Introduction
Fine particulate matter (PM2.5) indicates
atmospheric aerodynamic equivalent diameter less than or equal to
2.5 micron particles (1,2). Long-term and short-term exposure to
PM2.5 directly threatens public health (3,4).
PM2.5 has been related to respiratory disease and
cardiovascular disease (5–7). Exposure to PM2.5 increases
the risk of asthma and exacerbates established asthma (5,6).
PM2.5 induces inflammation and mucus hyperproduction in
the airway epithelium (7).
PM2.5 is necessary for the migration of human bronchial
smooth muscle cells, defining a novel role for PM2.5 in airway
remodelling in chronic obstructive pulmonary disease (8). Moreover, PM2.5 is
associated with the development of atherosclerosis in
ApoE−/− mice (9).
Many publications have demonstrated that
PM2.5 is associated with cancer incidence. Suggestive
evidence has shown an association between ambient air pollution and
the incidence of postmenopausal breast cancer in European women
(10). Ambient PM2.5
exposure may be a risk factor for hepatocellular carcinoma in the
United States (11).
PM2.5 was also involved in lung cancer burden (12). Enhanced ability of motility and
proliferation were observed after PM2.5 exposure of
non-small cell lung cancer cells (13). PM2.5 also induced
epithelial-mesenchymal transition of human lung cancer cells
(14). In addition, lung cancer
stem cell properties were induced by PM2.5 (15). However, the definitive relationship
between PM2.5 exposure and lung cancer has yet to be
explored.
Exosomes are vesicles smaller than 150 nm in
diameter that are enriched in endosome-derived components. Exosomes
have a bilayer lipid structure containing transmembrane proteins,
and they enclose soluble proteins, RNA and DNA (16,17).
Accumulating evidence has well recognized the important role of
exosomes as couriers to mediate communication between different
cells (18). Tumour-derived
exosomes (TEXs) are closely related to tumour development. TEXs
educated dendritic cells to promote tumour metastasis via the
HSP72/HSP105-TLR2/TLR4 pathway (19). Lnc-Sox2ot of TEXs promoted EMT and
stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma
(20). Tumour exosomal RNAs
promoted lung pre-metastatic niche formation by activating alveolar
epithelial TLR3 to recruit neutrophils (21). Wnt10b in cancer-associated
fibroblasts has been shown to promote breast cancer cell metastasis
(22). In addition, activation of
the Wnt signalling pathway was detected in PM2.5-induced
pulmonary arterial hypertension of rats (23). Therefore, exosomes from
PM2.5-treated lung cancer cells may affect tumour
progression through activation of Wnt signalling.
The present study demonstrated that Wnt3a was
enriched in exosomes from PM2.5-treated A549
(EXOPM2.5) cells (human epithelial cancer cells, which
activated Wnt/β-catenin signalling in A549 cells).
EXOPM2.5 significantly promoted A549 cell proliferation
in a Wnt3a-dependent fashion in vitro. Furthermore,
intratumoural injection of EXOPM2.5 accelerated tumour
growth and decreased survival rate of mice via Wnt3a. Therefore,
these results extend the knowledge of PM2.5 exposure and
lung cancer progression.
Materials and methods
Reagents
PM2.5 was purchased from the National
Institute of Standards and Technology (Gaithersburg, MD, USA).
Human Wnt3a siRNA, negative control (NC) siRNA and
antibodies against GRP94 (cat. no. sc-393402), CD63 (cat. no.
sc-59284), Tsg101 (cat. no. sc-136111), Alix (cat. no. sc-53540),
HSP70 (cat. no. sc-59570), Wnt3a (cat. no. sc-136163) and β-Actin
(cat. no. sc-517582) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Human Wnt1, Wnt3a, Wnt4, Wnt7a,
Wnt9a and Wnt10b primers were purchased from OriGene
Technologies, Inc. (Rockville, MD, USA). An antibody against
β-catenin (cat. no. ab6302) was purchased from Abcam (Cambridge,
MA, USA). LF3, a specific inhibitor of Wnt/β-catenin signalling
(24), was purchased from
Selleckchem (Houston, TX, USA). Cell Counting Kit-8 (CCK-8) was
purchased from Dojindo Molecular Technologies, Inc. (Tokyo, Japan).
Matrigel matrix basement membrane was purchased from BD Biosciences
(San Diego, CA, USA).
Mice and cell line
Female athymic nude mice (aged 6–8 weeks) were
purchased from Joint Ventures Sipper BK Experimental Animal Co.,
Ltd. (Shanghai, China). The mice were maintained in specific
pathogen-free facilities with temperature ranging from 22 to 24°C,
humidity ranging from 50 to 60% and 12 h of light/dark cycle at
Wenzhou Medical University (Wenzhou, China). Mice had free access
to food and water, and all experiments using mice were approved by
and performed according to the guidelines of the Animal Ethics
Committee of Wenzhou Medical University.
The A549 cell line, a human lung adenocarcinoma cell
line, was purchased from the American Type Culture Collection
(ATTCC; Manassas, VA, USA), and cultured in RPMI-1640 media
supplemented with 10% (v/v) fetal calf serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a 5%
CO2 incubator.
Preparation of PM2.5
PM2.5 (10 mg) was suspended in 1 ml of
normal saline, sonicated at 20% power for 3 pulses of 10 sec each
(waiting 5 sec between pulses), and then 4 ml of normal saline was
added to a final concentration of 2 mg/ml. The PM2.5
solution was aliquoted and stored at 4°C. Before use, the
PM2.5 solution was sonicated at 20% power for 3 pulses
of 10 sec each (waiting 5 sec between pulses).
PM2.5 treatment and exosome
isolation
A549 cells (2.5×105/ml) were seeded into
6-well plates in total volume of 2 ml/well. After 12 h, the
supernatant was discarded, and 2 ml of fresh RPMI-1640 media was
added with 100 µg/ml PM2.5. After 24 h, the supernatant
collected from all the wells (240 ml in total) was differentially
centrifuged at 300 × g for 10 min, 1,200 × g for 20 min, and 10,000
× g for 30 min at 4°C. The supernatants from the final
centrifugation were ultracentrifuged at 100,000 × g for 1 h at 4°C.
After removing the supernatants, the exosomal pellets were washed
in a large volume of ice-cold phosphate-buffered saline (PBS) and
centrifuged at 100,000 × g for an additional 1 h at 4°C. The final
pellets were resuspended in PBS. The amount of exosomal proteins
was assessed by a BCA assay (Thermo Fisher Scientific, Inc.).
Nanoparticle tracking analysis and
electronic microscopy
Nanoparticle tracking analysis of exosomes was
assessed by NanoSight NS300 Particle Size Analyzer (Malvern
Panalytical Ltd., Malvern, UK). To detect morphology of exosomes,
exosomes were isolated and diluted in 100 µl of PBS, and 20 µl of
the suspension was placed onto Formvar carbon-coated copper grids
(Beijing XXBR Technology Co., Ltd., Beijing, China) at room
temperature for 1 min. The excess suspension was removed using
filter paper. Exosomes were stained with 2% phosphotungstic acid at
room temperature for 5 min. The grids were then fixed with 2.5%
glutaraldehyde at room temperature for 5 min followed by rinsing
with PBS three times. Images were observed with a Philips Tecnai-10
transmission electron microscope operating at 80 kV (Phillips
Electronic Instruments, Inc., Mahway, NJ, USA).
