Introduction
Breast cancer is a malignancy occurring in breast
ductal epithelium. It is one of the most common tumors in females,
accounting for 7–10% in whole body malignancies (1). Approximately 1.5 million new breast
cancer cases are diagnosed annually worldwide, along with ~0.57
million cases who succumb to this disease (1). Developed western countries, such as
North America and Europe, are high-incidence areas (2). The morbidity in these areas is ~4
times that of Asia, Africa and Latin America (2). China is a low-incidence area of breast
cancer. However, the morbidity in the past 20 years has been
increasing, particularly in developed areas such as Beijing,
Shanghai and Guangzhou. Breast cancer has become a major hazard
threatening female health. It has surpassed cervical cancer and
ranks first among female malignancies (3).
miRNAs were first discovered in Caenorhabditis
elegans (4). miRNAs are known
to be extensively distributed in eukaryotes (4). It is one of the greatest gene families
accounting for ~1% of the genome. Each miRNA can regulate the
expression of hundreds of genes. In addition, multiple miRNAs can
act on a target in the same manner to downregulate the expression
of multiple proteins. miRNAs play a vital role in regulating gene
expression, biological growth and development (5). Therefore, some miRNAs may play
important roles in biological evolution processes, such as cell
proliferation, differentiation and apoptosis (5).
IGFs is a large family. It includes two types of
growth factors, namely, insulin-like growth factor-1 (IGF-1) and
insulin-like growth factor-2 (IGF-2) (6). IGF-1 and IGF-2 share high homology to
insulin (6). IGF-1 plays a key role
in growth and development, and exerts two types of biological
effects (7). The first one is
insulin-like metabolism. It can promote tissue uptake of glucose as
well as synthesis of glycogen, protein and fat. In addition, it
inhibits hepatic glycogen decomposition (8). The second effect is functioning as the
mediator of growth hormone. It can promote cells to enter stage
stage S from stage G1 and accelerate cell division (8). As a result, it can enhance cell
growth. IGF-1R can promote cell proliferation (8). Thus, it has attracted attention in
research on the genesis and development of cancer. These results
revealed that microRNA-320a suppresses tumor cell growth and
invasion of human breast cancer by targeting IGF-1R.
Materials and methods
Patient samples
Samples from patients with breast cancer (n=12, 67±8
years age) and healthy volunteers (n=12) were collected as well as
10 ml peripheral blood. Serum was collected after centrifugation at
2,000 × g for 10 min at 4°C and saved at −80°C for RT-qPCR or gene
expression microarrays. The present study was approved by the
Ethics Committee of Peking Union Medical College Hospital and
written informed consent of patients was obtained from all patients
from Peking Union Medical College Hospital.
RNA extraction and reverse
transcription-qPCR (RT-qPCR)
Total RNA was isolated from serum and cell samples
using TRIzol reagent (Invitrogen; Thermo Fisher Scientifc Inc.,
Waltham, MA, USA). Total RNA (500 ng) was reverse-transcribed into
cDNA using PrimeScript RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China). RT-qPCR was performed using SYBR Premix Ex
Taq (Takara Biotechnology Co., Ltd.). The cycling conditions were
as follows: 42°C for 5 min; 95°C for 30 sec; and 40 cycles at 95°C
for 25 sec, 55°C for 15 sec and 72°C for 30 sec. Each sample was
analyzed in triplicate. The expression of microRNA-320a was
analyzed using the 2−ΔΔCq method.
Gene expression microarrays
Total RNA was isolated from serum samples using
RNeasy Plus Micro Kit (Qiagen, Inc., Valencia, CA, USA). CDNA were
amplified using the Ovation PicoSL WTA System V2 kit (NuGEN
Technologies, San Carlos, CA, USA) and Cy3-labeled using the
SureTag DNA labeling kit (Agilent Technologies, Inc., Santa Clara,
CA, USA). SureScan Microarray Scanner (Agilent Technologies, Inc.)
was used to scan. Data was extracted using Feature Extraction
software v. 10.7.3.1 (Agilent Technologies, Inc.) and evaluated
using the software (RDevelopment Core Team, 2012).
Cell culture and transfection
MDA-MB-231 cells were purchased from the Cell Bank
of the Shanghai Institute for Biological Sciences, Chinese Academy
of Sciences (Shanghai, China) and cultured in Dulbecco's modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS; both from
Gibco; Thermo Fisher Scientific, Inc. Carlsbad, CA, USA).
MicroRNA-320a, anti-microRNA-320a, si-IGF-1R and negative mimics
were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
MicroRNA-320a (500 ng), IGF-1R plasmid anti-microRNA-320a,
si-IGF-1R and negative mimics were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientifc, Inc.) according to the manufacturer's protocol. After
transfection for 4 h, old DMEM was removed and new DMEM containing
the p-Akt inhibitor (MK 2206 dihydrochloride, 2.5 nM) was added
into the cells for 48 h.
Cell proliferation assay
Following transfection for 0, 24, 48 and 72 h,
transfected cells (1×103) were added to 96-well plates
and 20 µl MTT solution (5 mg/ml) was added to every well and
incubated for a further 4 h at 37°C. Subsequently, the culture
medium was removed and dimethyl sulfoxide (DMSO) was dissolved for
20 min at 37°C.
Transwell cell invasion assay
After transfection for 6 h, transfected cells
(1×105) were added to the upper chamber of the Transwell
plates (BD Biosciences, San Jose, CA, USA) and 500 µl culture
medium supplemented with 10% FBS was added to the lower portion of
the chamber. Non-invading cells were removed using cotton wool
after transfection for 48 h. Then, the Transwell membrane was fixed
with 95% ethanol on ice for 30 min, stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) and
quantified under a light microscope.
Apoptosis assay
Following transfection for 48 h, the cells were
washed with PBS and stained with Annexin V-fluorescein
isothiocyanate and propidium iodide (BD Biosciences, San Jose, CA,
USA) for 15 min in the dark. The apoptosis rate was assessed using
a C6 flow cytometer (BD Biosciences).
Western blot analysis
Total protein was isolated from the transfected
cells using RIPA buffer and determined using the Pierce™ BCA
Protein Assay kit (Thermo Fisher Scientifc, Inc.). Protein (50 µg)
was fractionated using 8–12% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked using 5% skimmed dry
milk in Tris-buffered saline and Tween-20 (TBST) at room
temperature for 1 h, followed by incubation with the following
primary antibodies: IGF-1R (1:500; cat. no. sc-7952), p-AKt (1:500;
cat. no. sc-7985-R), Bax (1:500; cat. no. sc-6236), cyclin D1
(1:500; cat. no. sc-717) and GAPDH (1:500; cat. no. sc-25778; all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at
4°C. The membranes were washed with TBST for 20 min and probed with
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat.
no. sc-2004; Santa Cruz Biotechnology, Inc.) at room temperature
for 1 h. The protein bands were visualized using a chemiluminescent
detection system (Thermo Fisher Scientifc, Inc.).
Immunofluorescence
Cells were fixed with 4% w/v paraformaldehyde in
phosphate-buffered saline (PBS) for 15 min at room temperature and
incubated with 0.3% Triton X-100 for 15 min at room temperature.
The cells were then blocked with 5% BSA in PBS for 1 h at room
temperature and incubated with the primary antibody IGF-1R at 4°C
overnight. After being washed with PBS, the sections were incubated
in a mixture of fluorescent secondary antibody (Alexa
555-conjugated antimouse immunoglobulin G) (1:100; cat. no.
sc-362272; Santa Cruz Biotechnology) for 1 h at room temperature.
