Introduction
Electrochemotherapy is an effective local ablative
technique that consists of the use of intratumoral or systemic
administration of chemotherapeutics (cisplatin or bleomycin) in
combination with electroporation. Electroporation is exposure of
cells to short, intense electric pulses that enable formation of
transient pores in the cell membrane which allow diffusion of
chemotherapeutics into the cells, to exert the antitumor effect
(1,2).
There are multiple mechanisms of action involved in
electrochemotherapy. The primary mechanism is the direct effect on
tumor cells, by increased drug accumulation in the cells after
electroporation (3). Furthermore,
due to the release of tumor antigens after electrochemotherapy and,
consequently, the immunogenic cell death, the immune response of
the organism is elicited (4,5). There
are also indirect effects of electrochemotherapy, the vascular lock
and the vascular disruptive effect (6).
Knowledge of the underlying mechanisms of
electrochemotherapy has facilitated its translation into clinical
practice for the treatment of various cutaneous and subcutaneous
tumors. The objective response rate is high, ~70–80% (7–9) and is
comparable to other local ablative treatment approaches (10). In the clinic, electrochemotherapy is
predominantly used in palliative treatment for cases of
recurrent/residual lesions after intensive previous treatments
using standard modalities. Furthermore, this method can be the
first treatment option when surgery or radiotherapy would have
caused an aesthetic or functional defect, e.g. in selected tumors
of the head and neck area (11–14).
However, recent studies indicate differential
effectiveness of electrochemotherapy in different cell types
(15,16). Moreover, it has been shown that the
effectiveness of electrochemotherapy depends on the tumor histology
(8,17), size (18,19)
and previous treatment (20). As
reported by Campana et al, the pre-treatment with radio- or
chemotherapy significantly lowered the response rate of the
electrochemotherapy-treated tumors (20,21).
One of the possible reasons for this is the endothelial cell
dysfunction (increased permeability and apoptosis) caused by
irradiation that contributes to post-irradiation inflammation and
tissue fibrosis. In cases with the intravenous administration of
the drug, the resulted reduction in blood supply can diminish
delivery of the drug to tumor cells (9,22). In
addition, pre-irradiated recurrent tumors consist of selected,
highly resistant malignant cells that survived previous treatment.
Intrinsic radioresistance of surviving tumor cells is one of the
main obstacles in radiotherapy that affects the curability of the
patients and could, together with impaired blood supply, contribute
to the lower responsiveness to salvage electrochemotherapy
(23,24).
In the head and neck area, many tumors treated by
electrochemotherapy have been previously irradiated, which pose
several questions: First of all, what is their sensitivity to
electrochemotherapy as a potential salvage treatment option; and
secondly, whether the differential sensitivity to
electrochemotherapy with bleomycin or cisplatin exists.
In order to assess the importance of intrinsic
radioresistance in salvage electrochemotherapy for recurrent
squamous cell carcinoma of the head and neck, we determined the
response rate of a cell line and tumor xenografts in SCID mice of
human hypopharyngeal squamous cell carcinoma with induced intrinsic
radioresistance to electrochemotherapy with cisplatin or bleomycin.
We compared its sensitivity to the parental tumors without the
induced radioresistance. Additionally, the uptake of
chemotherapeutics in the tumors after electrochemotherapy and
capacity of the cells to repair DNA damage after exposure to these
drugs were evaluated.
Materials and methods
Cell lines
In the present study, 2 isogenic cell lines were
used: parental FaDu [human squamous cell carcinoma cell line;
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA)] and the radioresistant subline FaDu-RR, which was
established in the laboratory of the Department of Experimental
Oncology (Institute of Oncology, Ljubljana, Slovenia) by
fractionated irradiation of the FaDu cell line. The cells received
4 cycles of irradiation (30 Gy/cycle; 2 Gy/day; 5 days a week for 3
weeks; the total dose was 120 Gy) with the Gulmay 225 X-ray system
(Gulmay Medical Ltd., Byfleet, UK) with 0.55 mm Cu and 1.8 mm Al
filtering, at a dose-rate 1.8 Gy/min. The radioresistance of the
newly established FaDu-RR cells was confirmed with clonogenic
assay: The effective dose at 50% survival (ED50) was
1.60±0.11 Gy for FaDu and 2.57±0.09 Gy for FaDu-RR (P<0.001)
(Table I). Both cell lines were
grown as a monolayer in Advanced Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Loughborough, UK),
supplemented with 5% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin (Grünenthal, Aachen,
Germany), 50 mg/ml gentamicin (Krka, Novo Mesto, Slovenia) and 10
mM L-glutamine (GlutaMAX; Gibco; Thermo Fisher Scientific, Inc.).
Cells were maintained in a humidified atmosphere at 37°C with 5%
CO2.
 | Table I.Radiosensitivity of FaDu and FaDu-RR
cell lines. |
Table I.
Radiosensitivity of FaDu and FaDu-RR
cell lines.
| Cell line | ED50 ±
SEM | DMF
(ED50) | ED90 ±
SEM | DMF
(ED90) |
|---|
| FaDu | 1.60±0.11 | 1 | 4.16±0.13 | 1 |
| FaDu-RR |
2.57±0.09a | 1.60 |
5.35±0.13a | 1.29 |
Animals and tumor models
In total, 120 6- to 8-week old female
immunodeficient SCID (C.B-17/IcrHsd-Prkdcscid)
mice were purchased from Envigo Laboratories (Udine, Italy). They
were kept at room temperature (21°C) with a 12-h light cycle in a
specific pathogen-free environment with food and water ad
libitum. The radioresistant and radiosensitive tumor models
were induced by subcutaneous injection of 2×106 FaDu or
FaDu-RR cells in 100 µl of 0.9% sodium chloride (B. Braun Melsungen
AG, Melsungen, Germany) solution. To prepare the cell solution, the
cells were trypsinized and centrifuged (470 × g for 5 min). The
cell pellet was then resuspended in 0.9% sodium chloride, at the
concentration of 20×106 cells/ml. To monitor tumor
growth, the volume of the tumors was measured using a Vernier
caliper, and calculated with the equation for an ellipsoid: V = (π
× a × b × c)/6 (where a, b and c are 3 perpendicular diameters of
the tumor). When the tumors reached 6 mm in the longest diameter
(~40 mm3), the mice were divided randomly into
experimental groups, consisting of 5–7 mice, and the treatment
started according to the protocol. The experiments were approved by
the Ministry of Agriculture, Forestry and Food of the Republic of
Slovenia (permit no. U34401-1/2015/16), and were in compliance with
the standards required by the UKCCCR guidelines and the EU
directive.
Histology
The radioresistant and radiosensitive tumors
(3/group) were induced as described above. When the tumors reached
~100 mm3 in size, they were excised, separated from the
skin, fixed in a zinc fixative [BD Pharmingen™ 10X Zinc Fixative
(Formalin free); BD Biosciences, San Diego, CA, USA] and embedded
in paraffin. From each paraffin block 2-µm-thick sections were cut
and stained with Masson trichrome, as well as immunohistochemically
(IHC) to determine proliferation (Ki-67; cat. no. RM9106S1; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), hypoxia (HIF-1α;
dilution 1:1,000; cat. no. ab2185; Abcam, Cambridge, UK) and the
number of tumor blood vessels (anti-CD31 antibody; dilution
1:1,500; cat. no. ab28364; Abcam). From the slides, 5 random parts
of each tumor were selected and captured with a DP72 CCD camera
(Olympus Corp., Tokyo, Japan) connected to an Olympus BX-51
fluorescence microscope (Olympus Corp.). Images were quantitatively
evaluated by 3 independent researchers, as previously described
(25).
Exposure to chemotherapeutics in
vitro
Cells were plated on Petri dishes (VWR International
GmbH, Wien, Austria). When the cells attached, various
concentrations of cisplatin (ranging from 1.67 to 8.33 µM) or
bleomycin (from 2.5 to 20 µM) suspensions were prepared out of a
stock cisplatin (1 mg/ml; Cisplatina Kabi; Fresenius Kabi AG, Bad
Homburg, Germany) or bleomycin (3 mg/ml; Heinrich Marck Nachf GmbH,
Illertissen, Germany) suspensions, respectively, and added to the
culture medium with the cells. After 2 h, the medium containing the
chemotherapeutics was replaced with fresh medium. The cell survival
was determined by clonogenic assay, where the plating efficiency
(ratio between the number of colonies and the number of plated
cells) and surviving fraction (ratio between the plating efficiency
of the treated groups and control group) of the cells were
calculated.
