Introduction
Breast cancer is the most common malignancy among
women (1). Most breast
cancer-associated mortalities result from tumor metastasis, which
frequently follows a specific dissemination pattern. Breast cancer
is a complex molecular disease in which gene mutations result in
uncontrolled cell growth and proliferation. These mechanisms mainly
involve cell growth and cell death pathways. As apoptosis has an
important role in tumor surveillance (2), the induction of apoptosis may result
in tumor suppression. Recently, there has been renewed interest in
finding natural therapeutic compounds that can induce apoptosis and
inhibit cell proliferation to treat cancer (3–5).
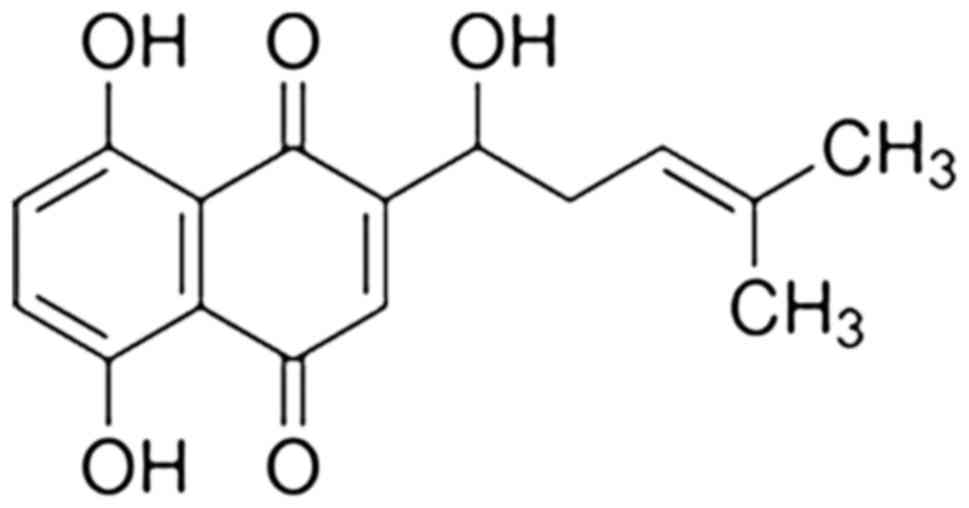
Shikonin is a natural naphthoquinone derivative
isolated from the traditional medical herb Lithospermum
erythrorhizon. Shikonin has anti-tumor, anti-inflammatory
(6), anti-microbial (7), and anti-thrombotic properties
(8). Prolonged exposure of shikonin
to cancer cells does not result in chemoresistance, and shikonin
inhibits the expression of inflammatory cytokines and associated
signaling pathways (9). Shikonin
decreases the expression of tumor necrosis factor (TNF)α,
interleukin (IL)12, IL6, IL1β, IL2, and interferon (IFN)γ (10), inhibits extracellular
signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK)
signaling (11), and reduces the
expression of nuclear factor (NF)-κB and signal transducer and
activator of transcription 3 (STAT3) transcription factors.
Shikonin inhibits the proteasome and modulates cancer cell
metabolism by inhibiting the tumor-specific pyruvate kinase
M214-16. Shikonin causes cell cycle arrest and induces necroptosis
in various cancer types. In addition, shikonin inhibits matrix
metalloproteinase (MMP)9 and integrin β1 expression, and decreases
the invasive potential of cancer cells. The p38 and JNK signaling
pathways are targeted by anti-cancer agents to induce apoptosis in
murine 4T1 cells (12,13). Collectively, shikonin modulates
various signaling pathways and elicits responses in various cancer
types.
In breast cancer, shikonin induces cell death and
inhibits migration (14,15), but the mechanisms are not yet well
understood. The importance of signaling pathways modulated by
shikonin in cancerous and non-cancerous models has previously been
examined with respect to breast carcinoma growth, metastasis and
tumorigenicity.
In the present study, the molecular mechanisms
underlying the effects of shikonin on the inhibition of 4T1 cell
proliferation and the induction of apoptosis were investigated
in vitro and in vivo.
Materials and methods
Compounds and reagents
Shikonin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) and dissolved in dimethyl sulfoxide
(DMSO); the final concentration was 100 mM. SB203580 and SP600125
inhibitors were purchased from Wako Pure Chemical Industries, Ltd.
(Osaka, Japan). A Cell Counting Kit-8 (CCK8) was purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). A
Caspase-3/7 Fluorescence Assay kit was purchased from Cayman
Chemical Company (Ann Arbor, MI, USA). Antibodies for total JNK,
phospho (p)-JNK, total p38, p-p38, and β-actin were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Caspase-3/7
inhibitor (N-Ac-DEVD-CHO) was purchased from Cayman Chemical
Company. Medetomidine, midazolam, and butorphanol were purchased
from Nippon Zenyaku Kogyo Co., Ltd. (Fukushima, Japan), Astellas
Pharma Inc (Tokyo, Japan) and Meiji Seika Pharma Co., Ltd. (Tokyo,
Japan), respectively.
Cell lines and cell culture
conditions
The 4T1 murine mammary cancer and MDA-MB-231 human
breast cancer cells (ATCC, Manassas, VA, USA) were cultured in
RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum
(FBS) (both from Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and 1% penicillin and streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. All assays were performed
when the cells reached 70% confluency.
