Introduction
Cancer cachexia is a complex syndrome that is
characterized by an ongoing loss of skeletal muscle mass (1). If a decrease in fat mass occurs,
conventional nutritional support is insufficient to overcome the
loss. Cachexia induced by malignant diseases can lead to severe
consequences, including poor prognosis, diminished quality of life,
and decreased radiochemotherapy efficacy (2–4).
Cachexia occurs in up to 80% of patients with advanced stage
cancer, and ~50% of patients have cachexia at the time of initial
cancer diagnosis. Furthermore, cachexia is responsible for more
than 20% of cancer-associated deaths (2). For these reasons, there is increasing
focus on cancer cachexia in the field of cancer therapy.
Currently, the underlying mechanisms of cachexia
remain largely unknown; correspondingly, therapeutic progress is
limited (5). Skeletal muscle loss
is the prominent characteristic of cachexia; however, at present,
there are no effective standard measures of cancer cachexia as it
is a complex clinical syndrome with unclear underlying mechanisms.
Therefore, the focus of treatment is on the inhibition of skeletal
muscle loss to mitigate the associated adverse affects.
The molecular mechanisms of skeletal muscle
atrophy/hypertrophy are intricate and remain poorly defined;
however, an opportunity for downstream intervention has been
identified that may circumvent the variations and redundancy in
upstream mediators, and these findings may ultimately translate
into new targeted therapies. Specifically, a number of studies
(6–8) have indicated that two signaling
mediators are required to upregulate the expression of the key E3
ligases, muscle RING finger-containing protein 1 (MuRF1) and muscle
atrophy F box protein (MAFbx, otherwise known as atrogin-1), which
predominantly mediate sarcomeric breakdown during muscle loss.
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and,
in particular, tumor necrosis factor (TNF)-α induce MuRF1
upregulation via NF-κB, resulting in the degradation of myosin
heavy chains (MyHC) (9).
The transcription factor NF-κB was first identified
over 20 years ago and is a regulator of the expression of the κB
light chain in B cells. A study by Rhoads et al (10) showed that there was a 25% increase
in p65 phosphorylation and a significant decrease in the expression
of IκBα in gastric cancer patients when compared with the levels in
controls, which suggest that the activation of the classical NF-κB
pathway in the muscle tissue of patients accompanies cancer
cachexia. Additionally, a report by Wysong et al (11) suggested that NF-κB inhibition via
the IκB complex is able to protect against cancer-associated
cardiac atrophy. According to these findings, it seems feasible
that skeletal muscle loss and cardiac atrophy could be inhibited by
disrupting the NF-κB pathway.
Compounds of natural origin can be used as new and
innovative therapeutic agents for the treatment of diseases.
Luteolin (3–5,7-tetrahydroxy flavone) is a natural flavonoid
present in several plants. A number of studies have reported its
potential anticancer activity and its inhibition of NF-κB
activation in cancer; moreover, suppression of NF-κB by luteolin
can activate TNF-α-induced apoptosis (12). Luteolin decreases NF-κB activation
at both the transcriptional and translational levels and inhibits
the production of the inflammatory mediators interleukin (IL)-6,
IL-8, and vascular endothelial growth factor (VEGF) by
TNF-triggered human keratinocytes (13).
However, to the best of our knowledge, there are no
reports indicating that luteolin can inhibit skeletal and cardiac
muscle loss. Therefore, in the present study, we investigated
whether luteolin inhibits cancer-induced skeletal muscle and
cardiac atrophy by inhibiting the NF-κB pathway in vivo. Our
results suggest that luteolin is a promising candidate to be
developed into an effective therapeutic agent for the treatment of
muscle loss associated with cancer cachexia.
Materials and methods
Cell culture and natural drug
preparation
Lewis lung cancer (LLW) cells were purchased from
Shanghai Institutes for Biological Sciences (Shanghai, China) and
were grown in Dulbecco's modified Eagle's medium (DMEM) (Biosera,
Kansas City, MO, USA) supplemented with 10% fetal bovine serum
(FBS), 100 U/ml penicillin, and 100 µg/ml streptomycin, in a
humidified incubator at 37°C with 5% CO2.
Luteolin was purchased from Nantong Feiyu Biological
Technology Co., Ltd. (>98%; FY14650427) and diluted in PBS for
use in the present study. The structure of luteolin is shown in
Fig. 1.
In vivo model of cancer cachexia
Cachexia induced by LLC is frequently used (14–16) as
a preclinical and experimental model as it resembles clinical
cachexia, including the resulting physiological and metabolic
characteristics; hence, this in vivo model has been used for
many years and is an established model of cancer-induced cachexia.
Mice were obtained from Shanghai S&P-Shall Kay Laboratory
Animal Co., Ltd. (specific pathogen-free certificate numbers: SCXK
Hu 2008–0016). The 30 male 4-to 6-week-old (18–22 g) C57BL/6 mice
obtained were housed in a uniform temperature room (26°C) under a
12-h light/dark cycle (light from 08:00 to 20:00). They were placed
in closed cages and given free access to chow and water. The feed
used and protocols carried out in this study were in accordance
with the regulations of the Institutional Animal Care and Use
Committee (IACUC) of Jiangsu University (Jiangsu, China). The
experimental mice were randomly divided into the following three
groups (10 mice per group) after 1 week of acclimation: A control
group, a model group, and a luteolin group. On the first day of the
experiment, 200 µl of PBS was injected subcutaneously into the
right flank of each mouse in the control group. Mice in the other
two groups were each injected subcutaneously at the same position
with ~1×107 LLW cells in 200 µl of PBS. From day 7 to
24, mice in the treatment group were treated with luteolin.
