Introduction
B-cell lymphoma 2 (Bcl2)-antagonist/killer protein
(Bak) is a member of the BH3-only Bcl-2 protein family. Bak is a
proapoptotic effector of canonical mitochondrial apoptosis
(1). BH3-only Bcl-2 proteins
promote apoptosis by directly activating Bak, and by suppressing
antiapoptotic proteins in the mitochondria and endoplasmic
reticulum (ER). These proteins are regulated by several mechanisms,
including transcription and post-translational modifications, in
order to prevent constitutive cell death (2). Bak exerts its proapoptotic activity
via hierarchical and highly regulated interactions with other
BH3-only Bcl-2 family members; in addition, it also operates as a
sensor of extrinsic and intrinsic cell death signals (3). Our previous study demonstrated that
high Bak expression was associated with favorable prognosis in
breast cancer; furthermore, the restoration of Bak sensitized
breast cancer cells to paclitaxel (4). Therefore, molecules that mimic the BH3
domain are proposed to be molecular targets for cancer therapy.
Therefore, investigations to obtain further knowledge regarding the
molecular mechanisms that regulate responsiveness to cancer therapy
using these molecules are warranted (5).
Bromodomain-containing protein 7 (BRD7), a member of
the bromodomain-containing protein family, has been identified as a
tumor suppressor in several types of cancer, including
nasopharyngeal carcinoma, breast and prostate cancers (6). BRD7 inhibits cancer cell growth and
metastasis, and promotes apoptosis by downregulating the
phosphatase and tensin homolog/protein kinase B (AKT),
mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase (ERK) and retinoblastoma/E2F transcription
factor signaling pathways (7–11).
Furthermore, it is involved in multiple physiological processes,
including the regulation of spermatogenesis and inflammation
(12,13). BRD7 acts as a co-activator for p53
and is involved in the acetylation of p53 target genes and in the
subsequent activation of functional p53 pathways (14,15).
The depletion of BRD7 in breast cells results in the loss of
estrogen receptor α (ERα) expression, and this loss of ERα is
reflected in resistance to the antiestrogen drug Fulvestrant
(16). Bak functions as a
downstream effector of p53 in the mitochondria (17). The accumulation of phospho-p53 in
the mitochondria activates Bak, which subsequently induces cell
apoptosis and death (18,19). However, the association between BRD7
and Bak remains unknown.
In the present study, the association and functions
of BRD7 and Bak in breast cancer tissues were investigated, and the
regulatory effects of BRD7 on Bak were also determined. BRD7 and
Bak were downregulated in breast cancer tissues, and there was a
positive correlation between their expression. BRD7 activated Bak
promoter activity and induced Bak expression in an indirect manner.
In addition, ectopic expression of BRD7 in breast cancer cells
inhibited cell proliferation, promoted apoptosis and sensitized
cancer cells to paclitaxel, while restoring the expression of Bak
abolished BRD7-mediated inhibitory effects on cell proliferation
and paclitaxel sensitization in breast cancer cells, whether in
vitro and in vivo. These results demonstrated that BRD7
inhibits cell proliferation and sensitizes breast cancer cells to
paclitaxel by activating Bak; thus, these results may provide a
target for the diagnosis and treatment of breast cancer.
Materials and methods
Tissue samples and clinical data
A total of 225 breast cancer tissues (age, 46±0.66
years; male/female patient ratio, 1/224) and 62 non-tumor breast
tissues (age, 48±0.84 years; n=62 female patients) were collected
from the Second Xiangya Hospital of Central South University
(Hunan, China) between November 2001 and September 2012. Patients
with diabetes, nephritis, hepatitis or cardiovascular disease were
excluded. Patient information was obtained from medical records.
The present study was approved by the Ethics Committee of Central
South University. Written informed consent was obtained from all of
the participants.
Cell culture
The human breast cancer cell lines MCF-7 and
MDA-MB-231 were obtained from American Type Culture Collection
(Manassas, VA, USA). All cells were cultured in high glucose
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained
in a humidified atmosphere of 5% CO2 in air at 37°C.
Cell treatment
To achieve BRD7 overexpression in MCF-7 and
MDA-MB-231 cells, cells at 80% confluence were transfected in
6-well plates with pIRES2-EGFP-3Flag/BRD7 or pIRES2-EGFP (Addgene,
Inc., Cambridge, MA, USA) using Lipofectamine 3000™ transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The cells transfected with
pIRES2-EGFP were used as a negative control. Then, to knockdown Bak
expression in MCF-7 and MDA-MB-231 cells, the cells were
transfected with Bak small interfering RNA (siRNA) oligonucleotides
or control siRNA oligonucleotides (siRNA sequence,
5′-CCGACGCUAUGACUCAGAGdTdT-3′; Guangzhou RiboBio Co., Ltd.,
Guangzhou, China; 50 nmol/l) using Lipofectamine 3000™ transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The cells transfected with control
siRNA oligonucleotides were used as a negative control. To
determine the chemosensitivity in MCF-7 and MDA-MB-231 cells 48 h
following BRD7 overexpression or Bak knockdown, the cells were
treated with paclitaxel (Corden Pharma Latina S.P.A, Sermoneta,
Latina, Italy; 400 nM for MCF-7 and 80 nM for MDA-MB-231) for 48 h
at 37°C.
Immunohistochemistry and evaluation of
staining
The tumor sections were fixed with 4%
paraformaldehyde at room temperature for 48 h, paraffin-embedded
and then serially cut at 4 µm. Slides were regularly deparaffinized
with xylene twice (each for 10 min), rehydrated through an ethanol
gradient (100, 95, 90, 80 and 70%, each for 5 min), and washed with
phosphate-buffered saline (PBS) twice. Slides were retrieved in
citric acid buffer (pH 6.0) by boiling for 20 min using a microwave
oven. Following cooling, the slices were washed with PBS and then
blocked with 100% normal goat serum (Boster Biological Technology,
Pleasanton, CA, USA), and incubated with the following primary
antibodies overnight at 4°C: Rabbit polyclonal anti-BRD7 (1:100
dilution; cat. no. 51009-2-AP; ProteinTech Group, Inc., Chicago,
IL, USA) and rabbit polyclonal anti-Bak (1:200 dilution; cat. no.
3814; Cell Signaling Technology, Inc., Danvers, MA, USA). The
sections were then washed with PBS and incubated with an
horseradish peroxidase (HRP)-conjugated anti-rabbit secondary
antibody (1:1 dilution; cat. no. KIT-5905; Fuzhou Maixin Biotech
Co., Ltd., Fujian, China) for 2 h at 37°C. Then the sections were
washed with PBS and stained using a 3,3′-diaminobenziden detection
kit (Maxim Biomedical, Inc., Rockville, MD, USA). Finally, the
sections were counterstained with hematoxylin for 30 sec at room
temperature. The evaluation of staining was performed as previously
described (4). Briefly, the slides
were evaluated by two independent pathologists blinded to
clinicopathological features and the clinical course, under a light
microscope (BX51; Olympus Corporation, Tokyo, Japan). The staining
intensity of BRD7 or Bak were scored as 0 (negative, -), 1 (weak,
+), 2 (moderate, ++) and 3 (strong, +++). The extent of staining
was scored as 0–1.0 (0–100%). The final staining score (0–3) was
calculated as the multiplication of the intensity score and extent
score. A final score of ≥1 was defined as high expression,
otherwise scores were defined as low expression.
Cell viability assay
MCF-7 and MDA-MB-231 cells transfected with BRD7 or
Bak were plated in 96-well plates at a density of 1,000 cells in
100 µl of the aforementioned DMEM+FBS media/well. The cells were
treated with paclitaxel (Corden Pharma Latina S.P.A; 400 nM for
MCF-7 and 80 nM for MDA-MB-231) for 48 h at 37°C with 5%
CO2, and the cell viability was assessed by Cell
Counting Kit-8 assay (cat. no. B34304; Bimake, Shanghai, China)
according to the manufacturers' instructions.
