Introduction
Cancer cachexia is a multifactorial syndrome
characterized by weight loss, muscle atrophy and anorexia, with or
without adipose tissue loss (1,2). In total,
50–80% of patients with advanced cancer develop cachexia and it is
widely accepted to be indirectly responsible for ≥20% of all
cancer-associated mortalities (3).
Furthermore, cancer cachexia can cause resistance to comprehensive
treatment, increase the incidence of adverse reactions, reduce
patient quality of life, and increase morbidity and mortality
(4,5).
The pathogenesis mechanism of cancer cachexia is complicated and
there are no effective means for preventing skeletal muscle
wasting. A number of studies have focused on early diagnosis
(6,7)
and the development of new drugs to delay cachexia progression
(8,9).
Muscle atrophy is the most specific characteristic
of cancer cachexia and is induced by an imbalance between muscle
protein synthesis and degradation (10). Skeletal muscle protein degradation is
primarily regulated by the ubiquitin-proteasome pathway under
wasting conditions (11,12). Two E3 ubiquitin ligases, muscle
RING-finger containing protein-1 (MuRF1) and muscle atrophy Fbox
protein (MAFbx), are specifically expressed in atrophying skeletal
muscle and mediate the degradation of muscle protein (13,14). The
expression of these E3 ubiquitin ligases is mediated by several
signaling pathways, including PI3K/Akt, NF-κB/IκB kinase and
myostatin/activin receptor type-2B signalling (15–18).
During muscle atrophy, PI3K/Akt pathway activity decreases, leading
to activation of the FoxO3α transcription factor and expression of
E3 ubiquitin ligases (19). In
addition, muscle protein synthesis is mediated by several myogenic
genes, including the myogenic differentiation antigen (MyoD) family
of transcription factors, including MyoD, myogenin (MyoG), myogenic
factor 5 (Myf5) and myogenic regulatory factor 4 (Mrf4) (20). Reagents that can block protein
degradation and/or activate protein synthesis are expected to be
beneficial therapies for skeletal muscle atrophy.
Matrine is approved by the China Food and Drug
Administration (CFDA) for the prevention and treatment of cancer
cachexia. As the main active component of Sophora
flavescens, matrine has been widely used in Traditional Chinese
Medicine for thousands of years. In recent decades, matrine has
been verified to exhibit broad efficacy against hepatitis (21), cardiac injury (22), fibrotic diseases (23) and cancer (24–27), which
occurs via regulation of Akt/FoxO3α, NF-κB, JAK-STAT and mTOR
pathways. Previously, it has been reported that matrine improves
cancer cachexia symptoms and partly preserves skeletal muscle mass
in mice. This effect was mostly attributed to matrine's
anti-inflammatory activity (28).
However, evidence demonstrates that inflammation is not the main
driving force of cancer cachexia-induced muscle wasting (29,30).
Therefore, an in-depth study of matrine's intrinsic mechanism for
preserving muscle mass is required.
The aim of the present study was to evaluate the
effect of matrine on skeletal muscle atrophy and clarify the
underlying mechanism. It was first evaluated whether
matrine-treatment could ameliorate skeletal muscle atrophy in an
in vivo cancer cachexia mouse model induced by CT26 colon
adenocarcinoma. Subsequently, it was elucidated whether
matrine-treatment could improve C2C12 myoblast differentiation and
alleviate myotube atrophy induced by dexamethasone (Dex), tumor
necrosis factor α (TNFα) or conditioned medium (CM). In addition,
the effect of matrine on E3 ubiquitin ligases and their associated
signaling pathways was analyzed.
Materials and methods
Cell culture and differentiation
The CT26 colon adenocarcinoma and C2C12 cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA). These cells were confirmed to be without mycoplasma
contamination by using a color one-step mycoplasma detection kit
[Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China]. The
cells were maintained in Dulbecco's modified Eagle's medium (DMEM;
Corning Life Sciences, Corning, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Zhejiang Tianhang
Biotechnology Co., Ltd., Hangzhou, China), 100 U/ml penicillin and
100 µg/ml streptomycin. The cells were cultured in an incubator at
37°C in a humidified atmosphere of 5% CO2. Myotubes were
induced by culturing the C2C12 cells in DMEM with 2% horse serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 3–5
days, replacing the medium every 24 h.
Establishment of C2C12 myotube atrophy
models and treatments
Three C2C12 myotube atrophy models were established,
according to previous studies (31–33).
Briefly, C2C12 myoblasts were differentiated to myotubes by
culturing in 2% horse serum at 37°C. Dex (National Institute for
the Control of Pharmaceutical and Biological Products, Beijing,
China) or TNFα (Novus Biologicals, LLC, Littleton, CO, USA) were
then added to the media for 48 h at 100 µM and 50 ng/ml,
respectively. For the third model, the myotubes were incubated for
48 h in CM consisting of 33% ‘cachexia liquid’ and 66% fresh DMEM
with 2% horse serum; this CM was replaced every 24 h. The ‘cachexia
liquid’ was acquired as follows; when CT26 cells reached 90%
confluence, their culture medium was replaced with 2% horse serum
(differentiation medium) and the supernatant was collected as
‘cachexia liquid’ after 48 h. For the investigation of myoblast
differentiation and myotube atrophy, matrine (Shanghai EFE
Biological Technology Co., Ltd., Shanghai, China) was added to the
culture medium at 0.1 and 0.2 mM for 48 h at 37°C. For the
signalling pathway investigation, 0.1 mM matrine was added for 48 h
at 37°C and 10 nM wortmannin (MedChemExpress, Monmouth Junction,
NJ, USA) was added to culture medium for 48 h at 37°C.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) assay was performed according to the
manufacturer's protocol. Briefly, CT26 or C2C12 cells were seeded
in 96-well plates at 3,000-5,000 cells/well. Matrine (97%) was
added at different concentrations (0.1, 0.3, 0.89, 2.68, 8.05 and
24.16 mM for CT26 cells, and 0.001, 0.004, 0.012, 0.037, 0.111,
0.333 and 1 mM for C2C12) for 48 h. Subsequently, 10 µg/ml CCK-8
regent was added and incubated at 37°C for 1 h. The absorbance was
measured at 490 nm with a Synergy™ HT Multi-Mode Microplate Reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Immunofluorescence and determination
of C2C12 myotube diameter and myotube fusion index
After being treated with or without matrine (100 µM)
and Dex (100 µM) for 48 h at 37°C, the myotubes were washed with
cold PBS three times and were fixed with 4% paraformaldehyde at
room temperature. The cells were permeabilized by treatment with
0.5% Triton X-100 for 20 min. The myotubes were washed with PBS and
blocked in 5% bovine serum albumin (Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China) for 1 h at room temperature
(25±2°C). The myotubes were incubated with primary antibodies
against myosin heavy chain (MHC; cat. no. sc-376157; 1:200), MyoD
(cat. no. sc-71629; 1:150), MuRF1 (cat. no. sc-398608; 1:150) and
MAFbx (cat. no. sc-166806; 1:150) overnight at 4°C, which were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The myotubes were then incubated with Alexa Fluor 488-conjugated
(cat. no. 4408S; 1:1,000) and Alexa Fluor 594-conjugated secondary
antibodies (cat. no. 8889S; 1:1,000) at 4°C overnight, which were
both from Cell Signalling Technology, Inc. (Danvers, MA, USA). DAPI
(5 µg/ml; Beijing Solarbio Science and Technology Co., Ltd.) was
used to stain the nuclei for 5 min at room temperature. Images of
the C2C12 myotubes were obtained under a fluorescence microscope
equipped with a digital camera (Olympus Corporation, Tokyo, Japan;
magnification, ×100 and ×200). A minimum of six representative
images were selected and 150 sets of myotube data were measured per
group by CellSens standard software (version 1.16; Olympus
Corporation).
Animal cancer cachexia model and
matrine treatment
All procedures involving animals and their care in
this study were approved by the Animal Care Committee of Shanghai
Sixth People's Hospital (Shanghai, China) in accordance with
institutional requirements and Chinese government guidelines for
animal experiments. Six-week-old male mice (weight, 20±1.5 g) were
housed in a controlled environment (25±2°C; 12 h light/12 h dark
cycle; relative humidity of ~50–60%) and provided standard
laboratory chow and water ad libitum. The mouse cancer
cachexia model bearing CT26 tumor was established as previously
described (34,35). Briefly, 40 mice were divided randomly
into four groups with ten mice per group. The normal control (NC)
group included mice without CT26 inoculation that received normal
saline treatment. The normal mice treated with matrine (NC + Mat)
group consisted of mice without CT26 inoculation that received
matrine-treatment. The cancer cachexia (CC) group included mice
with CT26 inoculation that received normal saline treatment. The
cancer cachexia mice treated with matrine (CC + Mat) group
consisted mice with CT26 inoculation that received
matrine-treatment. The CT26 tumor suspension (~2×106
cells) was injected subcutaneously into the right flanks of the
mice. Ten days after CT26 inoculation, 50 mg/kg matrine was given
via intraperitoneal injection. The food intake, body weights, and
longest and shortest tumor diameters were recorded every 2 days.
The tumor weights were calculated using the following formula:
Weight (mg)=0.52 × (L × S2), where L is the longest
diameter and S is the shortest diameter. The longest diameter of
the CT26 tumors did not exceed 1.5 cm during the experiment. A
total of 21 days after tumor inoculation, the mice were euthanized.
The heart, liver, spleen, lungs, kidneys, epididymal fat,
gastrocnemius muscle and tibialis anterior muscle were dissected,
weighed and snap frozen in liquid nitrogen.
Hematoxylin-eosin staining and
determination of the myofiber cross-sectional area (CSA)
A total of three fresh gastrocnemius muscles were
dissected randomly from each group, fixed with GD fixative
(v/v=1:19) (Servicebio, Wuhan, China; cat. no. G1111) for 12 h at
room temperature, embedded with paraffin, sectioned into 4 µm-thick
slices and stained with hematoxylin for 8 min (Servicebio; cat. no.
