Introduction
According to the National Cancer Institute (NCI)
report, approximately 22,240 new cases of ovarian cancer will be
diagnosed in the US in 2018, and 14,070 patients will die of this
disease (1). For stage I, II III
and IV epithelial ovarian cancer patients, the 5-year relative
survival rates are 89, 70, 36 and 17%, respectively, and the
10-year relative survival rates are 84, 59, 23 and 8%, respectively
(2).
In addition to conventional cytoreductive surgery
and platinum-based chemotherapy, tumor targeted therapy has also
been used in patients with advanced ovarian cancer, but many
patients still relapse after treatment. The survival rate has still
not improved, and the mechanisms of occurrence, development,
invasion and metastasis in ovarian cancer patients remain unclear.
Thus, a significant unmet medical need exists for the development
of efficacious therapies that can improve the survival rate of
these patients (3).
Therefore, exploring the biological characteristics
of tumor cells that are common but differ from normal cells and
seeking a specific intervention for this characteristic is the key
to improve the efficacy of tumor therapy.
Metabolic reprogramming is a hallmark of cancer
(4). Tumor cells exhibit an altered
metabolic phenotype characterized by increased glycolysis and
diminished oxidative phosphorylation that is called ‘aerobic
glycolysis’ or the ‘Warburg effect’ (5,6).
Increased glycolysis in cancer cells switches cellular metabolic
flux to produce more biological building blocks, thereby sustaining
rapid proliferation (7).
Intermediates of the tricarboxylic acid cycle and metabolites
relating to energy metabolism, amino acids, and gut microbial
metabolism are perturbed in breast and ovarian cancer cells
compared with normal cells (8). As
ovarian cancer progresses, complete oxidation of glucose and fatty
acids is significantly reduced and occurs concurrently with
increases in lactate excretion and 3H-deoxyglucose uptake by
late-stage cancer cells, shifting the cells towards a more
glycolytic phenotype; this notion is confirmed by metabolic changes
during ovarian cancer progression (9). A recent study demonstrated that
inhibition of glycolysis and glutaminolysis may be a promising
therapeutic strategy for the treatment of ovarian cancer (10).
YWHAZ (also known as 14-3-3zeta, 14-3-3ζ) is a
member of the 14-3-3 family proteins influencing diverse vital
cellular processes, such as metabolism, signal transduction,
apoptosis and cell cycle regulation. Clinical studies confirm that
overexpression of YWHAZ is negatively correlated with prognosis in
multiple tumors, including adenocarcinoma of the esophago-gastric
junction (AEG) (11), breast cancer
(12), head and neck (13) and hepatocellular carcinoma (14).
YWHAZ may contribute to the development of early
stage cancers and promote the transition to invasive cancers
(15). High YWHAZ expression in the
primary tumor was found to be significantly associated with earlier
time to recurrence and distant metastasis (16). As a phospho-serine/-threonine
binding protein, YWHAZ plays important roles in the genesis and
development of cancer via binding to different partners, such as
MEK/ERK (7), HIF-1α (13) or TGF-β/Smads (15). Thus, inhibiting YWHAZ (14-3-3ζ)
functional activities might prove to be of potential therapeutic
utility. The development of inhibitors for the 14-3-3 family has
been somewhat successful, but achieving selectivity for specific
family members has proven to be challenging. Therefore, in
14-3-3ζ-overexpressing tumors, intercepting downstream effector
targets of 14-3-3ζ may be an effective therapy. Importantly,
studies have demonstrated that 14-3-3 regulates cellular metabolic
processes. For example, 14-3-3 binding to the cardiac isoform of
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2)
suggests that 14-3-3 is involved in regulating glycolysis and
metabolism (17). In addition,
14-3-3ζ prevents BAD-BAX mitochondrial localization and protects β
cells from multiple stressors (18). Increasing 14-3-3ζ expression in
human mammary epithelial cells (hMECs) upregulated LDHA expression,
elevated glycolytic activity and promoted early transformation.
Knockdown of LDHA in 14-3-3ζ-overexpressing hMECs significantly
reduced glycolytic activity and inhibited transformation (7).
At present, whether YWHAZ regulates epithelial
ovarian cancer (EOC) metastasis through glycolysis has not yet been
studied. Kim et al evaluated the prognostic value of
quantitative metabolic parameters measured on F-18 FDG PET/CT (FDG
PET/CT) at the time of the first relapse in patients with relapsed
EOC (19) and motivated us to
explore whether glycolysis and its downstream effectors are
involved in YWHAZ-mediated ovarian cancer cell migration.
In the present study, it was found that YWHAZ is
upregulated in ovarian cancer and high expression of YWHAZ predicts
poor survival in ovarian cancer patients. Furthermore, we revealed
that YWHAZ promoted ovarian cancer metastasis by activating the
PI3K/Akt1/vimentin signaling pathway and upregulating glycolysis.
These data could help us understand the effect and the underlying
mechanism of YWHAZ in ovarian cancer metastasis.
Materials and methods
Cell culture and reagents
Human ovarian carcinoma cell lines ES-2, SKOV3,
293T, HEY, COV318 and OVCAR3 were provided by the Shanghai Cancer
Institute (Shanghai, China). ES-2 and SKOV3 cells were used in
assessing the function of YWHAZ on cellular behaviors and the
activation of glycolysis. Other cell lines were only used for the
detection of YWHAZ expression levels. All cells were cultured
according to the instructions of the American Type Culture
Collection (ATCC; Manassas, VA, USA). Antibodies used in this study
included YWHAZ (cat. no. ab51129; Abcam, Cambridge, MA, USA), AKT
(cat. no. ab18785; Abcam), p-AKT1 (cat. no. ab133458; Abcam),
PI3K-p85α (cat. no. 13666), vimentin (cat. no. 5741), LDHA (cat.
no. 2012), PFKP (cat. no. 5412), tubulin (cat. no. 2146; all were
from Cell Signaling Technology, Danvers, MA, USA).
RNA isolation and real-time qPCR
RNA isolation and real-time qPCR were performed as
previously described (6). Total RNA
of ES-2 and SKOV3 cells was extracted using TRIzol reagent (Takara
Bio Inc., Shiga, Japan). cDNA was synthesized by PrimeScript RT-PCR
kit (Takara Bio Inc.) based on the manufacturer's protocols.
Quantitative real-time PCR was run with SYBR Premix Ex Taq (Takara
Bio Inc.) on the 7500 Real-time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cDNA was
diluted at 1:20 before use. Primers used for YWHAZ were:
5′-AGGAGCCCGTAGGTCATCTT-3′ (forward) and 5′-TGCTTGTGAAGCATTGGGGA-3′
(reverse). Primers used for 18S were: 5′-TGCGAGTACTCAACACCAACA-3′
(forward) and 5′-GCATATCTTCGGCCCACA-3′ (reverse). The cycling
settings were as follows: 95°C for 10 min; 95°C for 10 sec, 60°C
for 30 sec for 45 cycles; 40°C for 30 sec. Expression level and
fold change were calculated using the ΔΔCq method (20).
Plasmid construction and
transfection
Two stable plasmids for opposite functions were
constructed. For stable YWHAZ interference, short hairpin RNA
(shRNA) targeting YWHAZ or control shRNA was constructed into the
PLKO.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.). The
sequences of the shRNAs were designed based on the sequence of
siRNA-1 of YWHAZ (si1). For stable overexpression of YWHAZ, the
cDNA encoding YWHAZ was subcloned into the pCDH-CMV-MCS-EF1-Puro
(CD510B-1) vector to generate the YWHAZ-overexpressing plasmid.
