Introduction
Ulcerative colitis (UC) is an idiopathic relapsing
inflammatory illness that leads to long term and occasionally
irreversible impairment of gastrointestinal structure and function
(1,2). Patients may suffer from abdominal
pain and bloody diarrhea as well as rectal excretion of mucus and
pus, which markedly affects quality of life (3). Therefore, it is vital to decrease UC
and colitis-associated colorectal cancer. UC is induced by genetic
risk factors, barrier dysfunction, and environmental and gut
microbiota factors (4).
Macrophages are widely distributed throughout the gastrointestinal
tract. They are found in all mucous membranes and play the most
important role in the gastrointestinal immune system (5). Macrophages are activated by
pathogen-associated molecular patterns and secrete proinflammatory
factors including TNF-α, IL-1, and IL-6, as well as the chemokines
CXCL9 and CXCL10 (6). These
factors are also involved in the activation of driving UC
pathogenesis. There is currently no drug available for the complete
cure of UC (7,8).
Specialized pro-resolving mediators (SPMs) are
widely regarded as having strong anti-inflammatory activities.
Previous studies demonstrated that intraperitoneal treatment with
17-HDHA in a UC mouse model alleviated dextran sulfate sodium
(DSS)-induced epithelial damage and macrophage infiltration
(9) and Resolvin E1 displayed
potent anti-inflammatory effects against colitis and attenuated
TNF-stimulated NF-κB activation (10). Resolvin D1 and Resolvin D2
prevented colitis by suppressing the secretion of TNF-α, IL-1β and
NF-κB (11). Resonvin D5 could
relieve intestinal ischemia reperfusion injury by reducing
neutrophil recruitment (12).
However, most commercial SPMs exhibit low activity, and the
synthesis methods are time-consuming and complicated. To circumvent
these problems, we developed an ecofriendly and cost effective
method using a microbial enzyme, lipoxygenase derived from
Oscillatoria nigroviridis PCC 7112, to generate the compound
7S,15R-dihydroxy-16S,17S-epoxy-Docosapentaenoic Acid (diHEP-DPA)
(13).
In the present study, we investigated the effect of
diHEP-DPA in a mouse UC model. The results indicate that diHEP-DPA
attenuates DSS-induced colitis in vitro and in vivo.
The changes in colitis were evaluated by the shortening of colon
length, MPO activity, histological damage, etc. Furthermore,
diHEP-DPA reduced inflammatory cytokine (IL-1β, IL-6 and TNF-α)
expression and NO production by the GPR32 receptor in vitro.
These results indicate that diHEP-DPA might improve DSS-induced
colitis via the NF-κB signaling pathway.
Materials and methods
Chemicals, kits, and antibodies
diHEP-DPA (purity >98%) was purified and obtained
from DHA as described previously (13). Cell activity was assessed using a
MTT assay kit (Promega). Lipopolysaccharide (LPS) and Phorbol
12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich.
DSS was obtained from MP biotechnology (Solon, OH, CA). The final
DMSO concentration was <0.1% and the control group was treated
with DMSO alone. A human monocytic cell line (THP1) was purchased
from the Korea Cell Line Bank (Seoul, Republic of Korea).
Cell viability assay
We conducted the MTT assay to determine cell
viability. THP1 cells were cultured in RPMI-1640 media containing
10% fetal bovine serum and 1% penicillin/streptomycin and seeded
into 96-well plates at a density of 10,000 cells/well. A range of
diHEP-DPA concentrations were added with or without LPS (1 µg/ml)
for 24 h. After that, MTT solution was added and the optical
density (OD490) was determined with a microplate reader
(Biotek).
Measurement of proinflammatory
cytokines and NO levels
THP1 macrophages were subjected to LPS-induced
inflammation according to a previously published protocol (14). Briefly, 100 µl of THP1 cell
suspension containing 2×105 cells was seeded into a
96-well-plate. The following day, the cells were treated with LPS
(1 µg/ml) with or without diHEP-DPA at various concentrations.
After 2 days, the supernatant was collected and centrifuged at
1,000 × g for 5 min. The levels of cytokines were measured using
the CBA human inflammatory cytokine assay kit (BD) according to the
manufacturer's instructions and the samples were analyzed using a
fluorescence-activated cell sorting (FACS) system (BD). The
quantification of cytokines was done using the FCAP Array program.
The levels of nitrite in the culture medium were determined using
the Griess reagent (Promega), according to the manufacturer's
instructions.
Small interfering RNA (siRNA) and
analysis of FACS
To identify the receptor for diHEP-DPA, we treated
the THP1 cell line with human siGPR32 (NM_001506.2; Bioneer),
siChemR23 (NM_001142343.1; Bioneer), siFPR2 (NM_001005738.1;
Bioneer) or universal negative siRNA (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, THP1 cells were seeded. GPR32 siRNA was
diluted in Opti-MEM Reduced Serum Medium (Thermo Fisher Scientific,
Inc.) and added to the culture medium at a final concentration of
20 nM. The transfection of siRNA into THP1-derived macrophages in
suspension was done using the TransIT-X2® system kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. To select the most effective siRNA concentration,
real-time quantitative polymerase chain reaction (RT-qPCR) and FACS
analysis were performed 48 h after transfection. The samples were
incubated with anti-GPR32 antibody (GTX71225; GeneTex) for 30 min
on ice. Cells were stained with Alexa Flour 488 goat anti-rabbit
antibody (ab15077; Abcam) for 30 min, then analyzed using a FACS
system (BD).
