Introduction
Lung cancer is the most common malignancy and the
leading cause of cancer-related deaths (1). Its occurrence and development is a
complex process involving oxygen stress, uncontrolled
proliferation, apoptosis, cell transformation and inflammation.
With the development of molecular biology, immunotherapy and
molecular targeted therapy, significant breakthroughs in the
molecular mechanisms of lung cancer have been achieved in recent
years. A better understanding of the molecular mechanisms of lung
cancer may provide an effective way of improving the prevention,
diagnosis, treatment and prognosis of patients with lung
cancer.
In clinical diagnosis, pathologists often assess the
tumor grade based on the morphology of the nucleus. Components of
the nuclear envelope have essential roles in many aspects of cell
function and may be involved in tumor development and progression.
A series of protein-protein interactions take place on the nuclear
envelope which affect tumor cell biology (2). The linker of nucleoskeleton and
cytoskeleton (LINC) complex has been suggested to anchor both the
nuclear membranes and nuclear lamina to the cytoskeleton (3–5). The
KASH domain of the Nesprin family of proteins and the SUN domain of
the SUN family of proteins interact in the perinuclear space, and
play a major role in the LINC complex (5–8). The
SUN family of proteins which are anchored on the inner nuclear
membrane connect with the lamina and chromosomes, and the Nesprin
family of proteins positioned on the outer nuclear membrane connect
with the cytoskeleton proteins (9,10). The
LINC complex functions in neurogenesis, gametogenesis and retina
formation, and LINC defects may contribute to human diseases, such
as muscular dystrophy, ataxia, and cancer (11,12).
Some LINC complex proteins have been reported to be
specifically expressed in tumors. The methylation of Nesprin1 gene
promoter may be associated with the development of lung cancer
(13,14). SUN1 and 2 are expressed at low
levels in breast cancer (15). Lv
et al demonstrated that SUN2 plays a tumor-suppressor role
by suppressing the Warburg effect in lung cancer (16). Sperm-associated antigen 4 (SPAG4),
first found in mammalian sperm tails, belongs to the SUN family of
proteins, interacts specifically with the outer dense fiber 27
(ODF27) protein (17), and plays
important roles in spermatogenesis and sperm motility. SPAG4 is
localized in the inner nuclear membrane and is a mediating protein
between the nucleoskeleton and cytoskeleton. Recently, Kennedy
et al reported that SPAG4 may be an independent cancer
marker (12). Knaup et al
revealed that SPAG4, an HIF1 target, was correlated with the
prognosis of renal cell carcinoma (18). From these findings, we speculated
that SPAG4 may be associated with the development of lung
cancer.
In the present study, we examined clinical samples
of lung cancer and found that SPAG4 was highly expressed. To
determine the possible mechanism of SPAG4 action in lung cancer,
experiments were designed to reveal that SPAG4 interacts with
Nesprin3, which influences the migration of lung tumor cells. This
SPAG4/Nesprin3 interaction may offer new strategies for the
diagnosis and treatment of lung cancer.
Materials and methods
Collection of tissue samples
This study was approved in December 2014 by the
Ethics Committee of the Third Xiangya Hospital, Central South
University, Changsha, China, and the collection of lung cancer
samples was performed in accordance with ethical standards. The
signing of the informed consent form was carried out by the members
of the test group and the subjects. When the informed consent was
signed, the subject was informed about the details of the clinical
trial. None of the patients had undergone preoperative intervention
therapy or chemotherapy. The tissues were removed during surgery,
and those used for western blotting were immediately stored in
liquid nitrogen, and those used for immunohistochemistry were
stored in 10% formalin at room temperature. Paired samples of lung
cancerous and paracarcinomatous tissues for immunohistochemistry
were obtained from 46 patients with lung cancer who underwent lung
lobectomy from December 2014 to June 2015 at the Department of
Cardiothoracic Surgery, Third Xiangya Hospital of Central South
University, Changsha, China. Sixteen of the 46 paired samples were
also used for western blotting. The adjacent tissues were obtained
from the edge of the lung lobe (≥5 cm from the carcinoma). These 46
patients included 29 males and 17 females with a mean age of 57.5
years (range, 32–74 years). No distant metastasis was found in the
selected patients before surgery. Detailed clinicopathological data
such as tumor size, clinical grade [according to
tumor-node-metastasis (TNM) stage], histological type,
differentiation degree and lymph node metastasis were obtained and
summarized. The tumor stage was defined according to the 8th
edition of the TNM classification of the Union for International
Cancer Control (UICC).
Construction of plasmids
The eukaryotic vector pEGFP-N1 carrying different
fragments of SPAG4 was used, and each of these fragments was
amplified by polymerase chain reaction (PCR) using the following
primers: forward, 5′-CCAAGCTTGCCACCAGGATGCGGCGAAGCTCCCG-3′
(the HindIII site was included) and reverse,
5′-GGGTCGACAAATGGGGCCCCTGTGCACTG-3′
(the SalI site was included) for integral SPAG4; forward,
5′-CCAAGCTTGCCACCAGGATGCGGCGAAGCTCCCG-3′
(HindIII) and reverse, 5′-GCGTCGACCGGATGGAACAGACCTCCCT-3′
(SalI) for the 1–165 amino acid (aa) fragment of SPAG4;
forward, 5′-GCAAGCTTATGCCTCCCCCGCGGGTGTTCAAG-3′
(HindIIId) and reverse, 5′-GGGTCGACGCATAGTCGGGCTTCCGCAC-3′
(SalI) for the 126–260aa fragment of SPAG4; forward,
5′-GCAAGCTTATGGATTTTGTGCGGAAGCCCGAC-3′
(HindIII) and reverse, 5′-GGGTCGACAAATGGGGCCCCTGTGCACTG-3′
(SalI) for the 232–438aa fragment of SPAG4.
The eukaryotic vector pCMV-C-flag was used to
construct the tagged expression plasmid pCMV-C-flag-SPAG4: forward,
5′-CCAAGCTTGCCACCAGGATGCGGCGAAGCTCCCG-3′
(the HindIII site was included) and reverse,
5′-GCCTCGAGATGGGGCCCCTGTGCACTGC-3′
(the XhoI site was included). The eukaryotic vector
pCMV-N-HA was used to construct pCMV-N-HA-Nesprin3: forward,
5′-CATAAGCTTATGAACTCAGCAGCCCCAGGAC-3′
(the HindIII site was included) and reverse,
5′-GGGATCCATCCTCCAAAAATCGGAT-3′
(the KpnI site was included). The products were sequenced to
confirm the identity. The procedure and conditions for PCR were:
95°C for 2 min and 30 sec, followed by 35 cycles at 94°C for 30
sec, 62°C for 30 sec, and 72°C for 1 min and 20 sec, and a final
extension at 72°C for 10 min. All finished vectors were sent to
Sangon Biotech Co., Ltd. (Shanghai, China) for gene sequencing.
Cell culture and electric transfection
methods
The lung cancer cell line A549 was purchased from
the Xiangya Cell Bank, Central South University, Changsha, China.
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with 10% fetal bovine serum (FBS) was used for cell
culture. For electric transfection, the cells were diluted with
electric transfection buffer (Eppendorf, Hamburg, Germany) at
0.5–1.5×106 cells/ml, mixed with plasmids 20–30 µg/ml,
and then placed in electric transfection dishes. The cell dishes
were installed on a Multiporator (Eppendorf Electroporator 2510),
with the parameters set at 600 V and 100 µsec. The transferred
cells were cultured in normal medium at 37°C in a 5% CO2
and 95% humidified air atmosphere. After 20 h, the cells were
observed under a fluorescence microscope and images were
obtained.