Mice and the orthotopic model of
murine mammary cancer cells
Female BALB/c mice (6 weeks old) were purchased from
Sankyo Labo Service Corporation, Inc. (Tokyo, Japan). This study
was carried out in strict accordance with the Guide for the Care
and Use of Laboratory Animals of University of Toyama (Toyama,
Japan). The protocol was approved by the Committee on the Ethics of
Animal Experiments of University of Toyama. All surgery was
performed under optimal anesthesia [a combination anesthetic with
medetomidine (0.3 mg/kg), midazolam (4.0 mg/kg) and butorphanol
(5.0 mg/kg)], and all efforts were made to minimize suffering.
The animals were grouped according to body weight,
and housed under controlled laboratory conditions, including a 12 h
light/12 h dark cycle and free access to standard diet and water.
For cancer induction, the mice received a single subcutaneous
injection of 1×105 4T1 breast cancer cells suspended in
phosphate-buffered saline (PBS) in the fourth mammary fat pad.
Tumors were inspected and measured every other day: Tumor volume
(mm3) = 1/2 [length (mm) × width2
(mm2)].
Cell proliferation assay
The 4T1 cells were cultured into 6-cm dishes with
RPMI-1640 medium, supplemented with 10% (v/v) FBS and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. After a 24 h incubation period,
adherent cells covered 70% of the dish surface. Cells were
stimulated with different concentrations of shikonin (0–8 µM) for
24 h and then imaged with a Type 1109 137 001 light microscope
(Leica, Wetzlar, Germany).
Cytotoxicity assay
Cytotoxicity in 4T1 and MDA-MB-231 cells was
measured with a CCK-8 assay, following the manufacturer's
instructions. Briefly, cells were seeded at a density of
1×105/100 µl/well in 96-well plates, and then incubated
at 37°C in a humidified atmosphere containing 5% CO2 for
12 h in RPMI-1640 medium. Cells were treated with different
concentrations of shikonin (0-4.0 µM). After a 24-h incubation
period, the wells were washed twice with cold PBS (−) (high
temperature sterilized) and then 100 µl RPMI-1640 medium and 10 µ
CCK-8 were added to each well; the cells were incubated for another
2 h. Optical density was detected at 450 nm with a Filter Max F5
Multi-Mode microplate reader (Molecular Devices, San Jose, CA,
USA).
Caspase-3/7 fluorescence assay and
inhibitor experiment
To examine apoptosis in 4T1 and MDA-MB-231 cells
after shikonin treatment, a caspase-3/7 fluorescence assay was
used. Cells were seeded in a 96-well plate at a density of
1×104/well in RPMI-1640 medium at 37°C in a humidified
atmosphere containing 5% CO2 overnight. The cells were
treated with shikonin for 12 h in an incubator at 37°C and 5%
CO2. The plate was centrifuged at 400 × g for 5 min. The
culture medium was aspirated, 200 µl assay buffer was added to each
well, the plate was centrifuged at 630 × g for 5 min, after which
the supernatant was aspirated. Subsequently, 50 µl cell-based assay
lysis buffer was added to each well. The cells were incubated with
gentle shaking in an orbital shaker for 30 min at room temperature
and then the plate was centrifuged at 630 × g for 10 min. Following
this, 45 µl supernatant was transferred from each well to a
corresponding well in a new black microtiter 96-well plate.
Afterward, 5 µl assay buffer was added to the wells for sample
activity measurement, and 5 µl caspase-3/7 inhibitor solution was
used to test assay specificity. Then, 50 µl caspase-3/7 substrate
solution was added to each well and the plate was incubated at 37°C
for 30 min. The fluorescence intensity of each well was measured at
an excitation wavelength of 485 nm and an emission wavelength of
535 nm. The 4T1 cells were pretreated with inhibitors of p38
(SB203850, 10 µM) and JNK (SP600125, 10 µM) for 15 min in a 37°C
incubator containing 5% CO2. Cells were washed with cold
PBS (−) twice after pretreatment, and then treated with 2 µM
shikonin for 12 h in an incubator. Afterward, the same protocol was
followed, but the caspase-3/7 inhibitor was removed. Instead, assay
buffer was added to all wells after the supernatant was transferred
to the corresponding well in a new black microtiter 96-well
plate.
Western blotting assay
4T1 cells were categorized into two groups: The
control group, which had 4T1 cells with/without 2 µM shikonin
treatment for 24 h, and the treatment group, which had 4T1 cells
treated with 2 µM shikonin for 24 h and pretreated with/without p38
and JNK inhibitors for 15 min. Whole cell lysates were prepared
with lysis buffer (20 mM HEPES-NaOH, 0.3 M NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 0.001% Triton X-100, 1 mM DTT, 1 mM
sodium orthovanadate, 20 mM β-glycerophosphate disodium salt
hydrate, 10 µg/ml aprotinin, 10 µg/ml leupeptin, and 1 mM PMSF).
Protein concentrations in lysates were quantified by a protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Total
protein was resolved via 12% SDS-PAGE, and then transferred to a
PVDF membrane at 20 mA for 30 min. The membranes were blocked with
non-fat milk for one night at 4°C. Primary antibodies [β-actin
(13E5) rabbit mAb, no. 4970; p38 MAPK antibody, no. 9212; p-p38
MAPK (Thr180/Tyr182) antibody, no. 9211; SAPK/JNK antibody, no.
9252; p-SAPK/JNK (Thr183/Tyr185) antibody, no. 9251; all from Cell
Signaling Technology, Danvers, MA, USA] were diluted 1:1,000, and
incubated for 90 min at room temperature. After washing with
Tris-buffered saline and Tween 20 three times (10 min each), the
secondary antibody (diluted 1:2,000) was added to the membrane and
incubated at room temperature for 45 min. β-actin was used as the
loading control and for normalization. The density of the blots was
quantified using Image Studio Lite v.5.2 software (LI-COR
Biosciences, Lincoln, NE, USA).