Animal experiments
Eighteen six-week-old male BALB/c mice (body weight:
23.26±1.15 g) were purchased from Orien (Seoul, Korea). All mice
used in the experiment were housed in an air-conditioned animal
room at 24°C±2°C, a relative humidity of 55%, a 12 h light-dark
cycle, and were provided tap water and a standard diet. After 7–10
days of acclimation, the mice were used for the experiments. The
mice were divided into three groups as follows: normal group (ND,
n=6), 4% DSS group (DSS, n=6) and DSS + diHEP-DPA (diHEP-DPA: 20
µg/kg body weight orally through diHEP-DPA, n=6). The body weight
of the animals was measured daily. The mice were maintained on 4%
DSS for 1 week and treated orally with diHEP-DPA once a day a 1
week. After feeding for 1 week, blood samples were collected by
cardiac extraction and serum cytokine levels were measured using a
kit (Abcam). The animals were sacrificed by cervical dislocation
after used the anaesthesia (isoflurane) and blood collection. For
histological analysis, colon tissues were excised, washed rapidly,
fixed in neutral-buffered formaldehyde, and stored until used. All
animal experiments were performed according to the guidelines for
animal handling and welfare at our facilities according to the
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience & Biotechnology (KRIBB-AEC-20310),
Daejeon, Korea.
Evaluation of disease activity
index
Animals were daily examined for body weight and
disease activity index (DAI). At the end of the intervention (day
14), the DAI was determined by the sum of the following scores:
body weight loss (scored as: 0, none; 1, 1–5%; 2, 5–10%; 3, 10–20%;
4, >20%), the presence or absence of fecal blood (scored as 0,
negative hemoccult test; 2, positive hemoccult test; 4, gross
bleeding), and stool consistency (scored as 0, well-formed pellets;
2, loose stools; 4, diarrhea). The colon tissue was isolated and
colon length was measured.
Hematoxylin and eosin (H&E)
staining and histopathology of colon tissue
Colon tissues were isolated at the end of culture
and the samples were fixed in formalin and embedded in paraffin.
Sections of 5 µm thickness were stained with H&E, then
evaluated for histological changes by light microscopy and imaging
(Leica). The histological scores are shown in Table SI.
Myeloperoxidase (MPO) activity
assay
MPO activity can be used to indicate the level of
neutrophil infiltration in UC (15). Colon tissues were homogenized in
ice-cold HTAB solution and 10-mM EDTA. After centrifuging the
homogenate, the supernatant was collected and insoluble material
removed. MPO activity was assayed using a myeloperoxidase
colorimetric activity assay kit (Sigma-Aldrich, St. Louis, MO, USA)
according to the manufacturer's instructions.
Western blot Analysis
Cells were collected and lysed with lysis buffer
containing protease and phosphatase inhibitors on ice for 45 min,
and centrifuged at 12,000 × g for 5 min. The primary antibodies
were all obtained from Abcam and included anti-TNF-α (ab255275),
anti-IL-6 (ab233706), anti-IkBα (ab32518), anti-pIkBα (ab133462),
anti-p65 (NF-κB) (ab16502), anti-p-p65 (ab76302), anti-iNOS
(ab178945), anti-COX2 (ab179800), anti-GAPDH (ab181602), and
anti-LaminB1 (ab16048). The membranes were washed three times with
TBST, incubated with secondary antibodies (ab205718), and developed
using the ECL Plus western blotting detection system (Pierce)
according to the manufacturer's protocol. The membranes were
exposed to CL-XPosureTM film (Thermo Fisher Scientific, Inc.). The
gray density of the scanned images was quantified with Image J
software (version 1.6).
Gene expression analysis
Total RNA was isolated using the TaKaRa MiniBEST kit
(Takara Bio, Inc.) according to the manufacturer's protocol. RT-PCR
was done using 100 ng of total RNA, a reaction volume of 50 µl, and
the specific primers listed in Table
SII. The PCR cycle conditions were as follows: 95°C for 30 sec,
56°C for 30 sec, and 72°C for 30 sec; 45 cycles, followed by a
10-min extension at 72°C. The relative expression levels were
calculated by the comparative CT method. β-actin was used as an
internal control.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were analyzed using one-way ANOVA and Tukey's test.
Categorical data were analyzed using Mann Whitney U test, or
Kruskal-Wallis and Dunn's post hoc test. A P-value less than 0.05
was considered statistically significant. All analyses were
performed using GraphPad Prism 8 Software (GraphPad Software
Inc.).
Results
Anti-inflammatory effect of diHEP-DPA
on LPS-stimulated THP1 cells
Fig. 1A shows the
structural change of every intermediate at each step in the
synthesis of diHEP-DPA. First, we investigated THP1 macrophage cell
viability when treated with or without diHEP-DPA. diHEP-DPA did not
cause any cytotoxic effects on THP1 cells as shown in Fig. 1B. The results (Fig. 1C) indicated a significant
reduction of IL-6 and TNF-α secretion at 10-µM diHEP-DPA treatment,
whereas IL-1β was decreased at 20 µM. Analysis over a range of
doses showed that the inhibitory effect of diHEP-DPA was
dose-dependent over a concentration range of 1–20 µM. In addition,
nitrite levels were measured and the results are shown in Fig. 1D. There was a significant
reduction in nitrites compared with the control group.
