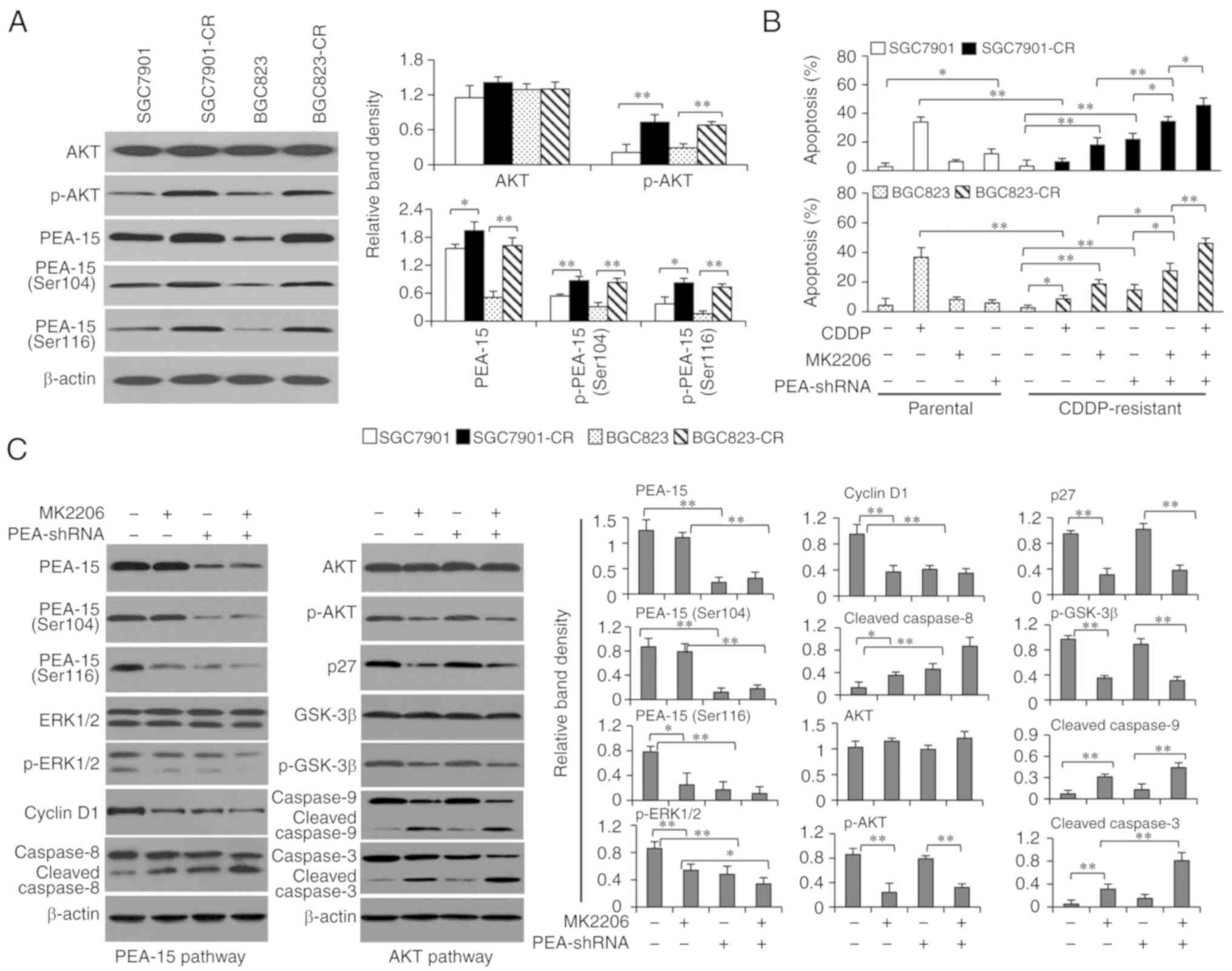
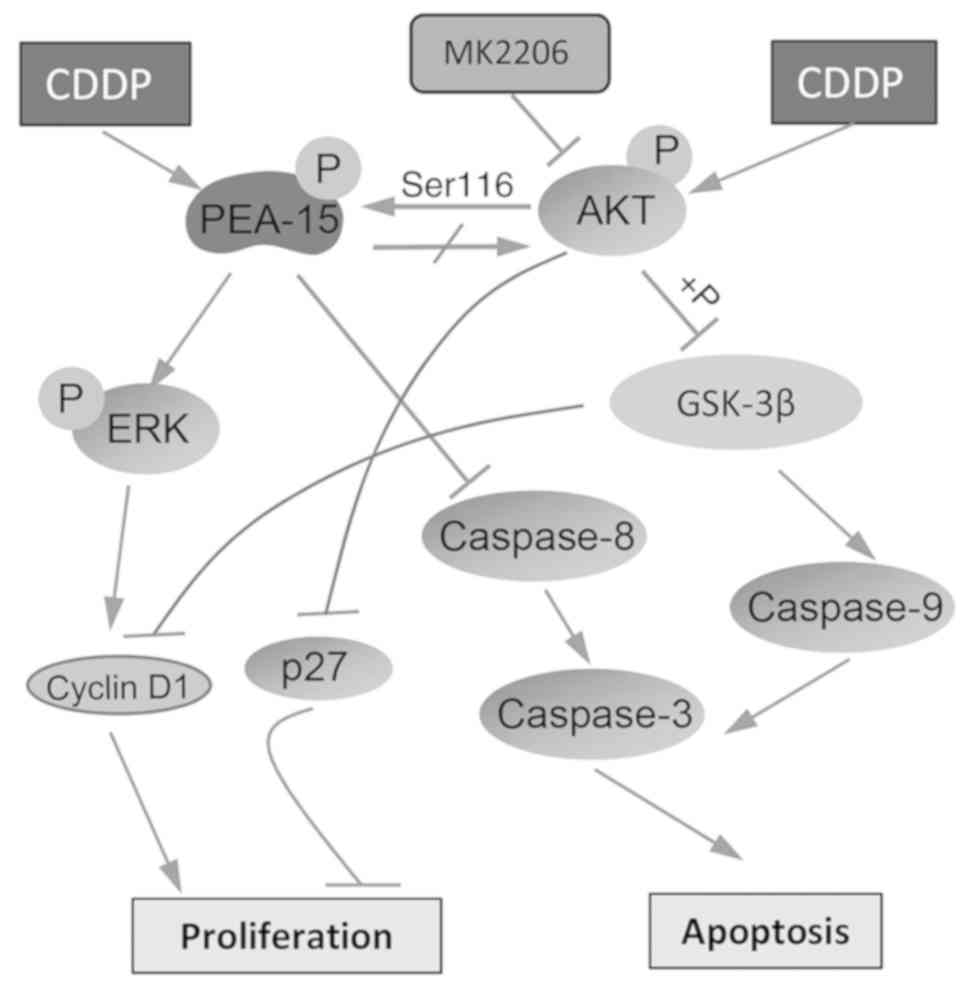
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cats A, Jansen EP, van Grieken NC,
Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot
H, Trip AK, Swellengrebel HA, et al: Chemotherapy versus
chemoradiotherapy after surgery and preoperative chemotherapy for
resectable gastric cancer (CRITICS): An international, open-label,
randomised phase 3 trial. Lancet Oncol. 19:616–628. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koizumi W, Tanabe S, Azuma M, Ishido K,
Nishimura K, Sasaki T, Nakatani K, Higuchi K, Nakayama N and Katada
C: Impacts of fluorouracil-metabolizing enzymes on the outcomes of
patients treated with S-1 alone or S-1 plus cisplatin for
first-line treatment of advanced gastric cancer. Int J Cancer.
126:162–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Araujo H, Danziger N, Cordier J, Glowinski
J and Chneiweiss H: Characterization of PEA-15, a major substrate
for protein kinase C in astrocytes. J Biol Chem. 268:5911–5920.