Real-time PCR
Total RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The following PCR conditions were
used: 1 cycle at 95°C for 30 sec; and 40 cycles of 5 sec at 95 and
60°C at 34 sec. Real-time PCR was performed using an Applied
Biosystems 7500 Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA). The following primers were used: Wnt1
forward, 5′-ctcttcggcaagatcgtcaacc-3′ and reverse, 5′-cga tgg aac
ctt ctg agc agg a-3′; Wnt3a forward, 5′-atg aac cgc cac aac
aac gag g-3′ and reverse, 5′-gtc ctt gag g aa gtc acc gat g-3′;
Wnt4 forward, 5′-gct gga gaa gtg cgg ctg tga-3′ and reverse,
5′-cca caa acg act gtg aga agg c-3′; Wnt7a forward, 5′-agg
aga agg ctc aca aat ggg c-3′ and reverse, 5′-cgg caa tga tgg cgt
agg tga a-3′; Wnt9b forward, 5′-cct gct tga gtg cca gtt tca
g-3′ and reverse, 5′-aca ccg cgt aca gga aag ctg t-3′;
Wnt10b forward, 5′-ctc ggg att tct tgg att cca gg-3′ and
reverse, 5′-gcc atg aca ctt gca ttt ccg c-3′.
Immunofluorescence staining
For detection of β-catenin nuclear translocation,
A549 cells were treated with 10 µg/ml exosomes for 0, 30 and 60
min. The cells were then fixed, permeabilized and incubated with
rabbit polyclonal antibodies against β-catenin (cat. no. ab6302;
Abcam) using a dilution of 1:1,000 at 4°C overnight. Subsequently,
the cells were incubated with Alexa Fluor® 647
conjugated goat anti-rabbit antibodies (cat. no. ab150079; Abcam)
using a dilution of 1:200 for 1 h. Cells were counterstained with
DAPI to indicate DNA. Stained cells were viewed using a confocal
microscope (SP2; Leica, Solms, Germany).
Migration assay
A549 cells (1×106/ml and
2.5×105/ml) were treated with 10 µg/ml exosomes from
A549 with mock treatment (EXOCtrl) or
EXOPM2.5 for 24 h at 37°C. Then, 2×104 cells
in 100 µl of serum-free media were seeded into the top chamber. The
bottom chamber was filled with 800 µl of medium containing 20%
serum. After being cultured for 18 h at 37°C, the cells were fixed
with methanol for 20 min and washed with PBS three times. The fixed
cells were stained with 10 µg/ml DAPI for 30 min and washed with
PBS. The stained cells were examined using a fluorescence
microscope.
Invasion assay
After rehydration using a 6-fold volume of
serum-free media, 50 µl of Matrigel was added on an 8-µm
polycarbonate membrane in 24-well Transwell plates, and the
Matrigel was solidified at 37°C. Then, 1×106 A549 tumour
cells were incubated with 10 µg/ml EXOCtrl or
EXOPM2.5 for 24 h at 37°C. Subsequently,
5×104 A549 cells in 100 µl of serum-free media were
seeded into the top chamber. The bottom chamber was filled with 800
µl of medium containing 20% FBS. After being cultured for 48 h at
37°C, the cells were fixed with methanol for 20 min and washed with
PBS three times. The fixed cells were stained with 10 µg/ml DAPI
for 30 min and washed with PBS. The stained cells were examined
using a fluorescence microscope.
In vitro proliferation assay
A549 cells (2×104) were seeded into
96-well plates at 200 µl/well, and 2 µg of exosomes was then added
for 24 h. To some of the wells, 10 µM LF3 was added. Four hours
before the end of culture, 20 µl of CCK-8 was added. The optical
density (OD) of each well was read at 450 nm using an automated
microplate reader (Sunrise; Tecan Group, Ltd., Mannedorf,
Switzerland).
Western blot analysis
Exosomes or crude proteins were extracted from cell
lysates by RIPA lysis buffer (Beyotime Institute of Biotechnology,
Shanghai, China) and then qualified by BCA Protein Assay kit
(Beyotime Institute of Biotechnology). A total of 20 µg of proteins
was separated by 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride polyvinylidene difluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA). The membrane was blocked with 5%
BSA in TBST, and then incubated with corresponding primary
antibodies overnight at 4°C. After incubating with HRP-coupled
secondary antibodies for 1 h at room temperature, the membranes
were scanned using a Tanon 4500 Gel Imaging System (Tanon Science
and Technology Co., Ltd., Shanghai, China). The following primary
antibodies and secondary antibodies were used: Rabbit monoclonal
antibodies against GRP94 (dilution 1:1,000; cat. no. ab108606),
rabbit monoclonal antibodies against Tsg101 (dilution 1:1,000; cat.
no. ab125011), mouse monoclonal antibodies against Alix (dilution
1:500; cat. no. ab117600), mouse monoclonal antibodies against
HSP70 (dilution 1:500; cat. no. ab47455), mouse monoclonal
antibodies against Wnt3a (dilution 1:1,000; cat. no. ab81614),
mouse monoclonal antibodies against CD63 (dilution 1:500; cat. no.
ab193349), rabbit monoclonal antibodies against β-catenin (dilution
1:2,000; cat. no. ab32572), mouse monoclonal antibodies against
β-actin (dilution 1:500; cat. no. ab8226;), HRP-coupled rabbit
polyclonal antibodies against mouse (dilution 1:2,000; cat. no.
ab6728) and HRP-coupled goat antibodies against rabbit (dilution
1:2,000; cat. no. ab6721; all were from Abcam).
RNA interference assay
For transient silencing of the Wnt3a gene, 40
nM siRNA was transfected into cells (2×105/well) using 3
µl of INTERFERin siRNA transfection reagent (Polyplus-Transfection,
New York, NY, USA) per well in a 24-well plate. The knockdown
efficiency of Wnt3a was confirmed by western blotting.
In vivo animal studies
To establish the tumour model, A549 cells
(5×106) were subcutaneously injected into nude mice. On
day 10, the mice were randomly divided into three groups (each
group with 5 mice/total 120 mice) and received intratumoural
injections of 5 µg of exosomes every other day (total 14
injections). For the survival study, when the largest tumour volume
reached 4,000 mm3 (60 mice), the mice were no longer
free to move around. For humanitarian reasons, the mice were
sacrificed by cervical dislocation after being intraperitoneally
injected with 50 mg/kg pentobarbital sodium approved by Animal
Ethics Committee of Wenzhou Medical University. The other 60 mice
were also sacrificed by this way on day 36. The length and width of
tumours were assessed every four days by vernier caliper and the
tumour was calculated according to the following formula: Volume =
(length × width2)/2.