Flow cytometry for apoptosis
analysis
An Annexin V-phycoerythrin (PE)/7-aminoactinmycin D
(AAD) Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ,
USA) was used to detect cell apoptosis. Following BRD7
overexpression and Bak siRNA treatment, 2×105 cells were
collected and washed twice with cold PBS by centrifugation at 1,000
× g for 5 min at room temperature, and then resuspended in 100 µl
of 1X binding buffer. A volume of 5 µl Annexin V-PE and 5 µl 7-AAD
were added to the cell suspension, mixed well and incubated for 10
min at room temperature, protected from light. Cells were analyzed
using the BD LSRFortessa flow cytometer (BD Biosciences) with
FlowJo software (version 10.5; FlowJo LLC, Ashland, OR, USA). Three
independent experiments were performed for this assay.
Western blotting
Cells were lysed in cold Radioimmunoprecipitation
Assay buffer and protein concentrations were determined using a BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 60
µg protein was separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), which were blocked by soaking in 5% non-fat milk for 1 h at
37°C. Then the membranes were incubated with the corresponding
primary antibodies overnight at 4°C. The following antibodies were
used: anti-Flag (1:2,000 dilution; cat. no. F7425; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), anti-GAPDH (1:2,000 dilution; cat.
no. 60004-1-Ig; ProteinTech Group, Inc.), anti-BRD7 (1:1,000
dilution; cat. no. 51009-2-AP; ProteinTech Group, Inc.), anti-Bak
(1:1,000 dilution; cat. no. 3814; Cell Signaling Technology, Inc.),
anti-total poly (adenosine diphosphate-ribose) polymerase (PARP;
1:1,000 dilution; cat. no. 9532; Cell Signaling Technology, Inc.)
and anti-cleaved PARP (1:1,000 dilution; cat. no. 9548; Cell
Signaling Technology, Inc.). Following washing with 1X TBST
(containing 0.05% Tween-20) 3 times for 8 min each, the membranes
were incubated with the appropriate secondary antibody [1:3,000
dilution; cat. no. SA00001-1 for HRP-conjugated AffiniPure Goat
Anti-Mouse IgG (H+L); 1:3,000 dilution; cat. no. SA00001-2 for
HRP-conjugated AffiniPure Goat Anti-rabbit IgG (H+L); ProteinTech
Group, Inc.] for 1 h at 37°C. Finally, the bands were visualized
using an Enhanced Chemiluminescence kit (EMD Millipore). Signals
were quantified by ImageJ software (version 1.8.0; National
Institutes of Health, Bethesda, MD, USA) and normalized to
GAPDH.
Luciferase reporter assay
To detect the promoter activity of Bak, the putative
promoter region (2,000 bp upstream of the Bak transcription
initiation site) was synthesized and inserted into a pGL3-enhancer
vector (Promega Corporation, Madison, WI, USA) to generate a
recombinant luciferase reporter gene (pGL3-Bak). MCF-7 and
MDA-MB-231 cells were transfected with pGL3-Bak and co-transfected
with a BRD7 expressing plasmid or vector control (Addgene, Inc.)
using Lipofectamine 3000™ transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. At 48 h post-transfection, cells were collected and
lysed with radioimmunoprecipitation (RIPA; cat. no. B100020,
ProteinTech Group, Inc.). A volume of 100 µl of the supernatants
were used to detect luciferase activities (normalized to
Renilla luciferase activity) using a luciferase reporter
gene assay kit (Promega Corporation) and the PARADIGM Detection
Platform (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reverse transcribed using a SuperScript™ IV First-Strand Synthesis
System (cat. no. 18091050; Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols to generate total
cDNA to serve as templates for Bak mRNA detection. To obtain cDNA,
10 µl 2X RT Reaction Mix, 2 µl RT Enzyme Mix, 100 ng RNA and
DEPC-treated water up to 20 µl were added into a tube, gently mixed
and then RT-PCR was conducted with the following temperature
protocol: 25°C for 10 min, 50°C for 30 min, 85°C for 5 min and then
chilled on ice. The mRNA expression of Bak was measured using
Bright Green 2X qPCR Master Mix (Applied Biological Materials,
Inc., Richmond, Canada) according to manufacturer's instructions.
The expression of β-actin was used as an endogenous control.
RT-qPCR was performed with the following thermocycling conditions:
95°C for 3 min, and then 39 cycles of 95°C for 10 sec and 60°C for
30 sec. Data were quantified using the 2−ΔΔCq method
(20). The primers used were as
follows: Bak forward, 5′-GCAGGCTGATCCCGTCC-3′ and reverse,
5′-CAAACAGGCTGGTGGCAATC-3′; β-actin forward,
5′-TTGTTACAGGAAGTCCCTTGCC-3′ and reverse,
5′-ATGCTATCACCTCCCCTGTGTG-3′.
Chromatin immunoprecipitation (ChIP)
assay
The EZ-Magna ChIP kit (EMD Millipore) was used for
the ChIP assays according to manufacturer's protocol. MCF-7 cells
at 100% confluency in 6-well plates were fixed with 4%
paraformaldehyde for 24 h at room temperature and incubated with
glycine for 10 min at room temperature to generate DNA-protein
crosslinks. Then, the cells were lysed with the cell lysis buffer
RIPA (cat. no. B100020; ProteinTech Group, Inc.) and sonicated to
generate chromatin fragments. Next, the lysates were
immunoprecipitated with Magnetic Protein A Beads conjugated with
Flag-specific antibodies (1:100 dilution; cat. no. F7425;
Sigma-Aldrich; Merck KGaA). The samples immunoprecipitated with
immunoglobulin G were used as a negative control, and baculoviral
IAP repeat containing 2 was used as a positive control, based our
previous results (21). Finally,
the precipitated DNA was analyzed by PCR under the following
thermocycling conditions: 95°C for 3 min, followed by 29 cycles of
95°C for 10 sec and 60°C for 30 sec to amplify different regions of
the Bak promoter segments. The promoter sequence was divided into
10 fragments based on the DNA walking method (22). All of these PCR products were
~200–240 bp in length. The primers of each of the promoter segment
pairs were as follows: F1 forward, 5′-CCCAGCAGGGTGAGCGCC-3′ and
reverse, 5′-CAGCAGTGGGGAAGGCACA-3′ (239 bp); F2 forward,
5′-TCTGTGCCTTCCCCACTGCT-3′ and reverse, 5′-GCTCTGGGAGGGGTGCAAA-3′
(239 bp); F3 forward, 5′-AGTTTGCACCCCTCCCAGA-3 and reverse,
5′-GTGGTCCAGCCCTCCTCCAC-3′ (239 bp); F4 forward,
5-GGTGGAGGAGGGCTGGACC-3′ and reverse, 5′-CATGCCCAGCTAATTTTTGTAT-3′
(237 bp); F5 forward, 5′-GAAACCCCATCTCTACTAAAAATAC-3′ and reverse,
5′-TGGGAGGCAAGCAAAACTCTT-3′ (214 bp); F6 forward,
5′-GAGTTTTGCTTGCCTCCCACC-3′ and reverse 5′-TGGATGGGGGAGGCAGAGC-3′
(232 bp); F7 forward, 5′-CCTAGCTCTGCCTCCCCCA-3′ and reverse,
5′-TGGGAGATGGGAGTGGAGGTC-3′ (214 bp); F8 forward,
5′-GGCTCTGACCTCCACTCCCAT-3′ and reverse 5′-CAGATCTCAGCAGCCCCAGC-3′
(238 bp); F9 forward, 5′-CTTGAGCTTCCCCTTCCCCA-3′ and reverse,
5′-GGAAACTGGGCTCCCACTCA-3′ (209 bp); and F10 forward,
5′-AGGGGCTGAGTGGGAGCC-3′ and reverse, 5′-CACCCTACAGGCTGTCGGC-3′
(215 bp). The PCR products were resolved electrophoretically on a
1.5% agarose gel and visualized using ethidium bromide
staining.
