. We
found that PVT1 knockdown induced lower viability of PaTu8988 and
BxPc-3 cells at 10, 24, 48 and 72 h after transfection (Fig. 2A and B). In contrast, PVT1
overexpression enhanced cell viability at 10, 24, 48 and 72 h in
SW1990 cells (Fig. 2C). In
addition, we found that PVT1 knockdown suppressed the ability of
adhesion in PaTu8988 and BxPc-3 cells (Fig. 2D and E) while PVT1 overexpression
enhanced the ability in SW1990 cells (Fig. 2F). Collectively, these results
revealed that PVT1 may promote viability of pancreatic cancer cells
and tumor cell adhererence to the extracellular matrix (ECM).
PVT1 enhances the migration ability of
pancreatic cancer cells
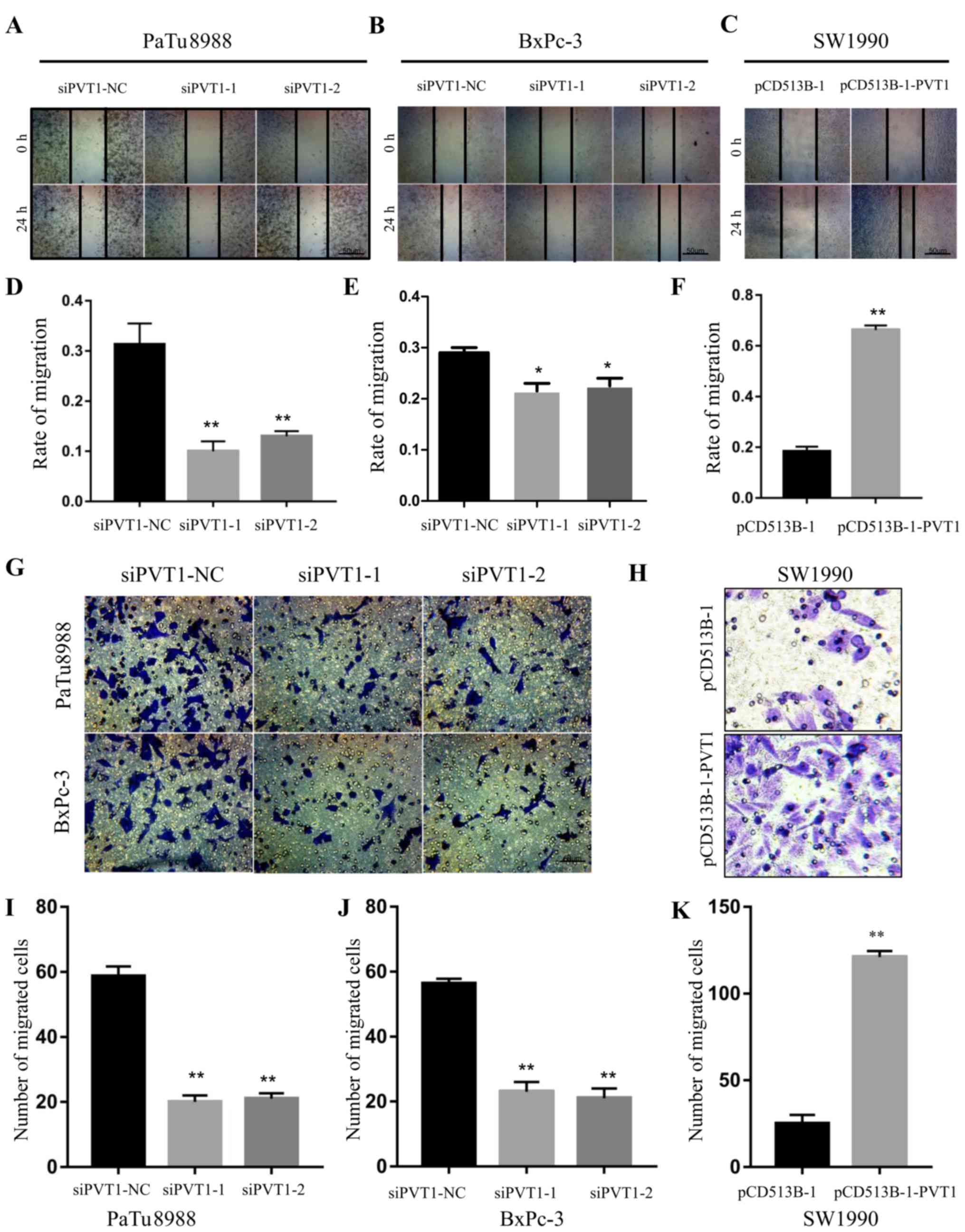
Next, we examined the ability of migration by wound
healing assays in pancreatic cancer cells. The migration rate was
0.292±0.053, 0.125±0.012 and 0.143±0.005 (P<0.01) in siPVT1-NC,
siPVT1-1 and siPVT1-2 PaTu8988 cells (Fig. 3A and D), and 0.291±0.002,
0.224±0.025 and 0.245±0.034 (P<0.05) in siPVT1-NC, siPVT1-1 and
siPVT1-2 BxPc-3 cells, respectively (Fig. 3B and E), while 0.185±0.015 and
0.664±0.011 in pCD513B-1 and pCD513B-1-PVT1 (P<0.01) SW1990