The cells were analyzed using an LSM 700 NLO confocal microscope
(Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences among groups were analyzed by a one-way analysis of
variance (ANOVA) followed by Bonferroni's correction for multiple
comparisons. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
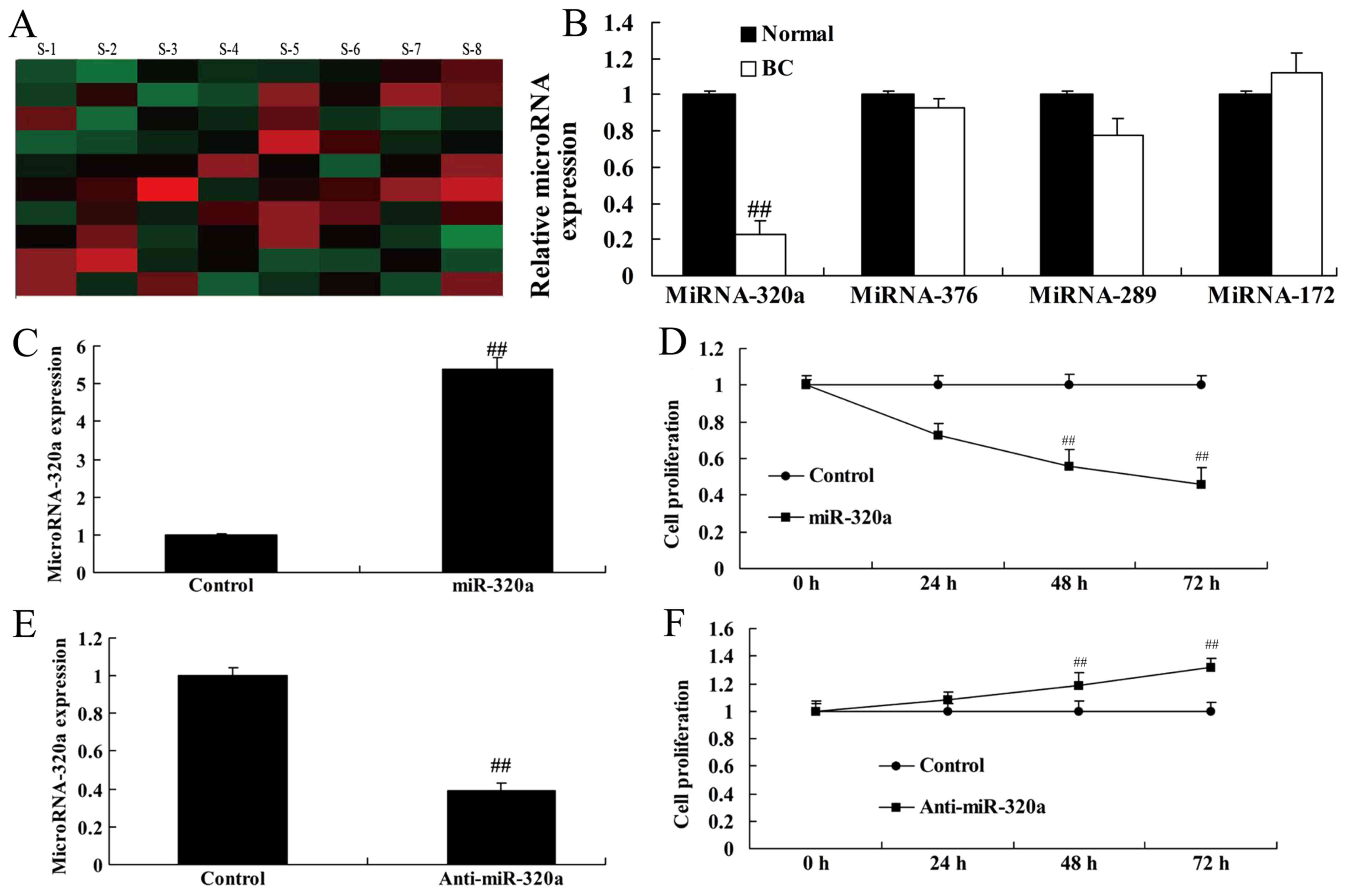
MicroRNA-320a expression is
downregulated in human breast cancer
To validate the role of microRNA-320a in the serum
of breast cancer patients and a healthy normal control group, we
analyzed the expression in microRNA-320a by real-time PCR. As
revealed in Fig. 1A and B,
microRNA-320a expression was downregulated in human breast cancer
patients, compared with the normal control. Then, we used
microRNA-320a and anti-microRNA-320a mimics to increase and inhibit
microRNA-320a expression in MDA-MB-231 cells, respectively
(Fig. 1C and E). The results
revealed that overexpression of microRNA-320a inhibited cell
proliferation of MDA-MB-231 cells, and downregulation of
microRNA-320a induced cell proliferation of MDA-MB-231 cells,
compared with the negative control groups (Fig. 1D and F).
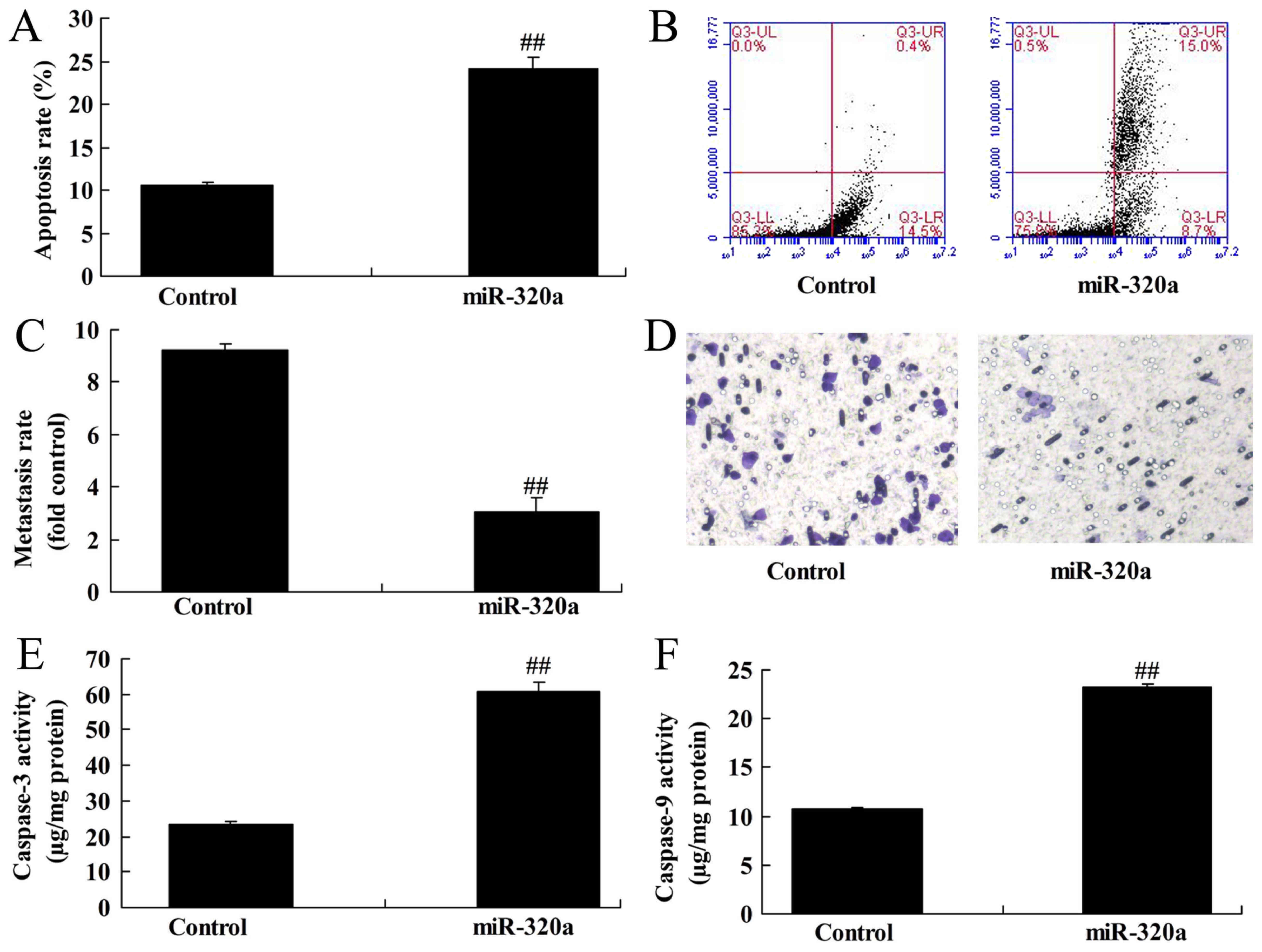
Overexpression of microRNA-320a
induces apoptosis and reduces invasion of MDA-MB-231 cells
Subsequently, it was demonstrated that
overexpression of microRNA-320a induced the apoptosis rate and
inhibited the Transwell cell invasion rate of MDA-MB-231 cells,
compared with the negative control groups (Fig. 2A-D). In addition, overexpression of
microRNA-320a induced caspase-3/-9 activity of MDA-MB-231 cells,
compared with the negative control groups (Fig. 2E and F).