Tumor xenograft in nude mice
Animal experiments were approved by the Ethical
Committee for Animal Research of Central South University. The
MCF-7 cells were transfected with BRD7 plasmids with or without Bak
siRNA as aforementioned for 36 h prior to injections. To assess
tumor growth and paclitaxel sensitization, 100 µl of the
transfected MCF-7 cells (1×106) was subcutaneously
injected into nude mice (total n=24 female mice; n=6 mice/group;
age, 2 months; weight, ~15 g); the mice were housed under a 12-h
light/dark cycle at 20–22°C with 50–60% humidity and had free
access to food and water. The mice were purchased from Shanghai
Laboratory Animal Center (Shanghai, China). The researchers who
injected the cells were blind to the treatment. On day 14 following
the injection of cells, when the smallest group of tumors was
produced, the mice were intraperitoneally injected with paclitaxel
(Corden Pharma Latina S.P.A) every 2 days, with 4 injections
administered in total (15 mg/kg/injection). The control mice were
injected with an equal volume of saline. The tumor sizes were
measured regularly and calculated using the following formula: 0.5
× L × W2, where L and W refer to the length and width of
the tumor, respectively. On day 26 following cell injections, the
mice were sacrificed by injecting an overdose of pentobarbital
sodium. The tumors were then excised and weighed. The inhibition
rate was calculated as follows: (Average tumor weight of control
group - tumor weight of the experimental group/average tumor weight
of control group) × 100, where the experimental group refers to
control+paclitaxel, BRD7+paclitaxel or BRD7+siBak+paclitaxel. The
tumor tissues were then fixed with 4% paraformaldehyde for 24 h at
room temperature and paraffin-embedded for the immunohistochemistry
assay as aforementioned.
Statistical analysis
In the present study, all experiments were repeated
at least three times, and data are expressed as the mean ± standard
error of the mean. SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA) was used to perform statistical analysis. Differences between
two groups were compared by an independent-samples t-test.
Differences among three or more groups were compared by one-way
analysis of variance with a post hoc Bonferroni test. The
correlation between BRD7 and Bak in breast cancer tissues was
analyzed by Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of BRD7 and Bak in breast
cancer
To investigate the role of BRD7 and Bak in breast
cancer, the present study first evaluated the expression of BRD7
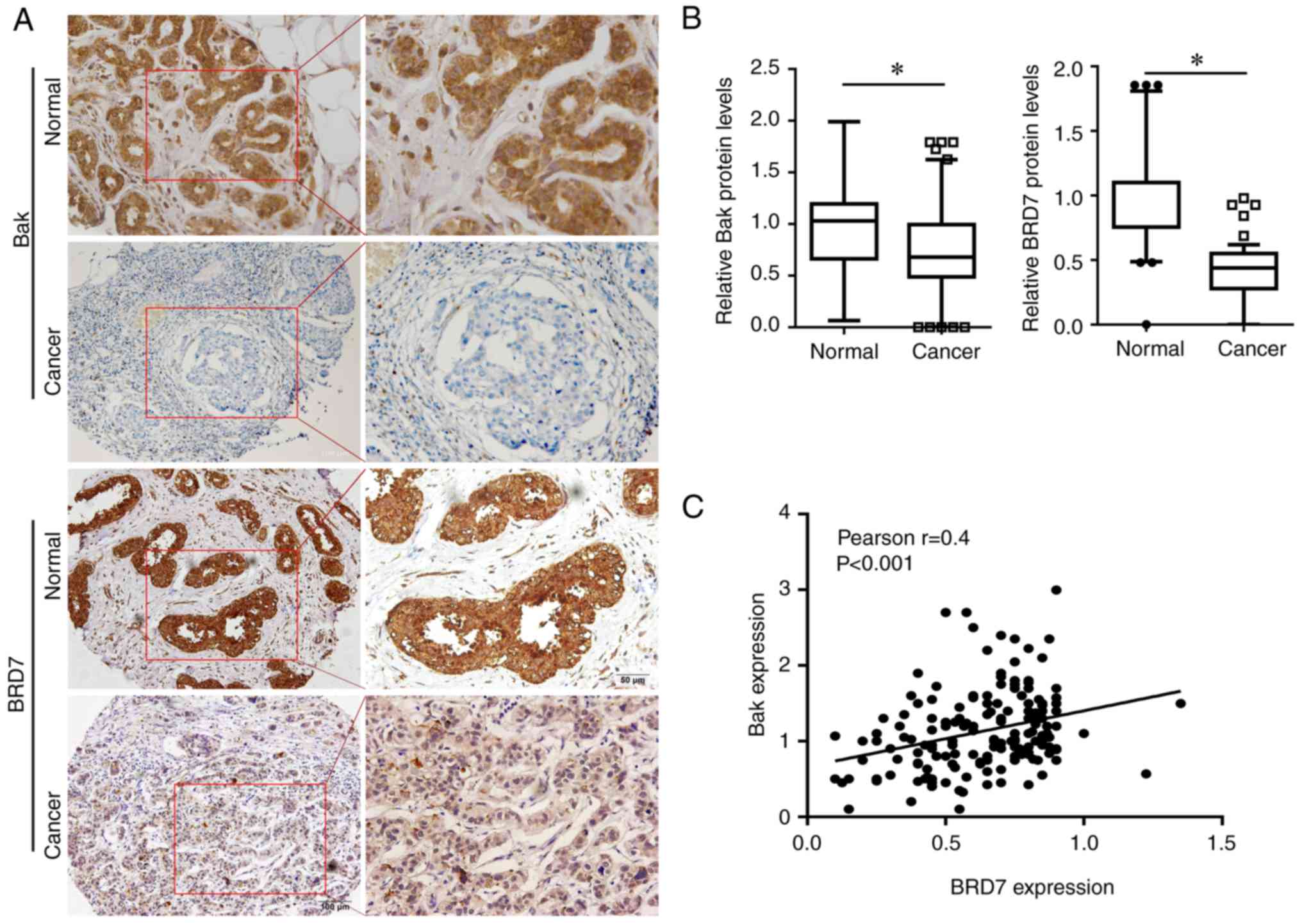