Bimolecular fluorescence
complementation (BiFC) assay
To construct recombinant BiFC plasmids, the Nesprin3
and SPAG4 cDNA sequences were respectively amplified (from A549
total mRNA) by PCR using the following primers: forward,
5′-CCAAGCTTGCCACCAGGATGCGGCGAAGCTCCCG-3′
(the HindIII site was included) and reverse,
5′-CCGGTACCATGGGGCCCCTGTGCACTGCCCTC-3′
(KpnI) for SPAG4; forward, 5′-CATAAGCTTATGAACTCAGCAGCCCCAGGAC-3′
(HindIII) and reverse, 5′-GGGATCCATCCTCCAAAAATCGGAT-3′
(KpnI) for Nesprin3. Fragments of YFP DNA sequence from the
PA7-YFP vector were truncated at residue 155, and were thus
designated as YN and YC DNA fragments, using the following primers:
forward, 5-′CGGGGTACCCGCTCCATCGCCACGATGGTGAGCAAGGGCGAG-3′
(KpnI was included) and reverse, 5′-CCGGAATTCTTAGGCCATGATATAGACGTTGT-3′
(EcoRI) for YN; forward, 5′-CGGGGTACCCGCCCGGCCTGCAAGATCCCGAACGACCTGAAACAGAAGGTCATGAACCACGACAAGCAGAAGAACGGCAT-3′
(KpnI) and reverse, 5′-CCGGAATTCTTACTTGTACAGCTCGTCCATGC-3′
(EcoRI) for YC. The YN DNA fragment was connected to the 3′
(C-terminus) of Nesprin3 cDNA, and the YC fragment was connected to
the 3′ (C-terminus) of SPAG4 cDNA. The connected fragments were
inserted into the pcDNA3.1(+) vector to generate
pcDNA3.1(+)-Nesprin3-YN and pcDNA3.1(+)-SPAG4-YC, respectively. All
constructs were verified by sequencing (Sangon Biotech Co.,
Ltd.).
For the BiFC assay, the cells were suspended in 300
µl transfer buffer (Eppendorf) and co-transfected with a mixture of
pcDNA3.1(+)-Nesprin3-YN and pcDNA3.1(+)-SPAG4-YC using the
electroporation method. Cells were maintained in a 5%
CO2 and 95% humidified air atmosphere at 37°C for 20 h.
Finally, the cells were analyzed using a fluorescence microscope
(Nikon Corp., Melville, NY, USA).
Establishment of the SPAG4 and
Nesprin3 silenced A549 cell model
The GV248 lentiviral vector (containing neomycin
resistant and EGFP genes; Shanghai GeneChem, Co., Ltd., Shanghai,
China) carrying three types of SPAG4-shRNA or three types of
Nesprin3-shRNA was used to screen for maximum silencing efficiency.
We electrically transfected the SPAG4-shRNA or Nesprin3-shRNA
vectors into A549 cells to construct the SPAG4/Nesprin3-silenced
A549 cell model, and the analogue vector was used as the control.
Twenty hours after electric transfection, the A549 cells were
cultured in medium with neomycin (1 mg/ml) for 9 days (5–7 days
after all cells in the untransfected group died). Each cell line
was then subjected to extensive culture. SPAG4 or Nesprin3
expression was determined for each group using western
blotting.
Western blotting
Tissues or cells were lysed in
radioimmunoprecipitation assay (RIPA; Beyotime Institute of
Biotechnology, Haimen, China) buffer with protease inhibitors
(cOmplete Mini; Roche Diagnostics, Basel, Switzerland) and
phenylmethanesulfonyl fluoride (PMSF) (KGP610; Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). After electrophoresis, the
proteins were transferred to polyvinylidene fluoride (PVDF)
membranes (IPVH10010; EMD Millipore, Billerica, MA, USA). The
Bio-Rad electrophoresis system and Wet blotting system (both from
Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used to perform
the aforementioned steps. Non-specific binding was blocked by
immersing the membrane in 5% non-fat milk for 2 h at room
temperature with shaking. Primary antibodies were applied overnight
at 4°C. The rabbit anti-SPAG4 antibody (cat. no. sc-135414; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) was used at a 1:500
dilution, the rabbit anti-Nesprin3 antibody (cat. no. ab74261;
Abcam, Cambridge, UK) was used at a 1:1,000 dilution, the mouse
anti-Flag antibody (cat. no. AF519; Beyotime Institute of
Biotechnology), the mouse HA-Tag antibody (cat. no. 66006-1-1g) and
mouse anti-GAPDH antibody (cat. no. 60001-1-1g) (both from
ProteinTech Group, Inc.: Wuhan Sanying Biotechnology, Wuhan, China)
were used at a 1:2,000 dilution. Horseradish peroxidase-conjugated
anti-mouse (cat. no. 7076s) or anti-rabbit secondary antibodies
(cat. no. 7074s) (both from Cell Signaling Technology, Inc.,
Danvers, MA, USA) were applied at 1:3,000 for 1 h at 37°C. Signals
were visualized using an enhanced chemiluminescent (ECL) system.
Protein band intensity was measured and normalized to GAPDH by
ImageJ software.
Immunohistochemical staining
Immunohistochemical staining for the SPAG4 protein
was carried out using an SP ready-to-use kit (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.: OriGene Technologies,
Beijing, China) according to the manufacturer's instructions. The
rabbit anti-hSPAG4 antibody was used as the primary antibody
according to the manufacturer's instructions. The SPAG4 antibody
was diluted at 1:50. Sections were then incubated with
biotin-labeled goat anti-rabbit/mouse IgG (Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd.: OriGene Technologies) for 10 min
and subsequently with streptavidin peroxidase for 10 min. The
slides were visualized using diaminobenzidine and counterstained
with hematoxylin before microscopy. Addition of the primary rabbit
anti-SPAG4 antibody was omitted from the protocols for the negative
controls.
Three random ×200 microscopic fields containing
tumor cells were evaluated by two independent experienced
investigators without prior knowledge of the patient disease data;
≥100 tumor cells were examined per field. Two scoring systems
including staining intensity and percentage of immunoreactive cells
were used. The staining intensity was scored on a semi-quantitative
4-point scale: 0, negative control; 1, weak cytoplasmic and nuclear
staining (slightly darker than the negative control); 2, moderate
staining; and 3, intense staining (equivalent to or darker than the
positive control). The percentage of stained cells was also scored
on a semiquantitative 4-point scale: 0 for <10%, 1 for 10–25%, 2
for 25–50%, and 3 for >50%. Finally, the scores of staining
intensity and the percentage of stained cells were combined and
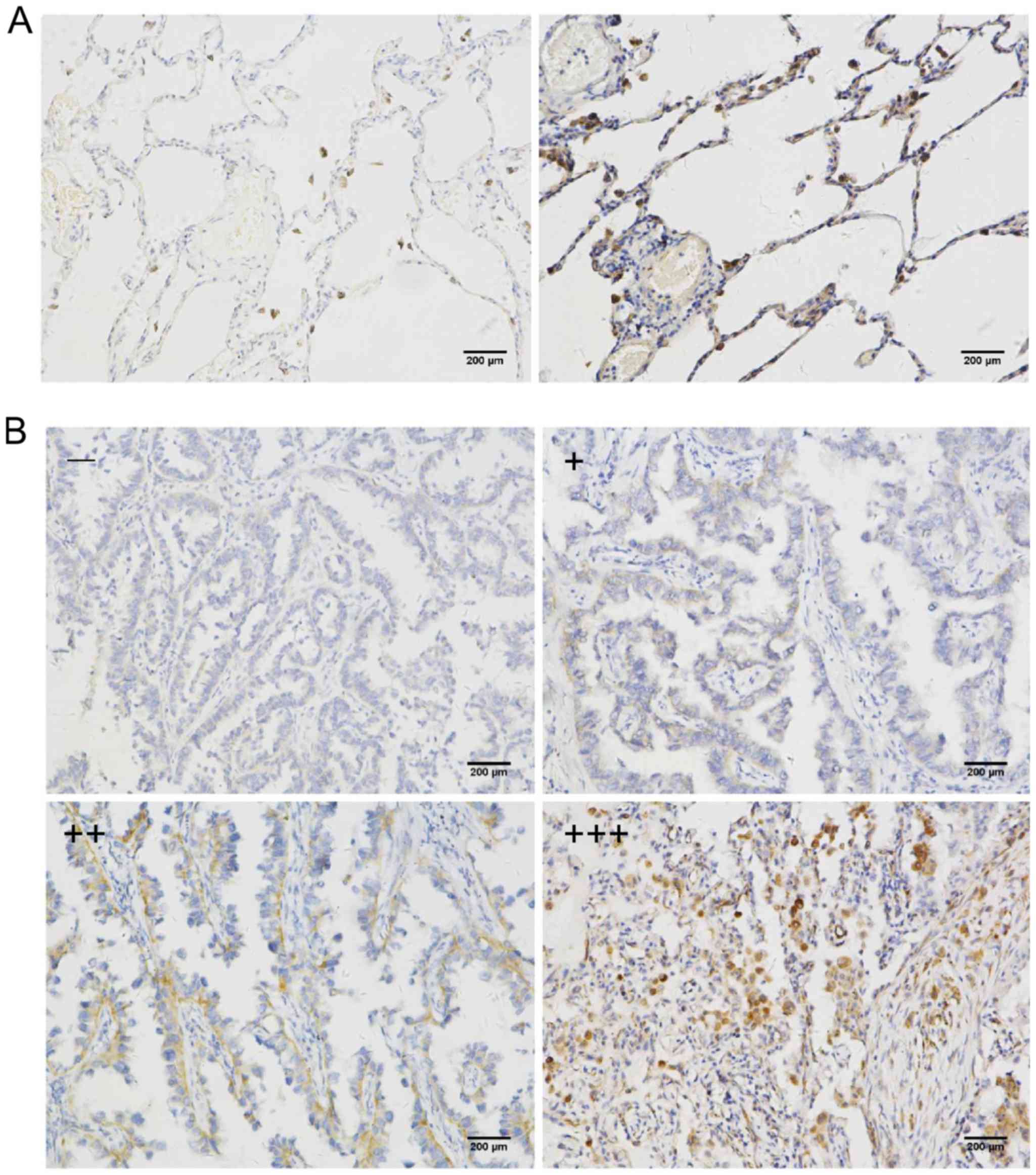
evaluated as follows: 0–1 as -, 2 as +, 3–4 as ++ and 5–6 as
+++.