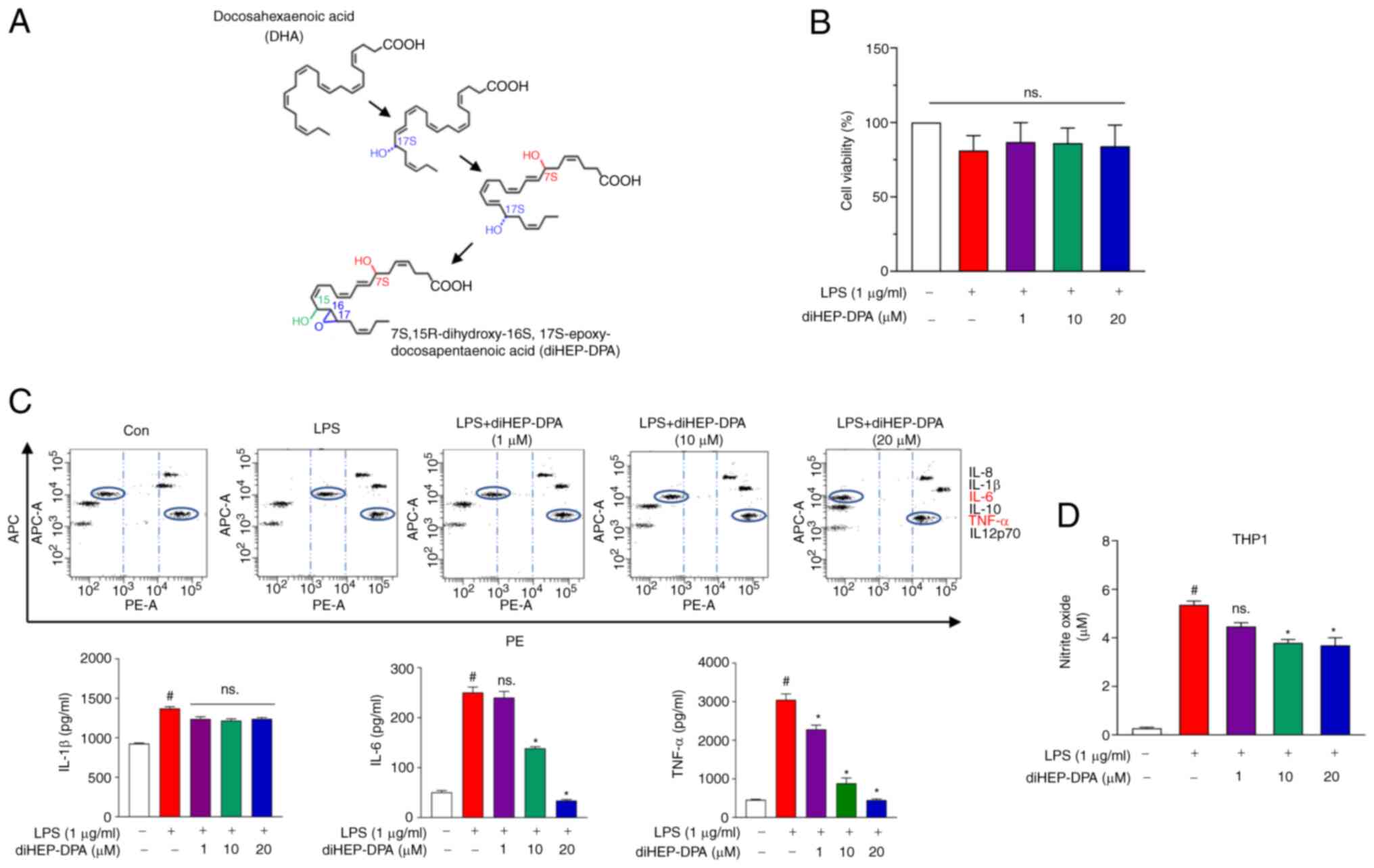 | Figure 1.diHEP-DPA attenuates inflammation
induced by LPS. (A) Structural changes during diHEP-DPA production.
(B) Cell viability of THP1 following LPS-induced inflammation
treated with or without diHEP-DPA at various concentrations. (C)
Secretion of inflammatory cytokines by inflamed macrophages were
inhibited by diHEP-DPA. The specific cytokine bead order from top
to bottom will be IL-8, IL-1β, IL-6, IL-10, TNF-α and IL12p70. (D)
Effect of diHEP-DPA on NO production induced by LPS in THP1
macrophages. diHEP-DPA reduced the NO production effectively at ≥10
µM. #P<0.05 vs. the DMSO-treated control group; *P<0.05 vs.
the LPS group. diHEP-DPA,
7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic; LPS,
lipopolysaccharide; NO, nitric oxide; DSS, dextran sulfate
sodium. |
The anti-inflammatory activity of
diHEP-DPA is dependent on the GPR32 receptor
At least six types of resolvins (Resolvin D1 through
D6) activate their target cells through GPR32 which led to the
renaming of GPR32 to the Resolvin D1 receptor (11,12,16). We determined whether a reduction
of GPR32 membrane expression could eliminate the anti-inflammatory
effect of diHEP-DPA on LPS-induced inflammation. Fig. 2A shows that negative siRNA or
siGPR32 did not alter cell viability. Transient transfection of
THP1 with siRNA specific for GPR32 resulted in a significant
decrease of GPR32 mRNA and ablated GPR32 expression after 48 h
(Fig. 2B and C). Furthermore, we
found that the inhibition of diHEP-DPA on inflammatory cytokine
secretion and NO production was abolished after transfection with
siGPR32 (Fig. 2D and E).