and Bak in breast cancer tissues. Bak was primarily expressed in
the cytoplasm, while BRD7 was mainly expressed in the nucleus
(Fig. 1A); their expressions were
significantly decreased in breast cancer tissues (n=225) when
compared with the normal control (n=62; Fig. 1B). In addition, the association
between BRD7 and Bak was evaluated, and the protein levels of BRD7
were revealed to be positively correlated with Bak levels in breast
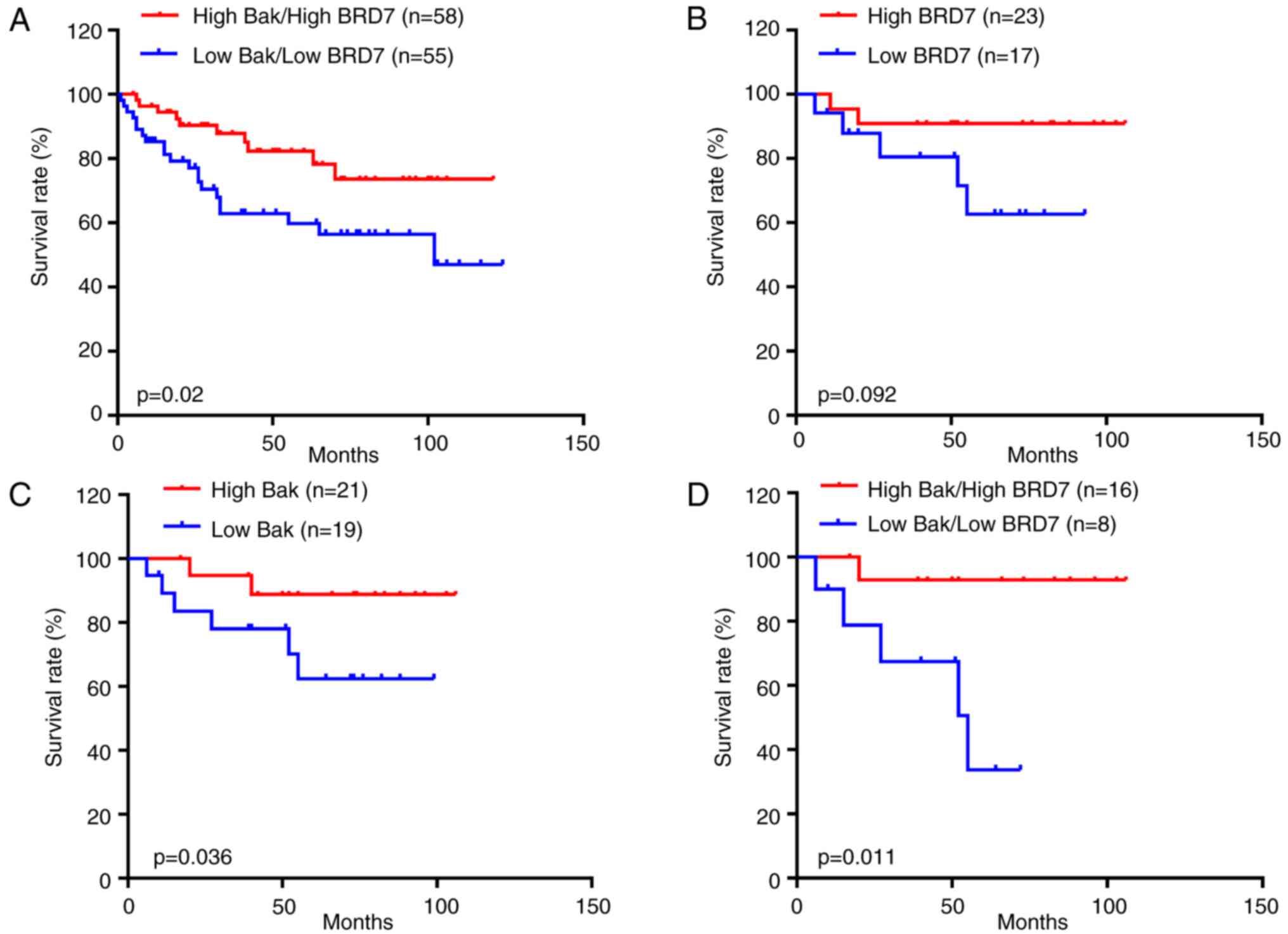
cancer tissues (n=225, Pearson's r=0.4, P<0.001; Fig. 1C). Notably, the patients with low
Bak and BRD7 expression had a lower survival rate than the patients
with high Bak and BRD7 expression (P=0.02; Fig. 2A).
Furthermore, to investigate the associations between
Bak/BRD7 and paclitaxel treatment in breast cancer, 40 cases that
had used therapeutic strategies including paclitaxel treatment were
selected from the 225 patients with breast cancer. As a result, the
patients treated with paclitaxel that had greater BRD7 expression
had higher overall survival than those with low BRD7 expression;
however, there was no statistically significant difference
(P=0.092; Fig. 2B). While high Bak
expression indicated a higher overall survival rate in patients
treated with paclitaxel compared with those of patients with low
Bak expression (P=0.036; Fig. 2C).
In addition, the patients treated with paclitaxel with low Bak and
BRD7 expression had a lower survival rate than those with high Bak
and BRD7 expression (P=0.011; Fig.
2D).
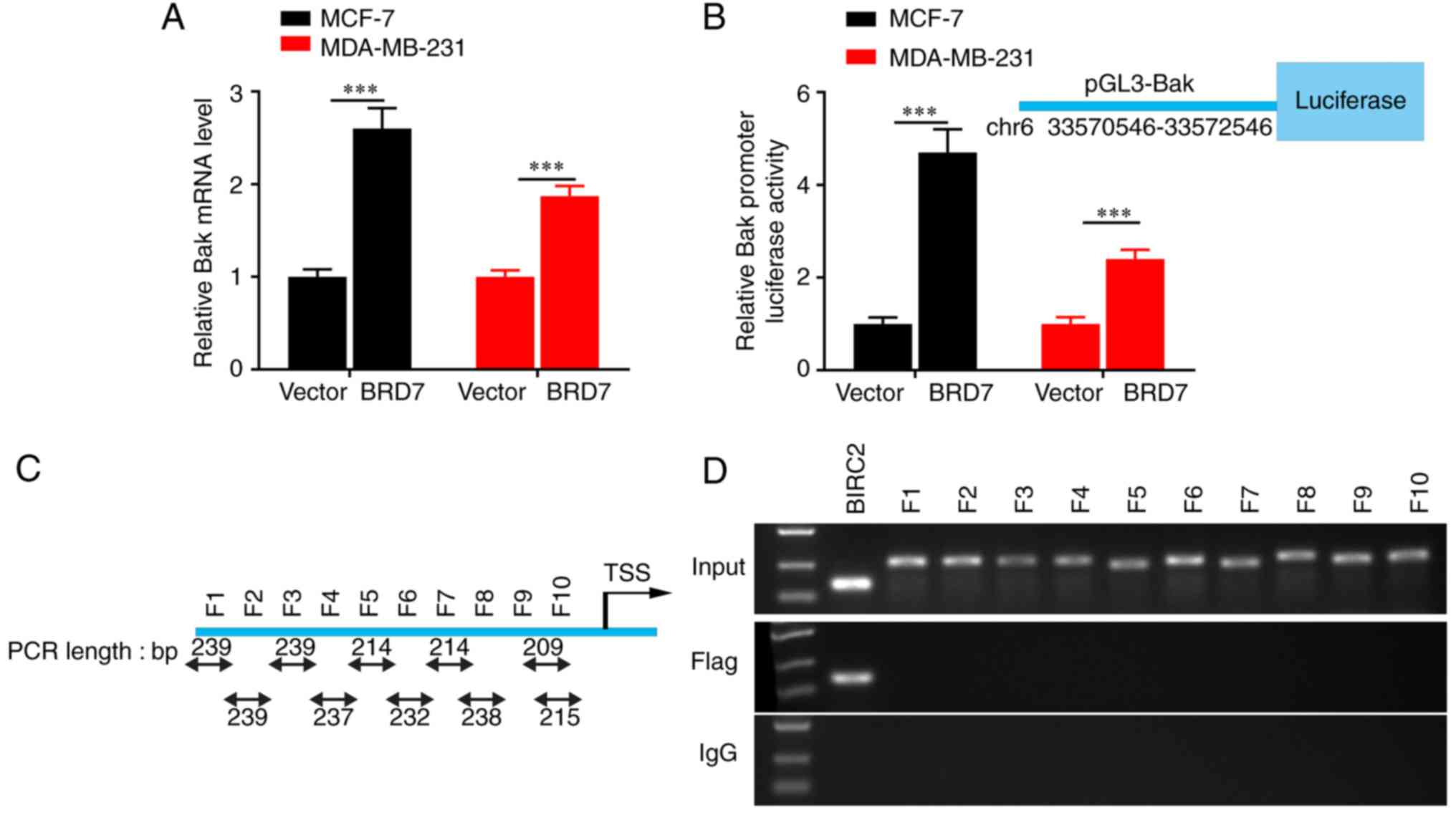
BRD7 activates promoter activity and
the expression of Bak in an indirect manner
As chromatin remodeling function is crucial for
BRD7-mediated transcriptional regulation (23) and BRD7 was positively correlated
with Bak expression at the protein level in breast cancer tissues,
the present study further investigated the effect and mechanism of
BRD7 on Bak transcriptional regulation and expression. The results
revealed that overexpression of BRD7 significantly upregulated the
expression of Bak mRNA as detected by RT-qPCR assays (Fig. 3A), and increased the activity of the
Bak promoter, as evaluated by dual-Luciferase reporter analysis
(Fig. 3B). In addition, the ChIP
assay was performed to investigate whether BRD7 binds to the Bak
promoter. The potential promoter region was first predicted and
then divided it into 10 fragments based on the DNA walking method
(Fig. 3C). The results demonstrated
that the Flag antibody couldn't pull down any of the fragments from
the Bak promoter (Fig. 3D). These
results indicated that BRD7 may control Bak expression at the
transcriptional level in an indirect manner.
