Introduction
Reactive oxygen species (ROS) are generated by
tightly regulated enzymes during normal cellular metabolic
processes, and are known to convert deoxyribonucleoside
triphosphates (dNTPs) into oxidised DNA bases in the dNTP pool when
they are overproduced (1–4). The damaged nucleotides are integrated
into the DNA double chains during the process of replication,
resulting in DNA structural damage that may ultimately lead to
apoptosis (5–7). MutT homolog 1 (MTH1) can hydrolyse the
oxidised forms of dGTP (8–10), thus, preventing 8-oxo-dGTP from
being incorporated into DNA and leading to G to T transversions
(11). The production and
elimination of ROS is tightly regulated in normal cells during
cellular metabolism, thus the role of MTH1 is non-essential for
normal cell survival (12,13). Unlike normal cells, tumours produce
high levels of oxidants due to chronically hyperactivated mitogenic
and pro-survival signalling, as well as metabolic alterations, and
have a greater reliance on MTH1 function. Thus, hMTH1 may play a
crucial role in the survival of many cancers, such as melanoma and
breast cancer (3,14), and the expression level of hMTH1 is
positively correlated with the malignant degree of lung cancer and
gastric carcinoma (15,16). Data were published in 2014 lending
further support to the hypothesis that MTH1 has a role in the
prevention of DNA damage and cancer cell survival. It was shown
that MTH1 inhibitors, such as TH287, TH588 and (S)-crizotinib, were
sufficient to produce DNA breaks and induce tumour suppressor
responses (13,17). Researchers have expressed
recombinant human MTH1 protein in Escherichia coli for
crystallisation (18–20) and inhibition experiments (13,17,21).
However, there have been few reports on the optimised expression of
hMTH1, as well as its purity and enzymatic activity, which are key
factors for inhibitor screening and the functional analysis of
hMTH1 protein. In addition, to generate an anti-hMTH1 specific mAb,
highly bioactive hMTH1 is required.
Since Köhler and Milstein (22) proposed the method of hybridoma
technology and prepared mouse monoclonal antibodies, many
antibodies specific to various antigens have been obtained
(23). They have applications in
the diagnosis and therapy of cancer and infectious diseases. At
present, the available therapeutic anticancer mAbs recognise
extracellular or cell surface proteins. However, most tumour
targets are nuclear or cytoplasmic tumour-associated proteins.
Therefore, generating therapeutic mAbs that recognise intracellular
tumour antigens will facilitate possible cancer target selection
and enhance therapeutic potency. Various efficient and safe methods
of delivering an antibody into a living cell have been introduced,
such as cell-penetrating peptides (CPPs), micelles, liposomes and
even pH-sensitive antibodies (24–27).
In our experiment, we optimised the gene sequence
for optimal expression in E. coli, and the optimal induction
conditions of OD600, inducer concentration, temperature
and time were obtained. The rhMTH1 was verified to have high purity
by high-performance liquid chromatography (HPLC) analysis. A series
of assays were used to determine the enzymatic activity of MTH1. We
injected soluble hMTH1 protein into mice as an antigen and produced
an anti-hMTH1 mAb. We showed that the anti-hMTH1 mAb had a high
binding affinity for hMTH1 by western blotting, ELISA and an
immunofluorescence assay. In summary, the bioactive rhMTH1 was
suitable for the selection of hMTH1 inhibitors and the MTH1 mAb may
be a novel tool for the detection of hMTH1 in tumour cells, with
potential for further functional investigation and clinical
applications.
Materials and methods
Strains, plasmids and culture
medium
The E. coli Transetta (DE3) chemically
competent cells were purchased from TransGen Biotechnology Co.,
Ltd. (Beijing, China). NcoI, EcoRI and T4 DNA ligases
were purchased from New England Biolabs, Ltd. (Beijing, China).
Isopropyl-β-D-thiogalactopyranoside (IPTG), kanamycin, malachite
green and ammonium molybdate were obtain from Sangon Biotechnology
Co., Ltd. (Shanghai, China). The dGTP and inorganic pyrophosphatase
were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Anti-His mouse monoclonal antibody (cat. no. D191001) and
goat anti-mouse IgG conjugated to horseradish peroxidase (cat. no.
D110087) were purchased from Sangon Biotechnology Co., Ltd.
Six-week-old female BALB/c mice were obtained from the College of
Animal Science and Technology Yangzhou University (Yangzhou,
China). Freund's adjuvant, bovine serum albumin (BSA), polyethylene
glycol (PEG4000, 50% w/v), hypoxanthine/aminopterin/thymidine (HAT)
and hypoxanthine/thymidine (HT) were all from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were from Invitrogen (Thermo
Fisher Scientific, Inc.). Mouse monoclonal antibody subtype
identification kit was purchased by ProteinTech Group Inc. (Wuhan,
China). The anti-mouse IgG (cat. no. A7028), FITC-conjugated
goat-anti-mouse IgG (cat. no. A0568) and DAPI were from Beyotime
Institute of Biotechnology (Shanghai, China). The MCF-7 cell line
and SP2/0 myeloma cell line was purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA).
Construction of recombinant hMTH1
expression plasmids
The hMTH1 sequence (GenBank accession number
D38594.2) was optimised by Nanjing GenScript Co., Ltd., (Nanjing,
China) to adapt the E. coli expression system, and the
GenBank accession number of the optimised sequence was MH193376.
The hMTH1 gene was amplified by PCR using forward
(5′-CCATGGGCATGGGTGCA-3′) and reverse primers
(5′-GAATTCGACCGTATCAACTTCG-3′), cleaved with
NcoI/EcoRI restriction enzymes, then ligated with
pET-280a plasmids to generate a series of recombinant expression
vectors. The constructed vectors were transformed into E.
coli Transetta (DE3) chemically competent cells, and the
negative control was pET28a in this experiment. The positive clones
were confirmed by colony PCR using the T7 forward and the reverse
primers.
Optimised expression and purification
of hMTH1 protein
For protein expression, pET-28a-MTH1/Transetta (DE3)
single bacterial colonies were cultured in LB medium supplemented
with 100 µg/ml kanamycin at 37°C in a shaking incubator at 200 rpm
until the OD600 of the culture medium reached 0.5. IPTG
(0.4 mM) was then added to the culture medium and incubated at 25°C
and 160 rpm for 12 h. The culture was centrifuged at 5,938 × g for
15 min, and the pellet of induced recombinant strains was
resuspended in cell lysis buffer (50 mM Tris, 500 mM NaCl, 5 mM
DTT, 0.1‰ PMSF; pH 7.5) and then placed in an ice bath for
ultrasonic lysis (Ningbo Scientz Biotechnology Co., Ltd., Ningbo,
China) for 30 min (250 Watts, 5 sec, 5 sec). The strain cell lysate
was centrifuged at 1,3361 × g for 20 min to separate the
precipitate and soluble parts. Supernatant and pellet were
subjected to 12% SDS-PAGE electrophoresis to assess the expression
levels and solubility of the recombinant protein.
To improve the soluble expression of rhMTH1 protein,
the induction conditions of the initial strain OD600
value (0.5/0.8), induction temperature (25/20/16°C), IPTG
concentration (0.1/0.2/0.4 mM) and induction time (12/16/20 h) were
optimised. The supernatant and precipitate of rhMTH1 (15 µl/lane)
at different conditions were detected by 12% SDS-PAGE and Tanon
Image software 1.0 (Tanon Science and Technology, Co., Ltd.,
Shanghai, China) was used for semi-quantitative analysis of the
percentage fraction of soluble rhMTH1 by densitometry. In this
experiment, the percentage of soluble rhMTH1 in the total protein
was calculated.
hMTH1 was purified using an Ni-NTA column (GE
Healthcare, Chicago, IL, USA) and eluted by different
concentrations of imidazole (50, 100 and 250 mM, respectively). The
target sample was identified by 12% SDS-PAGE and concentrated to 1
ml by ultrafiltration. SDS-certified samples were further purified
by Sephadex G-50 Gel filtration chromatography (GE Healthcare). The
LC system connected to the Ni-NTA and gel filtration columns was
provided by Nanjing University Puyang Institute of Scientific
Instruments (Nanjing, China). After adding the sample to a G-50
column, it was eluted by Tris-HCl elution buffer (25 mM Tris, 75 mM
NaCl and 10% glycerine) at a flow rate of 0.07 ml/min, and the
collected solution was detected by 12% SDS-PAGE.
Western blot analysis
For western blotting, rhMTH1 protein was separated
by 12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes using a wet transfer system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 300 mA for 30 min. The membrane was blocked
with 5% non-fat dried milk diluted in TBST at 37°C for 2 h and
incubated with anti-His antibody (dilution 1:5,000) in TBST plus 3%
non-fat milk at 4°C overnight, followed by horseradish peroxidase
(HRP)-conjugated goat anti-mouse antibody (dilution 1:5,000) for 2
h at 37°C. Visualisation via enhanced chemiluminescence was
performed in a gel imager (Tanon Science and Technology, Co.,
Ltd.).