Furthermore, we investigated the siChemR23 and siFPR2, both of them
which did not abolish the inhibitory effect of diHEP-DPA on
inflammatory secretion (Fig.
S1). These results indicate that the diHEP-DPA-mediated effects
on mouse macrophages are GPR32-dependent and GPR32 may be the
receptor, or at least one of the receptors for diHEP-DPA.
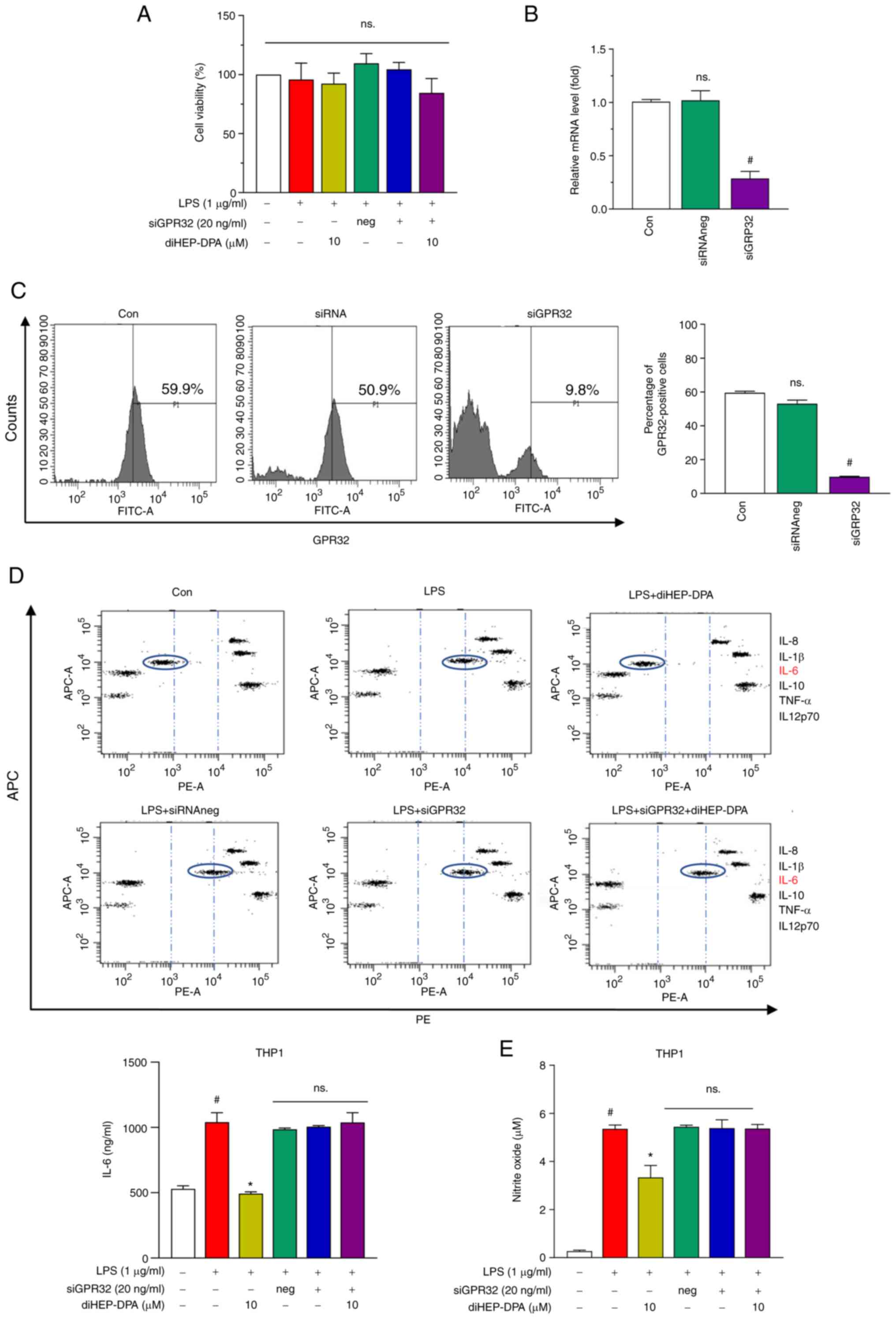 | Figure 2.Anti-inflammatory activity of
diHEP-DPA depends upon GPR32. (A) Effect of siGPR32 on cell
viability with or without siGPR32. siGPR32 silenced the expression
of GPR32 at the (B) gene and (C) protein level. The transcript
levels of GPR32 were measured by reverse trancription-quantititive
PCR. β-actin was used as an internal control. Protein expression
was determined using antibodies against GPR32 by FACS. (D) Cytokine
profile assay of the supernatant of siGPR32 system was determined.