cells (Fig. 3C and F). These
results indicated that knockdown of PVT1 significantly decreased
the migration ability of PaTu8988 and BxPc-3 cells. To confirm the
aforementioned results, we conducted Transwell assays to examine
migration ability. The number of migrated cells was 58±12, 16±10
and 18±9 (P<0.01) in siPVT1-NC, siPVT1-1 and siPVT1-2 PaTu8988
cells, and 57±6, 20±8 and 18±9 (P<0.01) in siPVT1-NC, siPVT1-1
and siPVT1-2 BxPc-3 cells, respectively (Fig. 3G, I and J). Furthermore, the number
of migrated cells was 20±10 and 127±12 (P<0.01) in pCD513B-1 and
pCD513B-1-PVT1 SW1990 cells (Fig. 3H
and K). All these data revealed that PVT1 promotes the ability
of migration in pancreatic cancer cells.
PVT1 promotes the invasion ability of
pancreatic cancer cells
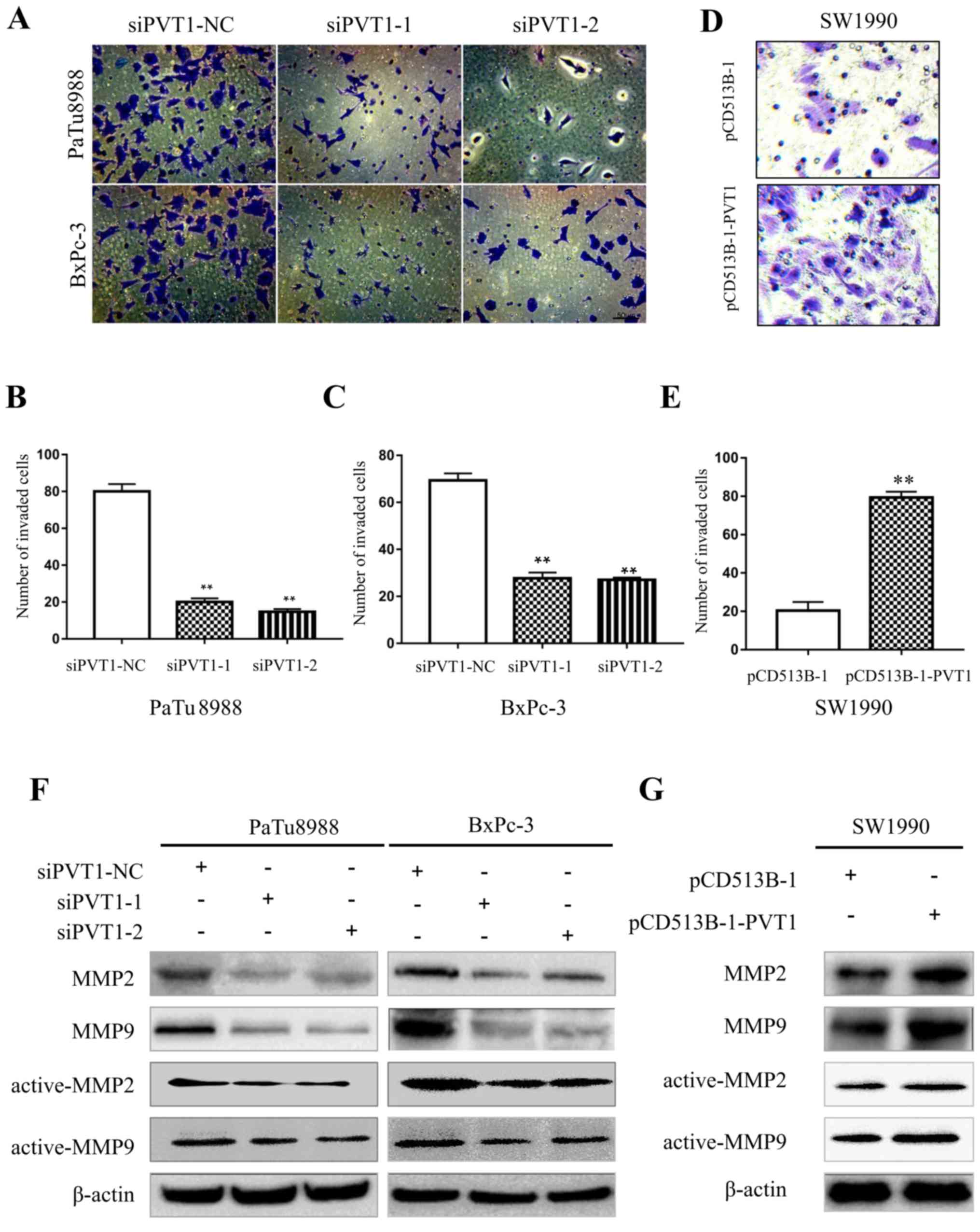
Subsequently, we examined the ability of invasion
using BD Matrigel invasion assays in pancreatic cancer cells.
PaTu8988 and BxPc-3 cells were transfected with siPVT1-NC, siPVT1-1
and siPVT1-2 plasmids, and SW1990 cells with pCD513B-1 and
pCD513B-1-PVT1 for 72 h. The number of invasive cells was 82±5,