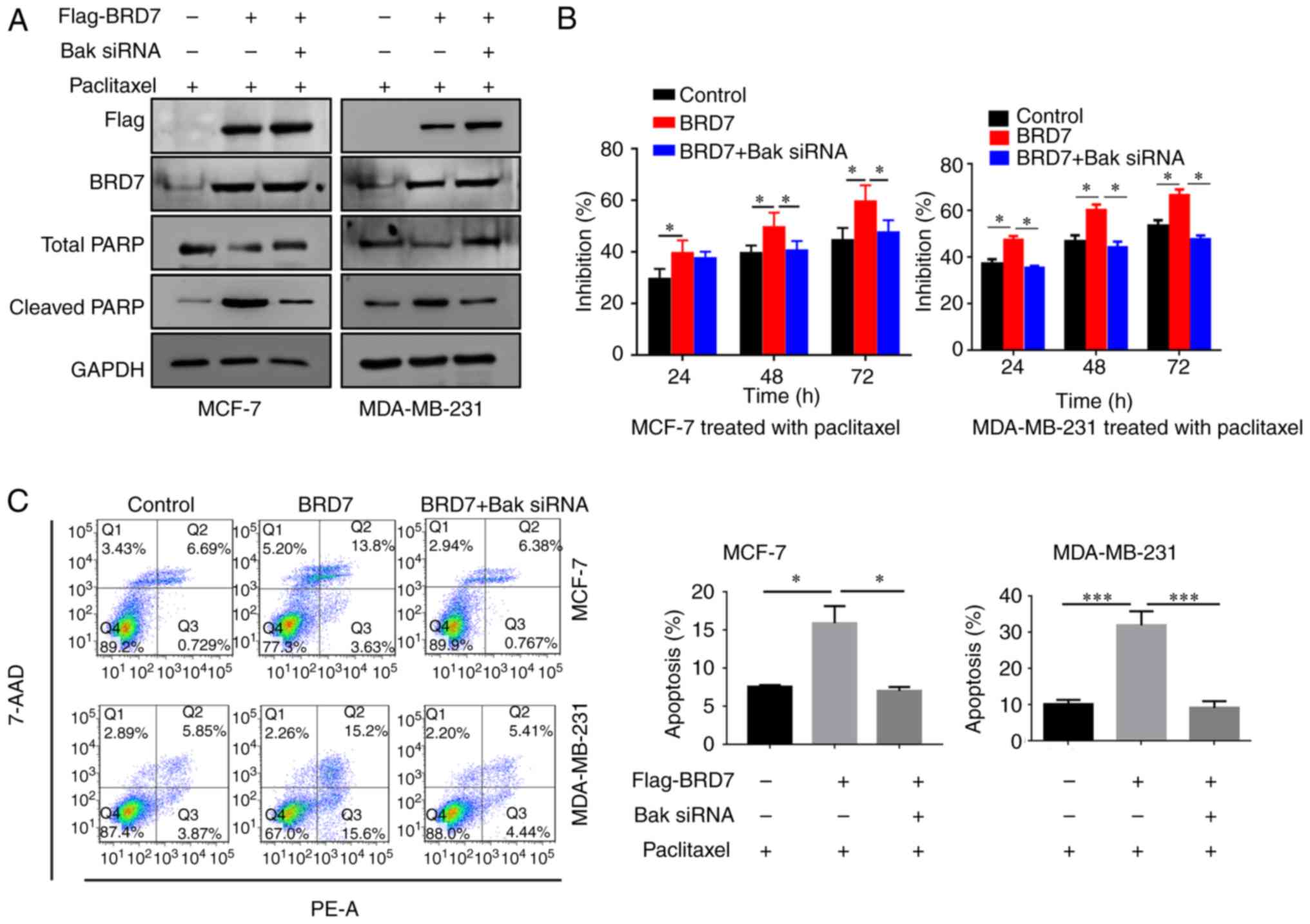
Silencing of Bak abolishes the
BRD7-mediated inhibition of breast cancer cell proliferation
As BRD7 positively regulates the expression of Bak,
the present study then investigated the effect of Bak on
BRD7-mediated inhibition of proliferation. Therefore, BRD7 was
overexpressed in the MCF-7 and MDA-MB-231 cells (Fig. 4A). Overexpression of BRD7 induced
the expression of Bak as well as the apoptotic marker cleaved PARP;
however, silencing of Bak by siRNA did not alter BRD7 expression
(Fig. 4A). In addition,
overexpression of BRD7 significantly inhibited cell proliferation
in MCF-7 and MDA-MB-231 cells, which was abolished by Bak silencing
(Fig. 4B). In addition, the
promotive effects of BRD7 on cell apoptosis were also attenuated by
Bak silencing (Fig. 4C).
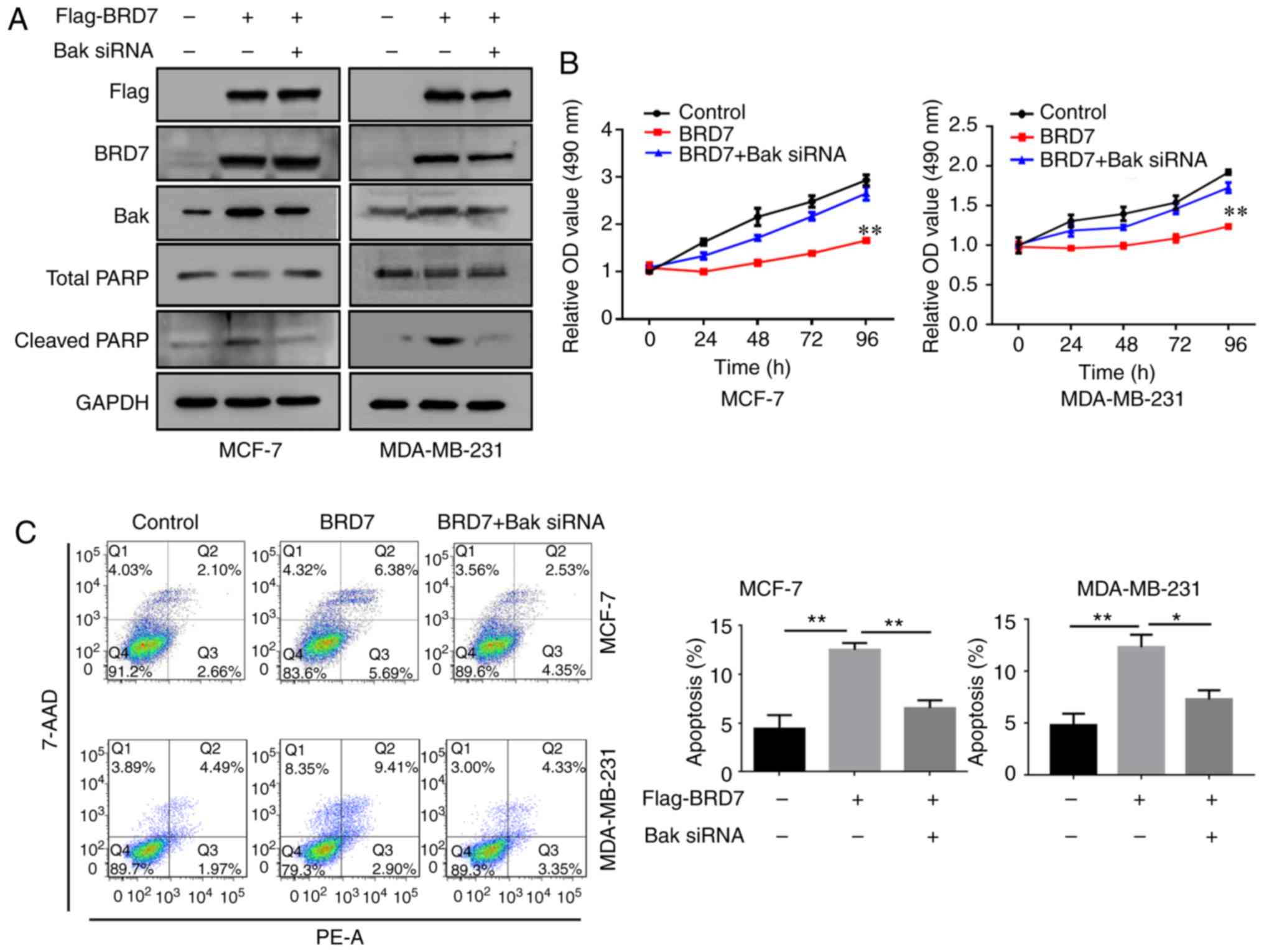 | Figure 4.BRD7 inhibits breast cancer cell
growth by activating Bak. (A) Western blotting analysis for Flag,
BRD7, Bak, total PARP and cleaved PARP following the indicated
treatments in MCF-7 and MDA-MB-231 cells. GAPDH was used as a
loading control. (B) Cell Counting Kit-8 was performed to measure
the cell viability of MCF-7 and MDA-MB-231 cells following the
indicated treatments. **P<0.01 vs. Control. (C) Flow cytometry
was conducted to measure the levels of cell apoptosis in MCF-7 and
MDA-MB-231 cells following the indicated treatments. *P<0.05 and
**P<0.01, as indicated. BRD7, bromodomain-containing protein 7;
Bak, B-cell lymphoma 2 antagonist/killer; PARP, poly (adenosine
diphosphate-ribose) polymerase; OD, optical density; 7-AAD,
7-aminoactinmycin D; PE, phycoerythrin; siRNA, small interfering
RNA. |
Silencing of Bak attenuates
BRD7-enhanced paclitaxel cytotoxicity in breast cancer cells
As the expression of BRD7 and Bak was associated
with the survival rate of patients treated with paclitaxel in
breast cancer (Fig. 2D), the
present study then detected the effect of Bak on BRD7-mediated
paclitaxel cytotoxicity. As expected, overexpression of BRD7
enhanced the inhibitory effects of paclitaxel on cell proliferation
and the promotion of cell apoptosis as supported by the significant