Then C510B-1-YWHAZ-Linker-HA plasmids which had been constructed
were packaged as required. Virus packaging was performed in 293T
cells after cotransfection of constructed plasmids with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.;
cat. no. 11668-019). Viruses were harvested at 48 and 72 h after
transfection, and virus titers were determined. ES-2 and SKOV3
cells, two target cell lines, were infected with 1×108
recombinant lentivirus-transducing units plus 8 µg/ml polybrene
(Sigma-Aldrich, Shanghai, China). Cells were selected and
maintained in the presence of 2 µg/ml puromycin. The transfection
efficiency was assessed by RT-PCR and western blotting at both the
mRNA and protein levels. Subsequent experiments were performed
after 2 weeks of puromycin selection.
siRNA transfection
Lipofectamine RNAiMAX transfection reagent
(Invitrogen Thermo Fisher Scientific, Inc.) was used for
transfecting the designed small interfering RNAs (siRNAs) into ES-2
and SKOV3 cells. The transfection steps were carried out following
the manufacturer's instructions. Diluted siRNA was added into
diluted Lipofectamine RNAiMAX reagent for a 5-min incubation at
room temperature. Then, the siRNA-lipid complex was added to the
targeted cells. The final siRNA applied per well was 25 pmol in one
6-well plate. The sequences were: GGAGAUUACUACCGUUACUdTdT
(hs-YWHAZ-si-1), GAGCUGAAUUAUCCAAUGAdTdT (hs-YWHAZ-si-2) and
CGUCUCAAGUAUUGAACAAdTdT (hs-YWHAZ-si-3). Non-targeting scrambled
siRNA was used as a control in the transfection. The silencing
effects were verified by qRT-PCR and western blot analysis.
Cell proliferation assay
Cell proliferation assay was performed as previously
described (21). ES-2 and SKOV3
cells were seeded in 96-well plates at the concentration of 5,000
cells in 100 µl complete medium/well before transfection. After the
cells were attached, transient transfection was performed according
to the protocols. At 24, 48 and 72 h after siRNA transfection, 10
µl of Cell Counting Kit-8 (CCK-8) reagents (Share-bio, Shanghai,
China) was added to each well for 1 h of incubation. The absorbance
was measured at 450 nm using a microplate reader. All experiments
were performed in triplicate.
Cell migration assay
ES-2 and SKOV3 cell migration was determined using
8-mm pore size cell culture inserts within a 24-well plate (Corning
Inc., Corning, NY, USA) based on the protocols. Cells
(2×104) in 100 µl serum-free medium were added into the
upper chamber with 700 µl serum medium in the lower chamber and
incubated for 24 h. The migrated cells were immobilized with 4%
paraformaldehyde and stained with 0.1% crystal violet. The quantity
of migrated cells was counted under a light microscope in six
random fields. Each experiment was performed in triplicate.
Apoptosis assay
ES-2 and SKOV3 cells were seeded on 6-well plates.
Cell death was assessed by double-labelling with Annexin V-FITC and
propidium iodide (PI) according to the protocols. After induction
of apoptosis by serum-free media, the required cells were harvested
and washed in cold phosphate-buffered saline (PBS) followed by
resuspending the cells in Annexin-binding buffer. After addition of
5 µl of Annexin V to each 100 µl of cell suspension, the cells were
incubated for 15 min at room temperature. Then propidium iodide
(PI) was added 5 min before detection on a flow cytometer
(Becton-Dickinson; BD Biosciences, San Jose, CA, USA).
Western blotting
Total protein was extracted from ES-2 and SKOV3
cells using a total protein extraction buffer (Beyotime Institute
of Biotechnology, Haimen, China). BCA Protein Assay kit was used to
measure the protein concentration. Total protein (50 µg) was added
into each sample well. Then the same amounts of proteins were
separated by 12% SDS-PAGE and transferred to NC membranes. The
membranes were blocked with 5% non-fat dry milk for 1 h and washed
twice with TBS wash buffer. Then the membranes were incubated at
4°C overnight with the following primary antibodies: YWHAZ
(dilution 1:1,000), p-Akt1 (dilution 1:1,000), tubulin (dilution
1:2,000), PI3K (dilution 1:2,000), vimentin (dilution 1:1,000),
LDHA (dilution 1:2,000) and PFKP (dilution 1:1,000). After washing
with TBS wash buffer, HRP-conjugated anti-rabbit (cat. no.
ab205718; Abcam) and anti-mouse (cat. no. ab205719; Abcam)
secondary antibodies were diluted (dilution 1:10,000) and added for
incubation for 1 h at room temperature. The protein bands were
detected by an ECL kit (Share-bio).
H&E staining
For H&E staining, the tissue samples were
stained with hematoxylin and eosin. The staining procedures were
performed routinely. Firstly, the sections which were pretreated
were rinsed in to distilled water. Then the nuclei were stained
with hematoxylin for 10 min. After washing in running tap water for
5 min, the sections were differentiated with 0.3% acid alcohol for
1 min. For bluing, the sections were inserted in 0.2% ammonia water
later following by a 5-min wash with water. Then the sections were
counterstained in eosin for 1 min. Finally, dehydration, clearing
and mounting of the sections were performed.
In vivo metastatic model
Mice were supplied by and cultivated in the SPF
Animal Laboratory of East China Normal University according to the
protocols approved by the Animal Care Commission and received
humane care according to the criteria outlined in the ‘Guide for
the Care and Use of Laboratory Animals’ prepared by the National
Academy of Sciences and published by the National Institutes of
Health. A total of 20 female BALB/C nude mice (6-weeks old) were
divided into four groups for the metastasis experiments. They lived
in standard housing conditions with 10 h of light and 14 h of dark
cycle. The indoor temperature was maintained at 26°C and the
relative humidity was maintained at 40–60%. The particles in the
air did not exceed >0.3 µm. The food and water were sterilized
by special treatment. We treated ES2 and SKOV3 cell lines with
stable transfection in advance. After a 2-week puromycin selection,
2×106 SKOV3 or ES2 cells expressing Lenti-shcontrol or
Lenti-shYWHAZ were injected into the tail vein of nude mice (n=5
each group). The mice were sacrificed by neck dislocation 4 weeks
later according to common protocols of the metastasis assay used in
numerous literatures and the number of metastatic nodules in the
lung was counted under an inverted microscope (Olympus Corporation,
Tokyo, Japan). The harvested lungs were fixed and stained by
H&E for histologic assessment.
Lactate production assay
Lactate production was detected as previously
described using the Lactate Assay kit (cat. no. ABIN411683;
BioVision Inc., Mountain View, CA, USA) (6). Lactate production was measured at the
absorbance (570 nm) with a microplate reader.
Seahorse XF analysis
The extracellular acidification rate (ECAR) of
control or YWHAZ-knockdown ovarian cancer cells was assayed on the
Seahorse Extracellular flux analyzer (Seahorse Bioscience,
Billerica, MA, USA) to evaluate glycolysis according to the
manufacturer's instructions (6).
SKOV3 and ES2 cells were seeded on a XF96-well plate at a density
of 4×104/well and allowed to attach overnight, followed
by transfection with the negative-control siRNA or siRNAs targeting
YWHAZ. After 24 h, cells were incubated in a non-CO2
37°C incubator with unbuffered medium for 1 h followed by a
sequential injection of 10 mM glucose, 1 µM oligomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 50 mM
2-deoxyglucose (2-DG; Sigma-Aldrich; Merck KGaA; D8375).
Measurements were normalized to total protein content and reported
as mpH/min for ECAR. Each datum was determined in triplicate.