Statistical analysis
All data are expressed as the means ± standard
deviation from three independent experiments. Image Studio Lite
v.5.2 software was used for analysis. Differences between two
groups were determined using a Student's t-test and Bonferroni's
correction, comparing the treated groups with the control.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell proliferation
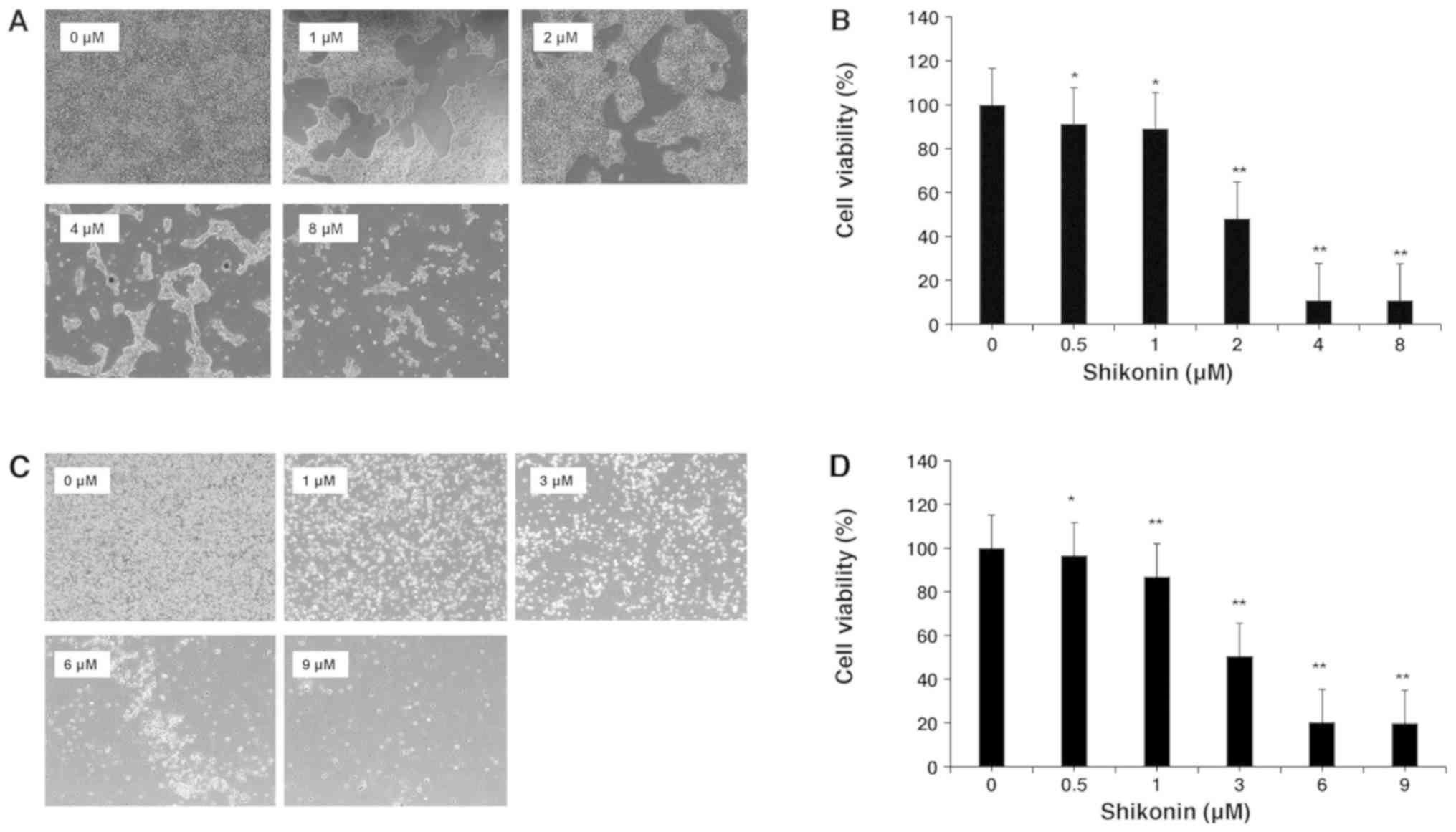
4T1 and MDA-MB-231 cells were used to investigate
the effect of shikonin on proliferation (Fig. 1). As presented in Fig. 2A, a confluent monolayer of 4T1 cells
was observed without shikonin treatment, but confluence was reduced
with increasing concentrations of shikonin (0–8 µM). Shikonin
significantly inhibited 4T1 cell proliferation in a dose-dependent
manner compared with that of the control, a cytotoxic effect that
was more evident after 24 h. The dose of shikonin that effectively