To analyse the specificity of the hMTH1 mAb by
western blotting as described above, the prepared anti-hMTH1 mAb
was used as the primary antibody.
Purity and yield determination of
recombinant hMTH1
The purity of the sample was determined via
high-performance liquid chromatography (HPLC; Shimadzu Corp.,
Tokyo, Japan). Tris-HCl buffer in the sample was replaced with 0.05
mol/l phosphate buffer (25 mM NaHPO4, 25 mM
NaH2PO4, 300 mM NaCl; pH 6.7) for HPLC
analysis by concentration and dilution. A total of 20 µl sample
(20.1 mg/ml) was loaded on the TSK-GW-4000 column (Tosoh Corp.,
Tokyo, Japan) and eluted with 0.05 mol/l phosphate buffer at a
speed of 0.8 ml/min.
The concentration of total soluble protein was
determined by bicinchoninic acid (BCA) assay and the purity of the
soluble rhMTH1 in the total soluble protein was assessed by HPLC or
SDS-PAGE.
Enzymatic activity determination
The enzymatic assay was based on the hydrolysis
reaction of dGTP catalysed by recombinant hMTH1 protein to form
dGMP and pyrophosphate. The inorganic phosphates of the enzyme
coupling reaction product can be quantitatively determined after an
excess of pyrophosphatase is added to the assay. The absorbance of
inorganic phosphate was measured with malachite green (82.2 µM
malachite green, 0.364 mM ammonium molybdate, 17% concentrated
sulfuric acid, 0.17% Tween-20) at OD630 nm (28).
The half-maximal effective concentration
(EC50) of hMTH1 was determined by malachite green
measurement. Human MTH1 was diluted in assay buffer (100 mM
Tris-acetate, 40 mM sodium chloride, 10 mM magnesium acetate, 1 mM
DTT and 0.005% Tween-20; pH 7.5) to generate eight different
concentrations (0, 0.006, 0.015, 0.045, 0.075, 0.12, 0.18 and 0.24
nM). In the assay, rhMTH1 (0-0.24 nM) and assay buffer were added
to 96-well plates and incubated with shaking for 15 min at 25°C.
dGTP and inorganic pyrophosphatase were added to a final
concentration of 0.1 mM and 0.2 U/ml, respectively, and incubated
with shaking for 45 min at 25°C. The malachite green assay reagent
was added, followed by incubation with shaking for 45 min at 25°C.
The absorbance of the assay plate was read at 630 nm using a
full-wavelength microplate reader (Thermo Fisher Scientific, Inc.).
The EC50 value was determined by fitting a dose-response
stimulation curve to the data points using non-linear regression
analysis in GraphPad Prism 5 software (GraphPad Software, Inc., La
Jolla, CA, USA), where y represents the absorbance at 630 nm and ×
is log(MTH1). Moreover, the concentration of MTH1 at which it
reached its maximum enzymatic activity was used for the
determination of the TH287 IC50 value.
TH287 IC50 value
determination by recombinant MTH1
The method of determining the IC50 value
of TH287 was similar to that of the enzymatic activity assay
described above. TH287 (provided by Professor Lai Yisheng, China
Pharmaceutical University, Nanjing, China), known to be an hMTH1
inhibitor, was diluted in assay buffer to generate eight different
concentrations from 100 to 0.4 nM. Briefly, the assay was developed
with a final hMTH1 concentration of 0.18 nM, according to the
calculation mentioned above. A dilution series of TH287 was
included on the assay plate as well as a negative control (lacking
enzyme) and a positive control (lacking inhibitor). The
IC50 value was determined by fitting a dose-response
inhibition curve to the data points using non-linear regression
analysis in GraphPad Prism 5 software (GraphPad Software, Inc.),
where y is the rate of inhibition and × is log(TH287).
Determination of Km and
kcat for hMTH1
To draw the standard curve of inorganic phosphate,
inorganic phosphate was diluted in the assay buffer to generate a
series of concentrations (0–24 µM) and incubated with malachite
green in the plate at 25°C for 45 min. The standard curve was made
and analysed using GraphPad Prism 5 software (GraphPad Software,
Inc.) to assess the linear relationship of the absorbance at 630 nm
with different concentrations (0, 3, 6, 9, 12, 15, 18 and 24 µM) of
inorganic phosphate. hMTH1 activity was assayed in a reaction
mixture containing assay buffer and various amounts of dGTP (0, 40,
80, 120 and 160 µM). The mixtures were incubated at 25°C for 3 min
with 1.5 nM hMTH1 protein. Malachite green assay reagent (50 µl)
was added, followed by incubation with shaking for 45 min at 25°C.
The hydrolysed products of nucleoside triphosphates were quantified
using the inorganic phosphate standard curve, and the
Lineweaver-Burk curve was plotted using GraphPad Prism 5.0
(GraphPad Software, Inc.). The Michaelis-Menten parameters
(Km and Vmax), catalytic constant
(kcat) and catalytic efficiency
(kcat/Km) were calculated using the following
equations:
1/V = Km/Vmax[S] +
1/Vmax and kcat =
Vmax/[S]hMTH1
Immunisation of mice and ELISA
determination
A total of 50 µg of the purified hMTH1 was
emulsified with an equal volume of Freund's complete adjuvant, and
then injected into the back and limbs of three six-week-old female
BALB/c mice, while the negative control was an unimmunised and
healthy BALB/c mouse. For the following two booster immunisations,
50 µg hMTH1 in Freund's incomplete adjuvant was injected into each
mouse at three-week intervals. Three days before cell fusion, the
mouse with the highest antibody titre was injected intravenously
with 50 µg hMTH1 without Freund's adjuvant. Procedures involving
animals and their care were conducted in conformity with NIH
guidelines (NIH Pub. no. 85–23, revised 1996) and were approved by
the Animal Care and Use Committee of the China Pharmaceutical
University (Nanjing, China).
The serum titres of immunised mice were monitored by
indirect ELISA as follows: rhMTH1 (1 µg/ml) in 0.1 M carbonate
coating buffer (15 mM Na2CO3, 34 mM
NaHCO3; pH 9.6) was used to coat plates, which were
incubated at 37°C for 2 h and blocked with 3% BSA at 37°C for 2 h.
Mouse serum was used as the primary antibody and was incubated for
2 h at 37°C. The negative control was incubated with serum from
non-immunised mice. Subsequently, the plate was incubated with
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
(1:5,000) at 37°C for 1 h. Finally, TMB was added to develop the
colour, and the result was measured using a full-wavelength
microplate reader at 450 nm after the reaction had been stopped by
2 M H2SO4.
Production and purification of the
monoclonal antibody against hMTH1
In this experiment, nine six-week-old female BALB/c
mice were used for immunisation and antibody production. They were
~18 g and lived in a room with constant temperature and luminosity.
In addition, we provided food and water on time every day. Spleen
cells were collected from the immunised mice and suspended in DMEM,
and then mixed with SP2/0 myeloma cells at a ratio of 10:1 in the
presence of PEG 1450. The feeder layer cells from BALB/c mice were
plated into 96-well plates to create an environment conducive to
the growth of fused cells. The selection of fused cells was
performed with HAT medium and positive hybridoma clones were
selected by indirect ELISA. A cell line with high affinity was
obtained after being subcloned three times via the limiting
dilution method. 0.5 ml sterile paraffin oil was injected
intraperitoneally into five adult female BALB/c mice before the
hybridoma injection. After 1 week, 1×106 hybridoma cells
were injected into each mouse. The anti-hMTH1 was purified from
mouse ascites by protein A Sepharose chromatography (GE Healthcare)
according to the manufacturer's protocol. The purified MTH1 mAb was
analysed by SDS-PAGE and western blotting.
Isotype analysis
The subtype of the hMTH1 mAb was detected with a
mouse monoclonal antibody (mAb) isotyping reagent (IgG1, IgG2a,
IgG2b, IgG2c, IgG3 and IgM, κ, λ) (ProteinTech, Inc., Chicago, IL,
USA). The heavy and light chain types of the hMTH1 mAb were
determined by the absorbance at OD450 nm.
hMTH1 monoclonal antibody affinity
constant determination
Indirect ELISA was performed to determine the
affinity constant (Kaff) of the hMTH1 mAb. The rhMTH1
was diluted to two concentrations [(Ag')=1 µg/ml and (Ag)=2 µg/ml]
with 0.1 M carbonate coating buffer (pH 9.6), and the plate was
coated. The primary antibody was serially diluted to
105, 104, 103, 102, 10,
1, 0.1 and 0.01 ng/ml and added to the 96-well plate, while the
negative control was incubated with 1% BSA. The subsequent
procedures were the same as for the indirect ELISA described above.
The EC50 values [(Ab')t and (Ab)t]
were determined by fitting a sigmoidal dose-response curve to the
data points in GraphPad Prism 5 software, where y represents the
absorbance at 450 nm and y is the concentration of the hMTH1
antibody. The affinity constant was finally determined using the
following equation: Kaff = (n-1)/2 [n(Ab')t -
(Ab)t], where n = (Ag)/(Ag').