Clinical samples
For immunohistochemical staining of YWHAZ and
analysis of its correlation with clinicopathological features, an
ovarian cancer tissue microarray containing 83 cases of ovarian
cancer samples and 18 cases of normal ovarian samples was used.
These tissues were obtained from the Department of Gynecology,
Obstetrics and Gynecology Hospital, Fudan University from May 2006
to January 2009. All human ovarian tissues were obtained with
informed consent and all protocols were approved by the Ethics
Review Committee of the Research Ethics Committee of the Fifth
People's Hospital of Shanghai, Fudan University Hospital.
Expression score was determined by staining intensity and
immunoreactive cell percentage. Scoring was conducted according to
the ratio and intensity of positive staining cells: 0–5% scored 0;
6–35% scored 1; 36–70% scored 2; and >70% scored 3. The final
score of YWHAZ expression was designated as low or high expression
group as follows: Low expression, score 0–1; and high expression,
score 2–3. All the scores of YWHAZ expression were performed in a
blinded manner and determined independently by two senior
pathologists.
Statistical analysis
All data were analyzed as the mean ± standard
deviation (SD). Statistical analyses were conducted using SPSS 14.0
software (SPSS Inc., Chicago, IL, USA). We performed the Pearson's
χ2 test in cross tables to assess the relationships
between the expression levels of YWHAZ and the clinicopathological
factors. Overall survival (OS) was calculated using the
Kaplan-Meier method. Comparison between groups was analyzed by
one-way analysis of variance (ANOVA) followed by a post hoc test
(Student-Newman-Keuls method). Values of P<0.05 were considered
to indicate a statistically significant result.
Results
YWHAZ is highly expressed in
epithelial ovarian cancer tissues
To investigate the relationship between YWHAZ and
the biological characteristics of ovarian cancer, we analyzed YWHAZ
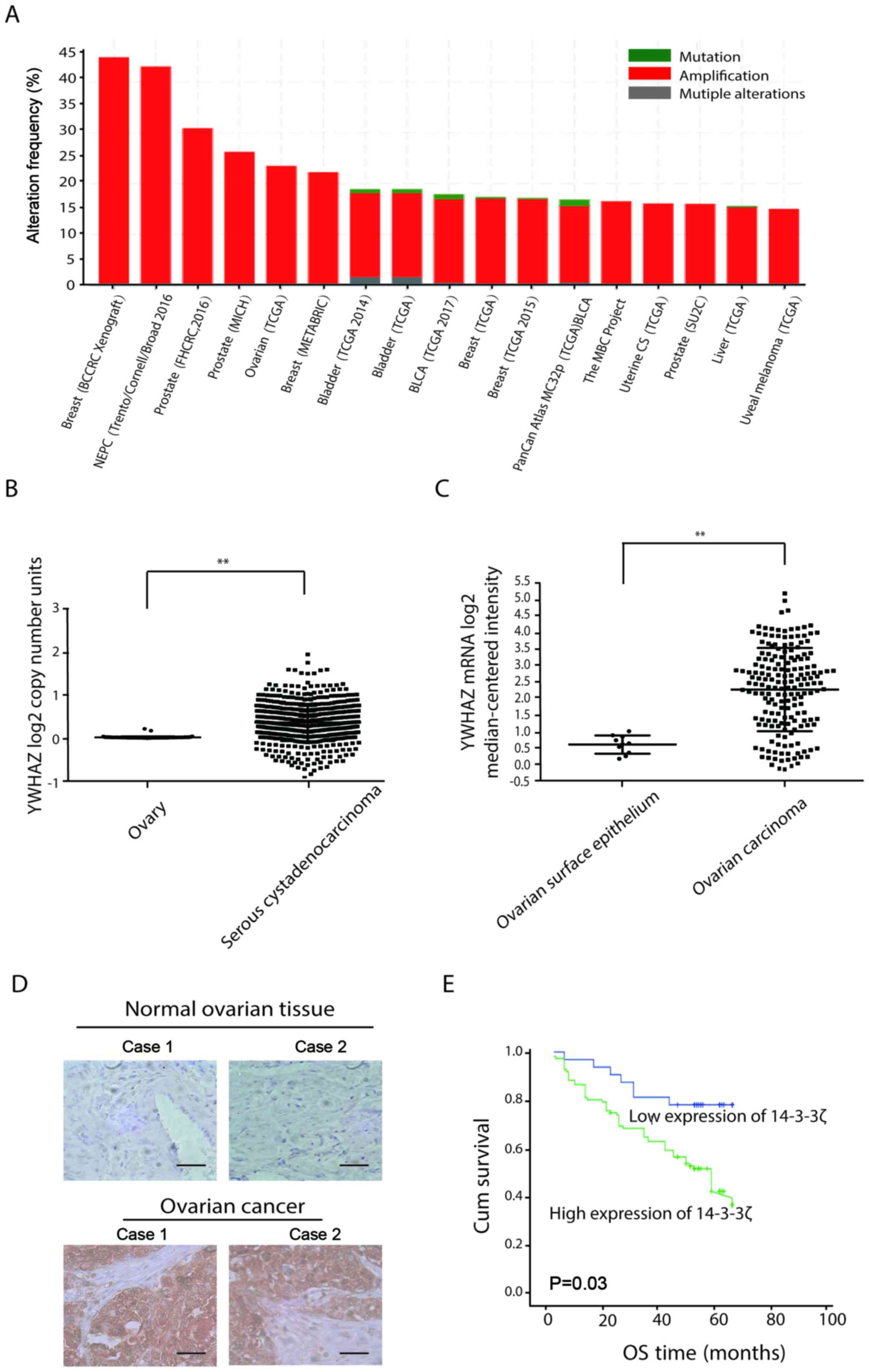
expression using the cBioPortal database (www.cbiopotal.org). The number of copies of the YWHAZ
gene was increased in most tumors, such as breast, prostate and
ovarian cancer (Fig. 1A). Using the
TCGA database (http://cancergenome.nih.gov/), we analyzed YWHAZ copy
number and mRNA levels in ovarian cancers compared with normal
ovarian tissue. We found that YWHAZ mRNA expression and copy number
in cancer tissues was similarly increased (Fig. 1B and C). Then, we performed an
immunohistochemical (IHC) analysis of a tissue microarray that
contained 83 epithelial ovarian cancer (EOC) tissue samples and 20
normal ovarian tissues. As shown in Fig. 1D, strong YWHAZ staining was observed
in most EOC samples (61.4 and 51/83), whereas staining was minimal
in normal ovarian tissues (16.7 and 3/18). Using Kaplan-Meier
analysis, patients with high YWHAZ expression were found to be
significantly associated with reduced overall survival (Fig. 1E).
Then, we analyzed the relevance of YWHAZ expression
based on patient clinicopathological parameters. We found that
patients with high YWHAZ expression exhibited higher tumor stage
and worse prognosis (Table I).
 | Table I.YWHAZ expression and
clinicopathological parameters in 83 ovarian cancer cases. |
Table I.