Statistical analysis
Results were expressed as the mean ± SEM. Statistics
were analysed by one-way or two-way analysis of variance (ANOVA)
with Newman-Keuls post hoc test. The survival curves were
calculated using the Kaplan-Meier method and the log-rank test was
used for survival analysis. All statistics were analysed by
GraphPad Prism 5.0 software (Graphpad Software, Inc., La Jolla, CA,
USA). A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
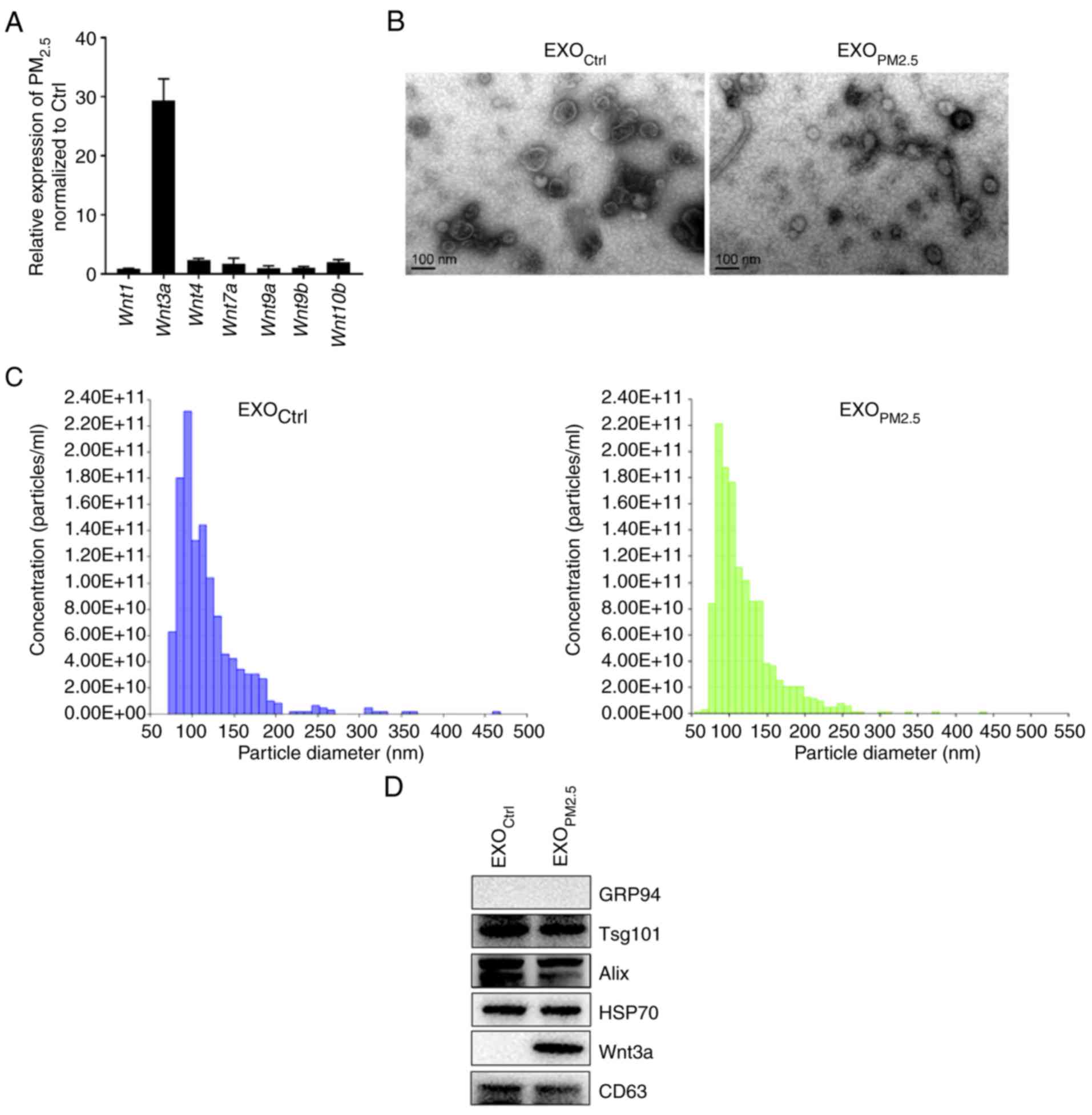
PM2.5 exposure increases
Wnt3a protein level in EXOPM2.5
To assess if PM2.5 treatment upregulated
expression of Wnt family members, the mRNA expression level of
Wnt family members in PM2.5-treated A549 cells
was assessed. The Wnt3a mRNA level was greatly increased
compared to control cells (Fig.
1A). To investigate if PM2.5 exposure affected Wnt3a
protein level in EXOPM2.5, EXOPM2.5 and
exosomes from A549 cells with mock treatment (EXOCtrl)
were isolated. Visualization using electron microscopy indicated
that EXOCtrl and EXOPM2.5 had similar
morphology, and both had diameters ranging from 50 to 150 nm
(Fig. 1B), indicating that
PM2.5 exposure did not affect exosomal morphology.
Nanoparticle tracking analysis revealed that the size distribution
of EXOCtrl and EXOPM2.5 was 119±41.7 and
119±41.3 nm (mean ± SD), respectively (Fig. 1C). The protein components of
EXOCtrl and EXOPM2.5 were examined. Both
exosomes were negative for the endoplasmic reticulum-residing
protein, GRP94, and positive for HSP70 as well as the
multivesicular body-related proteins, CD63, Tsg101 and Alix
(Fig. 1D). According to a previous
publication, CD63 was used as a loading control (25). Notably, Wnt3a was only detected in
EXOPM2.5 (Fig. 1D).
These results indicated that EXOPM2.5 has higher Wnt3a
protein levels than EXOCtrl.