Luteolin was diluted in PBS and delivered via intragastric
administration (20 mg/kg/day, 0.3 ml). The control and model groups
received daily sham treatments of PBS alone (0.3 ml). Body weight
was measured for each group using an electronic scale at the
beginning and end of the experiment, and tumor-free body weight was
estimated by subtracting the weight of the excised tumor. On day
24, blood samples were obtained from the orbital vein, centrifuged
at 3,000 rpm at 4°C for 20 min within 1 h, and then stored at
−80°C. Immediately after blood withdrawal, the mice were sacrificed
by cervical dislocation. The tumors, hearts, bilateral
gastrocnemius muscles and bilateral tibialis anterior muscles were
immediately harvested and weighed. The muscle tissues were quickly
frozen in liquid nitrogen and stored at −80°C.
Detection of cytokines
Using commercially available enzyme-linked
immunosorbent assay (ELISA) kits, the serum cytokines TNF-α and
IL-6 were detected. The detection kits contain pre-coated plates
(mouse IL-6: DKW12-2060; mouse TNF-α: DKW12-2,720; Dakewei Biotech.
Co., Ltd., Shenzheng, China) and were used in accordance with the
manufacturer's protocols. Serum from each animal (50 µl) was
assayed in duplicate. Standard curves were created using
recombinant mouse IL-6 and TNF-α to allow quantitative
calibration.
Western blot (WB) analysis
After muscle and cardiac tissues were homogenized by
adding a protein lysis buffer (Beyotime Institute of Biotechnology,
Haimen, Jiangsu, China), the homogenates were centrifuged for 10
min at 12,000 rpm at 4°C, and the supernatant was aspirated. A BCA
Protein Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used according to the manufacturer's protocol to determine
the protein concentrations. The proteins were separated by gel
electrophoresis (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore Corp., Bedford, PA, USA). The PVDF membranes were
incubated with the indicated primary antibodies overnight at 4°C
with gentle agitation (anti-atrogin-1: cat. no. ab74023,
anti-MuRF1: cat. no. ab172479, anti-phospho-p65: cat. no. ab86299,
anti-IKKβ: cat. no. ab32135, anti-p38: cat. no. ab170099,
anti-p-p38: cat. no. ab195049; Abcam, Cambridge, MA, USA) and with
anti-NF-κB p65 (cat. no. 8242; Cell Signaling Technology, Inc.,
Danvers, MA, USA). The diultions for all antibodies was 1:1,000
except anti-atrogin-1 and anti-phospho-p65 with 1:500. Using an
image scanning densitometer connected to a chemiluminescence
system, antibody binding was detected after incubation with a
horseradish peroxidase-conjugated secondary antibody (Cell
Signaling Technology, Inc.). Quantitative analysis of protein
expression levels was performed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA). The protein expression
levels were normalized to β-actin expression.
Real-time quantitative reverse
transcription PCR (qRT-PCR) analysis
We measured the mRNA expression of MuRF1 and
atrogin-1 in the gastrocnemius muscles and cardiac muscles of the
three groups by qRT-PCR analysis, as described in detail elsewhere.
In brief, total RNA was extracted from the whole gastrocnemius
muscle and cardiac muscle using a RiboPure™ Kit (Life Technologies
Japan Inc., Tokyo, Japan) according to the manufacturer's
instructions. First-strand cDNA was generated by reverse
transcription using a High-Capacity RNA-to-cDNA Kit (Takara Bio
Inc., Beijing, China), and the resulting cDNA was stored at −20°C
for later analysis. qRT-PCR was performed using a TaqMan Fast
Universal PCR Master Mix (Takara Bio Inc.) and a Thermal Cycler
Dice Real-Time System II (Takara Bio Inc.). The levels of mRNA were
determined using the primers shown in Table I. The expression of the genes was
normalized to the expression of β-actin, and the results are
expressed as relative differences.
 | Table I.List of primers used for PCR
analysis. |
Table I.
List of primers used for PCR
analysis.
|
| Primer sequence
(5′-3′) |
|---|
| Atrogin-1 | F:
TCCAGTGAGGAGCAGTTCAG |
|
| R:
CAGTGTGATTGGCATTTGGT |
| MuRF-1 | F:
ACGAGAAGAAGAGCGAGCTG |
|
| R:
CAAAGTCAATGGCCCTCAAG |
| β-actin | F:
TCAGTGCCGGCCTCGTCTCAT |
|
| R:
TGACCAGGCGGCCAATACGG |
Statistical analysis
All data represent the means ± standard deviation
(SD), and ANOVA with Tukey's post hoc comparison was used to
identify significant differences between the groups. This analysis
was performed with SPSS version 18.0 (SPSS, Inc., Chicago, IL,
USA), and a two-sided P-value <0.05 was considered to indicate
statistical significance.