YWHAZ expression and
clinicopathological parameters in 83 ovarian cancer cases.
|
|
| YWHAZ |
|
|---|
|
|
|
|
|
|---|
| Parameters | Total | Score <2 n
(%) | Score ≥2 n (%) | P-value |
|---|
| Ovarian cancer
group | 83 | 32 (38.6) | 51 (61.4) |
0.000a |
| Control group | 18 | 15 (83.3) | 3
(16.7) |
|
| Histologic
subgroups |
|
|
| 0.882 |
|
Serous | 60 | 14 (23.3) | 46 (76.7) |
|
|
Endometriods | 11 | 5
(45.4) | 6
(54.6) |
|
| Clear
cell | 12 | 5
(41.7) | 7
(58.3) |
|
| FIGO stage |
|
|
| 0.026a |
|
I–II | 39 | 15 (38.5) | 24 (61.5) |
|
|
III–IV | 44 | 4
(9.1) | 40 (90.9) |
|
| Lymph node
status |
|
|
| 0.017a |
|
Negative | 23 | 10 (43.4) | 13 (56.6) |
|
|
Positive | 60 | 11 (18.3) | 49 (81.7) |
|
| Tumor size
(cm) |
|
|
| 0.017a |
|
<2 | 32 | 15 (43.3) | 17 (56.7) |
|
| ≥2 | 51 | 10 (19.7) | 41 (80.3) |
|
YWHAZ expression in ES2 and SKOV3 cell
lines transfected with siRNAs against YWHAZ
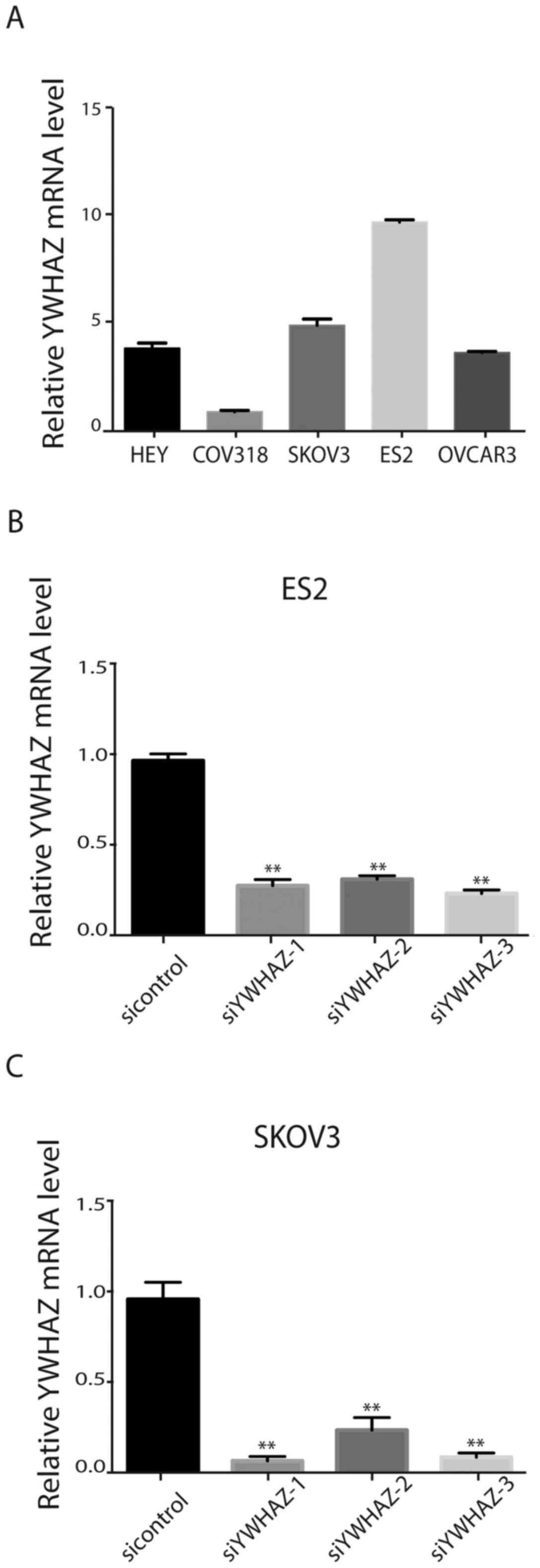
To select suitable cell lines for further research,
we assessed YWHAZ expression in five ovarian cell lines (HEY,
CAOV3, ES2, COV318 and SKOV3). The results showed that ES2 and
SKOV3 cells that exhibit high metastatic potential expressed the
highest levels of YWHAZ (Fig. 2A).
Therefore, ES2 and SKOV3 cells were transfected with siRNAs
targeting YWHAZ or a scrambled siRNA (labeled as control). RT-PCR
and western blotting were used to validate the silencing effects of
the siRNAs in the cell lines (Fig. 2B
and C). The results verified that YWHAZ expression levels were
significantly decreased by the three siRNAs against YWHAZ.
Silencing of YWHAZ suppresses ovarian
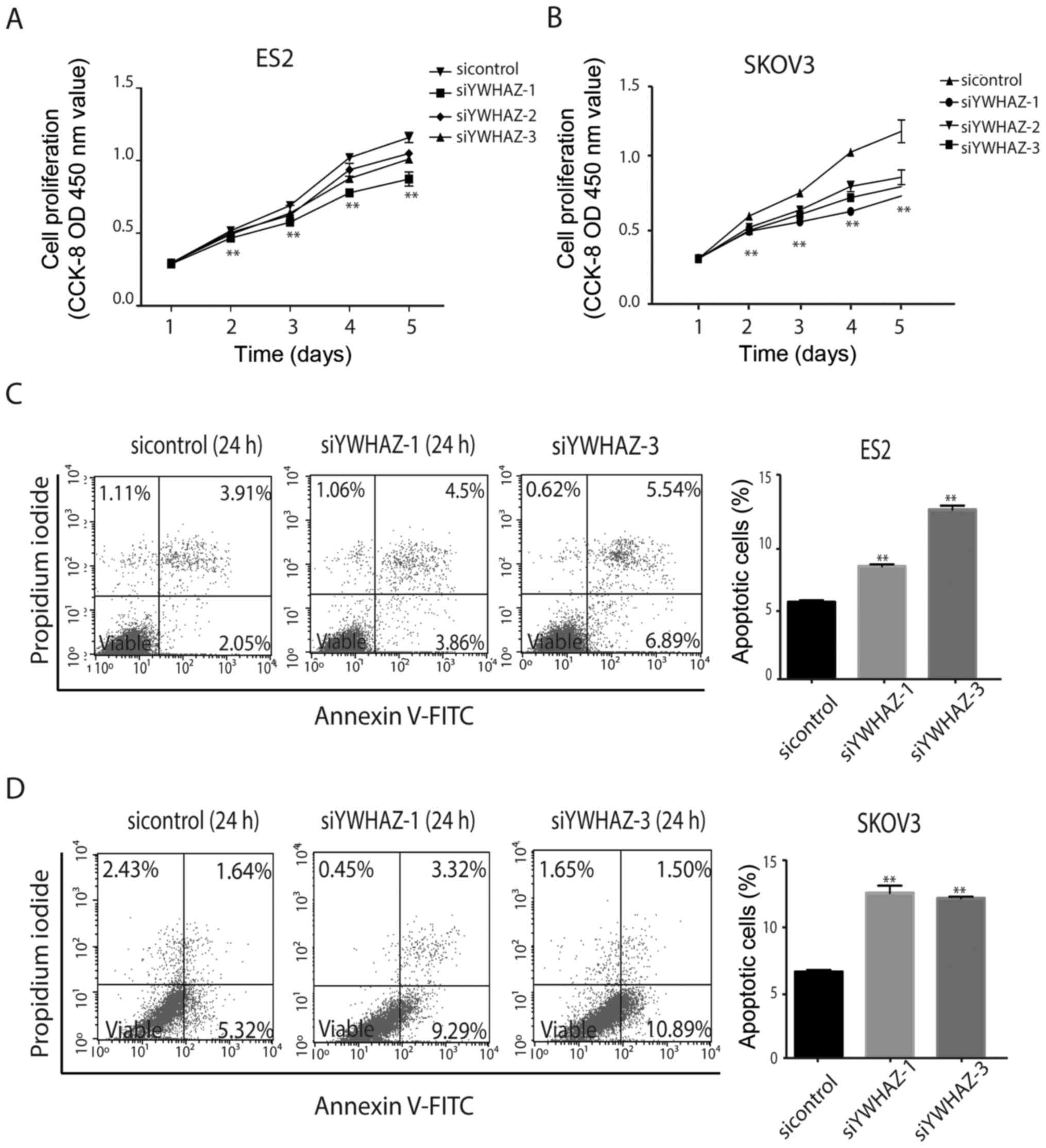
cancer cell proliferation in vitro
Next, we investigated the effect of YWHAZ on cell
viability. After transfection with siRNAs, the Cell Counting Kit-8
(CCK-8) assay was used to detect ES2 and SKOV3 proliferation. The
results showed that ES2 and SKOV3 cell proliferation was suppressed
by silencing of YWHAZ (Fig. 3A and
B).