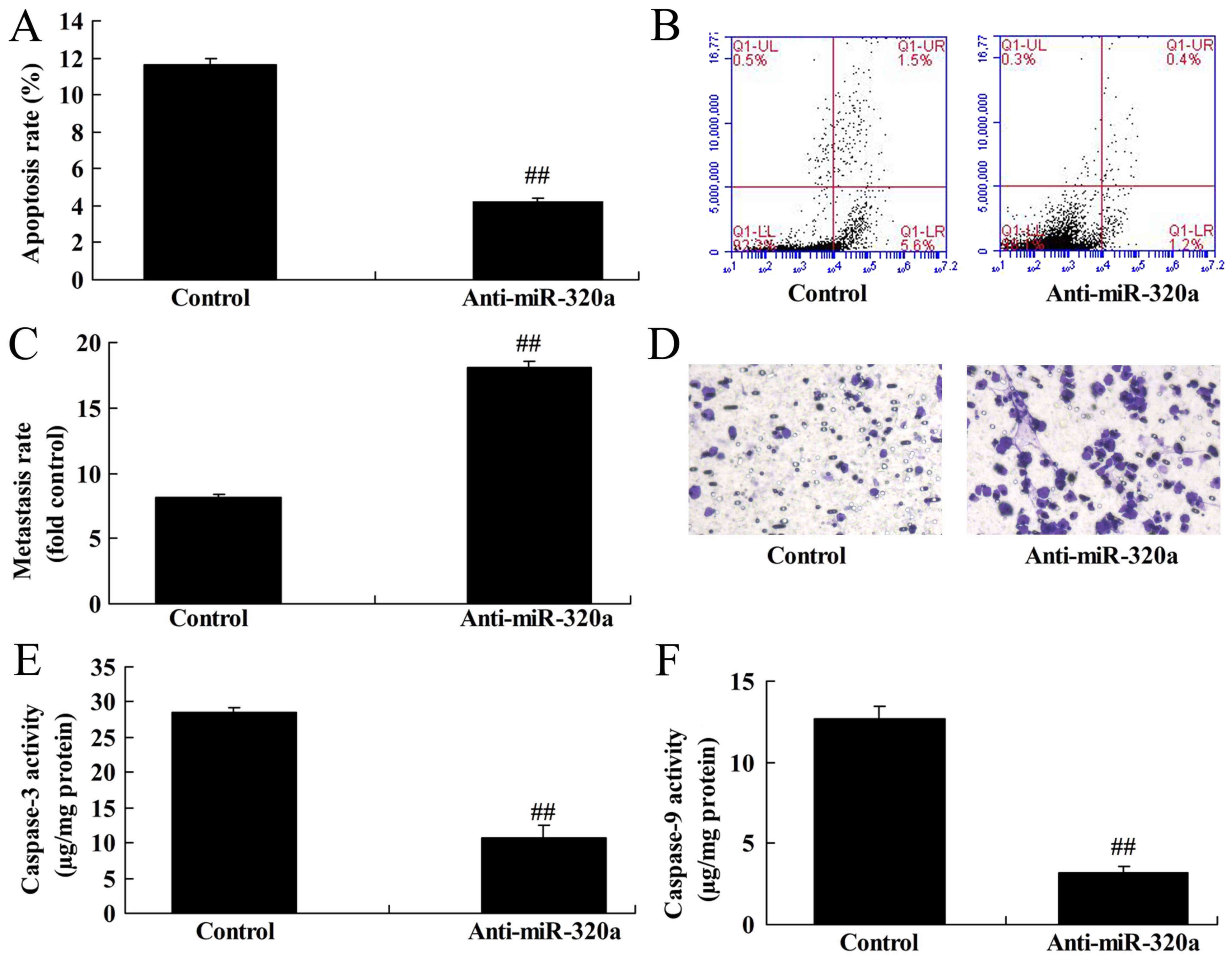
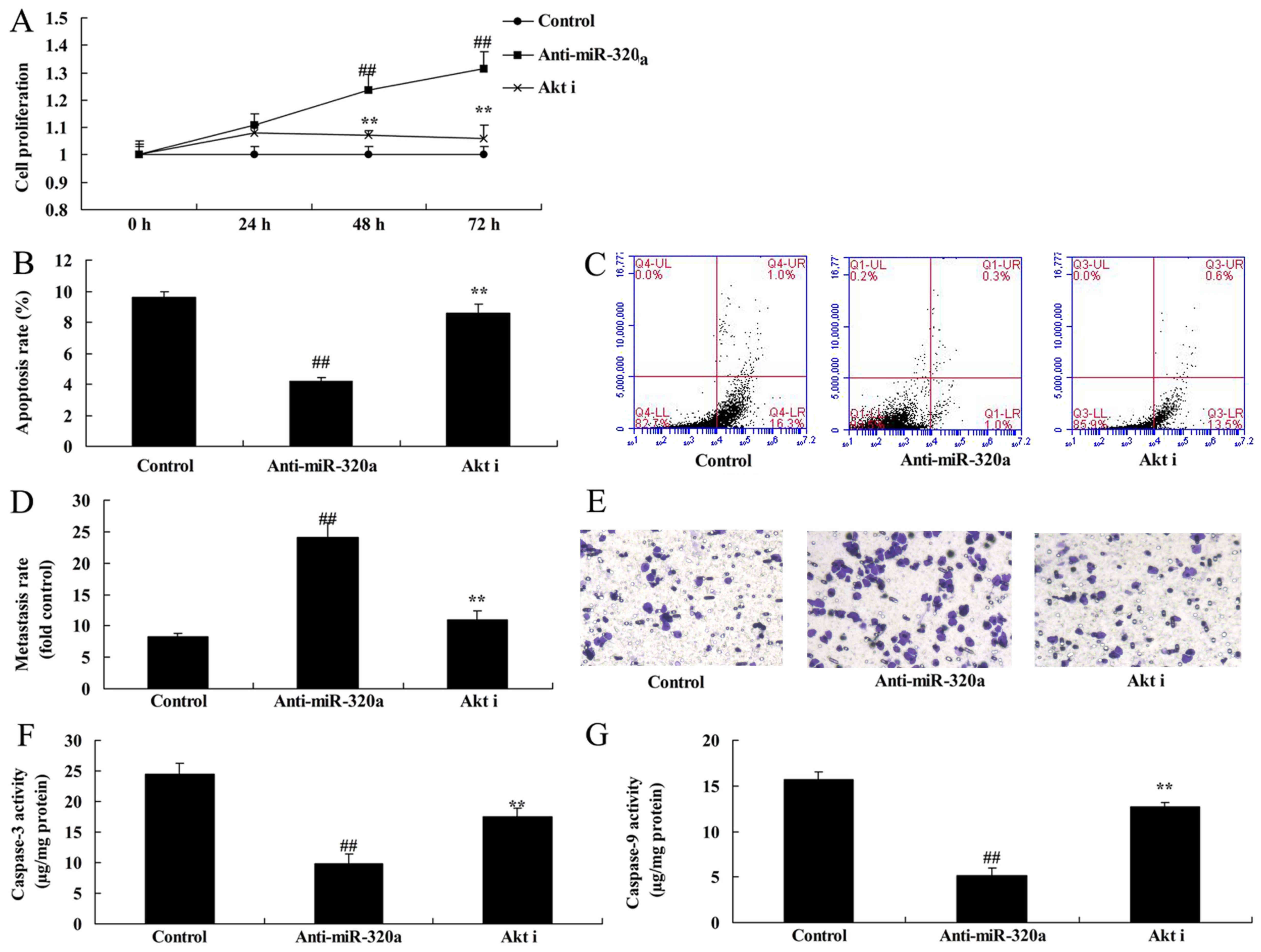
Downregulation of microRNA-320a
reduces apoptosis of MDA-MB-231 cells
Next, it was demonstrated that downregulation of
microRNA-320a inhibited the apoptosis rate and increased the
Transwell cell invasion rate of MDA-MB-231 cells, compared with the
negative control groups (Fig.