inhibited 50% of the growth of 4T1 cells after 24 h was 2 µM
(Fig. 2B); additionally, the IC50
of MDA-MB-231 cells was 3 µM (Fig. 2C
and D)
Involvement of caspase-3/7 in
shikonin-induced apoptosis
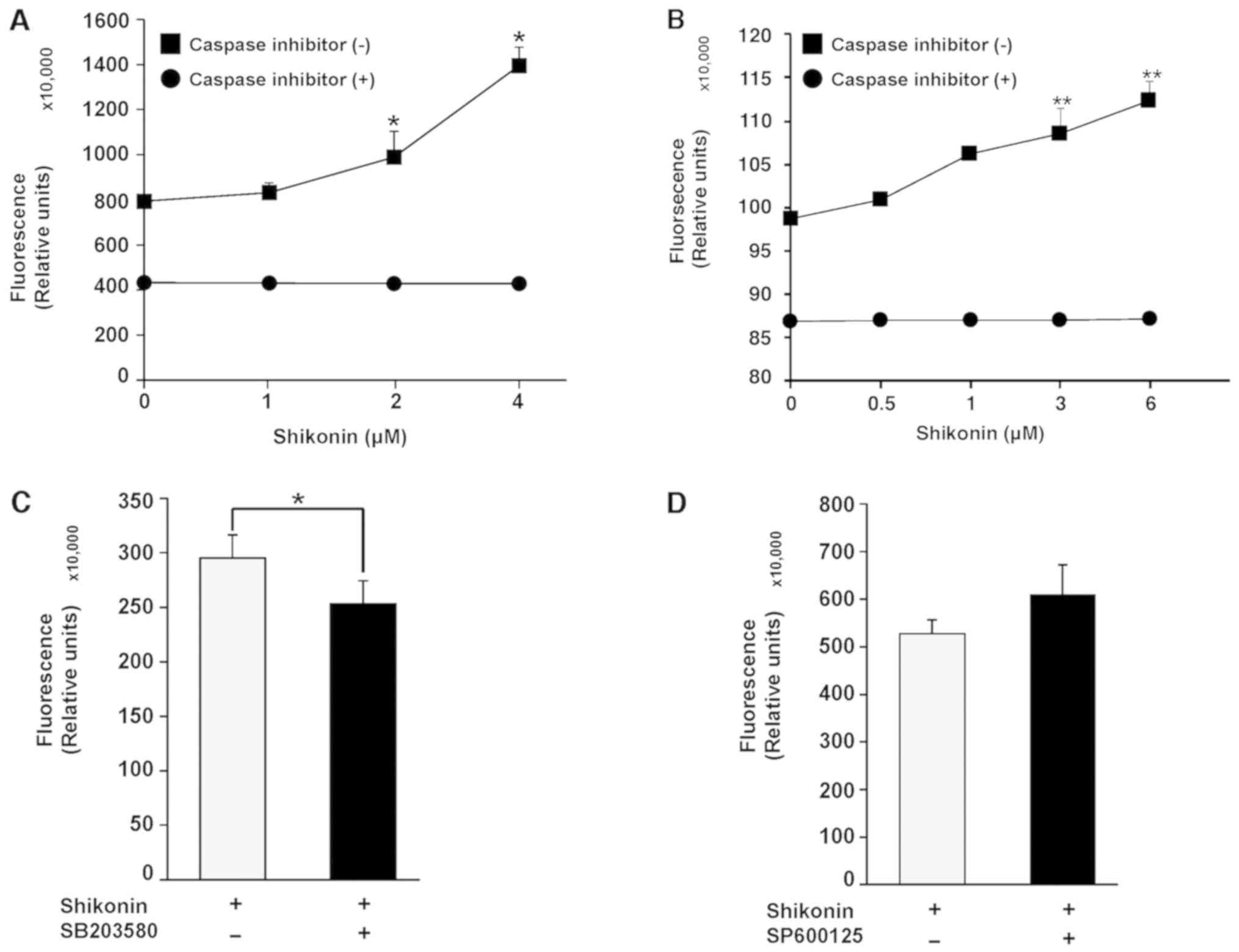
Treatment of 4T1 cells with 2 and 4 µM (Fig. 3A) shikonin resulted in a significant
increase in caspase-3/7 activity compared with the control.
Shikonin also increased caspase-3/7 activity in MDA-MB-231 cells
(Fig. 3B). The involvement of
caspase in shikonin-induced apoptosis was examined by pretreating
cells with SB203580 (p38 inhibitor, 10 µM) (Fig. 3C) or SP600125 (JNK inhibitor, 10 µM)
(Fig. 3D). SB203580, but not
SP600125, significantly inhibited shikonin-induced apoptosis in 4T1
cells by decreasing caspase activity. These results suggested that
shikonin-induced apoptosis in breast cancer cells is
caspase-dependent and regulated by the p38 signaling pathway.