(E) Effect of diHEP-DPA on NO production induced by LPS is
dependent upon GPR32. #P<0.05 vs. the control group; *P<0.05
vs. the LPS group. diHEP-DPA,
7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic; GPR32, G
protein-coupled receptor 32; si, short interfering; NO, nitric
oxide; LPS, lipopolysaccharide; con, control; neg, negative; DSS,
dextran sulfate sodium. |
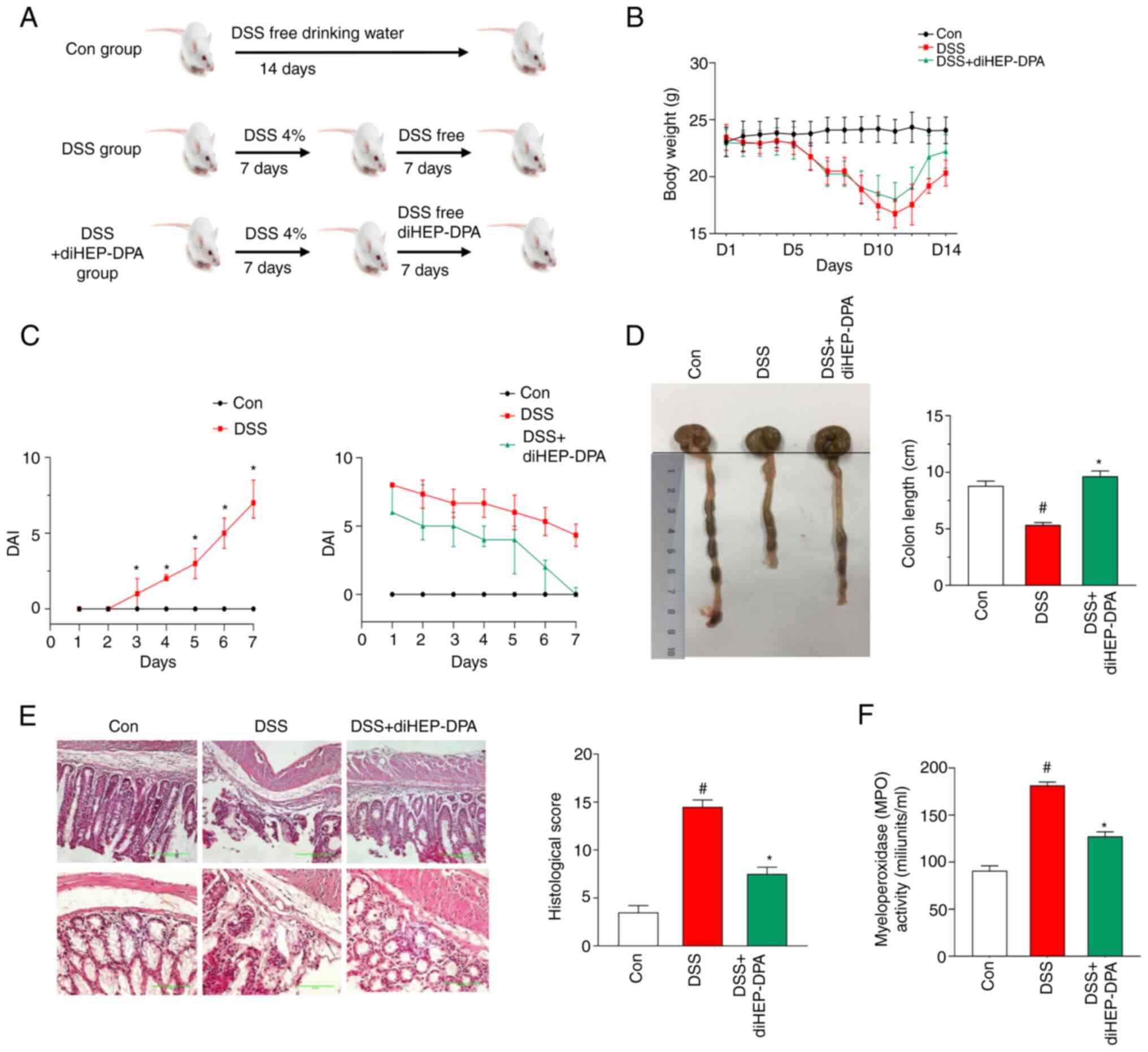
The effect of diHEP-DPA on symptoms of
DSS-induced colitis in mice
The DSS-induced colitis in vivo experiments
were performed according to the experimental design shown in
Fig. 3A. After the mice were fed
with drinking water (with 4% DSS), we assessed the colitis symptom
score (DAI) every day (Fig. 3B).
DSS induced a significant weight loss on day 7. After
administration of diHEP-DPA, weight recovered gradually and was
almost same as the control group at day 7. The results (Fig. 3C) indicated that the DAI score
gradually increased from day 3 of DSS induction and was
significantly higher compared with the control group from day 4. On
day 7, the mice exhibited colitis symptoms meaning that the
DSS-induced colitis model was successfully established. After
diHEP-DPA administration, the DAI scores of the DSS + diHEP-DPA
group remained higher than those of the control group (Fig. 3D). However, compared with the DSS
group, diHEP-DPA showed improvement in colitis symptoms on day 4 of
diHEP-DPA administration and a significant reduction in the DAI at
days 5–7 after diHEP-DPA administration.
diHEP-DPA prevents colonic shortening
and pathological damage in DSS-induced colitis
To investigate the effect of diHEP-DPA on colitis,
we measured the colon length in vivo. The results (Fig. 3D) show that colon length of the
DSS group was shortened, whereas diHEP-DPA attenuated colonic
shortening. As shown in Fig. 3E,
the colon tissue of DSS-induced mice showed obvious crypt
destruction compared with the control group. The histological score
of the DSS group was significantly higher than that of the control
group and the diHEP-DPA administration group was significantly
reduced compared with the DSS group, although it was still higher
than the control group. Myeloperoxidase activity (MPO) activity is
an indicator of neutrophil infiltration in UC. Fig. 3F shows that MPO activity in the
DSS-treated group was significantly increased compared with the
control group and following treatment with diHEP-DPA, the MPO
activity significantly decreased compared with the DSS-treated
group. These results indicate that diHEP-DPA improves DSS-induced
UC in vivo.
