Introduction
Gastric cancer is one of the most commonly diagnosed
cancers worldwide (1,2). Currently, while radical surgery is the
most common and effective treatment, the prognosis of patients with
advanced gastric cancer following curative resection remains poor.
The 5-year survival rate of patients with gastric cancer is 27.4%
after surgical resection (3). There
is no denying that proliferation, invasion and metastasis of cancer
cells lead to poor prognoses, which presents therapeutic
challenges. The major causes of death for gastric cancer patients
are liver metastases and peritoneal metastases (4). Prevention of tumour recurrence and
metastasis must urgently be improved. Available evidence has
demonstrated that certain nutrients and non-nutrients derived from
foods, such as phytochemicals (4–8) and
vitamins (9–11), have protective effects against
cancer.
Consumption of vitamin E, one of the most widely
consumed vitamins, is prevalent because of its antioxidant capacity
and multiple health benefits. Tocotrienols, isoforms of the vitamin
E family, can be derived from several plant species, including palm
oil, wheat germ, barley and bran oil. It should be noted that the
antitumour properties of vitamin E (8–10) have
been a hot topic in recent years, which may make tocotrienols a
promising group of drugs for future development (12,13).
Cyclooxygenase-2 (COX-2), an important isoform of
the COX family, is well known for its close relationship to
tumourigenesis, invasiveness, inflammatory response, angiogenesis
and tumour cell growth (14). It
has been reported that COX-2 is continuously expressed and is
closely associated with the invasiveness and metastasis of gastric
cancer cells (15). COX-2 promotes
the development and metastasis of gastric cancer cells.
Furthermore, there are several other types of tumours in which
COX-2 is highly expressed, such as oesophageal, liver, lung and
breast cancer, as well as other gastrointestinal cancers (16–20).
Matrix metalloproteinases (MMPs) can degrade nearly
all types of ECM proteins, and they destroy histologic barriers
during the processes of invasion and metastasis (15). In addition, MMP-9 and MMP-2 have
been reported to be overexpressed and associated with tumour
migration and invasion (21,22).
Notably, there is an intimate connection between MMPs and the
mechanism by which COX-2 regulates tumour metastasis (15).
Although recent studies have revealed that γ-T3
displays inhibitory effects on the migration and invasion of human
gastric cancer cells, the specific mechanism remains unclear
(9). Our present study demonstrated
that compared to γ-T3 alone, combination of γ-T3 and
N-[2-(cyclohexyloxy)-4-nitropheny]-methane sulphonamide
(NS-398, a highly selective inhibitor of COX-2) did not
significantly alter the exocrine levels of MMP-2 and MMP-9 in
gastric adenocarcinoma cell lines. Thus, it was concluded that the
reduction of COX-2 resulting from γ-T3 was likely to be the cause
that inhibited the invasion and migration of SGC-7901 and MGC-803
cells.
Materials and methods
Chemicals and cell lines
γ-Tocotrienol (γ-T3) (≥97%) was purchased from
Hygeia Industries Inc. (Wilmette, IL, USA). γ-T3 was dissolved in
ethanol (95%) to produce a 104 µmol/l solution and was stored at
−20°C. A Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). RPMI-1640 medium,
fetal bovine serum (FBS), 0.25% EDTA and penicillin/streptomycin
were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). anti-COX-2 (1:1,000; cat. no. 4842), anti-MMP-2 (1:1,000;
cat. no. 4022), anti-MMP-9 (1:1,000; cat. no. 3852) and
anti-β-actin antibodies, along with NS-398, were purchased from
Cell Signaling Technology Inc. (Danvers, MA, USA). NS-398 was
prepared in a range of concentrations.
Cell culture
Human gastric cancer cell lines SGC-7901 and MGC-803
were purchased from the Cancer Institute of the Chinese Academy of
Medical Sciences (Beijing, China). The two cell lines were cultured
at 37°C in a 5% CO2 incubator in 100-mm culture dishes
with RPMI-1640 medium supplemented with 10% FBS and 1%
penicillin/streptomycin. Upon reaching ~70–80% confluence, the
cells were trypsinized using a mixture of 0.25% trypsin and EDTA,
and then logarithmic phase cells were selected for subsequent
experiments.
CCK-8 assay
The proliferation of SGC-7901 and MGC-803 cells
treated with various concentrations of γ-T3 or NS-398 was detected
by CCK-8 assays. Briefly, 1×104 cells/well (100 µl) were
seeded in 96-well microtiter plates. After incubation overnight,
the cells were changed to fresh culture medium (2% FBS) containing
0, 15, 30, 45 and 60 µmol/l of γ-T3, or 0, 25, 50, 75 and 100
µmol/l NS-398. After incubation for 24, 48 or 72 h, 10 µl of CCK-8
was added to each well. After incubating for another 4 h, the
absorbance values in each well were measured at a wavelength of 450
nm on a microplate reader (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA). Cell viability was assessed with blank controls
as 100% to calculate the percentage of control.
Scratch wound healing assay
SGC-7901 or MGC-803 cells were seeded in 6-well
plates at 5×105 cells/well overnight in a humidified
incubator with an atmosphere of 5% CO2 at 37°C. After
pretreatment with 30 µmol/l of γ-T3 for 24 h, a sterile 200 µl
Eppendorf tip was used to scratch the cells in the plates, which
were then washed with PBS three times and placed in fresh
serum-free RPMI-1640 medium for 24 and 48 h. The distance of wound
healing was recorded. Three random fields were selected for
examination and were photographed with an inverted microscope
(magnification, ×100).
Transwell invasion assay
Cell invasion assays were conducted in Transwell
chambers (Corning, Acton, MA, USA) with 8-µm pores containing
Matrigel in a 24-well plate, as previously described (23). Briefly, SGC-7901 or MGC-803 cells
were treated with γ-T3 (0, 15, 30, 45 and 60 µmol/l) for 24 h and
were then trypsinized and resuspended in serum-free RPMI-1640
medium. The cells were added into the upper chamber at
2×105 cells/well in 200 µl serum-free RPMI-1640 medium.
RPMI-1640 medium containing 10% FBS (500 µl) was added into the
lower chamber. After incubation for 48 h, the non-invasive cells in
the inside membrane of the chamber were scraped off with a sterile
cotton swab. The cells in the outer membrane of the chamber, which
had passed through the Matrigel, were washed three times with PBS,
fixed with 4% formaldehyde for 20 min and stained with crystal
violet for 20 min. Cells located on the underside of the filter
were photographed and counted with an inverted microscope
(magnification, ×200). The results were expressed as the number of
cells that had invaded through the Matrigel within each field.