Silencing of YWHAZ promotes ovarian
cancer cell apoptosis in vitro
To explore whether YWHAZ affects ovarian cancer cell
apoptosis, ES2 and SKOV3 cells transfected with siRNAs against
YWHAZ were used to detect cell apoptosis by flow cytometry. The
results showed that YWHAZ silencing promoted ES2 and SKOV3 cell
apoptosis (Fig. 3C and D).
Silencing of YWHAZ suppresses ovarian
cancer cell migration in vitro and in vivo
To further study the functional role of YWHAZ in
ovarian cancer, we investigated the effects of YWHAZ on ovarian
cancer cell migration in vitro. Silencing of YWHAZ
expression significantly inhibited ES2 and SKOV3 cell migration
in vitro as assessed by Transwell migration assay (Fig. 4A and B), whereas YWHAZ
overexpression promoted ES2 and SKOV3 cell migration in
vitro (Fig. 4C and D) compared
with the control group. Then, we constructed stable ES-2 and SKOV3
cell lines expressing shYWHAZ. The knockdown efficiency was
determined by qRT-PCR and western blotting (Fig. 4E and F). Then, we injected these
cells into the tail vein of BALB/C nude mice. Thirty-five days
after injection, we examined tumor metastases in the mice by
histological H&E staining analyses. Compared with the control
group, the number of metastatic lung nodules was significantly
reduced in the interference group (Fig.
4G and H). Consistent with our findings in vitro,
genetic silencing of YWHAZ significantly reduced the tumor burden
of EOC in the xenograft metastasis model in nude mice (Fig. 4G and H).
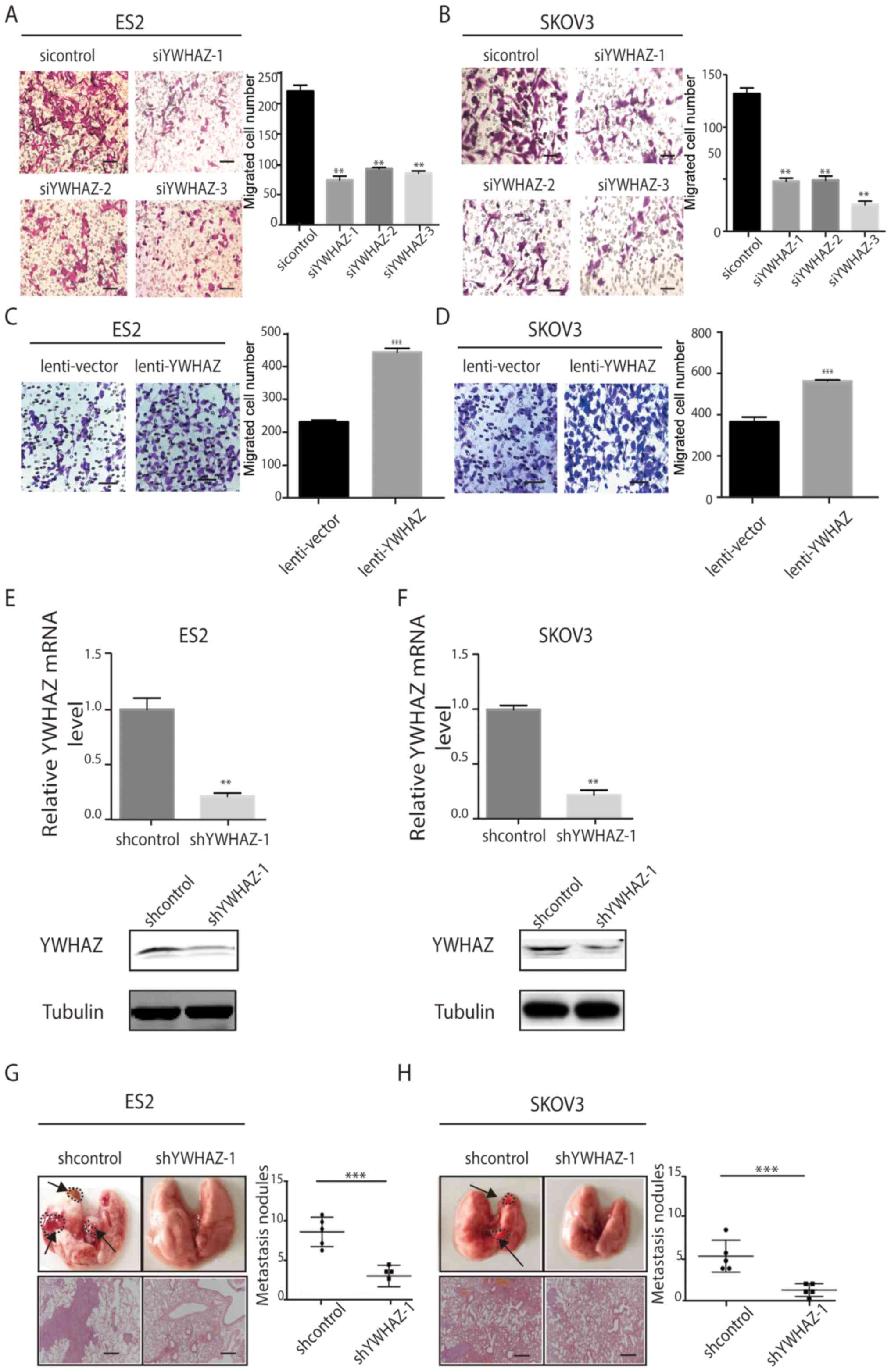 | Figure 4.YWHAZ promotes EOC cell migration
in vitro and in vivo. (A and B) Transwell migration
assays using ES2 and SKOV3 cells transfected with Lenti-shYWHAZ-(1,
2, 3) or NC (sicontrol). Scale bar, 100 µm. (C and D) Transwell
migration assays using ES-2 and SKOV3 cells with overexpression of
YWHAZ. Representative images are shown on the left, and the
quantification of 3 randomly selected fields is shown on the right
(original magnification, ×200). The results shown are mean ± SD.