Involvement of the p38 and JNK
pathways in shikonin-induced apoptosis
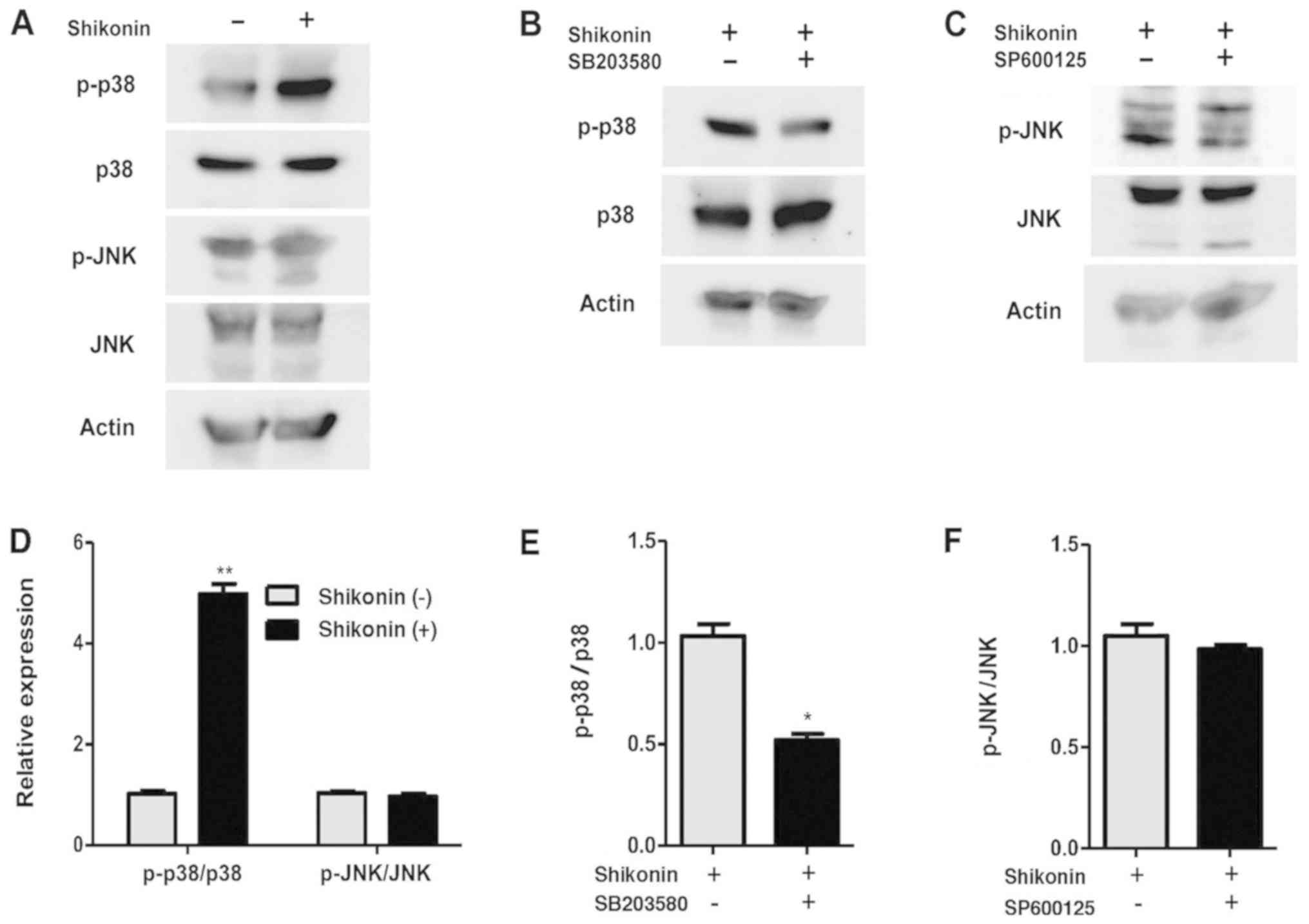
Mitogen-activated protein kinases, such as JNK and
p38, control cell proliferation and death (16), and shikonin can regulate JNK and p38
activation in prostate cancer. To determine the effect of shikonin
in 4T1 cells, the activation of p38 and JNK signaling pathways was
investigated via western blotting. Total p38 and JNK levels were
similar when compared with those in the non-treated group (Fig. 4A). As presented in Fig. 4B and C, both groups of 4T1 cells
were treated with 2 µM shikonin for 24 h. But p38 phosphorylation
increased with shikonin treatment (Fig.
4D) and p-JNK levels did not change (Fig. 4D). In the group pretreated with
SB203580, p-p38 levels were markedly suppressed (Fig. 4B and E). However, pretreatment with
SP600125 only slightly decreased p-JNK levels (Fig. 4C and F). Thus, the p38 signaling
pathway is associated with shikonin-induced apoptosis. Statistical
analysis through Image Studio Lite v.5.2 software from LI-COR and
showed a significant increase in the expression of p-p38 in groups
where shikonin was administered, and decrease in the expression of
p-p38 in groups which pretreated with SB203580.
Anti-tumor effect of shikonin on 4T1
cancer
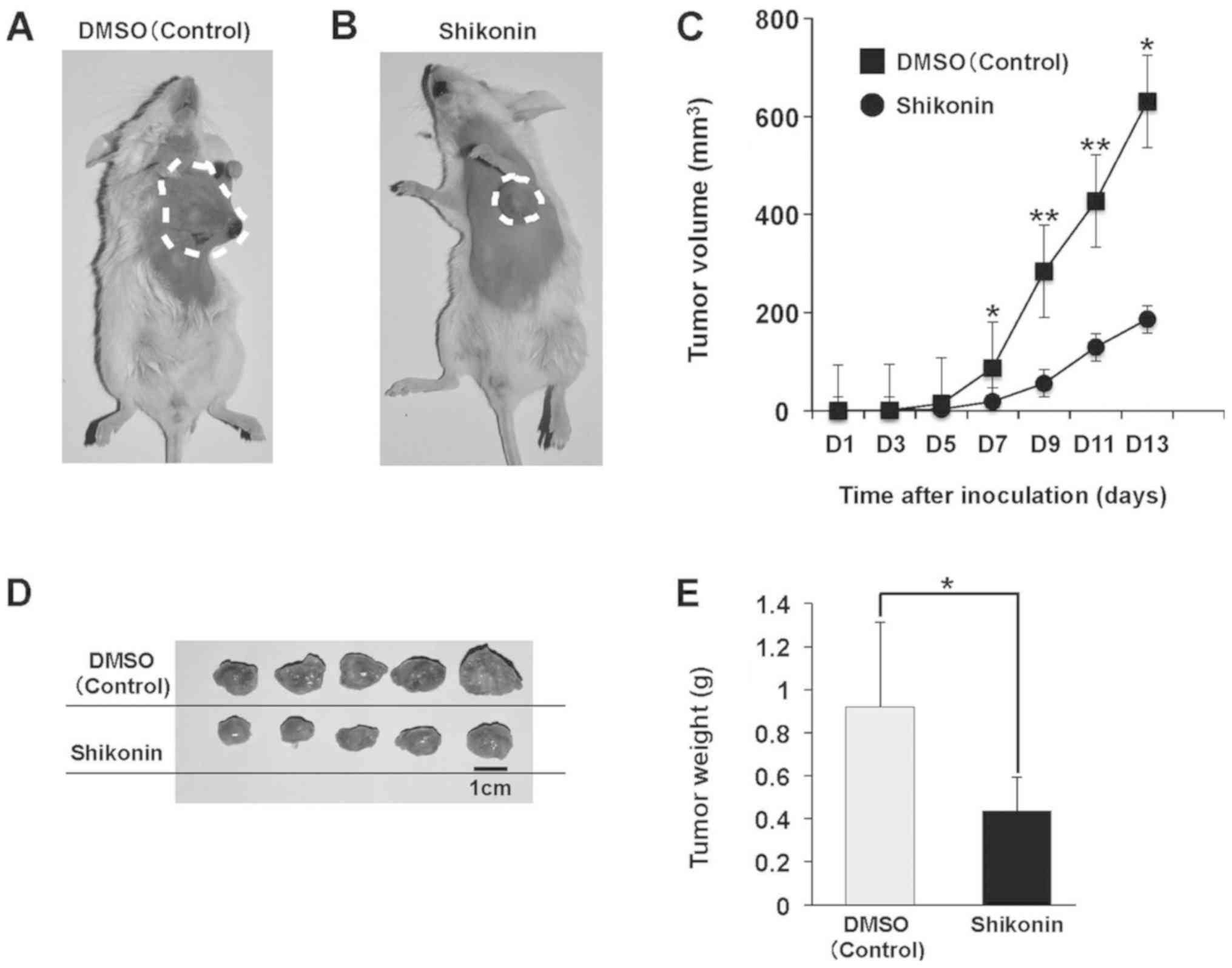
To determine whether shikonin inhibits tumor growth
in vivo, an orthotopic 4T1 model was used. A single
subcutaneous injection of 1×105 4T1 breast cancer cells
suspended in PBS was administered into the fourth mammary fat pad
in mice. Shikonin (2 mg/kg) or 1% DMSO was injected on the same day
as 4T1 cell implantation. Tumor volume was measured with a Vernier
caliper every other day at the same time as shikonin or DMSO
injection. Shikonin suppressed orthotopic 4T1 tumor growth in
vivo (Fig. 5). From day 7,
tumor volumes started to differ between the treatment and DMSO
groups, and there were significant differences up to day 13
(Fig. 5A). On day 13, mice were
sacrificed, and tumor sizes were measured (Fig. 5B).
The humane endpoints of the study (tumors did not
exceed 20 mm in diameter; feeding, drinking and walking behaviors
were not observed) were set in accordance with the Guide for the
Care and Use of Laboratory Animals of University of Toyama. Tumor
weights revealed the anti-tumor effect of shikonin on 4T1 breast
cancer (Fig. 5C).
Discussion
It has been found that shikonin exhibits
anti-proliferation and apoptosis-inducing effects on 4T1 murine
mammary cancer and MDA-MB-231 human breast cancer cells (Figs. 1–3)
(17,18). Various medicinal plants have been