G1004) and eosin-phloxine (Servicebio; cat. no. G1001) for 1 min at
room temperature. The muscle sections were observed under a light
microscope (Olympus Corporation; magnification, ×200). Myofiber
CSAs were calculated using CellSens software (version 1.16; Olympus
Corporation), and a minimum of six images and 180 sets of data were
acquired per group.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from mice gastrocnemius, tibialis anterior
and C2C12 myotubes were extracted using RNAiso reagent (Takara Bio
Inc., Otsu, Japan). cDNA was synthesized using HiScript II Q RT
SuperMix for qPCR (cat. no. R223-01; Vazyme Biotech Co. Ltd.,
Nanjing, Jiangsu, China) with the following procedure: 37°C for 15
min and 85°C for 5 sec. qPCR was performed with ChamQ™ Universal
SYBR qRT-PCR Master mix (cat. no. Q711-02; Vazyme Biotech Co.
Ltd.). qPCR and data analysis were conducted on a StepOnePlus™
Real-Time PCR system (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The qPCR amplifications consisted of
40 cycles of 95°C for 30 sec, 95°C for 15 sec and 62°C for 30 sec.
The 2−ΔΔCq method (36)
was used for quantification. All data were from three independent
experiments. GAPDH was used as the internal control gene. The
primers were designed, synthesized and ultra-purified by Sangon
Biotech Co. Ltd., Shanghai, China (Table
I).
 | Table I.Primer sequences used for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-polymerase chain reaction.
| Gene name | Direction | Sequence
(5′-3′) |
|---|
| MuRF1 | Forward |
TGCCTACTTGCTCCTTGTGC |
|
| Reverse |
CACCAGCATGGAGATGCAGT |
| MAFbx | Forward |
ACGTCGCAGCCAAGAAGAG |
|
| Reverse |
ATGGCGCTCCTTCGTACTTC |
| MHC I | Forward |
GCTGAGGCCCAGAAACAAG |
|
| Reverse |
TTCCACGATGGCGATGTTC |
| MHC IIa | Forward |
ACTTTGGCACTACGGGGAAAC |
|
| Reverse |
CAGCAGCATTTCGATCAGCTC |
| MHC IIb | Forward |
CTTTGCTTACGTCAGTCAAGGT |
|
| Reverse |
AGCGCCTGTGAGCTTGTAAA |
| MHC IIx | Forward |
GCGAATCGAGGCTCAGAACAA |
|
| Reverse |
GTAGTTCCGCCTTCGGTCTTG |
| Myostain | Forward |
CCAGGACCAGGAGAAGATGG |
|
| Reverse |
GGATTCCGTGGAGTGCTCAT |
| GAPDH | Forward |
TGGTCGTATTGGGCGCCTGGT |
|
| Reverse |
TCGCTCCTGGAAGATGGTGA |
Western blotting
Myotubes and gastrocnemius muscle tissue were lysed
using RIPA buffer (Thermo Fisher Scientific, Inc.). Total protein
was quantified with a BCA kit (Beyotime Institute of Biotechnology,
Haimen China). Subsequently, 50 µg denatured protein per lane was
subjected to SDS-PAGE on 10% polyacrylamide gels and then
transferred to polyvinylidene difluoride membranes. Following
blocking with 5% skim milk in TBS containing 0.05% Tween-20 for 1 h
at room temperature, the membranes were incubated with primary
antibodies against MuRF1 (cat. no. sc-398608; 1:500), MAFbx (cat.
no. sc-166806; 1:500), phosphorylated (p)-Akt (cat. no. sc-81433;
1:500), Akt (cat. no. sc-81434; 1:500), p-FoxO3α (cat. no.
sc-101683; 1:500), FoxO3α (cat. no. sc-48348; 1:500), p-mTOR (cat.
no. sc-293133; 1:250), mTOR (cat. no. sc-517464; 1:250; all from
Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. 5174S; 1:1,000;
Cell Signaling Technology, Inc.) at 4°C overnight. After washing
with PBS, the corresponding fluorescently labelled secondary
antibodies (catalog nos. 926-68020 and 926-68021, 1:3,000; LI-COR
Biosciences, Lincoln, NE, USA) were incubated with the membranes at
room temperature for 1.5 h. The membranes were imaged on an
Odyssey® CLx Infrared Imaging system (LI-COR
Biosciences). Image Studio 5.0 software (LI-COR Biosciences) was
used for measuring integrated optical densities.
Statistical analysis
Statistical analyses were performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). Significance was
determined using one-way ANOVA. For comparisons among multiple
groups, post-hoc pairwise comparisons were performed using Tukey's
multiple-comparison tests. All values are expressed as the mean ±
standard deviation (n≥3). P<0.05 was considered to indicate a
statistically significant difference.
Results
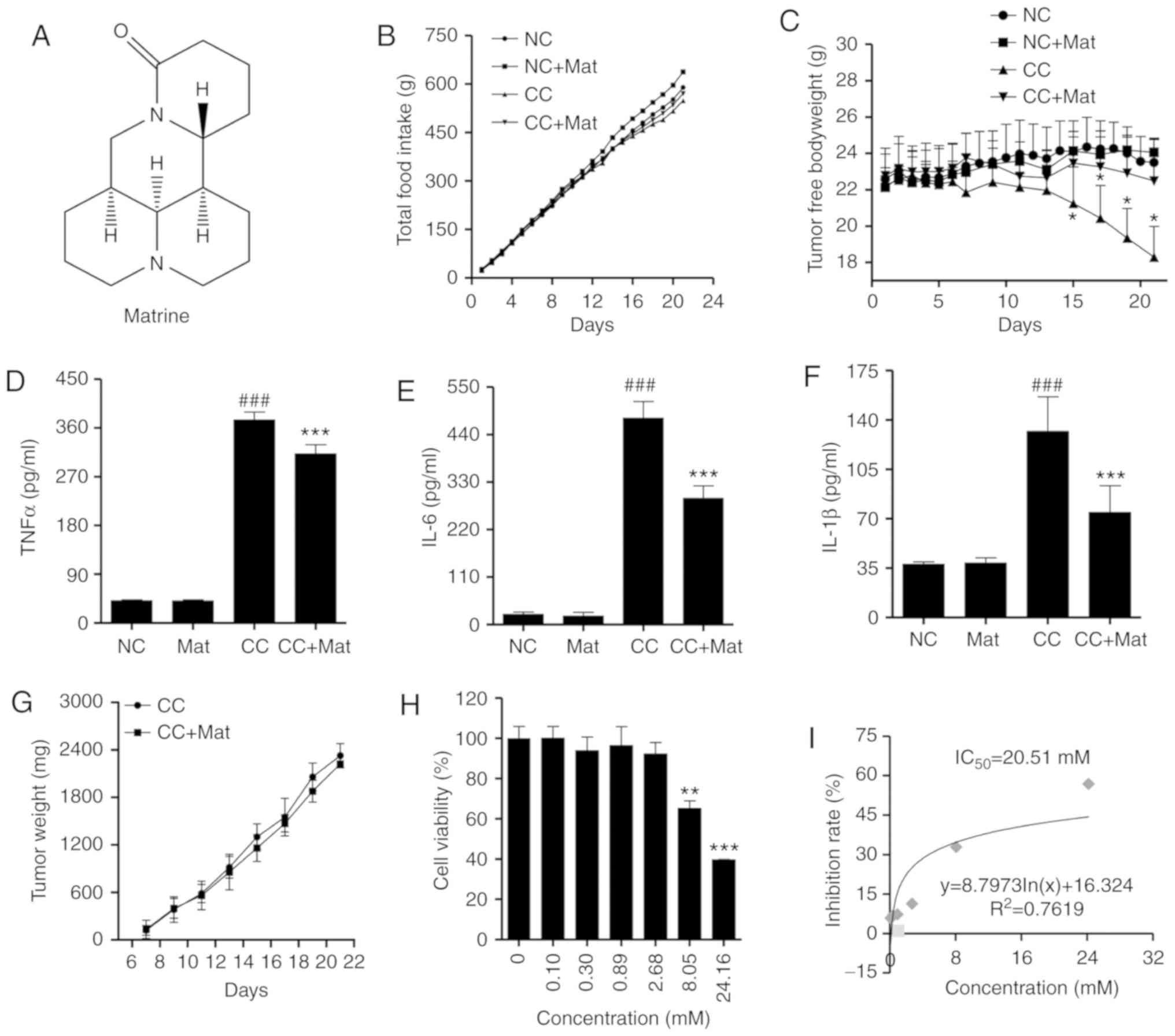
Matrine improves cancer cachexia
symptoms in CT26-bearing mice and exhibits limited effects on CT26
cell proliferation
To evaluate the anti-cachexia effect of matrine
(Fig. 1A), a classic CT26 colon
adenocarcinoma-bearing murine model was established. The tumors
were palpable by the seventh day post-inoculation. Mice bearing
CT26 tumors presented with early cancer cachexia symptoms,
including decreased food intake from day 12 (Fig. 1B). Total body weights were not
significantly different until day 18 in the CT26-bearing mice
compared with the negative control (data not shown). However, the
free-tumor body weights (total body weight minus tumor weight) were
significantly different from day 15 (P<0.05; Fig. 1C). Based on a previous study (28), the mice were intraperitoneally
injected with matrine at 50 mg/kg. Matrine-treatment demonstrated a
positive effect on the body weights but no influence on food
intake.
The organ and tissue weights also suggested less
wasting in the cancer cachexia matrine-treatment group compared
with in the cancer cachexia group (Table
II). The mass loss in the gastrocnemius and tibialis anterior
muscles was also reversed with matrine-treatment. In addition,
matrine significantly decreased the serum concentrations of
inflammatory cytokines, including TNFα (Fig. 1D), IL-6 (Fig. 1E), and IL-1β (Fig. 1F), which are known cachectins
(10).
 | Table II.Organ and tissue weights of mice in
different treatment groups. |
Table II.