Scale bar, 100 µm. (E and F) YWHAZ mRNA and protein expression
levels after YWHAZ gene silencing in ES2 and SKOV3. shcontrol,
lenti-shcontrol; shYWHAZ-1, lenti-shYWHAZ-1. (G and H)
Representative images of collected lungs and hematoxylin and eosin
staining of lung tissues in control and YWHAZ-interference groups
are shown on the left. Numbers of lung metastatic nodules were
counted and the results are shown on the right. shcontrol,
lenti-shcontrol; shYWHAZ-1, lenti-shYWHAZ-1. Values are means means
± SD. **P<0.01 and ***P<0.001. Scale bar, 100 µm. EOC,
epithelial ovarian cancer. |
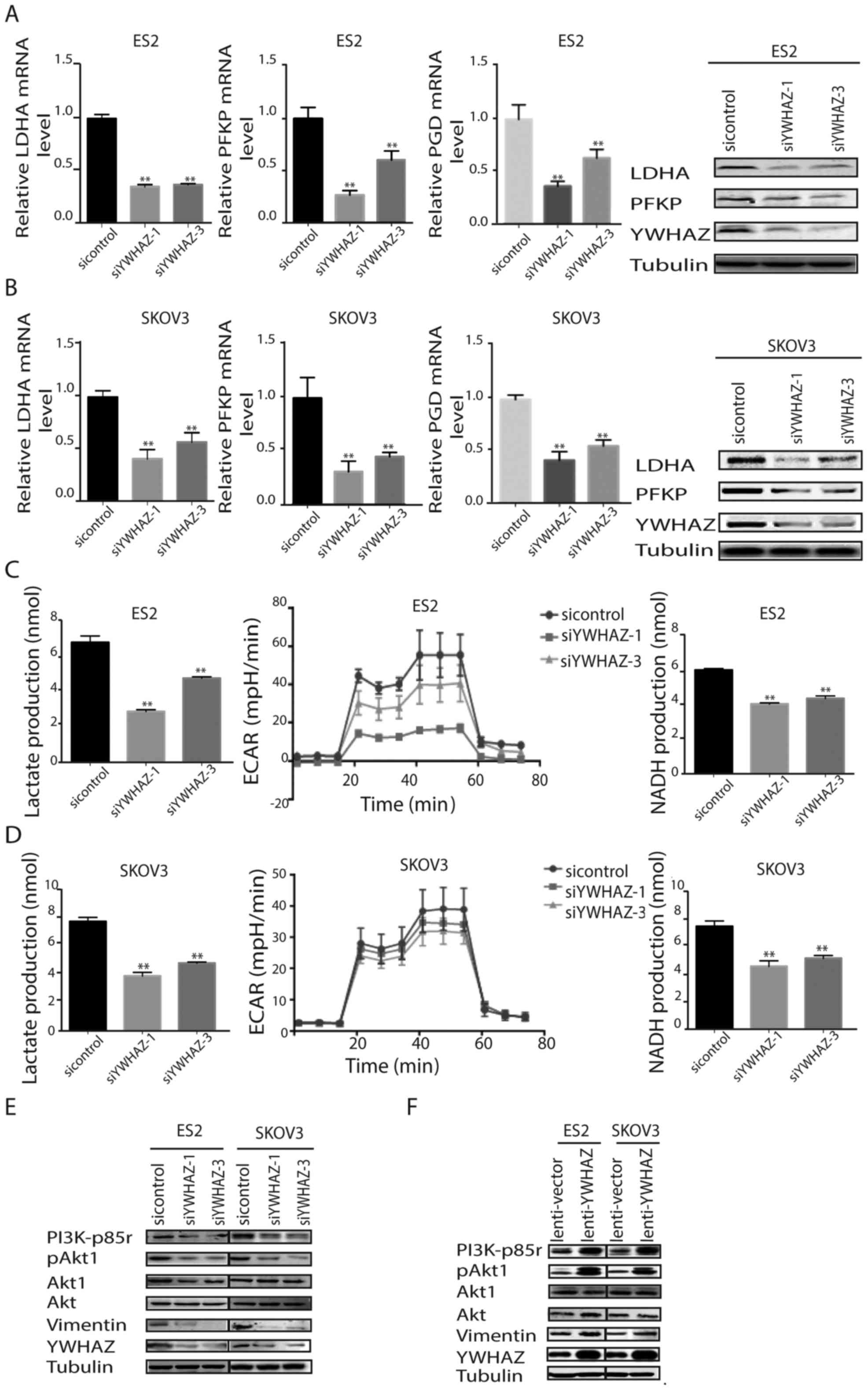
Effects of the silencing of YWHAZ on
glycolysis and the PI3K/AKT pathway
Considering that YWHAZ modulates cellular glycolysis
in other cancers, we next investigated whether YWHAZ is involved in
ovarian cancer glycolysis. First, we measured glycolysis-related
enzymes upon YWHAZ silencing in ovarian cancer cells. In
YWHAZ-silenced ES-2 and SKOV3 cells, the mRNA and protein
expression levels of glycolysis-related enzymes, including lactate
dehydrogenase A (LDHA), human platelet phosphofructokinase (PFKP)
and glyceraldehyde-3-dehydrogenase (PGD), were significantly
reduced (Fig. 5A and B). Then, we
examined lactate production, extracellular acidification rates
(ECAR) and NADH production to probe glycolysis. YWHAZ silencing
significantly reduced lactate, ECAR and NADH production in ES-2 and
SKOV3 cells (Fig. 5C and D). These
data suggest that YWHAZ can promote ovarian cancer cell glycolysis
by regulating the expression of related metabolic enzymes. YWHAZ
binds numerous effectors with a phosphoserine or phosphothreonine
motif and participates in cancer development and progression by
regulating a large spectrum of both general and specialized
signaling pathways. Importantly, YWHAZ interacts with Akt1
(22), a crucial factor involved in
the regulation of Warburg effect by targeting glycolytic enzymes
(6). This information prompted us
to explore the possibility that YWHAZ may stimulate the Warburg
effect through its regulation of Akt1 in EOC cells. As shown in
Fig. 5E, levels of phosphorylated
Akt1 were reduced after genetic inhibition of YWHAZ. In contrast,
YWHAZ overexpression upregulated Akt1 phosphorylation (Fig. 5F). Notably, YWHAZ also regulated the
phosphorylation of PI3K (Fig. 5F),
suggesting that YWHAZ also affected Akt1 through PI3K signaling.
Furthermore, the expression level of vimentin, a downstream
substrate of Akt1, was downregulated by YWHAZ silencing and
upregulated by YWHAZ overexpression. These data suggest that YWHAZ
promotes the Warburg effect by modulating PI3K/Akt1 signaling.
Collectively, these results demonstrated that YWHAZ
promotes ovarian cancer growth and metastasis, which is probably
mediated by regulating PI3K/Akt1/vimentin signaling and
glycolysis.
Discussion
Epithelial ovarian cancer (EOC) is the most lethal
of all gynecological malignancies. It is typically diagnosed at a
late stage, with a 5-year survival rate of less than 30%.
Metastasis plays a crucial role in promoting ovarian tumor
progression and decreasing patient survival rates (23). In addition, it is considered that
the development of ovarian cancer is related to diverse factors
including abnormality of chromosome, activation of oncogenes,
inactivation of tumor-suppressor genes and apoptosis inhibition
(24). However, the underlying
mechanisms by which ovarian cancer spreads have yet to be
thoroughly explored. Thus, it is critical to understand the
molecular mechanisms that promote the metastasis and progression in
EOC. In the present study, we aimed to address the contributions of
YWHAZ in EOC and explore its potential diagnostic and therapeutic
value.
Metabolic alterations have been suggested to play a
crucial role in cancer development. The key metabolic changes in
cancer include aerobic glycolysis and macromolecular synthesis,
causing anti-apoptosis in cancer cells. Activation of Akt enhances
glycolytic activity and is commonly observed in cancer cells,
including ovarian cancer. Considering the high incidence of
chemoresistance and the rapid spread of disease in ovarian cancer,
metabolic approaches represent rational strategies to improve the
poor prognosis of ovarian cancer.