Results
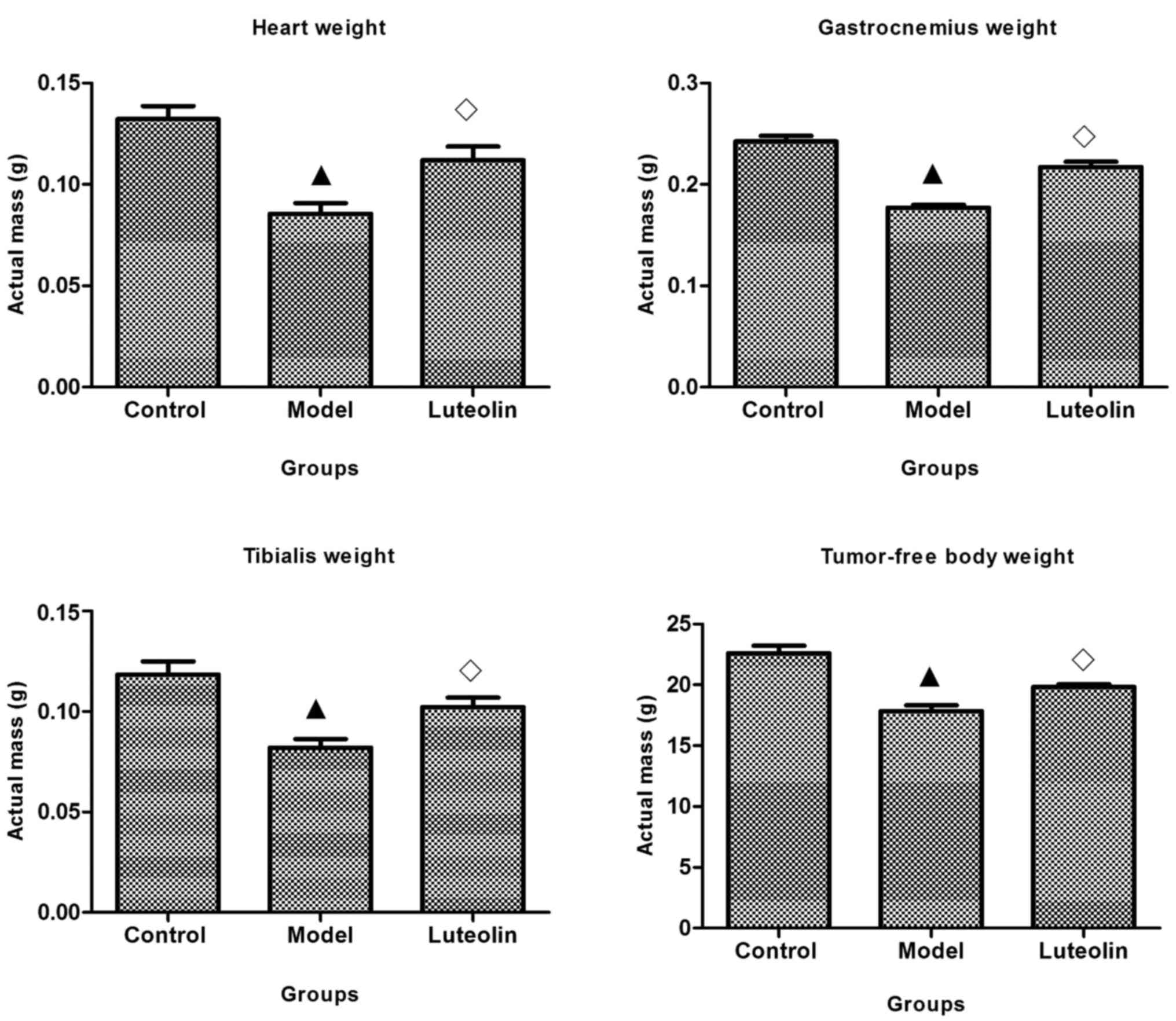
Effect of luteolin on tumor-free body
weight and weight of gastrocnemius muscles and heart
One of the main characteristics of cancer cachexia
is the loss of skeletal muscle. On day 21, the tumor-free body
weights of tumor-bearing mice were significantly lower than those
of the control and model mice. The masses of the heart muscle,
gastrocnemius muscle, and tibialis muscle were obviously reduced in
the model group compared with those of control mice; however, the
masses were significantly increased in model mice treated with
luteolin compared with those in the model group. Unfortunately, the
masses in the luteolin group remained lower than those of normal
mice (Fig. 2).