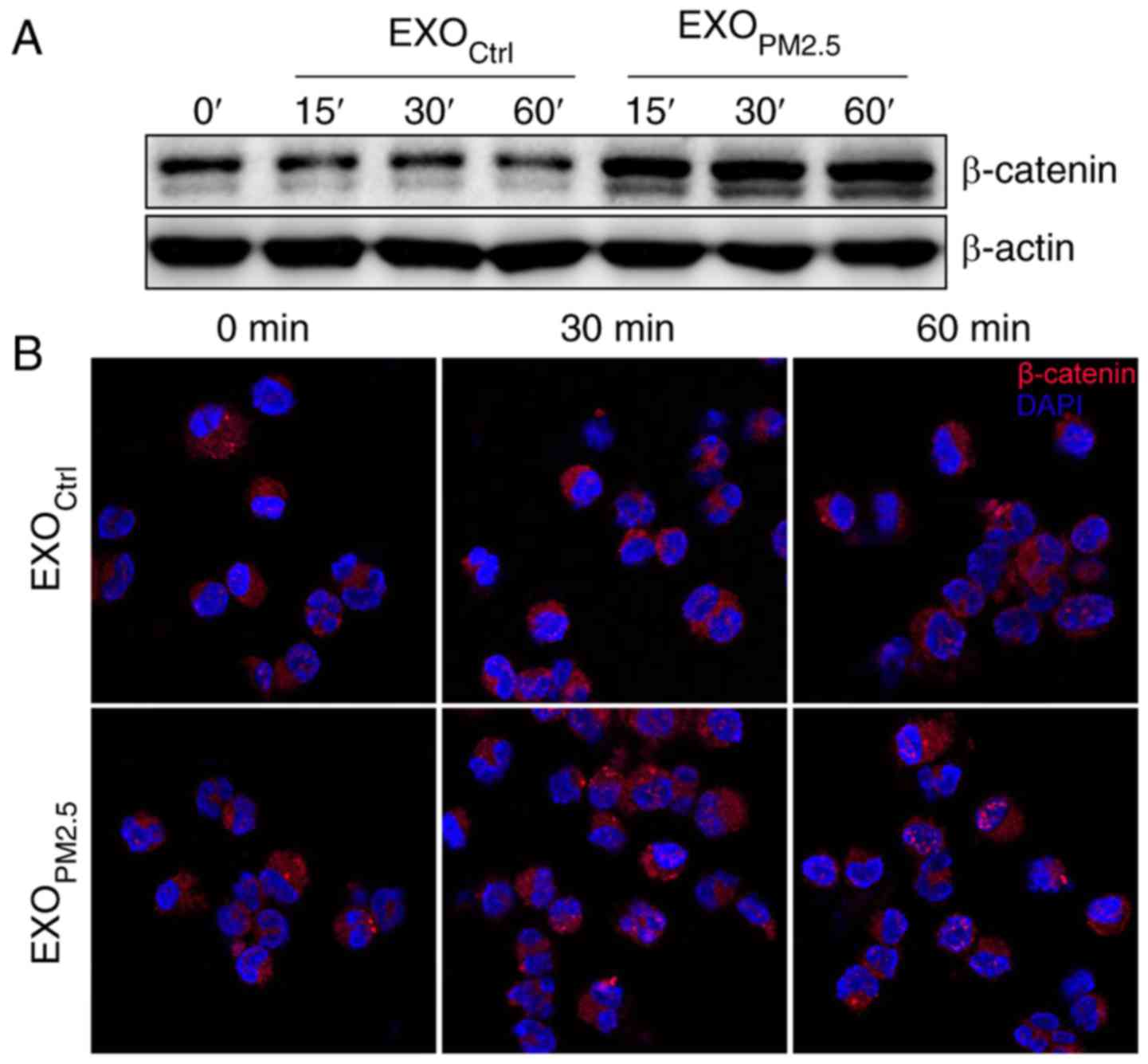
EXOPM2.5 induces activation
of the Wnt/β-catenin pathway
β-catenin is a key downstream effector in the Wnt
signalling pathway (26). In the
off-state, β-catenin is phosphorylated by CK1 and subsequently
phosphorylated by GSK-3β (27,28),
resulting in destabilization of β-catenin (29). In the on-state, a Wnt ligand binds
to a Frizzled receptor and then prevents β-catenin phosphorylation
by GSK-3β, leading to accumulation and increased nuclear import of
β-catenin (30). Since Wnt3a was
enriched in EXOPM2.5, we investigated whether
EXOPM2.5 activated Wnt/β-catenin signalling. Treatment
with EXOPM2.5 markedly increased total β-catenin protein
in A549 cells (Fig. 2A). In
addition, enhanced nuclear translocation of β-catenin was observed
in EXOPM2.5-treated A549 cells (Fig. 2B). These results indicated that
EXOPM2.5 activated the Wnt/β-catenin pathway.
EXOPM2.5 does not affect
A549 cell migration and invasion
Since the Wnt/β-catenin pathway has been implicated
in tumour migration and invasion (31,32),
we investigated whether EXOPM2.5 promoted A549 cell
migration. As revealed in Fig. 3A,
EXOPM2.5 treatment had no effect on A549 cell migration
(Fig. 3A and B). The role of
EXOPM2.5 on invasion of A549 cells was next examined. No
difference of invasive ability was observed in A549 cells with or
without EXOPM2.5 treatment (Fig. 3C and D). These results indicated
that EXOPM2.5 does not alter the migration and invasion
abilities of A549 cells.
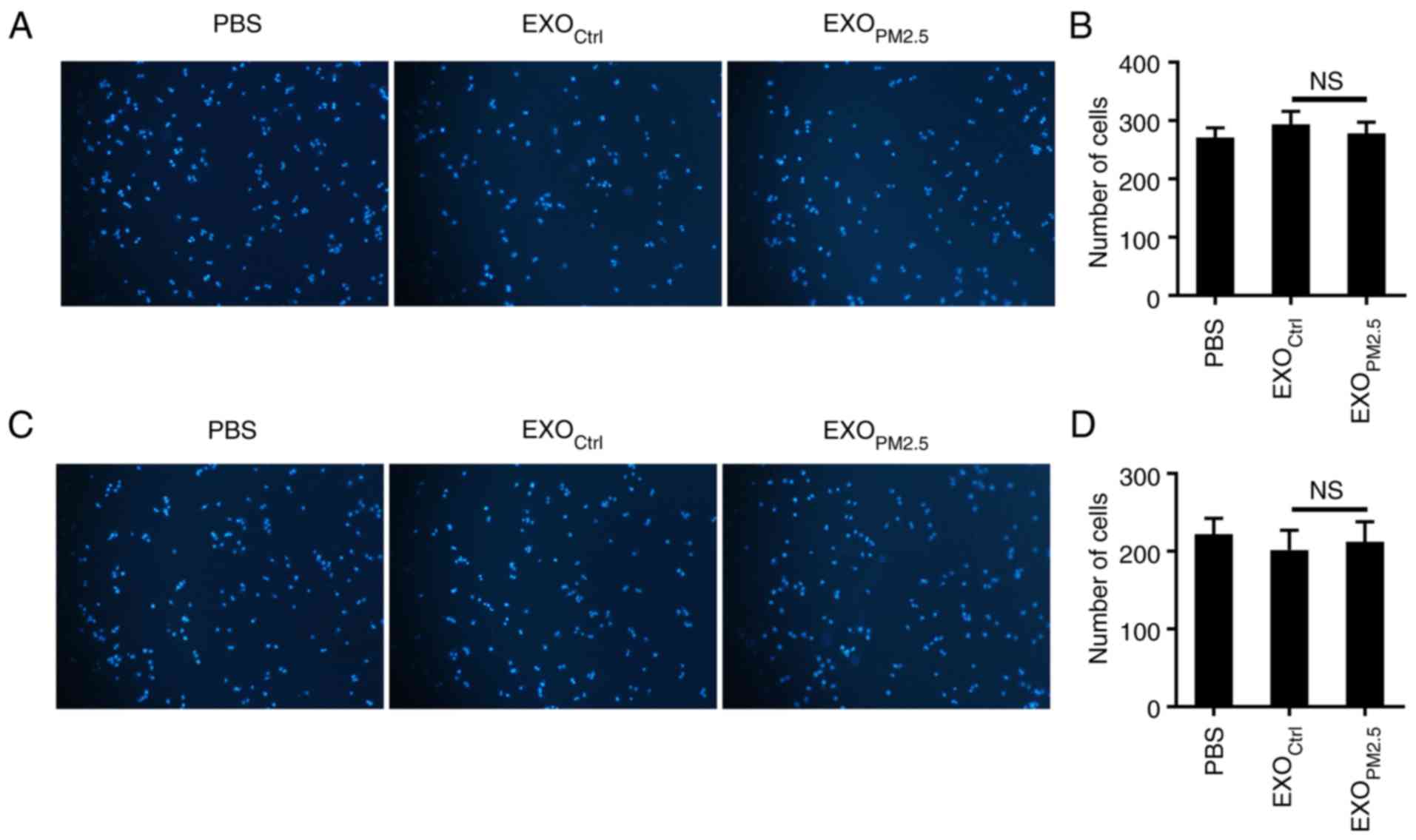 | Figure 3.EXOPM2.5 does not affect
A549 cell migration and invasion. (A) A549 tumour cells were
incubated with 10 µg/ml EXOCtrl or EXOPM2.5
for 24 h, and the cells were then plated in the top chamber of a
Transwell plate. After 18 h, the cells on the bottom of the
Transwell filter were imaged and quantified. Magnification, ×100.