24±9 and 20±5 in siPVT1-NC, siPVT1-1 and siPVT1-2 PaTu8988 cells,
and 68±3, 28±7 and 24±11 in siPVT1-NC, siPVT1-1 and siPVT1-2 BxPc-3
cells, respectively (Fig. 4A-C),
demonstrating that downregulation of PVT1 significantly inhibited
the invasion ability of PaTu8988 and BxPc-3 cells. In addition, the
number of invasive cells was 20±5 and 80±8 in pCD513B-1 and
pCD513B-1-PVT1 SW1990 cells, respectively (Fig. 4D and E) thus revealing that
upregulation of PVT1 significantly promoted the invasion ability of
SW1990 cells. Matrix-metalloproteinases (MMPs) have been confirmed
to play pivotal roles in tumor cell invasion through adherence and
degradation of the basement membrane and ECM (20). To determine whether PVT1 can
regulate the activity of MMPs, we investigated the expression level
of MMP2 and MMP9 through western blotting. In the PaTu8988 and
BxPc-3 cells, knockdown of PVT1 suppressed MMP2 and MMP9 (active
and total) protein levels (Fig.
4F). Conversely, in SW1990 cells overexpression of PVT1
increased MMP2 and MMP9 (active and total) protein levels (Fig. 4G). Collectively, these data revealed
that PVT1 promotes the invasion ability of pancreatic cancer
cells.