3A-D). Furthermore, downregulation of microRNA-320a suppressed
caspase-3/-9 activity of MDA-MB-231 cells, compared with the
negative control group (Fig. 3E and
F). Thus, these results revealed that microRNA-320a
participated in apoptosis in breast cancer cells.
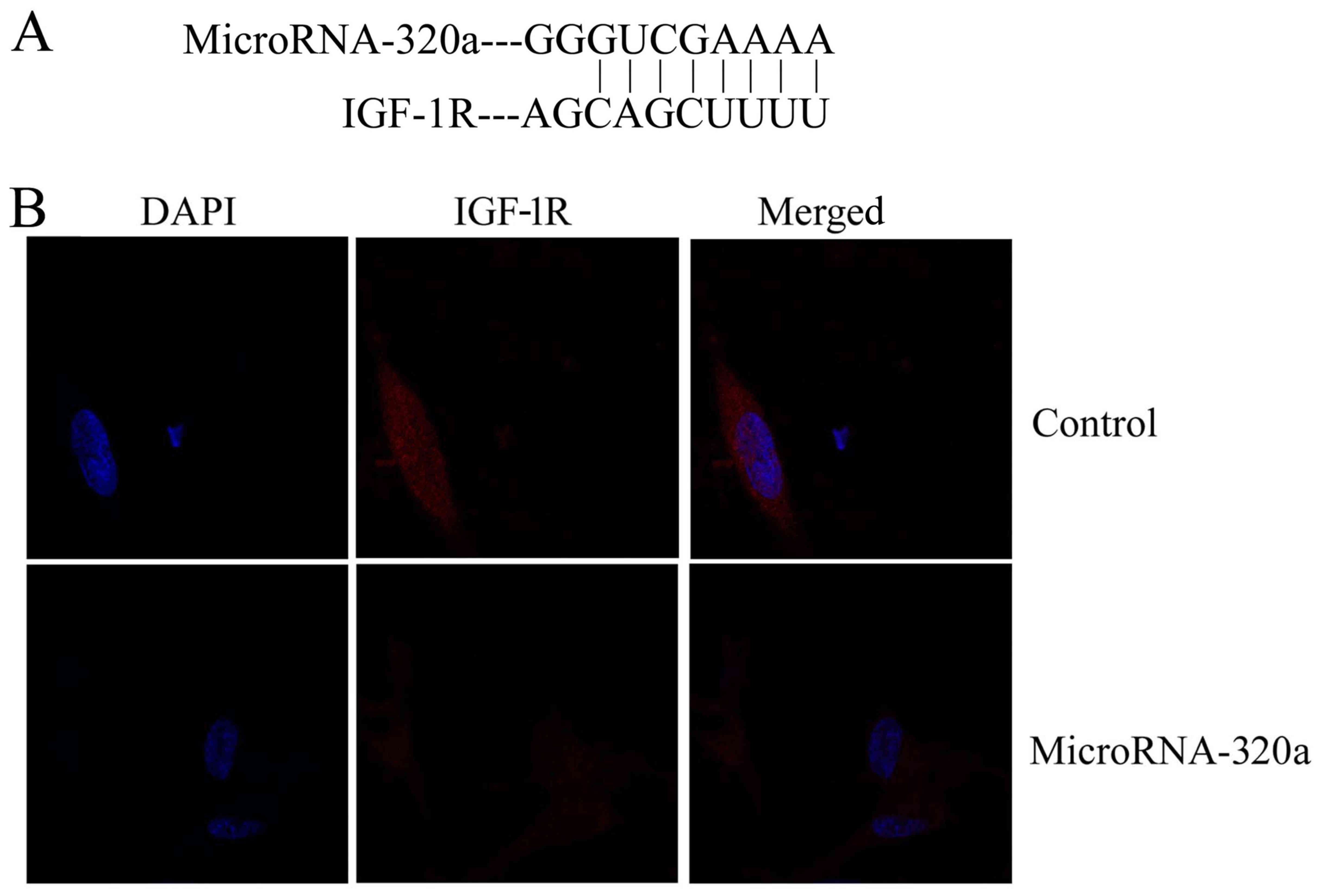
Effects of microRNA-320a on MDA-MB-231
cell apoptosis through IGF-1R expression
We searched for microRNA-320a target genes using
three computer-aided miRNA target prediction programs and IGF-1R
was revealed to be a target (Fig.
4A). Immunofluorescence revealed that overexpression of
microRNA-320a suppressed IGF-1R protein expression in MDA-MB-231
