Introduction
Breast cancer remains one of the most frequent
malignancies in women, accounting for ~350,000 annual mortalities
in recent years worldwide (1,2).
Surgical treatment is currently the preferred option for almost all
types of breast cancer. However, surgical incision procedures lead
to the loss of breast volume and distortion of shape, and follow-up
radiation therapy often results in breast tissue fibrosis and poor
wound healing (3,4). Among the plastic surgery techniques
currently available to reconstruct the breast, fat grafting is
gaining major interest given its ease to harvest, low morbidity and
capacity to improve the tissue quality, particularly in
breast-conserving surgery (5–9).
However, the possibility that breast cancer cells may still reside
in patients with breast cancer after the surgical treatment cannot
be completely excluded.
Adipose tissue is a multifunctional organ mainly
consisted of mature adipocytes and the adipose mesenchymal stromal
cells (MSCs)/stromal vascular fraction. Adipose MSCs are
heterogeneous and contain several populations, including
adipose-derived stem cells (ADSCs), endothelial progenitor cells,
pre-adipocytes, lymphocytes, mast cells, pericytes, and
adipose-resident macrophages (10,11).
Transplantation of adipose tissue, consisting of ADSCs that are
metabolically active and secrete various cytokines, does not simply
behave as an inert filler but it tends to influence the cancer
microenvironment (12,13). Hence, whether fat grafting can be
applied to patients following breast cancer surgery is still a
controversial issue.
It is widely recognized that multipotent ADSCs with
their regenerative features, such as pro-angiogenic,
anti-apoptotic, pro-proliferative and multipotent differentiation
characteristics, within the transferred fat mainly contribute to
the restorative and reconstructive qualities of autologous fat
grafting (14–16). Unfortunately, these regenerative
features are also assumed to be associated with tumor initiation
and metastasis, causing safety concerns in clinical utilization.
Notably, the majority of studies investigate the interaction
between ADSCs and breast cancer cells and are performed in a
two-dimensional (2D) context in vitro (17–20),
which does not fully recapitulate the in vivo condition.
Hence, an in vitro 3D culture system was established and
applied an in vivo animal model to investigate the
interaction between ADSCs and MCF-7 breast cancer cells in tumor
development in the present study, mainly focusing on the tropism of
ADSCs towards the breast cancer cells and the potential mechanism
of ADSCs on promoting MCF-7 cells progression.
Materials and methods
Ethics approval
All procedures performed in the present study
involving human participants were approved by the Southern Medical
University Institutional Review Board (Guangzhou, China) and the
patient provided written informed consent to donate remaining
tissues after liposuction. All procedures performed involving
animal experiments were approved by the Nanfang Hospital animal
ethic committee (permit no. NFYY201679) and was conducted in
accordance with the ethical standards of the National Health and
Medical Research Council China.
Cell preparation and
identification
Human ADSCs were isolated from abdominal liposuction
aspirates of a 28-year-old female patient during an abdominoplasty
procedure with informed consent under approval from the Southern
Medical University Institutional Review Board. Briefly, fat
aspirate was washed with PBS, centrifuged at 800 × g at 25°C for 5
min and digested with 0.1% collagenase at 37°C for 2 h. The
dispersed material was centrifuged (170 × g; 25°C) for 5 min, and
the pellet was resuspended in Dulbecco's modified Eagle's medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin,
and seeded in flasks. Next day, non-adherent cells were removed,
and the remaining cells were cultured until 80% confluency. Passage
3 ADSCs were used in the following experiments. For the senescence
evaluation of used cells, passage 3 ADSCs were further subjected to
replicative senescence experiments. For a control culture, the same
senescence experiments were conducted on ADSCs at passage 10.
MCF-7 cells were obtained from the Research
Laboratory Collaboration Alliance of Nan Fang Hospital (Guangzhou,
China). All cells used in the present study were maintained in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin, in a humidified (85%) atmosphere with 5%
CO2 at 37°C.
To induce multilineage differentiation, ADSCs were
cultured in adipogenic, osteogenic, and chondrogenic medium as
previously described (21). Fat,
bone and cartilage cells differentiated from ADSCs were identified
by staining with Oil Red O (15 min at 25°C), Alizarin red (5 min at
25°C) or Alcian blue (30 min at 25°C), respectively.
Senescence-associated β-galactosidase
assay
β-Galactosidase assay was used for assessing
senescence of used cells using a Senescence-associated
β-galactosidase Staining kit (cat. no. C0602; Beyotime Institute of
Biotechnology, Haimen, China) as previously described (22,23).
Briefly, passage 3 and 10 ADSCs were washed in PBS, fixed for 10
min (room temperature) in 2% formaldehyde, washed, and incubated
with the working solution containing 0.05 mg/ml
5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside (X-gal). After
incubation at 37°C for 12 h in the dark, the nucleus was
counterstained with nuclear fast red (cat. no. N8002;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and positive cells
were observed under a light microscope at ×200 magnification. The
percentage of senescent cells was calculated by the number of blue,
β-galactosidase-positive cells out of all cells in 6 different
microscope fields. Senescence assays were performed in
triplicate.
Preparation of co-culture conditioned
media
To study the effects of cytokines from a co-culture
system on MCF-7 cells, ADSCs and MCF-7 co-culture conditioned media
(AM-CM) was prepared. The same amount (4×105) of ADSCs
and MCF-7 cells were plated in a flask and co-cultured to 80%
confluency. Serum-free DMEM was added to the flask and cultured for
48 h at 37°C after being washed with PBS twice. The AM-CM was
filtered and stored at −80°C for a week, until further use.
Cell membrane labeling and co-culture
in Matrigel
To track the interaction between cells, ADSCs and
MCF-7 cells were stained with Vybrant® DiI Cell-Labeling
Solution and DiO Cell-Labeling Solution, respectively (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The same amount (4×104) of ADSCs and MCF-7
cells were mixed uniformly and seeded in Growth-factor-reduced
Matrigel (cat. no. 356230; BD Biosciences, Franklin Lakes, NJ, USA)
to fabricate a 3D culture system. The interactions between ADSCs
and MCF-7 cells was observed continuously in Matrigel for 96 h at
37°C and 5% CO2 using a confocal laser-scanning
microscope (FV10i-W; Olympus Corporation, Tokyo, Japan). Co-culture
assays were performed in quadruplicate.
Scanning electron microscopy
(SEM)
For scanning electron microscopy, the same amount
(4×104) of ADSCs and MCF-7 cells were co-cultured at
37°C in Matrigel on round glass coverslips (Carl Roth GmbH &
Co. KG, Karlsruhe, Germany) in 12-well plates. After 2 days,
co-culture samples were fixed in PBS (1 ml) with 2% glutaraldehyde
and incubated at 25°C for 60 min. Samples were dehydrated in
increasing concentrations of acetone, critical-point dried, fixed
to stubs with colloidal silver, sputtered with gold using a MED 010
coater and examined under a S-3000N scanning electron microscope
(Hitachi Ltd., Tokyo, Japan). An acceleration voltage of 20 kV was
used, and images were observed using S-3000N scanning electron
microscope (Hitachi, Ltd.).
In vitro Transwell migration
assay
Adipose stromal cells (ASC) migration assays were
performed in triplicate using Transwell migration chambers (8-µm
pore size; BD Biosciences). ADSCs (3×103) were plated in
the upper wells, whereas MCF-7 cells (3×103) or AM-CM
(600 µl) were dispensed in the lower chamber. Controls were
represented by serum-free DMEM. After 24 h of incubation at 37°C,
cells that remained on the top of the filter were scrubbed off, and
cells that had migrated to the underside of the filter were fixed
in methanol and stained with DAPI for 30 min at 25°C. Migrated
cells were manually counted under a fluorescence microscope.
Migration assays were performed in triplicate.
To further investigate the mechanisms underlying the
cells migration in this co-culture system, MCF-7 cells
(1×105) were co-cultured with ADSCs cells
(1×105) in Matrigel. MCF-7 alone served as the control.
Cells in both groups were collected for gene analysis at day 1, 5,
9.
Tumorsphere formation in vitro
To investigate the tumorsphere formation capacity of
MCF-7 cells under the influence of either contact or secretion
signals of ADSCs, MCF-7 cells (1×105) were either
co-cultured with ADSCs (1×105) or treated with 1 ml
AM-CM in Matrigel in 6-wells plates. MCF-7 cells (1×105)
alone served as the control. The diameters of tumorspheres in four
random fields of each well were counted and imaged. Tumorsphere
formation assays were performed in triplicate.