Specificity of the purified monoclonal
antibody
The specificity and affinity of this mAb against
hMTH1 was determined by cell ELISA, which was similar to the
indirect ELISA. First, 1×104 MCF-7 cells were seeded in
96-well plates and cultured at 37°C with 5% CO2 until
they reached 90% cell density. Subsequently, the cells were fixed
with 4% paraformaldehyde for 20 min. Triton X-100 (0.5%) was used
to disrupt the cell membrane at room temperature for 20 min. The
rest of the steps were the same as for the ELISA. The prepared
anti-hMTH1 was used as the primary antibody and the negative
control was 1% BSA. The absorbance of the plate was measured at a
wavelength of 450 nm.
Immunofluorescence assay
To analyse the specificity of the MTH1 mAb to MTH1
expressed on cells, a tumour cell line, MCF-7, that overexpresses
MTH1 was grown on 6-well plates at 37°C with 5% CO2
until 50% cell density was achieved. The plate was washed with PBS
between each step. The plate was fixed in 4% paraformaldehyde for
20 min at 25°C, blocked with 10% FBS and 0.5% Triton X-100 for 2 h
at 25°C, and incubated with prepared anti-hMTH1 mAb (100 µg/ml) or
anti-mouse IgG (100 µg/ml) at 4°C overnight. The negative control
was incubated in PBS with 10% FBS. Thereafter, the cells were
incubated with FITC-conjugated goat anti-mouse IgG (dilution 1:500)
for 1 h at 25°C and counterstained with DAPI (dilution 1:10) for 10
min at 25°C. The cells were then observed under a fluorescence
microscope (Olympus Corp., Tokyo, Japan).
Results
Human MTH1 gene optimisation and
expression plasmid construction
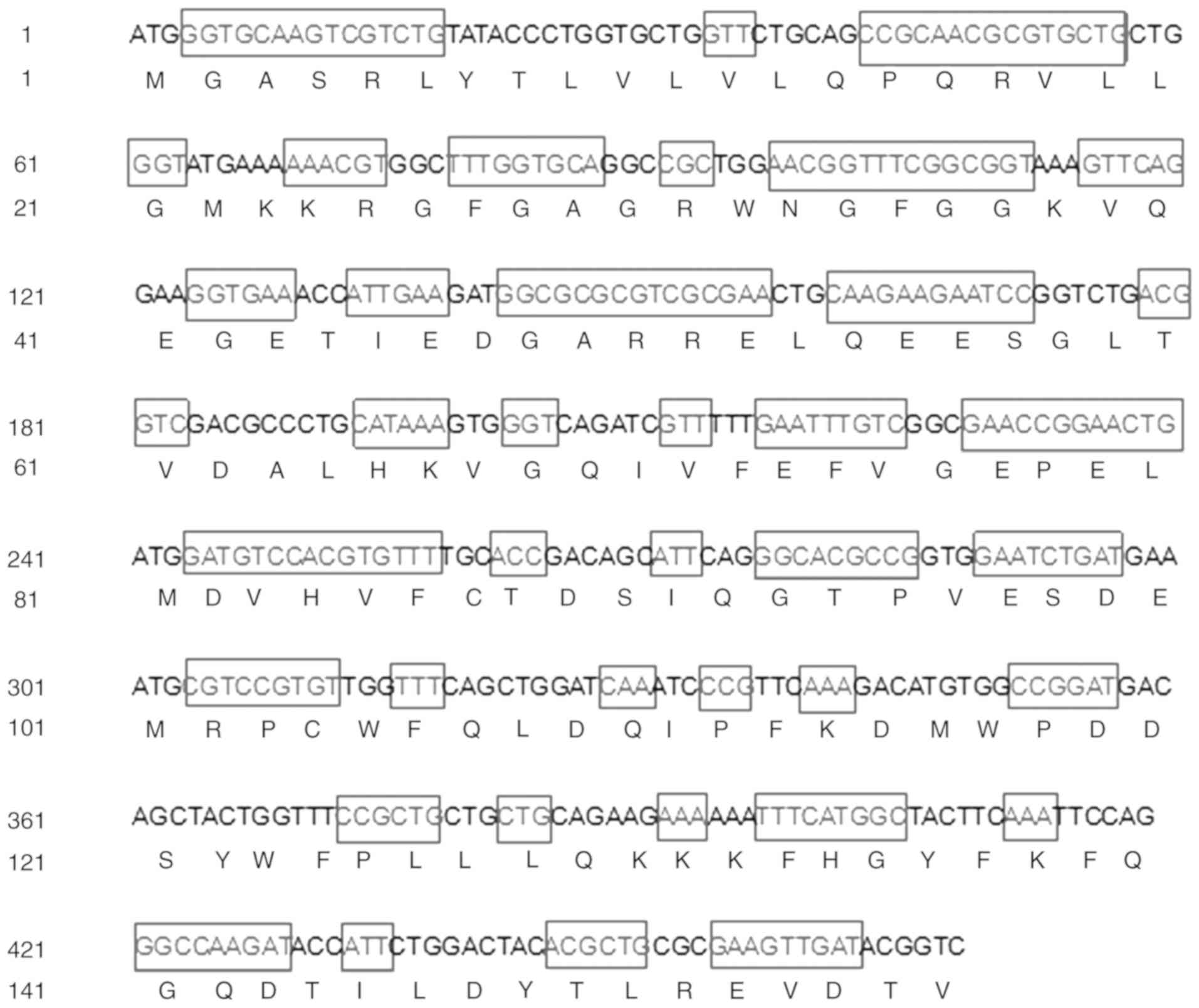
To improve the expression level, the MTH1 DNA
sequence was analysed and the codon was optimised according to the
ideal codon for E. coli (GenBank accession no. MH193376)
(Fig. 1). The hMTH1 gene was
inserted into the C-terminal His-tag on the backbone of pET-28a, as
shown in Fig. 2A. The 691 bp gene
band was obtained by colony PCR using the T7 forward and reverse
primers, which demonstrated that the hMTH1 gene was subcloned into
the pET-28a plasmid (Fig. 2B).
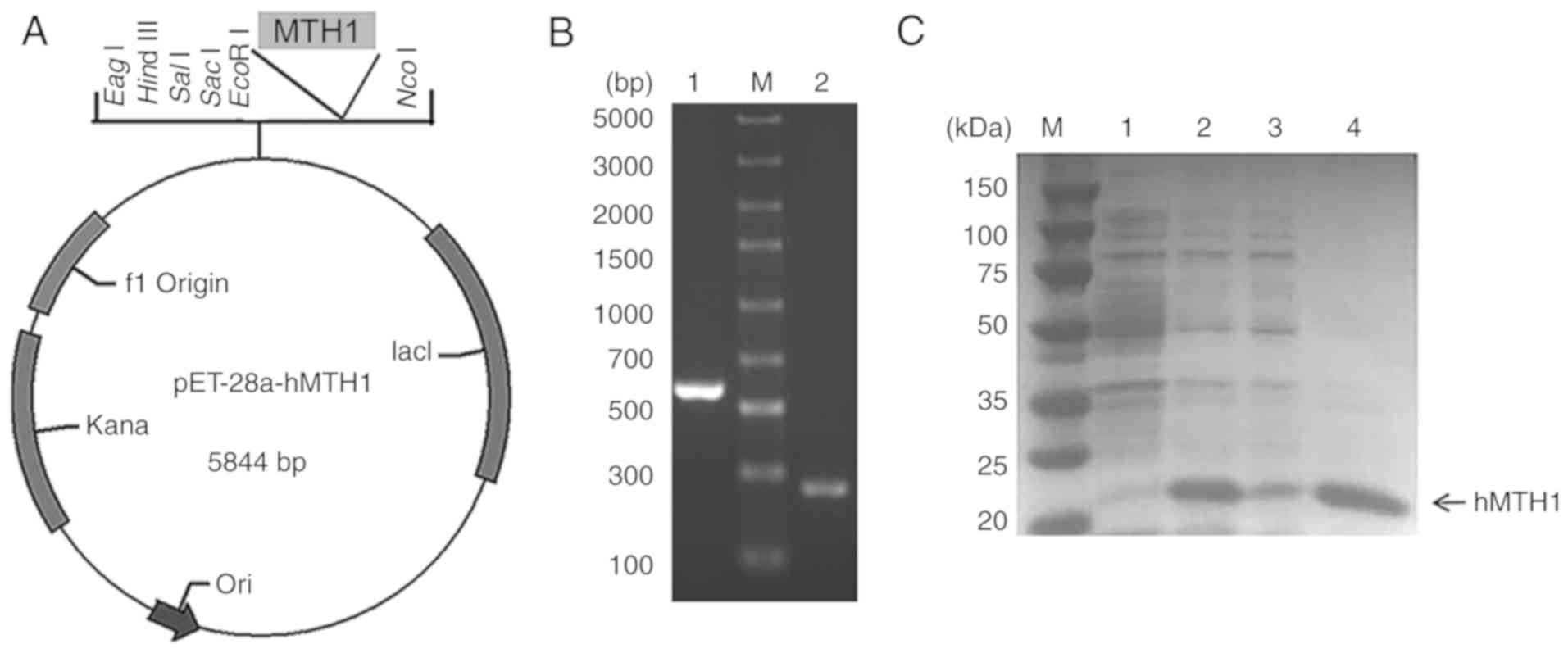 | Figure 2.(A) Construction of the pET-28a-hMTH1
expression vector. The hMTH1 gene was inserted between NcoI
and EcoRI sites, and thus was fused at the C-terminal
His-tag. (B) Results of colony PCR. Lane M, DNA marker; lane 1, PCR