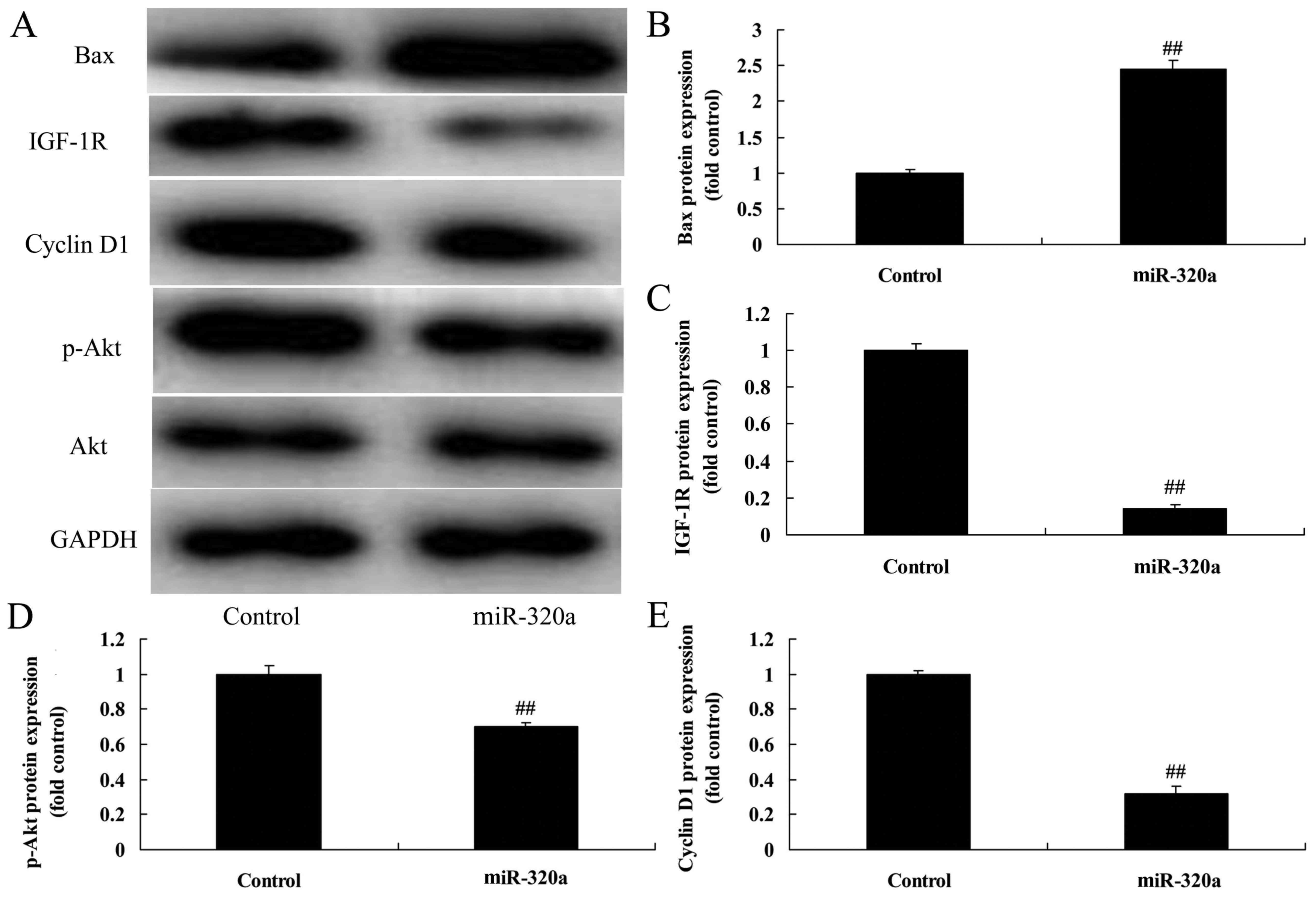
cells, compared with the negative control group (Fig. 4B). Next, we found that
overexpression of microRNA-320a suppressed IGF-1R, p-Akt and cyclin
D1 protein expression, and induced Bax protein expression in
MDA-MB-231 cells, compared with the negative control groups
(Fig. 5). The suppression of
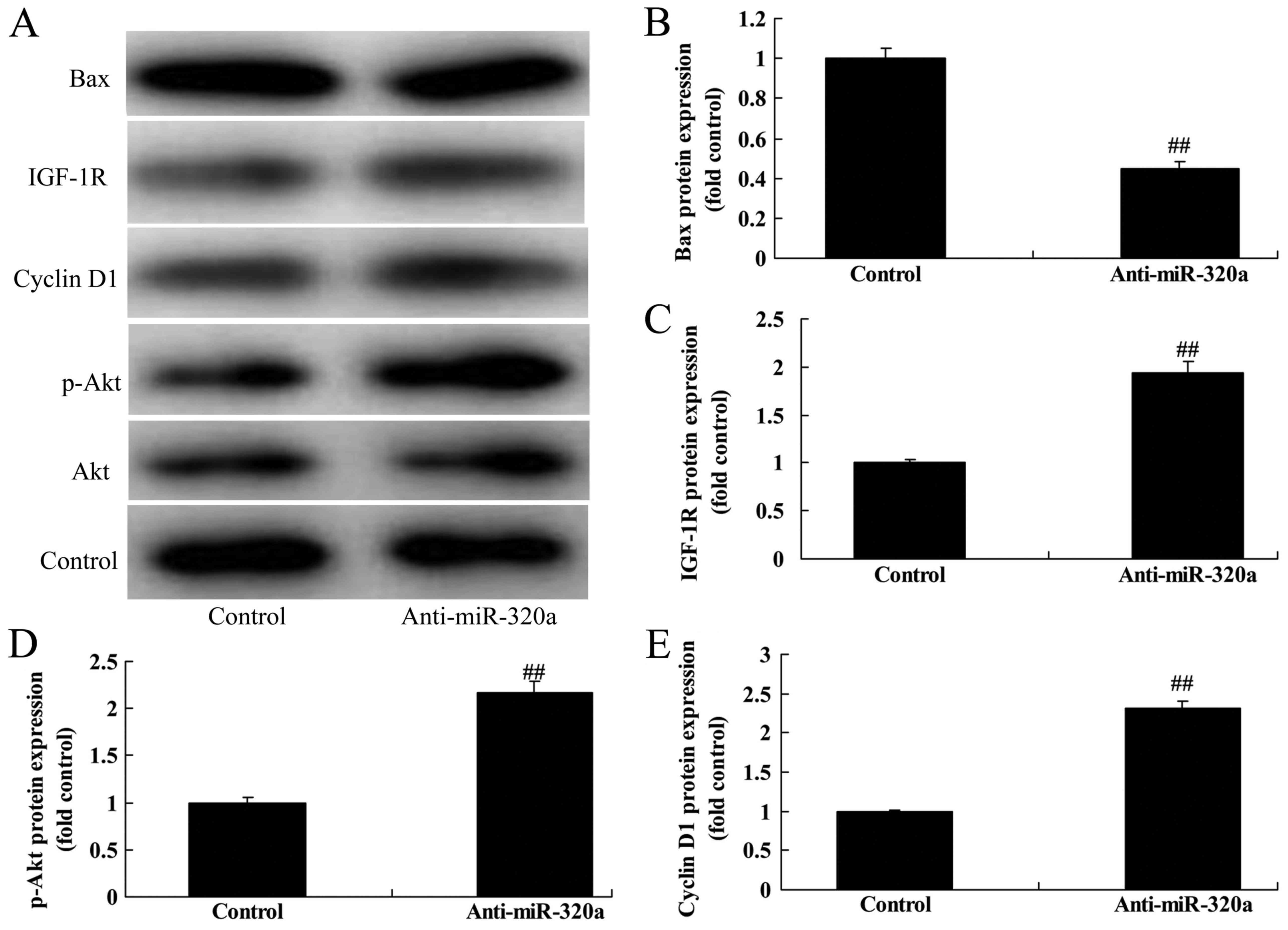
microRNA-320a induced IGF-1R, p-Akt and cyclin D1 protein
expression, and suppressed Bax protein expression in MDA-MB-231
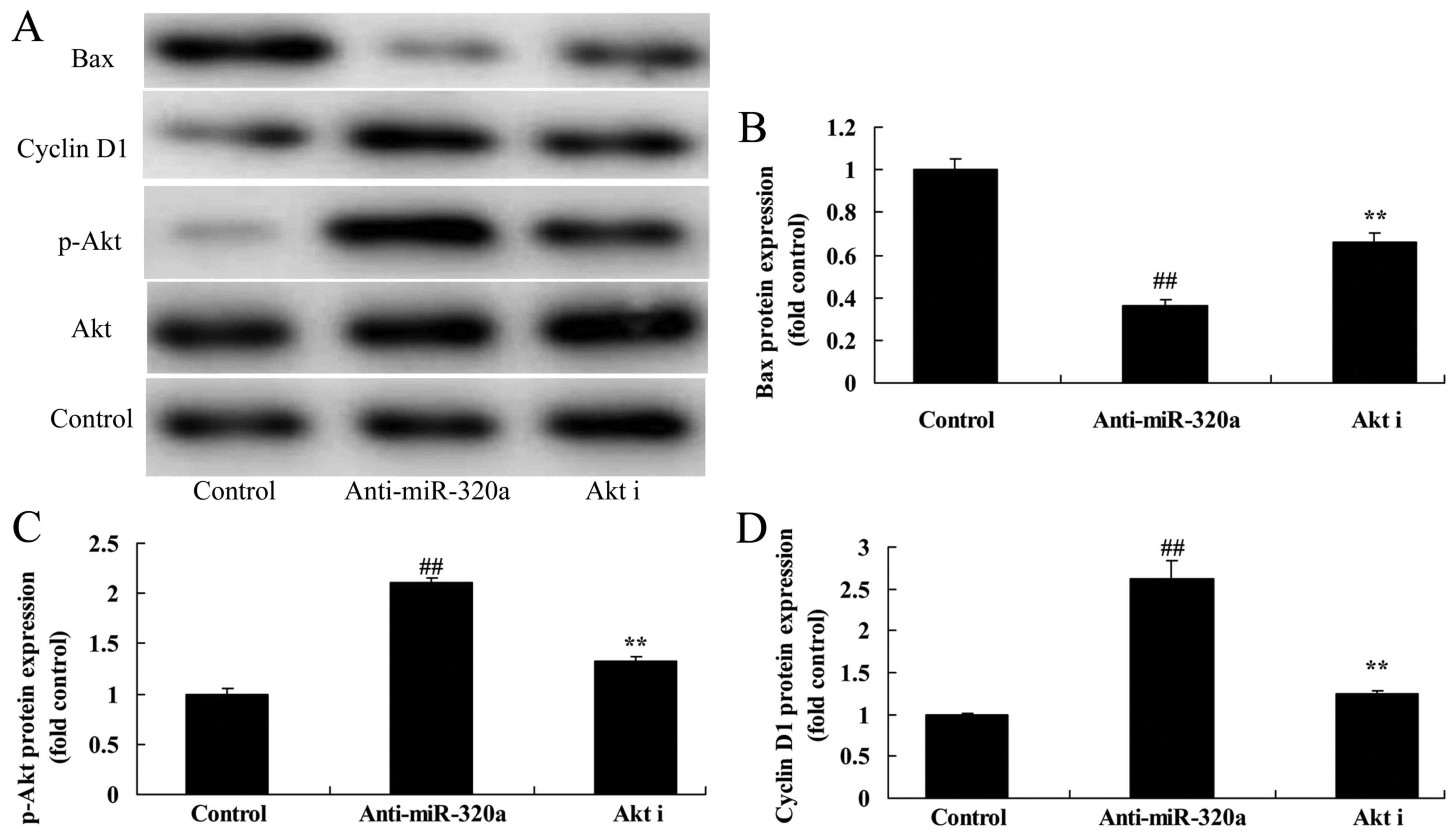
cells, compared with the negative control group (Fig. 6).