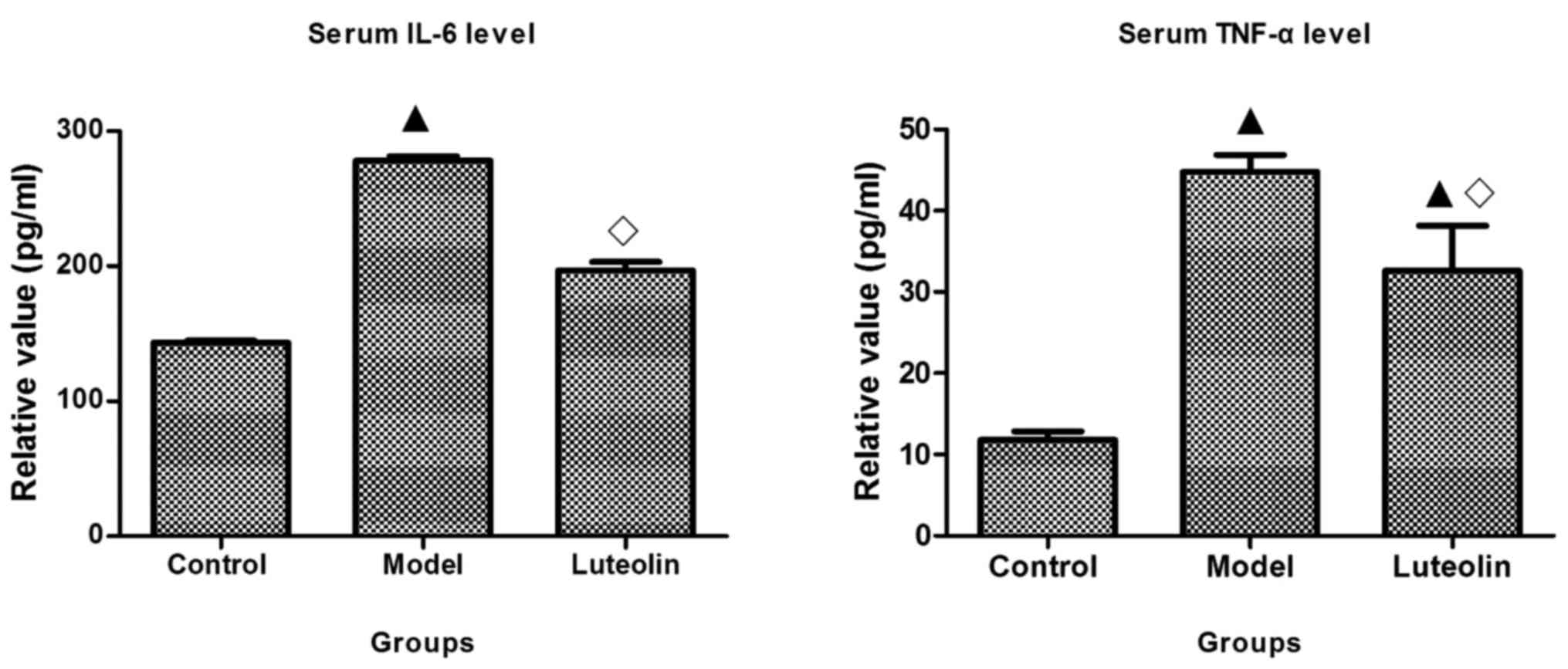
Effect of luteolin on TNF-α and
IL-6
Cytokines, especially TNF-α and IL-6, are released
at significantly increased levels by tumors, and trigger and
promote the progression of cachexia (8,17,18).
In the present study, we first detected TNF-α and IL-6 in the sera
of mice using ELISAs. In the model mice, these two cytokines were
obviously elevated compared with their levels in the control mice,
while their levels were significantly reduced by luteolin treatment
compared with those in the model mice. The level of TNF-α remained
significantly higher in the luteolin-treated mice than that in the
control mice (Fig. 3).
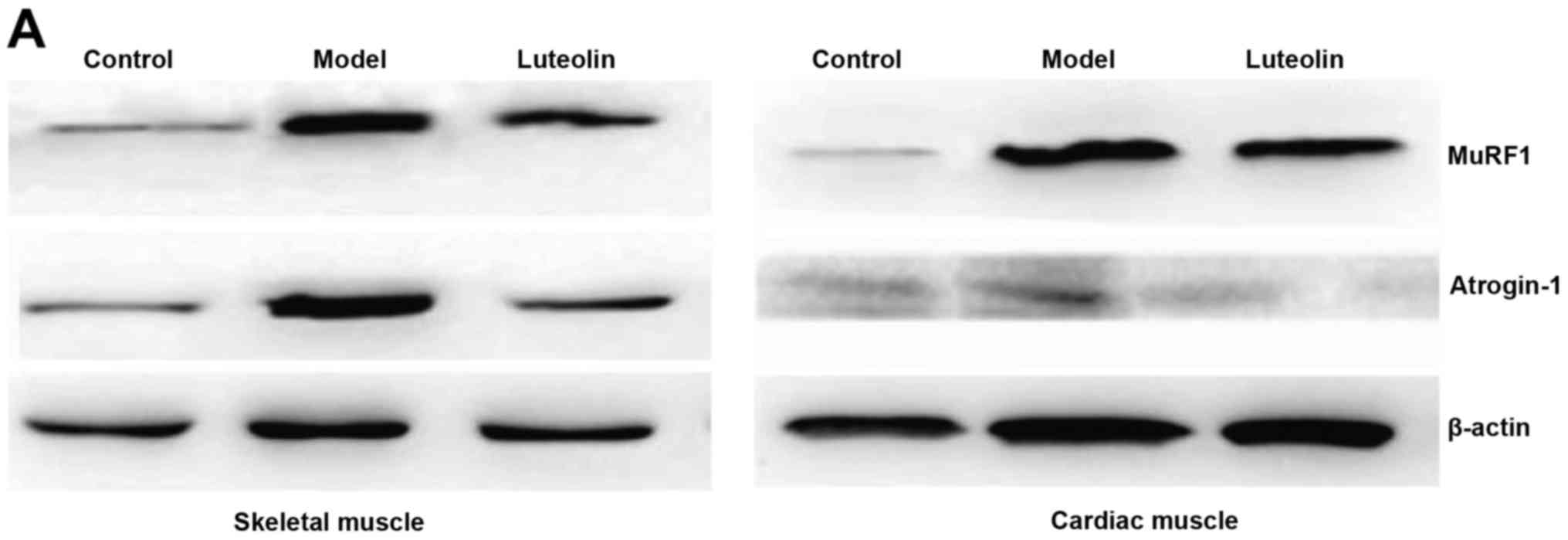
Effect of luteolin on atrogin-1 and
MuRF-1 expression
MuRF1 and atrogin-1 have been identified as
important markers of muscle degradation, and the upregulation of
these proteins has been reported during the degradation of skeletal
muscle protein (6,19,20).