verification of pET-28a-hMTH1; lane 2, PCR verification of pET-28a
vector control. (C) 12% SDS-PAGE electrophoresis analysis of
expression. Lane M, protein marker; lane 1, total protein of
recombinant E. coli cells without adding IPTG; lane 2, total
protein of recombinant E. coli cells with 0.4 mM IPTG; lane
3, supernatant of sonicated cells; lane 4, precipitate of sonicated
cells. hMTH1, human MutT homolog 1; IPTG,
isopropyl-β-D-thiogalactopyranoside. |
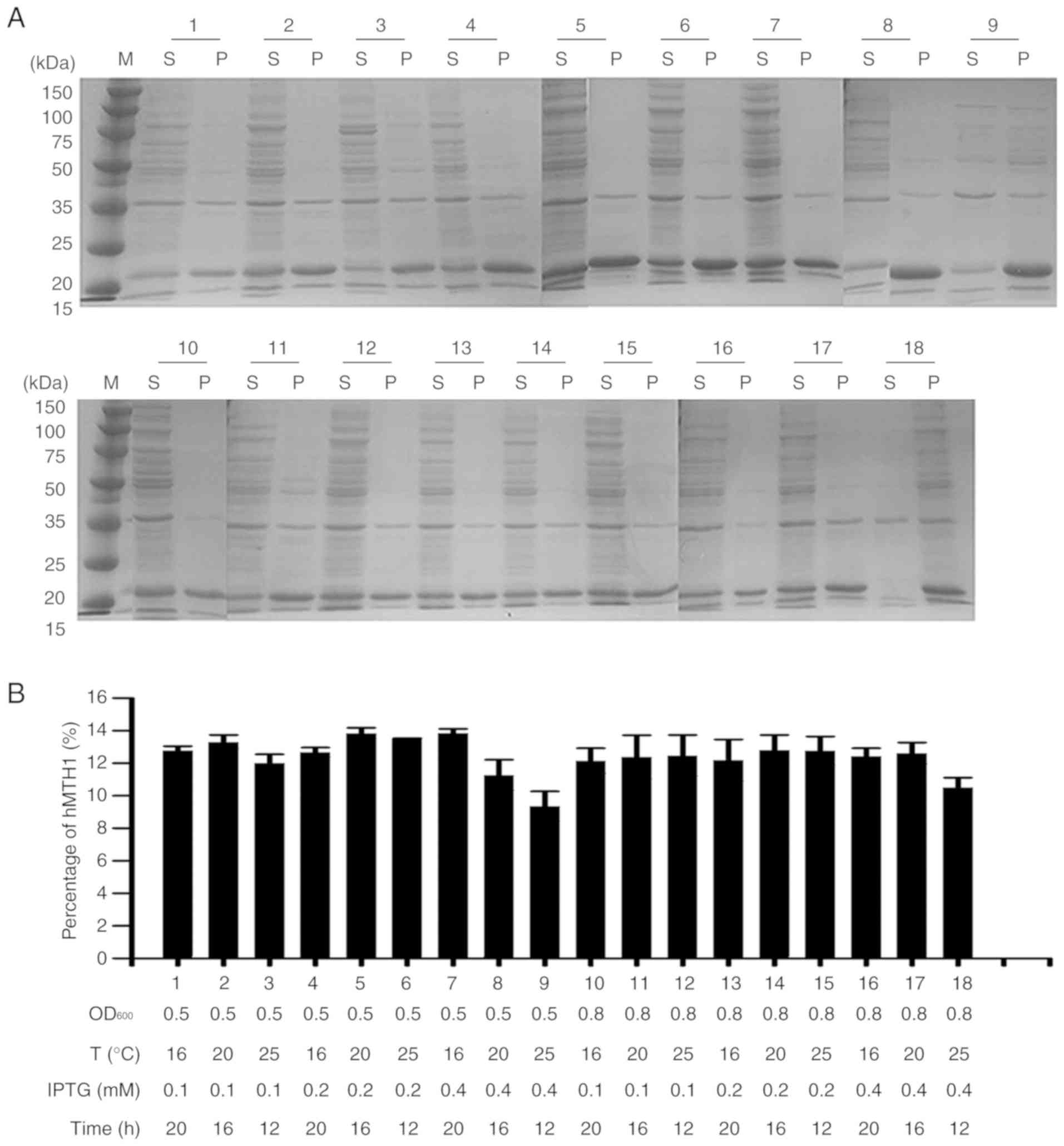
Expression optimisation of hMTH1
The plasmid pET28a-hMTH1 was transformed into
competent E. coli Transetta (DE3) cells for protein
expression. After inducing with 0.4 mM IPTG for 12 h at 25°C and
160 rpm, the recombinant protein of 21 kDa in size was successfully
expressed, with a 9.37% percentage of soluble MTH1 in the total
protein (Fig. 2C). As previously
reported, the amounts of various recombinant products in the
soluble fraction may be improved effectively through optimisation
of the induction conditions (29).
Therefore, four parameters, including the OD600 value,
IPTG concentration, induction temperature and induction time were
investigated in a univariate analysis. The percentage fraction of
hMTH1 was determined through densitometric semi-quantitative
analysis using TanonImage software (version 1.0). The results
showed that the optimal conditions were OD600 = 0.5 with
induction at 16°C for 20 h with 0.4 mM IPTG, and the percentage of
soluble hMTH1 in the total protein expressed in the E. coli
increased to 13.86% (Fig. 3A and
B).
Purification and determination of
hMTH1
The human MTH1 protein we designed contained
His-tags, thus an Ni-NTA column was used for purification and the
eluents were detected by 12% SDS-PAGE (Fig. 4A). A total of 100 and 250 mM
imidazole eluting solution was treated with Sephadex G-50 (GE
Healthcare) for further purification after ultra-filtering to 1 ml.
There were no other bands apart from the target protein in the 12%
SDS-PAGE (Fig. 4B). In the western
blot experiment, anti-His antibody was used to target proteins with
His-tags, and the black band on the PVDF membrane proved that the
purified protein we obtained was hMTH1 (Fig. 4A and B). In addition, HPLC was used
to assess the sample purity, with the results revealing that the
retention time of the protein was 14.556 min and the purity was
>98% (Fig. 4C).