IGF-1R decreases the effects of
microRNA-320a on the apoptosis of MDA-MB-231 cells
To explore the tumor-suppressive role of IGF-1R in
the effects of microRNA-320a on the apoptosis of MDA-MB-231 cells,
the IGF-1R plasmid and microRNA-320a were co-transfected with
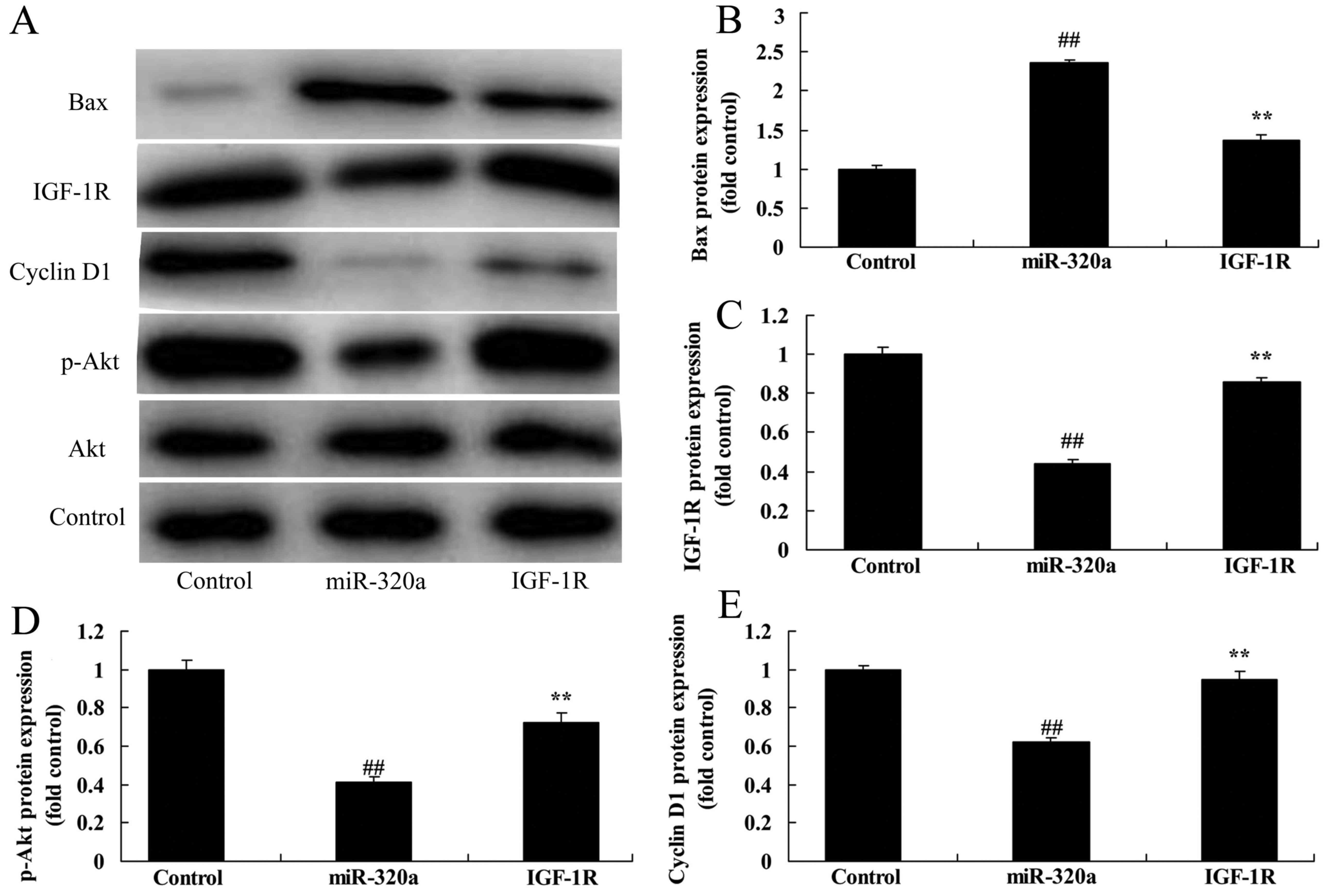
MDA-MB-231 cells. As revealed in Fig.
7A and C, IGF-1R induced the expression of IGF-1R protein
expression, and decreased the effects of microRNA-320a on the
suppression of p-Akt and cyclin D1 protein expression, and
induction of Bax protein expression in MDA-MB-231 cells, compared
with the negative control groups (Fig.
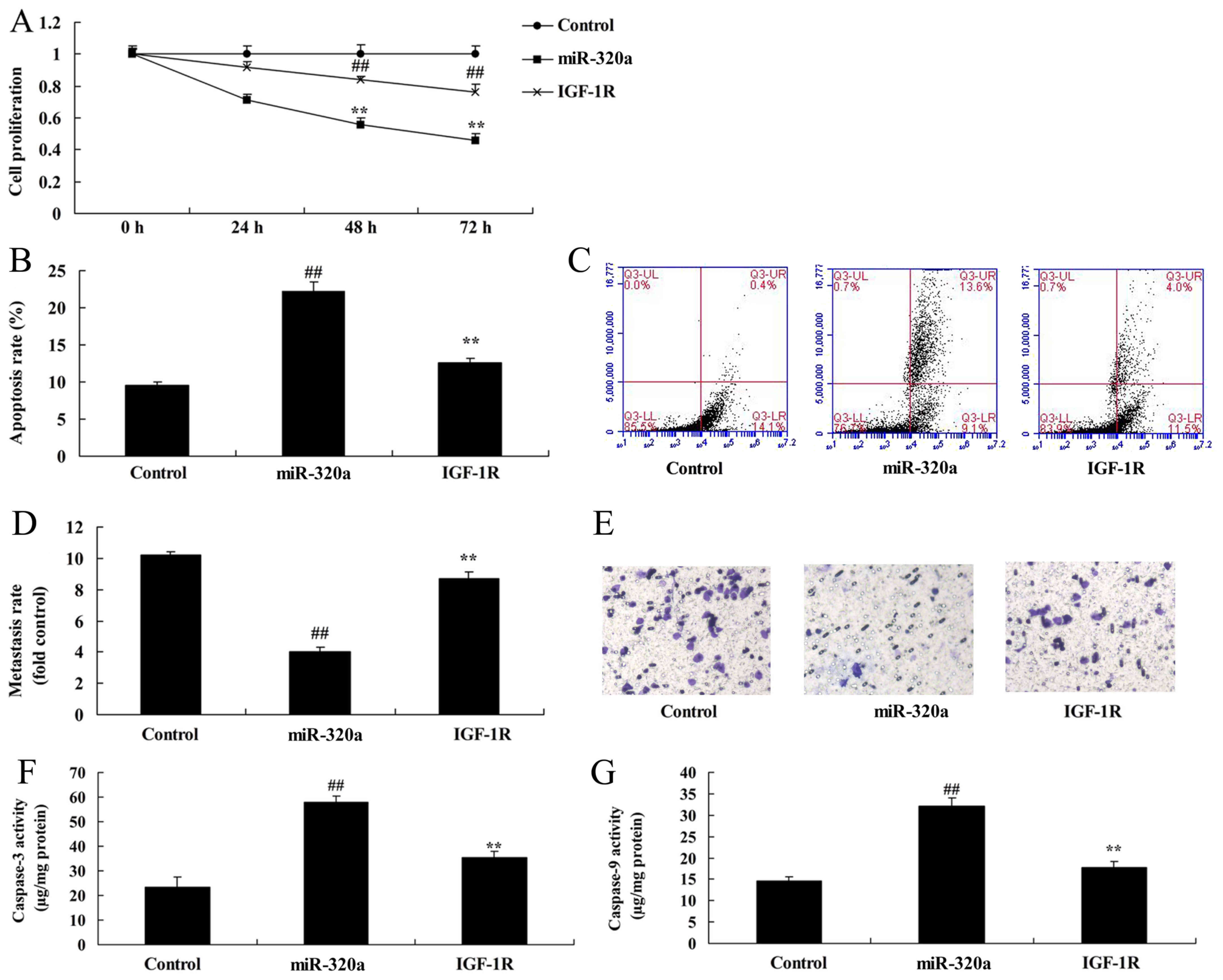
7A-E). In addition, the activation of IGF-1R decreased the
effects of microRNA-320a on the inhibition of cell proliferation
and Transwell cell invasion rate, and promotion of apoptosis of
MDA-MB-231 cells (Fig. 8A-E). The
microRNA-320a-induced Bax expression and caspase-3/-9 activities
were decreased in MDA-MB-231 cells by IGF-1R, compared with the
microRNA-320a group (Fig. 8F and
G).