Co-immunoprecipitation (Co-IP)
Protein (200 µg) extracted from the transfected
cells, 30 µl sepharose beads (Santa Cruz Biotechnology, Inc.) and
cell lysates [treated with 1 mM DL-dithiothreitol (DTT), 1 mM PMSF,
1 µg/ml aprotinin, l µg/ml leupeptin protease inhibitor, 0.5% NP40]
were mixed to a volume of 400 µl. The cell lysates obtained from
the cells were precleared by incubation for 2 h at 4°C on a shaker.
The clear supernatant was incubated overnight with control IgG,
anti-HA (cat. no. 66006-1-1g; ProteinTech Group, Inc.: Wuhan
Sanying Biotechnology) or anti-FLAG antibody (AF519; Beyotime
Institute of Biotechnology). The samples were then washed with 0.5%
NP40 cell lysate three times. Immobilized protein complexes were
eluted by denaturation in 2X SDS sample buffer at 95°C for 10 min
for subsequent western blotting.
Immunofluorescence
For immunofluorescence, the cells were washed with
phosphate-buffered saline (PBS) solution (4°C) and immediately
fixed in cold methanol for 15 min and permeabilized for 10 min in
0.2% Triton X-100 (Beyotime Institute of Biotechnology). Staining
antibodies included mouse anti-Nesprin3 at a 1:200 dilution and
rabbit anti-SPAG4 at a 1:100 dilution. After washing, the sections
were incubated with Cy3-labeled goat anti-rabbit IgG (cat. no.
KGAB019; Nanjing KeyGen Biotech Co., Ltd.). Nuclei were
counterstained using DAPI (Invitrogen: Thermo Fisher Scientific,
Inc., Carlsbad, CA, USA). After staining, the cells were imaged on
a Nikon TE300 Eclipse inverted microscope (Nikon Corp.).
Statistical analysis
Statistical analyses were carried out using SPSS
version 24.0. Analysis of the association between the expression of
SPAG4 and clinicopathological features was performed using
Pearson's Chi-square test or Fisher's exact test. All values from
the in vitro assays were expressed as the mean ± SEM or SD
of at least three independent experiments or replicates. P<0.05
was considered statistically significant in all tests.
Results
Increased expression of SPAG4 in lung
cancer tissue
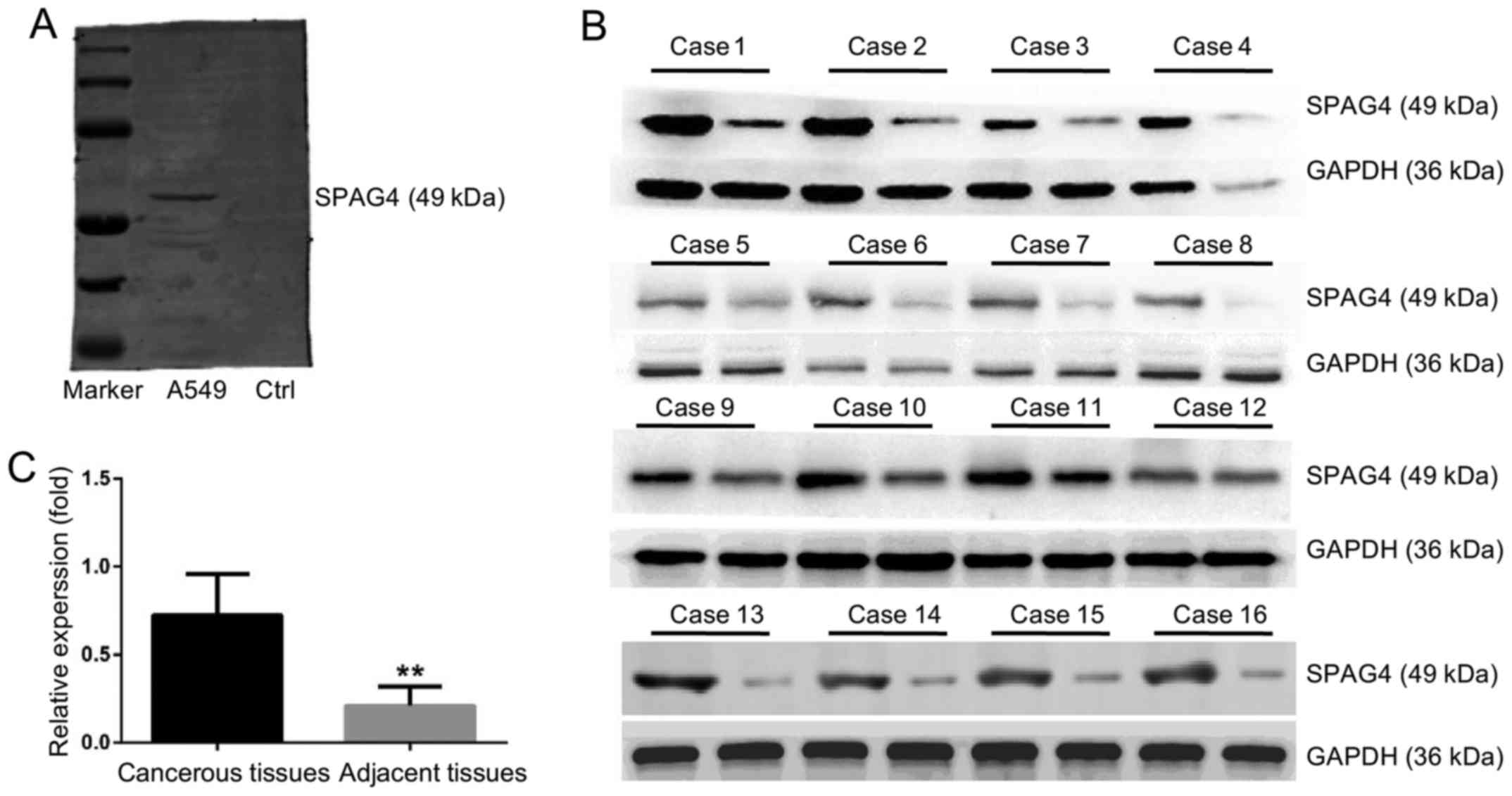
Fig. 1A shows the
specificity of the anti-SPAG4 antibody. No non-specific bands were
detected and the antibody was qualified for immunohistochemistry
studies. Fig. 1B and C reveals the
high SPAG4 expression in lung carcinoma tissues. Based on the
assessment by pathologists, SPAG4 expression as revealed by
immunohistochemistry was divided into four levels. The cancer and
paracancerous tissue sampling areas are shown in Fig. 2A and B, respectively. The staining
of the SPAG4 protein was predominantly localized in the cytoplasm
of lung cancer cells. Precise localization of SPAG4 is shown in the
immunofluorescence results. The positive rate of SPAG4 expression
in cancerous tissues was 69.6% (32/46), and only 21.7% (10/46) in
the corresponding adjacent non-cancerous tissues (Table I). The ratio of SPAG4 positivity was
significantly different between the cancerous and adjacent tissues
(P<0.001).