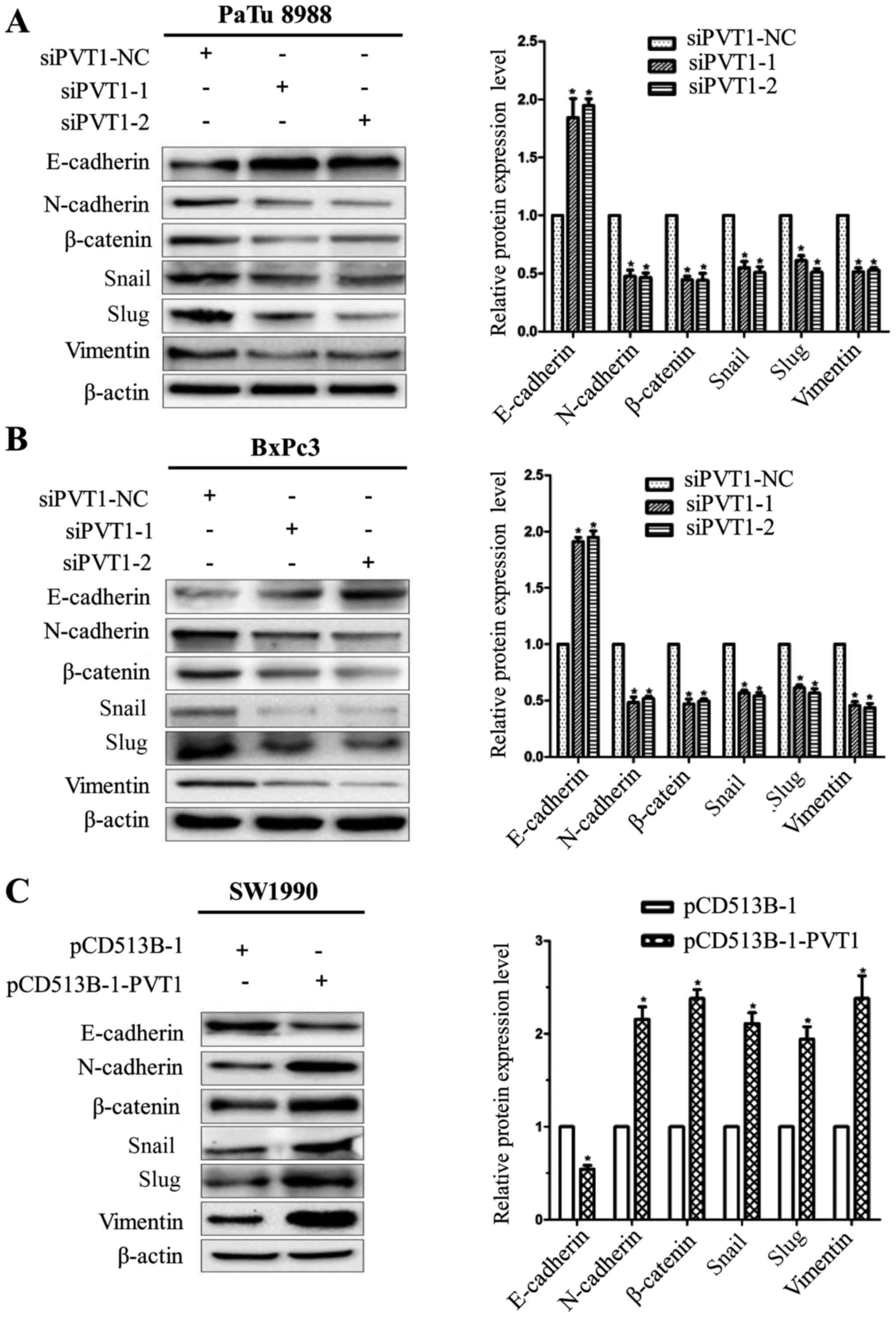
PVT1 enhances epithelial-mesenchymal
transition in pancreatic cancer cells
During tumor development, tumor cells constantly
communicate with the surrounding microenvironment, which can guide
tumor cells to undergo a phenomenon termed EMT. EMT is regarded as
a pivotal step for the promotion of tumor invasion and metastasis
and plays a potential role in the progression of pancreatic cancer
(21). Thus, we assessed whether
PVT1 was involved in EMT to influence cancer metastasis. We
examined the expression of EMT marker genes and EMT-related
transcription factors using western blotting. PVT1-siRNAs increased
E-cadherin expression and decreased vimentin, N-cadherin,
β-catenin, Snail and Slug expression in PaTu8988 and BxPc-3 cells
(Fig. 5A and B). Conversely,
ectopic expression of PVT1 decreased E-cadherin expression and
increased vimentin, N-cadherin, β-catenin, Snail and Slug
expression in SW1990 cells (Fig.