Akt inhibitor reduces the effects of
anti-microRNA-320a on the apoptosis of MDA-MB-231 cells
Next, to clarify whether Akt participated in the
effects of microRNA-320a on the apoptosis of MDA-MB-231cells, p-Akt
inhibitor (MK 2206 dihydrochloride, 2.5 nM) was used in
MDA-MB-231cells following downregulation of microRNA-320a. As
revealed in Fig. 9A-D, the p-Akt
inhibitor suppressed p-Akt and cyclin D1 protein expression, and
induced Bax protein expression in the MDA-MB-231 cells following
downregulation of microRNA-320a, compared with the
anti-microRNA-320a group. The p-Akt inhibitor following
downregulation of microRNA-320a decreased cell proliferation and
the Transwell cell invasion rate, and increased the apoptosis rate,
Bax expression and caspase-3/-9 activities in the MDA-MB-231 cells
compared with the anti-microRNA-320a group (Fig. 10).
Discussion
Breast cancer is mainly treated with traditional
surgical treatment, supplemented by local or systemic radiotherapy,
chemotherapy and endocrine therapy postoperatively (9). Molecular biological and molecular
immunological research has been rapidly developed in recent years
(10). In addition, research on
tumor gene theories has also advanced rapidly (9). These have provided opportunities for
the early diagnosis and treatment of breast cancer. Breast cancer
research on gene therapy includes inhibiting oncogene function,
recovering tumor suppressor gene function, gene immunotherapy, and
suicide gene therapy (3). In
addition, it also includes multidrug resistance gene therapy,
antitumor angiogenesis gene therapy, apoptotic gene therapy and
application of an oncolytic virus (3). We found that microRNA-320a expression
was downregulated in human breast cancer.
It is currently known that miRNAs are extensively
distributed in eukaryotes. It is one of the greatest gene families,
accounting for ~1% of the genome. miRNAs play a vital role in
regulating gene expression, biological growth and development
(11,12). In the present study, it was found
that overexpression of microRNA-320a induced apoptosis of breast
cancer. Yu et al reported that microRNA-320a inhibited
breast cancer metastasis by targeting metadherin (13). Therefore, all these studies provide
evidence that microRNA-320a is a tumor-suppressive microRNA in
human breast cancer.
IGF-1R is excessively expressed in multiple tumors
(14). They include pancreatic,
prostate, non-small cell lung and breast cancer (14). IGF-1R is markedly and excessively
expressed in breast cancer cells compared with normal breast
epithelium and benign tumor tissues. Moreover, research has
indicated that the IGF-1R expression level is related to the
recurrence, metastasis and prognosis of breast cancer (15). The present study also revealed that
overexpression of microRNA-320a suppressed IGF-1R, p-Akt and cyclin
D1 protein expression, and induced Bax protein expression in
MDA-MB-231 cells. Guo et al revealed that microRNA-320a
suppresses glioma cells by targeting IGF-1R expression (16).
IGF-1R can bind with corresponding ligands (17). Subsequently, its tyrosine kinase
will produce auto-phosphorylation to phosphorylate its substrate
(17). Furthermore, it can activate
the PI3K/Akt or MAPK signaling pathways. In addition, it has been
revealed to promote phosphorylation inactivation of the BAD protein
in the Bcl-2 family (18). Thus, it
can prevent cells from apoptosis. The PI3K/Akt signaling pathway
can be activated in numerous human malignancies (18). They include breast, colorectal,
ovarian, pancreatic and endometrial cancer. We found that IGF-1R
decreased the effects of microRNA-320a on the apoptosis of
MDA-MB-231 cells. This may at least provide certain insights into
the tumor-suppressive mechanism of microRNA-320a in human breast
cancer.
Existing studies have indicated that the
PI3K/Akt/mTOR signaling pathway can be activated in numerous
malignancies (19). Such signaling
pathways are activated in 70% of breast cancer cases (19). There are studies reporting the
correlation of the PI3K/Akt/mTOR signaling pathway with breast
cancer (20). A series of signaling
pathways, including the PI3K/Akt/mTOR signal transduction pathway,
can induce tumor angiogenesis (21). Moreover, they can regulate processes
such as tumor cell differentiation, proliferation, apoptosis and
metastasis (21). Our study
revealed that the Akt inhibitor reduced the effects of
anti-microRNA-320a on the apoptosis of MDA-MB-231 cells. Zhu et
al revealed that microRNA-320a suppressed chronic myeloid
leukemia by targeting PI3K/AKT expression (16).