To further investigate the effects of cytokines from
a co-culture system on tumorsphere formation of MCF-7 cells, MCF-7
cells (1×105) were treated with 1 ml AM-CM (replaced
every 3 days) in Matrigel in 6-wells plates. MCF-7 cells
(1×105) alone served as the control. On day 1, 5 and 9,
the Matrigel cultures were made into single-cell suspensions using
Dispase (10 mg/ml; Roche Diagnostics, Basel, Switzerland) and cells
in both groups were collected for subsequent gene analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The co-cultures and MCF-7 cells alone that were
cultured on Matrigel were made into single-cell suspensions using
Dispase (10 mg/ml; Roche Diagnostics). Sample RNA was extracted
using TRIzol® Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and used for cDNA synthesis with the DBI-2220
Bestar® qPCR RT Kit (DBI Bioscience, Ludwigshafen,
Germany) according to the manufacturer's protocols. qPCR was
conducted using a customized All-in-One™ qPCR Primer Array
(GeneCopoeia, Inc., Rockville, MD, USA) and the ABI 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primers used in the present study were: Macrophage
inflammatory protein (MIP)-1δ, forward
5′-CCACTCAACATACTGCCTTCTA-3′, and reverse
5′-AGTGTAGGAACCCTGCATTAC-3′; MIP-3α, forward
5′-CCAAAGAACTGGGTACTCAACA-3′, and reverse
5′-GAGTAGCAGCACTGACATCAA-3′; SOX2, forward
5′-GAGAGAGAAAGAAAGGGAGAGAAG-3′, and reverse
5′-GAGAGAGGCAAACTGGAATCA-3′; OCT4, forward
5′-GGAGGAAGCTGACAACAATGA-3′, and reverse
5′-CTCTCACTCGGTTCTCGATACT-3′; E-Cadherin, forward
5′-CTCGACACCCGATTCAAAGT-3′, and reverse 5′-CCAGGCGTAGACCAAGAAAT-3′;
Vimentin, forward 5′-GATTCACTCCCTCTGGTTGATAC-3′, and reverse
5′-GTCATCGTGATGCTGAGAAGT-3′; and β-actin, forward
5′-GGACCTGACTGACTACCTCAT-3′, and reverse
5′-CGTAGCACAGCTTCTCCTTAAT-3′. Thermocycling conditions were as
follows: 95°C for 10 min; followed by 40 cycles of 10 sec at 95°C
and 20 sec at 55°C. PCR specificity was assessed by the
2−ΔΔCq method (24);
β-actin was used as an endogenous reference gene and for
normalization.
Western blot analysis
The Matrigel cultures were made into single-cell
suspensions using Dispase (10 mg/ml; Roche Diagnostics). Protein
was extracted from cells (1×106) using M-PER Mammalian
Protein Extraction Reagent (Thermo Fisher Scientific, Inc.) and
quantification of protein lysates was conducted with the Bradford
method. Protein products (60 µg/lane) were separated using 10%
SDS-PAGE and subsequently transferred overnight onto a
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked in 5% milk for 1 h at 25°C and
incubated with the following primary antibodies: Anti-E-Cadherin
[1:500; Cell Signaling Technology (CST), Inc., Danvers, MA, USA;
cat. no. 3195]; or anti-Vimentin antibody (1:500; CST, Inc.; cat.
no. 5741). The membranes were cultured with horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G secondary
antibody (1:10,000; cat. no. 111-035-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 30 min at 25°C, and
proteins were visualized with the WesternBreeze Chemiluminescent
Detection kit (Thermo Fisher Scientific, Inc.). β-actin (1:1,000;
cat. no. 4970; CST, Inc.) served as an internal control.
Tumor-bearing mice preparation and in
vivo imaging of ADSCs homing to tumors
Female nude mice (n=9; age, 4–5 weeks; weight, 13–15
g) were purchased from the Southern Medical University Laboratory
Animal Center and were maintained in microisolator cages at the
Animal Experiment Center of Nanfang Hospital. Mice were housed in a
pathogen-free animal facility (25±2°C; 55% humidity) with ad
libitum access to standard food and water, and a 12-h
light/dark cycle. Tumor-bearing models were prepared as previously
described (25). Briefly, MCF-7
cells (2×106), resuspended in 0.5 ml PBS, were
subcutaneously injected into each side of groin adipose pad of the
nude mouse. Tumor formation was observed at the groin area 2–3
weeks following the MCF-7 cells injection and the tumor-bearing
mice were used in subsequent in vivo experiments. A cell
tracer Vybrant® DiI was used to trace ADSCs in
vivo; DiI labeling solution was prepared following the
manufacturer's protocol. Briefly, 5×105 ADSCs in 1 ml of
PBS were mixed with 5 µl of DiI labeling solution and incubated for
15 min at 37°C. After labeling, cells were washed and injected into
the tail veins of tumor-bearing mice. Bioluminescence imaging of
mice and excised organs (lung, heart, liver and kidney) and tumors
was conducted to trace the ADSCs using an in vivo
Multispectral Imaging Systems FX, bioluminescence imaging system
(Carestream Health, Inc., Rochester, NY, USA) at 4 weeks following
ADSC injection.
Xenograft assays in nude mice and
frozen sectioning
Female nude mice (age, 4–5 weeks; weight, 13–15 g;
n=30) were purchased from the Southern Medical University
Laboratory Animal Center and were maintained as aforementioned. For
xenograft experiments, MCF-7 cells (1×106) mixed with
ADSCs (1×106) were injected into the right groin adipose
pad, whereas MCF-7 cells (1×106) alone injected into the
left groin adipose pad served as a control. Tumor volumes were
measured using Vernier calipers every 4 days for 32 consecutive
days and calculated using the following formula: Tumor volume =
(length × width2)/2, following previously published
protocols (26,27). The mice were sacrificed at 32 days
post-inoculation by cervical dislocation after being anesthetized
by 1% pentobarbital (40 mg/kg), and the tumor samples were removed
for further analysis.
For frozen sections, fresh excised tumor samples
were embedded in frozen section embedding medium optimal cutting
temperature (OCT) compound and were frozen rapidly at −20°C. The
frozen samples were then subjected to hematoxylin and eosin
(H&E) staining, following a previously published protocol
(28). Briefly, the frozen samples
were cryosectioned into 5-µm slices and mounted onto charged
microscope slides. The slices were washed with distilled water and
30% isopropanol to remove the OCT compound, and stained with the
H&E working solution (hematoxylin for 4 min and eosin for 30
sec) at 25°C. Following staining, the sections were mounted with
neutral balata (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
for 30 min at 25°C and observed under a light microscope.
Statistical analysis
Quantitative results were expressed as the means ±
standard deviation. The comparison between co-culture or AM-CM
treated samples at different time points with MCF-7 cells was
examined using the Student's t-test with SPSS 22.0 software (IBM
Corp., Armonk, NY, USA). Multiple comparisons of migrated ADSCs in
Transwell migration assay and tumorspheres diameter in the
tumorsphere formation assays were performed using one-way analysis
of variance and the least significant difference post hoc analysis
was performed to ascertain significance between groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
ADSCs characterization and senescence
evaluation
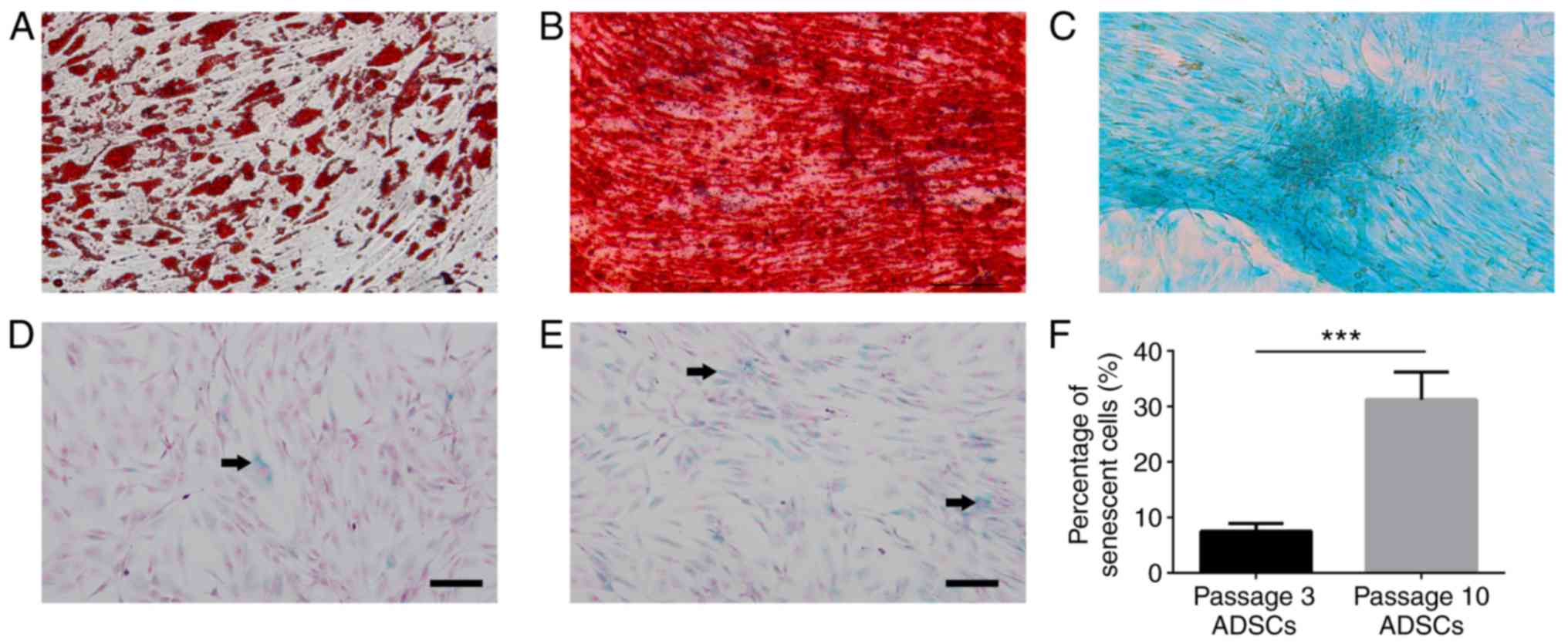
The isolated ADSC cells expressed lipid droplets,
matrix mineralization, cartilage-specific proteoglycans and were
positive for Oil Red O (Fig. 1A),
Alizarin red (Fig. 1B), and Alcian
blue (Fig. 1C). Senescence degree
of ADSCs was evaluated by in situ
senescence-associated-β-galactosidase assay. Few senescent cells
were observed in passage 3 ADSCs (Fig.