5C). These observations strongly demonstrated that PVT1
promoted a transition from epithelial to mesenchymal phenotype.
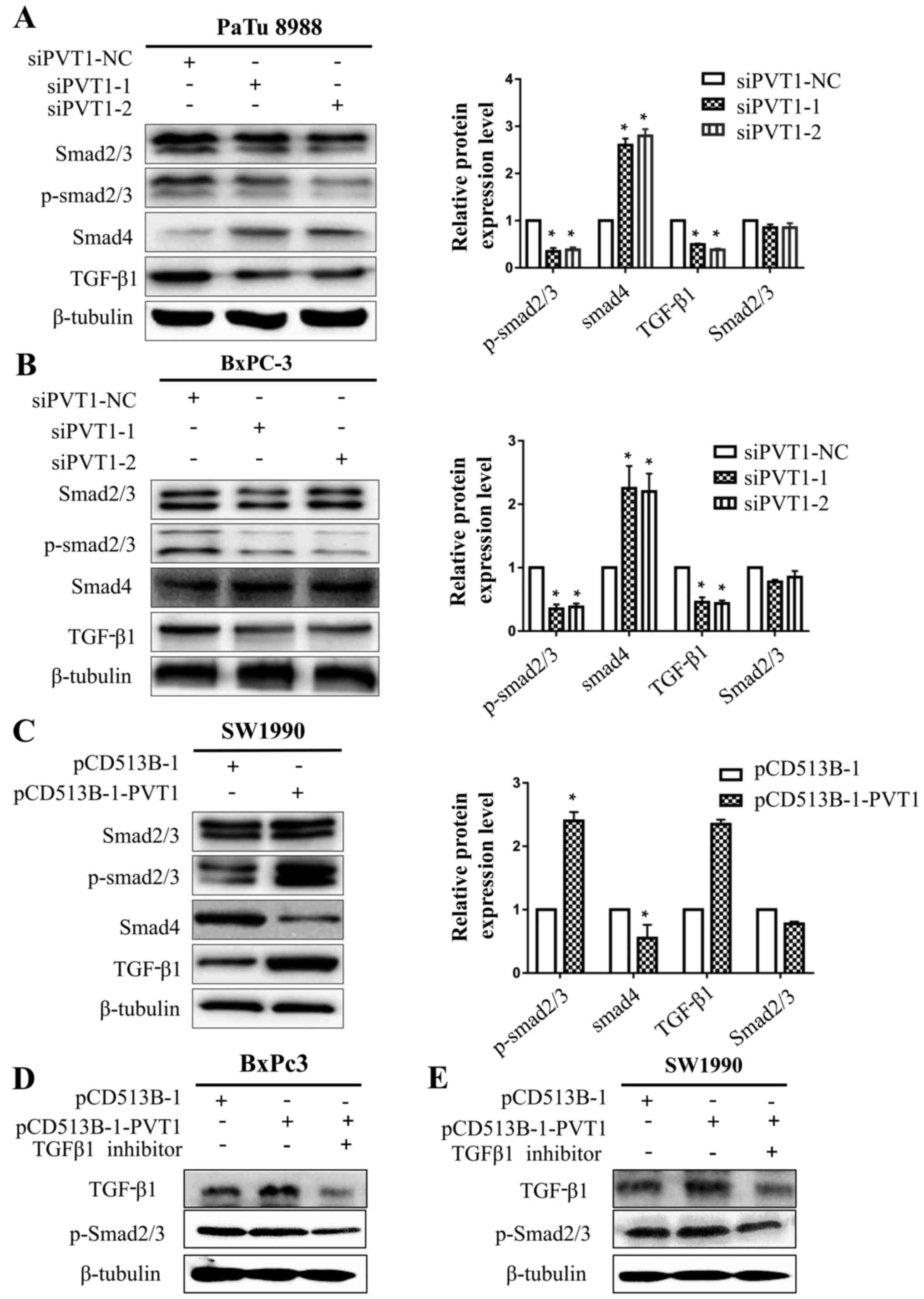
PVT1 activates EMT via TGF-β/Smad
signaling in human pancreatic cancer cells
Multiple studies have suggested that the TGF-β/Smad
signaling pathway is a central regulator in cancer cell viability,
metastasis and the EMT process. Thus, we speculated that PVT1 could
regulate TGF-β/Smad signal transduction in human pancreatic cancer
cells, focusing on p-Smad2/3, Smad4 and TGF-β1 since they are
pivotal signaling molecules in the TGF-β/Smad signaling pathway.
PVT1 knockdown increased Smad4 expression and decreased p-Smad2/3
and TGF-β1 expression in PaTu8988 and BxPc-3 cells (Fig. 6A and B). Conversely, SW1990 cells
transfected with PVT1 expression plasmid decreased Smad4 expression
and increased p-Smad2/3 and TGF-β1 expression (Fig. 6C). However, TGF-β1 knockdown
downregulated p-Smad2/3 after we transfected BxPc-3 and SW1990
cells with pCD513B-1-PVT1 (Fig. 6D and
E), indicating that PVT1 upregulates p-Smad2/3 mainly through
TGF-β1 upregulation. Knockdown or overexpression of PVT1 had no
influence on total Smad2/3 protein. These data revealed that PVT1
promoted EMT in pancreatic cancer cells possibly via TGF-β/Smad
signaling.
Discussion
In the present study, we determined that PVT1 may
function as an oncogene in pancreatic cancer. We revealed for the
first time, to the best of our knowledge, that PVT1-induced
expression of p-Smad2/3 represents a novel and critical role for
controlling the growth and EMT in pancreatic cancer cells. All
these findings revealed that PVT1 may be a potential candidate for
the diagnosis of pancreatic cancer.
A previous study demonstrated that frequent mutation
of the q24 band of chromosome 8(8q24) was associated with PVT1
expression in breast cancer (14).