 | Table I.Differential expression of SPAG4 in
cancerous and corresponding adjacent tissues. |
Table I.
Differential expression of SPAG4 in
cancerous and corresponding adjacent tissues.
|
|
| SPAG4
expression |
|
|---|
|
|
|
|
|
|---|
| Tissues | No. of cases | Negative (−/+) |
Positive(++/+++) | Positive rate
(%) |
|---|
| Cancerous | 46 | 14 | 32 | 69.6a |
| Adjacent | 46 | 36 | 10 | 21.7 |
Notably, according to these data, SPAG4 expression
was not significantly correlated with age, histological type or
tumor size, but was strongly correlated with tumor differentiation
grade (P=0.014), lymph node metastasis (P=0.035) and clinical stage
(P=0.008) (Table II). These
results indicated that SPAG4 was upregulated in lung cancer and its
high expression was closely related to tumor cell migration in lung
cancer.
 | Table II.Association between SPAG4 expression
and clinicopathological features. |
Table II.
Association between SPAG4 expression
and clinicopathological features.
|
|
| SPAG4
expression |
|
|---|
|
|
|
|
|
|---|
|
|
| Negative | Positive |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
data | Patients | − | + | ++ | +++ | P-value |
|---|
| Total | 46 | 5 | 9 | 18 | 14 |
|
| Age (years) |
|
|
|
|
| 0.403 |
|
<60 | 22 | 4 | 4 | 8 | 6 |
|
|
>60 | 24 | 1 | 5 | 10 | 8 |
|
| Histological
type |
|
|
|
|
| 0.766 |
|
Squamous carcinoma | 15 | 1 | 4 | 5 | 5 |
|
|
Adenocarcinoma | 31 | 4 | 5 | 13 | 9 |
|
| Tissue size
(cm) |
|
|
|
|
| 0.447 |
|
D<3 | 16 | 2 | 4 | 6 | 4 |
|
|
D>3 | 30 | 3 | 5 | 12 | 10 |
|
| Clinical grade |
|
|
|
|
| 0.008a |
| I | 17 | 3 | 6 | 6 | 2 |
|
| II | 16 | 2 | 3 | 5 | 6 |
|
|
III | 13 | 0 | 0 | 7 | 6 |
|
| Differentiation
degree |
|
|
|
|
| 0.014a |
|
Undifferentiated | 8 | 0 | 0 | 3 | 5 |
|
| Poorly
differentiated | 13 | 0 | 2 | 5 | 6 |
|
|
Moderately/well-differentiated | 25 | 5 | 7 | 10 | 3 |
|
| Lymph node
metastasis |
|
|
|
|
| 0.035a |
|
Yes | 30 | 2 | 4 | 13 | 11 |
|
| No | 16 | 3 | 5 | 5 | 3 |
|
SPAG4 promotes the migration of lung
cancer cells
Given that SPAG4 expression was upregulated in lung
cancer tissues, we then determined whether SPAG4 was involved in
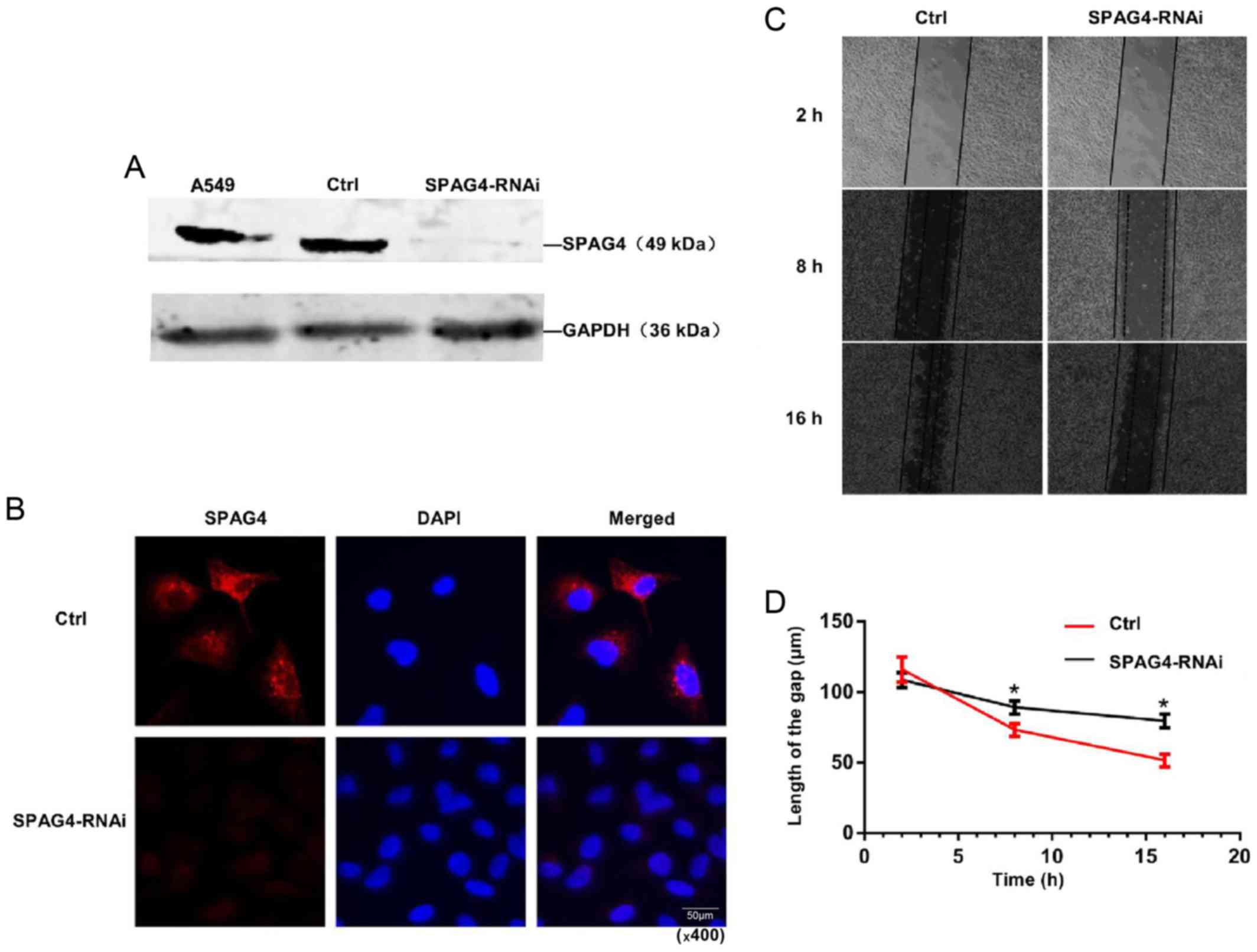
regulating the biological behavior of lung cancer cells. Marked
downregulation of SPAG4 expression was observed in
SPAG4-RNAi-treated A549 cells (Fig.