1D), whereas numerous senescent cells were observed in passage
10 ADSCs (Fig. 1E). Quantification
analysis revealed that there was a significantly decreased
percentage of blue, β-galactosidase-positive cells in passage 3
compared with that in the passage 10 ADSCs (Fig. 1F).
Tumor tropism of ADSCs, chemokine
expression and cell interactions
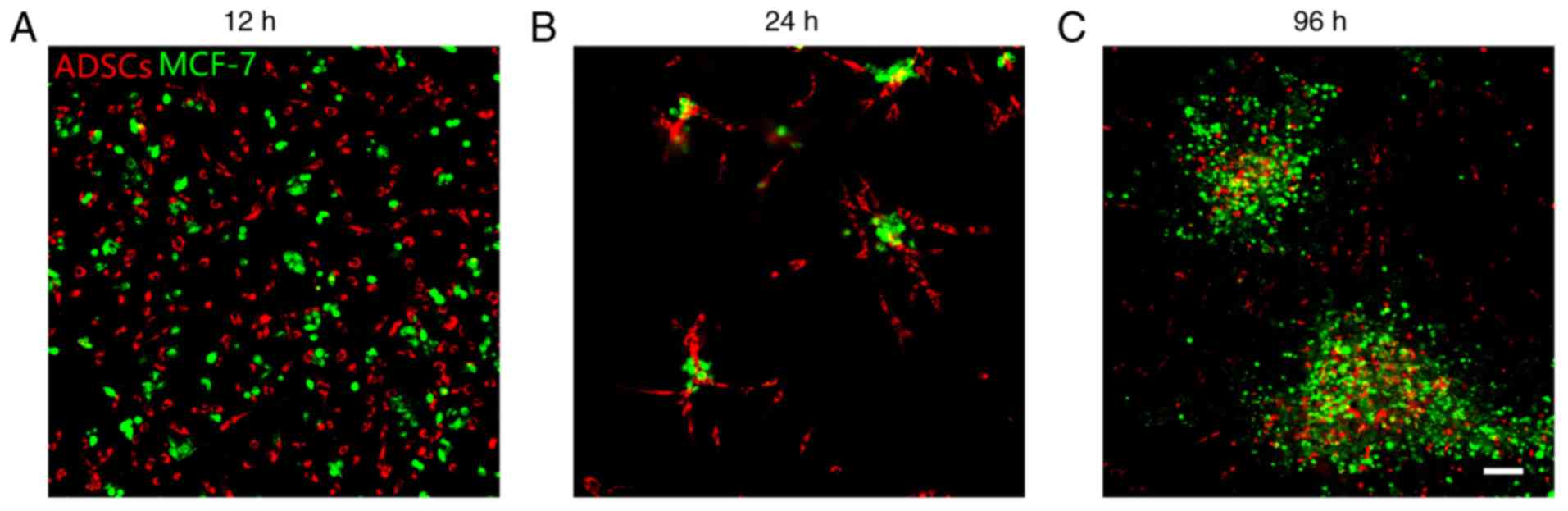
ADSCs and MCF-7 cells were evenly distributed at 12
h after co-culture (Fig. 2A). At 24
h, ADSCs were touching and surrounding MCF-7 cells (Fig. 2B). ADSCs were observed inside MCF-7
tumorspheres at 96 h after co-culture (Fig. 2C). Based on this observation, it was
hypothesized that MCF-7 cells may exert a tropism effect on ADSCs.
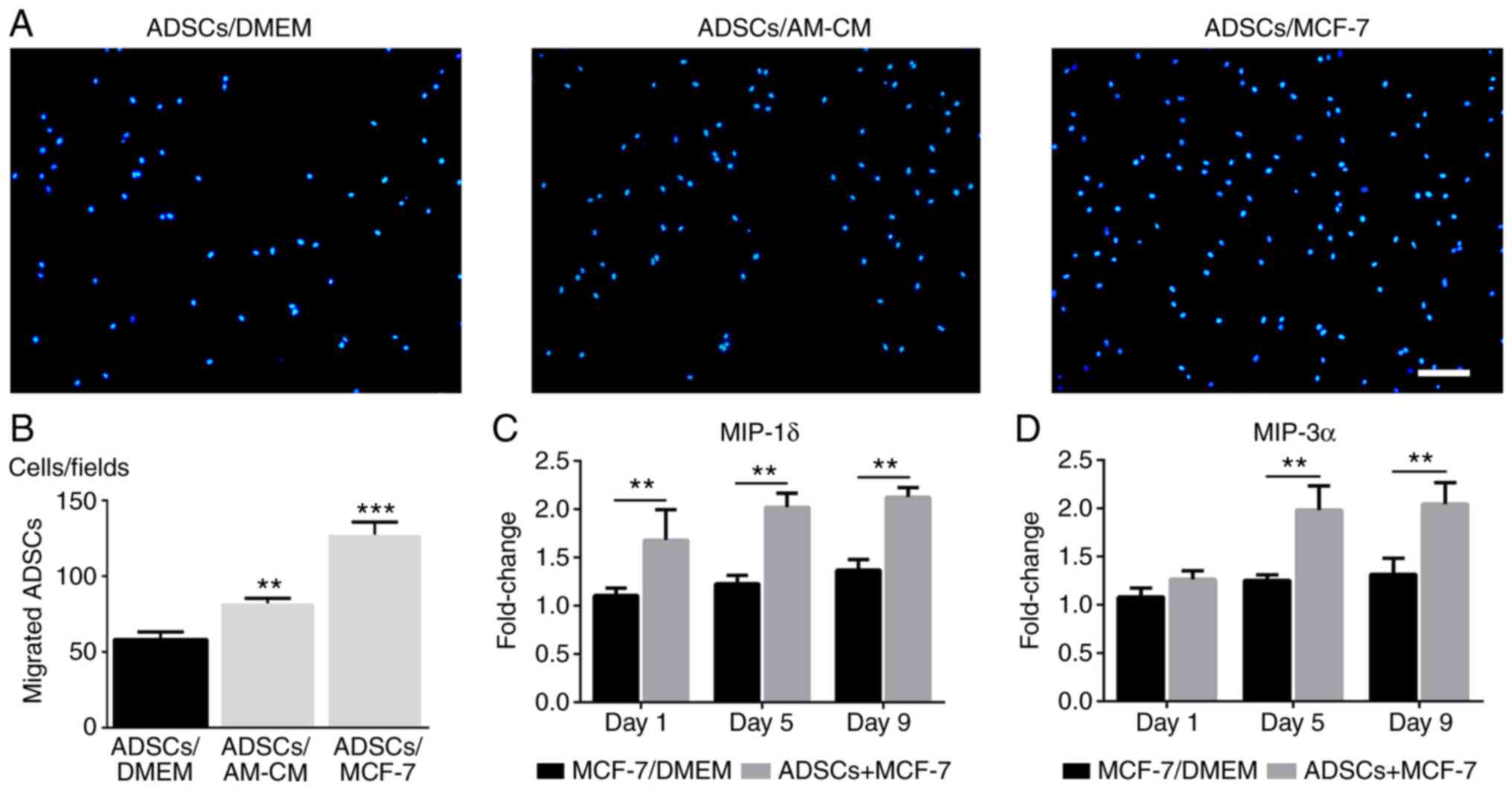
The migration of ADSCs towards MCF-7 cells was investigated in
vivo and in vitro. In the Transwell system,
quantification of migrated cells revealed that the migration
activity of ADSCs from the upper wells was significantly promoted
by MCF-7 cells in the lower chambers and, to a lesser extent, by
the putative secreted cytokines from the co-culture system compared
with the control (Fig. 3A and B).