PVT1 expression is required for high Myc protein levels in
8q24-amplified human cancer cells (12). Our study confirmed that PVT1
expression was significantly increased in pancreatic cancer tissues
compared to adjacent normal tissues, corresponding with the
findings of Huang et al (19), which indicated that the reduced PVT1
expression may play an important role in the development of
pancreatic cancer and may be a biomarker for the detection of
cancer development and progression.
PVT1 can promote cervical cancer progression by
epigenetically silencing miR-200b (22). Kong et al found that PVT1
promoted gastric cancer cell viability by epigenetically regulating
p15 and p16 (23). In the present
study, we confirmed that PVT1 stimulated oncogenic activities
including the viability, adhesion, migration and the ability of
invasion in pancreatic cancer cells consistent with previous
studies in other tumors further supporting its oncogenic potential.
Carcinoma cells can transition from an epithelial to mesenchymal
differentiation state through a process known as EMT (24). The process of EMT is characterized
by alterations in the pattern of gene expression, loss of
epithelial cell junction proteins, such as E-cadherin, and an
upregulation of mesenchymal markers, such as vimentin and
N-cadherin (25). Loss of
E-cadherin expression is considered as a key event during the
induction of EMT (26). The present
study confirmed that knockdown of PVT1 upregulated E-cadherin and
downregulated vimentin, N-cadherin, β-catenin, Snail and Slug while
PVT1 overexpression resulted in the downregulation of E-cadherin
and upregulation of vimentin, N-cadherin, β-catenin, Snail and
Slug. All these data revealed that PVT1 may drive typical
epithelial phenotype cell transformation into spindle-shaped
mesenchymal phenotype cells, promoting the ability of viability,
migration and invasion in human pancreatic cancer cells.