increase in the rate of inhibition (Fig. 5B) and apoptosis (Fig. 5C) when compared with the negative
control. Overexpression of BRD7 also increased the expression of
cleaved PARP (Fig. 5A). However,
silencing of Bak in cells expressing BRD7 decreased cleaved PARP
expression, as well as the rate of inhibition (Fig. 5B) and apoptosis when compared with
the BRD7 only group (Fig. 5C).
BRD7 enhances paclitaxel cytotoxicity
by activating Bak in vivo
As BRD7 could inhibit cell proliferation and
sensitize cancer cells to paclitaxel through Bak activation in
breast cancer cells, the present study then chose to further
validate the roles and mechanism of Bak in paclitaxel sensitization
in vivo by tumor xenograft in nude mice. The results
revealed that paclitaxel treatment suppressed tumor growth in
vivo and BRD7 overexpression enhanced the inhibitory role of
paclitaxel, while Bak silencing by siRNA reversed BRD7-mediated
chemosensitivity as determined by evaluating tumor volume, tumor
inhibition rate and tumor growth curves (Fig. 6A-C). The immunohistochemistry
results demonstrated that BRD7 expression was significantly
increased in BRD7 plus paclitaxel and BRD7 plus siBak and
paclitaxel groups when compared with the control groups, and Bak
expression was induced by BRD7 but silenced by siBak transfection
(Fig. 6D). The expression of the
apoptotic marker cleaved PARP was positively regulated by BRD7 and
the expression of the proliferative marker Ki67 was negatively
regulated by BRD7; while Bak silencing reversed the effect of BRD7
on the expression cleaved PARP and Ki67 (Fig. 6D). These results support the notion
that BRD7 enhances paclitaxel cytotoxicity through the activation
of Bak in vivo.
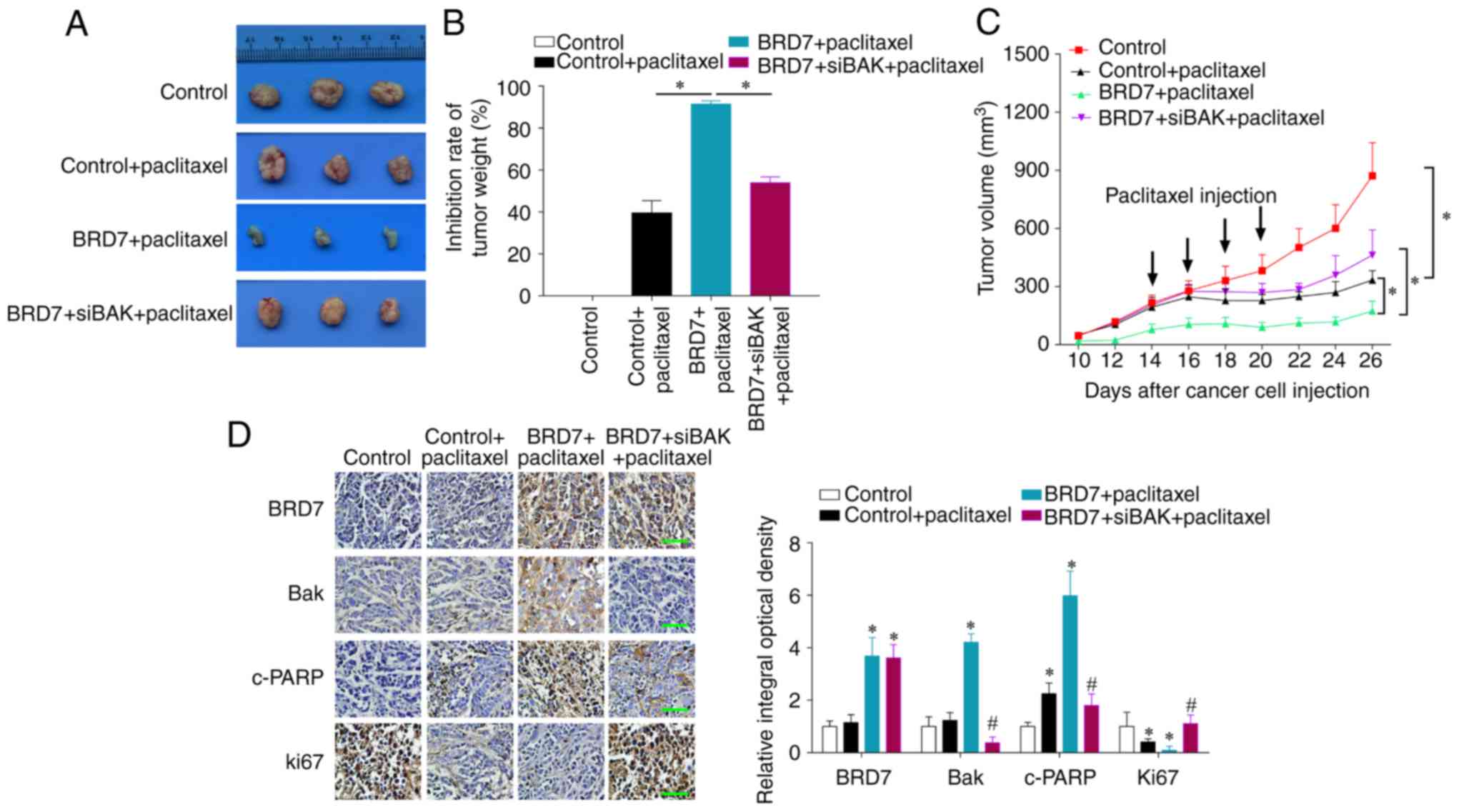 | Figure 6.Knockdown of Bak reverses
BRD7-enhanced chemosensitivity in vivo. The MCF-7 cells
transfected with BRD7 with or without Bak siRNA were injected into
nude mice. On day 14 following cell injections, the mice were
administered four paclitaxel injections, one every two days. (A)
Mice were sacrificed and the tumors were obtained from mice on day
26 post-injections. (B) The tumor weight inhibition rate.