The expression levels of the inflammatory chemokines MIP-1δ and
MIP-3α in the co-culture system (ADSCs + MCF-7) were significantly
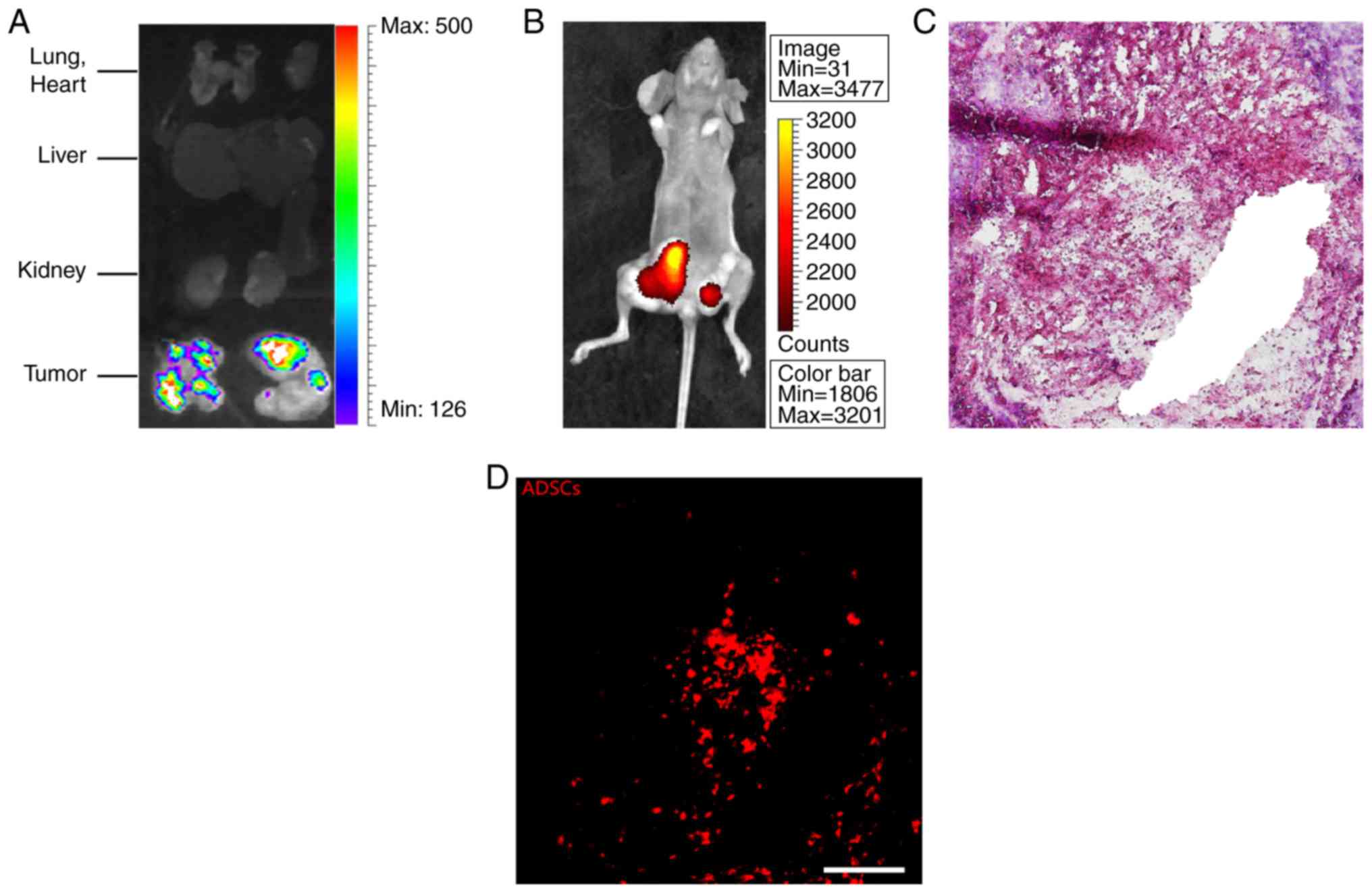
increased compared with the control (MCF-7/DMEM; Fig. 3C and D). To trace
fluorescence-labeled ADSCs, mice were first observed under a
bioluminescence imaging live fluorescence system and subsequently
the tumors and important organs (lung, heart, liver and kidney)
were carefully dissected from mice and observed under the same
system. The results revealed that the fluorescence signal was
mainly concentrated in tumor tissue and nearly no signal was
observed in other important organs (Fig. 4A and B). H&E staining and
DiI-labelled ADSCs were observed in sections (Fig. 4C and D, respectively). Collectively,
these results suggested that ADSCs mainly migrated to tumor site
following intravenous injection, possibly through the circulatory
system.
The morphological appearance of co-cultures of ADSCs
and MCF-7 cells was observed by a scanning electron microscope.
Spherical MCF-7 cells grew into tumorspheres with irregular
surfaces in Matrigel substrate (Fig.
5A). Tumorspheres were surrounded by ADSCs, which were
recognized by their flatly spread morphology (Fig. 5B). At higher magnification, the
construction of connections between ADSCs and MCF-7 cells were
observed (Fig. 5C).
ADSCs enhance tumorsphere formation,
cancer stem cell (CSC) marker expression, and in vivo tumor
formation
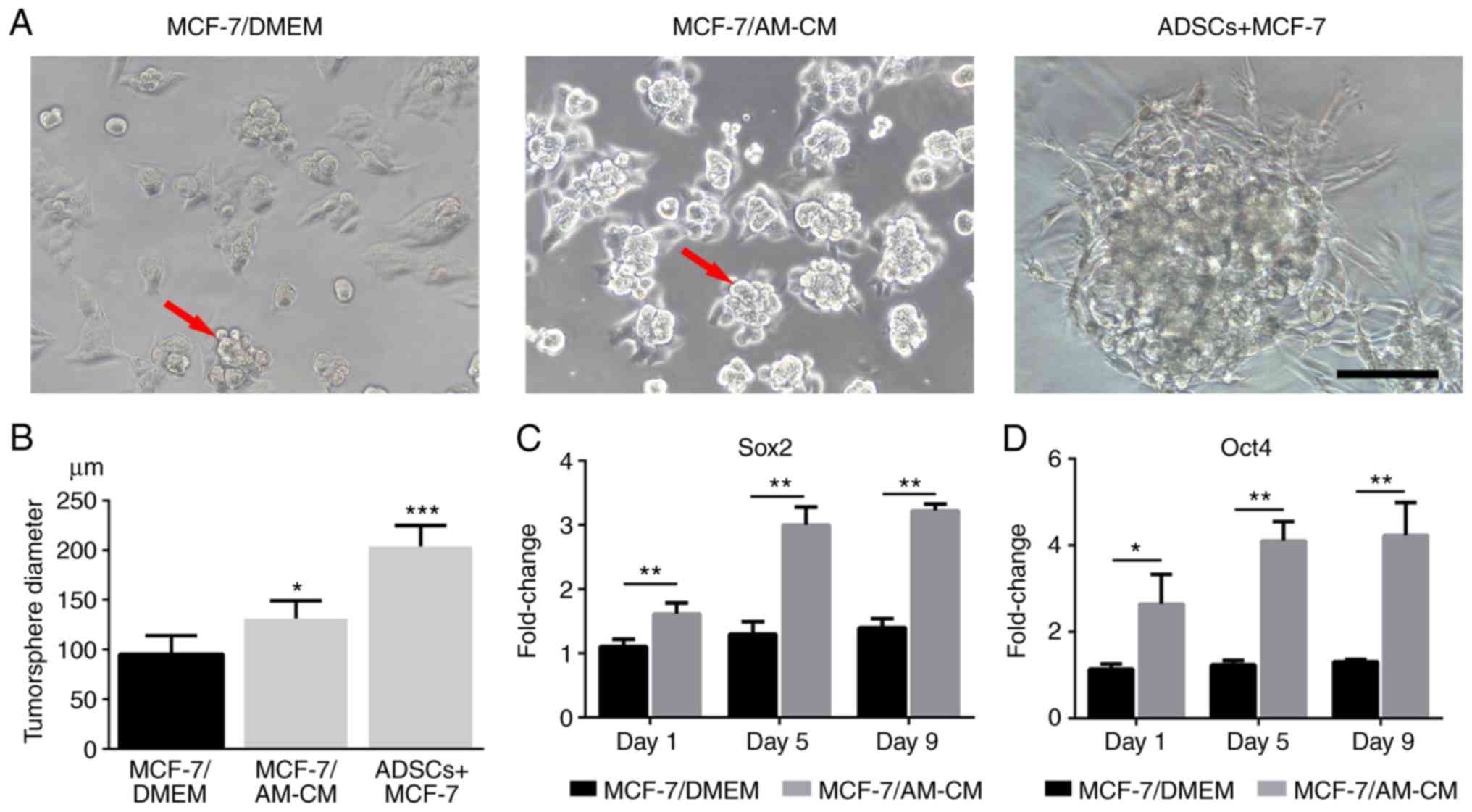
Tumorsphere formation, which is a property of cancer
stem cells, was observed in our 3D culture system. MCF-7 cells
co-cultured with ADSCs exhibited a significant capacity to form
tumorspheres, whereas the AM-CM also exhibited a marked effect on
tumorsphere formation (Fig. 6A).