The 14-3-3ζ gene, which is also known as YWHAZ on
8q22, is often amplified in numerous tumor types, such as breast
and prostate cancer (15,25), and high expression of 14-3-3ζ in the
primary tumor is significantly associated with earlier time to
recurrence and with distant metastasis (26). We examined YWHAZ expression in EOC
and this study demonstrated that the YWHAZ-positive tumor rate was
significantly increased in EOC. We analyzed the correlation between
YWHAZ expression and ovarian cancer clinicopathological parameters
and found that YWHAZ expression levels were closely related to
tumor stage, tumor size and metastasis. Kaplan-Meier analysis
revealed that ovarian cancer patients with YWHAZ-positive tumors
had shorter overall survival than YWHAZ-negative patients.
Therefore, we identified that YWHAZ may represent a potential
predictor of poor prognosis in ovarian cancer patients, which is
consistent with a previous study (26).
Upregulation of YWHAZ is very common in many
aggressive human types of cancers as it is linked to tumor cell
proliferation and migration. In this study, when YWHAZ was
silenced, the proliferation and migration of the EOC cell lines
were significantly inhibited, whereas the apoptosis of the EOC cell
lines was promoted. YWHAZ/14-3-3ζ integrates numerous signaling
pathways by activating the MEK-ERK-CREB axis to promote early
breast cancer transformation (7);
playing a crucial role during leukemogenesis through the
c-Myc/miR-451/YWHAZ/AKT cascade (27); promoting dynamic interactions with
the core autophagy regulator Atg9A to modulate autophagy (28); by activating Rac1-GTPase to enhance
prostate cancer cell-matrix interactions, motility and
transendothelial migration (29);
by activating JNK/p38/MAPK signaling pathway to enhance the
anticancer effect of cis-diammine dichloroplatinum (CDDP) in
hepatoma cell lines (30); by
activating the TGFβ/Smad pathway leading to ZFHX1B/SIP-1
upregulation, E-cadherin loss and epithelial-mesenchymal transition
(EMT) reduced cell adhesion (15);
and reducing receptor tyrosine kinase (HER2 and EGFR) signaling
(HER2/EGFR) to reverse endocrine resistance in breast cancer
(16).
Although the functional roles of YWHAZ in tumor cell
proliferation, migration and apoptosis have been well established,
the underlying mechanisms by which YWHAZ promotes ovarian cancer
cell proliferation, migration and apoptosis inhibition remain
unclear. From a therapeutic perspective, targeting YWHAZ is of
particular interest as the present study revealed increased YWHAZ
expression in EOC which was found to be associated with poor
prognosis, consistent with the results of He et al (26).
Tumor cells often exhibit an altered metabolic
phenotype. As ovarian cancer progresses, complete oxidation of
glucose and fatty acids is significantly decreased and occurs
concurrently with increases in lactate excretion and
3H-deoxyglucose uptake by late-stage cancer cells, shifting the
cells towards a more glycolytic phenotype, confirming the metabolic
changes during ovarian cancer progression (8). An altered metabolism during ovarian
cancer progression allows for increased macromolecular synthesis
and unrestrained growth of the tumor-initiating cell population or
cancer stem cells (CSCs/TICs). Ovarian cancer CSCs/TICs exhibit
reductions in glucose and fatty acid oxidation with a concomitant
increase in lactate secretion (31). In recent years, mounting evidence
indicates that numerous multifaceted factors are involved in
glycolysis. Altered energy metabolism has been confirmed to be as
widespread in cancer cells as many of the other cancer-associated
traits that have been accepted as hallmarks of cancer (4). These results suggest that the
therapeutic targets in the glycolysis pathway could be utilized as
anticancer strategies.
Studies have confirmed that YHWHAZ participates in
glucose metabolism. YWHAZ can protect β cells from multiple
stresses by preventing BAD-BAX mitochondrial localization (18). YWHAZ gene knockout mice (14-3-3ζKO)
exhibited elevated fasting insulin levels while maintaining normal
β cell responsiveness to glucose when compared with wild-type
littermate controls (32). As a
response to intratumoral hypoxia, HIF-1α also mediates metabolic
alterations that drive cancer progression (22). Under hypoxic conditions in
vitro, 14-3-3ζ knockdown inhibits hypoxia-induced HCC invasion
by the HIF-1α/EMT pathway (14).
In addition, YWHAZ expression is strongly correlated
with the expression of canonical glycolytic genes, particularly
lactate dehydrogenase A (LDHA). Thus, we aimed to ascertain whether
downregulation of YWHAZ could influence glycolysis in ovarian
cancer. Our results showed that upon silencing of YWHAZ, the
expression of glycolysis metabolites (lactate, ECAR and NADH) and
enzymes (LDHA) were decreased, which is consistent with Chang et
al (7).
Existing data indicated that PI3K/Akt1 participates
in glycolysis (33). In this study,
we found that phosphorylation of Akt1 and its upstream kinase PI3K
was reduced after YWHAZ knockdown in ovarian cancer cells. Notably,
Akt1 phosphorylates Ser-58 on YWHAZ both in vitro and in
intact cells (22). Moreover, the
Ser-58 phosphorylated form inhibits its interaction with TP53 and
p53 transcriptional activity (34).
Collectively, we can infer that YWHAZ is a substrate of Akt1 and
that YWHAZ promotes PI3K/Akt1/vimentin signaling, forming a
feedback functional loop to promote cancer glycolysis,
proliferation and migration. An important issue that needs future
investigation is how YWHAZ regulates PI3K/Akt1 signaling. One
possibility is by interacting with an upstream factor with a
phosphoserine or phosphothreonine motif.
In this study, we determined that high YWHAZ
expression was associated with poor prognosis in ovarian cancer.
Regarding the biological significance of YWHAZ, it promoted
proliferation and migration of ovarian cancer cells. Additionally,
the silencing of YWHAZ reduced the glycolytic capability of EOC
cells. Investigation into the molecular mechanisms revealed that
YWHAZ silencing downregulated the phosphorylation of PI3K/Akt1 in
EOC cell lines. Taken together, our results suggest a role for
YWHAZ in the progression of ovarian cancer and identify YWHAZ as a
potentially important molecule for human ovarian cancer
progression. Further research is needed to clarify which signaling
pathway is involved in YWHAZ-mediated regulation of PI3K/Akt1
expression, thereby affecting glycolysis in ovarian cancer cells
and promoting cell proliferation and metastasis.
Acknowledgements
We thank Dr Lei Zhu, Dr Jun Li and Dr Ya-Hui Wang
for technical help and advice.
Funding
The present study was supported by the Natural
Science Foundation, Minhang, Shanghai (no. 2016MHZ02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XMY and LWZ conceived and designed the study,
analyzed and interpreted the data, and provided a critical review
of the manuscript. JS and JY collected and analyzed the data and
wrote the manuscript. JS, JY, SHJ, HF, YPC and ZYW prepared the
experimental materials and performed the in vitro assays.
SHJ and ZYW performed the in vivo assays. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of Fudan University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baldwin LA, Huang B, Miller RW, Tucker T,
Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR
and Seamon LG: Ten-year relative survival for epithelial ovarian
cancer. Obstet Gynecol. 120:612–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Damia G and Sessa C: Successes and
limitations of targeted cancer therapy in ovarian cancer. Prog
Tumor Res. 41:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu S, Balakrishnan A, Bok RA, Anderton B,
Larson PE, Nelson SJ, Kurhanewicz J, Vigneron DB and Goga A:
13C-pyruvate imaging reveals alterations in glycolysis that precede
c-MYC induced tumor formation and regression. Cell Metab.