Electrochemotherapy in vitro
In this experiment, 90 µl of cell suspension
[2.2×107 cells/ml in electroporation buffer (125 mM
sucrose, 10 mM K2HPO4, 2.5 mM
KH2PO4 and 2 mM MgCl2 ×
6H20)] (26) was mixed
with 10 µl of culture medium, containing increasing concentrations
of cisplatin (from 1.67 to 33.32 µM) or bleomycin (from
10−5 to 1 µM). One-half of the mixture was exposed to
electric pulses and the other half served as control for treatment
with the chemotherapeutics alone. After that, the cells were
incubated for 5 min at room temperature in ultra-low attachment
plates (Costar® 24-Well Plate, Ultra-Low Attachment
Surface; Corning Inc., Corning, NY, USA), then diluted in the cell
culture medium and plated on Petri dishes for the clonogenic assay.
The parameters of electrical pulses were 8 square wave electric
pulses, amplitude over distance ratio of 1,300 V/cm and pulse
duration of 100 µsec at a frequency of 1 Hz. The pulses were
delivered by electroporator GT-01 (Faculty of Electrical
Engineering, University of Ljubljana, Ljubljana, Slovenia) using 2
parallel stainless steel plate electrodes with 2 mm of inner
distance.
As a part of this experiment, the
electropermeabilization of these 2 cell lines was determined,
measuring the propidium iodide (PI) uptake immediately after
electroporation (using electric field intensities from 400 to 1,600
V/cm). The increase in uptake of PI after electroporation was used
as an indicator of electropermeability of cells. The measurement of
median fluorescence and percentage of PI-positive cells was carried
out with FACSCanto II flow cytometer (BD Biosciences) as described
by Prevc et al (27).
Electrochemotherapy in vivo
The mice bearing either radioresistant or
radiosensitive tumors were divided into the following experimental
groups (5–7 mice/group): the control (received no therapy), the
electroporation-only, the chemotherapy and the electrochemotherapy
group. The mice in the chemotherapy group received cisplatin- or
bleomycin-based chemotherapy. They were injected with 4 mg/kg
cisplatin (concentration 1,142.8 µg/ml) or 5 mg/kg bleomycin
(concentration 1,492.5 µg/ml), dissolved in 0.9% sodium chloride,
into the retro-orbital sinus. Selection of the chemotherapeutic
dosage for electrochemotherapy was based on previous studies
(28,29) and was in the range where complete
responses of different tumor models were expected. For
electrochemotherapy-treated tumors, electric pulses (8 electrical
pulses of 100 µ sec duration at 1 Hz, the electric field intensity
was 1,300 V/cm) were applied 3 min after the mice were i.v.
injected with cisplatin or bleomycin. The electric pulses were
delivered by ELECTRO Cell B10 electric pulse generator (Leroy
Biotech, Saint-Orens-de-Gameville, France) using 2 stainless steel
plate electrodes with 6-mm inner distance. When the tumors reached
250 mm3 in size, the mice were sacrificed with cervical
dislocation that followed anesthesia with 3% isoflurane. Survival
(Kaplan-Meier) curves were drawn. Growth delay (GD) was calculated
as the difference in tumor doubling time (DT) of the treated groups
and DT of the corresponding control group. Due to the difference in
the growth rate of control tumors (FaDu vs. FaDu-RR), also the
normalized GD (nGD) was calculated for each treated group (30).
Platinum determination in vitro and in
vivo
The uptake of cisplatin was evaluated after chemo-
and electrochemotherapy, both in vitro and in vivo.
To determine and compare the cisplatin uptake in vitro, the
cells were first treated with chemotherapy (exposure to 5 µM
cisplatin for 5 min, or 2 h; n=3) or with electrochemotherapy
(using the same cisplatin concentration, the exposure time was 5
min; n=3). Then, the cells were centrifuged at 470 × g for 5 min
and the cell pellet was stored at −20°C until further analysis.
The protocol for the in vivo measurement was
adapted from our previous study, described by Kranjc et al
(31). Briefly, the mice were first
treated with chemotherapy or electrochemotherapy with cisplatin (6
mice/group). One hour after the treatment (32), the blood of the treated mice was
collected with a glass capillary from the intra-orbital sinus and
centrifuged at 1,811 × g for 10 min. Then, the serum was collected
and stored at −20°C. After the blood collection, the mice were
sacrificed with cervical dislocation that followed anesthesia with
3% isoflurane; the tumors were excised and separated from the
overlying skin, weighed and stored at −20°C until further
analysis.
All the collected samples were first digested in 1:1
mixture of 65% nitric acid (Merck KGaA, Darmstadt, Germany) and 30%
hydrogen peroxide (Merck KGaA) at 90°C for 48 h. Before analyses,
digested samples were diluted with Milli-Q water (Direct-Q 5
Ultrapure water system; EMD Millipore, Watertown, MA, USA).
Platinum content was determined by inductively coupled plasma mass
spectrometry (7,700× ICP-MS; Agilent Technologies Japan Ltd.,
Tokyo, Japan) by monitoring the 195Pt and
194Pt isotopes (33,34).
The measured platinum content in samples (given in ng) obtained
from tumors was then divided by the mass of the tumor (g); the
serum samples were divided by the volume of isolated serum (ml);
the samples from the in vitro experiment were normalized to
number of cells in the pellet (ng/106 cells).
Bleomycin determination in vivo
The samples for bleomycin determination were
obtained in the same way as for platinum determination after in
vivo chemo- and electrochemotherapy, using 6 mice/group. For
analysis, the tumor samples were ground to fine powder under liquid
nitrogen, sonicated, centrifuged and filtered. After the
purification with solid phase extraction the bleomycin
concentration was determined by liquid chromatography coupled to
tandem mass spectrometry (LC-MS/MS) on Nexera ultra high
performance LC (Shimadzu Corp., Kyoto, Japan) coupled to
QTRAP® 4500 MS/MS system (AB Sciex Germany GmbH,
Darmstadt, Germany) (35). The
measured bleomycin concentration in each sample was then normalized
to the mass of the tumor or to the volume of the isolated serum, as
described above.
γH2AX immunofluorescent staining
For determination of DNA double-strand breaks (DSB)
after exposure to cisplatin or bleomycin, the cells were first
plated on coverslips in 6-well plates and then exposed to 3.33 µM
of cisplatin or 5 µM of bleomycin in cell medium for 2 h. At
different time-points after the exposure, the cells were fixed in a
mixture of 4% paraformaldehyde [Thermo Fisher (Kendel) GmbH,
Karlsruhe, Germany] and 0.1% Triton X-114 (Sigma-Aldrich; Merck
KGaA), and then permeabilized in 0.5% Triton X-114 and after that
blocked in 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA). Cells were then incubated overnight at 4°C in mouse
monoclonal anti-γ H2A.X (phospho S 139) antibody [9F3] (cat. no.
ab26350; Abcam, Cambridge, MA, USA) at dilution 1:3,000 in a 5% BSA
and 0.1% Triton X-114 mixture. The cells were then washed
thoroughly with phosphate-buffered saline (PBS) and incubated in
secondary donkey anti-mouse IgG H&L (Alexa Fluor 488) antibody
(cat. no. ab150105; Abcam) at dilution 1:2,000 in 0.1% Triton X-114
for 1 h at room temperature. The cells were then washed with PBS
and distilled water, and the coverslips were mounted on microscope
slides with Fluoroshield with DAPI (Sigma-Aldrich; Merck KGaA) for
nuclei counterstaining, dried and viewed under the Olympus BX-51
fluorescence microscope (Olympus Corp.) equipped with a camera DP72
(Olympus Corp). Filter U-MWIB (Olympus Corp.) was used for γH2AX
foci and U-MWU2 (Olympus Corp.) was used for nuclei counterstaining
at 100-fold magnification. The number of γH2AX foci/nuclei was
evaluated by image analysis using Fiji software (ImageJ image
processing program; National Institutes of Health, Bethesda, MD,
USA) (36). For the analysis, γH2AX
foci were determined in control groups as well (i.e. cells received
no treatment) in both cell lines to assess the baseline number of
foci/nucleus. In both control groups, >90% of nuclei had 3 or
fewer foci; therefore, in the analysis of the treated cells, nuclei
with >3 foci were considered as γH2AX-positive.