Quantitative analysis revealed that tumorspheres in co-culture
groups (ADSCs + MCF-7) were larger than those treated with AM-CM
(MCF-7/AM-CM). As a control, MCF-7 cells alone (MCF-7/DMEM) in
Matrigel exhibited the weakest tumorsphere formation capacity
(Fig. 6B). RT-qPCR analysis further
revealed a higher expression of key CSC markers SOX2 and OCT4 in
AM-CM treated MCF-7 (MCF-7/AM-CM) than in the MCF-7 cells alone
(MCF-7/DMEM; Fig. 6C and D).
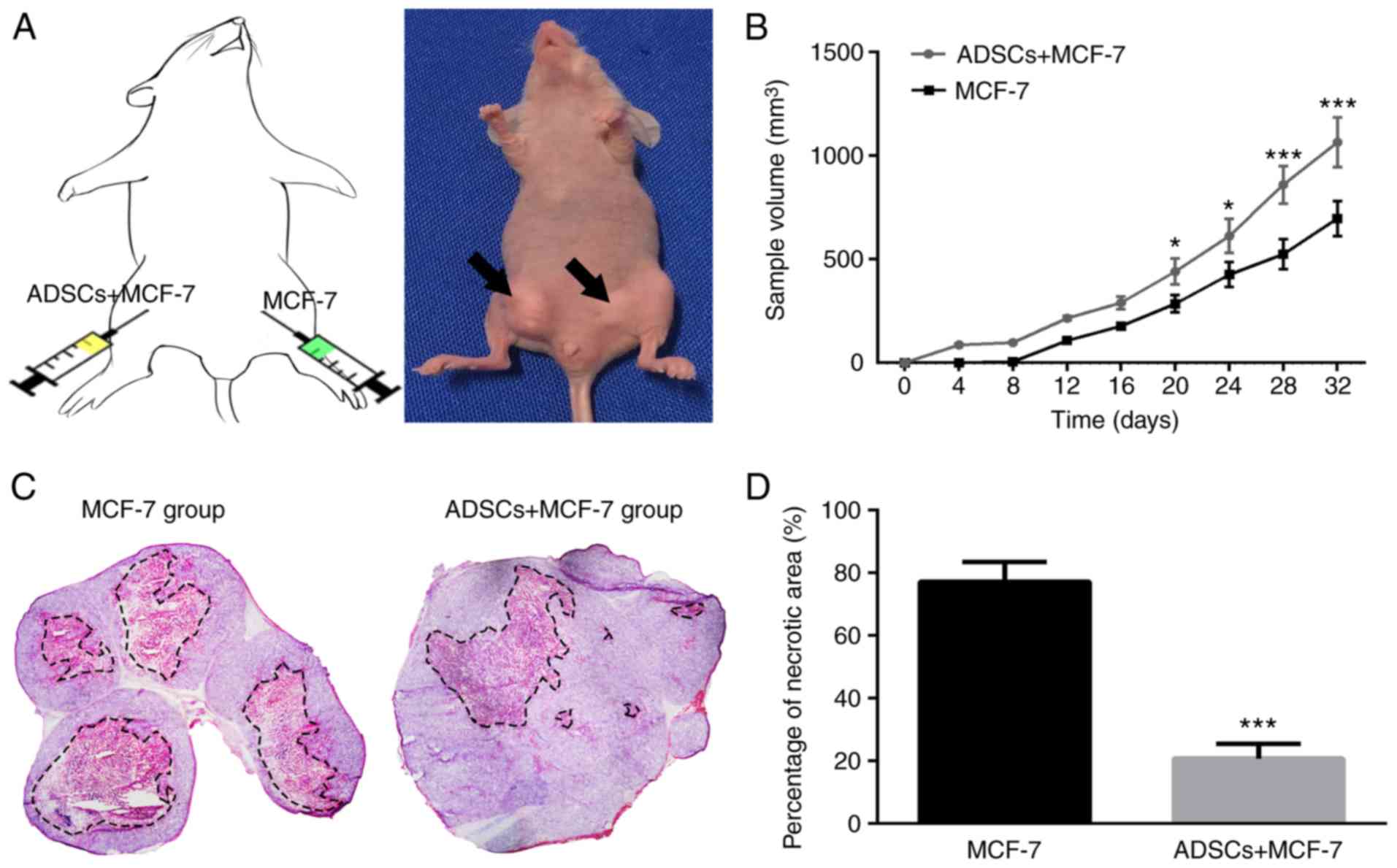
To further evaluate the effect of ADSCs on tumor
growth of MCF-7 cells in vivo, a xenograft model we
established, in which MCF-7 cells were mixed with or without ADSCs
and were then inoculated subcutaneously into nude mice. As shown in
Fig. 7A and B, the tumor volume of
the ASC-treated group was markedly increased compared with that of
the control group. H&E staining revealed that the necrotic area
in ASC-treated tumor tissue was considerably reduced compared with
that in controls (Fig. 7C and D).
These results indicate that ADSCs may enhance the stemness
expression and tumor-promoting properties of MCF-7 cells.
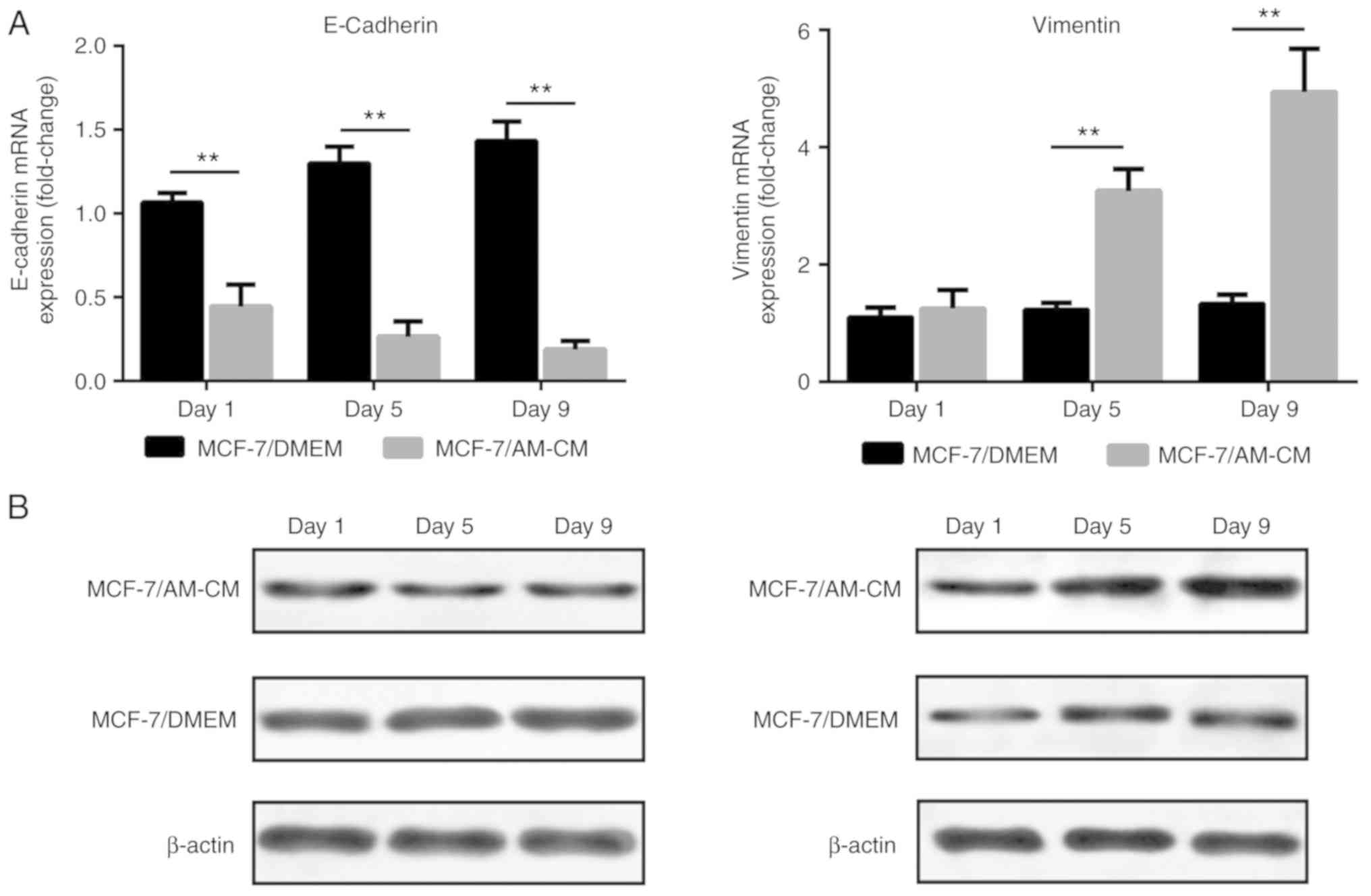
Expression of epithelial and
mesenchymal markers
In view of the morphological changes of MCF-7 cells
in tumorspheres, whether the acquisition of stemness properties is
associated with the epithelial to mesenchymal transition (EMT) was
investigated. Representative EMT markers were analyzed in the AM-CM
treated MCF-7 cells group (MCF-7/AM-CM) and the control MCF-7 cells
group (MCF-7/DMEM). Results revealed reduced E-Cadherin (epithelial
marker) and increased Vimentin (mesenchymal marker) mRNA expression
in the AM-CM treated MCF-7 cells compared with the control MCF-7
cells (Fig. 8A). Protein expression
analysis revealed a similarly decreased E-Cadherin and increased
Vimentin level in AM-CM treated MCF-7 cells compared with control
MCF-7 cells (Fig. 8B), indicating
that the ADSCs may induce the EMT in MCF-7 cells.