Western blot analysis
To determine the levels of COX-2, MMP-2 and MMP-9
protein expression, total protein was extracted from SGC-7901 and
MGC-803 cells using cell lysis buffer containing protease
inhibitors (both from Cell Signaling Technology, Inc.). Total
protein determination and gel electrophoresis were performed as
described in our previous studies (24). The blots were incubated with
anti-COX-2 (1:1,000; cat. no. 4882) anti-MMP-2 (1:1,000; cat. no.
4042), anti-MMP-9 (1:1,000; cat. no. 3852) or anti-β-actin primary
antibodies, and goat anti-rabbit horseradish peroxidase (HRP)
secondary antibodies (1:8,000; cat. no. A9169) (Sigma-Aldrich, St.
Louis, MO, USA). Target protein bands in membranes were visualized
using enhanced chemiluminescence (Thermo Fisher Scientific, Inc.).
The relative expression of the three aforementioned proteins was
assessed using β-actin as an internal standard.
Enzyme-linked immunosorbent assay
(ELISA)
To further determine whether γ-T3, NS-398 or γ-T3
combined with NS-398 inhibited exocrine functions of MMP-9 and
MMP-2 in SGC-7901 and MGC-803 cells, culture supernatants were
examined using ELISA assays. Supernatants were collected from
SGC-7901 and MGC-803 cells after treatment with γ-T3 (0, 15, 30, 45
or 60 µmol/l), NS-398 (100 µmol/l) or γ-T3 combined with NS-398 (30
and 100 µmol/l, respectively) for 24 h. The concentrations of MMP-9
and MMP-2 in the supernatants were detected according to the ELISA
kit protocol. Optical density values were acquired using a
microplate reader. Final values were calculated by subtracting the
optical density at 570 nm from that at 450 nm. Each sample was
assessed at least three times. Concentrations of MMP-9 and MMP-2
were calculated with a standard curve.
Statistical analysis
All experiments were independently performed at
least three times, and data were expressed as the means ± SD.
Differences between γ-T3-treated and control groups were analysed
using Student's t-test or one-way analysis of variance (ANOVA)
followed by Dunnett's post hoc test using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). The grey values of protein bands were analysed
by ImageJ software. Differences were considered statistically
significant at P<0.05.
Results
γ-T3 inhibits the proliferation of
SGC-7901 and MGC-803 cells
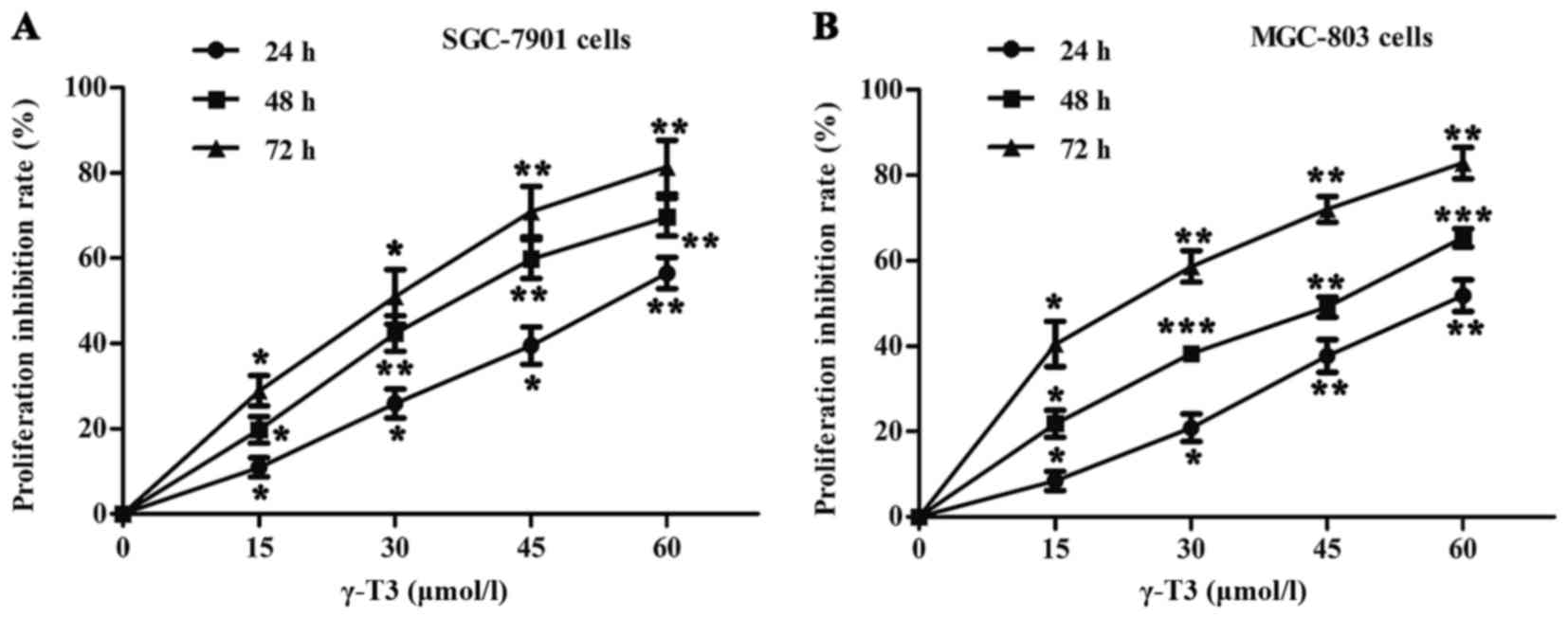
As shown in Fig. 1,
the rates of cell proliferation were determined by CCK-8 assays in
SGC-7901 and MGC-803 cells. γ-T3 significantly inhibited the
proliferation of SGC-7901 and MGC-803 cells treated with different
concentrations of γ-T3 (0, 15, 30, 45 or 60 µmol/l) in a time- and
dose-dependent manner (P<0.05, P<0.01 and P<0.001,
respectively) (Fig. 1). The
IC50 values were also calculated for cells treated with
γ-T3 for 24, 48 or 72 h (Table
I).
 | Table I.The IC50 values in
SGC-7901 and MGC-803 cells treated with γ-T3 for different
durations. |
Table I.