(B) The results of A were statistically analysed (n=5). (C) A549
tumour cells were incubated with 10 µg/ml EXOCtrl or
EXOPM2.5 for 24 h, and cells were then plated in the top
chamber, which was precoated with 50 µl of Matrigel. After 48 h,
the cells on the bottom of the Transwell filter were imaged and
quantified. Magnification, ×100. (D) The results of C were
statistically analysed (n=5). Data are representative of three
independent experiments. NS, not significant; EXOPM2.5,
exosomes from PM2.5-treated A549 cells;
EXOCtrl, exosomes from A549 cells with mock
treatment. |
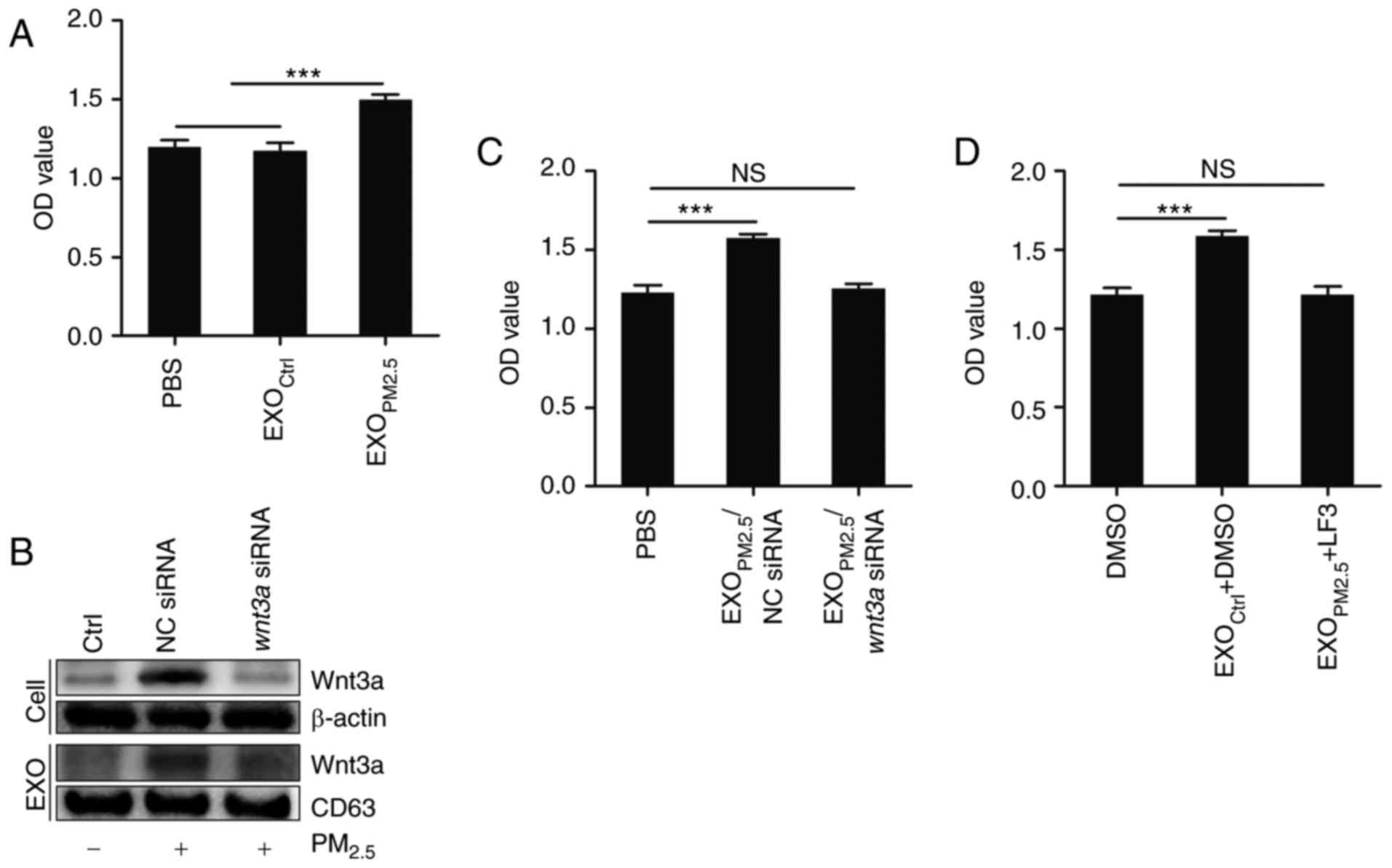
EXOPM2.5 promotes A549 cell
proliferation in a Wnt3a/β-catenin-dependent manner
β-catenin is implicated in tumourigenesis (33), and Wnt3a/β-catenin signalling has
also been demonstrated to induce tumour cell proliferation
(34). Thus, the effect of
EXOPM2.5 on A549 cell proliferation was investigated.
EXOPM2.5, but not EXOCtrl, significantly
promoted A549 cell proliferation (Fig.
4A). To elucidate the role of Wnt3a in this effect, Wnt3a was
knocked down in PM2.5-treated A549 cells by Wnt3a
siRNA. Exosomes with low amounts of Wnt3a protein were obtained
from PM2.5-treated A549 cells (Fig. 4B). Exosomes from
PM2.5-treated A549 cells transfected with NC siRNA
(EXOPM2.5/NC siRNA) promoted A549 cell proliferation
(Fig. 4C). However, exosomes from
PM2.5-treated A549 cells transfected with Wnt3a
siRNA (EXOPM2.5/Wnt3a siRNA) had no effect on
A549 cell proliferation (Fig. 4C).