examined for anti-tumor compounds (19). Here, it was found that shikonin
triggers apoptosis and diminishes the growth, size and weight of
tumors in an orthotopic BALB/c mouse model. Inhibition of 4T1 tumor
growth has also been observed in vivo (20,21).
Scrophularia oxysepala extract decreases the growth of
breast cancer cells and reduces tumor mass without significantly
affecting body weight, which suggests that the plant is not toxic
(22). It was found that tumor
volume and size were significantly greater in the control (DMSO)
group than in the shikonin-treated group (Fig. 5), which suggests that shikonin
induces apoptosis in tumor cells. Thus, shikonin may be an ideal
therapeutic tool for breast cancer.
Reactive oxygen species are the most important
mediators of shikonin-induced apoptosis in Bcr/Abl-positive chronic
leukemia cells through JNK activation. Shikonin also inhibits Akt
and receptor-interacting protein/NF-κB pathways in hepatocellular
carcinoma cells (23,24). However, the mechanism of
shikonin-induced apoptosis in various cancer cells is unclear.
Shikonin pretreatment attenuates ConA-induced acute liver injury by
inhibiting apoptosis and autophagy through suppression of the JNK
pathway (25). Apoptosis, the cell
cycle, and migration are closely associated with the p38 pathway
(26). The p38 signaling pathway is
also involved in pulmonary fibrosis, and p38 expression
significantly increases in lung homogenates from patients with
idiopathic pulmonary fibrosis in epithelial, endothelial,
fibroblasts and smooth muscle cells (27,28).
The p38 and JNK pathways were investigated to
understand how shikonin induces 4T1 tumor apoptosis (Fig. 4A and D). Apoptosis limits cancer
propagation, and affects the development and progression. Apoptosis
is modulated by many anti-apoptotic and pro-apoptotic factors. JNK
and p38 are critical for 4T1 cell apoptosis (29,30).
4T1 cells were pretreated with p38 and JNK inhibitors (SB203580 and
SP600125, respectively) for 15 min before cells were incubated with
shikonin, and several proteins were examined, including p38, p-p38,
JNK and p-JNK. Shikonin-treated cells increased p-p38 expression
while maintaining the same level of p38 and JNK expression. After
the p38 signaling pathway was blocked with SB203580, the expression
of p-p38 decreased compared with that of cells not pretreated with
inhibitor (Fig. 4B and E). The
expression of p-JNK was not changed by pretreatment with SP600125
(Fig. 4C and F). Thus, the p38
signaling pathway is a major regulator of shikonin-induced
apoptosis in 4T1 cells.