14:131–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang SH, Li J, Dong FY, Yang JY, Liu DJ,
Yang XM, Wang YH, Yang MW, Fu XL, Zhang XX, et al: Increased
serotonin signaling contributes to the Warburg effect in pancreatic
tumor cells under metabolic stress and promotes growth of
pancreatic tumors in mice. Gastroenterology. 153:277–291.e19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CC, Zhang C, Zhang Q, Sahin O, Wang
H, Xu J, Xiao Y, Zhang J, Rehman SK, Li P, et al: Upregulation of
lactate dehydrogenase a by 14-3-3ζ leads to increased glycolysis
critical for breast cancer initiation and progression. Oncotarget.
7:35270–35283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slupsky CM, Steed H, Wells TH, Dabbs K,
Schepansky A, Capstick V, Faught W and Sawyer MB: Urine metabolite
analysis offers potential early diagnosis of ovarian and breast
cancers. Clin Cancer Res. 16:5835–5841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson AS, Roberts PC, Frisard MI,
McMillan RP, Brown TJ, Lawless MH, Hulver MW and Schmelz EM:
Metabolic changes during ovarian cancer progression as targets for
sphingosine treatment. Exp Cell Res. 19:1431–1442. 2013. View Article : Google Scholar
|
|
10
|
Sun L, Yin Y, Clark LH, Sun W, Sullivan
SA, Tran AQ, Han J, Zhang L, Guo H, Madugu E, et al: Dual
inhibition of glycolysis and glutaminolysis as a therapeutic
strategy in the treatment of ovarian cancer. Oncotarget.
8:63551–63561. 2017.PubMed/NCBI
|
|
11
|
Watanabe N, Komatsu S, Ichikawa D, Miyamae
M, Ohashi T, Okajima W, Kosuga T, Konishi H, Shiozaki A, Fujiwara
H, et al: Overexpression of YWHAZ as an independent prognostic
factor in adenocarcinoma of the esophago-gastric junction. Am J
Cancer Res. 6:2729–2736. 2016.PubMed/NCBI
|
|
12
|
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT,
Lu J, Danes CG, Guo H, Lan KH, Ensor J, et al: 14-3-3zeta
overexpression defines high risk for breast cancer recurrence and
promotes cancer cell survival. Cancer Res. 69:3425–3432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin M, Morrison CD, Jones S, Mohamed N,
Bacher J and Plass C: Copy number gain and oncogenic activity of
YWHAZ/14-3-3zeta in head and neck squamous cell carcinoma. Int J
Cancer. 125:603–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang Y, Liu S, Li N, Guo W, Shi J, Yu H,
Zhang L, Wang K, Liu S and Cheng S: 14-3-3ζ promotes hepatocellular
carcinoma venous metastasis by modulating hypoxia-inducible
factor-1α. Oncotarget. 7:15854–15867. 2016.PubMed/NCBI
|
|
15
|
Lu J, Guo H, Treekitkarnmongkol W, Li P,
Zhang J, Shi B, Ling C, Zhou X, Chen T, Chiao PJ, et al: 14-3-3zeta
cooperates with ErbB2 to promote ductal carcinoma in situ
progression to invasive breast cancer by inducing
epithelial-mesenchymal transition. Cancer Cell. 16:195–207. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergamaschi A, Frasor J, Borgen K,
Stanculescu A, Johnson P, Rowland K, Wiley EL and Katzenellenbogen
BS: 14-3-3ζ as a predictor of early time to recurrence and distant
metastasis in hormone receptor-positive and -negative breast
cancers. Breast Cancer Res Treat. 137:689–696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pozuelo Rubio M, Peggie M, Wong BH,
Morrice N and MacKintosh C: 14-3-3s regulate
fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated
cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J.
2:3514–3523. 2003. View Article : Google Scholar
|
|
18
|
Lim GE, Piske M and Johnson JD: 14-3-3
proteins are essential signaling hubs for beta cell survival.
Diabetologia. 56:825–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim CY, Jeong SY, Chong GO, Son SH, Jung
JH, Kim DH, Lee SW, Ahn BC and Lee J: Quantitative metabolic
parameters measured on F-18 FDG PET/CT predict survival after
relapse in patients with relapsed epithelial ovarian cancer.
Gynecol Oncol. 136:498–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang XM, Cao XY, He P, Li J, Feng MX,
Zhang YL, Zhang XL, Wang YH, Yang Q, Zhu L, et al: Overexpression
of Rac GTPase activating protein 1 contributes to proliferation of
cancer cells by reducing hippo signaling to promote cytokinesis.
Gastroenterology. 155:1233–1249.e22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Powell DW, Rane MJ, Chen Q, Singh S and
McLeish KR: Identification of 14-3-3zeta as a protein kinase B/Akt
substrate. J Biol Chem. 277:21639–21642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A review in the theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Rev Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murata T, Takayama K, Urano T, Fujimura T,
Ashikari D, Obinata D, Horie-Inoue K, Takahashi S, Ouchi Y, Homma Y
and Inoue S: 14-3-3ζ, a novel androgen-responsive gene, is
upregulated in prostate cancer and promotes prostate cancer cell
proliferation and survival. Clin Cancer Res. 18:5617–5627. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Wu X, Liu X, Yan G and Xu C:
LC-MS/MS analysis of ovarian cancer metastasis-related proteins
using a nude mouse model: 14-3-3 zeta as a candidate biomarker. J
Proteome Res. 9:6180–6190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su R, Gong JN, Chen MT, Song L, Shen C,
Zhang XH, Yin XL, Ning HM, Liu B, Wang F, et al: c-Myc suppresses
miR-451_ǀYWTAZ/AKT axis via recruiting HDAC3 in acute myeloid
leukemia. Oncotarget. 7:77430–77443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weerasekara VK, Panek DJ, Broadbent DG,
Mortenson JB, Mathis AD, Logan GN, Prince JT, Thomson DM, Thompson
JW and Andersen JL: Metabolic-stress-induced rearrangement of the
14-3-3ζ interactome promotes autophagy via a ULK1- and
AMPK-regulated 14-3-3ζ interaction with phosphorylated Atg9. Mol
Cell Biol. 34:4379–4388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goc A, Abdalla M, Al-Azayzih A and
Somanath PR: Rac1 activation driven by 14-3-3ζ dimerization
promotes prostate cancer cell-matrix interactions, motility and
transendothelial migration. PLoS One. 7:e405942012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi JE, Hur W, Jung CK, Piao LS, Lyoo K,
Hong SW, Kim SW, Yoon HY and Yoon SK: Silencing of 14-3-3ζ
over-expression in hepatocellular carcinoma inhibits tumor growth
and enhances chemosensitivity to cis-diammined dichloridoplatium.
Cancer Lett. 303:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anderson AS, Roberts PC, Frisard MI,
Hulver MW and Schmelz EM: Ovarian tumor-initiating cells display a
flexible metabolism. Exp Cell Res. 328:44–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim GE, Piske M, Lulo JE, Ramshaw HS,
Lopez AF and Johnson JD: Ywhaz/14-3-3ζ deletion improves glucose
tolerance through a GLP-1-dependent mechanism. Endocrinology.
157:2649–2659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taylor C, Mannion D, Miranda F,
Karaminejadranjbar M, Herrero-Gonzalez S, Hellner K, Zheng Y,
Bartholomeusz G, Bast RC Jr and Ahmed AA: Loss of PFKFB4 induces
cell death in mitotically arrested ovarian cancer cells.
Oncotarget. 8:17960–17980. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Danes CG, Wyszomierski SL, Lu J, Neal CL,
Yang W and Yu D: 14-3-3 zeta down-regulates p53 in mammary
epithelial cells and confers luminal filling. Cancer Res.
68:1760–1767. 2008. View Article : Google Scholar : PubMed/NCBI
|