The IC50 values in
SGC-7901 and MGC-803 cells treated with γ-T3 for different
durations.
|
| IC50
(µmol/l) |
|---|
|
|
|
|---|
| Cells | 24 h | 48 h | 72 h |
|---|
| SGC-7901 | 54.14±1.06 | 35.77±1.05 | 27.07±1.08 |
| MGC-803 | 58.34±1.05 | 42.59±1.05 | 21.10±1.09 |
γ-T3 inhibits the migration and
invasion of SGC-7901 and MGC-803 cells
According to the IC50 values, 30 µmol/l
of γ-T3 evidently did not affect proliferation of these cells.
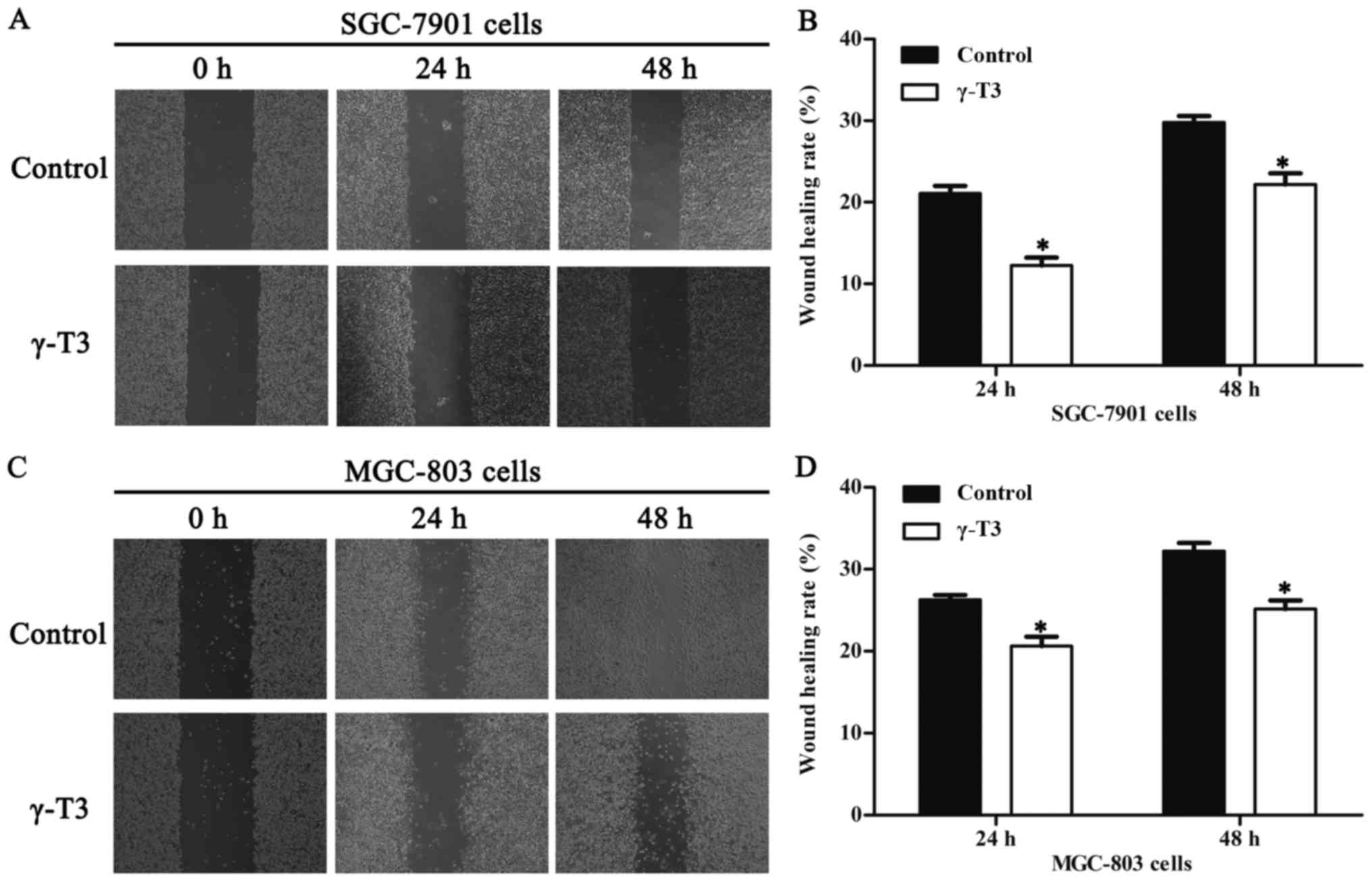
Consequently, 30 µmol/l γ-T3 was chosen to test cell migration in
wound healing assays. The results revealed that γ-T3 notably
inhibited the migration ability of SGC-7901 and MGC-803 cells after
treatment for 24 and 48 h compared with that of the control group
(P<0.05, Fig. 2). To analyse the
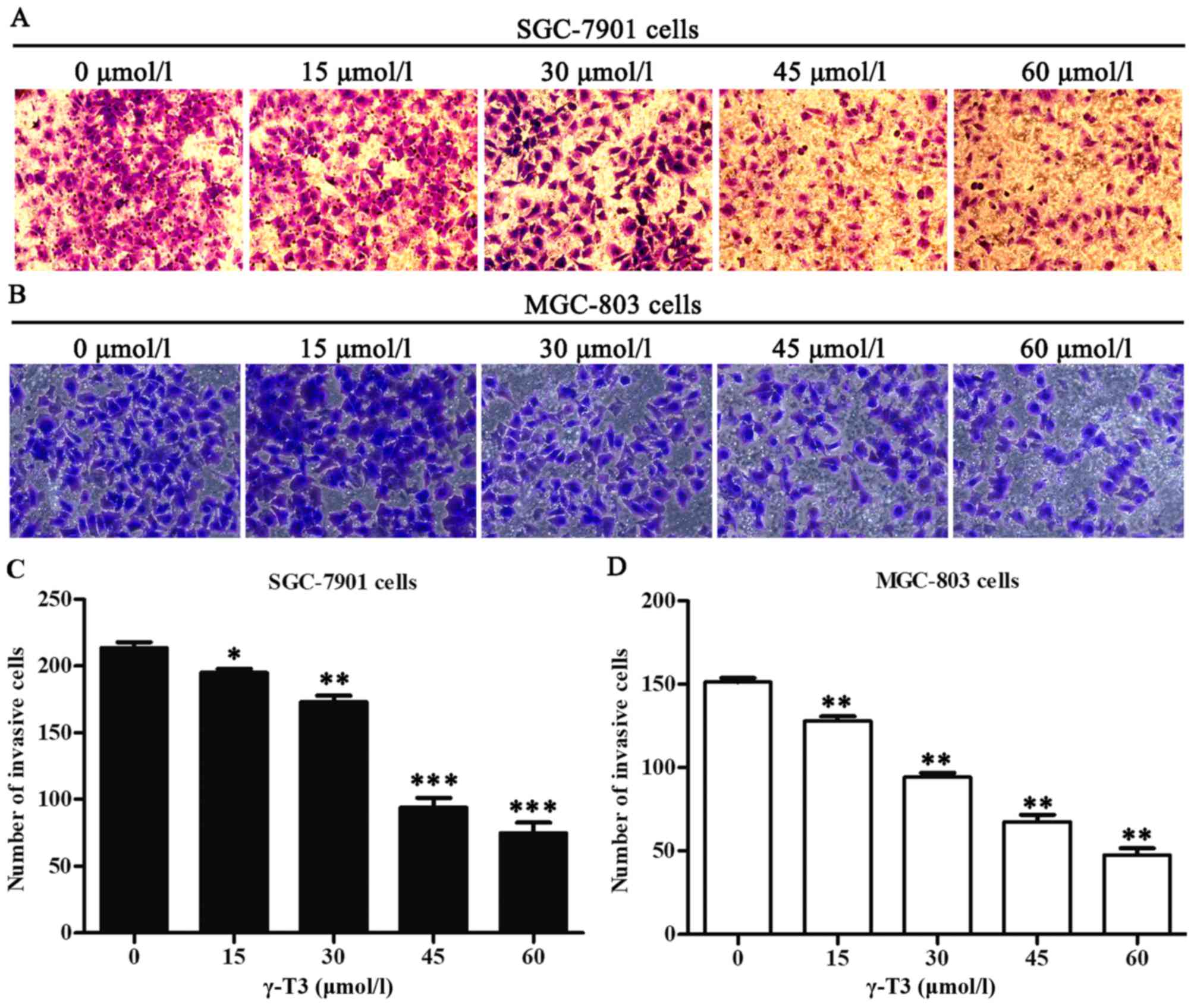
effects of γ-T3 on gastric cancer cell invasion, the Transwell