Statistical analysis
Statistical analysis and graphical representation of
the results were performed by SigmaPlot Software (version 13;
Systat Software, Hounslow, UK). All data were tested for normal
distribution using Shapiro-Wilk test and the arithmetic mean (AM)
and standard error of the mean (SEM) were calculated. Statistical
difference between the experimental groups (chemo- and
electrochemotherapy in vitro, electropermeabilization,
platinum and bleomycin uptake) was determined with a t-test and
one-way analysis of variance (one-way ANOVA) followed by a
Holm-Sidak test. In the γH2AX test, the data are presented as the
median and quartiles and the Mann-Whitney rank sum test was used to
assess the statistically significant differences. For the
statistical analysis of the in vivo growth delay data, the
growth delay of tumors in experimental groups was normalized to the
doubling time of tumors in the control group of each model due to
the difference between growth rates of the 2 models. For the
survival of mice, Kaplan-Meier survival analysis was performed. The
difference between experimental groups was considered significant
if the P-value was <0.05.
Results
Characterization of the cell
lines
The 2 cell lines differed in growth characteristics;
the doubling time in FaDu cells was 25±1.2 h, while in FaDu-RR
cells, the doubling time was 34.6±2.9 h. The FaDu cell line and its
radioresistant subline FaDu-RR differed in sensitivity to a 2-h
exposure to cisplatin. The radioresistant cell line FaDu-RR was
more resistant to cisplatin compared to the parental FaDu cell line
(IC50 for FaDu was 2.55±0.56 and 4.36±0.61 µM for
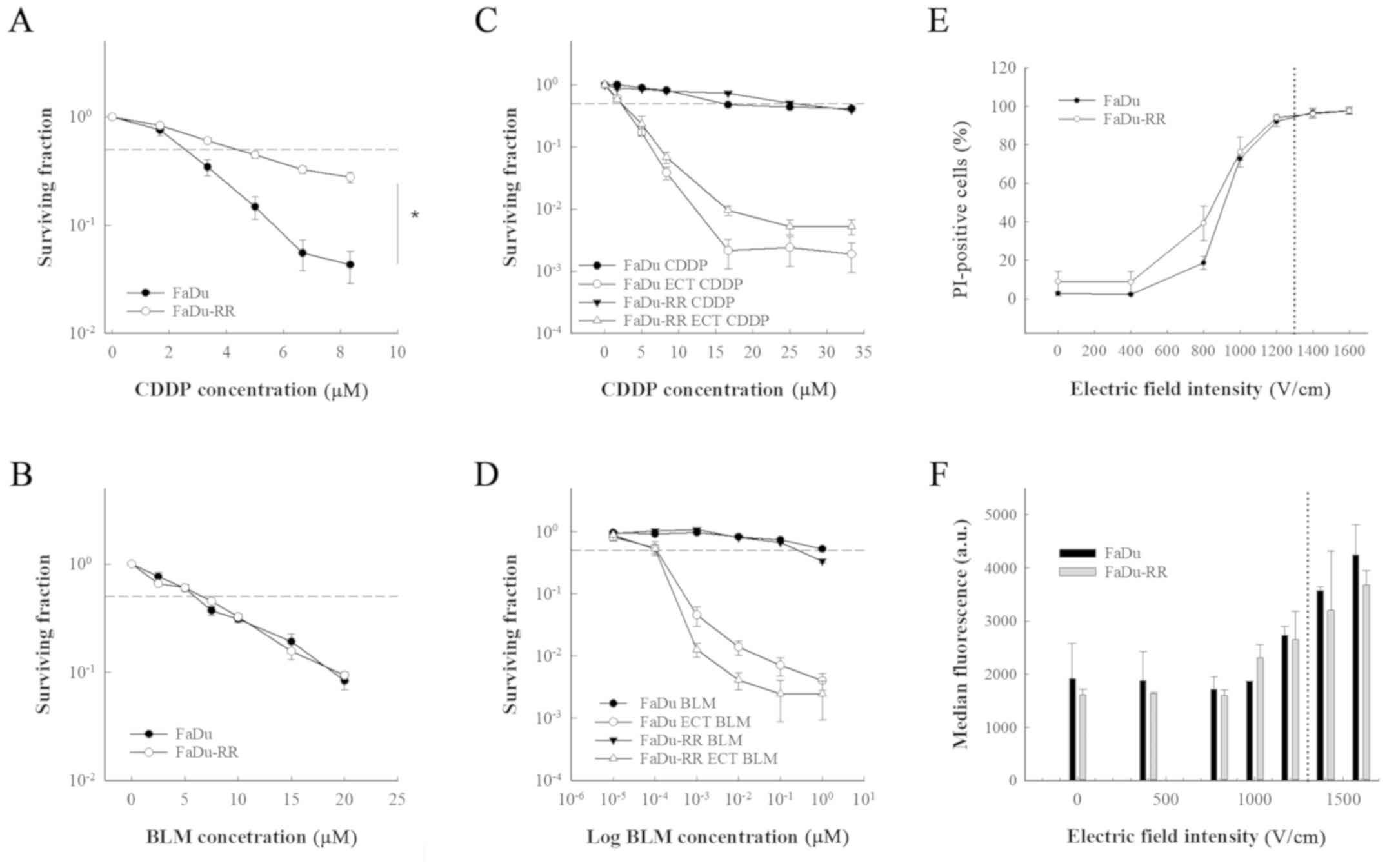
FaDu-RR; P<0.001) (Fig. 1A).
However, the 2 cell lines were equally sensitive to a 2-h exposure
to bleomycin (IC50 for FaDu was 5.93±0.34 and 6.63±0.47
µM for FaDu-RR; P=0.540) (Fig.
1B).
While the exposure of the cells to electric pulses
significantly increased the response of the cell lines to both
chemotherapeutics (P<0.001), there was no difference between the
cell lines in sensitivity to electrochemotherapy with cisplatin or
with bleomycin (Fig. 1C and D).
Furthermore, the cells did not differ in membrane
electropermeabilization, which was measured with the PI uptake. The
percentage of PI-positive cells at 1,300 V/cm (i.e. electric field
intensity that is used in electrochemotherapy) was 95% in both cell
lines (Fig. 1E and F), indicating
that the resistance of FaDu-RR cells to cisplatin may be due to the
impaired influx of cisplatin. By cell electroporation, however,
this restriction was overcome and both cell lines were equally
sensitive to electrochemotherapy with cisplatin.
Characterization of the tumor
models
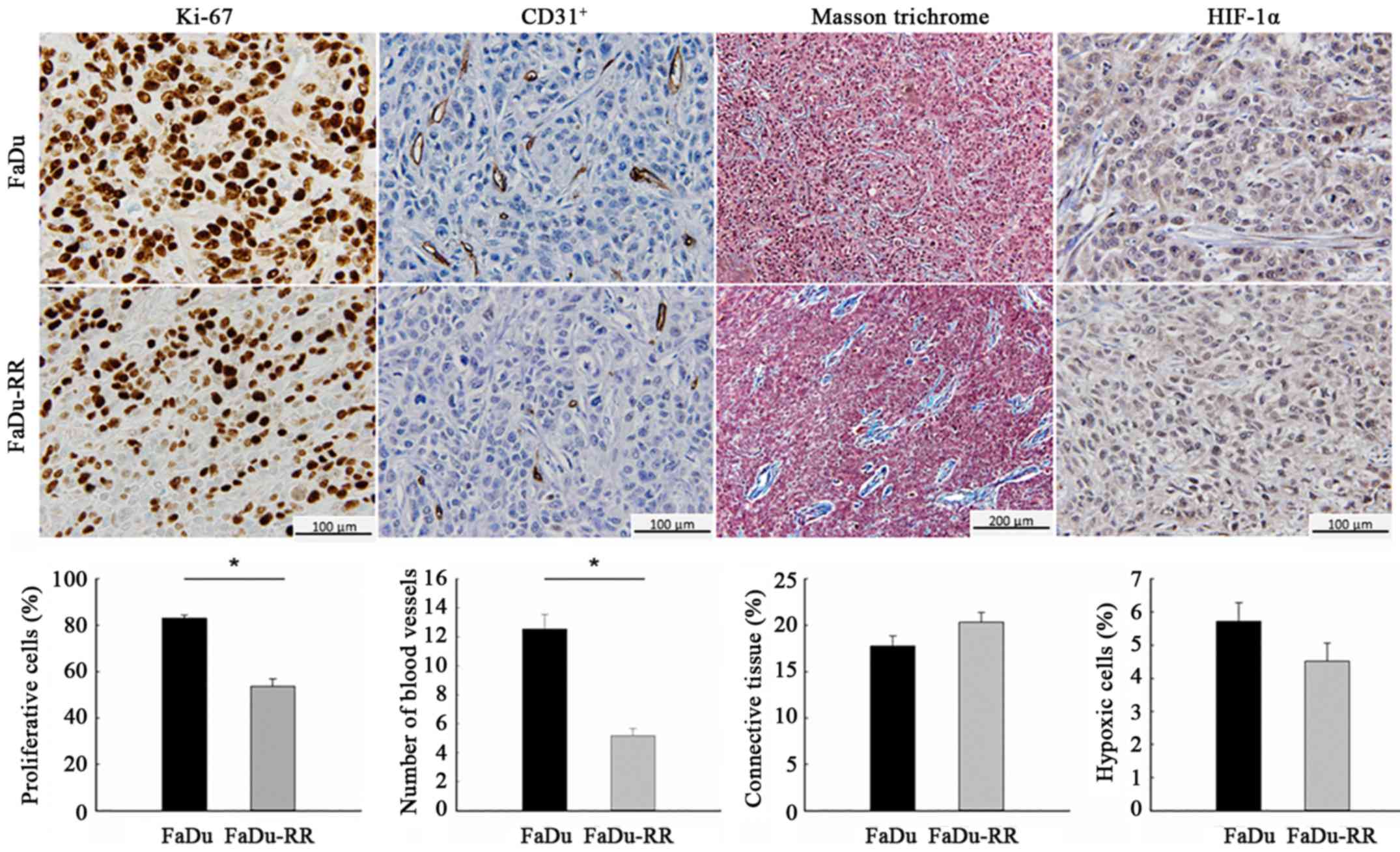
Characteristics of the radiosensitive and
radioresistant tumors differed significantly with respect to growth
rate and histological properties. The radioresistant tumors grew
slower (DT of control groups was 1.8±0.2 days for FaDu and 2.6±0.4
days for FaDu-RR; P=0.067), which was reflected in the lower
proliferation rate of radioresistant tumors: The percentage of the
proliferative cells, obtained with IHC staining for the
proliferative marker Ki-67, was 83.1±1.3% in FaDu and 53.6±3.3% in
FaDu-RR (P<0.001). Furthermore, radioresistant tumors were less
vascularized, with a lower average number of blood vessels/field
(60-fold magnification), obtained with IHC staining for endothelial
cell marker CD31, compared to radiosensitive tumors (12.6±1.0 in
FaDu vs. 5.2±0.5 in FaDu-RR; P<0.001). The difference in tumor
structure was also noticeable: in FaDu tumors the connective tissue
was evenly distributed between the cells while in FaDu-RR tumors,
it was less organized in individual bundles. Despite the
difference, the percentage of connective tissue was similar in both
tumor models (17.3±1.1 in FaDu vs. 20.3±1.0% in FaDu-RR; P=0.094).