Organ and tissue weights of mice in
different treatment groups.
| Organ or
tissue | NC | NC + Mat | CC | CC + Mat |
|---|
| Heart, mg | 112.67±7.93 | 107.75±6.33 |
70.66±5.45a |
97.15±7.55c |
| Liver, mg |
1,144.65±120.15 |
1,025.17±362.05 |
1,403.86±131.29a |
1,379.98±153.37 |
| Spleen, mg | 101.68±10.34 | 91.16±8.46 |
328.76±43.98a |
263.30±47.78c |
| Lung, mg | 137.54±9.89 | 136.17±13.71 | 128.66±11.89 | 126.41±9.93 |
| Kidney, mg | 355.46±25.84 | 361.17±23.66 |
315.55±33.13a |
354.42±25.32c |
| Epididymal fat,
mg | 576.74±58.72 | 611.58±71.64 |
131.59±48.42b |
271.56±84.27d |
| Gastrocnemius,
mg | 298.42±25.10 | 310.56±19.86 |
202.52±28.50b |
263.05±29.34e |
| Tibialis anterior,
mg | 99.77±11.03 | 116.41±19.06 |
66.05±15.58b |
94.49±12.93d |
CT26 tumor growth was not significantly inhibited
following matrine-treatment in mice (Fig.
1G). To determine the anticancer activity of matrine in CT26
cells, a CCK-8 assay was performed to determine the viability of
CT26 cells with or without matrine-treatment. The data demonstrated
that when the cells were treated with ≥8.05 mM matrine for 48 h,
the cell viability significantly decreased in a
concentration-dependent manner (Fig.
1H). The calculated IC50 of matrine was 20.51 mM in
CT26 cells (Fig. 1I). All of these
results indicate a limited anticancer effect of matrine. Thus, the
anti-cachexia effect of matrine appears to occur predominantly
through the alleviation of body weight and muscle wasting, rather
than anticancer effects.
Matrine improves skeletal muscle
atrophy in cachexia by inhibiting E3 ubiquitin ligase
expression
To determine whether the beneficial effect of
matrine on muscle wasting resulted from alleviation of skeletal
muscle atrophy, histological analysis of gastrocnemius muscle was
performed. Compared with that of the NC group, the CC group
exhibited myofiber atrophy, presenting as random loose fiber
arrangements and large inter-fiber gaps (Fig. 2A). The gastrocnemius myofibers of the
cachexia mice also demonstrated a sharp decrease in CSA, with most
myofibers having a value of 200–300 µm2. Following
matrine-treatment, the shape and arrangement of the myofibers
normalized, and there was a marked CSA increase, with most
myofibers exhibiting a value of 400–500 µm2 (Fig. 2B). Compared with that in the CC group,
there was a 70% increase in CSA in the CC + Mat group (Fig. 2C), which supported the muscle weight
increases. No negative influence of matrine on the gastrocnemius
muscle of normal mice was observed.
 | Figure 2.Matrine alleviates mice myofibers
atrophy by down-regulating the expression of E3 ubiquitin ligase.
(A) Hematoxylin-eosin staining of myofibers in mice gastrocnemius.
(B) The statistical results of myofiber CSA distribution in each
group (n>180 per group). (C) Mean muscle fibers CSA. (D) Reverse
transcription-quantitative polymerase chain reaction detected MAFbx
and MuRF1 mRNA expression in mice Ga (upper panels) and TA (lower
panels) (n=6). (E) Representative western blot images of MuRF1 and
MAFbx in mice Ga. (F and G) Statistical results of the western blot
analysis (n=3). Data are presented as the mean ± standard
deviation. Statistical significance was determined by one-way
ANOVA. ##P<0.01, ###P<0.001, vs. NC.
*P<0.05, **P<0.01, ***P<0.001 vs. CC. NC, negative
control; Mat, matrine; CC, cancer cachexia; CSA, cross-sectional
area; Ga, gastrocnemius; TA, tibialis anterior; MuRF1, muscle
RING-finger containing protein-1; MAFbx, muscle atrophy Fbox
protein. |
Subsequently, the mRNA levels of two E3 ubiquitin
ligases, MAFbx and MuRF1, in both gastrocnemius and tibia anterior
muscle were quantified by RT-qPCR. The mRNA expression levels of
both genes were significantly elevated more than ten-fold in the
cachexia mice compared with the NC group. Notably,
matrine-treatment significantly downregulated the mRNA levels of
both E3 ubiquitin ligases (Fig. 2D).
Consistent with the mRNA expression data, western blots
demonstrated that the protein expression levels of MuRF1 and MAFbx
in gastrocnemius muscle were significantly inhibited by
matrine-treatment compared with the CC group (Fig. 2E). Although there were differences
between mice, matrine significantly decreased the levels of the two
E3 ubiquitin ligases in the cachexia skeletal muscle.
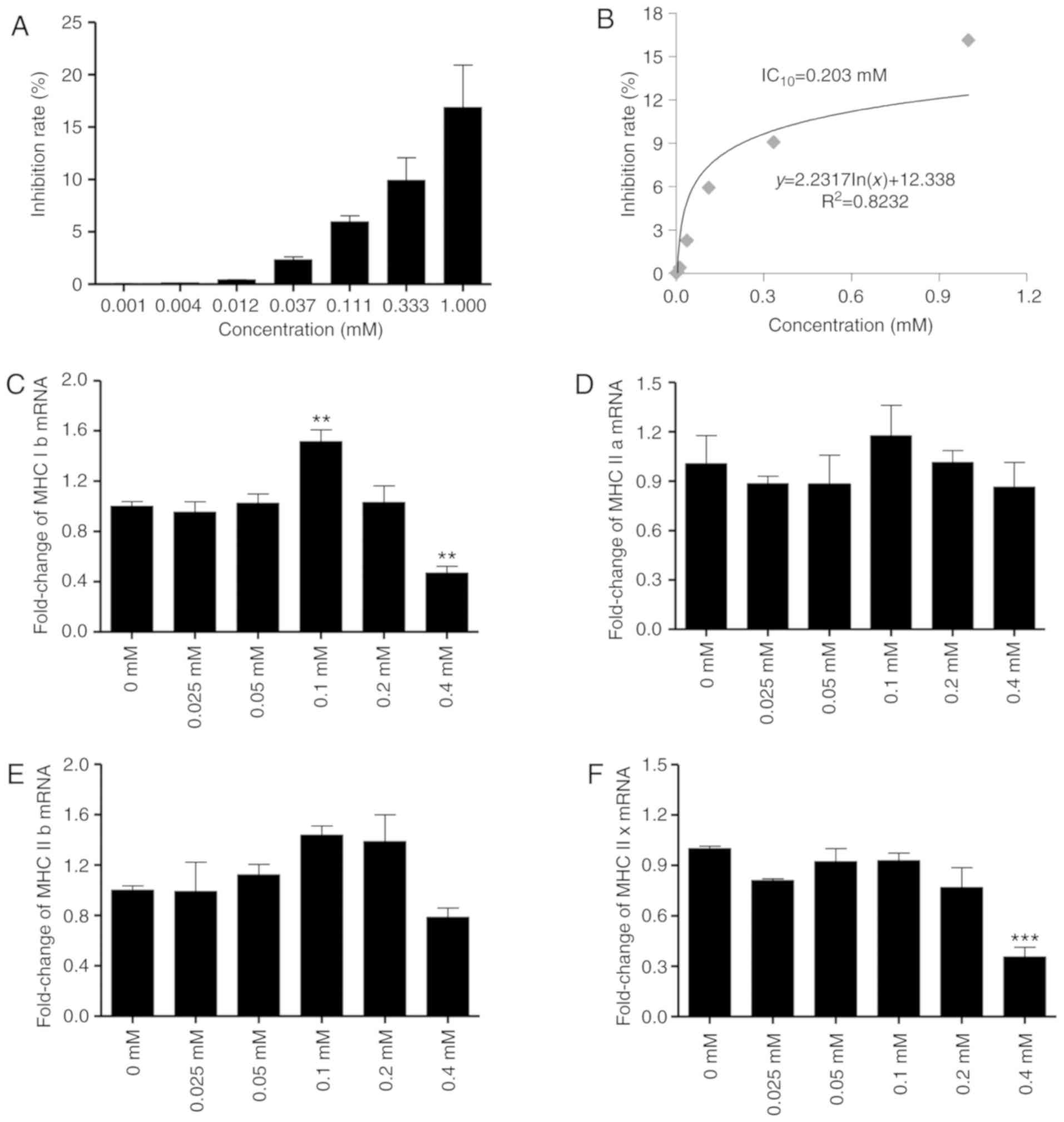
Matrine demonstrates no toxicity to
C2C12 myocytes and myotubes <0.2 mM
In order to determine the concentrations of matrine
that are non-toxic to C2C12 myocytes and myotubes, a CCK-8 assay
was performed. The data demonstrated that the IC10 of
matrine toward C2C12 myocytes is 0.203 mM (Fig. 3A and B), indicating no significant
toxicity at <0.203 mM. In addition, RT-qPCR analysis indicated
that 0.2 mM matrine exhibited no significant overall influence on
the mRNA expression of MHC, a major structural protein of myotubes.
However, matrine treatment at 0.1 mM significantly upregulated the
expression of MHC Ib (Fig. 3C). For
MHC IIa (Fig. 3D) and IIb (Fig. 3E), there were no significant changes
with matrine-treatment at any concentration. In addition, matrine
(0.4 mM) significantly inhibited the expression of MHC Ib and MHC
IIx (Fig. 3C and F).