chamber assay was also employed for this experiment. The invasive
ability of SGC-7901 and MGC-803 cells was quantified as the number
of tumour cells passing through the microporous membrane. γ-T3
significantly decreased the number of invasive cells (P<0.05,
P<0.01 and P<0.001; Fig. 3),
which indicated that γ-T3 inhibited the invasion of gastric cancer
cells.
γ-T3 inhibits the expression of COX-2,
matrix metalloproteinases-2 and −9 (MMP-2 and MMP-9) in SGC-7901
and MGC-803 cells
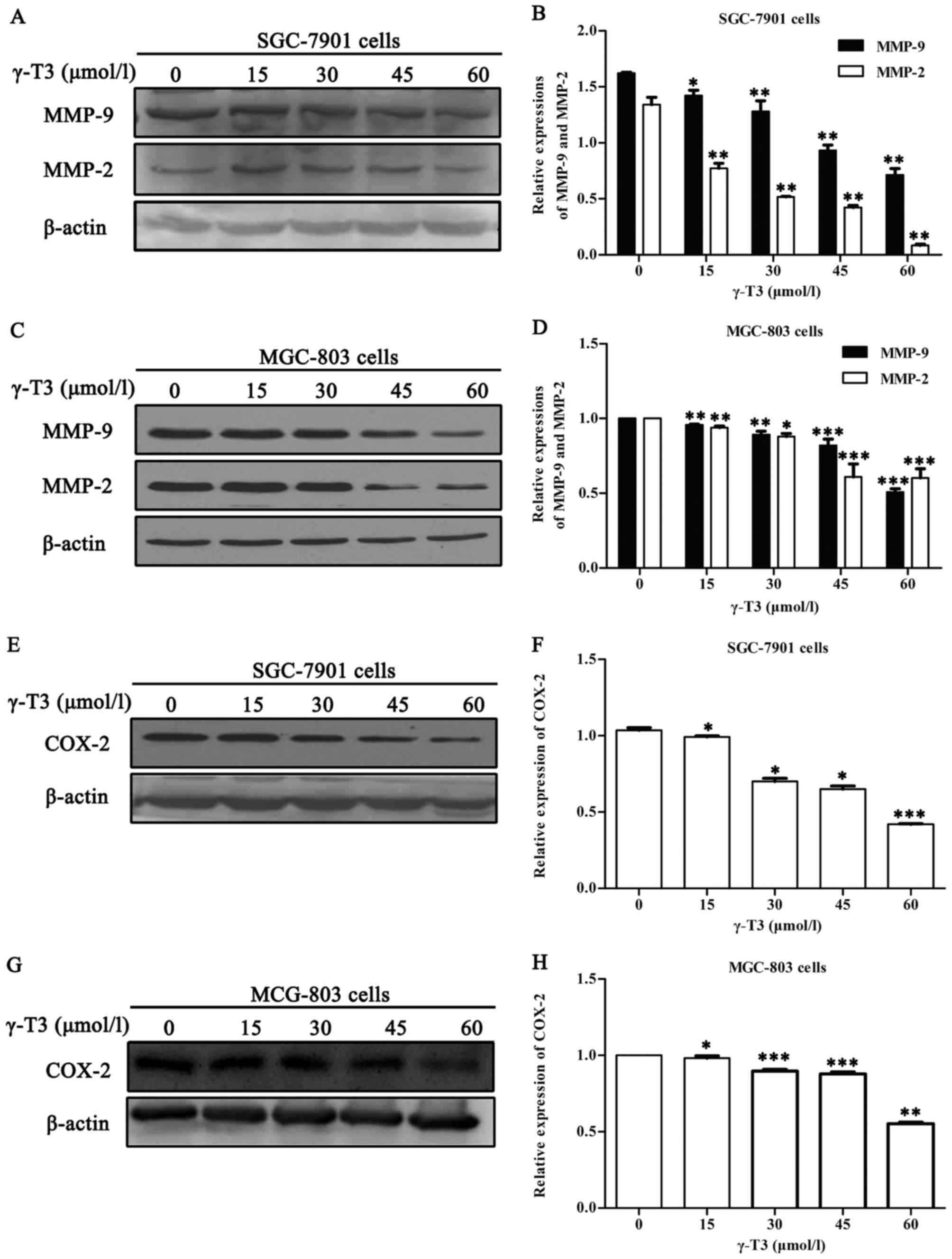
To assess the effects of γ-T3 on the expression of
COX-2, MMP-2 and MMP-9, western blot analyses were performed with
proteins from SGC-7901 and MGC-803 cells treated with different
concentrations of γ-T3 for 24 h. As shown in Fig. 4, γ-T3 significantly decreased the
expression of COX-2, MMP-2 and MMP-9 in gastric cancer cells in a
dose-dependent manner (P<0.05 or P<0.01 and P<0.001).