Caspase-3/7 activation is responsible for the final
step of apoptosis (31,32) and reflects the degree of apoptosis
in various cancer cells (33). To
determine whether the anti-proliferative effect of shikonin was
associated with the induction of apoptosis, a fluorescence
quantification assay was used. Treatment of 4T1 cells with 2 µM
shikonin significantly increased caspase-3/7 activity (Fig. 3A), which suggests that shikonin
inhibits 4T1 cell proliferation and induces apoptosis by activating
caspase-3/7. In addition, SB203580 reduced shikonin-induced 4T1
cell apoptosis by decreasing caspase-3/7 activity (Fig. 3C), though SP600125 did not have a
significant effect (Fig. 3C). In
the present study, shikonin exerted an anti-tumor effect by
inhibiting proliferation and inducing 4T1 and MDA-MB-231 cell
apoptosis. Shikonin was previously found to decrease the migration
(34) and invasion (15) ability of MCF-7 and MDA-MB-231 human
breast cancer cells. Therefore, the results suggest that shikonin
can inhibit human breast cancer metastasis. The effect of shikonin
on 4T1 cancer cells will be investigated in a future study. Further
research on the anti-tumor mechanisms of shikonin is needed to
determine whether there are other ligands involved in
immunosuppression, the immune response, and the delay of disease
progression.
In conclusion, shikonin induced p38-dependent
apoptosis in 4T1 and MDA-MB-231 cells and markedly suppressed in
vitro proliferation. Furthermore, shikonin delayed 4T1 cell
growth in an orthotopic mouse model. Thus, shikonin may be a
candidate drug to treat breast cancer.
Acknowledgements
The authors thank Yoshiki Takeshita and Takeshi Eto
for providing technical support.
Funding
This research was partially supported by a
Grant-in-Aid for the Cooperative Research Project from the
Institute of Natural Medicine, University of Toyama in 2014, 2015
and 2017 (grant nos. 2014S, 2015S and 2017A1).
Availability of data and materials
Requests for materials should be addressed to KK
(kkoizumi@inm.u-toyama.ac.jp).
Authors' contributions
JX and KK designed the research; JX, ML, YM, MS, HI,
AI, MF, NS and YS performed the experiments and prepared all the
figures. JX, KK, NS and YS wrote the paper. All authors discussed
and agreed on the results, read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Committee on the
Ethics of Animal Experiments of University of Toyama (Toyama,
Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing financial
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wakimoto R, Ono M, Takeshima M, Higuchi T
and Nakano S: Differential anticancer activity of pterostilbene
against three subtypes of human breast cancer cells. Anticancer
Res. 37:6153–6159. 2017.PubMed/NCBI
|
|
3
|
Chavoshi H, Vahedian V, Saghaei S,
Pirouzpanah MB, Raeisi M and Samadi N: Adjuvant therapy with
silibinin improves the efficacy of paclitaxel and cisplatin in
MCF-7 breast cancer cells. Asian Pac J Cancer Prev. 18:2243–2247.
2017.PubMed/NCBI
|
|
4
|
Zhao Y, Tian B, Wang Y and Ding H:
Kaempferol sensitizes human ovarian cancer cells-OVCAR-3 and SKOV-3
to tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL)-induced apoptosis via JNK/ERK-CHOP pathway and
up-regulation of death receptors 4 and 5. Med Sci Monit.
23:5096–5105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu WS, Chien CC, Liu KH, Chen YC and Chiu
WT: Evodiamine prevents glioma growth, induces glioblastoma cell
apoptosis and cell cycle arrest through JNK activation. Am J Chin
Med. 45:879–899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bi Y, Zhu Y, Zhang M, Zhang K, Hua X, Fang
Z, Zhou J, Dai W, Cui Y, Li J, et al: Effect of shikonin on spinal
cord injury in rats via regulation of HMGB1/TLR4/NF-kB signaling
pathway. Cell Physiol Biochem. 43:481–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan
Q, Luo J, Zen K and Yang J: Inhibiting aerobic glycolysis
suppresses renal interstitial fibroblast activation and renal
fibrosis. Am J Physiol Renal Physiol. 313:F561–F575. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andújar I, Ríos JL, Giner RM and Recio MC:
Pharmacological properties of shikonin-a review of literature since
2002. Planta Med. 79:1685–1697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Wang J, Yang Q, Wu S, Yang Z, Zhu
H, Zheng M, Liu W, Wu W, He J, et al: Shikonin inhibits the
lipopolysaccharide-induced release of HMGB1 in RAW264.7 cells via
IFN and NF-κB signaling pathways. Int Immunopharmacol. 19:81–87.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang SY, Yang XJ, Yang SS, Wang W, Tian
YY, Cao FL and Zhou J: Association analysis of cytokine
polymorphisms and plasma level in Northern Chinese Han patients
with paroxysmal nocturnal hemoglobinuria. Chin Med J (Engl).