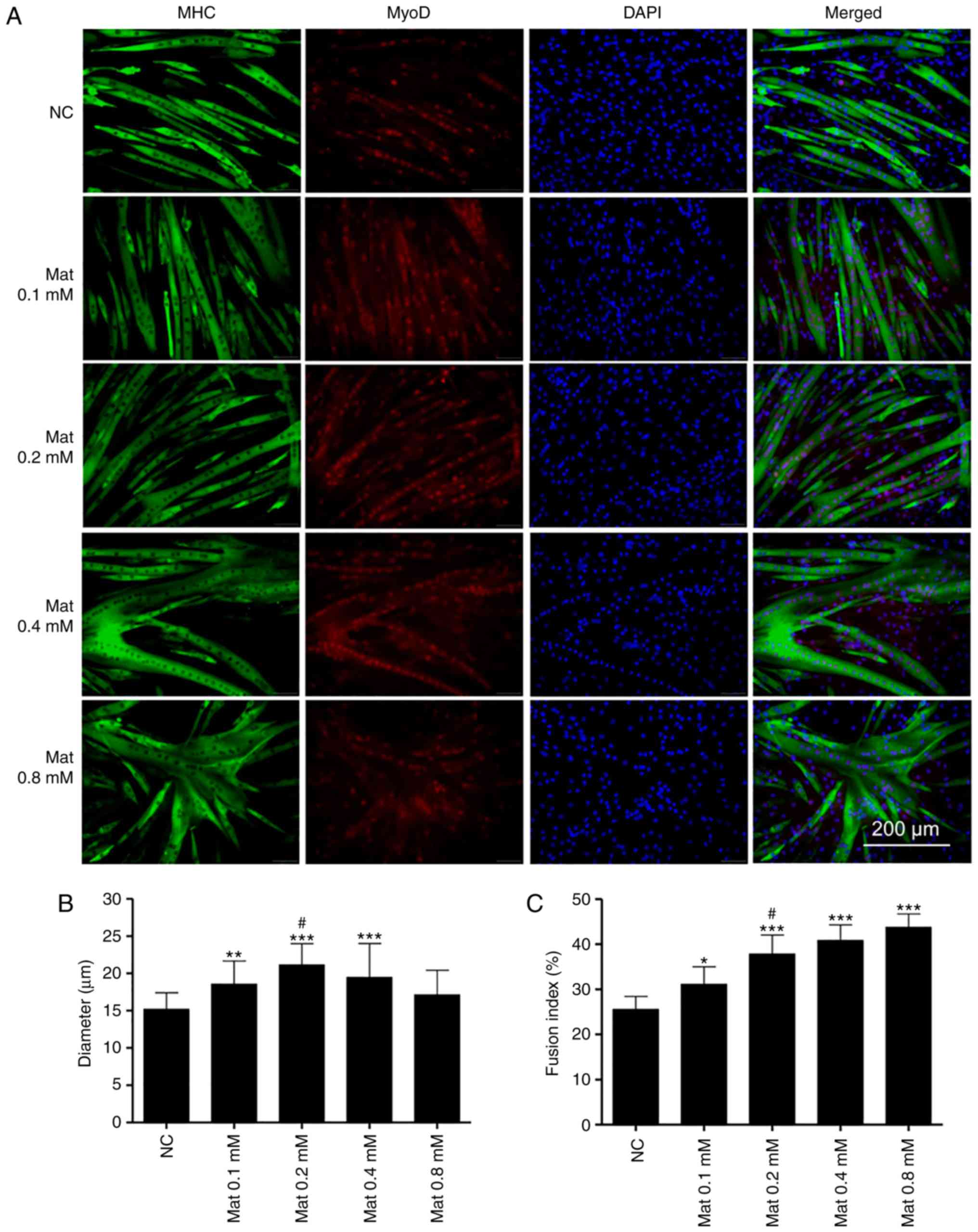
Matrine promotes C2C12 myoblast
differentiation with/without Dex
Double immunofluorescence staining of MHC and MyoD
was used to investigate the effect of matrine on normally
differentiated or Dex-stimulated C2C12 myoblasts. As presented in
Fig. 4A, the myotubes, but not the
undifferentiated myoblasts, were stained for MHC and MyoD.
Furthermore, matrine increased myotube diameters at 0.1–0.4 mM
(Fig. 4B). Notably, the myoblasts
fused into giant and atypical myotubes when matrine was used at 0.4
and 0.8 mM, with higher matrine concentrations demonstrating
increased fusion (Fig. 4C). However,
compared with that in the other matrine-treatment groups, MyoD
expression was lower in the 0.8 mM matrine-treatment group.
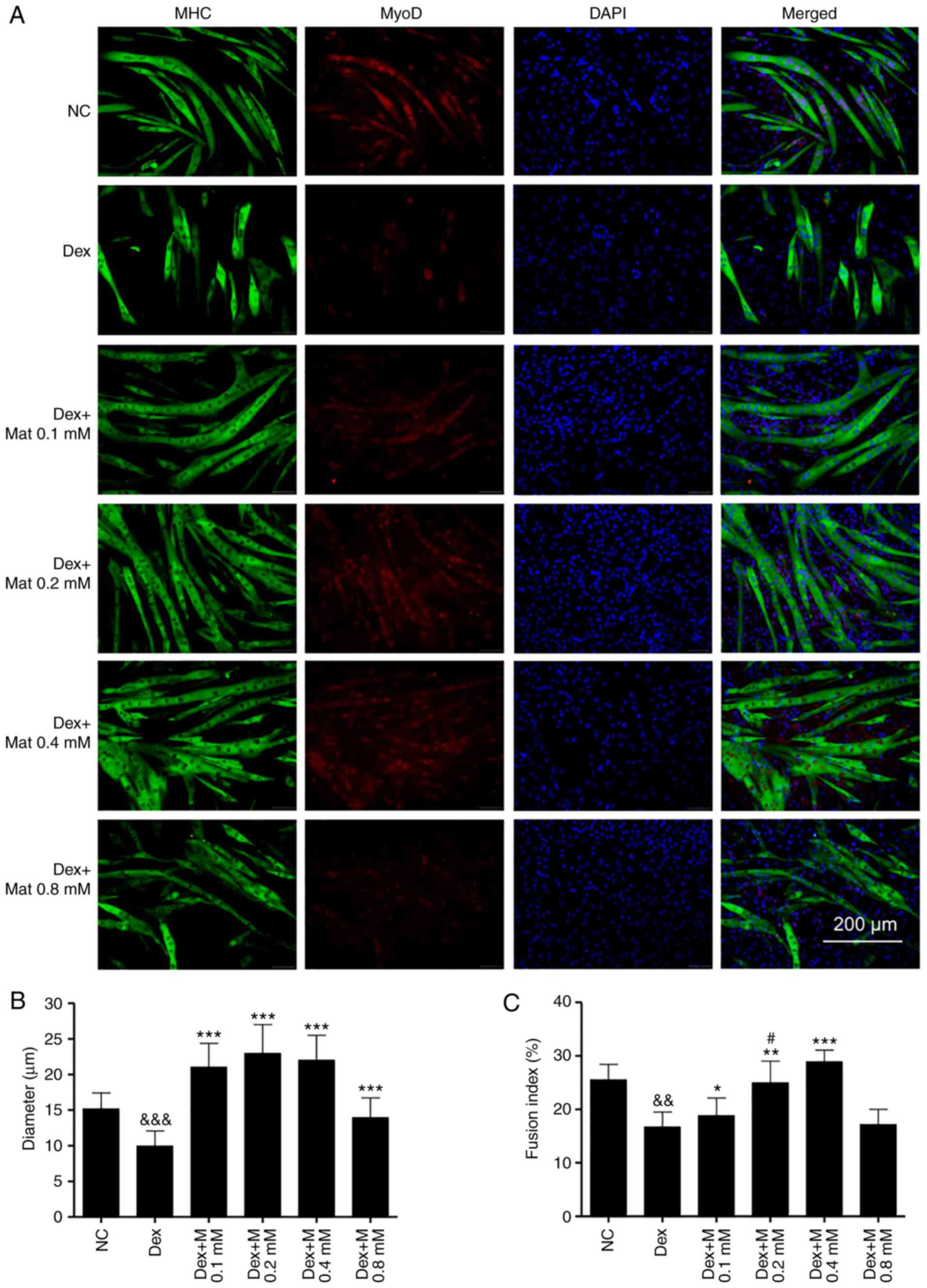
It was identified that Dex can significantly inhibit
differentiation of C2C12 myoblasts, since there was less myotube
formation in the Dex-treated groups. Furthermore, the formed
myotubes in the Dex-treated groups were shorter and exhibited fewer
nuclei (Fig. 5A). With
matrine-treatment at 0.1–0.4 mM, the myotube diameters and fusion
indexes increased (Fig. 5B and C).
These results indicate that matrine can increase the
differentiation of C2C12 myoblasts, even in the presence of Dex,
which impairs their differentiation. However, there was no
significant difference in fusion index between the Dex + Mat 0.8 mM
and Dex groups. In addition, compared with that of the other
matrine-treatment groups, the Dex + Mat 0.8 mM group demonstrated
lower MyoD expression. This suggests matrine can increase myoblast
differentiation when used up to 0.4 mM.
Matrine increases atrophic C2C12
myotube diameters and inhibits Dex-induced upregulation of E3
ubiquitin ligases
Despite having confirmed that matrine promotes C2C12
myoblast differentiation, whether it can stabilize myotubes in the
presence of impairment factors remains unknown. After 3–5 days of
differentiation and myotube formation, 100 µM Dex, 50 ng/ml TNFα or
CM were added for 48 h to induce myotube apoptosis and atrophy.