Furthermore, the tumors did not differ in the percentage of hypoxic
cells (5.7±0.6 in FaDu vs. 4.5±0.6% in FaDu-RR; P=0.119), most
likely due to the small size of the tumors at the time of the
histological analyses (~100–150 mm3) (Fig. 2).
Electrochemotherapy in vivo
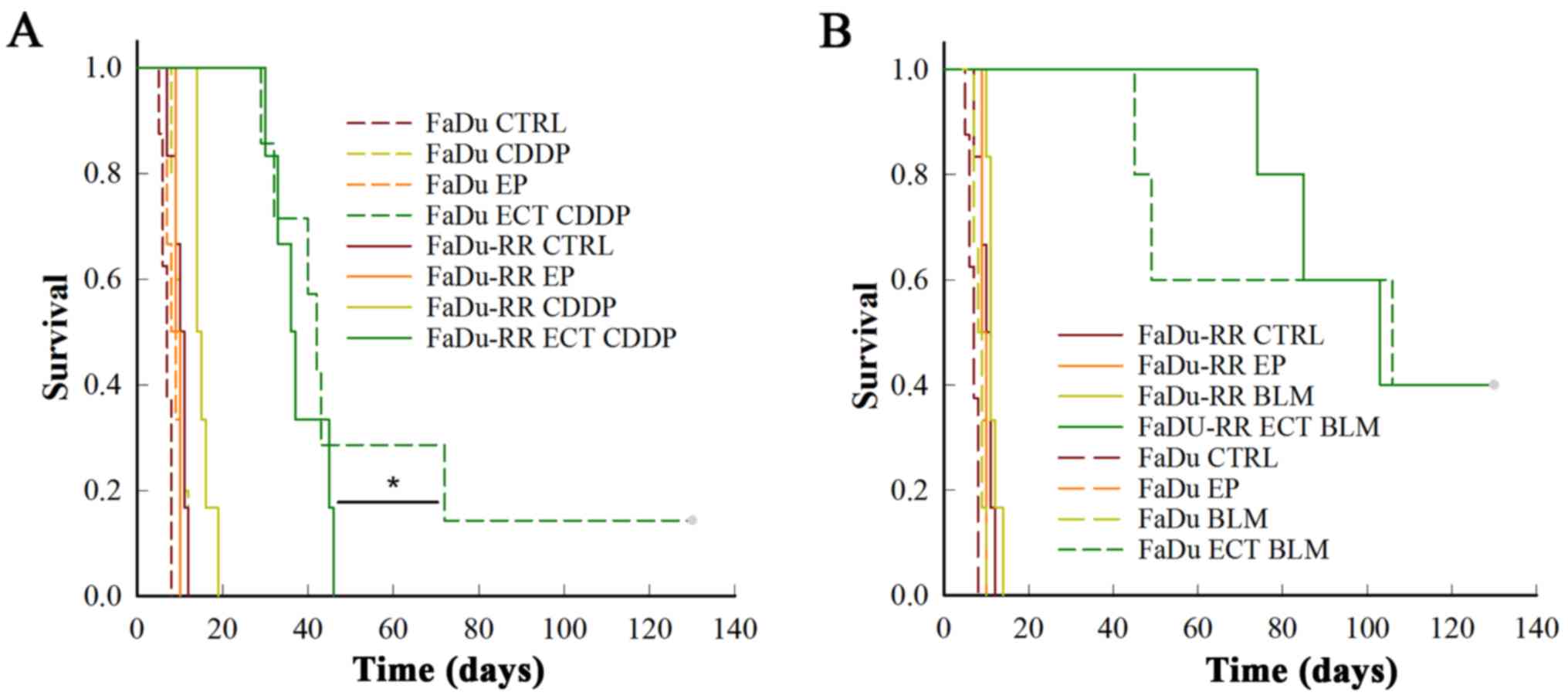
Treatment of FaDu or FaDu-RR tumors with cisplatin
or bleomycin alone had no effect on their growth, due to relatively
low doses of the chemotherapeutics that were used. However, the
FaDu-RR tumors were more resistant to electrochemotherapy with
cisplatin, compared to FaDu tumors: FaDu-RR tumors had shorter nGD
(10.3±0.9 in FaDu-RR, 18.6±3.2 in FaDu; P=0.026) and had no
complete responses (CR), while in FaDu tumors there was 16.7% of CR
(P=0.015) (Fig. 3A).
After electrochemotherapy of radiosensitive FaDu and
radioresistant FaDu-RR tumors with bleomycin, there was no
difference in the survival curves (P=0.900), with 40% of CR and
comparable nGD (30.9±5.8 in FaDu vs. 27.9±2.1 in FaDu-RR tumors;
P=0.787) obtained in the 2 groups (Fig.
3B).