125:1576–1580. 2012.PubMed/NCBI
|
|
11
|
Chen Y, Zheng L, Liu J, Zhou Z, Cao X, Lv
X and Chen F: Shikonin inhibits prostate cancer cells metastasis by
reducing matrix metalloproteinase-2/-9 expression via AKT/mTOR and
ROS/ERK1/2 pathways. Int Immunopharmacol. 21:447–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ning L, Ma H, Jiang Z, Chen L, Li L, Chen
Q and Qi H: Curcumol suppresses breast cancer cell metastasis by
inhibiting MMP-9 via JNK1/2 and Akt-dependent NF-κB signaling
pathways. Integr Cancer Ther. 15:216–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scherberich A, Tucker RP, Degen M,
Brown-Luedi M, Andres AC and Chiquet-Ehrismann R: Tenascin-W is
found in malignant mammary tumors, promotes alpha8
integrin-dependent motility and requires p38MAPK activity for BMP-2
and TNF-alpha induced expression in vitro. Oncogene. 24:1525–1532.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su Y, Huang N, Chen D, Zhang L, Dong X,
Sun Y, Zhu X, Zhang F, Gao J, Wang Y, et al: Successful in vivo
hyperthermal therapy toward breast cancer by Chinese medicine
shikonin-loaded thermosensitive micelle. Int J Nanomedicine.
12:4019–4035. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang SY, Lee JK, Jang EH, Jeong SY and Kim
JH: Shikonin blocks migration and invasion of human breast cancer
cells through inhibition of matrix metalloproteinase-9 activation.
Oncol Rep. 31:2827–2833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W, Tian W, Ijaz M and Wang F:
Inhibition of EGF-induced migration and invasion by sulfated
polysaccharide of Sepiella maindroni ink via the suppression of
EGFR/Akt/p38 MAPK/MMP-2 signaling pathway in KB cells. Biomed
Pharmacother. 95:95–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thakur R, Trivedi R, Rastogi N, Singh M
and Mishra DP: Inhibition of STAT3, FAK and Src mediated signaling
reduces cancer stem cell load, tumorigenic potential and metastasis
in breast cancer. Sci Rep. 5:101942015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nie Y, Yang Y, Zhang J, Cai G, Chang Y,
Chai G and Guo C: Shikonin suppresses pulmonary fibroblasts
proliferation and activation by regulating Akt and p38 MAPK
signaling pathways. Biomed Pharmacother. 95:1119–1128. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takaku S, Shimizu M and Takahashi H:
Japanese Kampo medicine ninjin'yoeito synergistically enhances
tumor vaccine effects mediated by CD8+ T cells. Oncol
Lett. 13:3471–3478. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borhani S, Mozdarani H, Babalui S,
Bakhshandeh M and Nosrati H: In vitro radiosensitizing effects of
temozolomide on U87MG cell lines of human glioblastoma multiforme.
Iran J Med Sci. 42:258–265. 2017.PubMed/NCBI
|
|
21
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27, and p53, release of cytochrome
c, and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nam KN, Son MS, Park JH and Lee EH;
Shikonins attenuate microglial inflammatory responses by inhibition
of ERK, Akt, and NF-kappa B, : Neuroprotective implications.
Neuropharmacology. 55:819–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baradaran PC, Mohammadi A, Mansoori B,
Baradaran SC and Baradaran B: Growth inhibitory effect of
Scrophularia oxysepala extract on mouse mammary carcinoma 4T1 cells
in vitro and in vivo systems. Biomed Pharmacother. 85:718–724.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong K and Li W: Shikonin, a Chinese
plant-derived naphthoquinone, induces apoptosis in hepatocellular
carcinoma cells through reactive oxygen species: A potential new
treatment for hepatocellular carcinoma. Free Radic Biol Med.
51:2259–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu T, Xia Y, Li J, Li S, Feng J, Wu L,
Zhang R, Xu S, Cheng K, Zhou Y, et al: Shikonin attenuates
concanavalin A-induced acute liver injury in mice via inhibition of
the JNK pathway. Mediators Inflamm 2016. 27483672016.
|
|
27
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-Mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18:10882017. View Article : Google Scholar :
|
|
28
|
Yoshida K, Kuwano K, Hagimoto N, Watanabe
K, Matsuba T, Fujita M, Inoshima I and Hara N: MAP kinase
activation and apoptosis in lung tissues from patients with
idiopathic pulmonary fibrosis. J Pathol. 198:388–396. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khalil N, Xu YD, O'Connor R and Duronio V:
Proliferation of pulmonary interstitial fibroblasts is mediated by
transforming growth factor-beta1-induced release of extracellular
fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK.
J Biol Chem. 280:43000–43009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn J, Won M, Choi JH, Kim YS, Jung CR, Im
DS, Kyun ML, Lee K, Song KB and Chung KS: Reactive oxygen
species-mediated activation of the Akt/ASK1/p38 signaling cascade
and p21(Cip1) downregulation are required for shikonin-induced
apoptosis. Apoptosis. 18:870–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shahsavari Z, Karami-Tehrani F and Salami
S: Shikonin induced necroptosis via reactive oxygen species in the
T-47D breast cancer cell line. Asian Pac J Cancer Prev.
16:7261–7266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lan W, Wan S, Gu W, Wang H and Zhou S:
Mechanisms behind the inhibition of lung adenocarcinoma cell by
shikonin. Cell Biochem Biophys. 70:1459–1467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nguyen QD, Challapalli A, Smith G, Fortt R
and Aboagye EO: Imaging apoptosis with positron emission
tomography: ‘bench to bedside’ development of the caspase-3/7
specific radiotracer [(18)F]ICMT-11. Eur J Cancer. 48:432–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong D, Jang SY, Jang EH, Jung B, Cho IH,
Park MJ, Jeong SY and Kim JH: Shikonin as an inhibitor of the
LPS-induced epithelial-to-mesenchymal transition in human breast
cancer cells. Int J Mol Med. 36:1601–1606. 2015. View Article : Google Scholar : PubMed/NCBI
|