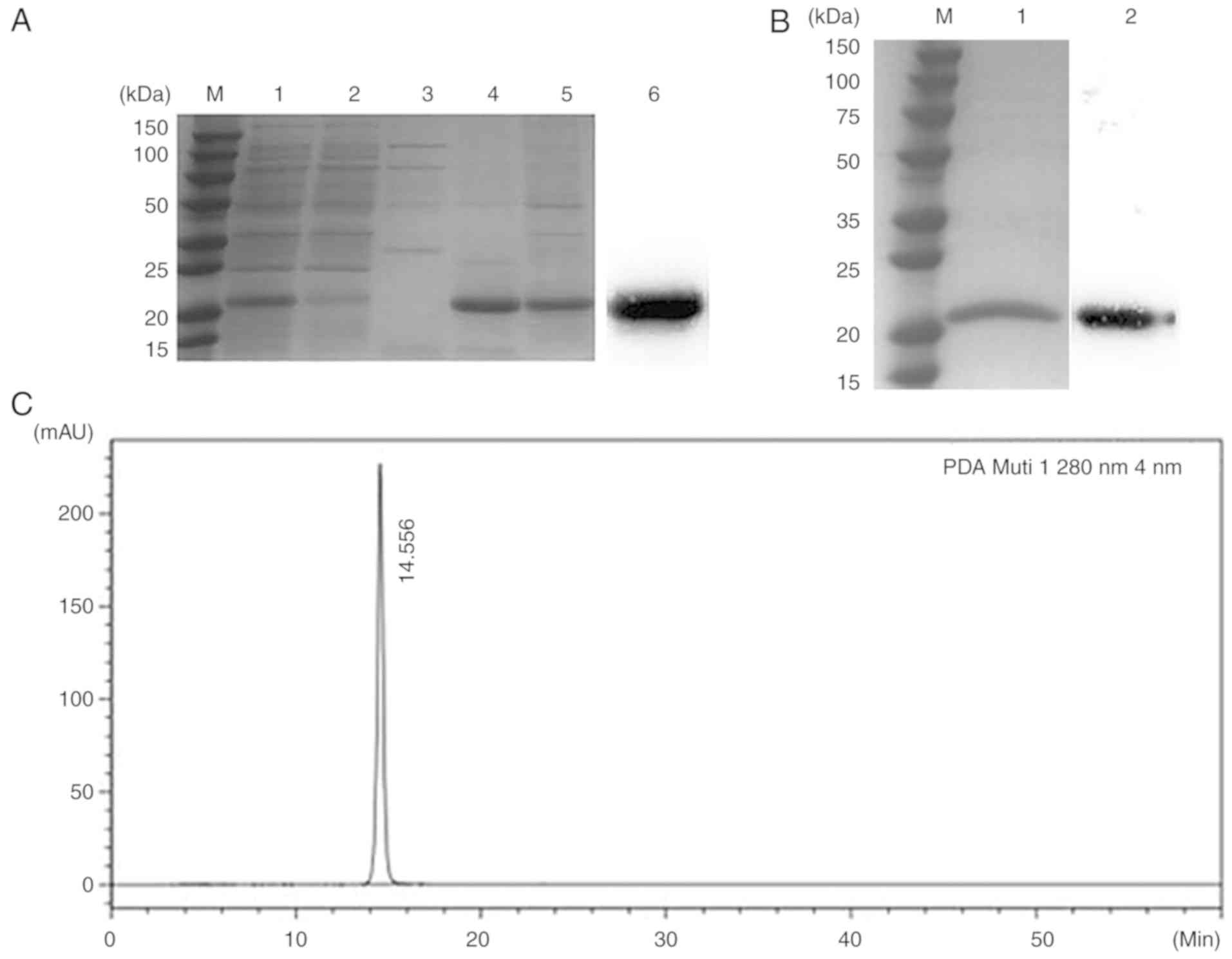 | Figure 4.Purity analysis. (A) Results of 12%
SDS-PAGE after purification by Ni-NTA affinity chromatography. Lane
M, protein maker; lane 1, supernatant of sonicated cells; lane 2,
flow-through collected from the Ni-NTA column; lane 3, 50 mM
imidazole eluent; lane 4, 100 mM imidazole eluent; lane 5, 250 mM
imidazole eluent; lane 6, western blot analysis for recombinant
hMTH1. The primary antibody used in the western blot analysis was
the anti-His monoclonal antibody. (B) Results of 12% SDS-PAGE after
purification by G-50 Gel filtration chromatography. Lane M, protein
marker; lane 1, the eluent collected by G-50 Gel filtration
chromatography; lane 2, the recombinant protein hMTH1 that was
analysed by western blot analysis. The primary antibody was the
anti-His monoclonal antibody. (C) HPLC analysis of rhMTH1. Only one
peak was detected. hMTH1, human MutT homolog 1; HPLC,
high-performance liquid chromatography; SDS-PAGE, sodium dodecyl
sulfate-polyacrylamide gel electrophoresis; rhMTH1, recombinant
hMTH1 protein. |
After two critical steps of purification, including
purification by Ni column and Sephadex G-50, 20.1 mg hMTH1 was
obtained with >98% purity from 1 l medium and the total yield
was calculated to be 12.2% (Table
I).
 | Table I.Purity and yield of hMTH1. |
Table I.
Purity and yield of hMTH1.
| Purification
step | Total soluble
protein (mg)a | Soluble hMTH1
(mg)a | Purity of hMTH1
(%)b | Yield (%) |
|---|
| Cell lysate | 410.0 | 164.0 | 40.0 | 100.0 |
| Supernatant | 162.0 | 81.0 | 50.0 | 49.4 |
| Ni column | 57 | 42.8 | 75.0 | 26.1 |
| G-50 | 20.1 | 20.1 | >98% | 12.2 |
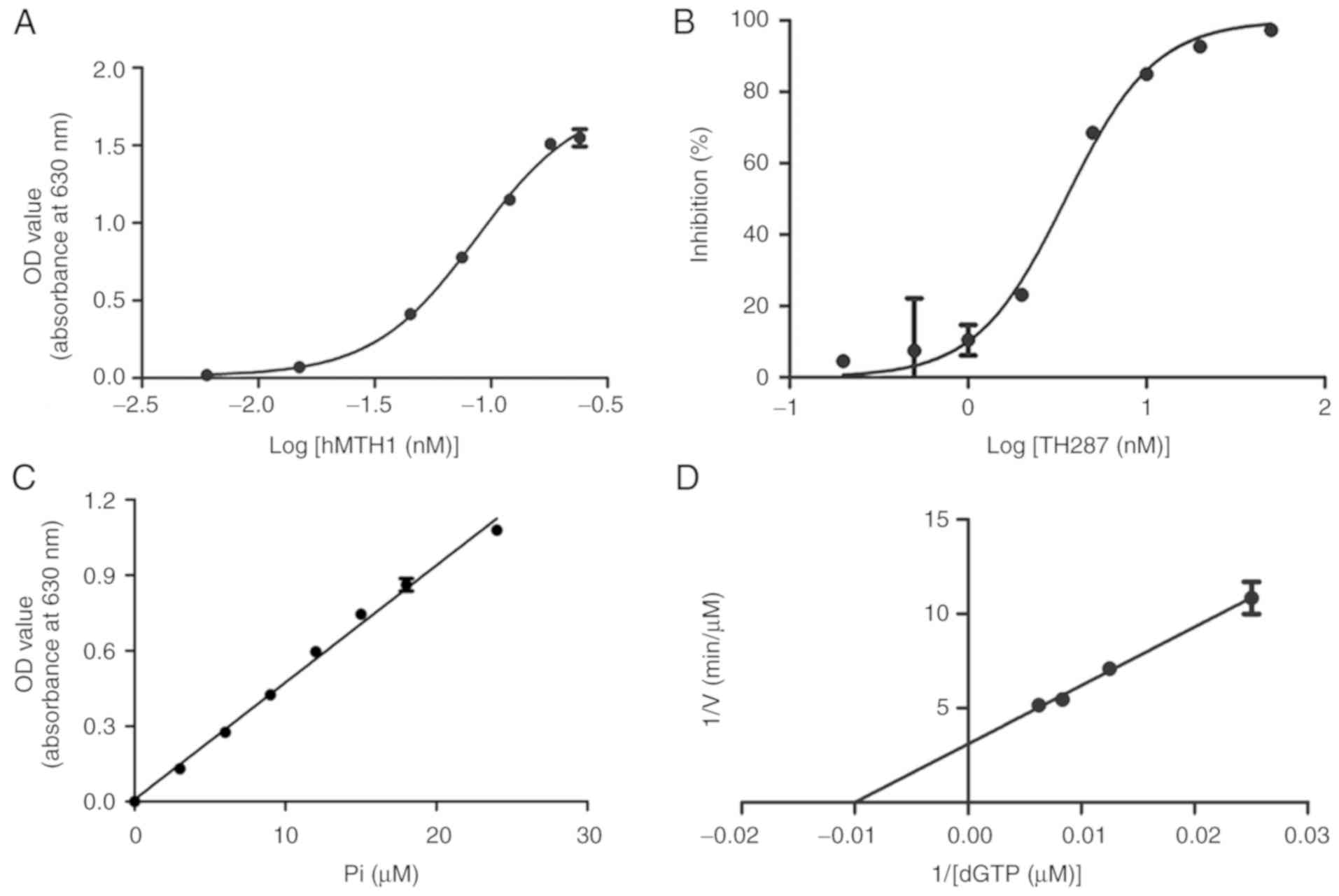
Recombinant hMTH1 enzyme activity and
TH287 IC50 value determination
The enzymatic assay was based on the hydrolysis
reaction of dGTP catalysed by the recombinant hMTH1 protein to form
dGMP and pyrophosphate. As shown in Fig. 5A, the concentration required for the
50% maximal effect (EC50) of rhMTH1 was 0.08543 nM.
TH287 IC50 was determined using 0.18 nM rhMTH1, which
reached its maximum enzymatic activity, and this concentration was
consistent with the reference value (13). The IC50 curve of TH287
represented a clear S-type trend with no apparent data offset
(Fig. 5B). The IC50 of
TH287 was 3.53±0.47 nM, similar to the reference value (13). It was confirmed that rhMTH1 protein
with high activity was obtained and could accurately verify the
IC50 value of TH287 in an hMTH1 inhibitor screening
model, as well as having the potential to be used to screen other
MTH1 inhibitors.
Kinetic parameters of hMTH1 for
dGTP
According to the linear equation of the standard
curve, y = 0.0465× + 0.0082, with a correlation coefficient
(R2) of 0.9947 (Fig.