Dex-treatment resulted in the apoptosis and atrophy of formed
myotubes, since discontinuous MHC staining was visible. TNFα- and
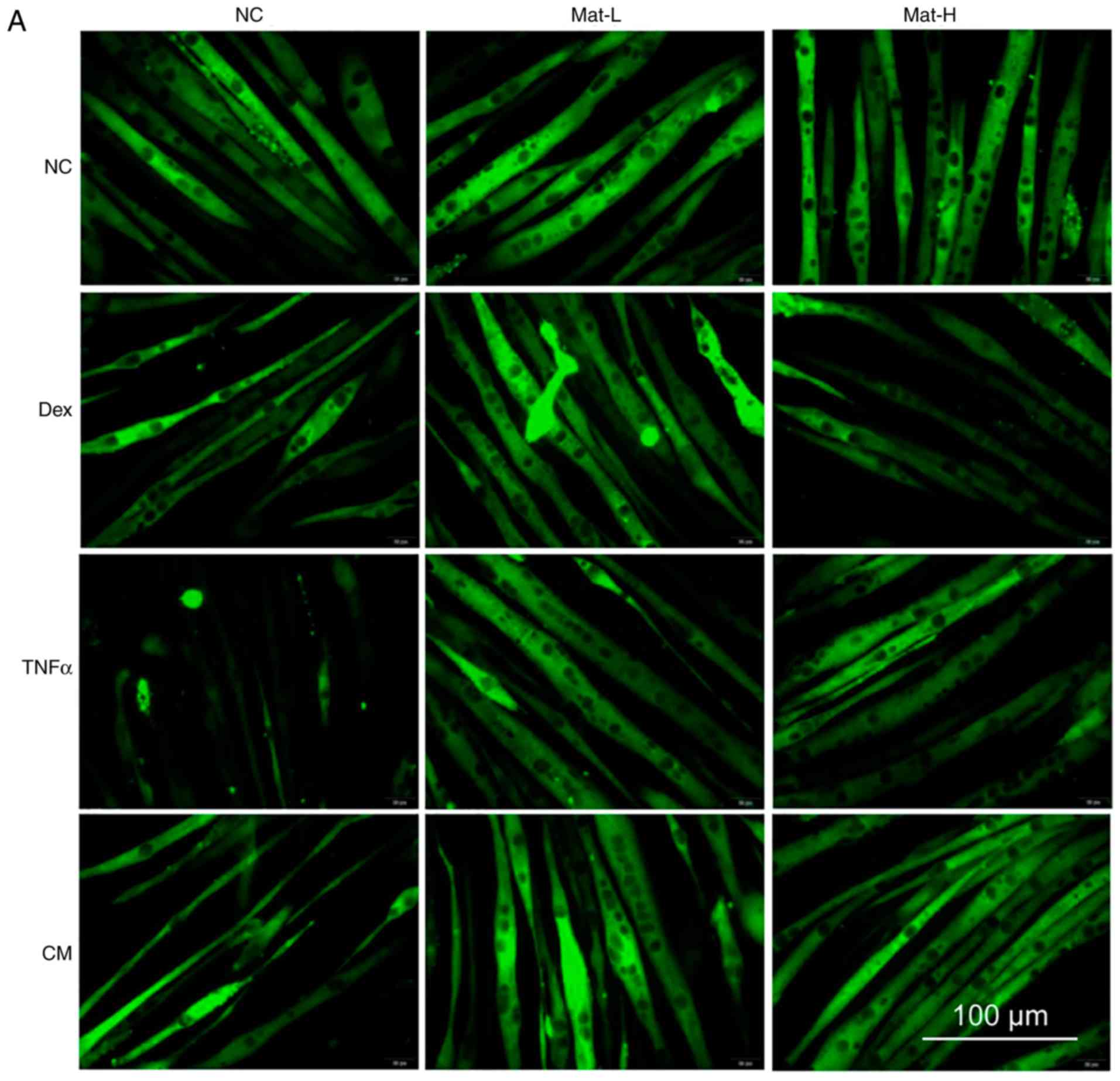
CM- treatment also resulted in extensive myotube damage. Matrine
demonstrated protective effects at both 0.1 mM (Mat-L) and 0.2 mM
(Mat-H; Fig. 6A). The diameters of
most normal myotubes ranged between 9–15 µm, while they ranged
between 5–11 µm after being treated with Dex, TNFα or CM (Fig. 6B and C). With matrine co-treatment,
the myotube diameters significantly improved, with values of 7–15
µm. There was no effect of matrine on the morphology of normal
myotubes, indicating the absence of toxicity on formed
myotubes.
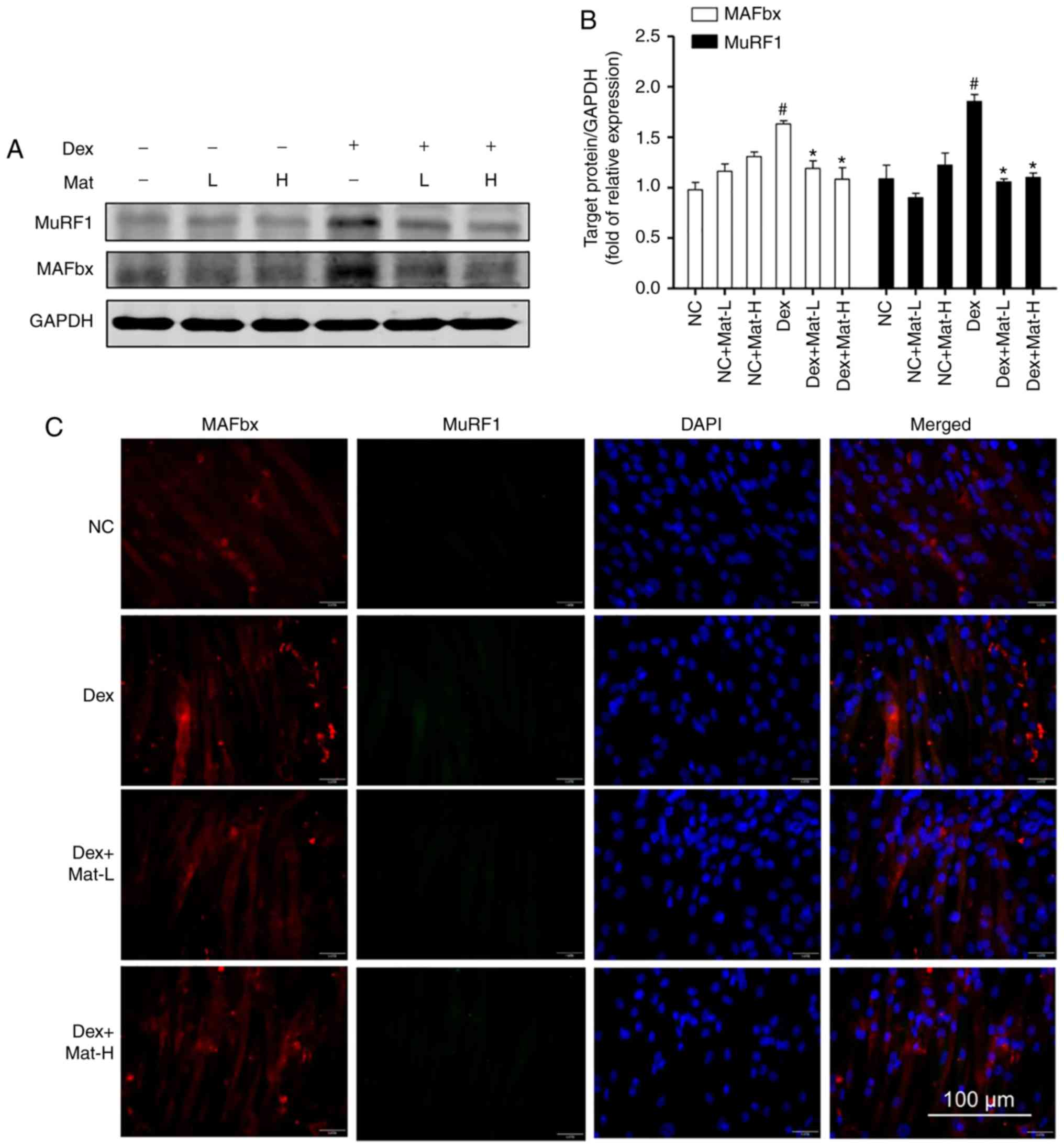
Western blotting and immunofluorescence for MAFbx
and MuRF1 (Fig. 7) also supported the
ability of matrine to protect myotubes under Dex-induced myotube
conditions. Dex-treatment significantly upregulated MAFbx and MuRF1
(Fig. 7A). Matrine (0.1 mM) decreased
expression of MAFbx and MuRF1 by 27 and 43%, respectively. These
results are consistent with the in vivo experimental
findings where matrine downregulated the expression of MAFbx and
MuRF1 in cachexia mice skeletal muscle. In addition, matrine
exhibited no significant influence on the expression of these two
E3 ubiquitin ligases in normal myotubes. Furthermore, it was
identified that matrine significantly decreased the mRNA level of
myostatin (Fig. S1), an upstream
regulator of E3 ubiquitin ligases, which was significantly
upregulated by following treatment with Dex.
The western blotting data was then corroborated
using immunofluorescence. The images revealed that MAFbx and MuRF1
could not be detected in normal C2C12 myotubes. However,
particularly with the Dex-treatment group, both E3 ubiquitin
ligases demonstrated higher fluorescence. Co-treatment with matrine
substantially decreased the expression of both E3 ubiquitin
ligases, with little observable difference between the two
concentrations of matrine.
Effect of matrine on the
Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes
When myotubes were treated with 100 µM Dex for 48 h,
accompanied by the upregulated expression of the two E3 ubiquitin
ligases, a significant decrease was also identified in the relative
phosphorylation of Akt, mTOR and FoxO3α. Treatment with matrine at
0.1 mM for 48 h reversed this effect on Akt, FoxO3α, and mTOR
phosphorylation (P<0.05; Fig. 8).
However, compared with the treatment with Dex and matrine, the
additional treatment with 10 nM wortmannin (a PI3K inhibitor) for
48 h diminished the effect of matrine by decreasing the
phosphorylation of Akt, mTOR and FoxO3α, accompanied by the
upregulation of MAFbx and MuRF1. This indicates that the
anti-atrophic effect of matrine was attenuated by inhibition of the
Akt/mTOR/FoxO3α signaling pathway. Therefore, these results suggest
that matrine-treatment upregulates anabolism and downregulates
catabolism of muscle-specific proteins.