3A). Immunofluorescence further demonstrated an evident
reduction in SPAG4 expression in SPAG4-RNAi-treated A549 cells,
demonstrating that the expression of SPAG4 was successfully reduced
by the RNAi method (Fig. 3B).
As shown in Table
II, SPAG4 may be closely associated with tumor cell migration.
The scratch wound assay revealed that cell migration of
SPAG4-silenced cells was slower than that of the control (Fig. 3C and D). These results indicated
that SPAG4 plays an important role in the migration of lung cancer
cells, and a higher level of SPAG4 expression is highly related to
stronger migration of cancer cells.
Transmembrane helix plays a major role
in SPAG4 positioning
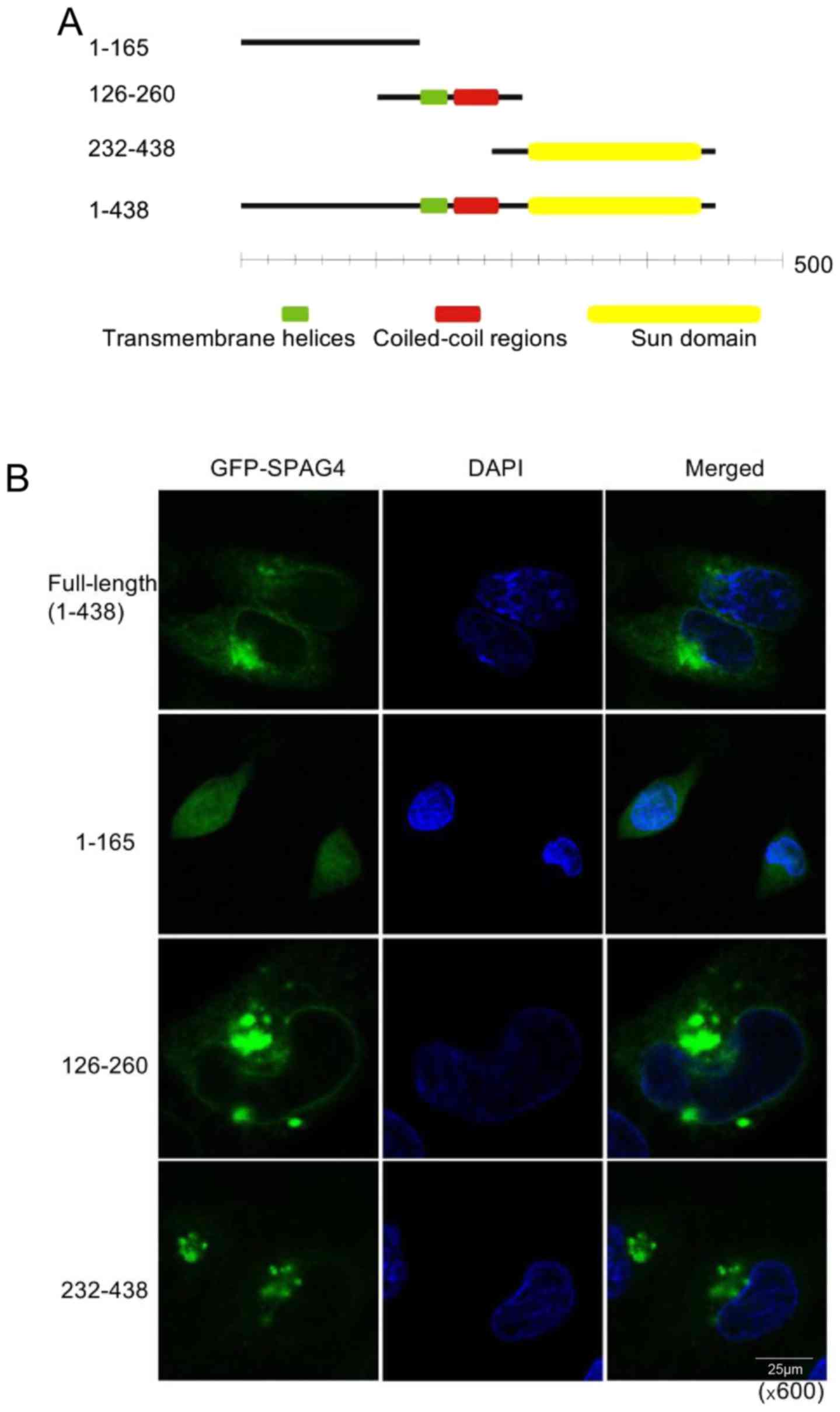
To determine the location of SPAG4 protein in lung
cancer cells, we generated the pEGFP-N1-SPAG4 plasmid encoding the
SPAG4-GFP fusion protein, and then a series of GFP-SPAG4 deletion
mutants were generated to identify the region that is critical for
localization of SPAG4 to the nuclear envelope or cytoplasm.
According to the gene information in NCBI (Fig. 4A), we constructed the full-length
and three fragments of SPAG4 into the pEGFP-N1 plasmid,
respectively. Each of the four plasmids was transfected into A549
cells, and confocal laser scanning microscopy was used to obtain
images (Fig. 4B). The green
fluorescence from the full-length SPAG4-transfected cells was
located evenly around the nucleus and on one side of the nucleus in
the cytoplasm, while the fragment 1-165aa was located in the
cytoplasm and not on the nuclear membrane. The fragment 126-260aa,
containing the transmembrane helices and the coiled-coil region,
was positioned in the membrane structure of the cells. However, its
agglomeration was not only on one side of the nucleus but also
occurred elsewhere in the cytoplasm. The fragment 232-438aa,
containing only the SUN domain, was not only located in the
membrane structure, but also on one side of the nucleus in the
cytoplasm. It is evident that the C-terminus (SUN domain) of SPAG4
most probably guides it to the nuclear envelope, while the
transmembrane helices help it anchor on the inner nuclear
envelope.
Identification of Nesprin3 as an
interacting protein of SPAG4
Since it has long been known that Nesprin3 silencing
greatly attenuates the migratory flow response with cells moving
significantly more slowly in the direction of flow (19), we suspected that SPAG4 may be
closely related to Nesprin3, which is located on the outer nuclear
membrane and is also a component of the LINC complex.