Therefore, we detected the expression of MuRF1 and atrogin-1 at the
protein and transcript levels by WB analysis and qRT-PCR,
respectively, in gastrocnemius muscle samples. The protein levels
of MuRF1 and atrogin-1 were obviously upregulated in the model
mice; however, this upregulation was attenuated by luteolin
treatment (Fig. 4A and B).
Correspondingly, the mRNA levels of MuRF1 and atrogin-1 were
significantly elevated in the model mice, and this elevation was
inhibited by luteolin treatment in the skeletal muscle (Fig. 4C). In addition, we detected MuRF1
and atrogin-1 expression in cardiac muscle to assess their
correlation with the reduction of heart mass. The levels of MuRF1
and atrogin-1 were obviously upregulated in the model mice, but
only the increase in MuRF1 was significantly inhibited by luteolin
treatment (Fig. 4A-C).
Effect of luteolin on NF-κB signaling
and p38 mitogen-activated protein kinase (MAPK)
In view of the established critical relationship
between increased cytokines in serum and NF-κB signaling (11,21),
we speculated that luteolin exerts therapeutic effects by
regulating NF-κB signaling. Based on WB analysis, IκB kinase β
(IKKβ) and p-p65 were observed to be significantly upregulated in
skeletal (Fig. 5A and B) and
cardiac muscles (Fig. 5C and D) in
the model mice, and the expression levels of these proteins were
suppressed by luteolin.
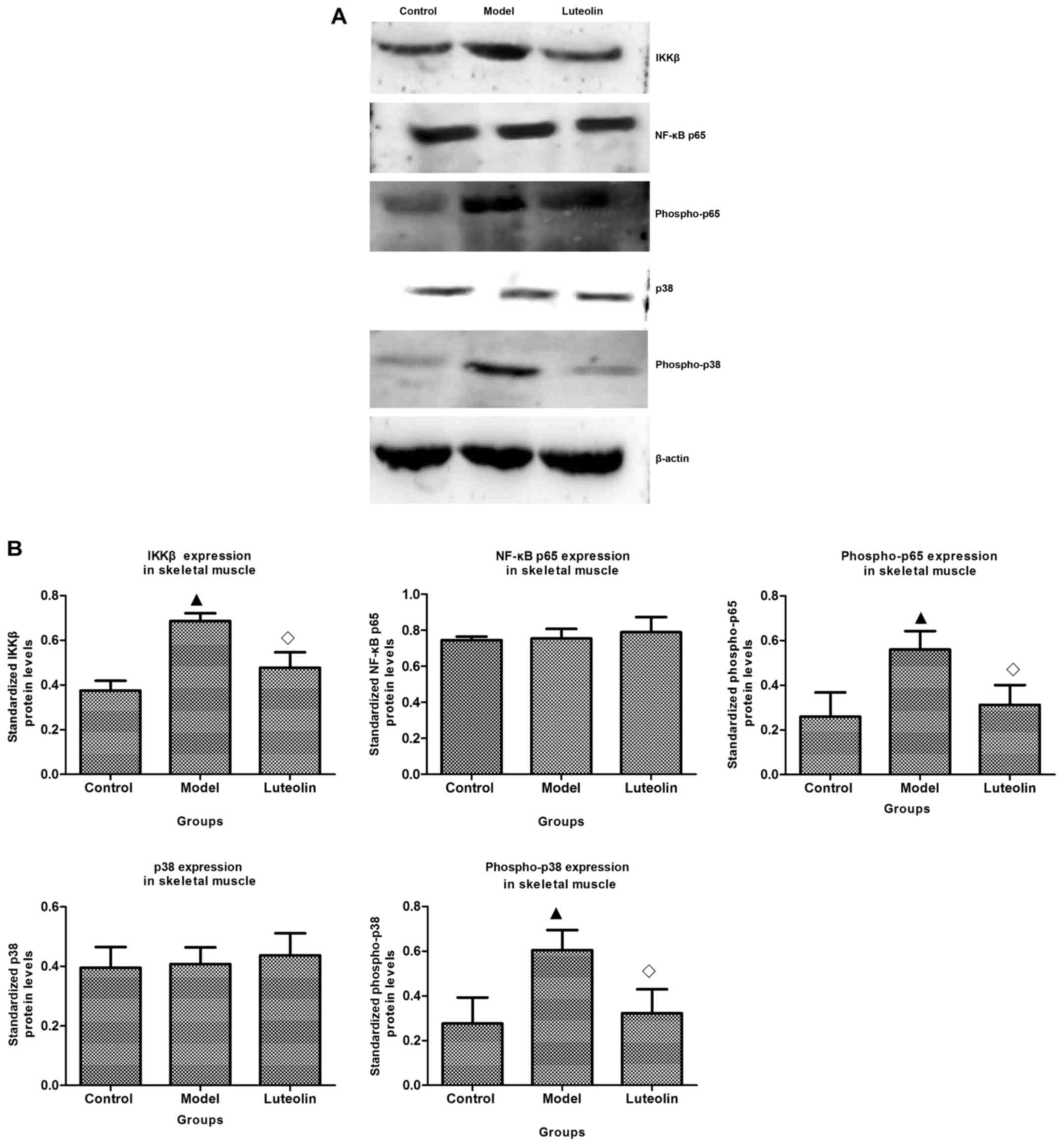 | Figure 5.Effects of luteolin on NF-κB signaling
and the p38 MAP kinase pathway. NF-κB p65, phospho-p65, IKKβ, p38,
and phospho-p38 were detected by western blot (WB) analysis as
described in Materials and methods. (A) The luteolin group had
lower phospho-p65, IKKβ, and phospho-P38 expression in skeletal
muscle and the same level of NF-κB, p65 and p38 expression compared
with the model group. (B) The luteolin group had significantly
different levels of phospho-p65, IKKβ, and phospho-p38 in skeletal
muscle, but exhibited no difference in NF-κB p65 and p38 expression
compared with the model group. (C) The luteolin group had lower
phospho-p65 and IKKβ expression in cardiac muscle compared with
that in model mice, while the expression of NF-κB p65, p38, and
phospho-p38 was similar between the model and luteolin groups. (D)
The luteolin group had significantly different levels of
phospho-p65 and IKKβ in cardiac muscle, but exhibited no difference
in NF-κB p65, p38, and phospho-p38 expression compared with the
model group. Statistical significance vs. the control group
(▲P<0.05); statistical significance vs. the model
group (◊P<0.05). |
In addition, proinflammatory cytokines can activate
the NF-κB pathway and p38 MAPK. For this reason, we assayed the
levels of p38 and phospho (p)-p38. The levels of p38 were
approximately equivalent in all three groups in the skeletal muscle
(Fig. 5A and B) and in the cardiac
muscle (Fig. 5C and D); however,
there were obvious differences in the levels of p-p38. There was