1993.PubMed/NCBI
|
|
5
|
Fiory F, Formisano P, Perruolo G and
Beguinot F: Frontiers: PED/PEA-15, a multifunctional protein
controlling cell survival and glucose metabolism. Am J Physiol
Endocrinol Metab. 297:E592–E601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greig FH and Nixon GF: Phosphoprotein
enriched in astrocytes (PEA)-15: A potential therapeutic target in
multiple disease states. Pharmacol Ther. 143:265–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammed HN, Pickard MR and
Mourtada-Maarabouni M: The protein phosphatase 4-PEA15 axis
regulates the survival of breast cancer cells. Cell Signal.
28:1389–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Funke V, Lehmann-Koch J, Bickeboller M,
Benner A, Tagscherer KE, Grund K, Pfeifer M, Herpel E, Schirmacher
P, Chang-Claude J, et al: The PEA-15/PED protein regulates cellular
survival and invasiveness in colorectal carcinomas. Cancer Lett.
335:431–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quintavalle C, Hindupur SK, Quagliata L,
Pallante P, Nigro C, Condorelli G, Andersen JB, Tagscherer KE, Roth
W, Beguinot F, et al: Phosphoprotein enriched in diabetes
(PED/PEA15) promotes migration in hepatocellular carcinoma and
confers resistance to sorafenib. Cell Death Dis. 8:e31382017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buonomo R, Giacco F, Vasaturo A, Caserta
S, Guido S, Pagliara V, Garbi C, Mansueto G, Cassese A, Perruolo G,
et al: PED/PEA-15 controls fibroblast motility and wound closure by
ERK1/2-dependent mechanisms. J Cell Physiol. 227:2106–2116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stassi G, Garofalo M, Zerilli M,
Ricci-Vitiani L, Zanca C, Todaro M, Aragona F, Limite G, Petrella G
and Condorelli G: PED mediates AKT-dependent chemoresistance in
human breast cancer cells. Cancer Res. 65:6668–6675. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun XP, Dong X, Lin L, Jiang X, Wei Z,
Zhai B, Sun B, Zhang Q, Wang X, Jiang H, et al: Up-regulation of
survivin by AKT and hypoxia-inducible factor 1α contributes to
cisplatin resistance in gastric cancer. FEBS J. 281:115–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trencia A, Perfetti A, Cassese A,
Vigliotta G, Miele C, Oriente F, Santopietro S, Giacco F,
Condorelli G, Formisano P, et al: Protein kinase B/Akt binds and
phosphorylates PED/PEA-15, stabilizing its antiapoptotic action.
Mol Cell Biol. 23:4511–4521. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi N, Peacock JW, Beraldi E, Zoubeidi
A, Gleave ME and Ong CJ: Hsp27 silencing coordinately inhibits
proliferation and promotes Fas-induced apoptosis by regulating the
PEA-15 molecular switch. Cell Death Differ. 19:990–1002. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Jiang X, Zhang Q, Dong X, Gao Y, He
Y, Qiao H, Xie F, Xie X and Sun X: Neuropilin-1 is associated with
clinicopathology of gastric cancer and contributes to cell
proliferation and migration as multifunctional co-receptors. J Exp
Clin Cancer Res. 35:162016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
17
|
Shin M, Lee KE, Yang EG, Jeon H and Song
HK: PEA-15 facilitates EGFR dephosphorylation via ERK sequestration
at increased ER-PM contacts in TNBC cells. FEBS Lett.
589:1033–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Böck BC, Tagscherer KE, Fassl A, Krämer A,
Oehme I, Zentgraf HW, Keith M and Roth W: The PEA-15 protein
regulates autophagy via activation of JNK. J Biol Chem.
285:21644–21654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L,
Zhang Q, Wu Y, Jiang H and Sun X: STAT3 interacts with Skp2/p27/p21
pathway to regulate the motility and invasion of gastric cancer
cells. Cell Signal. 25:931–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L, Li G, Zhu H, Dong X, Zhao D, Jiang
X, Li J, Qiao H, Ni S and Sun X: 2-Methoxyestradiol synergizes with
sorafenib to suppress hepatocellular carcinoma by simultaneously
dysregulating hypoxia-inducible factor-1 and −2. Cancer Lett.
355:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu
B, Pan S, Dong X, Tan G, Wei Z, et al: Inhibition of Akt reverses
the acquired resistance to sorafenib by switching protective
autophagy to autophagic cell death in hepatocellular carcinoma. Mol
Cancer Ther. 13:1589–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han P, Li H, Jiang X, Zhai B, Tan G, Zhao
D, Qiao H, Liu B, Jiang H and Sun X: Dual inhibition of Akt and
c-Met as a second-line therapy following acquired resistance to
sorafenib in hepatocellular carcinoma cells. Mol Oncol. 11:320–334.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
25
|
Eckert A, Böck BC, Tagscherer KE, Haas TL,
Grund K, Sykora J, Herold-Mende C, Ehemann V, Hollstein M,
Chneiweiss H, et al: The PEA-15/PED protein protects glioblastoma
cells from glucose deprivation-induced apoptosis via the ERK/MAP
kinase pathway. Oncogene. 27:1155–1166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stottrup C, Tsang T and Chin YR:
Upregulation of AKT3 confers resistance to the AKT inhibitor MK2206
in breast cancer. Mol Cancer Ther. 15:1964–1974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hindupur SK, Balaji SA, Saxena M, Pandey
S, Sravan GS, Heda N, Kumar MV, Mukherjee G, Dey D and Rangarajan
A: Identification of a novel AMPK-PEA15 axis in the
anoikis-resistant growth of mammary cells. Breast cancer Res.
16:4202014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zanca C, Garofalo M, Quintavalle C, Romano
G, Acunzo M, Ragno P, Montuori N, Incoronato M, Tornillo L,
Baumhoer D, et al: PED is overexpressed and mediates TRAIL
resistance in human non-small cell lung cancer. J Cell Mol Med.
12:2416–2426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Zhu XY, Wang L and Lin Y:
Expression and significance of CDC25B, PED/PEA-15 in esophageal
carcinoma. Cancer Biother Radiopharm. 30:139–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartholomeusz C, Rosen D, Wei C, Kazansky
A, Yamasaki F, Takahashi T, Itamochi H, Kondo S, Liu J and Ueno NT:
PEA-15 induces autophagy in human ovarian cancer cells and is
associated with prolonged overall survival. Cancer Res.
68:9302–9310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee J, Bartholomeusz C, Krishnamurthy S,
Liu P, Saso H, Lafortune TA, Hortobagyi GN and Ueno NT: PEA-15
unphosphorylated at both serine 104 and serine 116 inhibits ovarian
cancer cell tumorigenicity and progression through blocking
β-catenin. Oncogenesis. 1:e222012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh M, Chaudhry P, Fabi F and Asselin E:
Cisplatin-induced caspase activation mediates PTEN cleavage in
ovarian cancer cells: A potential mechanism of chemoresistance. BMC
Cancer. 13:2332013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radisavljevic Z: AKT as locus of cancer
multidrug resistance and fragility. J Cell Physiol. 228:671–674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li CW, Xia W, Lim SO, Hsu JL, Huo L, Wu Y,
Li LY, Lai CC, Chang SS, Hsu YH, et al: AKT1 inhibits
Epithelial-to-mesenchymal transition in breast cancer through
phosphorylation-dependent Twist1 degradation. Cancer Res.
76:1451–1462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Serova M, de Gramont A, Tijeras-Raballand
A, Dos Santos C, Riveiro ME, Slimane K, Faivre S and Raymond E:
Benchmarking effects of mTOR, PI3K, and dual PI3K/mTOR inhibitors
in hepatocellular and renal cell carcinoma models developing
resistance to sunitinib and sorafenib. Cancer Chemother Pharmacol.
71:1297–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maiso P, Huynh D, Moschetta M, Sacco A,
Aljawai Y, Mishima Y, Asara JM, Roccaro AM, Kimmelman AC and
Ghobrial IM: Metabolic signature identifies novel targets for drug
resistance in multiple myeloma. Cancer Res. 75:2071–2082. 2015.
View Article : Google Scholar : PubMed/NCBI
|