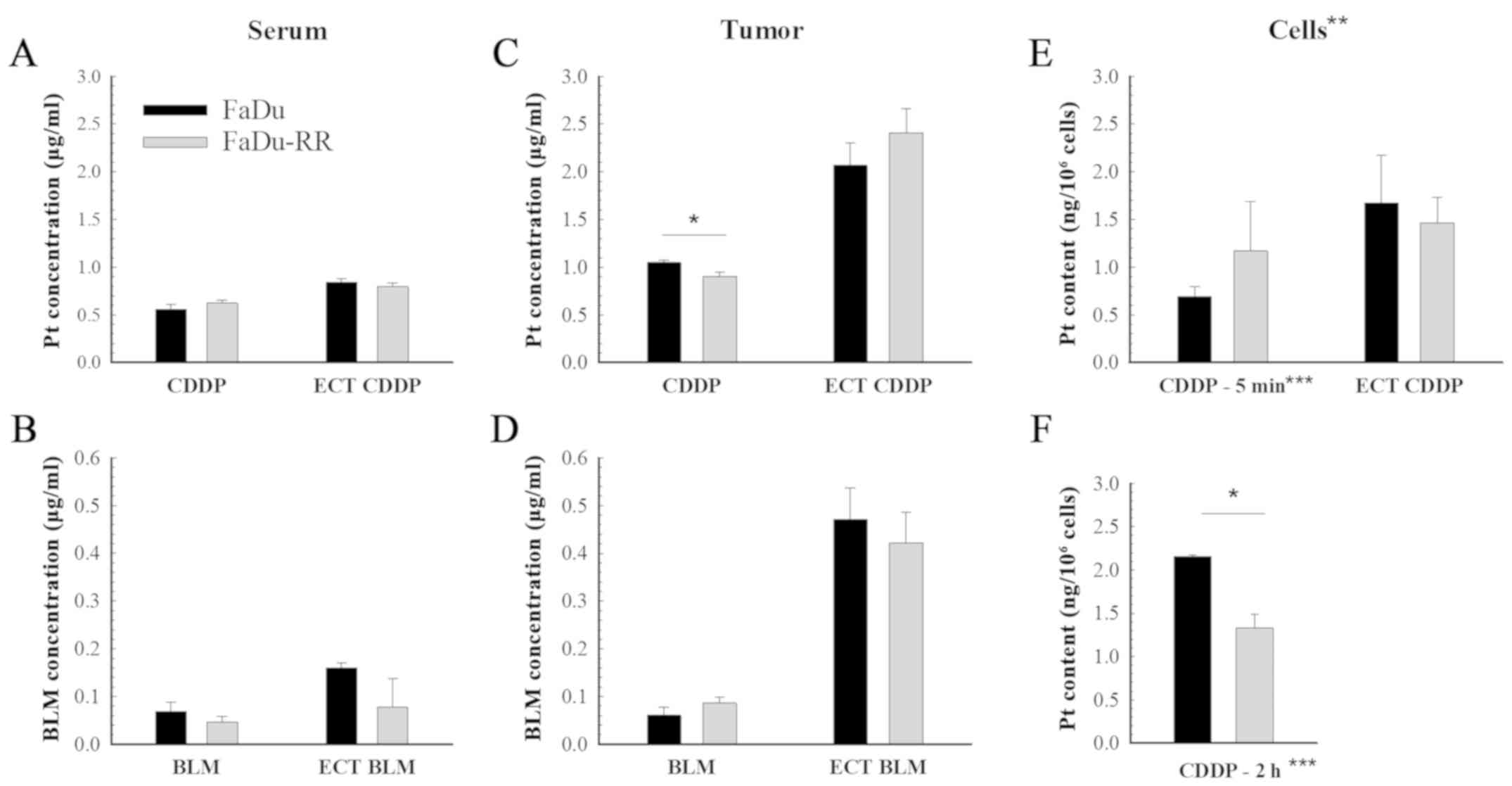
Platinum and bleomycin
accumulation
The content of both chemotherapeutics in the tumors
and serum was measured 1 h after the treatment with chemotherapy
and electrochemotherapy. While there was no difference in platinum
or bleomycin accumulation in the serum of mice with FaDu or FaDu-RR
tumors (Fig. 4A and B), the tumor
uptake of cisplatin was lower in the radioresistant tumors compared
to the radiosensitive ones (1.05±0.02 µg/g in FaDu vs. 0.90±0.04
µg/g in FaDu-RR; P=0.010). Nevertheless, after the
electrochemotherapy, there was no difference in the uptake of
chemotherapeutics between the 2 tumor models: The measured
bleomycin concentration in tumors was 0.47±0.06 µg/g in FaDu and
0.42±0.06 µg/g in FaDu-RR (P=0.602); the platinum concentration was
2.07±0.23 µg/g in FaDu and 2.41±0.25 µg/g in FaDu-RR (Fig. 4C and D). Tumor uptake of bleomycin
was comparable between FaDu and FaDu-RR tumors, either in case of
drug administration only or after adding electric pulses. The
potentiation of the drug content in tumors after application of the
electrical pulses was higher after electrochemotherapy with
bleomycin (7.6-fold in FaDu and 4.7-fold in FaDu-RR) than with
cisplatin (2-fold in FaDu and 2.7-fold in FaDu-RR).
The in vitro experiment (Fig. 4E) showed no difference between the
cell lines in regards to platinum accumulation after a 5-min
exposure to cisplatin (0.69±0.11 ng/106 cells in FaDu
vs. 1.17±0.52 ng/106 cells in FaDu-RR; P=0.210) or after
electrochemotherapy (1.67±0.50 ng/106 cells in FaDu vs.
1.47±0.26 ng/106 cells in FaDu-RR; P=0.734).
With the additional experiment, where the exposure
time to cisplatin alone was prolonged to 2 h (Fig. 4F), the in vivo results were
confirmed, demonstrating lower platinum accumulation in FaDu-RR
cells (2.16±0.02 ng/106 cells in FaDu vs. 1.33±0.16
ng/106 cells in FaDu-RR; P=0.006).
The bleomycin uptake in vitro was not
performed due to insufficient sensitivity of the analytical method
for determination of bleomycin in the cells.
DNA double-strand breaks: Level and
repair rate
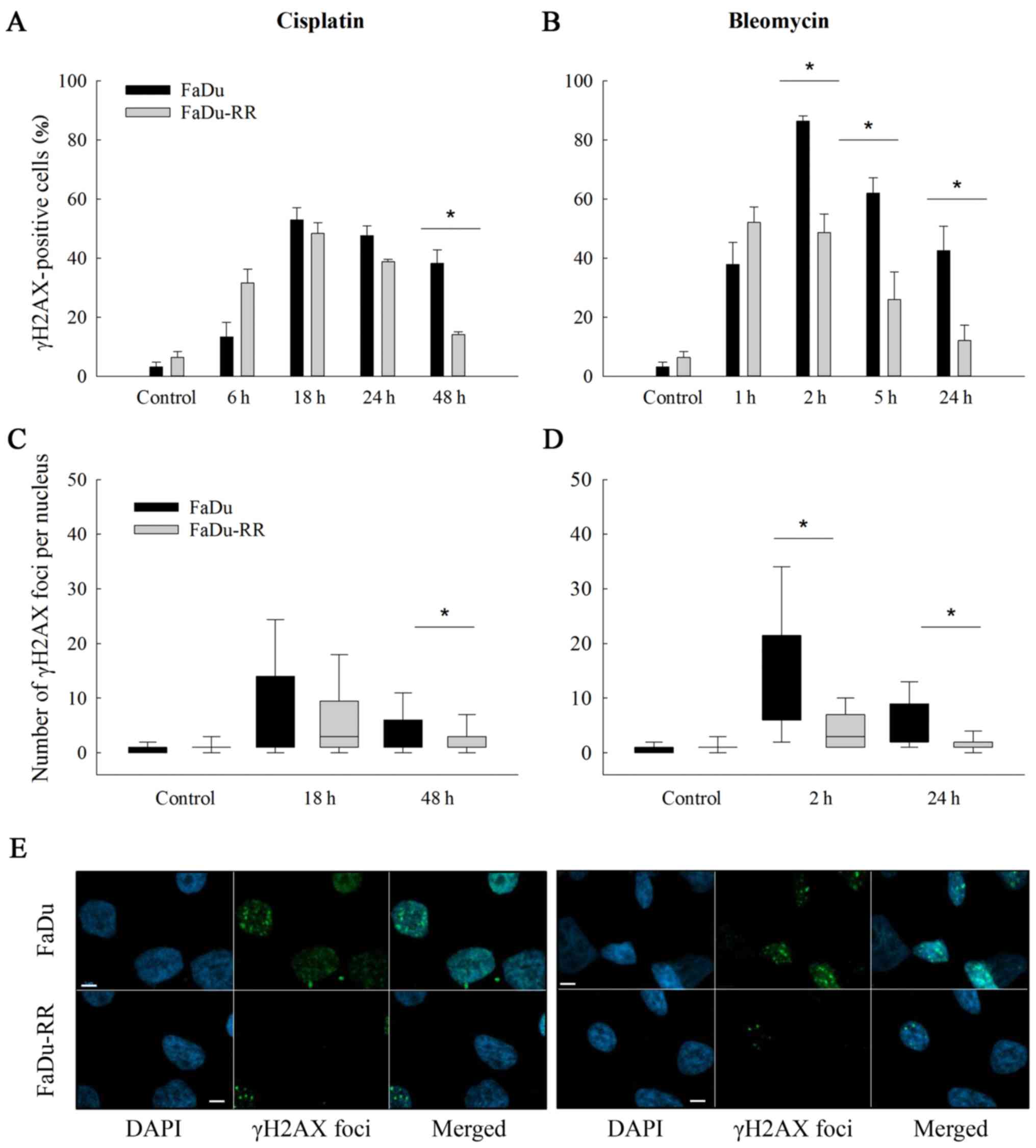
The γ-H2AX foci were determined at several
time-points (i.e., 6–48 h after exposure to cisplatin and 1–24 h
after exposure to bleomycin) with intention to assess the
differences in the repair rate of DNA DSBs. The efficiency of the
repair was evaluated at the last time-point (48 h after cisplatin
and 24 h after bleomycin) as the residual foci indicate the
capacity of cells to repair the DNA damage and consequently cell
survival (37). Our results suggest
that the radioresistant cells repaired DSBs faster and more
effectively than the parental cells, regardless of the drug that
the cells were exposed to. The percentage of γH2AX-positive nuclei
at the last time point after exposure to cisplatin or to bleomycin
was significantly lower in FaDu-RR cells (after cisplatin: 38.2±4.6
in FaDu vs. 14.1±0.9% in FaDu-RR; P=0.007; after bleomycin:
42.5±8.3 in FaDu vs. 12.0±5.2% in FaDu-RR; P=0.036). Furthermore,
the FaDu cells exhibited a higher level of DNA DSBs after exposure
to chemotherapeutics, which was demonstrated by a higher median
number of foci/nucleus in FaDu cells; the highest level of foci was
detected 18 h after exposure to cisplatin (4 foci/nucleus in FaDu
vs. 3 foci/nucleus in FaDu-RR; P=0.128) and 2 h after exposure to
bleomycin (12 foci/nucleus in FaDu vs. 3 foci/nucleus in FaDu-RR;
P<0.001) (Fig. 5C and D).