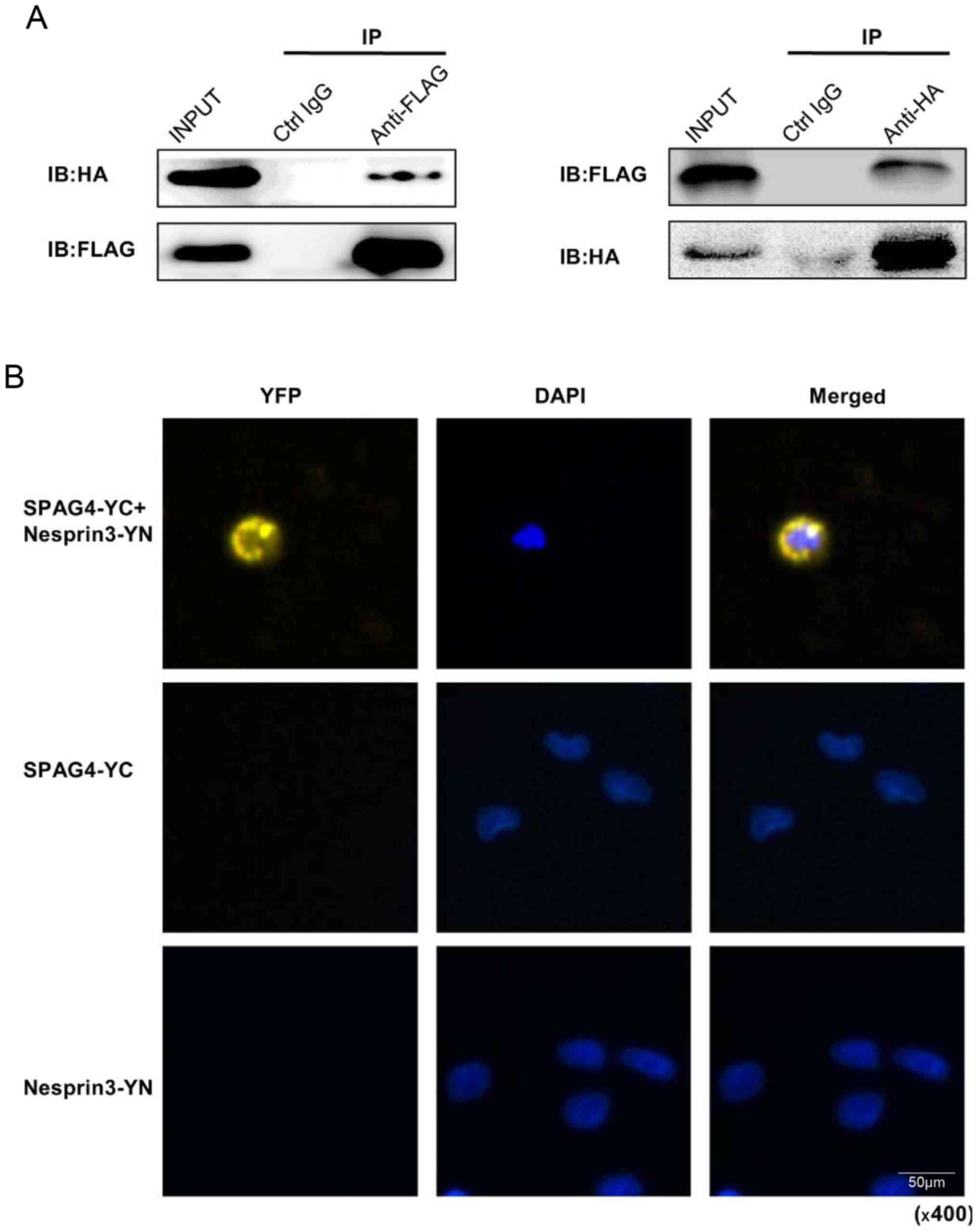
The Co-IP assay was used to successfully confirm the
interaction between SPAG4 and Nesprin3 (Fig. 5A). Following co-transfection with
the pCMV-C-flag-SPAG4 and pCMV-N-HA-Nesprin3 vectors, the cell
lysates were subjected to the Co-IP assay. In addition, we
consolidated this conclusion using the BiFC assay (20). Cells were co-transfected with
pcDNA3.1-SPAG4-YC and pcDNA3.1-Nesprin3-YN, and the yellow image
around the nuclear envelope indicated the interaction between SPAG4
and Nesprin3 (Fig. 5B).
SPAG4 exerts migration-promoting
functions by regulating Nesprin3 in lung cancer
The interaction between SPAG4 and Nesprin3 was
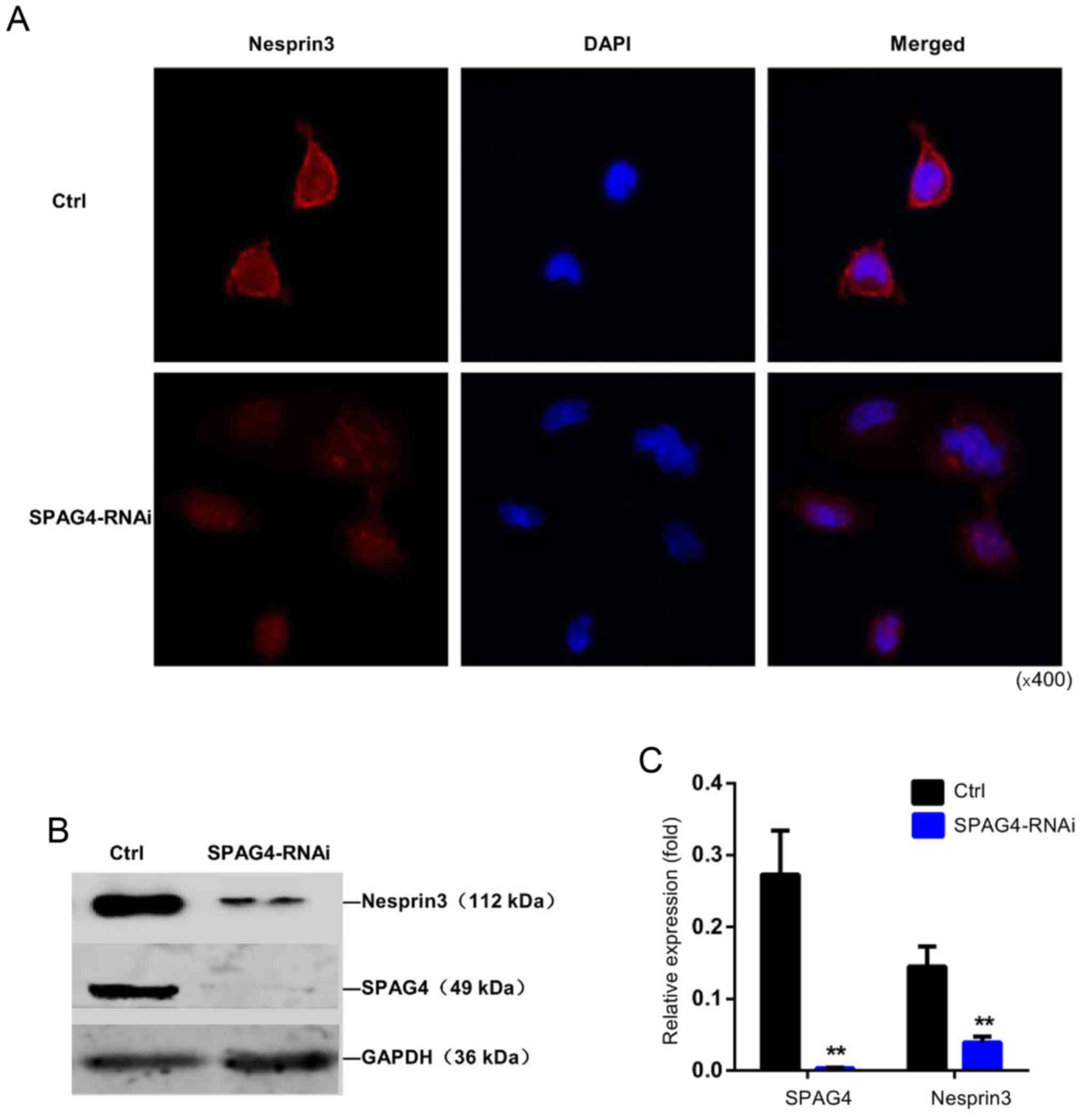
further confirmed by the distribution of Nesprin3 (Fig. 6A). Immunofluorescence staining
revealed that Nesprin3 exhibited strong nuclear membrane
localization in A549 cells. When SPAG4 was silenced, Nesprin3 was
not completely distributed around the nuclear membrane, and its
expression appeared to decrease. As shown in Fig. 6B and C, SPAG4 silencing
significantly reduced the expression of Nesprin3.