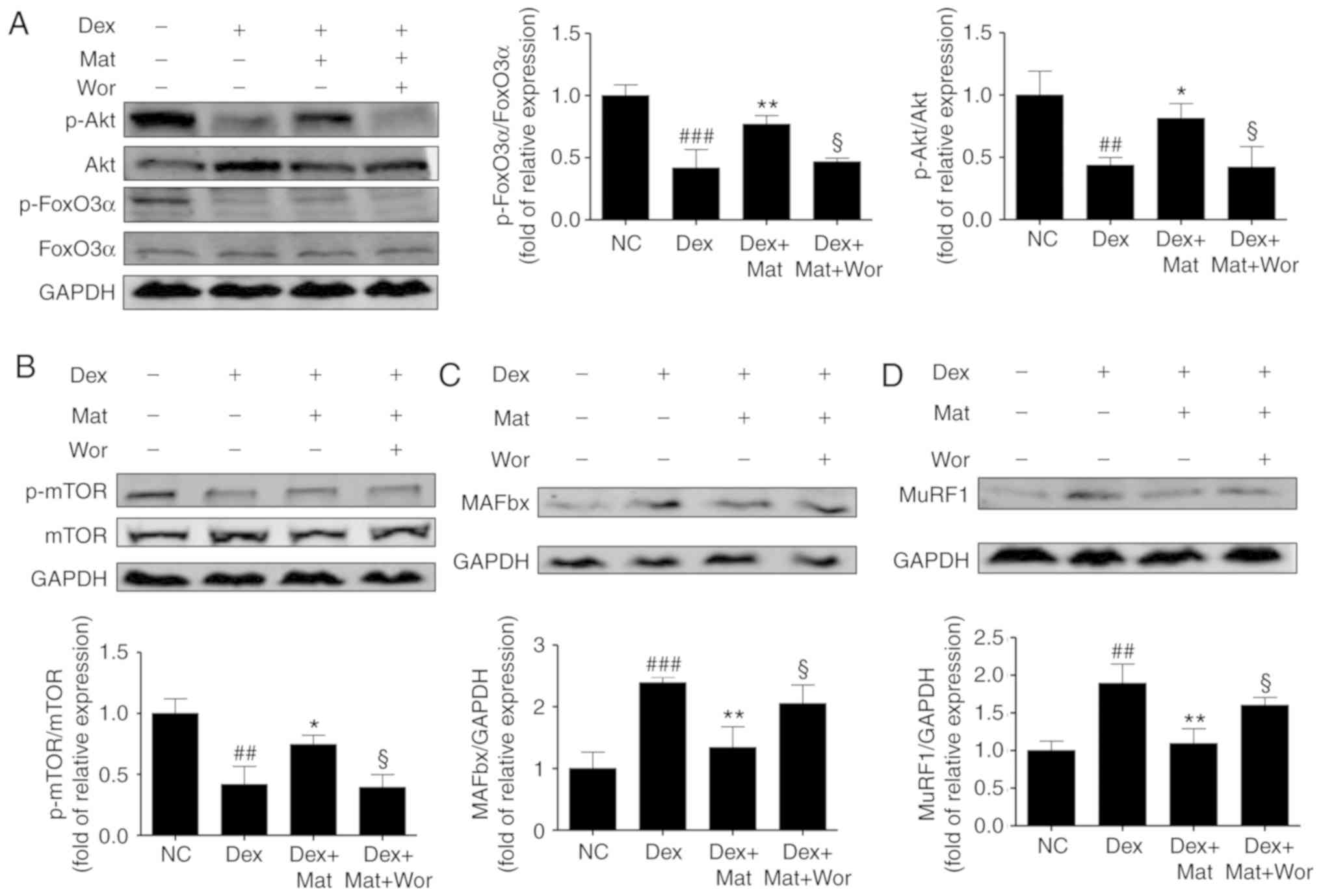 | Figure 8.Effect of matrine on the
Akt/mTOR/FoxO3α signalling pathway in C2C12 myotubes.
Representative western blot images and densitometric quantification
of the associated phosphorylated levels of (A) FoxO3α, Akt, (B)
mTOR, (C) MAFbx and (D) MuRF1 in C2C12 myotubes. Dex, matrine and
wortmannin were added to culture medium for 48 h at 100 µM, 0.1 mM
and 10 nM, respectively. Data are presented as the mean ± standard
deviation (n=3). Statistical significance was determined by one-way
ANOVA. *P<0.05, **P<0.01 vs. Dex. ##P<0.01,
###P<0.001 vs. NC. §P<0.05 vs. Dex +
Mat. NC, negative control; Dex, dexamethasone; Mat, matrine; Wor,
wortmannin; p, phosphorylated; MuRF1, muscle RING-finger containing
protein-1; MAFbx, muscle atrophy Fbox protein. |
Discussion
Body composition analysis has demonstrated that
patients with cancer cachexia lose 30% of their body weight and
their muscle mass decreases by ~75% (37). Skeletal muscle atrophy has been
associated with poor prognosis and suboptimal responses (5,38).
However, until recently, there has been no effective medicine to
treat cancer cachexia-induced muscle atrophy. Matrine is approved
by the CFDA for the prevention and treatment of cancer cachexia;
however, to the best of our knowledge, its mechanism of activity on
skeletal muscle remains unknown. The present study investigated the
anti-muscle atrophy effects and mechanisms of matrine activity both
in vitro and in vivo. Matrine significantly improved
the diameter and fusion index of C2C12 myotubes. Matrine also
normalized multiple factors that induced C2C12 myotube atrophy and
cachexia-induced skeletal muscle wasting. Notably and to the best
of our knowledge, this is the first time that matrine has been
demonstrated to downregulate expression of MuRF1 and MAFbx in C2C12
myotubes and skeletal muscle, and that activation of the PI3K/Akt
signaling pathway is a potential mechanism of matrine activity in
skeletal muscle.
Cancer cachexia has typical characteristics,
including loss of body weight, muscle and adipose wasting,
weakness, and anorexia (1,2). In previous studies, inflammatory
infiltration has been suggested as the main cause of muscle wasting
(39–41). However, there is evidence that
inflammation does not associate well with cancer cachexia-induced
muscle wasting and prolongation of life expectancy, and the Evans
publication (30,42) to further establish a comprehensive
definition did not include cytokines. Inflammatory factors are
derived from tumor cells and activated immune cells (43). In the present study, serum
inflammatory cytokines were downregulated by matrine, which was
probably one of its benefits. However, the most important finding
was that matrine decreased the expression of E3 ubiquitin ligases
in skeletal muscle, which is direct evidence of its anti-muscle
atrophy effect and is consistent with the increase in skeletal
muscle mass and myofiber CSA. Furthermore, matrine ameliorated
other cancer cachexia symptoms by increasing body, fat and organ
weights. In summary, the results demonstrated that matrine is a
potential drug for preventing cancer cachexia symptoms,
particularly for skeletal muscle atrophy in mice.
Matrine has been reported to exhibit acute toxicity
in Kunming mice at 80 mg/kg/day (44), and developmental toxicity and
neurotoxicity in zebrafish embryos (45). However, matrine was verified to
exhibit beneficial effects on hepatitis, cardiac injury and
fibrotic diseases (21–23). Therefore, appropriate concentrations
for the treatment of C2C12 myoblasts and myotubes need to be
investigated. The current CCK-8 assay results demonstrated that
matrine exhibited no significant toxicity on C2C12 myocytes below
0.203 mM. Myocytes and myotubes are two different types of C2C12
cells and they have different sensitivities to stimulation
(46). Levels of MHC, the main
structural protein of myotubes, are mediated by MuRF1, which
interacts with MHC and controls its half-life (47). The present RT-qPCR results revealed
that <0.2 mM matrine exhibited no negative influence on MHC
subfamily mRNA expression levels in myotubes. However, 0.1 mM
matrine upregulated mRNA expression of MHC Ib, while it
downregulated MHC Ib andIIx at 0.4 mM. C2C12 multinucleate myotubes
are formed by myocyte fusion along the axis direction. Myogenic
genes, including MyoD, MyoG, Myf5 and Mrf4, serve an important role
in this process (20). Dex degrades
MyoD by upregulation of atrogin-1, and this decreases protein
synthesis and increases skeletal muscle wasting (48). The expression levels of myogenic genes
reflect the differentiation potency of myocytes. In the current
study, matrine promoted MyoD expression with or without Dex
treatment, and this effect mostly reflected its influence on
myotube diameter and fusion index. One difference is that a higher
concentration of matrine (0.8 mM) produced the highest fusion
index, while decreasing the expression of MyoD. This suggests that
giant and irregular myotube formation may not reflect healthy
myocyte differentiation. These results revealed that matrine may
benefit C2C12 cells in vitro at <0.4 mM.
Typically, after culture in 2% horse serum for 3–5
days, C2C12 myocytes fuse to form myotubes. Subsequently, although
the differentiation medium is replaced daily, the myotubes
gradually atrophy a few days later (data not shown). MHC is
distributed widely in myotubes (49).
Therefore, MHC immunostaining reveals the outline of the myotubes
and decreased MHC is a marker of muscle atrophy. Dex, TNFα and CM
are often used to establish muscle or myotube atrophy models
(31–33). In the present study, decreases in
apoptosis and diameter of myotubes caused by these stimulants were
completely reversed by matrine. This suggests that matrine serves
an important role in modulating a common pathway of the different
factors that cause myotube atrophy. Dex-induced skeletal muscle
atrophy is caused by upregulation of atrogin-1 and MuRF1 via
specific regulators, including FoxO and NF-κB (50), and Dex upregulates the activity and
mRNA expression of myostatin (30).