Discussion
In the present study, we compared the in
vitro and in vivo response of 2 isogenic head and neck
cell lines with different radiosensitivity to electrochemotherapy
with cisplatin or bleomycin to explore the influence of intrinsic
radiosensitivity to the outcome of electrochemotherapy. In
addition, the difference in the DNA-damage response after exposure
to cisplatin or bleomycin, as well as the difference in the uptake
of these drugs, were explored.
The in vivo results indicate that
radioresistant tumors were also resistant to electrochemotherapy
with cisplatin, but were equally sensitive to electrochemotherapy
with bleomycin. Due to the higher complete response rate after
electrochemotherapy with bleomycin than with cisplatin, the results
favor bleomycin over cisplatin-based electrochemotherapy for
treatment of radioresistant tumors and/or tumors that regrow after
radiotherapy.
To explore the role of intrinsic radioresistance of
tumor cells in the response to treatment with electrochemotherapy,
we selected the model where the radioresistant cells were derived
from the parental cells, and selected pharyngeal squamous cell
carcinoma, since many head and neck tumors that are treated by
electrochemotherapy, are pre-irradiated tumors (20,38).
We established an isogenic radioresistant cell subline from the
head and neck FaDu cell line through repeated exposure to
irradiation. The so-called ‘isogenic models of radioresistance’
(39) have already been widely used
to study molecular response to irradiation in numerous human cancer
cell lines where the selection of a radioresistant subline was
achieved through repeated exposure of parental cells to
fractionated irradiation of variable overall total dose and
treatment time (40–44). Several differences have been
observed in radioresistant sublines compared to their parental cell
lines, including higher cellular levels of glutathione (45), reduced induction of apoptosis
(46) and increased ability to
repair DNA damage (47).
Furthermore, cross-resistance with DNA-damaging agents, especially
with cisplatin, was observed (42,46,48).
Addiitonally, in the clinic, observations indicate that development
of radioresistance correlates with the chemoresistance of the
tumors and vice versa. Moreover, the tumor response to induction
chemotherapy is considered the most reliable in vivo assay
of tumor chemosensitivity and radiosensitivity (or resistance)
currently available in the clinical setting (49). The in vitro results of this
study are in line with these observations. While a significant
resistance of the radioresistant subline to cisplatin was observed,
there was no difference between the cells in sensitivity to
bleomycin. Moreover, the repair of DNA double-strand breaks after
exposure to cisplatin or bleomycin was faster and more effective in
the radioresistant subline, regardless of the agent they were
exposed to. The explanation why the latter does not correlate with
the cell survival after exposure to bleomycin may lie in different
drug mechanisms of action that may be involved (50–52).
We must point out that the aim of γH2AX immunofluorescent staining
was to assess the difference between the 2 cell lines in the amount
and repair-rate of DNA double-strand breaks after exposure to
chemotherapeutics, used in electrochemotherapy. Since the
electroporation (as a delivery system) only facilitates the passage
of the drugs into the cells and does not interfere with the action
of the drug on the DNA, we avoided adding the electroporation in
this experiment.
When the exposure to cisplatin was combined with
electrical pulses (electrochemotherapy), the difference in response
between the cell lines was less obvious and not statistically
significant. One of the possible reasons for this observation could
be the facilitated passage of the drug through the cell membrane
during the electroporation. Our results of the cisplatin uptake
indicate that the radioresistant cells develop mechanisms to efflux
cisplatin, influencing either active transport or passive diffusion
(53,54). The application of electrical pulses
overcame this problem, as the uptake of cisplatin in radioresistant
cells after electrochemotherapy is comparable to the uptake in the
parental cells.
In vivo, the tumors established from the head
and neck isogenic cell lines differed in histological
characteristics. Radioresistant tumors were less vascularized and
had lower proliferation rate, which can both adversely influence
the response to salvage non-surgical therapies. Furthermore, the
tumor models differed in response to electrochemotherapy. The
radiosensitive tumors responded better to electrochemotherapy with
cisplatin than the radioresistant ones. On the other hand, there
was no difference between the tumor models in response to
electrochemotherapy with bleomycin. Electrochemotherapy with
bleomycin was in fact more effective than with cisplatin in both
tumor models, but the difference was greater in FaDu-RR tumors,
since they were more resistant to electrochemotherapy with
cisplatin than FaDu tumors and equally sensitive to
electrochemotherapy with bleomycin.
However, we must point out that the difference in
response between the 2 tumor models was small and biologically not
significant (there was no CR in radioresistant tumors and only one
CR in radiosensitive tumors). Because the 2 tumor models did not
differ substantially in tumorigenicity, indeed only a small or no
difference was expected (43). The
intracellular accumulation of cisplatin or bleomycin after
electrochemotherapy was similar between the tumor models,
indicating that the observed cisplatin-resistance of radioresistant
tumors to electrochemotherapy is not due to the drug uptake, but
rather relates to intrinsic mechanisms, such as more efficient DNA
double-strand break repair, as indicated by faster resolution of
γH2AX foci.
The specific histological characteristics of
radioresistant tumors, such as the altered vasculature and
distribution of the components of the extracellular matrix, may
also contribute to the differential response to electrochemotherapy
with cisplatin (55). Moreover,
there are other possible mechanisms of cisplatin resistance that
were not addressed in this study, such as intracellular
inactivation of cisplatin by thiol-containing molecules, specific
DNA-repair pathway mechanisms (especially the nucleotide excision
repair and mismatch repair), altered apoptosis induction and
epithelial-mesenchymal transition (EMT) of the cells after
fractionated irradiation (56–58).
In this study, we focused only on the intrinsic
resistance of tumor cells to electrochemotherapy. However, the
differences in the response rate of pre-irradiated tumors to
non-surgical treatment modalities could also be the result of
changes in the tumor microenvironment after irradiation that may
promote tumor invasion and spread through the effects on tumor
vasculature, stroma and immune system (22). The most likely reason for the worse
outcome of previously irradiated tumors treated by
electrochemotherapy in patients, could be the radiotherapy-induced
damaged vasculature and proliferation of fibrous tissue in the
tumor bed that could compromise the chemotherapeutic delivery in
sufficient concentration to the site of electroporation (20,59).
The data in the literature, though, do not report on the vascular
lock after the delivery of electric pulses, which in the case of
hampered tumor vascularization after irradiation could be less
expressed.
There are limitations of the present study that
should be addressed. As described above, only intrinsic
radioresistance of the cells was evaluated. Due to the nature of
the tumor cells, the xenografts could only be induced in
immuno-compromised SCID mice; consequently, the adaptive immune
system that could also play a role in response to
electrochemotherapy, was excluded. Exploring the role of the immune
system in the response of such tumors to electrochemotherapy would
demand a different design of the study, performing other or
additional experiments (4,5,60). As
this study deals mainly with intrinsic radioresistance of tumor
cells, including these experiments would be beyond the scope of the
research.
In order to fully understand the impact of previous
irradiation of the tumors on the response to salvage
electrochemotherapy, further studies should be employed, such as
experiments on in vivo established recurrent tumors after
previous (chemo)radiotherapy in immuno-competent mice where the
tumor bed effect and immune response could be evaluated as well.