In the presence of LF3, a specific inhibitor of Wnt/β-catenin
signalling, EXOPM2.5 did not promote A549 cell
proliferation (Fig. 4D). These
results demonstrated that the effect of EXOPM2.5 on the
enhanced A549 cell proliferation was dependent on Wnt3a/β-catenin
signalling.
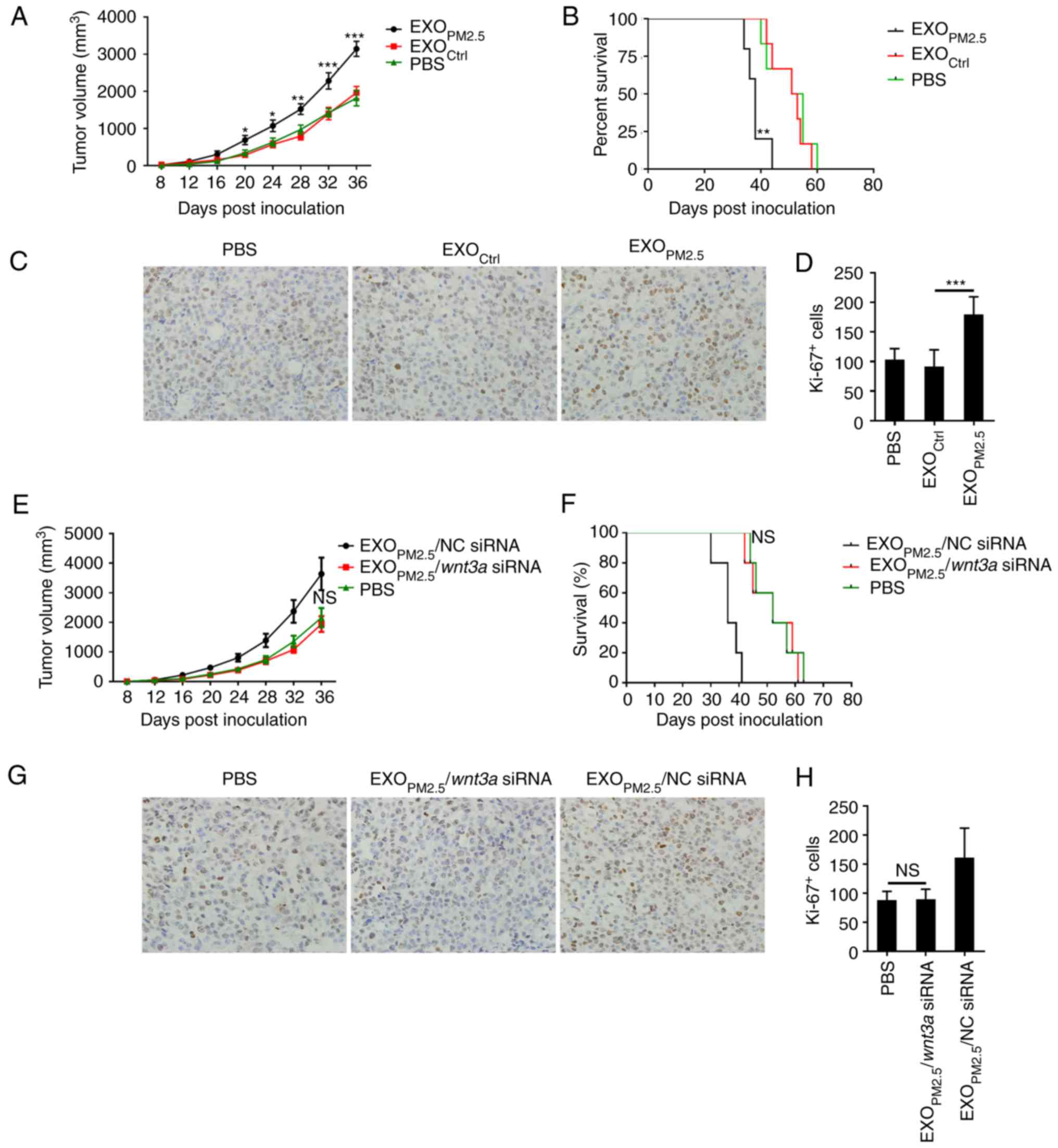
EXOPM2.5 promotes A549 cell
growth in vivo via Wnt3a
Finally, the effect of EXOPM2.5 on A549
cell progression in vivo was assessed. An A549 tumour model
in nude mice was established, and intratumoural injection of
EXOPM2.5 was performed every other day.
EXOPM2.5 increased A549 cell growth (Fig. 5A) and reduced the survival rate of
tumour-bearing mice (Fig. 5B).
Immunohistochemical staining of Ki-67 revealed that
EXOPM2.5 significantly promoted tumour cell
proliferation (Fig. 5C and D). To
dissect the role of Wnt3a in EXOPM2.5 in this process,
intratumoural injection of EXOPM2.5/NC siRNA or
EXOPM2.5/Wnt3a siRNA was performed every other
day. EXOPM2.5/NC siRNA, but not
EXOPM2.5/Wnt3a siRNA, increased tumour growth and
reduced the survival rate of tumour-bearing mice (Fig. 5E and F). Immunohistochemical results
also revealed that EXOPM2.5/Wnt3a siRNA did not
promote tumour cell proliferation (Fig.
5G and H). In the survival study, when the largest tumour
volume reached 4,000 mm3, for humanitarian reasons, the
mice were sacrificed. However, in Fig.
5B, compared with EXOCtrl or PBS-treated mice, the
tumour volume of EXOPM2.5-treated mice reached 4,000
mm3 much earlier. Similarly, in Fig. 5F, compared with
EXOPM2.5/Wnt3a siRNA or PBS-treated mice, the
tumour volume of EXOPM2.5/NC siRNA-treated mice reached
4,000 mm3 much earlier. Therefore, these results
reflected the real tendency of survival time of tumour mice with
different treatments. Altogether, these results indicated that
EXOPM2.5-induced tumour growth in vivo was
dependent on Wnt3a.
Discussion
Since humans are required to breathe in air, the
PM2.5 pollution in atmosphere directly affects the
physiological environment of the respiratory tract, especially the
lungs. PM2.5 is implicated in increased risk of lung
cancer (35). However, there is no
direct evidence of the effect of PM2.5 exposure on lung
cancer cells. The present study revealed that
PM2.5-treated A549 lung cancer cells produced exosomes
containing high levels of Wnt3a, which promoted A549 cell
proliferation by activating Wnt/β-catenin signalling. We also
detected Wnt3a by flow cytometry after adsorbing exosomes onto
latex, but we did not detect Wnt3a this way (data not shown) which
suggests that Wnt3a does not exist in exosomes. IL-10 and TGF-β1
are in exosomes, where they can exert immunosuppressive functions
via the expression of their receptors on the cell membrane
(36,37). Therefore, even if Wnt3a is in
exosomes, it still can activate β-catenin signalling. In tumour
patients who inhale PM2.5, PM2.5 stimulates
lung epithelial cells. PM2.5 may also induce lung
epithelial cells to secrete exosomes containing Wnt3a, which
activates Wnt/β-catenin signalling in tumour cells, leading to
tumour progression.