Consistent with these previous findings, both western blots and
double immunostaining suggested that Dex upregulated MuRF1 protein,
MAFbx protein and myostatin mRNA in C2C12 myotubes (Fig. S1). Co-treatment with matrine and Dex
demonstrated that MuRF1 and MAFbx protein, and myostatin mRNA were
downregulated to much lower levels, which suggests that the
progression of myotube atrophy was inhibited.
Several signaling pathways are associated with
skeletal muscle hypertrophy and atrophy, involving the control of
protein synthesis and degradation (51). In general, the Akt/mTOR and MAPK
pathways are inhibited during muscle atrophy, while FoxO and NF-κB
are activated (17,52). Upon dephosphorylation, FoxO
translocates to the nucleus as a transcription factor, thereby
upregulating E3 ubiquitin ligase (52). When FoxO activation is inhibited by
RNAi in mouse muscles in vivo, both atrogin-1 induction
during starvation and myotube atrophy induced by glucocorticoids
are prevented (19). Akt is a
downstream target of PI3K, which in turn leads to activation of
mTOR, followed by activation of p70S6K and PHAS-1/4E-BP1; this
promotes protein synthesis through increases in translation
initiation and elongation (15).
Consistent with this, in the present study of C2C12 myotubes, Dex
significantly decreased the relative levels of p-Akt, p-mTOR and
p-FoxO3α. However, matrine-treatment largely reversed the
phosphorylation of these signal proteins, which could be attenuated
by wortmannin, a PI3K inhibitor frequently used for blocking the
PI3K/Akt pathway. Combined with the upregulation of the two E3
ligases with wortmannin-treatment, matrine possibly exerts an
important effect on the Akt/mTOR/FoxO3α signaling pathway. There is
another report that matrine inhibits the phosphorylation of FoxO3α,
which induces apoptosis of prostate cancer cells (27). Therefore, the effects of matrine on
C2C12 myotubes and cancer cells may be different, which helps
explain why matrine alleviated the CT26 tumor burden in the current
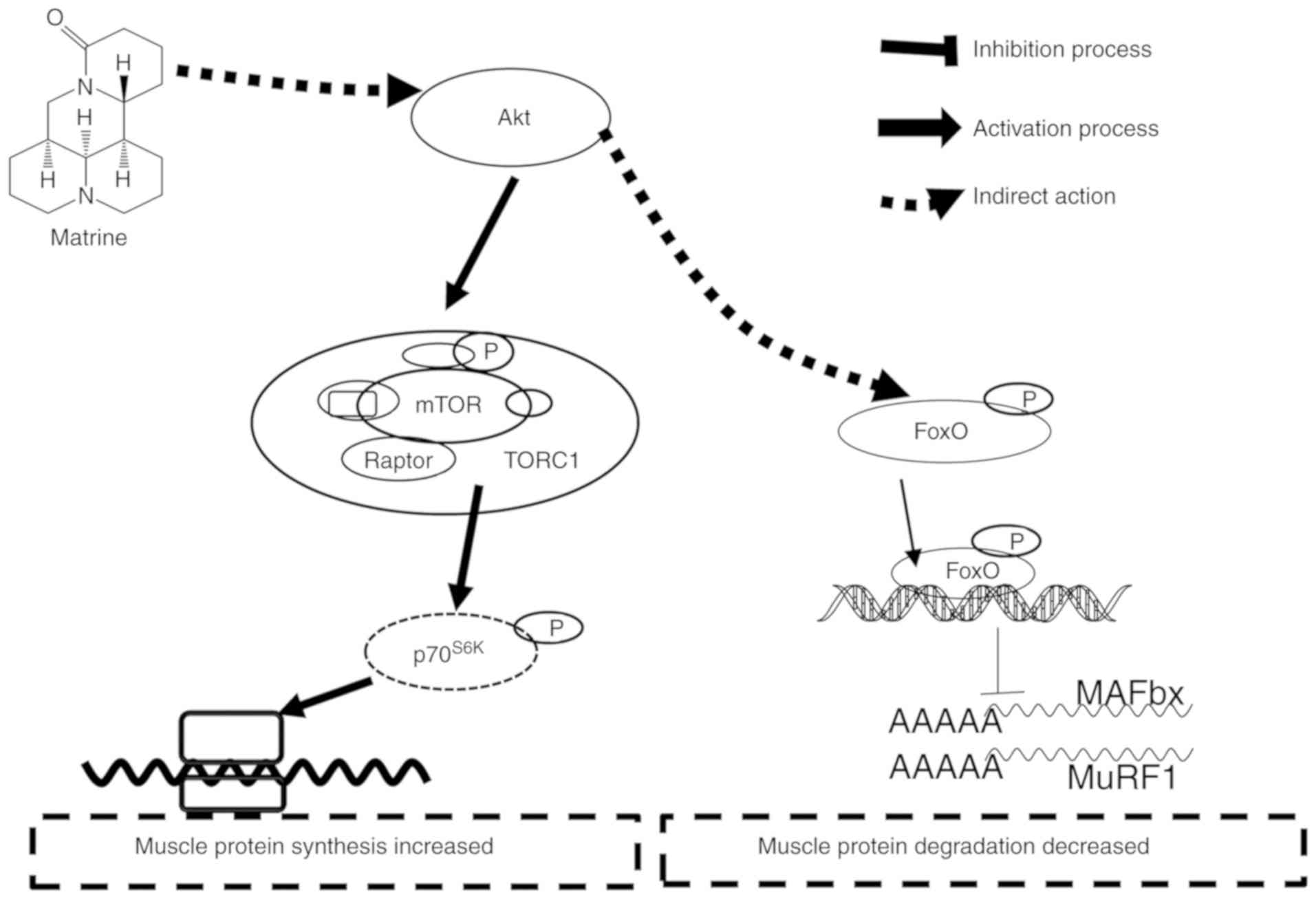
study. However, more in-depth studies are required. Collectively,
the anti-muscle atrophy effects of matrine appear to involve the
Akt/mTOR/FoxO3α signaling pathway (Fig.
9).
However, there are certain limitations of the
current study. First, the effect of matrine was only identified on
CT26 tumor cachexia. Future research should focus on the effect of
matrine on more cancer cachexia models. Second, the effect of
matrine on patients with cancer cachexia would be more convincing.
However, a small number of patients with matrine-treated cancer
cachexia receive surgery; therefore, few clinical samples would be
accessible. Along with the increasing popularity of treating cancer
cachexia with matrine, we hope to obtain more clinical samples and
perform more in-depth research in the future.
In conclusion, the present study predominantly
investigated the anti-muscle atrophy effects and mechanism of
matrine in C2C12 myotubes, and the effects of matrine were verified
in CT26-induced cachexia mice. Matrine substantially improved CT26
colon adenocarcinoma-induced skeletal muscle atrophy by preserving
the mass and CSA of myofibers. Matrine-treatment also significantly
increased C2C12 myoblast differentiation and attenuated myotube
atrophy. Notably, to the best of our knowledge, matrine was
demonstrated for the first time to downregulate expression of MuRF1
and MAFbx in cancer cachexia skeletal muscle, and matrine's effect
on the Akt/mTOR/FoxO3α signaling pathway was identified to be
involved in this phenomenon.
Supplementary Material
Supporting Data
Acknowledgements
The authors wish to thank Ms Jingxian Zhang and Ms
Dan Feng (Department of Pharmacy, Shanghai Jiao Tong University
Affiliated Sixth People's Hospital, Shanghai, China), who provided
kind assistance with the animal experiments.
Funding
This work was supported by grants from the National
Science Foundation of China (grant nos. 81873042 and 81872494).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LiC, YH, QY JH and CG participated in the research
design. LiC, LinC and BX performed the experiments. JLi, JLu and BX
contributed the reagents, materials and analysis tools. LiC, JLi,
QY, JLu, LW and CG acquired and analysed the data. JLu and JH
interpreted the data. LiC, LW, JLu and JH wrote and proofread the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures involving animals and their care in
the current study were approved by the Animal Care Committee of
Shanghai Jiao Tong University Affiliated Sixth People's Hospital
(Shanghai, China) in accordance with institutional requirements and
Chinese government guidelines for animal experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MuRF1
|
muscle RING-finger containing
protein-1
|
|
MAFbx
|
muscle atrophy Fbox protein
|
|
CFDA
|
China Food and Drug Administration
|
|
Dex
|
dexamethasone
|
|
TNFα
|
tumor necrosis factor α
|
|
CSA
|
cross-sectional area
|
|
CM
|
conditioned medium
|
References
|
1
|
Baracos VE, Martin L, Korc M, Guttridge DC
and Fearon KCH: Cancer-associated cachexia. Nat Rev Dis Primers.
4:171052018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Argiles JM, Busquets S, Stemmler B and
Lopez-Soriano FJ: Cancer cachexia: Understanding the molecular
basis. Nat Rev Cancer. 14:754–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daly LE, Ní Bhuachalla ÉB, Power DG,
Cushen SJ, James K and Ryan AM: Loss of skeletal muscle during
systemic chemotherapy is prognostic of poor survival in patients
with foregut cancer. J Cachexia Sarcopenia Muscle. 9:315–325. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon K, Arends J and Baracos V:
Understanding the mechanisms and treatment options in cancer
cachexia. Nat Rev Clin Oncol. 10:90–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang QJ, Zhao JR, Hao J, Li B, Huo Y, Han
YL, Wan LL, Li J, Huang J, Lu J, et al: Serum and urine
metabolomics study reveals a distinct diagnostic model for cancer
cachexia. J Cachexia Sarcopenia Muscle. 9:71–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quanjun Y, Genjin Y, Lili W, Bin L, Jin L,
Qi Y, Yan L, Yonglong H, Cheng G and Junping Z: Serum metabolic
profiles reveal the effect of formoterol on cachexia in
tumor-bearing mice. Mol Biosyst. 9:3015–3025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen T, Li B, Xu Y, Meng S, Wang Y and
Jiang Y: Luteolin reduces cancerinduced skeletal and cardiac muscle
atrophy in a Lewis lung cancer mouse model. Oncol Rep.