diHEP-DPA attenuates the level of
inflammatory factors in serum and colon tissue
We further characterize the inflammation by
detecting the expression of cytokines in the serum, including
TNF-α, IL-6, and IL-1β. The results showed that diHEP-DPA
suppressed the production of inflammatory cytokines as shown in
Fig. 4A. We also measured the
expression of these inflammatory factors in colon tissue at the
mRNA and protein level (Fig. 4B and
C) and the results indicated that diHEP-DPA significantly
attenuated DSS-induced inflammatory factor (TNF-α and IL-6)
expression. As shown in Fig. 4D,
colon tissue from DSS-induced mice had significantly increased
levels of p-p65 and p-IκBα protein in the nucleus compared with the
control group. DiHEP-DPA-treated mice exhibited decreased
accumulation of nuclear p65 compared with DSS-induced mice.
DiHEP-DPA did not have a significant effect on IκBα expression in
the cytosol, whereas it decreased p65 levels compared with the
DSS-treated group. As shown in Fig.
4E and F, the expression of iNOS was significantly higher in
the DSS-treated group than in the control group and diHEP-DPA
significantly reversed this effect. COX2 showed no significant
changes following DSS treatment, whereas decreased COX2 expression
was observed in the diHEP-DPA-treated group.
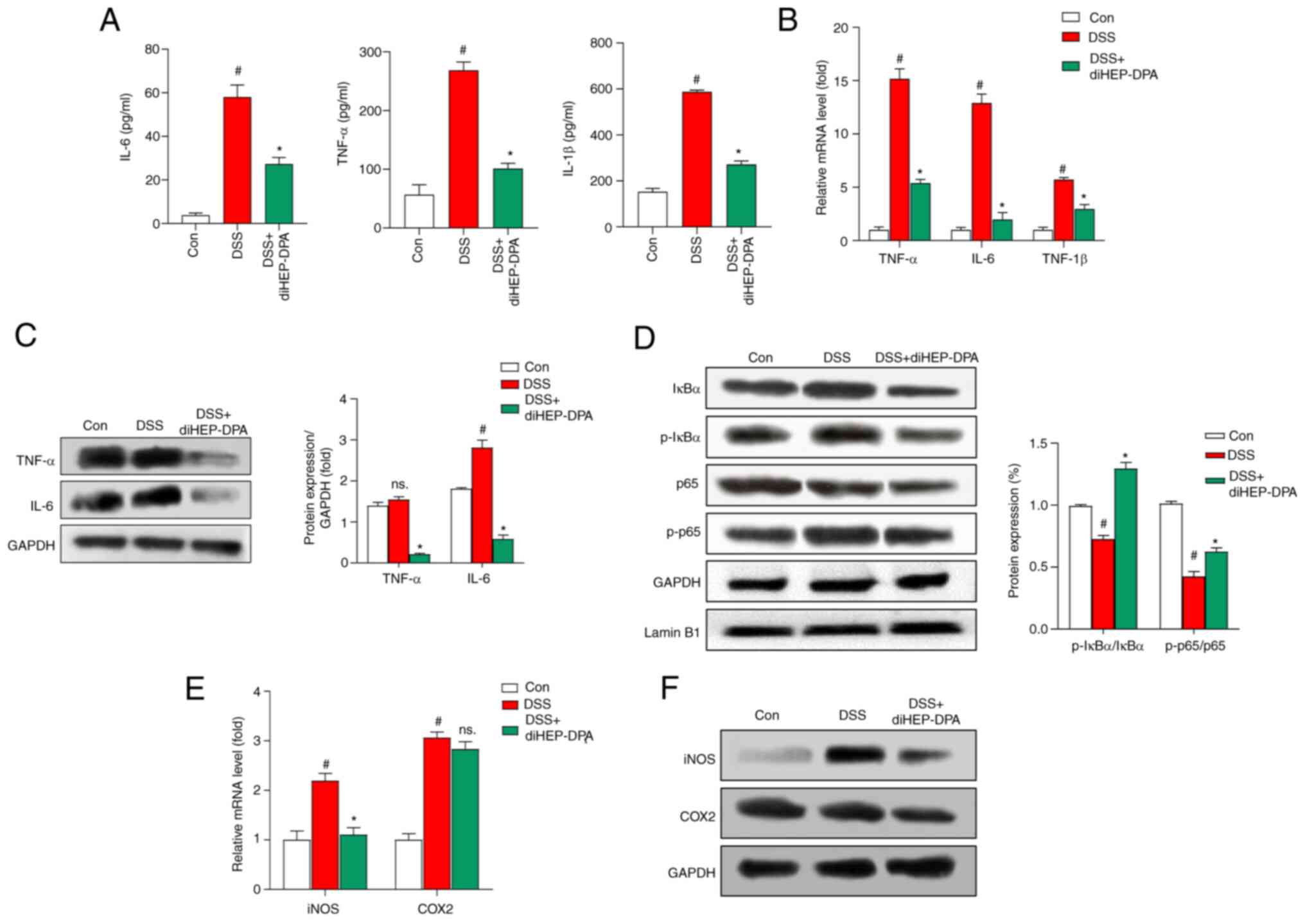 | Figure 4.Effect of diHEP-DPA on inflammatory
factors induced by DSS in vivo. (A) After feeding for 1 week, blood
samples were collected from the mice retro-orbitally before the
animals were sacrificed and the inflammatory cytokines were
measured by ELISA. (B) mRNA and (C) protein transcript levels of
IL-6, TNF-α and IL-1β were measured using RT-qPCR and western
blotting, respectively. (D) Expression levels of IkBα, p-IkBα, p65
and p-p65 in colon tissue as determined using specific antibodies.