significant upregulation of p-p38 in both skeletal muscle and
cardiac muscle in the model mice (Fig.
5A-D), which was inhibited by luteolin treatment in the
skeletal muscle, while being unaffected by luteolin treatment in
the cardiac muscle.
Discussion
The primary characteristic of tumor-associated
cachexia is muscle atrophy, and loss of skeletal muscle in cachexia
results from decreased protein synthesis combined with increased
protein degradation. The expression of the E3 ligase E3a-II is also
reported to be significantly induced at the onset and during the
progression of muscle wasting (20,22).
Meanwhile, E3a-II has been shown to be induced in myotubes by
treatment with TNF-α or IL-6 (17,23).
Other studies confirm the importance of the IKKβ/NFκB pathway in
the induction of the ubiquitin-proteasome pathway (21).
Compounds of natural origin could be used as novel,
innovative therapeutic agents for the treatment of cancer. Previous
studies have reported that a large number of phytochemical
compounds may represent new anticancer compounds. For example,
ginsenoside Rg3 has significant cancer-inhibitory activity; its
suggested mechanisms of action include the induction of apoptosis,
the inhibition of proliferation, metastasis and angiogenesis, and
the promotion of immunity (24). As
another example, we also reported that baicalin alleviated anorexia
and inhibit skeletal muscle atrophy in experimental models of
cancer cachexia (25). No effective
treatments currently exist for the clinical treatment of cancer
cachexia. Thus, inadequacies remain in the clinical management of
cachexia due to the complex nature of the condition. However, as
pathways continue to be identified, there is increased potential
for more effective treatment of muscle wasting in cancer
patients.
In the present study, we successfully replicated a
cachexia model by subcutaneously injecting LLW cells into C57BL/6
mice according to procedures outlined in the literature (14–16).
As muscle loss is the main characteristic of cachexia, which leads
to insufficient muscle function, we first observed variation in
muscle weight. Significant reductions in the gastrocnemius and
heart muscle masses were observed in the tumor-bearing group. Such
muscle mass loss was diminished in tumor-bearing mice that were
administered luteolin; however, their muscle mass remained lower
than that in the normal group. Moreover, the key markers of
degradation in muscle, MuRF1 and atrogin-1, were upregulated in
tumor-bearing mice, whereas this was suppressed by luteolin
treatment.
In many cancers, TNF-α plays a paramount role in
activating the NFκB pathway to induce a signaling cascade that
promotes the progression of tumorigenesis. We measured the level of
TNF-α in mouse serum, and the results confirmed increased TNF-α
expression in the model group. However, TNF-α was restored to
control group levels by luteolin intervention.