Discussion
Although stem cells are a promising source for cell
therapy in regenerative medicine, the potential pro- or
anti-tumoral actions of these cells remain controversial (29). Adipose tissue is an abundant,
accessible and rich source of ADSCs, and adipose transplantation is
gaining increasing interest among the plastic surgery techniques
currently available to reconstruct the breast after mastectomy for
breast cancer. However, recent scientific attention has turned to
whether grafted ADSCs within adipose tissue may increase the risk
of cancer recurrence (14,19,30–33).
In the present study, ADSCs and MCF-7 cells were
co-cultured in a 3D model to investigate the impacts of ADSCs on
breast cancer cells. It was found that the co-culture system
resulted in migration of ADSCs to MCF-7 cells and simultaneously
promoted tumor progression. Similar to other immune cells, MSCs
exhibit tropism for sites of tissue damage and the tumor
microenvironment (34–36). Various studies indicated that MSCs
migrated to sites of inflammation and diseased tissues when
injected systemically (37,38). In contrast, other studies have
reported that MSC migration can be induced by conditioned medium
from colorectal cancer (39),
gliomas (40,41), and breast cancer (42) cells in vitro. To date, the
majority of the studies on MSC tumor tropism were performed with
bone marrow-derived MSCs, and limited data are available regarding
the ADSCs from adipose tissue. In the present study, it was
observed that MCF-7 cells induced efficient ASC tropism. MSC
migration to tumors is thought to be due to chemokines secreted by
tumor cells, but this needs to be validated. Chemokines were
originally identified as potent attractants for leukocytes, such as
neutrophils and monocytes, and were generally regarded as mediators
of acute and chronic inflammation (inflammatory chemokines)
(43). Additionally, chemokines and
their receptors have been identified as actors promoting MSC tumor
tropism and initiation or cancer progression (43–48).
Among these inflammatory chemokines, MIP-1δ and MIP-3α are
considered as key considered factors in inducing MSC migration
(39). MIP-1δ and MIP-3α are
cytokines that mainly regulate immune cell migration (49) and were recently considered serum
biomarkers for hepatocellular carcinoma (50). Lejmi et al (36) investigated the migration of human
bone marrow-derived MSCs induced by conditioned medium of Huh-7
hepatoma cells, detecting increased levels of MIP-1δ and MIP-3α in
Huh-7-CM using a human cytokine antibody array. Transwell migration
assay showed that recombinant MIP-1δ and MIP-3α increased bone
marrow-derived MSC migration and that inhibition of antibodies
against MIP-1δ and MIP-3α slightly decreased MSCs migration.
Consistent with these results, increased expression levels of
MIP-1δ and MIP-3α were detected in the co-culture system compared
with the control, indicating that these two inflammatory chemokines
may participate in ADSCs migration in the present study.
Along with the migration of ADSCs to MCF-7 cells,
aggregation and sphere-like structure formation of MCF-7 cells are
also important features of the 3D co-culture system. Previous
studies have reported that sphere-like structure formation of
cancer cells in vitro indicates the acquisition of CSC
properties in cancer cell subpopulations (51–53).
CSCs are identified by high self-renewal capability, the capacity
to grow as tumorspheres in vitro and tumor growth promotion
in vivo (54,57). In xenograft breast cancer models,
MCF-7 and ADSCs co-injection induced notably faster tumor growth
and fewer necrotic areas in tumor tissues than MCF-7 injection
alone, suggesting the cancer-promoting activity of ADSCs in
vivo. Prompted by these results, it could be assumed that
tumorsphere and tumor tissue formation is associated with CSC
generation. The CSC markers SOX2 and OCT4 has been reported to
inhibit apoptosis and promote biological activity in CSCs (55,56).
SOX2 and OCT4 expression levels were increased in MCF-7 cells after
treatment with AM-CM on Matrigel substrates. This result indicated
that the tumorsphere formation in the co-culture system is likely
associated with the stem-like transfer of MCF-7 cells influenced by
the secretory cytokines from ADSCs.
Candidate CSCs have been identified in a variety of
human malignancies, including leukemias, and a number of solid
tumors, such as glioblastomas, medulloblastomas and carcinomas
(57–60). The contributions of the EMT program
in promoting cancer cells with stem-like properties have been well
documented in many types of carcinoma. Mani et al (61) demonstrated a direct link between the
EMT and epithelial stem cell properties. Using different EMT
inducers, they showed that the induction of the EMT in human breast
cancer cells accounted for the acquisition of their stem-like
characteristics. The EMT is a key program during embryonic
development, tissue remodeling, and cancer progress (62) and is characterized by the loss of
epithelial characteristics coupled with the gain of mesenchymal
properties. Furthermore, EMT process is associated with a
mesenchymal-like breast cancer phenotype, including acquisition of
invasive properties and the loss of cell-cell adhesion. These
features are associated with a more aggressive phenotype and a poor
prognosis (63). In direct
co-culture systems, malignant tumorsphere formation was observed,
indicating loss of contact inhibition. Hence, we hypothesized that
co-culturing with ADSCs promoted the EMT of MCF-7 cells. As
expected, gene expression analysis revealed a significant
upregulation of the mesenchymal marker Vimentin and the
downregulation of the epithelial marker E-Cadherin in AM-CM treated
MCF-7 cells compared with the control, indicating the potential
acquisition of EMT process in MCF-7 cells. Altogether, the present
results indicated that the stem-like transfer of MCF-7 cells may be
a consequence of the EMT induced by secretory cytokines from
ADSCs.
Matrigel-based 3D co-culture system may be a
convenient and rapid platform for studying cell interaction in
tumor development (64,65). Using this platform, it was
demonstrated that the interaction between ADSCs and MCF-7 cells
stimulated the expression of the chemokines MIP-1δ and MIP-3α,
which may act as a regulator inducing the migration activity of
ADSCs toward breast cancer cells and to establish direct cell-cell
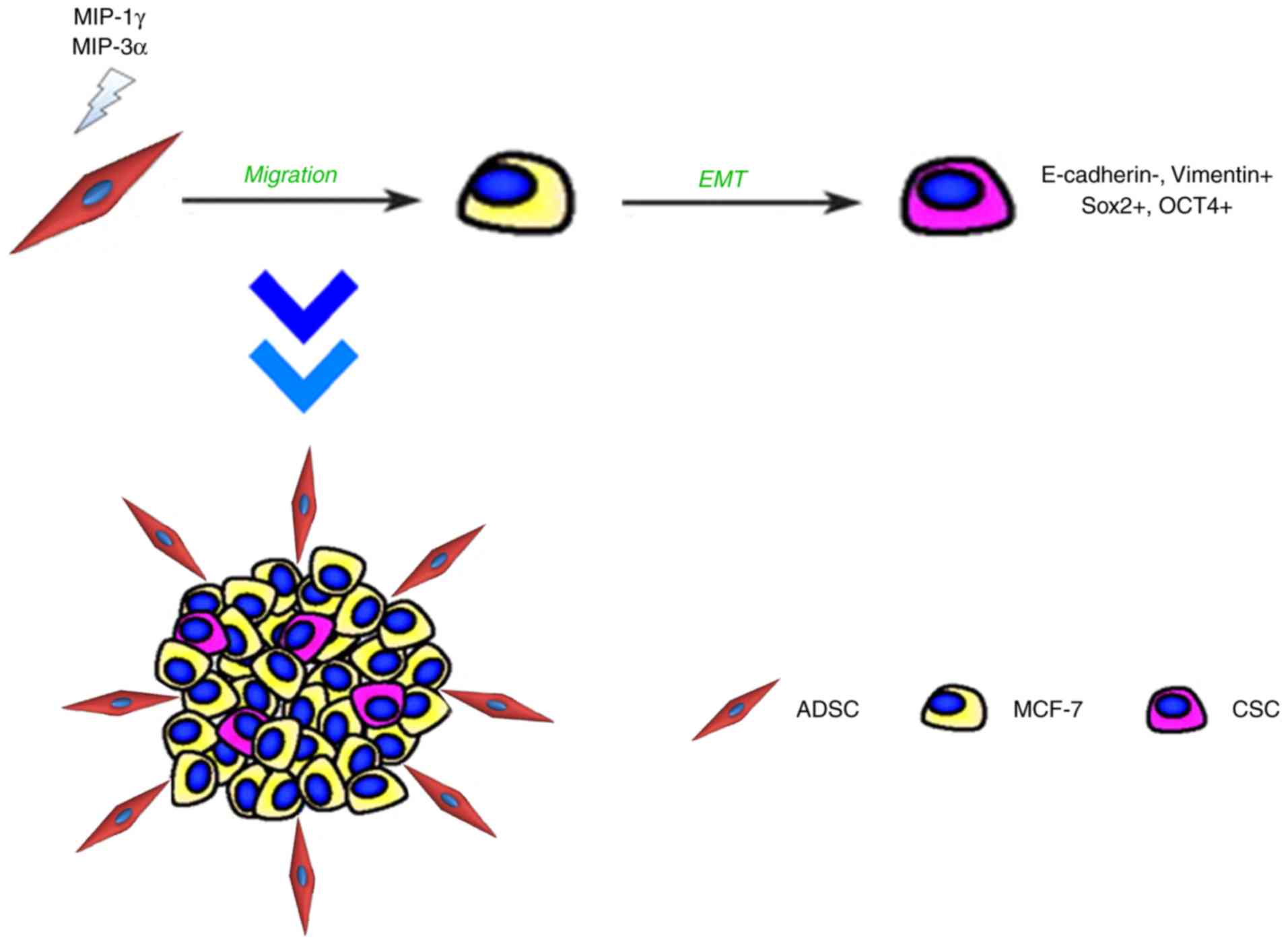
contacts. Furthermore, it was demonstrated that ADSCs serve
pro-malignant roles in MCF-7 cells through promoting the
tumorsphere formation of MCF-7 cells, which are likely associated
with CSC properties through the EMT process (Fig. 9). The promoted tumorigenicity was
further confirmed in the in vivo xenotransplantation model.