5C), the inorganic phosphate (Pi) which was the hydrolysed
product of nucleoside triphosphates was able to be quantified. In
the enzymatic reaction, the reaction mixtures, containing various
concentrations of substrates (0–160 µM for dGTP), were incubated
with 1.5 nM rhMTH1 for 48 min at 25°C. According to the
1/(dGTP)-1/V curve that was plotted using GraphPad Prism 5.0,
kinetic analysis of the dGTP substrate with rhMTH1 revealed a
Km of 103.63±34.64 µM and a kcat of 3.64±0.58
sec−1 as well as a catalytic efficiency
(kcat/Km) of 0.037±0.0064
sec−1•µM−1 (Fig.
5D).
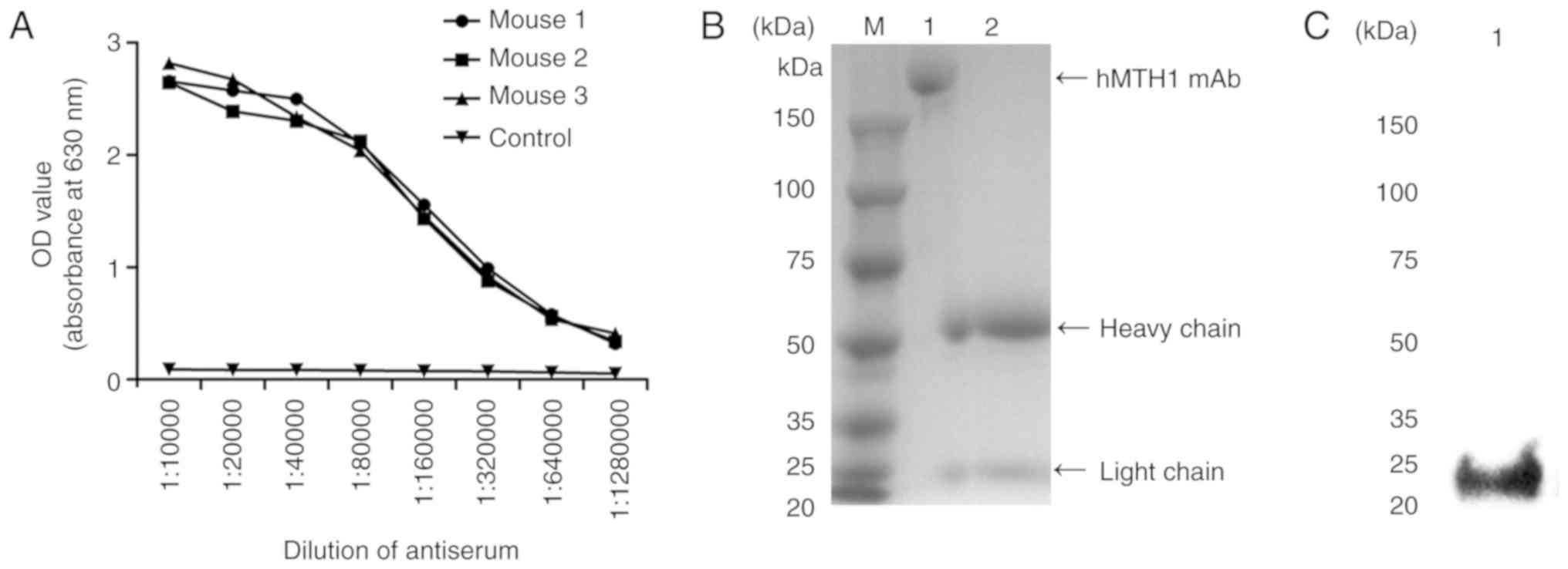
Production, purification and
identification of the hMTH1 monoclonal antibody
Indirect ELISA was used to screen the highest titre
among the mouse antiserum samples. The serum of three immunised
mice was harvested and diluted into a series of concentrations
(1:10,000; 1:20,000; 1:40,000; 1:80,000; 1:160,000; 1:320,000;
1:640,000; and 1:1,280,000) after three immunisations. The titre
results in Fig. 6A show that the
antiserum titre of mouse three, was the highest, therefore spleen
cells from this mouse were used to fuse with SP2/0 myeloma cells to
generate antibody hybridoma cells. After subcloning three times, a
positive cell line, named 4A4, with a relatively high binding
affinity to hMTH1, was successfully obtained. After that,
1×106 hybridoma cells were injected into BALB/c mice
intraperitoneally to induce the formation of ascites containing mAb
directly against hMTH1 protein. Within 7 to 10 days, ascites were
collected and purified via protein A column (GE Healthcare). The
hMTH1 mAb was identified by SDS-PAGE. The integrated MTH1 mAb was
~170 kDa with one clear heavy chain at 55 kDa and a light chain at
23 kDa, with a purity >90% (Fig.
6B). The specificity against hMTH1 was identified by western
blot analysis. The results demonstrated that the hMTH1 mAb was able
to specifically combine with the recombinant hMTH1 (Fig. 6C).
Specificity and affinity of the
purified monoclonal antibody
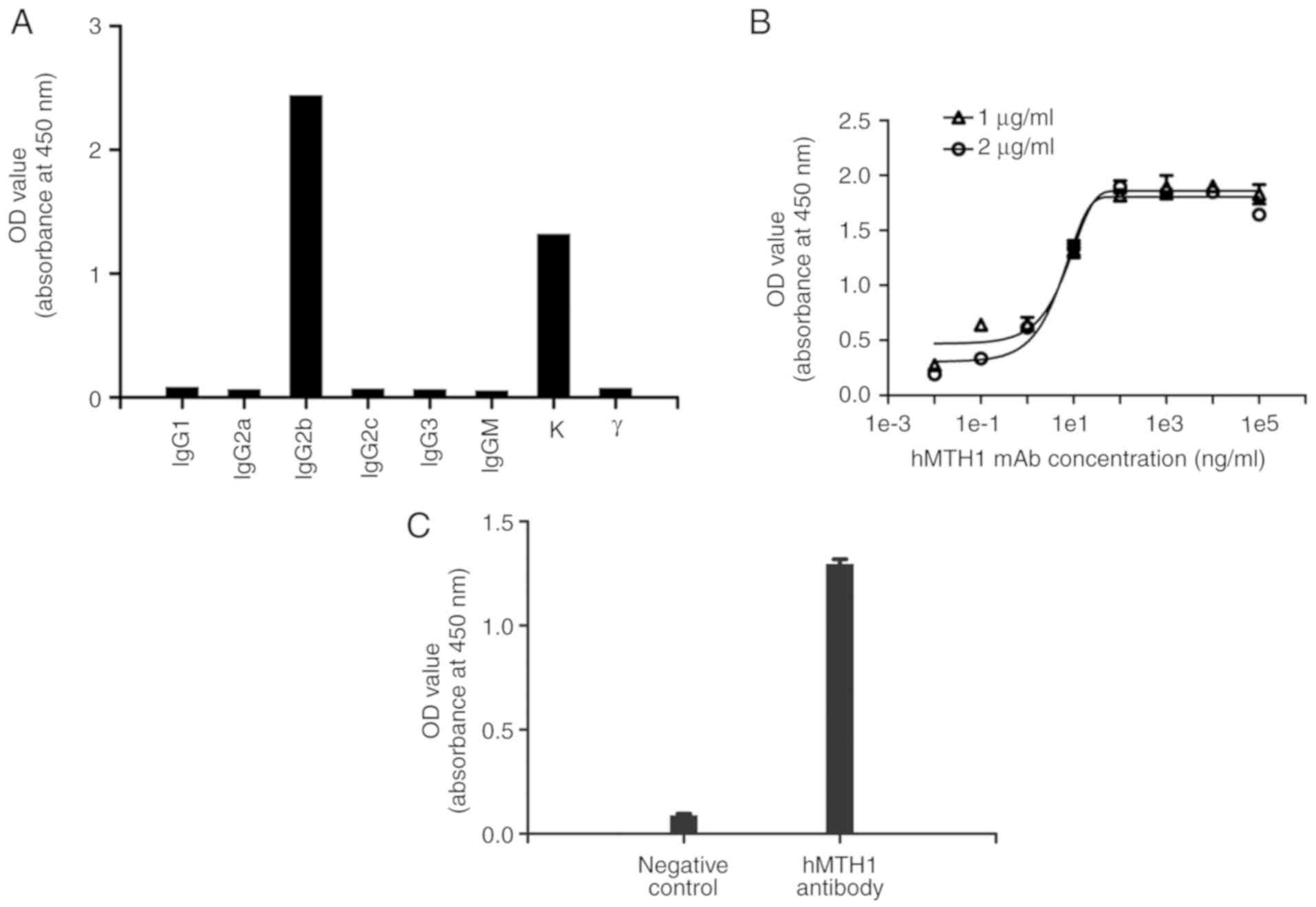
The isotype of the hMTH1 mAb was detected using a
subtyping kit. The results demonstrated that the type of heavy
chain was IgG2b and the type of light chain was kappa (κ), as shown
in Fig. 7A. Sigmoid curves were
plotted using the OD values against the antibody concentrations
(1e-2, 1e-1, 1e1, 1e2, 1e3, 1e4, 1e5 ng/ml) in two different
antigen concentrations, as shown in Fig. 7B. The EC50 of the
antibody at concentrations of 1 and 2 µg/ml of rhMTH1 in this
experiment were 8.739 and 4.377 ng/ml, respectively. Therefore, the
affinity constant of the anti-hMTH1 antibody was
8.73×10−9 M.