In developed countries, the concentration of
PM2.5 in the atmosphere is generally less than 10
µg/m3, and in developing countries it is generally above
35 µg/m3, and the highest is likely to reach 200–300
µg/m3. An adult breathes more than 20,000 times a day,
inhaling ~20 m3 of air. Therefore, an adult inhales at
least 200 µg/day of PM2.5. The concentration we used was
100 µg/ml in 2 ml and the quality of PM2.5 was 200 µg.
Thus, in humans, it is likely to be exposed to such a
concentration. Notably, at a concentration of 100 µg/ml, we did not
observe increased apoptosis of A549 cells (data not shown). On the
contrary, PM2.5 promoted lung tumor cell proliferation
by inducing the cells to secrete exosomes with high levels of
Wnt3a. With PM2.5 treatment, A549 cells notably
upregulated Wnt3a expression. The mechanism of the effect of
PM2.5 was not investigated in this study. Environmental
ultrafine particulate matter has been reported to activate NF-κB
and AP-1 (7). Wnt10a and Wnt10b are
the target genes of NF-κB (38).
Bioinformatics analysis predicted binding sites of NF-κB and AP-1
in the Wnt3a promoter, indicating that PM2.5 may
promote Wnt3a transcription by activating NF-κB and AP-1. If
the mechanism is unveiled in the future, it will be beneficial to
identify the specific target to prevent lung cancer progression
caused by PM2.5 exposure. Wnt signalling through its
receptors (Frizzled) activate β-catenin signalling, which is often
called the canonical pathway (39).
As a ligand of the canonical pathway, the downstream effector of
Wnt3a in EXOPM2.5 is β-catenin, which was supported by
the increased protein level and nuclear translocation of β-catenin
in EXOPM2.5-treated A549 cells. Use of the LF3 inhibitor
confirmed that Wnt3a contained in EXOPM2.5 promoted A549
cell proliferation through activation of β-catenin signalling in
vitro. However, the role of β-catenin in
EXOPM2.5-mediated tumour inhibition was not ascertained
in vivo, but the findings did indicate that all of the
effects were Wnt3a-dependent.
The present study demonstrated that
EXOPM2.5 significantly promoted A549 cell proliferation
in vitro. Exosomes isolated from Wnt3a knockdown
EXOPM2.5-treated A549 cells had extremely low levels of
Wnt3a and did not induce A549 cell proliferation in vitro.
These results indicated that Wnt3a was responsible for
EXOPM2.5-mediated A549 cell proliferation in
vitro. Inhibition of β-catenin signalling in A549 cells
prevented EXOPM2.5-induced A549 cell proliferation in
vitro, indicating that Wnt3a contained in EXOPM2.5
activated β-catenin signalling in A549 cells. The mouse tumour
model revealed that EXOPM2.5 promoted A549 cell growth
and decreased the survival rate of tumour-bearing mice. In the
mouse tumour model, EXOPM2.5 did promote A549 cell
proliferation. The Wnt/β-catenin pathway was also involved in
tumour cell migration and invasion by mediating
epithelial-mesenchymal transition of tumour cells (40). However, the migration and invasion
promoting effect of EXOPM2.5 on A459 cells in
vitro could not be observed.
In summary, PM2.5 exposure induced high
expression of Wnt3a in A549 lung cancer cells. Isolated exosomes
with a high level of Wnt3a activated β-catenin signalling in A549
cells and promoted their proliferation in vitro.
Furthermore, these exosomes also promoted tumour progression in
vivo. Therefore, these results indicated that inhibition of the
Wnt/β-catenin pathway or exosome secretion may prevent
PM2.5-mediated lung cancer progression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Medicines Health Science and Technology Program (no. 2016KYB193),
the Key Research Project of Shandong Province (nos. 2016GSF201028
and 2017GSF218056) and the National Natural Science Foundation of
China (no. 81770029).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HX, XJ and YW performed the real-time PCR, the
immunofluorescent staining, migration, invasion, cell proliferation
and the animal experiments. SL and LC performed the cell culture,
the exosome isolation and the western blotting experiments. LD
conceived and designed the study. HX wrote the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experiments using mice were approved by and
performed according to the guidelines of the Animal Ethics
Committee of Wenzhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PM2.5
|
fine particulate matter
|
|
TEXs
|
tumour-derived exosomes
|
|
EXOPM2.5
|
exosomes from
PM2.5-treated A549
|
|
EXOCtrl
|
exosomes from A549 with mock
treatment
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ceRNA
|
competing endogenous RNAs
|
|
OD
|
optical density
|
References
|
1
|
Huang L, Pu Z, Li M and Sundell J:
Characterizing the indoor-outdoor relationship of fine particulate
matter in non-heating season for urban residences in Beijing. PLoS
One. 10:e01385592015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho SM: Environmental epigenetics of
asthma: An update. J Allergy Clin Immunol. 126:453–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Honda T, Pun VC, Manjourides J and Suh H:
Anemia prevalence and hemoglobin levels are associated with
long-term exposure to air pollution in an older population. Environ
Int. 101:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zanobetti A and Schwartz J: The effect of
fine and coarse particulate air pollution on mortality: A national
analysis. Environ Health Perspect. 117:898–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirabelli MC, Vaidyanathan A, Flanders WD,
Qin X and Garbe P: Outdoor PM2.5, ambient air temperature, and
asthma symptoms in the past 14 days among adults with active
asthma. Environ Health Perspect. 124:1882–1890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song J, Kang J, Lin B, Li J, Zhu Y, Du J,
Yang X, Xi Z and Li R: Mediating role of TRPV1 Ion channels in the
co-exposure to PM2.5 and formaldehyde of Balb/c mice asthma model.
Sci Rep. 7:119262017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZH, Wu YF, Wang PL, Wu YP, Li ZY,
Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, et al: Autophagy is
essential for ultrafine particle-induced inflammation and mucus
hyperproduction in airway epithelium. Autophagy. 12:297–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye X, Hong W, Hao B, Peng G, Huang L, Zhao
Z, Zhou Y, Zheng M, Li C, Liang C, et al: PM2.5 promotes human
bronchial smooth muscle cell migration via the sonic hedgehog
signaling pathway. Resp Res. 19:372018. View Article : Google Scholar
|
|
9
|
Du X, Jiang S, Zeng X, Zhang J, Pan K,
Zhou J, Xie Y, Kan H, Song W, Sun Q and Zhao J: Air pollution is
associated with the development of atherosclerosis via the
cooperation of CD36 and NLRP3 inflammasome in ApoE−/−
mice. Toxicol Lett. 290:123–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen ZJ, Stafoggia M, Weinmayr G,
Pedersen M, Galassi C, Jørgensen JT, Oudin A, Forsberg B, Olsson D,
Oftedal B, et al: Long-term exposure to ambient air pollution and
incidence of postmenopausal breast cancer in 15 European cohorts
within the ESCAPE project. Environ Health Perspect. 125:1070052017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
VoPham T, Bertrand KA, Tamimi RM, Laden F
and Hart JE: Ambient PM2.5 air pollution exposure and
hepatocellular carcinoma incidence in the United States. Cancer
Causes Control. 29:563–572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao Y, Xu L, Lin X and Hao YT: Temporal
trend in lung cancer burden attributed to qmbient fine particulate
matter in Guangzhou, China. Biomed Environ Sci. 30:708–717.