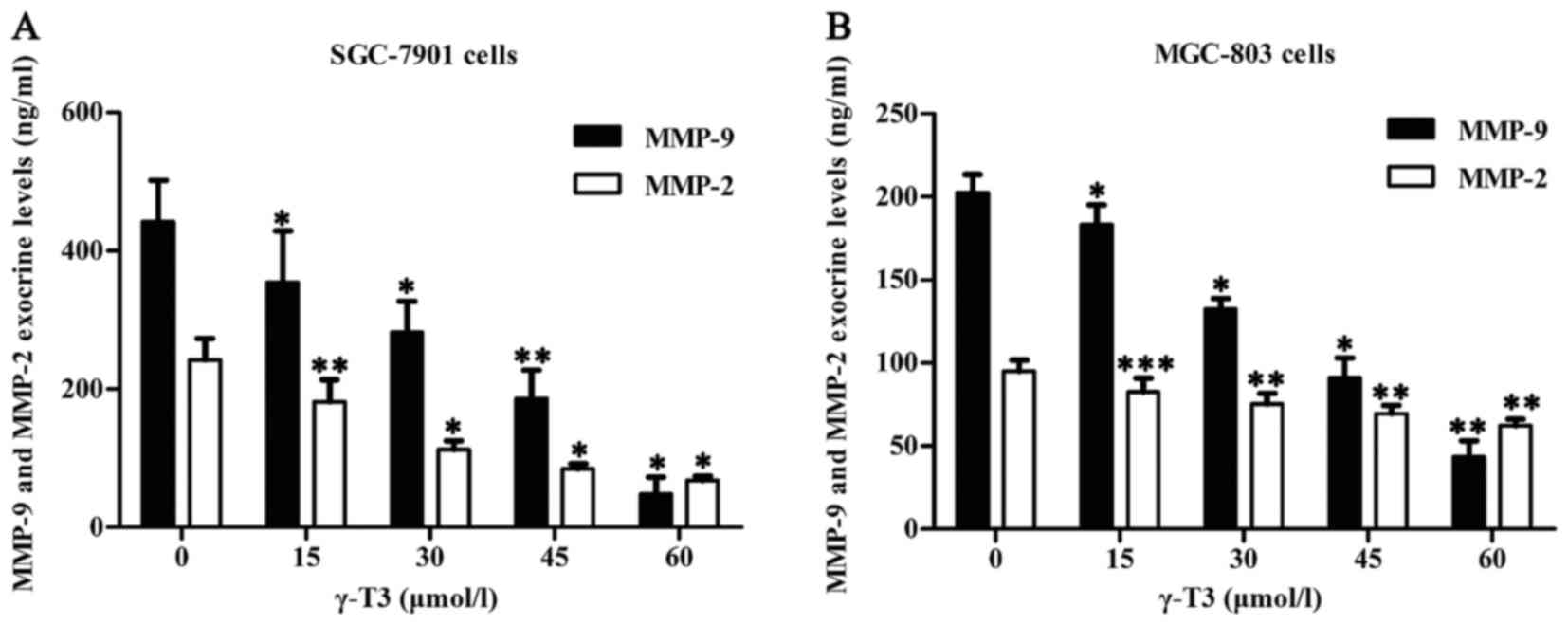
γ-T3 inhibits exocrine levels of MMP-2
and MMP-9 in SGC-7901 and MGC-803 cells
To further detect exocrine levels of MMP-2 and
MMP-9, cell supernatants were explored by ELISA. The results
revealed that γ-T3 significantly inhibited secretion of MMP-2 and
MMP-9 in SGC-7901 and MGC-803 cells (P<0.05 or P<0.01 and
***P<0.001) (Fig. 5). Thus, γ-T3
inhibited not only intracellular expression of MMP-2 and MMP-9, but
also secretion of MMP-2 and MMP-9 in SGC-7901 and MGC-803
cells.