In addition, IL-6 has been reported to have a
regulatory role in muscle wasting during cachexia (23); however, an increased IL-6 level is
not the most important activator of NF-κB. In this study, IL-6
levels were high in the model mice, and were reduced by luteolin
treatment; however, the difference was not significant.
The purpose of the present study was to determine
whether luteolin functions by targeting NF-κB signaling proteins
and NF-κB transcription factors, which function in cancer-induced
muscle wasting. The existing literature indicates the involvement
of the NF-κB signaling protein, IκBα, in LLC tumor-bearing mice,
and the overexpression of a super-repressor form of IκBα in
skeletal muscle was found to be associated with a 50% inhibition of
muscle fiber atrophy (26). We
detected the expression levels of MuRF1 and atrogin-1 in skeletal
tissue, and observed that their levels were decreased by luteolin
treatment, as compared with those in tumor-bearing mice
administered vehicle treatment. The inhibition of classical NF-κB
signaling is sufficient to significantly decrease tumor-induced
muscle loss, at least in mice, in part by inhibiting the
upregulation of MuRF1. As one component of the complex, it is
generally accepted that the activation of NF-κB requires the
activation of either IKKα or IKKβ (27); and the activation of IKKβ has been
reported to cause profound muscle wasting that resembles clinical
cachexia (21). For this reason, we
also detected the expression of IKKβ, and identified that its
expression matched that of NF-κB.
It has been reported that MuRF1 activity is
necessary to induce cardiac atrophy, and the significant
dexamethasone-induced atrophy induced in the heart tissues of
wild-type mice is essentially absent in MuRF1−/− mice
(28). In addition, the present
study observed that, in heart muscle tissue, the expression of
MuRF-1 was upregulated in model mice, whereas this upregulation was
attenuated in tumor-bearing mice administered luteolin. Therefore,
we speculate that luteolin can inhibit the expression of MuRF1
through inhibition of classical NF-κB signaling based on the
current findings.
Although the expression of atrogin-1 in skeletal
muscle tissue was also downregulated by luteolin treatment, its
expression in heart tissue was the same as that in the model mice.
Proinflammatory cytokines, such as TNF-α, TWEAK and IL-1, stimulate
two established pathways: the NF-κB and the p38 MAPK pathways
(29). Correspondingly, the
expression levels of p38 and p-p38 were detected in skeletal muscle
and heart muscle, and the results indicated that atrogin-1
expression was closely correlated with p38, which regulated partly
the atrogin-1 expression. Moreover, MuRF1 and atrogin-1 are also
regulated by other transcription factors and pathways (30–32);
therefore, it is possible that luteolin reduces MuRF1 and atrogin-1
by mechanisms other than influencing NF-κB signaling in skeletal
and heart muscle. Nonetheless, the current study indicated that
luteolin limits the loss of cardiac and skeletal muscle and acts by
inhibiting NF-κB activation.
In conclusion, the present results clearly indicated
that the natural flavonoid luteolin inhibited the production of the
inflammatory mediators TNF-α and IL-6 in a cancer cachexia model.
To the best of our knowledge, this study is the first to report
that luteolin inhibits the expression of MuRF1 by decreasing NF-κB
activation at both the transcriptional and translational levels. In
addition, luteolin may alleviate the effects of atrogin-1
upregulation by reducing the expression of p38. Therefore, luteolin
has the potential to be developed as a safe and effective
alternative therapy for the treatment of cancer cachexia.
Acknowledgements
We acknowledge Dr Feng Shi (College of Pharmacy,
Jiangsu University) for assisting in the assessment of this project
at the beginning of the experiment.
Funding
The present study was supported by the Liaoning
Natural Science Foundation (Grant no. 2015010571-301).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
BL conceived and designed the experiment. BL, TC and
YX performed the experiments. YJ analyzed the data. SM contributed
the reagents/materials/analysis tools. BL and YW wrote the paper.
All authors agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Animal research protocols carried out in this study
were in accordance with the regulations of the Institutional Animal
Care and Use Committee (IACUC) of Jiangsu University.
Consent for publication
Not applicable.
Competing interests
The authors declare that no competing interests
exist.
References
|
1
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classifi cation of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Argilés JM, Busquets S, Stemmler B and
López-Soriano FJ: Cancer cachexia: Understanding the molecular
basis. Nat Rev Cancer. 14:754–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans WJ, Morley JE, Argilés J, Bales C,
Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H,
Mantovani G, et al: Cachexia: A new definition. Clin Nutr.
27:793–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bilir C, Engin H, Can M, Temi YB and
Demirtas D: The prognostic role of inflammation and hormones in
patients with metastatic cancer with cachexia. Med Oncol.
32:562015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaughan VC, Martin P and Lewandowski PA:
Cancer cachexia: impact, mechanisms and emerging treatments. J
Cachexia Sarcopenia Muscle. 4:95–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tisdale MJ: Mechanisms of cancer cachexia.