Cell ELISA was used to further validate the
specificity of the purified mAb against hMTH1 in human breast
cancer MCF-7 cells. hMTH1 mAb purified from ascites had a specific
binding capacity to the natural hMTH1 expressed on MCF-7 cells
(Fig. 7C).
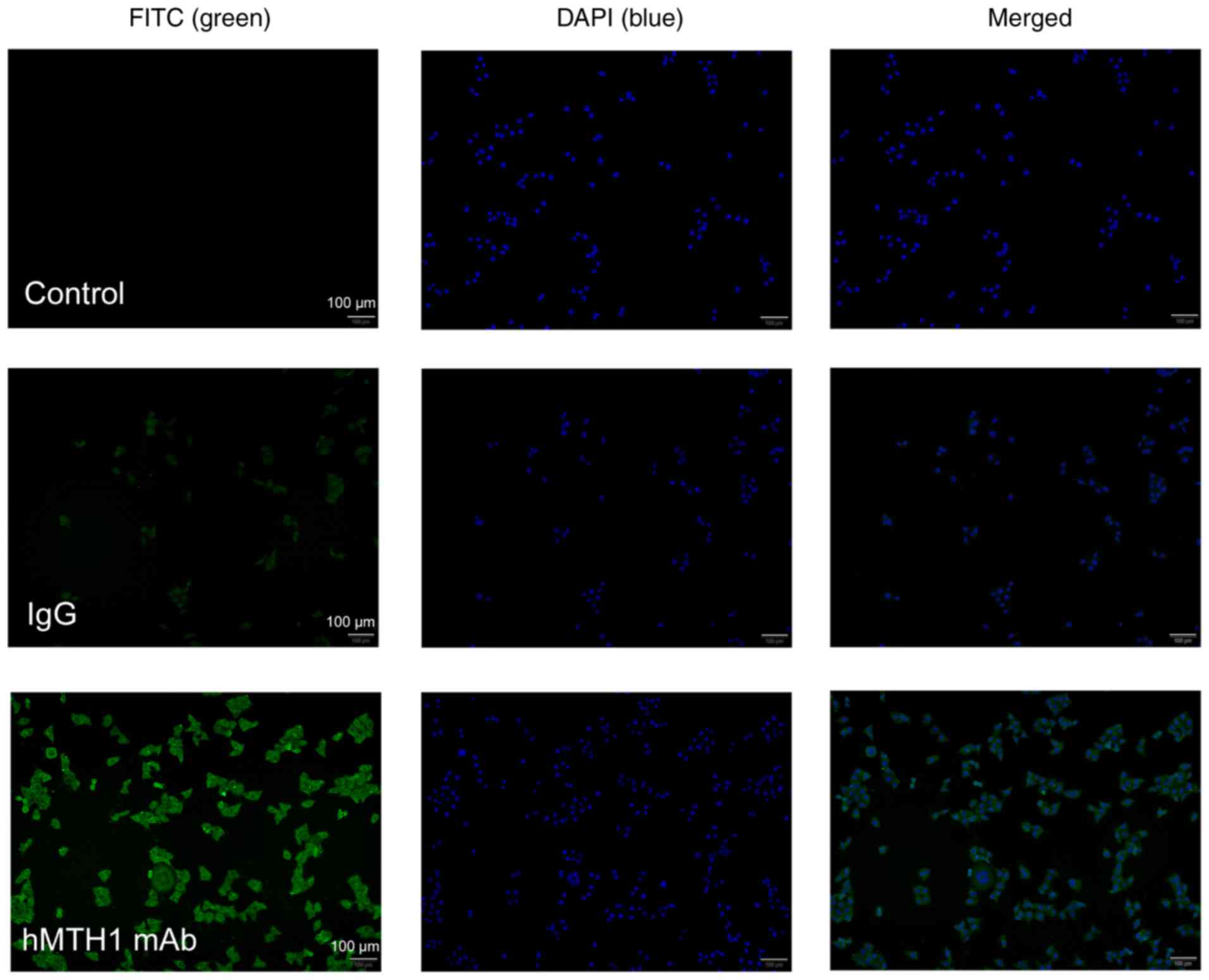
Immunofluorescence assay
It has been reported that MCF-7 cells express a high
level of MTH1, as previously measured using mass spectrometry
(30) and novel probes (31). Therefore, we performed an
immunofluorescence experiment to further confirm the binding to
natural hMTH1, as shown in Fig. 8.
hMTH1 mAb purified from ascites served as the primary antibody to
incubate with the MCF-7 cells. After incubation with
FITC-conjugated goat-anti-mouse IgG and counterstaining with DAPI,
the hMTH1 mAb-treated cells displayed obvious green fluorescence
compared with the negative control and mouse IgG treatment. The
results also showed that green fluorescence was mainly accumulated
in the cytosol of the MCF-7 cells, with a minor proportion at the
mitochondria and nuclei, which corresponds with the distribution of
hMTH1. This indicates that the purified MTH1 mAb was able to
specifically combine with hMTH1 expressed on the MCF-7 cells.
Discussion
MutT homolog 1 (MTH1) protein is a homologous enzyme
of MutT and is widely distributed in mitochondria and the nucleus
(32). The human MTH1 gene
is located in the second sub-band of the second band of the short
arm of the seventh chromosome, with a total length of ~12 kb and a
total of six exons. It is an essential sanitiser of the free
nucleotide pool that prevents lethal DNA damage in cancer cells,
which has been known to maintain the growth of tumours and promotes
their development.
In the present study, we developed a brief protocol
to produce hMTH1 in a cost- and time-effective manner through an
optimised E. coli expression system and generated an
antibody against hMTH1. The present study showed that the
percentage of soluble hMTH1 in the total protein was 9.37% without
any optimisation. After optimisation, the percentage improved to
13.86%. We were thus able to acquire 20.1 mg hMTH1 protein from
1,000 ml medium with over 98% purity, and the total yield was
12.2%. In the analysis of enzyme activity determination, the
EC50 of rhMTH1 was 0.08543 nM and the IC50 of
TH287 was 3.53±0.47 nM, which were similar to the reference values
(13). It was demonstrated that we
obtained rhMTH1 protein with high purity and activity, which
accurately verified the IC50 of TH287 in the hMTH1
inhibitor screening model and may be used to screen other MTH1
inhibitors. Fujikawa et al reported that the purified hMTH1
has a Km of 258 µM and kcat of 15.7
sec−1 (8,33) after purification by DEAE Sephacel
column, HiTrap Butyl Sepharose 4FF column and HiPrep 26/60
Sephacryl S-100 HR gel filtration chromatography (34). In the present study, the Michaelis
constant (Km) and the catalytic constant
(kcat) were 103.63±34.64 µM and 3.64±0.58
sec−1, respectively. From this perspective, our protein
appears to have greater enzymatic activity.
To the best of our knowledge, the success rate of
hybridoma cell construction may be affected by many factors, such
as the growth state of the myeloma cells, the concentration of PEG,
the ratio of spleen cells to myeloma cells, and the number of
feeder layer cells (35). In the
present study, 7% PEG was used for cell fusion at 1 min of
incubation. Additionally, 1×105 feeder layer cells were
plated into each well of the 96-well plates to provide growth
factors for the cells to survive and proliferate in vitro.
Western blotting and ELISA analysis showed that this mAb had a high
affinity for hMTH1. In addition, the results of the
immunofluorescence assay determined that the hMTH1 mAb purified
from ascites had specific binding capacity to the natural hMTH1
expressed on the human breast cell line MCF-7. Though hMTH1 is a
cytoplasmic protein, various methods have been reported to
transduce antibodies into cells (24–27).
MTH1 is a non-essential enzyme in normal cells that is
overexpressed in tumours, so its antibodies could play a role in
targeted therapy for the broad-spectrum treatment of cancers.
To summarise, we reported an expression and
purification method for human recombinant hMTH1 protein, by which
milligrams of high purity (>98%) protein with bioactivity could
be produced in 5 working days. The ability to produce milligram
quantities of bioactive hMTH1 with simple steps will certainly
facilitate in vitro or in vivo studies. This
expression and purification strategy should also have reference
value for the production of hMTH1 on a large scale. Using the
rhMTH1, the anti-hMTH1 mAb was obtained via a hybridoma technique,
and it showed high affinity and binding ability. Considering that
this mAb has good selectivity to its corresponding antigen, it is
expected to have potential for the functional and mechanistic study
of hMTH1 in tumours, and for antitumour drug development in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fundamental
Research Funds for the National Natural Science Foundation of China
(grant no. 81603017), the Natural Science Foundation of Jiangsu
Province (grant no. BK20160765) and the Innovation Ability
Construction Plan of Hainan Province (grant nos. SQ2017JSKF0027 and
SQ2018JSKF0001).