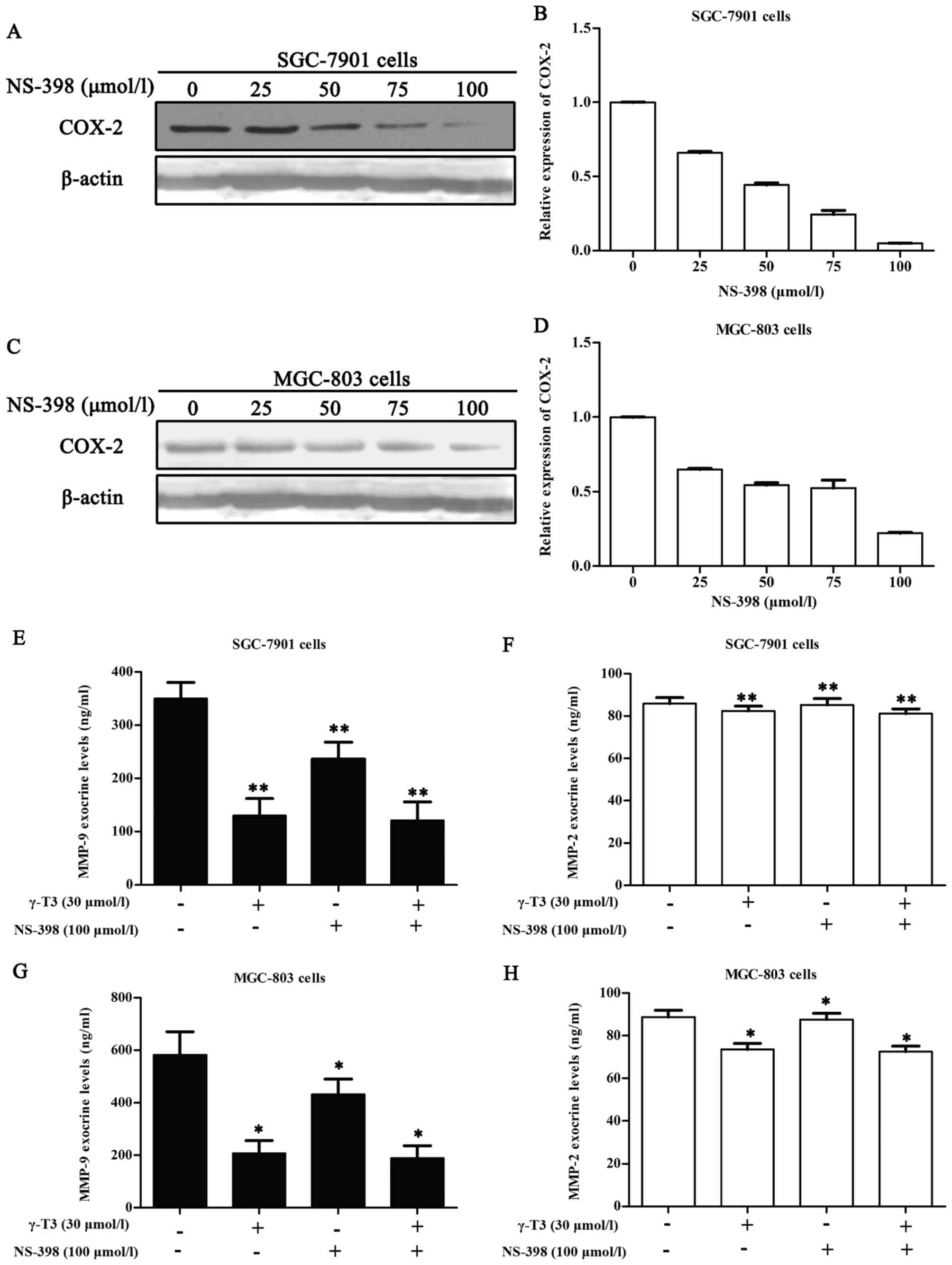
γ-T3 (30 µmol/l) combined with NS-398
(100 µmol/l) does not significantly alter the exocrine levels of
MMP-9 and MMP-2 in SGC-7901 and MGC-803 cells
In light of the aforementioned data, we utilized
ELISA assays to further investigate whether γ-T3 could affect the
exocrine levels of MMP-9 and MMP-2 in SGC-7901 and MGC-803 cells
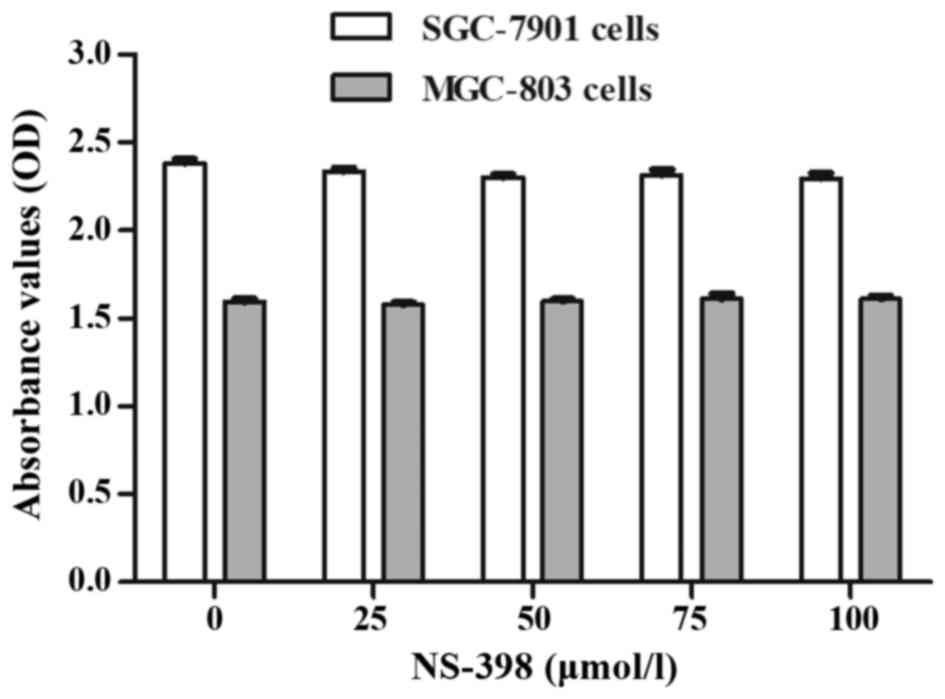
when combined with 100 µmol/l NS-398. This concentration of NS-398
maximized the inhibitory effect on COX-2 (Fig. 7A and C) and did not have adverse
effects on the cells according to the instructions and the CCK-8
assay (Fig. 6). In addition, 30
µmol/l γ-T3 in 24 h had no evident actions on the proliferation of
SGC-7901 and MGC-803 cells (Table
I). Thus, we selected 100 µmol/l NS-398 to ascertain whether
combination with 30 µmol/l γ-T3 affected the exocrine levels of
MMP-9 and MMP-2 in SGC-7901 and MGC-803 cells. Compared to the
control, the 30 µmol/l γ-T3 group, 100 µmol/l NS-398 group, and 30
µmol/l γ-T3 + 100 µmol/l NS-398 group exhibited a significant
declining trend (P<0.05 or P<0.01). However, compared to the
30 µmol/l γ-T3 group alone, the 30 µmol/l γ-T3 + 100 µmol/l NS-398
group exhibited no statistical significance (P>0.05).
Discussion
Gastric cancer is the second leading cause of
malignant tumours worldwide (25).
Chemotherapy is an important treatment method, but it frequently
leads to poor effects or failure due to multidrug resistance (MDR).
As a result, the discovery of novel therapeutic methods to solve
these problems is extremely urgent. Furthermore, since invasion and
metastasis are two important characteristics that can determine the
effects of cancer diagnosis and prognosis (26), it has become crucial to take
effective measures to increase prevention of gastric cancer.