*P<0.05, as indicated. (C) The tumor volumes were measured every
2-days. *P<0.05, as indicated. (D) The expression of BRD7, Bak,
c-PARP and Ki67 in tumor sections was evaluated by
immunohistochemistry. Magnification, ×400; Scale bars, 100 µm.
*P<0.05 vs. control; #P<0.05 vs. BRD7+paclitaxel
group. BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma
2 antagonist/killer; c-PARP, cleaved poly (adenosine
diphosphate-ribose) polymerase; si-, small interfering RNA. |
Discussion
The present study revealed that Bak was
significantly decreased in breast cancer tissues when compared with
the normal control. Our previous study demonstrated that low Bak
expression was associated with a poorer prognostic outcome for
patients with breast cancer (4).
The present results revealed that patients with low Bak and BRD7
expression had lower survival rates than the patients with high Bak
and BRD7 expression. Furthermore, greater Bak expression was
associated with a higher overall survival rate in patients treated
with paclitaxel compared with those of patients with low Bak
expression. Our previous study demonstrated that Bak is a direct
target of miR-125b, which mediated paclitaxel resistance in breast
cancer cells (24,25), suggesting that Bak may be associated
with drug resistance. In addition, decreased Bak expression was
also observed in doxorubicin-resistant Ewing sarcoma cells
(26), cisplatin-resistant ovarian
cancer and non-small cell lung cancer (27,28),
and docetaxel-resistant gastric cancer (29). Bak is a key effector for the
apoptotic pathway; Bak autoactivation serves a key role in
regulating the intrinsic apoptotic pathway in intact cells
(30). Thus, activating Bak would
be a promising strategy for triggering cancer cell apoptosis and
overcoming drug-resistance. Some candidate anti-cancer drugs
exhibit potent activity against multidrug-resistance by activating
Bak, such as coumarin (31) and
Leelamine in breast cancer (32).
In addition, human Bak protein integrated in liposomes was designed
to activate the mitochondrial apoptotic pathway in colon cancer
cells and glioblastoma (33).
In the present study, BRD7 activated the Bak
promoter and induced its expression, but it did not directly bind
to its promoter. BRD7 is a multifunctional gene, exhibiting its
functions through several pathways (6). As a tumor suppressor, BRD7 inhibited
cancer cell growth via phosphoinositide 3-kinase (PI3K)/Akt, ER
stress, ERK and hypoxia-inducible factor α (HIF1α)/lactate
dehydrogenase A signaling (10,34–36).
Furthermore, BRD7 regulated glucose homeostasis through glycogen
synthase kinase 3β and X-box binding protein 1 (37,38).
By binding to p53, BRD7 acetylates p53 in turn activating
downstream targets (14,15). In the present study, ectopic
expression of BRD7 in breast cancer cells inhibited cell
proliferation, promoted apoptosis and sensitized cancer cells to
paclitaxel, while Bak silencing abolished the BRD7-mediated
inhibitory effects on breast cancer cell growth and paclitaxel
sensitization. In line with these previous results, the present
study also indicated that there may be a positive correlation
between BRD7 and Bak expression in breast cancer tissues.
The present study also investigated BRD7 as a p53
co-activator and how it regulates Bak expression. Since MCF-7
contains wild-type p53 and MDA-MB-231 contains mutant p53, these
two types of cancer cells were selected to investigate whether BRD7
regulation of Bak expression is p53 dependent. Previous studies
have demonstrated that p53 upregulated modulator of apoptosis
(PUMA), a target gene of p53, can directly activate proapoptotic
Bak to permeabilize mitochondria, leading to caspase activation and
apoptosis (39). Antagonizing the
PI3K-AKT signaling pathway triggered PUMA promoter (40) and Bak release from myeloid cell
leukemia sequence-1/Bcl-2/Bcl-xL, resulting in Bak activation and
apoptosis (41). BRD7 interacts
with p85α and facilitates the nuclear translocation of p85α to
inhibit PI3K signaling (10,38).
In addition, via the acetylation its promoter BRD7, as a
co-activator of p53, can activate the downstream targets of p53,
including Bak (42–44). These results suggest that BRD7 may
activate Bak by regulating PI3K/Akt signaling, potentially in a
p53-dependent and independent manner.
In conclusion, the present study demonstrated that
BRD7 sensitizes breast cancer cells to paclitaxel by activating
Bak, which may occur via an indirect pathway. These results support
the function of BRD7 as a tumor suppressor and provide a novel
mechanism by which BRD7 enhances chemotherapy in breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81572748,
81772990 and 81802668), the Natural Science Foundation of Hunan
Province (grant no. 2018JJ3776), the Fundamental Research Funds for
the Central Universities of Central South University (grant nos.
2018zzts823 and 2018zzts233) and the Open-End Fund for the Valuable
and Precision Instruments of Central South University (grant no.
CSUZC201743).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL, RG, YLu and MZ made substantial contributions to
the design of the study. JM, WN, XW, YZ, JG, HW and FL analyzed and
interpreted the patient data. YLi, JG, WX, ZZ, SF, XL and XN
performed the cell biological experiments. All authors contributed
to writing the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethic
Committee of Central South University (Changsha, China). All
subjects provided written informed consent to participate in the
present study.
Patient consent for publication
All patients provided written informed consent for
the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lomonosova E and Chinnadurai G: BH3-only
proteins in apoptosis and beyond: An overview. Oncogene. 27 Suppl
1:S2–S19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shamas-Din A, Brahmbhatt H, Leber B and
Andrews DW: BH3-only proteins: Orchestrators of apoptosis. Biochim
Biophys Acta 1813. 508–520. 2011.
|
|
3
|
Ghiotto F, Fais F and Bruno S: BH3-only
proteins: The death-puppeteer's wires. Cytometry A. 77:11–21.
2010.PubMed/NCBI
|
|
4
|
Luo Y, Wang X, Wang H, Xu Y, Wen Q, Fan S,
Zhao R, Jiang S, Yang J, Liu Y, et al: High Bak expression is
associated with a favorable prognosis in breast cancer and
sensitizes breast cancer cells to paclitaxel. PLoS One.
10:e1389552015. View Article : Google Scholar
|
|
5
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu X, Li Z and Shen J: BRD7: A novel tumor
suppressor gene in different cancers. Am J Transl Res. 8:742–748.
2016.PubMed/NCBI
|
|
7
|
Liu Y, Zhao R, Wang H, Luo Y, Wang X, Niu
W, Zhou Y, Wen Q, Fan S, Li X, et al: miR-141 is involved in
BRD7-mediated cell proliferation and tumor formation through
suppression of the PTEN/AKT pathway in nasopharyngeal carcinoma.
Cell Death Dis. 7:e21562016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CL, Wang Y, Pan QZ, Tang Y, Wang QJ,
Pan K, Huang LX, He J, Zhao JJ, Jiang SS, et al:
Bromodomain-containing protein 7 (BRD7) as a potential tumor
suppressor in hepatocellular carcinoma. Oncotarget. 7:16248–16261.
2016.PubMed/NCBI
|
|
9
|
Zhang Q, Wei L, Yang H, Yang W, Yang Q,
Zhang Z, Wu K and Wu J: Bromodomain containing protein represses
the Ras/Raf/MEK/ERK pathway to attenuate human hepatoma cell
proliferation during HCV infection. Cancer Lett. 371:107–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiu YH, Lee JY and Cantley LC: BRD7, a
tumor suppressor, interacts with p85α and regulates PI3K activity.
Mol Cell. 54:193–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zhao R, Wei Y, Li M, Wang H, Niu W,
Zhou Y, Qiu Y, Fan S, Zhan Y, et al: BRD7 expression and c-Myc
activation forms a double-negative feedback loop that controls the
cell proliferation and tumor growth of nasopharyngeal carcinoma by
targeting oncogenic miR-141. J Exp Clin Cancer Res. 37:642018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao R, Liu Y, Wang H, Yang J, Niu W, Fan
S, Xiong W, Ma J, Li X, Phillips JB, et al: BRD7 plays an
anti-inflammatory role during early acute inflammation by
inhibiting activation of the NF-κB signaling pathway. Cell Mol
Immunol. 14:830–841. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Zhao R, Guo C, Jiang S, Yang J, Xu
Y, Liu Y, Fan L, Xiong W, Ma J, et al: Knockout of BRD7 results in
impaired spermatogenesis and male infertility. Sci Rep.