Unfortunately, there is currently no similar murine tumor model
(39).
In conclusion, our pre-clinical study of
radioresistant head and neck tumor model confirms that intrinsic
radioresistance of tumor cells significantly affects the outcome of
treatment of such tumors to electrochemotherapy with cisplatin, but
not to electrochemotherapy with bleomycin. Due to the higher
complete response rate obtained after electrochemotherapy with
bleomycin compared to cisplatin, the results favor bleomycin over
cisplatin-based electrochemotherapy for treatment of radioresistant
tumors and/or tumors that regrow after radiotherapy.
Acknowledgements
The authors would like to thank Mira Lavric
(Institute of Oncology Ljubljana, Slovenia) for her help with the
cell culturing, Ilija Vojvodic (Institute of Oncology Ljubljana,
Slovenia) for his contribution with the irradiation of the cells,
Andreja Brozic (Institute of Oncology Ljubljana, Slovenia) for her
help with flow cytometric measurements, Maja Ota (Institute of
Oncology Ljubljana, Slovenia) for her help with histological
staining and Barbara Staresinic (Institute of Oncology Ljubljana,
Slovenia) for her help with the imaging on confocal microscope.
Funding
The present study was financially supported by the
Slovenian Research Agency (program nos. P3-0003 and P3-0307) and
performed within the scope of LEA-EBAM (French-Slovenian European
Associated Laboratory: Pulsed Electric Fields Applications in
Biology and Medicine).
Availability of data and materials
The data that support the findings of this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MNZ contributed to the writing of the manuscript,
performed the experiments and the statistical analysis; AP and SK
performed the in vivo experiments, platinum and bleomycin
accumulation; AP contributed to the protocol for membrane
electropermeabilization measurement with PI; VT contributed to the
establishment of the radioresistant subline; VT and BG contributed
to the protocol for γH2AX immunofluorescent staining; MS performed
the histological analysis of the tumors; MC, PS and GS conceived
the study and wrote the manuscript; JS and TK contributed to the
analysis of platinum and bleomycin accumulation. All the authors
discussed the results and contributed to the review and writing of
the final manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiments were approved by the Ministry
of Agriculture, Forestry and Food of the Republic of Slovenia,
permit no. U34401-1/2015/16, and are in compliance with the
standards required by the UKCCCR guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yarmush ML, Golberg A, Serša G, Kotnik T
and Miklavčič D: Electroporation-based technologies for medicine:
Principles, applications and challenges. Annu Rev Biomed Eng.
16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mir LM, Orlowski S, Belehradek J Jr,
Teissie J, Rols PM, Sersa G, Miklavcic D, Gilbert R and Heller R:
Biomedical applications of electric pulses with special emphasis on
antitumor electrochemotherapy. Bioelectrochem Bioenerg. 38:203–207.
1995. View Article : Google Scholar
|
|
3
|
Sersa G, Bosnjak M, Cemazar M and Heller
R: Preclinical studies on electrochemotherapy. Handbook of
Electroporation. Miklavčič D: Springer International Publishing;
Cham, Switzerland: pp. 2153–2169. 2017
|
|
4
|
Calvet CY, Famin D, André FM and Mir LM:
Electrochemotherapy with bleomycin induces hallmarks of immunogenic
cell death in murine colon cancer cells. Oncoimmunology.
3:e281312014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Gennaro P, Gerlini G, Urso C, Sestini
S, Brandani P, Pimpinelli N and Borgognoni L:
CD4+FOXP3+ T regulatory cells decrease and
CD3+CD8+ T cells recruitment in TILs from
melanoma metastases after electrochemotherapy. Clin Exp Metastasis.
33:787–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sersa G, Jarm T, Kotnik T, Coer A,
Podkrajsek M, Sentjurc M, Miklavcic D, Kadivec M, Kranjc S, Secerov
A, et al: Vascular disrupting action of electroporation and
electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer.
98:388–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mali B, Jarm T, Snoj M, Sersa G and
Miklavcic D: Antitumor effectiveness of electrochemotherapy: A
systematic review and meta-analysis. Eur J Surg Oncol. 39:4–16.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertino G, Sersa G, De Terlizzi F, Occhini
A, Plaschke CC, Groselj A, Langdon C, Grau JJ, McCaul JA, Heuveling
D, et al: European research on electrochemotherapy in head and neck
cancer (EURECA) project: Results of the treatment of skin cancer.
Eur J Cancer. 63:41–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plaschke CC, Bertino G, McCaul JA, Grau
JJ, de Bree R, Sersa G, Occhini A, Groselj A, Langdon C, Heuveling
DA, et al: European Research on Electrochemotherapy in Head and
Neck Cancer (EURECA) project: Results from the treatment of mucosal
cancers. Eur J Cancer. 87:172–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spratt DE, Gordon Spratt EA, Wu S, DeRosa
A, Lee NY, Lacouture ME and Barker CA: Efficacy of skin-directed
therapy for cutaneous metastases from advanced cancer: A
meta-analysis. J Clin Oncol. 32:3144–3155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mevio N, Bertino G, Occhini A, Scelsi D,
Tagliabue M, Mura F and Benazzo M: Electrochemotherapy for the
treatment of recurrent head and neck cancers: Preliminary results.
Tumori. 98:308–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Groselj A, Kos B, Cemazar M, Urbancic J,
Kragelj G, Bosnjak M, Veberic B, Strojan P, Miklavcic D and Sersa
G: Coupling treatment planning with navigation system: A new
technological approach in treatment of head and neck tumors by
electrochemotherapy. Biomed Eng Online. 14 Suppl 3:S22015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seccia V, Muscatello L, Dallan I,
Bajraktari A, Briganti T, Ursino S, Galli L, Falcone A and
Sellari-Franceschini S: Electrochemotherapy and its controversial
results in patients with head and neck cancer. Anticancer Res.
34:967–972. 2014.PubMed/NCBI
|
|
14
|
De Virgilio A, Ralli M, Longo L, Mancini
P, Attanasio G, Atturo F, De Vincentiis M and Greco A:
Electrochemotherapy in head and neck cancer: A review of an
emerging cancer treatment (Review). Oncol Lett. 16:3415–3423.
2018.PubMed/NCBI
|
|
15
|
Landström F, Ivarsson M, Von Sydow AK,
Magnuson A, Von Beckerath M and Möller C:
Electrochemotherapy-evidence for cell-type selectivity in vitro.
Anticancer Res. 35:5813–5820. 2015.PubMed/NCBI
|
|
16
|
Frandsen SK and Gehl J: A review on
differences in effects on normal and malignant cells and tissues to
electroporation-based therapies: A focus on calcium
electroporation. Technol Cancer Res Treat. 17:1533033818788072018.
View Article : Google Scholar
|
|
17
|
Plaschke CC, Gothelf A, Gehl J and Wessel
I: Electrochemotherapy of mucosal head and neck tumors: A
systematic review. Acta Oncol. 55:1266–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mali B, Miklavcic D, Campana LG, Cemazar
M, Sersa G, Snoj M and Jarm T: Tumor size and effectiveness of
electrochemotherapy. Radiol Oncol. 47:32–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kunte C, Letulé V, Gehl J, Dahlstroem K,
Curatolo P, Rotunno P, Muir T, Occhini A, Bertino G, Powell B, et
al: Electrochemotherapy in the treatment of metastatic malignant
melanoma: A prospective cohort study by InspECT. Br J Dermatol.
176:1475–1485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campana LG, Mali B, Sersa G, Valpione S,
Giorgi CA, Strojan P, Miklavcic D and Rossi CR: Electrochemotherapy
in non-melanoma head and neck cancers: A retrospective analysis of
the treated cases. Br J Oral Maxillofac Surg. 52:957–964. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campana LG, Testori A, Curatolo P,
Quaglino P, Mocellin S, Framarini M, Borgognoni L, Ascierto PA,
Mozzillo N, Guida M, et al: Treatment efficacy with
electrochemotherapy: A multi-institutional prospective
observational study on 376 patients with superficial tumors. Eur J
Surg Oncol. 42:1914–1923. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krause M, Dubrovska A, Linge A and Baumann
M: Cancer stem cells: Radioresistance, prediction of radiotherapy
outcome and specific targets for combined treatments. Adv Drug
Deliv Rev. 109:63–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peitzsch C, Kurth I, Kunz-Schughart L,
Baumann M and Dubrovska A: Discovery of the cancer stem cell
related determinants of radioresistance. Radiother Oncol.