40:1129–1137. 2018.PubMed/NCBI
|
|
9
|
Chen X, Wu Y, Yang T, Wei M, Wang Y, Deng
X, Shen C, Li W, Zhang H, Xu W, et al: Salidroside alleviates
cachexia symptoms in mouse models of cancer cachexia via activating
mTOR signalling. J Cachexia Sarcopenia Muscle. 7:225–232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Argiles JM: The 2015 ESPEN Sir david
cuthbertson lecture: Inflammation as the driving force of muscle
wasting in cancer. Clin Nutr. 36:798–803. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lecker SH, Jagoe RT, Gilbert A, Gomes M,
Baracos V, Bailey J, Price SR, Mitch WE and Goldberg AL: Multiple
types of skeletal muscle atrophy involve a common program of
changes in gene expression. FASEB J. 18:39–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crossland H, Constantin-Teodosiu D,
Gardiner SM, Constantin D and Greenhaff PL: A potential role for
Akt/FOXO signalling in both protein loss and the impairment of
muscle carbohydrate oxidation during sepsis in rodent skeletal
muscle. J Physiol. 586:5589–5600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glass DJ: Signaling pathways perturbing
muscle mass. Curr Opin Clin Nutr Metab Care. 13:225–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bodine SC, Stitt TN, Gonzalez M, Kline WO,
Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC,
Glass DJ and Yancopoulos GD: Akt/mTOR pathway is a crucial
regulator of skeletal muscle hypertrophy and can prevent muscle
atrophy in vivo. Nat Cell Biol. 3:1014–1019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai D, Frantz JD, Tawa NJ, Melendez PA, Oh
BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ and
Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle
wasting in mice. Cell. 119:285–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McFarlane C, Plummer E, Thomas M, Hennebry
A, Ashby M, Ling N, Smith H, Sharma M and Kambadur R: Myostatin
induces cachexia by activating the ubiquitin proteolytic system
through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell
Physiol. 209:501–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zimmers TA, Fishel ML and Bonetto A: STAT3
in the systemic inflammation of cancer cachexia. Semin Cell Dev
Biol. 54:28–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sandri M, Sandri C, Gilbert A, Skurk C,
Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH and Goldberg
AL: Foxo transcription factors induce the atrophy-related ubiquitin
ligase atrogin-1 and cause skeletal muscle atrophy. Cell.
117:399–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitzmann M, Carnac G, Vandromme M, Primig
M, Lamb NJ and Fernandez A: The muscle regulatory factors MyoD and
myf-5 undergo distinct cell cycle-specific expression in muscle
cells. J Cell Biol. 142:1447–1459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
22
|
Li X, Wang X, Guo Y, Deng N, Zheng P, Xu
Q, Wu Y and Dai G: Regulation of endothelial nitric oxide synthase
and asymmetric dimethylarginine by matrine attenuates
isoproterenol-induced acute myocardial injury in rats. J Pharm
Pharmacol. 64:1107–1118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Wang B, Zhou C and Bi Y: Matrine
induces apoptosis in angiotensin II-stimulated hyperplasia of
cardiac fibroblasts: Effects on Bcl-2/Bax expression and caspase-3
activation. Basic Clin Pharmacol Toxicol. 101:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shao H, Yang B, HU R and Wang Y: Matrine
effectively inhibits the proliferation of breast cancer cells
through a mechanism related to the NF-κB signaling pathway. Oncol
Lett. 6:517–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Cheng B, Li H, Xu W, Zhai B, Pan
S, Wang L, Liu M and Sun X: Matrine inhibits proliferation and
induces apoptosis of human colon cancer LoVo cells by inactivating
Akt pathway. Mol Biol Rep. 41:2101–2108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Lai Y, Wang C, Xu G, He Z, Shang X,
Sun Y, Zhang F, Liu L and Huang H: Matrine inhibits the
proliferation, invasion and migration of castration-resistant
prostate cancer cells through regulation of the NF-κB signaling
pathway. Oncol Rep. 35:375–381. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai S, Chen T, Yu X, Luo M, Chen X, Lin C,
Lai Y and Huang H: The specific killing effect of matrine on
castration-resistant prostate cancer cells by targeting the
Akt/FoxO3a signaling pathway. Oncol Rep. 37:2819–2828. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Wang S, Li Y, Xiao Z, Hu Z and
Zhang J: Sophocarpine and matrine inhibit the production of
TNF-alpha and IL-6 in murine macrophages and prevent
cachexia-related symptoms induced by colon26 adenocarcinoma in
mice. Int Immunopharmacol. 8:1767–1772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Donohoe CL, Ryan AM and Reynolds JV:
Cancer cachexia: Mechanisms and clinical implications.
Gastroenterol Res Pract. 2011:6014342011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muscaritoli M, Anker SD, Argiles J, Aversa
Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P,
et al: Consensus definition of sarcopenia, cachexia and
pre-cachexia: Joint document elaborated by Special Interest Groups
(SIG) ‘cachexia-anorexia in chronic wasting diseases’ and
‘nutrition in geriatrics’. Clin Nutr. 29:154–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim H, Jang M, Park R, Jo D, Choi I, Choe
J, Oh WK and Park J: Conessine treatment reduces
dexamethasone-induced muscle atrophy by regulating MuRF1 and
atrogin-1 expression. J Microbiol Biotechnol. 28:520–526.
2018.PubMed/NCBI
|
|
32
|
Jackman RW, Floro J, Yoshimine R, Zitin B,
Eiampikul M, El-Jack K, Seto DN and Kandarian SC: Continuous
release of tumor-derived factors improves the modeling of cachexia
in muscle cell culture. Front Physiol. 8:7382017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xi QL, Zhang B, Jiang Y, Zhang HS, Meng
QY, Chen Y, Han YS, Zhuang QL, Han J, Wang HY, et al: Mitofusin-2
prevents skeletal muscle wasting in cancer cachexia. Oncol Lett.
12:4013–4020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aulino P, Berardi E, Cardillo VM, Rizzuto
E, Perniconi B, Ramina C, Padula F, Spugnini EP, Baldi A, Faiola F,
et al: Molecular, cellular and physiological characterization of
the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC
Cancer. 10:3632010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Q, Wan L, Zhou Z, Li Y, Yu Q, Liu L,
Li B and Guo C: Parthenolide from parthenium integrifolium reduces
tumor burden and alleviate cachexia symptoms in the murine CT-26
model of colorectal carcinoma. Phytomedicine. 20:992–998. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neefjes ECW, van den Hurk RM,
Blauwhoff-Buskermolen S, van der Vorst MJDL, Becker-Commissaris A,
de van der Schueren MAE, Buffart LM and Verheul HMW: Muscle mass as
a target to reduce fatigue in patients with advanced cancer. J
Cachexia Sarcopenia Muscle. 8:623–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carr RM, Enriquez-Hesles E, Olson RL,
Jatoi A, Doles J and Fernandez-Zapico ME: Epigenetics of
cancer-associated muscle catabolism. Epigenomics. Sep 25–2017.(Epub
ahead of print). doi: 10.2217/epi-2017-0058 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haddad F, Zaldivar F, Cooper DM and Adams
GR: IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985).
98:911–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel HJ and Patel BM: TNF-α and cancer
cachexia: Molecular insights and clinical implications. Life Sci.
170:56–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scheede-Bergdahl C, Watt HL, Trutschnigg
B, Kilgour RD, Haggarty A, Lucar E and Vigano A: Is IL-6 the best
pro-inflammatory biomarker of clinical outcomes of cancer cachexia?
Clin Nutr. 31:85–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fearon KC, Glass DJ and Guttridge DC:
Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell
Metab. 16:153–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang XY, Liang L, Chang JL, Yang MH and Li
ZG: Toxicity of matrine in Kunming mice. Nan Fang Yi Ke Da Xue Xue
Bao. 30:2154–2155. 2010.(In Chinese). PubMed/NCBI
|
|
45
|
Lu ZG, Li MH, Wang JS, Wei DD, Liu QW and
Kong LY: Developmental toxicity and neurotoxicity of two
matrine-type alkaloids, matrine and sophocarpine, in zebrafish
(Danio rerio) embryos/larvae. Reprod Toxicol. 47:33–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Burattini S, Ferri P, Battistelli M, Curci
R, Luchetti F and Falcieri E: C2C12 murine myoblasts as a model of
skeletal muscle development: Morpho-functional characterization.
Eur J Histochemistry. 48:223–233. 2004.
|
|
47
|
Clarke BA, Drujan D, Willis MS, Murphy LO,
Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E
and Glass DJ: The E3 Ligase MuRF1 degrades myosin heavy chain
protein in dexamethasone-treated skeletal muscle. Cell Metab.
6:376–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Castillero E, Alamdari N, Lecker SH and
Hasselgren PO: Suppression of atrogin-1 and MuRF1 prevents
dexamethasone-induced atrophy of cultured myotubes. Metabolism.
62:1495–1502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Adams GR, Hather BM, Baldwin KM and Dudley
GA: Skeletal muscle myosin heavy chain composition and resistance
training. J Appl Physiol (1985). 74:911–915. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Desler MM, Jones SJ, Smith CW and Woods
TL: Effects of dexamethasone and anabolic agents on proliferation
and protein synthesis and degradation in C2C12 myogenic cells. J
Anim Sci. 74:1265–1273. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schiaffino S, Dyar KA, Ciciliot S, Blaauw
B and Sandri M: Mechanisms regulating skeletal muscle growth and
atrophy. FEBS J. 280:4294–4314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lokireddy S, McFarlane C, Ge X, Zhang H,
Sze SK, Sharma M and Kambadur R: Myostatin induces degradation of
sarcomeric proteins through a Smad3 signaling mechanism during
skeletal muscle wasting. Mol Endocrinol. 25:1936–1949. 2011.
View Article : Google Scholar : PubMed/NCBI
|