These results are important for safety concerns regarding the
clinical application of ASC-based strategies, such as fat grafting,
in post-oncologic breast reconstruction, interestingly because
microscopic tumor cells may remain after tumor resection.
However, the present study is small and data is
limited, several drawbacks should be resolved to fully clarify the
precise mechanisms of ADSCs facilitating breast cancer development.
First, although the procedure for ADSCs isolation and expansion is
considered the most widely used method (66), these cultured cells are still
heterogeneous, containing stem cells with different multipotential
properties, committed progenitors, and differentiated cells, which
in turn may affect the biological properties of the total
population. Therefore, using a purified stem cell population from
adipose tissue would help improve the study design and strengthen
our conclusion. Second, MIP chemokines were not directly shown to
act on the migration activity of ADSCs towards MCF-7 cell due to
current limited conditions. According to previous studies, MIP
cytokines mainly regulate immune cell migration (53) and were recently found to be
important chemoattractants that induce MSC migration and further
favor its differentiation (36).
Using recombinant chemokines MIP-1δ and MIP-3α, Lejmi et al
(36) revealed that MSC migration
was induced whereas addition of anti-MIP-1δ and anti-MIP-3α
antibodies decreased the MSC migration activity. These results may
help to demonstrate that MIP-1δ and MIP-3α may be involved in the
migration of ADSCs to MCF-7 cells in the present study but using
recombinant chemokines MIP-1δ and MIP-3α or knockdown of these
genes to solidify this conclusion is still necessary in further
studies. Third, interaction between the other types of breast
cancer cells and ADSCs should be investigated in further studies to
fully clarify the potential impacts of fat grafting on breast
cancer cells in the clinic.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81471881,
81372083), Key Clinical Specialty Discipline Construction Program,
Health Collaborative Innovation major projects of Guangzhou (grant
no. 7414275040815), Natural Science Foundation of Guangdong
Province of China (grant no. 2014A030310155), Entry Point Project
of Guangdong Province of China (grant no. PY2014N036), Innovative
project of Guangdong Province of China (grant no. 2014KQNCX046) and
Administrator Foundation of Nanfang Hospital (grant no.
2014B009).
Availability of data and materials
The data sets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YH conceived, designed the study and wrote the
manuscript. YC and XW performed the experiments and analyzed the
results. JG and FL designed and coordinated the study. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were approved by the Southern Medical
University Institutional Review Board (Guangzhou, China) and the
patient provided written informed consent to donate remaining
tissues after liposuction. All procedures performed involving
animal experiments were approved by the Nanfang Hospital animal
ethic committee (permit no. NFYY201679) and was conducted in
accordance with the ethical standards of the National Health and
Medical Research Council China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nelson HD, Zakher B, Cantor A, Fu R,
Griffin J, O'Meara ES, Buist DS, Kerlikowske K, van Ravesteyn NT,
Trentham-Dietz A, et al: Risk factors for breast cancer for women
aged 40 to 49 years: A systematic review and meta-analysis. Ann
Intern Med. 156:635–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porter PL: Global trends in breast cancer
incidence and mortality. Salud Publica Mex. 51 (Suppl 2):S141–S146.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGuire KP, Eisen S, Rodriguez A, Meade T,
Cox CE and Khakpour N: Factors associated with improved outcome
after surgery in metastatic breast cancer patients. Am J Surg.
198:511–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munhoz AM, Montag E, Filassi JR and
Gemperli R: Current approaches to managing partial breast defects:
The role of conservative breast surgery reconstruction. Anticancer
Res. 34:1099–1114. 2014.PubMed/NCBI
|
|
5
|
Longaker MT, Aston SJ, Baker DC and
Rohrich RJ: Fat Transfer in 2014: What we do not know. Plast
Reconstr Surg. 133:1305–1307. 2014.PubMed/NCBI
|
|
6
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The Fate of Adipocytes after nonvascularized fat
grafting. Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung MT, Paik KJ, Atashroo DA, Hyun JS,
McArdle A, Senarath-Yapa K, Zielins ER, Tevlin R, Duldulao C, Hu
MS, et al: Studies in fat grafting: Part I. Effects of injection
technique on in vitro fat viability and in vivo volume retention.
Plast Reconstr Surg. 134:29–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atashroo D, Raphel J, Chung MT, Paik KJ,
Parisi-Amon A, McArdle A, Senarath-Yapa K, Zielins ER, Tevlin R,
Duldulao C, et al: Studies in fat grafting: Part II. Effects of
injection mechanics on material properties of fat. Plast Reconstr
Surg. 134:39–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Z, Peng Z, Chang Q, Zhan W, Zeng Z,
Zhang S and Lu F: The angiogenic and adipogenic modes of adipose
tissue after free fat grafting. Plast Reconstr Surg. 135:556e–567e.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bellei B, Migliano E, Tedesco M, Caputo S,
Papaccio F, Lopez G and Picardo M: Adipose tissue-derived
extracellular fraction characterization: Biological and clinical
considerations in regenerative medicine. Stem Cell Res Ther.
9:2072018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshimura K, Shigeura T, Matsumoto D, Sato
T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I,
et al: Characterization of freshly isolated and cultured cells
derived from the fatty and fluid portions of liposuction aspirates.
J Cell Physiol. 208:64–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pearl RA, Leedham SJ and Pacifico MD: The
safety of autologous fat transfer in breast cancer: Lessons from
stem cell biology. J Plast Reconstr Aesthet Surg. 65:283–288. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Lehuédé C, Laurent V, Dirat B,
Dauvillier S, Bochet L, Le Gonidec S, Escourrou G, Valet P and
Muller C: Adipose tissue and breast epithelial cells: A dangerous
dynamic duo in breast cancer. Cancer Lett. 324:142–151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolle SF, Fischer-Nielsen A, Mathiasen AB,
Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M,
Rasmussen BS, Talman ML, et al: Enrichment of autologous fat grafts
with ex-vivo expanded adipose tissue-derived stem cells for graft
survival: A randomised placebo-controlled trial. Lancet.
382:1113–1120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doi K, Ogata F, Eto H, Kato H, Kuno S,
Kinoshita K, Kanayama K, Feng J, Manabe I and Yoshimura K:
Differential contributions of graft-derived and host-derived cells
in tissue regeneration/remodeling after fat grafting. Plast
Reconstr Surg. 135:1607–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suga H, Eto H, Aoi N, Kato H, Araki J, Doi
K, Higashino T and Yoshimura K: Adipose tissue remodeling under
ischemia: Death of adipocytes and activation of stem/progenitor
cells. Plast Reconstr Surg. 126:1911–1923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuhbier JW, Bucan V, Reimers K, Strauss S,
Lazaridis A, Jahn S, Radtke C and Vogt PM: Observed changes in the
morphology and phenotype of breast cancer cells in direct
co-culture with adipose-derived stem cells. Plast Reconstr Surg.