It appears that SPAG4 affects the migration of lung
cancer cells by regulating Nesprin3. Thus, we studied the effect of
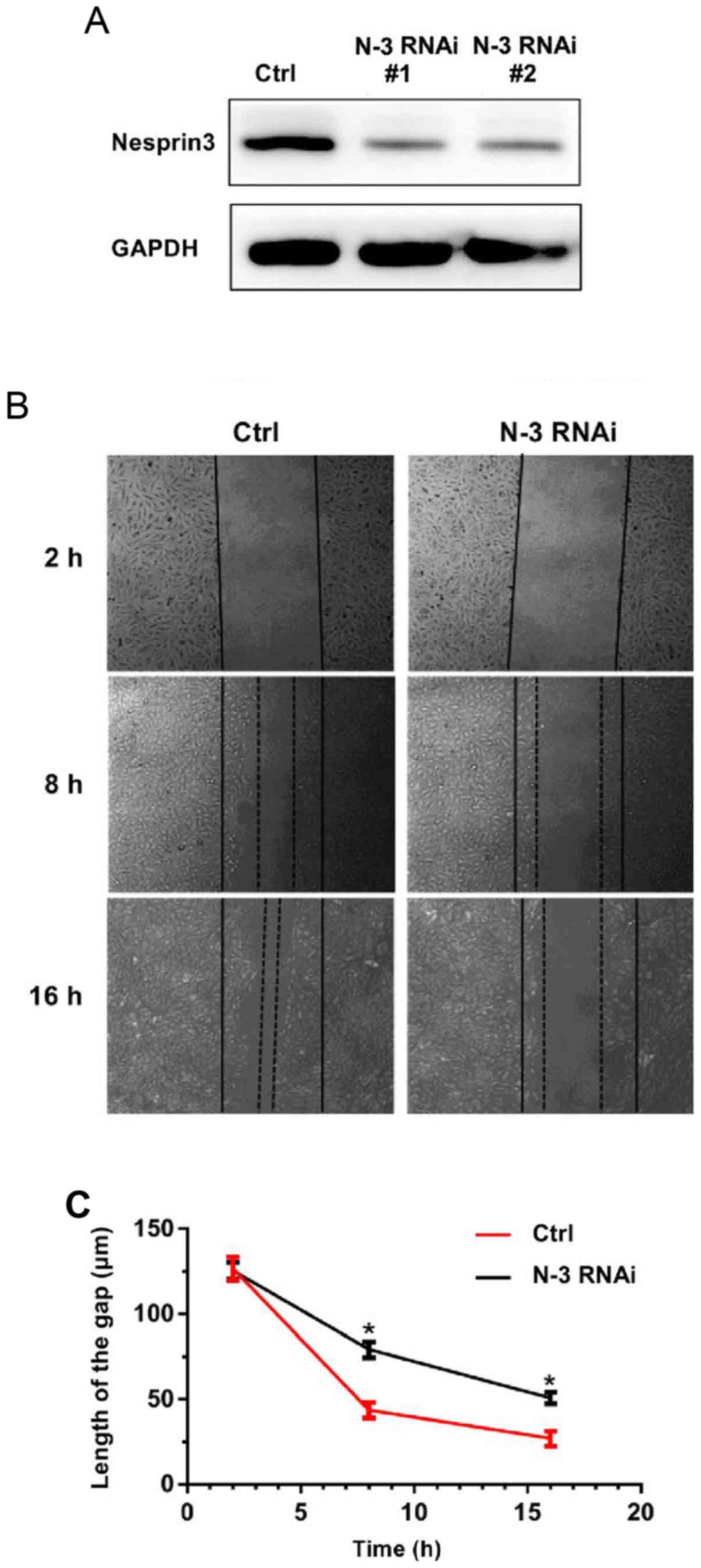
Nesprin3 on the migration of lung cancer cells. We performed the
scratch wound assay, and found that Nesprin3-silenced A549 cells
(verified by western blotting as shown in Fig. 7A) had slower migration, and
exhibited the same trend as SPAG4 (Fig.
7B and C). These results indicated that SPAG4, acting as a
positive regulator of Nesprin3, promoted cell migration in lung
carcinoma.
Discussion
SPAG4 is a new potential tumor biomarker, and was
found to be significantly increased in lung carcinoma tissues. The
present study indicated that downregulation of SPAG4 expression
suppressed cell migration. In addition, we validated the
interaction between SPAG4 and Nesprin3, and the role of Nesprin3 in
migration conformed with the possible link between the two
proteins. We found that SPAG4 may regulate the localization and
expression of Nesprin3 at the protein level and further promote the
development of lung cancer. This SPAG4/Nesprin3 axis may be useful
for the development of new treatment strategies for patients with
lung cancer. In the present study, we focused on the following
aspects to determine the relationship between SPAG4, Nesprin3 and
lung cancer.
Firstly, nuclear heteromorphism is an important
index in determining the degree of tumor differentiation (2). The downregulation of SUN2, Nesprin1
and 2 may render the nucleus easy to deform; thus worsening the
tumor cell differentiation (21).
Previous studies have revealed that LINC complex proteins may
regulate the proliferation and migration of cancer cells, and that
Nesprin1 and 2 affect cell proliferation (15). In addition, Nesprin3 expression is
required for flow-induced MTOC polarization and directional
migration (19), and SUN2 inhibits
tumor cell proliferation and migration (16). Although the expression of many LINC
complex proteins is downregulated in cancer, SPAG4 was found to be
upregulated in renal carcinoma (18). We found that the expression of SPAG4
was significantly higher in pulmonary carcinoma than in adjacent
tissues. In addition, the results of immunohistochemistry revealed
that SPAG4 expression was associated with tumor differentiation
grade. Thus, SPAG4 may serve as a new biomarker for lung cancer
diagnosis.
Secondly, the LINC complex plays a significant role
in cell growth, such as cell migration, nuclear positioning, and
the cell cycle (22). Knaup et
al indicated that SPAG4 overexpression promoted cell
proliferation (18), and our
results from the scratch wound assay revealed that SPAG4 silencing
inhibited the migration of cancer cells. These findings
demonstrated that SPAG4 acts as a facilitator in tumor progression.
Shoji et al revealed that the knockdown of SPAG4 led to
tetraploidy of cancer cells, resulting in subsequent suppression of
cell proliferation due to cell division disturbance (23). In the present study, we provided
evidence to demonstrate that SPAG4 is a positive regulator of
Nesprin3. Nesprin3, an outer nuclear membrane protein, has been
reported to influence the directional migration of cells (19) and was revealed to play an important
role in promoting cell migration in our study. We also examined the
distribution and structure of SPAG4, and observed that SPAG4 can
anchor to the inner nuclear envelope and is polarized in the
cytoplasm. The SUN domain cannot be folded correctly without
transmembrane helices, and may be found in the endoplasmic
reticulum first, and then anchored to the inner nuclear envelope by
transmembrane helices.
Thirdly, the LINC complex is known to be the link
between the nuclear skeleton and cytoplasmic skeleton. The KASH and
SUN family of proteins have a variety of functions in the LINC
complex (24). While Nesprin1 and 2
are related to actin filaments and Nesprin4 connects to
microtubules, Nesprin3 is closely related to MTOC (11). The MTOC/nucleus connectivity affects
a series of cell functions including migration. It has been
reported that Nesprin3 has a structural role by organizing
perinuclear cytoskeletal architecture as well as a functional role
by modulating flow-induced MTOC migration (19,25).
These results reveal the possible relationship between SPAG4 and
Nesprin3. We confirmed the interaction between SPAG4 and Nesprin3
by immunoprecipitation and BiFC assays. The silencing of SPAG4
resulted in the loose localization of Nesprin3 and a decrease in
Nesprin3 expression. Therefore, we suggest that the roles of SPAG4
and Nesprin3 in cell migration are synergistic. Previous studies
have revealed that Nesprin3 silencing reduces both the cell
migration velocity and the directionality of migration (19). The scratch wound assay with
Nesprin3-silenced cancer cells supports this view. Without the link
between SPAG4 and Nesprin3, the MTOC/nuclear connectivity may be
weakened and chromosomes may move slowly, further reducing the
migration of lung cancer cells.