γ-T3, a member of the vitamin E family, has been
demonstrated to inhibit proliferation and induce apoptosis, as well
as exhibit superior inhibition of migration and invasion in tumour
cells (8,9). These antitumour properties explain why
γ-T3 has received so much attention in recent years. In this study,
we also obtained evidence that γ-T3 inhibits the invasion and
migration of human gastric cancer cells.
COX-2 is an induced form of cyclooxygenase that is
often elevated in cancerous tissues (27). It is well known for accelerating the
invasion and migration of tumour cells, making it a research hot
topic in recent years (28). Recent
studies have confirmed that COX-2 plays an important role in the
invasion and metastasis of gastric cancer cells, and it has been
suggested that reducing the expression of COX-2 in tumour cells may
attenuate invasion and metastasis (29,30).
MMP-9, also known as 92-kDa type IV collagenase, is
an enzyme belonging to the family of zinc-metalloproteinases. MMP-9
is involved in the breakdown of the extracellular matrix in normal
physiological processes, such as angiogenesis, wound healing and
cell migration, as well as in metastasis. MMP-2, also known as
72-kDa type IV collagenase and gelatinase A, is associated with
processes such as cancer progression, cancer cell invasion, cell
signalling, neovascularization and lymphangiogenesis. Metastasis
and invasion are among the leading causes of death among gastric
cancer patients (31). MMPs can be
activated to destroy the basement membrane, resulting in the
degradation of the extracellular matrix, thus opening channels for
invasion and metastasis (15,32).
Both SGC-7901 (moderately differentiated) and
MGC-803 (undifferentiated or poorly differentiated) belong to
gastric adenocarcinoma cell lines. COX-2 can be expressed in the
two cell lines, and the level of COX-2 in SGC-7901 cells is higher
than in MGC-803 cells (33,34). In the present study, scratch wound
healing and Transwell invasion assays demonstrated that γ-T3
treatment markedly inhibited the migration and invasion of SGC-7901
and MGC-803 cells (Figs. 2 and
3). Western blot analyses in the
present study revealed that the expression of COX-2 and
intracellular MMPs was decreased (Fig.
4). ELISA data revealed that the exocrine concentrations of
MMPs in cell culture supernatants also exhibited a decreasing trend
after SGC-7901 and MGC-803 cells were pretreated with γ-T3 for 24 h
(Fig. 5). Notably, it has been
revealed that overexpression of COX-2 is involved in the induction
of MMPs (15). Thus, in light of
our data, we speculated whether the decreases of COX-2 resulted in
the exocrine levels of MMPs. Next, to determine whether these
inhibitory effects on migration and invasion were associated with
COX-2, we utilized NS-398 for verification. NS-398, a highly
selective inhibitor of COX-2, was chosen for joint treatment with
γ-T3, and the results were determined using ELISA assays. We
noticed that the exocrine level of MMP-2 in Fig. 5A was higher than that in Fig. 7F. The cause of this is that cancer
cells from different generations may exert different experimental
outcomes. Compared to γ-T3 alone, γ-T3 in combination with 100
µmol/l NS-398 did not significantly alter the exocrine expression
of MMP-9 and MMP-2 (P>0.05, Fig.
7). These findings indicated that the decrease in the exocrine
levels of MMPs were likely to result from downregulation of COX-2,
but the exact mechanism involved in the downregulation of MMPs by
COX-2 remained unknown. The ELISA assay results were compatible
with our hypothesis that the decreases of COX-2 were involved in
the induction of MMPs in human gastric cancer cells. The data
gathered in the present study suggest, at least in part, that the
inhibitory effects on migration and invasion in treated SGC-7901
and MGC-803 cells (γ-T3 alone or combined with NS-398) may be
associated with downregulation of COX-2.
In conclusion, the present study demonstrated that
γ-T3 significantly inhibited migration and invasion of human
gastric cancer cells via downregulation of COX-2 expression, making
it a promising novel drug for future cancer treatment. The
antitumour efficacy of γ-T3 may make a difference in the treatment
and prevention of gastric cancer. Although we have explored the
inhibitory potential of γ-T3 on migration and invasion in human
gastric cancer cells in vitro, these experiments are
insufficient to explain the mechanism by which γ-T3 acts. This
drawback is also a limitation of our study that can be improved in
future research. Accordingly, further research must be performed in
mouse models in vivo to illustrate our results obtained in
SGC-7901 and MGC-803 cells in vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81273061).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHZ and KM carried out all the experiments and YHZ
was responsible for drafing the manuscript. WGS was responsible for
the study design. JRL, HXW, WXT and YHT were responsible for data
collection and analysis. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
All authors carefully and critically reviewed the
submitted manuscript and agreed to the final version for
publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
γ-T3
|
γ-tocotrienol
|
|
COX-2
|
cyclooxygenase-2
|
|
CCK-8
|
Cell Counting Kit-8
|
|
MMP-2 and MMP-9
|
matrix metalloproteinases-2 and −9
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu X: Risk factors and prognosis of liver
metastasis from gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
17:108–111. 2014.(In Chinese). PubMed/NCBI
|
|
5
|
Chen HS, Liu M, Shi LJ, Zhao JL, Zhang CP,
Lin LQ, Liu Y, Zhang SJ, Jin JC, Wang L, et al: Effects of
raspberry phytochemical extract on cell proliferation, apoptosis,
and serum proteomics in a rat model. J Food Sci. 76:T192–T198.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong HW, Zhang S, Sun WG, Liu Q, Ibla JC,
Soriano SG, Han XH, Liu LX, Li MS and Liu JR: β-Ionone arrests cell
cycle of gastric carcinoma cancer cells by a MAPK pathway. Arch
Toxicol. 87:1797–1808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Q, Dong HW, Sun WG, Liu M, Ibla JC,
Liu LX, Parry JW, Han XH, Li MS and Liu JR: Apoptosis initiation of
β-ionone in SGC-7901 gastric carcinoma cancer cells via a PI3K-AKT
pathway. Arch Toxicol. 87:481–490. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Liu M, Li B, Zhao JL, Zhang CP, Lin
LQ, Chen HS, Zhang SJ, Jin JC, Wang L, et al: Fresh raspberry
phytochemical extract inhibits hepatic lesion in a Wistar rat
model. Nutr Metab (Lond). 7:842010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HK, Wang Q, Li Y, Sun WG, Liu JR, Yang
YM, Xu WL, Sun XR and Chen BQ: Inhibitory effects of
gamma-tocotrienol on invasion and metastasis of human gastric
adenocarcinoma SGC-7901 cells. J Nutr Biochem. 21:206–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang JS, Li DM, Ma Y, He N, Gu Q, Wang
FS, Jiang SQ, Chen BQ and Liu JR: γ-Tocotrienol induces
paraptosis-like cell death in human colon carcinoma SW620 cells.