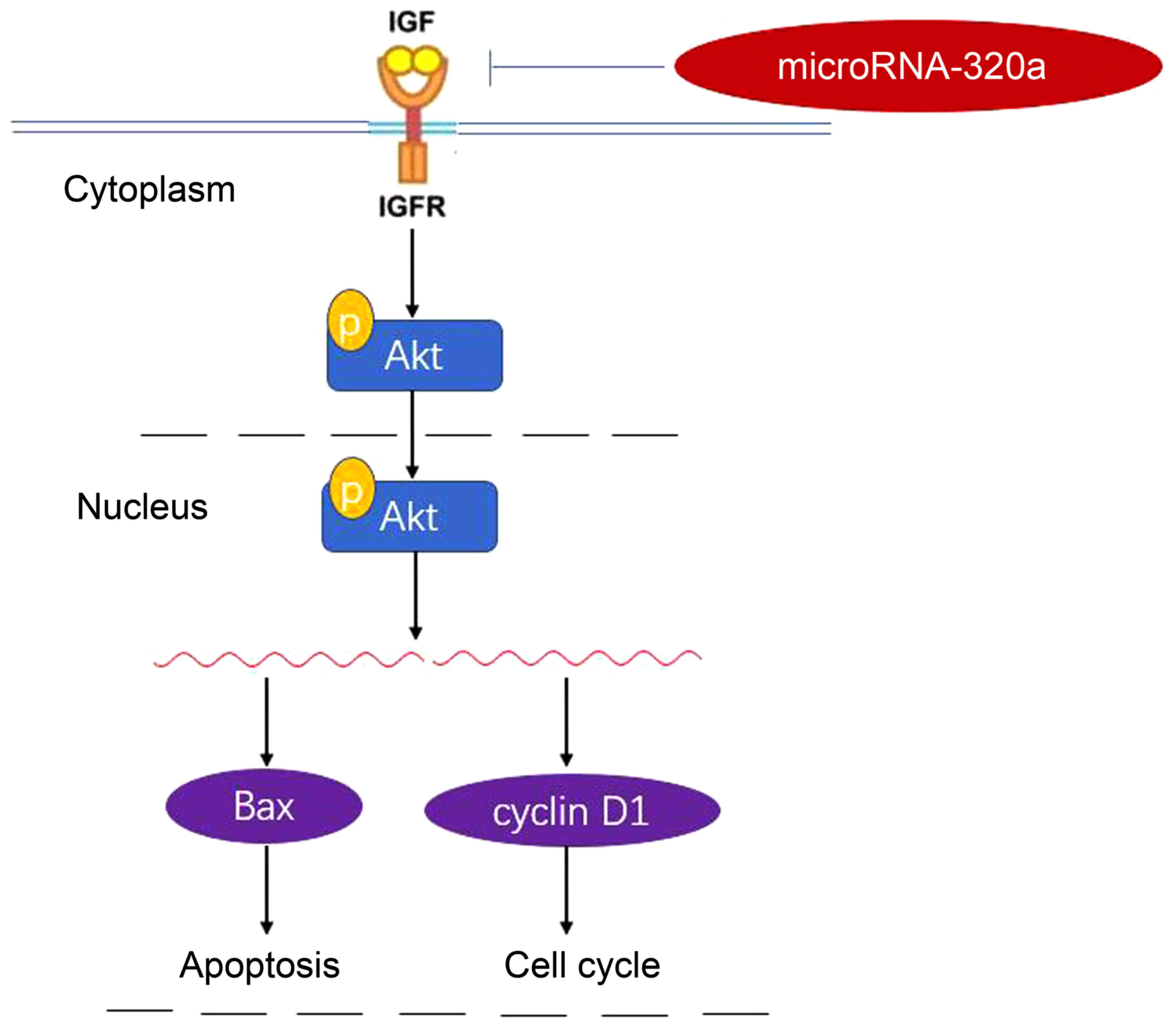
In conclusion, the present study demonstrated that
microRNA-320a expression was downregulated in human breast cancer
cells. MicroRNA-320a suppressed tumor cell growth and invasion of
human breast cancer cells through the PI3K/Akt signaling pathway by
targeting IGF-1R (Fig. 11). In the
future, the aberrant expression of microRNA-320a may be utilized in
interventions for patients with breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QS designed the experiment; JG, YZ, FM, YL, SS and
YZ performed the experiment. JG and QS analyzed the data; QS wrote
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Peking Union Medical College Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chirico A, D'Aiuto G, Penon A, Mallia L,
DE Laurentiis M, Lucidi F, Botti G and Giordano A: Self-efficacy
for coping with cancer enhances the effect of reiki treatments
during the pre-surgery phase of breast cancer patients. Anticancer
Res. 37:3657–3665. 2017.PubMed/NCBI
|
|
2
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gokal K, Wallis D, Ahmed S, Boiangiu I,
Kancherla K and Munir F: Effects of a self-managed home-based
walking intervention on psychosocial health outcomes for breast
cancer patients receiving chemotherapy: A randomised controlled
trial. Support Care Cancer. 24:1139–1166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu H, Dai M, Chen X, Chen X, Qin S and
Dai S: Integrated analysis of the potential roles of miRNAmRNA
networks in triple negative breast cancer. Mol Med Rep.
16:1139–1146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka H, Hazama S, Iida M, Tsunedomi R,
Takenouchi H, Nakajima M, Tokumitsu Y, Kanekiyo S, Shindo Y,
Tomochika S, et al: miR-125b-1 and miR-378a are predictive
biomarkers for the efficacy of vaccine treatment against colorectal
cancer. Cancer Sci. 108:2229–2238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy JP and Pinto DM: Temporal proteomic
analysis of IGF-1R signalling in MCF-7 breast adenocarcinoma cells.
Proteomics. 10:1847–1860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kucab JE and Dunn SE: Role of IGF-1R in
mediating breast cancer invasion and metastasis. Breast Dis.
17:41–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heskamp S, van Laarhoven HW,
Molkenboer-Kuenen JD, Franssen GM, Versleijen-Jonkers YM, Oyen WJ,
van der Graaf WT and Boerman OC: ImmunoSPECT and immunoPET of
IGF-1R expression with the radiolabeled antibody R1507 in a
triple-negative breast cancer model. J Nucl Med. 51:1565–1572.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalder M, Kyvernitakis I, Albert US,
Baier-Ebert M and Hadji P: Effects of zoledronic acid versus
placebo on bone mineral density and bone texture analysis assessed
by the trabecular bone score in premenopausal women with breast
cancer treatment-induced bone loss: Results of the ProBONE II
substudy. Osteoporos Int. 26:353–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barbie TU, Alexe G, Aref AR, Li S, Zhu Z,
Zhang X, Imamura Y, Thai TC, Huang Y, Bowden M, et al: Targeting an
IKBKE cytokine network impairs triple-negative breast cancer
growth. J Clin Invest. 124:5411–5423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Danza K, Summa S, Pinto R, Pilato B,
Palumbo O, Carella M, Popescu O, Digennaro M, Lacalamita R and
Tommasi S: TGFbeta and miRNA regulation in familial and sporadic
breast cancer. Oncotarget. 8:50715–50723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piperigkou Z, Franchi M, Gotte M and
Karamanos NK: Estrogen receptor beta as epigenetic mediator of
miR-10b and miR-145 in mammary cancer. Matrix Biol. 64:94–111.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Wang JG, Zhang L, Yang HP, Wang L,
Ding D, Chen Q, Yang WL, Ren KH, Zhou DM, et al: MicroRNA-320a
inhibits breast cancer metastasis by targeting metadherin.
Oncotarget. 7:38612–38625. 2016.PubMed/NCBI
|
|
14
|
Ren JG, Seth P, Ye H, Guo K, Hanai JI,
Husain Z and Sukhatme VP: Citrate suppresses tumor growth in
multiple models through inhibition of glycolysis, the tricarboxylic
acid cycle and the IGF-1R pathway. Sci Rep. 7:45372017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li ZH, Xiong QY, Xu L, Duan P, Yang QO,
Zhou P and Tu JH: miR-29a regulated ER-positive breast cancer cell
growth and invasion and is involved in the insulin signaling
pathway. Oncotarget. 8:32566–32575. 2017.PubMed/NCBI
|
|
16
|
Guo T, Feng Y, Liu Q, Yang X, Jiang T,
Chen Y and Zhang Q: MicroRNA-320a suppresses in GBM patients and
modulates glioma cell functions by targeting IGF-1R. Tumour Biol.
35:11269–11275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin Y, Deng W, Pang J, Kemper T, Hu J, Yin
J, Zhang J and Lu M: The microRNA-99 family modulates hepatitis B
virus replication by promoting IGF-1R/PI3K/Akt/mTOR/ULK1
signaling-induced autophagy. Cell Microbiol. 19:2017. View Article : Google Scholar
|
|
18
|
Ma Y, Fu S, Lu L and Wang X: Role of
androgen receptor on cyclic mechanical stretch-regulated
proliferation of C2C12 myoblasts and its upstream signals:
IGF-1-mediated PI3K/Akt and MAPKs pathways. Mol Cell Endocrinol.
450:83–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iskender B, Izgi K, Sakalar C and Canatan
H: Priming hMSCs with a putative anti-cancer compound,
myrtucommulone-a: A way to harness hMSC cytokine expression via
modulating PI3K/Akt pathway? Tumour Biol. 37:1967–1981. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashimoto K, Tsuda H, Koizumi F, Shimizu
C, Yonemori K, Ando M, Kodaira M, Yunokawa M, Fujiwara Y and Tamura
K: Activated PI3K/AKT and MAPK pathways are potential good
prognostic markers in node-positive, triple-negative breast cancer.
Ann Oncol. 25:1973–1979. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Liu Z, Li X, Tang X, He J and Lu S:
MicroRNA-1297 contributes to tumor growth of human breast cancer by
targeting PTEN/PI3K/AKT signaling. Oncol Rep. 38:2435–2443. 2017.
View Article : Google Scholar : PubMed/NCBI
|