Physiol Rev. 89:381–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fearon KC, Glass DJ and Guttridge DC:
Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell
Metab. 16:153–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schcolnik-Cabrera A, Chávez-Blanco A,
Domínguez-Gómez G and Dueñas-González A: Understanding tumor
anabolism and patient catabolism in cancer-associated cachexia. Am
J Cancer Res. 7:1107–1135. 2017.PubMed/NCBI
|
|
9
|
Li H, Malhotra S and Kumar A: Nuclear
factor-kappa B signaling in skeletal muscle atrophy. J Mol Med.
86:1113–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhoads MG, Kandarian SC, Pacelli F,
Doglietto GB and Bossola M: Expression of NF-kappaB and IkappaB
proteins in skeletal muscle of gastric cancer patients. Eur J
Cancer. 46:191–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wysong A, Couch M, Shadfar S, Li L,
Rodriguez JE, Asher S, Yin X, Gore M, Baldwin A, Patterson C, et
al: NF-κB inhibition protects against tumor-induced cardiac atrophy
in vivo. Am J Pathol. 178:1059–1068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tuorkey MJ: Molecular targets of luteolin
in cancer. Eur J Cancer Prev. 25:65–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weng Z, Patel AB, Vasiadi M, Therianou A
and Theoharides TC: Luteolin inhibits human keratinocyte activation
and decreases NF-κB induction that is increased in psoriatic skin.
PLoS One. 9:e907392014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Merriman RL, Shackelford KA, Tanzer LR,
Campbell JB, Bemis KG and Matsumoto K: Drug treatments for
metastasis of the Lewis lung carcinoma: lack of correlation between
inhibition of lung metastasis and survival. Cancer Res.
49:4509–4516. 1989.PubMed/NCBI
|
|
15
|
Au ED, Desai AP, Koniaris LG and Zimmers
TA: The MEK-inhibitor selumetinib attenuates tumor growth and
reduces IL-6 expression but does not protect against muscle wasting
in Lewis lung cancer cachexia. Front Physiol. 7:6822017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Wu Y, Yang T, Wei M, Wang Y, Deng
X, Shen C, Li W, Zhang H, Xu W, et al: Salidroside alleviates
cachexia symptoms in mouse models of cancer cachexia via activating
mTOR signalling. J Cachexia Sarcopenia Muscle. 7:225–232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Todorov P, Cariuk P, McDevitt T, Coles B,
Fearon K and Tisdale M: Characterization of a cancer cachectic
factor. Nature. 379:739–742. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albrecht JT and Canada TW: Cachexia and
anorexia in malignancy. Hematol Oncol Clin North Am. 10:791–800.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mueller TC, Bachmann J, Prokopchuk O,
Friess H and Martignoni ME: Molecular pathways leading to loss of
skeletal muscle mass in cancer cachexia - can findings from animal
models be translated to humans? BMC Cancer. 16:752016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai D, Frantz JD, Tawa NE Jr, Melendez PA,
Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and
Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle
wasting in mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clarke BA, Drujan D, Willis MS, Murphy LO,
Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E
and Glass DJ: The E3 Ligase MuRF1 degrades myosin heavy chain
protein in dexamethasone-treated skeletal muscle. Cell Metab.
6:376–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carson JA and Baltgalvis KA: Interleukin 6
as a key regulator of muscle mass during cachexia. Exerc Sport Sci
Rev. 38:168–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun M, Ye Y, Xiao L, Duan X, Zhang Y and
Zhang H: Anticancer effects of ginsenoside Rg3 (Review). Int J Mol
Med. 39:507–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li B, Wan L, Li Y, Yu Q, Chen P, Gan R,
Yang Q, Han Y and Guo C: Baicalin, a component of Scutellaria
baicalensis, alleviates anorexia and inhibits skeletal muscle
atrophy in experimental cancer cachexia. Tumour Biol.
35:12415–12425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Gammeren D, Damrauer JS, Jackman RW
and Kandarian SC: The IkappaB kinases IKKalpha and IKKbeta are
necessary and sufficient for skeletal muscle atrophy. FASEB J.
23:362–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willis MS, Rojas M, Li L, Selzman CH, Tang
RH, Stansfield WE, Rodriguez JE, Glass DJ and Patterson C: Muscle
ring finger 1 mediates cardiac atrophy in vivo. Am J Physiol Heart
Circ Physiol. 296:H997–H1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egerman MA and Glass DJ: Signaling
pathways controlling skeletal muscle mass. Cric Rev Biochem Mol
Biol. 49:59–68. 2014. View Article : Google Scholar
|
|
30
|
Waddell DS, Baehr LM, van den Brandt J,
Johnsen SA, Reichardt HM, Furlow JD and Bodine SC: The
glucocorticoid receptor and FOXO1 synergistically activate the
skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol
Endocrinol Metab. 295:E785–E797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stitt TN, Drujan D, Clarke BA, Panaro F,
Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD and Glass DJ: The
IGF-1/PI3K/Akt pathway prevents expression of muscle
atrophy-induced ubiquitin ligases by inhibiting FOXO transcription
factors. Mol Cell. 14:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sacheck JM, Ohtsuka A, McLary SC and
Goldberg AL: IGF-I stimulates muscle growth by suppressing protein
breakdown and expression of atrophy-related ubiquitin ligases,
atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 287:E591–E601.
2004. View Article : Google Scholar : PubMed/NCBI
|