PLoS One. 8:e577792013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang JS, Zhang SJ, Li Q, Liu YH, He N,
Zhang J, Zhou PH, Li M, Guan T and Liu JR: Tocotrienol-rich
fraction (TRF) suppresses the growth of human colon cancer
xenografts in Balb/C nude mice by the Wnt pathway. PLoS One.
10:e01221752015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling MT, Luk SU, Al-Ejeh F and Khanna KK:
Tocotrienol as a potential anticancer agent. Carcinogenesis.
33:233–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gagic Z, Ivkovic B, Srdic-Rajic T,
Vucicevic J, Nikolic K and Agbaba D: Synthesis of the vitamin E
amino acid esters with an enhanced anticancer activity and in
silico screening for new antineoplastic drugs. Eur J Pharm Sci.
88:59–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Qu L and Yan S: Cyclooxygenase-2
promotes tumor growth and suppresses tumor immunity. Cancer Cell
Int. 15:1062015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun WH, Sun YL, Fang RN, Shao Y, Xu HC,
Xue QP, Ding GX and Cheng YL: Expression of cyclooxygenase-2 and
matrix metalloproteinase-9 in gastric carcinoma and its correlation
with angiogenesis. Jpn J Clin Oncol. 35:707–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang JX, Xiao W, Chen WC, Lin MS, Song
ZX, Chen P, Zhang YL, Li FY, Qian RY and Salminen E: Relationship
between COX-2 and cell cycle-regulatory proteins in patients with
esophageal squamous cell carcinoma. World J Gastroenterol.
16:5975–5981. 2010.PubMed/NCBI
|
|
17
|
Wu BW, Li DF, Ke ZF, Ma D, Li YJ, Gang D,
Zheng ZG, Zhang KJ and Zhang YH: Expression characteristics of
heparanase in colon carcinoma and its close relationship with
cyclooxygenase-2 and angiogenesis. Hepatogastroenterology.
57:1510–1514. 2010.PubMed/NCBI
|
|
18
|
Krishnamachary B, Stasinopoulos I, Kakkad
S, Penet MF, Jacob D, Wildes F, Mironchik Y, Pathak AP, Solaiyappan
M and Bhujwalla ZM: Breast cancer cell cyclooxygenase-2 expression
alters extracellular matrix structure and function and numbers of
cancer associated fibroblasts. Oncotarget. 8:17981–17994. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang F, Lin C, Shi YH and Kuerban G:
MicroRNA-101 inhibits cell proliferation, invasion, and promotes
apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma
cells. Asian Pac J Cancer Prev. 14:5915–5920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Sun D, Lv Z, Wei Y, Zheng L, Zeng T
and Zhao J: Insulin-like growth factor binding protein-4 inhibits
cell growth, migration and invasion, and downregulates COX-2
expression in A549 lung cancer cells. Cell Biol Int. 41:384–391.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ram M, Sherer Y and Shoenfeld Y: Matrix
metalloproteinase-9 and autoimmune diseases. J Clin Immunol.
26:299–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Han L, Dong Y, Tan Y, Li Y, Zhao M,
Xie H, Ju H, Wang H, Zhao Y, et al: EGFRvIII/integrin β3
interaction in hypoxic and vitronectinenriching microenvironment
promote GBM progression and metastasis. Oncotarget. 7:4680–4694.
2016.PubMed/NCBI
|
|
24
|
Sun W, Wang Q, Chen B, Liu J, Liu H and Xu
W: Gamma-tocotrienol-induced apoptosis in human gastric cancer
SGC-7901 cells is associated with a suppression in
mitogen-activated protein kinase signalling. Br J Nutr.
99:1247–1254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu W, Yang Q, Liu B and Zhu Z: Serum
proteomics for gastric cancer. Clin Chim Acta. 431:179–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, You K, Li J, Wang Y, Xu H, Gao B and
Wang J: Madecassoside suppresses proliferation and invasiveness of
HGF-induced human hepatocellular carcinoma cells via
PKC-cMET-ERK1/2-COX-2-PGE2 pathway. Int Immunopharmacol. 33:24–32.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun WH, Zhu F, Chen GS, Su H, Luo C, Zhao
QS, Zhang Y, Shao Y, Sun J, Zhou SM, et al: Blockade of
cholecystokinin-2 receptor and cyclooxygenase-2 synergistically
induces cell apoptosis, and inhibits the proliferation of human
gastric cancer cells in vitro. Cancer Lett. 263:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang
K, Li X and Sun W: Harmine induces apoptosis and inhibits tumor
cell proliferation, migration and invasion through down-regulation
of cyclooxygenase-2 expression in gastric cancer. Phytomedicine.
21:348–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun K, Tang XH and Xie YK: Paclitaxel
combined with harmine inhibits the migration and invasion of
gastric cancer cells through downregulation of cyclooxygenase-2
expression. Oncol Lett. 10:1649–1654. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin NR, Jeong EH, Choi CI, Moon HJ, Kwon
CH, Chu IS, Kim GH, Jeon TY, Kim DH, Lee JH, et al: Overexpression
of Snail is associated with lymph node metastasis and poor
prognosis in patients with gastric cancer. BMC Cancer. 12:5212012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrixmetalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 1:44–49. 2010.(In
Bulgarian).
|
|
33
|
Liu XJ, Chen ZF, Li HL, Hu ZN, Liu M, Tian
AP, Zhao D, Wu J, Zhou YN and Qiao L: Interaction between
cyclooxygenase-2, Snail, and E-cadherin in gastric cancer cells.
World J Gastroenterol. 19:6265–6271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang H, Zhou Y, Liao Q and Ouyang H:
Helicobacter pylori infection promotes the invasion and
metastasis of gastric cancer through increasing the expression of
matrix metalloproteinase-1 and matrix metalloproteinase-10. Exp
Ther Med. 8:769–774. 2014. View Article : Google Scholar : PubMed/NCBI
|