(E) mRNA and (F) protein transcript levels of iNOS and COX2 were
measured using RT-qPCR and western blotting, respectively. The data
from triplicate experiments are presented as the mean ± SD.
#P<0.05 vs. the control group; *P<0.05 vs. the DSS group.
diHEP-DPA, 7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic; DSS,
dextran sulfate sodium; RT-qPCR, reverse transcription-quantitative
PCR; IkBα, inhibtor κB protein α; p-, phosporylated; iNOS,
inducible nitric oxide synthase; COX2, cytochrome c oxidase subunit
2; con, control. |
Discussion
DSS-induced colitis is a pathological syndrome
similar to human UC and is associated with body weight loss,
diarrhea, blood in the stool, and inflammatory cell infiltration;
thus, it is a widely used in vivo model for UC (17). We have developed a novel SPM,
diHEP-DPA, which was synthesized in our previous work by a
biosynthetic method (Fig. 1A).
The product displayed anti-inflammatory activity in LPS-induced
human macrophage cells and in a DSS-induced UC mouse model. The
results showed that diHEP-DPA attenuates LPS-induced inflammatory
cytokines (TNF-α, IL-6, IL-1β) and NO production by GPR32 in human
macrophages and alleviates the inflammation in DSS-induced colitis
in mice. Furthermore, the underlying mechanism may also involve the
inhibition of NF-κB signaling.
We demonstrated the anti-inflammatory activity of
diHEP-DPA in an LPS-induced THP1 macrophage inflammation model.
Treatment with diHEP-DPA did not reduce the viability of THP1
macrophages even at high concentrations (Fig. 1B), whereas the levels of TNF-α,
IL-6, and IL-1β were markedly reduced under in vitro and
in vivo conditions (Figs.
1C, 4E, and C). A beneficial
effect of NO derived from constitutive NOS and a detrimental effect
of NO produced by inducible NOS (iNOS) may be observed during the
development of colitis (18).
Therefore, we investigated the effect of diHEP-DPA on NO production
and the results showed that diHEP-DPA decreased NO production in
vitro (Fig. 1D) and inhibited
the expression of iNOS protein in vivo (Fig. 4F). GPR32 is an important receptor
in mediating the effects of resolvin in human macrophages (19). To identify the receptor of
diHEP-DPA, we treated THP1 macrophages with siGPR32, siFPR2, and
siChemR23. The results (Figs. 2
and S1) indicated that siGPR32
silenced the expression of GPR32 markedly and the positive effect
of diHEP-DPA on LPS-induced inflammation was eliminated in
siGPR32-treated THP1 macrophages. Thus we suspected that GPR32
might be one of the receptor for diHEP-DPA. For more solid
evidence, we will confirm the binding of GPR32 and diHEP-DPA in
further study.
We also observed that the diHEP-DPA-treated group
showed improved conditions than the DSS-treated group, such as
reduced body weight loss, retained colon length, and reduced DAI
score as shown in Fig. 3.
Moreover, diHEP-DPA prevented any macroscopic damage to the colon
tissue by reducing the accumulation of neutrophils (Fig. 3F). Additionally, diHEP-DPA reduced
the secretion and expression of inflammatory cytokines. Increased
secretion of inflammatory cytokines causes severe colitis
hallmarked by active NF-κB signaling (20). Our findings indicate that the
novel SPM, diHEP-DPA, also has the ability to ameliorate
DSS-induced colitis by upregulating antioxidant defenses by
suppressing NF-κB activation. The expression levels of iNOS and
p-IκBα proteins were significantly increased in colitis-induced
mice. Reports suggest that the production of proinflammatory
cytokines could cause the disruption of tight junctions and
intestinal homeostasis in colitis (21), and that there is a strong
association between iNOS-induced proinflammatory cytokines and the
condition of mucosal inflammation in UC pathogenesis. Therefore,
diHEP-DPA inhibits NF-κB activation in UC mice by reducing the
expression of proinflammatory cytokines and inhibiting NF-κB
translocation by disrupting the phosphorylation and degradation of
IkB-α (Fig. 4). In summary, oral
administration of diHEP-DPA effectively suppressed the pathogenesis
of UC in a mouse model. These results suggest a role for the
dietary component, diHEP-DPA, as an anti-inflammatory agent for UC
prevention and treatment.
In conclusion, diHEP-DPA is a novel SPM which was
previously synthesized by our group. In the present study, we
explored its potential application in UC treatment. The results
indicated that diHEP-DPA effectively reduces inflammatory cytokine
secretion in vitro and attenuates the DSS damage to the
colon in vivo. Mechanistically, diHEP-DPA inhibits the
activation of the NF-κB signaling pathway.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research was funded by the Ministry of Oceans and
Fisheries, Korea (grant no. 20210285) and the KRIBB Research
Initiative Program (grant no. KGM5482113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW performed all the experiments and wrote the
manuscript. HC helped design all the experiments and supervised the
preparation of the manuscript. YS helped with animal experiments
and some western blotting experiments. BL, SHJ and JC helped by
providing the fluorescence microscope, FACS protocol, they also
helped perform the related experiments and analyzed the data. YJ
and JS supervised the study, reviewed the manuscript, and provided
the funding and conception of this study. All authors have read and
approved the final version of the manuscript. LW, YJ and JS
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved bythe
Institutional Animal Care and Use Committee of the Korea Research
Institute of Bioscience and Biotechnology (approval no.