To maintain the core function of the LINC complex in
connecting the genome and nucleoskeleton to cytoplasmic filaments
and the extracellular matrix, the molecular structure of the
SUN-KASH interface would need to provide a tight binding interface
impervious to disruption (26). The
loss of these nuclear membrane proteins leads to an incomplete
nuclear membrane structure. Therefore, since a series of LINC
complex proteins such as SUN2 and Nesprin2 are downregulated in
tumors (13,15,16),
we speculate that SPAG4/Nesprin3 complexes may increase
compensatorily in order to complement missing LINC complexes to
maintain the total number of LINC complexes. It is possible that
tumor cells have alternative and cross-coordinated mechanisms to
maintain the characteristics of high invasion, once one becomes
deficient, the other compensates for the lack of activity.
Finally, although this interaction was confirmed,
the exact relationship between SPAG4 upregulation and the
downregulation of other LINC complex proteins is still unclear. We
did not determine the relationship between SPAG4 expression and the
prognosis of lung cancer patients. In addition, whether the
SPAG4/Nesprin3 complex complements the decreasing LINC complex in
lung cancer requires further validation. Such knowledge will, in
turn, provide a theoretical basis for new strategies in lung cancer
diagnosis and treatment.
Acknowledgements
The authors thank Wei Li for technical
assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472774 and
81170615).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XX and LJ conceived the study and revised the
manuscript. YJ and JJ designed and performed the experiments. LH
analyzed the data and revised the manuscript. ZZ and WF assisted in
the experiments. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the study are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in the studies involving
human participants were in accordance with the ethical standards of
the Institutional and/or National Research Committee and the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. This study was conducted with the approval of the Ethics
Committee of the Third Xiangya Hospital, Central South University,
Changsha, China (S071 2014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kratz JR, He J, Van Den Eeden SK, Zhu ZH,
Gao W, Pham PT, Mulvihill MS, Ziaei F, Zhang H, Su B, et al: A
practical molecular assay to predict survival in resected
non-squamous, non-small-cell lung cancer: Development and
international validation studies. Lancet. 379:823–832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zink D, Fischer AH and Nickerson JA:
Nuclear structure in cancer cells. Nat Rev Cancer. 4:677–687. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nery FC, Zeng J, Niland BP, Hewett J,
Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A and Breakefield XO:
TorsinA binds the KASH domain of nesprins and participates in
linkage between nuclear envelope and cytoskeleton. J Cell Sci.
121:3476–3486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ketema M and Sonnenberg A: Nesprin-3: A
versatile connector between the nucleus and the cytoskeleton.
Biochem Soc Trans. 39:1719–1724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tapley EC and Starr DA: Connecting the
nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear
envelope. Curr Opin Cell Biol. 25:57–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Link J, Leubner M, Schmitt J, Göb E,
Benavente R, Jeang KT, Xu R and Alsheimer M: Analysis of meiosis in
SUN1 deficient mice reveals a distinct role of SUN2 in mammalian
meiotic LINC complex formation and function. PLoS Genet.
10:e10040992014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel JT, Bottrill A, Prosser SL,
Jayaraman S, Straatman K, Fry AM and Shackleton S: Mitotic
phosphorylation of SUN1 loosens its connection with the nuclear
lamina while the LINC complex remains intact. Nucleus. 5:462–473.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu
B, Xu T, Zhuang Y, Xu R and Han M: SUN1 and SUN2 play critical but
partially redundant roles in anchoring nuclei in skeletal muscle
cells in mice. Proc Natl Acad Sci USA. 106:10207–10212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thomsen C, Udhane S, Runnberg R, Wiche G,
Ståhlberg A and Aman P: Fused in sarcoma (FUS) interacts with the
cytolinker protein plectin: Implications for FUS subcellular
localization and function. Exp Cell Res. 318:653–661. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vlcek S and Foisner R: Lamins and
lamin-associated proteins in aging and disease. Curr Opin Cell
Biol. 19:298–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fridkin A, Penkner A, Jantsch V and
Gruenbaum Y: SUN-domain and KASH-domain proteins during
development, meiosis and disease. Cell Mol Life Sci. 66:1518–1533.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kennedy C, Sebire K, de Kretser DM and
O'Bryan MK: Human sperm associated antigen 4 (SPAG4) is a potential
cancer marker. Cell Tissue Res. 315:279–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marmé A, Zimmermann HP, Moldenhauer G,
Schorpp-Kistner M, Müller C, Keberlein O, Giersch A, Kretschmer J,
Seib B, Spiess E, et al: Loss of Drop1 expression already at early
tumor stages in a wide range of human carcinomas. Int J Cancer.
123:2048–2056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tessema M, Willink R, Do K, Yu YY, Yu W,
Machida EO, Brock M, Van Neste L, Stidley CA, Baylin SB, et al:
Promoter methylation of genes in and around the candidate lung
cancer susceptibility locus 6q23-25. Cancer Res. 68:1707–1714.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto A, Hieda M, Yokoyama Y, Nishioka
Y K, Tsujimoto M and Matsuura N: Global loss of a nuclear lamina
component, lamin A/C, and LINC complex components SUN1, SUN2, and
nesprin-2 in breast cancer. Cancer Med. 4:1547–1557. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv XB, Liu L, Cheng C, Yu B, Xiong L, Hu
K, Tang J, Zeng L and Sang Y: SUN2 exerts tumor suppressor
functions by suppressing the Warburg effect in lung cancer. Sci
Rep. 5:179402015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tarnasky H, Gill D, Murthy S, Shao X,
Demetrick DJ and van der Hoorn FA: A novel testis-specific gene,
SPAG4, whose product interacts specifically with outer dense fiber
protein ODF27, maps to human chromosome 20q11.2. Cytogenet Cell
Genet. 81:65–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Knaup KX, Monti J, Hackenbeck T,
Jobst-Schwan T, Klanke B, Schietke RE, Wacker I, Behrens J, Amann
K, Eckardt KU, et al: Hypoxia regulates the sperm associated
antigen 4 (SPAG4) via HIF, which is expressed in renal clear cell
carcinoma and promotes migration and invasion in vitro. Mol
Carcinog. 53:970–978. 2014.PubMed/NCBI
|
|
19
|
Morgan JT, Pfeiffer ER, Thirkill TL, Kumar
P, Peng G, Fridolfsson HN, Douglas GC, Starr DA and Barakat AI:
Nesprin-3 regulates endothelial cell morphology, perinuclear
cytoskeletal architecture, and flow-induced polarization. Mol Biol
Cell. 22:4324–4334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yeh CH, Kuo PL, Wang YY, Wu YY, Chen MF,
Lin DY, Lai TH, Chiang HS and Lin YH: SEPT12/SPAG4/LAMINB1
complexes are required for maintaining the integrity of the nuclear
envelope in postmeiotic male germ cells. PLoS One. 10:e01207222015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cartwright S and Karakesisoglou I:
Nesprins in health and disease. Semin Cell Dev Biol. 29:169–179.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isermann P and Lammerding J: Nuclear
mechanics and mechanotransduction in health and disease. Curr Biol.
23:R1113–R1121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shoji K, Murayama T, Mimura I, Wada T,
Kume H, Goto A, Ohse T, Tanaka T, Inagi R, van der Hoorn FA, et al:
Sperm-associated antigen 4, a novel hypoxia-inducible factor 1
target, regulates cytokinesis, and its expression correlates with
the prognosis of renal cell carcinoma. Am J Pathol. 182:2191–2203.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jungwirth MT, Kumar D, Jeong DY and
Goodchild RE: The nuclear envelope localization of DYT1 dystonia
torsinA-ΔE requires the SUN1 LINC complex component. BMC Cell Biol.
12:242011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Revach OY, Weiner A, Rechav K, Sabanay I,
Livne A and Geiger B: Mechanical interplay between invadopodia and
the nucleus in cultured cancer cells. Sci Rep. 5:94662015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meinke P and Schirmer EC: LINC'ing form
and function at the nuclear envelope. FEBS Lett. 589 (19 Pt
A):2514–2521. 2015. View Article : Google Scholar : PubMed/NCBI
|