Availability of data and materials
All data generated or analysed during the present
study are included in this published article.
Authors' contributions
XJ and YLi conceived and designed the study; YLa
provided and characterised the hMTH1 inhibitor; CC and XL wrote the
manuscript, optimised the hMTH1 expression, prepared the hMTH1
antibody and performed the statistical analyses; MG, RD and NK
expressed and purified hMTH1; LW prepared the hMTH1 inhibitor. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Procedures involving animals and their care were
conducted in conformity with NIH guidelines (NIH Pub. no. 85-23,
revised 1996) and were approved by the Animal Care and Use
Committee of the China Pharmaceutical University (Nanjing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo M, He H, Kelley MR and Georgiadis MM:
Redox regulation of DNA repair: Implications for human health and
cancer therapeutic development. Antioxid Redox Signal.
12:1247–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rai P: Human Mut T Homolog 1 (MTH1): A
roadblock for the tumor-suppressive effects of oncogenic
RAS-induced ROS. Small GTPases. 3:120–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puigvert JC, Sanjiv K and Helleday T:
Targeting DNA repair, DNA metabolism and replication stress as
anti-cancer strategies. FEBS J. 283:232–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakabeppu Y: Cellular levels of
8-oxoguanine in either DNA or the nucleotide pool play pivotal
roles in carcinogenesis and survival of cancer cells. Int J Mol
Sci. 15:12543–12557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao T, Gu S, Liu F, Li L, Wang Z, Yang J
and Li G: Investigation of MTH1 activity via mismatch-based DNA
chain elongation. Anal Chim Acta. 905:66–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakabeppu Y, Ohta E and Abolhassani N:
MTH1 as a nucleotide pool sanitizing enzyme: Friend or foe. Free
Radic Biol Med. 107:151–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujikawa K, Kamiya H, Yakushiji H,
Nakabeppu Y and Kasai H: Human MTH1 protein hydrolyzes the oxidized
ribonucleotide, 2-hydroxy-ATP. Nucleic Acids Res. 29:449–454. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bialkowski K, Szpila A and Kasprzak KS:
Up-regulation of 8-oxo-dGTPase activity of MTH1 protein in the
brain, testes and kidneys of mice exposed to 137Cs γ
radiation. Radiat Res. 172:187–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waz S, Nakamura T, Hirata K, Koga-Ogawa Y,
Chirifu M, Arimori T, Tamada T, Ikemizu S, Nakabeppu Y and Yamagata
Y: Structural and kinetic studies of the human nudix hydrolase MTH1
reveal the mechanism for its broad substrate specificity. J Biol
Chem. 292:2785–2794. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maki H and Sekiguchi M: MutT protein
specifically hydrolyses a potent mutagenic substrate for DNA
synthesis. Nature. 355:273–275. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuzuki T, Egashira A and Kura S: Analysis
of MTH1 gene function in mice with targeted mutagenesis.
Mutat Res. 477:71–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gad H, Koolmeister T, Jemth AS, Eshtad S,
Jacques SA, Ström CE, Svensson LM, Schultz N, Lundbäck T,
Einarsdottir BO, et al: MTH1 inhibition eradicates cancer by
preventing sanitation of the dNTP pool. Nature. 508:215–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JY, Liu GZ, Wilmott JS, La T, Feng
YC, Yari H, Yan XG, Thorne RF, Scolyer RA, Zhang XD and Jin L:
Skp2-mediated stabilization of MTH1 promotes survival of melanoma
cells upon oxidative stress. Cancer Res. 77:6226–6239. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kennedy CH, Cueto R, Belinsky SA, Lechner
JF and Pryor WA: Overexpression of hMTH1 mRNA: A molecular
marker of oxidative stress in lung cancer cells. FEBS Lett.
429:17–20. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borrego S, Vazquez A, Dasí F, Cerdá C,
Iradi A, Tormos C, Sánchez JM, Bagán L, Boix J, Zaragoza C, et al:
Oxidative stress and DNA damage in human gastric carcinoma:
8-Oxo-7′8-dihydro-2′-deoxyguanosine (8-oxo-dG) as a possible tumor
marker. Int J Mol Sci. 14:3467–3486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber KV, Salah E, Radic B, Gridling M,
Elkins JM, Stukalov A, Jemth AS, Göktürk C, Sanjiv K, Strömberg K,
et al: Stereospecific targeting of MTH1 by (S)-crizotinib as an
anticancer strategy. Nature. 508:222–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Svensson LM, Jemth AS, Desroses M, Loseva
O, Helleday T, Högbom M and Stenmark P: Crystal structure of human
MTH1 and the 8-oxo-dGMP product complex. FEBS Lett. 585:2617–2621.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koga Y, Inazato M, Nakamura T, Hashikawa
C, Chirifu M, Michi A, Yamashita T, Toma S, Kuniyasu A, Ikemizu S,
et al: Crystallization and preliminary X-ray analysis of human MTH1
with a homogeneous N-terminus. Acta Crystallogr Sect F Struct Biol
Cryst Commun. 69:45–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carter M, Jemth AS, Hagenkort A, Page BD,
Gustafsson R, Griese JJ, Gad H, Valerie NC, Desroses M, Boström J,
et al: Crystal structure, biochemical and cellular activities
demonstrate separate functions of MTH1 and MTH2. Nat Commun.
6:78712015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ellermann M, Eheim A, Rahm F, Viklund J,
Guenther J, Andersson M, Ericsson U, Forsblom R, Ginman T,
Lindström J, et al: Novel class of potent and cellularly active
inhibitors devalidates MTH1 as broad-Spectrum cancer target. ACS
Chem Biol. 12:1986–1992. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Köhler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan X, He C, Jing W, Zhou X, Chen R, Cao
L, Zhu M, Jia R, Wang H and Guo Y: Intracellular osteopontin
inhibits toll-like receptor signaling and impedes liver
carcinogenesis. Cancer Res. 75:86–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cornelissen B, Hu M, McLarty K, Costantini
D and Reilly RM: Cellular penetration and nuclear importation
properties of 111In-labeled and 123I-labeled
HIV-1 tat peptide immunoconjugates in BT-474 human breast cancer
cells. Nucl Med Biol. 34:37–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee Y, Ishii T, Kim HJ, Nishiyama N,
Hayakawa Y, Itaka K and Kataoka K: Efficient delivery of bioactive
antibodies into the cytoplasm of living cells by
charge-conversional polyion complex micelles. Angew Chem Int Ed
Engl. 49:2552–2555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada Y, Perez SM, Tabata M, Abe J,
Yasuzaki Y and Harashima H: Efficient and high-speed transduction
of an antibody into living cells using a multifunctional
nanocarrier system to control intracellular trafficking. J Pharm
Sci. 104:2845–2854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shin SM, Choi DK, Jung K, Bae J, Kim JS,
Park SW, Song KH and Kim YS: Antibody targeting intracellular
oncogenic Ras mutants exerts anti-tumour effects after systemic
administration. Nat Commun. 8:150902017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baykov AA, Evtushenko OA and Avaeva SM: A
malachite green procedure for orthophosphate determination and its
use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem.
171:266–270. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malik A, Alsenaidy AM, Elrobh M, Khan W,
Alanazi MS and Bazzi MD: Optimization of expression and
purification of HSPA6 protein from Camelus dromedarius in
E. coli. Saudi J Biol Sci. 23:410–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coskun E, Jaruga P, Jemth AS, Loseva O,
Scanlan LD, Tona A, Lowenthal MS, Helleday T and Dizdaroglu M:
Addiction to MTH1 protein results in intense expression in human
breast cancer tissue as measured by liquid
chromatography-isotope-dilution tandem mass spectrometry. DNA
Repair. 33:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji D, Beharry AA, Ford JM and Kool ET: A
Chimeric ATP-Linked nucleotide enables luminescence signaling of
damage surveillance by MTH1, a cancer target. J Am Chem Soc.
138:9005–9008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakabeppu Y: Regulation of intracellular
localization of human MTH1, OGG1, and MYH proteins for repair of
oxidative DNA damage. Prog Nucleic Acid Res Mol Biol. 68:75–94.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujikawa K, Kamiya H, Yakushiji H, Fujii
Y, Nakabeppu Y and Kasai H: The oxidized forms of dATP are
substrates for the human MutT homologue, the hMTH1 protein. J Biol
Chem. 274:18201–18205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yakushiji H, Maraboeuf F, Takahashi M,
Deng ZS, Kawabata S, Nakabeppu Y and Sekiguchi M: Biochemical and
physicochemical characterization of normal and variant forms of
human MTH1 protein with antimutagenic activity. Mutat Res.
384:181–194. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ling S, Xiao S, Xie C, Wang R, Zeng L,
Wang K, Zhang D, Li X and Wang S: Preparation of monoclonal
antibody for brevetoxin 1 and development of Ic-ELISA and colloidal
gold strip to detect brevetoxin 1. Toxins. 10:E752018. View Article : Google Scholar : PubMed/NCBI
|