KRIBB-AEC-20310; Jeongup, Republic of Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: Cause and immunobiology. Lancet. 369:1627–1640.
2007. View Article : Google Scholar
|
|
2
|
Bouma G and Strober W: The immunological
and genetic basis of inflammatory bowel disease. Nat Rev Immunol.
3:521–533. 2003. View
Article : Google Scholar
|
|
3
|
Greuter T and Vavricka SR: Extraintestinal
manifestations in inflammatory bowel disease-epidemiology,
genetics, and pathogenesis. Expert Rev Gastroenterol Hepatol.
13:307–317. 2019. View Article : Google Scholar
|
|
4
|
Bonen DK and Cho JH: The genetics of
inflammatory bowel disease. Gastroenterology. 124:521–536. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flannigan KL, Geem D, Harusato A and
Denning TL: Intestinal antigen-presenting cells: Key regulators of
immune homeostasis and inflammation. Am J Pathol. 185:1809–1819.
2015. View Article : Google Scholar
|
|
6
|
Kmieć Z, Cyman M and Ślebioda TJ: Cells of
the innate and adaptive immunity and their interactions in
inflammatory bowel disease. Adv Med Sci. 62:1–16. 2017. View Article : Google Scholar
|
|
7
|
Spencer EA and Dubinsky MC: Therapeutic
drug monitoring in inflammatory bowel disease: History and future
directions. Pediatr Clin North Am. 64:1309–1326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dulai PS and Jairath V: Acute severe
ulcerative colitis: Latest evidence and therapeutic implications.
Ther Adv Chronic Dis. 9:65–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiu CY, Gomolka B, Dierkes C, Huang NR,
Schroeder M, Purschke M, Manstein D, Dangi B and Weylandt KH:
Omega-6 docosapentaenoic acid-derived resolvins and
17-hydroxydocosahexaenoic acid modulate macrophage function and
alleviate experimental colitis. Inflamm Res. 61:967–976. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arita M, Bianchini F, Aliberti J, Sher A,
Chiang N, Hong S, Yang R, Petasis NA and Serhan CN: Stereochemical
assignment, antiinflammatory properties, and receptor for the
omega-3 lipid mediator resolvin E1. J Exp Med. 201:713–722. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bento AF, Claudino RF, Dutra RC, Marcon R
and Calixto JB: Omega-3 fatty acid-derived mediators 17(R)-hydroxy
docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2
prevent experimental colitis in mice. J Immunol. 187:1957–1969.
2011. View Article : Google Scholar
|
|
12
|
Gobbetti T, Dalli J, Colas RA, Federici
Canova DF, Aursnes M, Bonnet D, Alric L, Vergnolle N, Deraison C,
Hansen TV, et al: Protectin D1n-3 DPA and resolvin
D5n-3 DPA are effectors of intestinal protection. Proc
Natl Acad Sci USA. 114:3963–3968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi JJ, Heo SY, Ju JH, Oh BR, Son WS and
Seo JW: Synthesis of two new lipid mediators from docosahexaenoic
acid by combinatorial catalysis involving enzymatic and chemical
reaction. Sci Rep. 10:188492020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Choi HS, Su Y, Lee B, Song JJ,
Jang YS and Seo JW: 7S,15R-Dihydroxy-16S,17S-Epoxy-Docosapentaenoic
Acid, a Novel DHA Epoxy Derivative, Inhibits Colorectal Cancer
Stemness through Repolarization of Tumor-Associated Macrophage
Functions and the ROS/STAT3 Signaling Pathway. Antioxidants
(Basel). 10:14592021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serhan CN, Chiang N, Dalli J and Levy BD:
Lipid mediators in the resolution of inflammation. Cold Spring Harb
Perspect Biol. 7:a0163112014. View Article : Google Scholar
|
|
16
|
Spite M, Norling LV, Summers L, Yang R,
Cooper D, Petasis NA, Flower RJ, Perretti M and Serhan CN: Resolvin
D2 is a potent regulator of leukocytes and controls microbial
sepsis. Nature. 461:1287–1291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar
|
|
18
|
Rumi G, Tsubouchi R, Okayama M, Kato S,
Mózsik G and Takeuchi K: Protective effect of lactulose on dextran
sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci.
49:1466–1472. 2004. View Article : Google Scholar
|
|
19
|
Schmid M, Gemperle C, Rimann N and
Hersberger M: Resolvin D1 polarizes primary human macrophages
toward a proresolution phenotype through GPR32. J Immunol.
196:3429–3437. 2016. View Article : Google Scholar
|
|
20
|
Seong MA, Woo JK, Kang JH, Jang YS, Choi
S, Jang YS, Lee TH, Jung KH, Kang DK, Hurh BS, et al: Oral
administration of fermented wild ginseng ameliorates DSS-induced
acute colitis by inhibiting NF-κB signaling and protects intestinal
epithelial barrier. BMB Rep. 48:419–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peterson LW and Artis D: Intestinal
epithelial cells: Regulators of barrier function and immune
homeostasis. Nat Rev Immunol. 14:141–153. 2014. View Article : Google Scholar
|