6:217762016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burrows AE, Smogorzewska A and Elledge SJ:
Polybromo-associated BRG1-associated factor components BRD7 and
BAF180 are critical regulators of p53 required for induction of
replicative senescence. Proc Natl Acad Sci USA. 107:14280–14285.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drost J, Mantovani F, Tocco F, Elkon R,
Comel A, Holstege H, Kerkhoven R, Jonkers J, Voorhoeve PM, Agami R,
et al: BRD7 is a candidate tumour suppressor gene required
for p53 function. Nat Cell Biol. 12:380–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harte MT, O'Brien GJ, Ryan NM, Gorski JJ,
Savage KI, Crawford NT, Mullan PB and Harkin DP: BRD7, a subunit of
SWI/SNF complexes, binds directly to BRCA1 and regulates
BRCA1-dependent transcription. Cancer Res. 70:2538–2547. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matissek KJ, Okal A, Mossalam M and Lim
CS: Delivery of a monomeric p53 subdomain with mitochondrial
targeting signals from pro-apoptotic Bak or Bax. Pharm Res.
31:2503–2515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Guo W, Zhou H, Luo N, Nie C, Zhao
X, Yuan Z, Liu X and Wei Y: Mitochondrial p53 phosphorylation
induces Bak-mediated and caspase-independent cell death.
Oncotarget. 6:17192–17205. 2015.PubMed/NCBI
|
|
19
|
Nieminen AI, Eskelinen VM, Haikala HM,
Tervonen TA, Yan Y, Partanen JI and Klefström J: Myc-induced
AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial
apoptosis. Proc Natl Acad Sci USA. 110:E1839–E1848. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu K, Xiong W, Zhou M, Wang H, Yang J, Li
X, Chen P, Liao Q, Deng H, Li X, et al: Integrating ChIP-sequencing
and digital gene expression profiling to identify BRD7 downstream
genes and construct their regulating network. Mol Cell Biochem.
411:57–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shapter FM and Waters DL: Genome walking.
Methods Mol Biol 1099. 133–146. 2014. View Article : Google Scholar
|
|
23
|
Liu H, Zhou M, Luo X, Zhang L, Niu Z, Peng
C, Ma J, Peng S, Zhou H, Xiang B, et al: Transcriptional regulation
of BRD7 expression by Sp1 and c-Myc. BMC Mol Biol. 9:1112008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi
Y, Xiong W, Li G, Lu J, Fodstad O, et al: MicroRNA-125b confers the
resistance of breast cancer cells to paclitaxel through suppression
of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J
Biol Chem. 285:21496–21507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Y, Wang X, Niu W, Wang H, Wen Q, Fan
S, Zhao R, Li Z, Xiong W, Peng S, et al: Elevated microRNA-125b
levels predict a worse prognosis in HER2-positive breast cancer
patients. Oncol Lett. 13:867–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iida K, Fukushi J, Matsumoto Y, Oda Y,
Takahashi Y, Fujiwara T, Fujiwara-Okada Y, Hatano M, Nabashima A,
Kamura S, et al: miR-125b develops chemoresistance in Ewing
sarcoma/primitive neuroectodermal tumor. Cancer Cell Int.
13:212013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai Y, Jin S, Li X and Wang D: The
involvement of Bcl-2 family proteins in AKT-regulated cell survival
in cisplatin resistant epithelial ovarian cancer. Oncotarget.
8:1354–1368. 2017.PubMed/NCBI
|
|
28
|
Ma J, Zhao Z, Wu K, Xu Z and Liu K: MCL-1
is the key target of adjuvant chemotherapy to reverse the
cisplatin-resistance in NSCLC. Gene. 587:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kubo T, Kawano Y, Himuro N, Sugita S, Sato
Y, Ishikawa K, Takada K, Murase K, Miyanishi K, Sato T, et al: BAK
is a predictive and prognostic biomarker for the therapeutic effect
of docetaxel treatment in patients with advanced gastric cancer.
Gastric Cancer. 19:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai H, Ding H, Meng XW, Peterson KL,
Schneider PA, Karp JE and Kaufmann SH: Constitutive BAK activation
as a determinant of drug sensitivity in malignant
lymphohematopoietic cells. Genes Dev. 29:2140–2152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang J, Wang Z, Liu Q, Li X, Sun J, Fung
KP and Liu F: DMFC
(3,5-dimethyl-7H-furo[3,2-g]chromen-7-one) regulates Bim
to trigger Bax and Bak activation to suppress drug-resistant human
hepatoma. Apoptosis. 22:381–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sehrawat A, Kim SH, Hahm ER, Arlotti JA,
Eiseman J, Shiva SS, Rigatti LH and Singh SV: Cancer-selective
death of human breast cancer cells by leelamine is mediated by bax
and bak activation. Mol Carcinog. 56:337–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liguori L, Pastorino F, Rousset X, Alfano
S, Cortes S, Emionite L, Daga A and Ponzoni M: Anti-tumor effects
of Bak-proteoliposomes against glioblastoma. Molecules.
20:15893–15909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Wang B and Gao S: BRD7 Acts as a
tumor suppressor gene in lung adenocarcinoma. PLoS One.
11:e1567012016.
|
|
35
|
Li D, Yang Y, Zhu G, Liu X, Zhao M, Li X
and Yang Q: MicroRNA-410 promotes cell proliferation by targeting
BRD7 in non-small cell lung cancer. FEBS Lett. 589:2218–2223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu W, Luo Y, Wang X, Zhou Y, Li H, Wang
H, Fu Y, Liu S, Yin S, Li J, et al: BRD7 inhibits the Warburg
effect and tumor progression through inactivation of HIF1α/LDHA
axis in breast cancer. Cell Death Dis. 9:5192018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Golick L, Han Y, Kim Y and Park SW: BRD7
regulates the insulin-signaling pathway by increasing
phosphorylation of GSK3β. Cell Mol Life Sci. 75:1857–1869. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SW, Herrema H, Salazar M, Cakir I,
Cabi S, Basibuyuk Sahin F, Chiu YH, Cantley LC and Ozcan U: BRD7
regulates XBP1s' activity and glucose homeostasis through its
interaction with the regulatory subunits of PI3K. Cell Metab.
20:73–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang L, Li A, Liao G, Yang F, Yang J,
Chen X and Jiang X: Curcumol triggers apoptosis of p53 mutant
triple-negative human breast cancer MDA-MB 231 cells via activation
of p73 and PUMA. Oncol Lett. 14:1080–1088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bean GR, Ganesan YT, Dong Y, Takeda S, Liu
H, Chan PM, Huang Y, Chodosh LA, Zambetti GP, Hsieh JJ, et al: PUMA
and BIM are required for oncogene inactivation-induced apoptosis.
Sci Signal. 6:ra202013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahmani M, Aust MM, Attkisson E, Williams
DJ Jr, Ferreira-Gonzalez A and Grant S: Dual inhibition of Bcl-2
and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in
human myeloid leukemia cells through a GSK3- and Bim-dependent
mechanism. Cancer Res. 73:1340–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Huang K, O'Neill KL, Pang X and
Luo X: Bax/Bak activation in the absence of Bid, Bim, Puma, and
p53. Cell Death Dis. 7:e22662016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren D, Tu HC, Kim H, Wang GX, Bean GR,
Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ and Cheng EH: BID,
BIM, and PUMA are essential for activation of the BAX- and
BAK-dependent cell death program. Science. 330:1390–1393. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jones NA, Turner J, McIlwrath AJ, Brown R
and Dive C: Cisplatin- and paclitaxel-induced apoptosis of ovarian
carcinoma cells and the relationship between bax and bak
up-regulation and the functional status of p53. Mol Pharmacol.
53:819–826. 1998.PubMed/NCBI
|