2017.PubMed/NCBI
|
|
13
|
Yang B, Chen DM, Zhao H and Xiao CL: The
effects for PM2.5 exposure on non-small-cell lung cancer induced
motility and proliferation. Springerplus. 5:20592016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang D, Ma MY, Zhou WC, Yang BA and Xiao
CL: Inhibition of miR-32 activity promoted EMT induced by PM2.5
exposure through the modulation of the Smad1-mediated signaling
pathways in lung cancer cells. Chemosphere. 184:289–298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei H, Liang F, Cheng W, Zhou R, Wu X,
Feng Y and Wang Y: The mechanisms for lung cancer risk of PM2.5:
Induction of epithelial-mesenchymal transition and cancer stem cell
properties in human non-small cell lung cancer cells. Environ
Toxicol. 32:2341–2351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kowal J, Arras G, Colombo M, Jouve M,
Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M and Théry
C: Proteomic comparison defines novel markers to characterize
heterogeneous populations of extracellular vesicle subtypes. Proc
Natl Acad Sci USA. 113:E968–E977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sansone P, Savini C, Kurelac I, Chang Q,
Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly
L, et al: Packaging and transfer of mitochondrial DNA via exosomes
regulate escape from dormancy in hormonal therapy-resistant breast
cancer. Proc Natl Acad Sci USA. 114:E9066–E9075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang
Y, Wang P, Wang J and Cai Z: Tumor-derived exosomes educate
dendritic cells to promote tumor metastasis via
HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology. 6:e13625272017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Jiang P, Li J, Peng M, Zhao X, Zhang
X, Chen K, Zhang Y, Liu H, Gan L, et al: Tumor-derived exosomal
lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in
pancreatic ductal adenocarcinoma. Oncogene. 37:3822–3838. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z,
Zhang X, Huang B, Xu X, Zheng J and Cao X: Tumor exosomal RNAs
promote lung pre-metastatic niche formation by activating alveolar
epithelial TLR3 to recruit neutrophils. Cancer Cell. 30:243–256.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Zeng C, Zhan Y, Wang H, Jiang X
and Li W: Aberrant low expression of p85α in stromal fibroblasts
promotes breast cancer cell metastasis through exosome-mediated
paracrine Wnt10b. Oncogene. 36:4692–4705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cong LH, Du SY, Wu YN, Liu Y, Li T, Wang
H, Li G and Duan J: Upregulation of Klotho potentially inhibits
pulmonary vascular remodeling by blocking the activation of the Wnt
signaling pathway in rats with PM2.5-induced pulmonary arterial
hypertension. J Cell Biochem. 119:5581–5597. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang L, Zhu Q, Neuenschwander M, Specker
E, Wulf-Goldenberg A, Weis WI, von Kries JP and Birchmeier W: A
small-molecule antagonist of the β-catenin/TCF4 interaction blocks
the self-renewal of cancer stem cells and suppresses tumorigenesis.
Cancer Res. 76:891–901. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB,
Xiang X, Cheng Z, Liu C, Wang J, Zhang L, et al: Thymus
exosomes-like particles induce regulatory T cells. J Immunol.
181:5242–5248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cadigan KM and Nusse R: Wnt signaling: A
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amit S, Hatzubai A, Birman Y, Andersen JS,
Ben-Shushan E, Mann M, Ben-Neriah Y and Alkalay I: Axin-mediated
CKI phosphorylation of beta-catenin at Ser 45: A molecular switch
for the Wnt pathway. Genes Dev. 16:1066–1076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yost C, Torres M, Miller JR, Huang E,
Kimelman D and Moon RT: The axis-inducing activity, stability, and
subcellular distribution of beta-catenin is regulated in
Xenopus embryos by glycogen synthase kinase 3. Genes Dev.
10:1443–1454. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sha J, Han Q, Chi C, Zhu Y, Pan J, Dong B,
Huang Y, Xia W and Xue W: PRKAR2B promotes prostate cancer
metastasis by activating Wnt/β-catenin and inducing
epithelial-mesenchymal transition. J Cell Biochem. 119:7319–7327.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang T, Ma Z, Liu L, Sun J, Tang H, Zhang
B, Zou Y and Li H: DDX39 promotes hepatocellular carcinoma growth
and metastasis through activating Wnt/beta-catenin pathway. Cell
Death Dis. 9:6752018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polakis P: The oncogenic activation of
beta-catenin. Curr Opin Genet Dev. 9:15–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li
Q, Wei S, Li BW, Xu JH, Chen L, et al: miR-324-3p promotes gastric
cancer development by activating Smad4-mediated Wnt/beta-catenin
signaling pathway. J Gastroenterol. 53:725–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang H, Li S, Sun L, Zhang X, Hou J and
Wang Y: Effects of the ambient fine particulate matter on public
awareness of lung cancer risk in China: Evidence from the
internet-based big data platform. JMIR Public Health Surveill.
3:e642017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SH, Lechman ER, Bianco N, Menon R,
Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A and Robbins PD:
Exosomes derived from IL-10-treated dendritic cells can suppress
inflammation and collagen-induced arthritis. J Immunol.
174:6440–6448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu L, Yang F, Jiang L, Chen Y, Wang K, Xu
F, Wei Y, Cao X, Wang J and Cai Z: Exosomes with
membrane-associated TGF-β1 from gene-modified dendritic cells
inhibit murine EAE independently of MHC restriction. Eur J Immunol.
43:2461–2472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Voutilainen M, Lindfors PH, Lefebvre S,
Ahtiainen L, Fliniaux I, Rysti E, Murtoniemi M, Schneider P,
Schmidt-Ullrich R and Mikkola ML: Ectodysplasin regulates
hormone-independent mammary ductal morphogenesis via NF-κB. Proc
Natl Acad Sci USA. 109:5744–5749. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu J, Cui CF, Yang L, Wang L and Jiang XH:
Emodin inhibits colon cancer cell invasion and migration by
suppressing epithelialmesenchymal transition via the Wnt/β-catenin
pathway. Oncol Res. Jan 4–2018.(Epub ahead of print). doi:
10.3727/096504018X15150662230295. View Article : Google Scholar
|