108:378–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stimac M, Dolinsek T, Lampreht U, Cemazar
M and Sersa G: Gene electrotransfer of plasmid with tissue specific
promoter encoding shRNA against endoglin exerts antitumor efficacy
against murine TS/A tumors by vascular targeted effects. PLoS One.
10:e01249132015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dolinsek T, Prosen L, Cemazar M, Potocnik
T and Sersa G: Electrochemotherapy with bleomycin is effective in
BRAF mutated melanoma cells and interacts with BRAF inhibitors.
Radiol Oncol. 50:274–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prevc A, Bedina Zavec A, Cemazar M,
Kloboves-Prevodnik V, Stimac M, Todorovic V, Strojan P and Sersa G:
Bystander effect induced by electroporation is possibly mediated by
microvesicles and dependent on pulse amplitude, repetition
frequency and cell type. J Membr Biol. 249:703–711. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sersa G, Miklavcic M, Cemazar M,
Belehradek J Jr, Jarm T and Mir LM: Electrochemotherapy with CDDP
on LPB sarcoma: Comparison of the anti-tumor effectiveness in
immunocompetent and immunodefficient mice. Bioelectrochem Bioenerg.
43:279–283. 1997. View Article : Google Scholar
|
|
29
|
Cemazar M, Miklavcic D and Sersa G:
Intrinsic sensitivity of tumor cells to bleomycin as an indicator
of tumor response to electrochemotherapy. Jpn J Cancer Res.
89:328–333. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Begg A: Principles and practices of the
tumor growth delay assay. Rodent Tumor Models in Experimental
Cancer Therapy. Kallman RF: Pergamon Press; New York, NY: pp.
114–121. 1987
|
|
31
|
Kranjc S, Kranjc M, Scancar J, Jelenc J,
Sersa G and Miklavcic D: Electrochemotherapy by pulsed
electromagnetic field treatment (PEMF) in mouse melanoma B16F10 in
vivo. Radiol Oncol. 50:39–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cemazar M, Miklavcic D, Scancar J, Dolzan
V, Golouh R and Sersa G: Increased platinum accumulation in SA-1
tumour cells after in vivo electrochemotherapy with cisplatin. Br J
Cancer. 79:1386–1391. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kranjc S, Cemazar M, Sersa G, Scancar J
and Grabner S: In vitro and in vivo evaluation of
electrochemotherapy with trans-platinum analogue
trans-[PtCl2(3-Hmpy)2]. Radiol Oncol.
51:295–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinčič A, Cemazar M, Sersa G, Kovač V,
Milačič R and Ščančar J: A novel method for speciation of Pt in
human serum incubated with cisplatin, oxaliplatin and carboplatin
by conjoint liquid chromatography on monolithic disks with UV and
ICP-MS detection. Talanta. 116:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kosjek T, Krajnc A, Gornik T, Zigon D,
Groselj A, Sersa G and Cemazar M: Identification and quantification
of bleomycin in serum and tumor tissue by liquid chromatography
coupled to high resolution mass spectrometry. Talanta. 160:164–171.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open source platform for biological image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Banáth JP, Klokov D, MacPhail SH, Banuelos
CA and Olive PL: Residual gammaH2AX foci as an indication of lethal
DNA lesions. BMC Cancer. 10:42010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Landström FJ, Reizenstein J, Adamsson GB,
Von Beckerath M and Möller C: Long-term follow-up in patients
treated with curative electrochemotherapy for cancer in the oral
cavity and oropharynx. Acta Otolaryngol. 135:1070–1078. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mcdermott N, Meunier A, Lynch TH,
Hollywood D and Marignol L: Isogenic radiation resistant cell
lines: Development and validation strategies. Int J Radiat Biol.
90:115–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Borràs-Fresneda M, Barquinero JF, Gomolka
M, Hornhardt S, Rössler U, Armengol G and Barrios L: Differences in
DNA repair capacity, cell death and transcriptional response after
irradiation between a radiosensitive and a radioresistant cell
line. Sci Rep. 6:270432016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukuda K, Sakakura C, Miyagawa K, Kuriu Y,
Kin S, Nakase Y, Hagiwara A, Mitsufuji S, Okazaki Y, Hayashizaki Y,
et al: Differential gene expression profiles of radioresistant
oesophageal cancer cell lines established by continuous
fractionated irradiation. Br J Cancer. 91:1543–1550. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gomez-Casal R, Epperly MW, Wang H, Proia
DA, Greenberger JS and Gomez-Casal VL: Radioresistant human lung
adenocarcinoma cells that survived multiple fractions of ionizing
radiation are sensitive to HSP90 inhibition. Oncotarget.
6:44306–44322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kurth I, Hein L, Mäbert K, Peitzsch C, Koi
L, Cojoc M, Kunz-Schughart L, Baumann M and Dubrovska A: Cancer
stem cell related markers of radioresistance in head and neck
squamous cell carcinoma. Oncotarget. 6:34494–34509. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Llobet LI, Baro M, Figueras A, Modolell
I, Da Silva MV, Muñoz P, Navarro A, Mesia R and Balart J:
Development and characterization of an isogenic cell line with a
radioresistant phenotype. Clin Trans Oncol. 15:189–197. 2013.
View Article : Google Scholar
|
|
45
|
Lynam-Lennon N, Reynolds JV, Pidgeon GP,
Lysaght J, Marignol L and Maher SG: Alterations in DNA repair
efficiency are involved in the radioresistance of esophageal
adenocarcinoma. Radiat Res. 174:703–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xie L, Song X, Yu J, Wei L, Song B, Wang X
and Lv L: Fractionated irradiation induced radio-resistant
esophageal cancer EC109 cells seem to be more sensitive to
chemotherapeutic drugs. J Exp Clin Cancer Res. 28:682009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mihatsch J, Toulany M, Bareiss PM, Grimm
S, Lengerke C, Kehlbach R and Rodemann HP: Selection of
radioresistant tumor cells and presence of ALDH1 activity in vitro.
Radiother Oncol. 99:300–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eichholtz-Wirth H, Reidel G and Hietel B:
Radiation-induced transient cisplatin resistance in murine
fibrosarcoma cells associated with elevated metallothionein
content. Br J Cancer. 67:1001–1006. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Strojan P, Haigentz M Jr, Bradford CR,
Wolf GT, Hartl DM, Langendijk JA, Rinaldo A, Eisbruch A, Mendenhall
WM, Forastiere AA, et al: Chemoradiotherapy vs. total laryngectomy
for primary treatment of advanced laryngeal squamous cell
carcinoma. Oral Oncol. 49:283–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hecht SM: Bleomycin: New perspectives on
the mechanism of action. J Nat Prod. 63:158–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Q, Cui K, Espin-Garcia O, Cheng D,
Qiu X, Chen Z, Moore M, Bristow RG, Xu W, Der S, et al: Resistance
to bleomycin in cancer cell lines is characterized by prolonged
doubling time, reduced DNA damage and evasion of G2/M arrest and
apoptosis. PLoS One. 8:e823632013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zuckerman JE, Raffin TA, Brown JM, Newman
RA, Etiz BB and Sikic BI: In vitro selection and characterization
of a bleomycin-resistant subline of B16 melanoma. Cancer Res.
46:1748–1753. 1986.PubMed/NCBI
|
|
53
|
Gately DP and Howell SB: Cellular
accumulation of the anticancer agent cisplatin: A review. Br J
Cancer. 67:1171–1176. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kilari D, Guancial E and Kim ES: Role of
copper transporters in platinum resistance. World J Clin Oncol.
7:106–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mesojednik S, Pavlin D, Sersa G, Coer A,
Kranjc S, Grosel A, Tevz G and Cemazar M: The effect of the
histological properties of tumors on transfection efficiency of
electrically assisted gene delivery to solid tumors in mice. Gene
Ther. 14:1261–1269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fuertes MA, Alonso C and Pérez JM:
Biochemical modulation of cisplatin mechanisms of action:
Enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2002. View Article : Google Scholar
|
|
58
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Milas L, Ito H, Hunter N, Jones S and
Peters LJ: Retardation of tumor growth in mice caused by
radiation-induced injury of tumor bed stroma: Dependency on tumor
type. Cancer Res. 46:723–727. 1986.PubMed/NCBI
|
|
60
|
Falk H, Lambaa S, Johannesen HH, Wooler G,
Venzo A and Gehl J: Electrochemotherapy and calcium electroporation
inducing a systemic immune response with local and distant
remission of tumors in a patient with malignant melanoma-a case
report. Acta Oncol. 56:1126–1131. 2017. View Article : Google Scholar : PubMed/NCBI
|