It has been demonstrated that TGF-β acts as a potent
driver of cancer progression through the induction of EMT (27). Upon stimulation by TGF-β1, Smad2/3
is activated by phosphorylation and along with Smad4, they are
translocated into the nucleus to activate transcription of cancer
cells, promoting EMT progression (28). Our results revealed that PVT1
promoted the growth and EMT through driving TGF-β/Smad signaling.
As anticipated, PVT1 knockdown downregulated TGF-β1 and p-Smad2/3
while PVT1 overexpression upregulated active-TGF-β1, and then
activated p-Smad2/3. Conversely, Smad4, a well-known
tumor-suppressor gene and a major mediator of intracellular TGF-β
signaling (29), was upregulated by
knockdown of PVT1. These results demonstrated that knockdown of
PVT1 promoted EMT via TGF-β signaling activation in pancreatic
cancer cells. Notably, it is suggested that PVT1 plays an important
role in treating cancer and may be a potential therapeutic target
candidate in pancreatic cancer.
In conclusion, PVT1 plays a pivotal role in cell
viability, adhesion, migration and invasion in pancreatic cancer
cells. PVT1 may function as a key regulator of EMT in pancreatic
cancer cells through the TGF-β/Smad signaling pathway. Therefore,
PVT1 may prove to be clinically useful for developing a prognostic
biomarker and therapeutic target for pancreatic cancer.
Acknowledgements
Thanks for all members from Lab 2508, Department of
Cell Biology, School of Medicine, Jiangsu University.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81672402 and
81472333), the Provincial Natural Science Foundation of Jiangsu,
China (no. BK20171305), the Research Programs of Jiangsu Provincial
Commission of Health and Family Planning, China (no. H201434), the
‘Six one Project’ Research Projects of High-level Medical Personnel
of Jiangsu Province (no. LGY2016054), the ‘Six Talents Peak’
High-level Talent Selection and Training Project of Jiangsu
Province (no. 2014-WSW-038), the Science and Technology Support
Program Project of Zhenjiang, China (nos. SH2014024) and the Qing
Lan Project of Jiangsu Provincial Education Department.
Revitalizing the key talent's subsidy project in science and
education, Jiangsu, China (no. ZDRCC2016026) and the Natural
Science Foundation of Jiangsu Province (no. BK20130474).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MX, XZ, WF and AG conceived, designed, coordinated
the study and wrote the paper. MX, XZ and WF participated in all
experiments. JZ performed and analyzed experiments shown in
Fig. 1. LG, YZ, WP and DW performed
and analyzed experiments in Figs.
2–4. WF and JZ performed and
analyzed experiments shown in Figs.
5 and 6. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Signed informed consent was obtained from all
patients, and the study was approved by the Ethics Committee of
Changhai Hospital of Shanghai, Second Military Medical
University.
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer. World J Gastroenterol. 22:9694–9705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma J, Siegel R and Jemal A: Pancreatic
cancer death rates by race among US men and women, 1970–2009. J
Natl Cancer Inst. 105:1694–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Limani P, Samaras P, Lesurtel M, Graf R,
DeOliveira ML, Petrowsky H and Clavien PA: Pancreatic cancer- a
curable disease. Praxis (Bern 1994). 104:453–460. 2015.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohammed S, Van Buren G II and Fisher WE:
Pancreatic cancer: Advances in treatment. World J Gastroenterol.
20:9354–9360. 2014.PubMed/NCBI
|
|
7
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beuran M, Negoi I, Paun S, Ion AD, Bleotu
C, Negoi RI and Hostiuc S: The epithelial to mesenchymal transition
in pancreatic cancer: A systematic review. Pancreatology.
15:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergmann JH and Spector DL: Long
non-coding RNAs: Modulators of nuclear structure and function. Curr
Opin Cell Biol. 26:10–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui M, You L, Ren X, Zhao W, Liao Q and
Zhao Y: Long non-coding RNA PVT1 and cancer. Biochem Biophys Res
Commun. 471:10–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Graham M and Adams JM: Chromosome 8
breakpoint far 3′ of the c-myc oncogene in a Burkitt's lymphoma 2;8
variant translocation is equivalent to the murine pvt-1 locus. EMBO
J. 5:2845–2851. 1986.PubMed/NCBI
|
|
12
|
Tseng YY, Moriarity BS, Gong W, Akiyama R,
Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell
TC, et al: PVT1 dependence in cancer with MYC copy-number
increase. Nature. 512:82–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guan Y, Kuo WL, Stilwell JL, Takano H,
Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, et al:
Amplification of PVT1 contributes to the pathophysiology of ovarian
and breast cancer. Clin Cancer Res. 13:5745–5755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zhu Z, Zhang B, Li W, Li X, Wu X,
Wang L, Fu L, Fu L and Dong JT: Frequent mutation of rs13281615 and
its association with PVT1 expression and cell proliferation
in breast cancer. J Genet Genomics. 41:187–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meyer KB, Maia AT, O'Reilly M, Ghoussaini
M, Prathalingam R, Porter-Gill P, Ambs S, Prokunina-Olsson L,
Carroll J and Ponder BA: A functional variant at a prostate cancer
predisposition locus at 8q24 is associated with PVT1
expression. PLoS Genet. 7:e10021652011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS
and Feng XJ: Increased expression of the lncRNA PVT1 promotes
tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6929–6935. 2014.PubMed/NCBI
|
|
17
|
Ding J, Li D, Gong M, Wang J, Huang X, Wu
T and Wang C: Expression and clinical significance of the long
non-coding RNA PVT1 in human gastric cancer. OncoTargets
Ther. 7:1625–1630. 2014. View Article : Google Scholar
|
|
18
|
You L, Chang D, Du HZ and Zhao YP:
Genome-wide screen identifies PVT1 as a regulator of Gemcitabine
sensitivity in human pancreatic cancer cells. Biochem Biophys Res
Commun. 407:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang C, Yu W, Wang Q, Cui H, Wang Y,
Zhang L, Han F and Huang T: Increased expression of the lncRNA PVT1
is associated with poor prognosis in pancreatic cancer patients.
Minerva Med. 106:143–149. 2015.PubMed/NCBI
|
|
20
|
Xu T, Jing C, Shi Y, Miao R, Peng L, Kong
S, Ma Y and Li L: microRNA-20a enhances the
epithelial-to-mesenchymal transition of colorectal cancer cells by
modulating matrix metalloproteinases. Exp Ther Med. 10:683–688.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Zhang G and Liu J: Long noncoding
RNA PVT1 promotes cervical cancer progression through
epigenetically silencing miR-200b. APMIS. 124:649–658. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong R, Zhang EB, Yin DD, You LH, Xu TP,
Chen WM, Xia R, Wan L, Sun M, Wang ZX, et al: Long noncoding RNA
PVT1 indicates a poor prognosis of gastric cancer and
promotes cell proliferation through epigenetically regulating p15
and p16. Mol Cancer. 14:822015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bogachek MV, De Andrade JP and Weigel RJ:
Regulation of epithelial-mesenchymal transition through SUMOylation
of transcription factors. Cancer Res. 75:11–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heldin CH, Miyazono K and ten Dijke P:
TGF-beta signalling from cell membrane to nucleus through SMAD
proteins. Nature. 390:465–471. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Sheng J, Dai D, Liu T and Qi F:
Smad4 acts as tumor suppressor by antagonizing lymphangiogenesis in
colorectal cancer. Pathol Res Pract. 211:286–292. 2015. View Article : Google Scholar : PubMed/NCBI
|