134:414–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamat P, Schweizer R, Kaenel P, Salemi S,
Calcagni M, Giovanoli P, Gorantla VS, Eberli D, Andres AC and Plock
JA: Human adipose-derived mesenchymal stromal cells may promote
breast cancer progression and metastatic spread. Plast Reconstr
Surg. 136:76–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kucerova L, Skolekova S, Matuskova M,
Bohac M and Kozovska Z: Altered features and increased
chemosensitivity of human breast cancer cells mediated by adipose
tissue-derived mesenchymal stromal cells. Bmc Cancer. 13:5352013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Daquinag A, Traktuev DO,
Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W and
Kolonin MG: White adipose tissue cells are recruited by
experimental tumors and promote cancer progression in mouse models.
Cancer Res. 69:5259–5266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eterno V, Zambelli A, Pavesi L, Villani L,
Zanini V, Petrolo G, Manera S, Tuscano A and Amato A:
Adipose-derived mesenchymal stem cells (ASCs) may favour breast
cancer recurrence via HGF/c-Met signaling. Oncotarget. 5:613–633.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alessio N, Bohn W, Rauchberger V, Rizzolio
F, Cipollaro M, Rosemann M, Irmler M, Beckers J, Giordano A and
Galderisi U: Silencing of RB1 but not of RB2/P130 induces cellular
senescence and impairs the differentiation potential of human
mesenchymal stem cells. Cell Mol Life Sci. 70:1637–1651. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gary RK and Kindell SM: Quantitative assay
of senescence-associated beta-galactosidase activity in mammalian
cell extracts. Anal Biochem. 343:329–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong
Y, Mao Y, Hu T, Zhang B and Song G: Involvement of
Wnt/β-catenin signaling in the mesenchymal stem cells
promote metastatic growth and chemoresistance of
cholangiocarcinoma. Oncotarget. 6:42276–42289. 2015.PubMed/NCBI
|
|
26
|
Zhou M, Liu S, Jiang Y, Ma H, Shi M, Wang
Q, Zhong W, Liao W and Xing MM: Doxorubicin-loaded single wall
nanotube thermo-sensitive hydrogel for gastric cancer
chemo-photothermal therapy. Adv Funct Mater. 25:4730–4739. 2015.
View Article : Google Scholar
|
|
27
|
Naito S, von Eschenbach AC, Giavazzi R and
Fidler IJ: Growth and metastasis of tumor cells isolated from a
human renal cell carcinoma implanted into different organs of nude
mice. Cancer Res. 46:4109–4115. 1986.PubMed/NCBI
|
|
28
|
Choi JS, Kim BS, Kim JY, Kim JD, Choi YC,
Yang HJ, Park K, Lee HY and Cho YW: Decellularized extracellular
matrix derived from human adipose tissue as a potential scaffold
for allograft tissue engineering. J Biomed Mater Res A. 97:292–299.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rhee K, Lee J and Eom Y: Mesenchymal stem
cell-mediated effects of tumor support or suppression. Int J Mol
Sci. 16:30015–30033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waked K, Colle J, Doornaert M, Cocquyt V
and Blondeel P: Systematic review: The oncological safety of
adipose fat transfer after breast cancer surgery. Breast.
31:128–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Massa M, Gasparini S, Baldelli I,
Scarabelli L, Santi P, Quarto R and Repaci E: Interaction between
breast cancer cells and adipose tissue cells derived from fat
grafting. Aesthet Surg J. 36:358–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito S, Kai Y, Masuda T, Tanaka F,
Matsumoto T, Kamohara Y, Hayakawa H, Ueo H, Iwaguro H, Hedrick MH,
et al: Long-term outcome of adipose-derived regenerative
cell-enriched autologous fat transplantation for reconstruction
after breast-conserving surgery for Japanese women with breast
cancer. Surg Today. 47:1500–1511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bielli A, Scioli MG, Gentile P,
Agostinelli S, Tarquini C, Cervelli V and Orlandi A: Adult
adipose-derived stem cells and breast cancer: A controversial
relationship. Springerplus. 3:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ponte AL, Marais E, Gallay N, Langonne A,
Delorme B, Herault O, Charbord P and Domenech J: The in vitro
migration capacity of human bone marrow mesenchymal stem cells:
Comparison of chemokine and growth factor chemotactic activities.
Stem Cells. 25:1737–1745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: Defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lejmi E, Perriraz N, Clément S, Morel P,
Baertschiger R, Christofilopoulos P, Meier R, Bosco D, Bühler LH
and Gonelle-Gispert C: Inflammatory chemokines MIP-1δ and MIP-3α
are involved in the migration of multipotent mesenchymal stromal
cells induced by hepatoma cells. Stem Cells Dev. 24:1223–1235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chamberlain G, Smith H, Rainger GE and
Middleton J: Mesenchymal stem cells exhibit firm adhesion,
crawling, spreading and transmigration across aortic endothelial
cells: Effects of chemokines and shear. PLoS One. 6:e256632011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Cao F, De A, Cao Y, Contag C,
Gambhir SS, Wu JC and Chen X: Trafficking mesenchymal stem cell
engraftment and differentiation in tumor-bearing mice by
bioluminescence imaging. Stem Cells. 27:1548–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Menon LG, Picinich S, Koneru R, Gao H, Lin
SY, Koneru M, Mayer-Kuckuk P, Glod J and Banerjee D: Differential
gene expression associated with migration of mesenchymal stem cells
to conditioned medium from tumor cells or bone marrow cells. Stem
Cells. 25:520–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ho IA, Chan KY, Ng WH, Guo CM, Hui KM,
Cheang P and Lam PY: Matrix metalloproteinase 1 is necessary for
the migration of human bone marrow-derived mesenchymal stem cells
toward human glioma. Stem Cells. 27:1366–1375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Egea V, von Baumgarten L, Schichor C,
Berninger B, Popp T, Neth P, Goldbrunner R, Kienast Y, Winkler F,
Jochum M, et al: TNF-α respecifies human mesenchymal
stem cells to a neural fate and promotes migration toward
experimental glioma. Cell Death Differ. 18:853–863. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dwyer RM, Potter-Beirne SM, Harrington KA,
Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T and Kerin MJ:
Monocyte chemotactic protein-1 secreted by primary breast tumors
stimulates migration of mesenchymal stem cells. Clin Cancer Res.
13:5020–5027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lazennec G and Richmond A: Chemokines and
chemokine receptors: New insights into cancer-related inflammation.
Trends Mol Med. 16:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zernecke A, Weber KS, Erwig LP, Kluth DC,
Schroppel B, Rees AJ and Weber C: Combinatorial model of chemokine
involvement in glomerular monocyte recruitment: Role of CXC
chemokine receptor 2 in infiltration during nephrotoxic nephritis.
J Immunol. 166:5755–5762. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ali S and Lazennec G: Chemokines: Novel
targets for breast cancer metastasis. Cancer Metastasis Rev.
26:401–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Escobar P, Bouclier C, Serret J, Bieche I,
Brigitte M, Caicedo A, Sanchez E, Vacher S, Vignais ML, Bourin P,
et al: IL-1β produced by aggressive breast cancer cells
is one of the factors that dictate their interactions with
mesenchymal stem cells through chemokine production. Oncotarget.
6:29034–29047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Griffith JW, Sokol CL and Luster AD:
Chemokines and chemokine receptors: Positioning cells for host
defense and immunity. Annu Rev Immunol. 32:659–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li Y, Wu J, Zhang W, Zhang N and Guo H:
Identification of serum CCL15 in hepatocellular carcinoma. Br J
Cancer. 108:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weiswald L, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu A, Yu X and Liu S: Pluripotency
transcription factors and cancer stem cells: Small genes make a big
difference. Chin J Cancer. 32:483–487. 2013.PubMed/NCBI
|
|
56
|
Santini R, Pietrobono S, Pandolfi S,
Montagnani V, D'Amico M, Penachioni JY, Vinci MC, Borgognoni L and
Stecca B: SOX2 regulates self-renewal and tumorigenicity of human
melanoma-initiating cells. Oncogene. 33:4697–4708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Turhan AG, Lemoine FM, Debert C, Bonnet
ML, Baillou C, Picard F, Macintyre EA and Varet B: Highly purified
primitive hematopoietic stem cells are PML-RARA negative and
generate nonclonal progenitors in acute promyelocytic leukemia.
Blood. 85:2154–2161. 1995.PubMed/NCBI
|
|
58
|
Holyoake TL, Jiang X, Drummond MW, Eaves
AC and Eaves CJ: Elucidating critical mechanisms of deregulated
stem cell turnover in the chronic phase of chronic myeloid
leukemia. Leukemia. 16:549–558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marsden CG, Wright MJ, Pochampally R and
Rowan BG: Breast tumor-initiating cells isolated from patient core
biopsies for study of hormone action. Methods Mol Biol.
590:363–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vega SL, Kwon MY and Burdick JA: Recent
advances in hydrogels for cartilage tissue engineering. Eur Cell
Mater. 33:59–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kondiah PJ, Choonara YE, Kondiah PP,
Marimuthu T, Kumar P, du Toit LC and Pillay V: A review of
injectable polymeric hydrogel systems for application in bone
tissue engineering. Molecules. 21(pii): E15802016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|