The COVID-19 pandemic caused by the severe acute
respiratory syndrome (SARS)-CoV-2 virus, which was first reported
on 31st of December in Wuhan, China, has quickly spread to 6
continents and hundreds of countries, and is the first pandemic
caused by a coronavirus (CoV) (1).
CoV are a large family of viruses that can cause
disease in humans and animals. They are RNA based viruses, exhibit
positive polarity, and are enveloped and non-segmented, belonging
to the Orthocoronavirinae subfamily (2). The total genome length of CoV is ~30
Kb. There are different regions in the genome, including a
5'-terminal noncoding region, an open reading box 1a/b-coding
region, an S region encoding, a spike glycoprotein (S protein), an
E region encoding the envelope protein (E protein), an M region
encoding the membrane protein (M protein), an N region encoding the
nucleocapsid protein (N protein) and a -3'-terminal non-coding
region (3). Genomic sequence
analysis of COVID-19 shows 88% similarity with two bat-derived
SARS-like coronaviruses, suggesting its origins in a species other
than humans (4,5).
COVID-19 may pass through mucous membranes,
particularly the nasal and larynx mucosa, and then enters the lungs
through the respiratory tract. SARS-CoV-2 requires the
angiotensin-converting enzyme 2 (ACE2), similar to how SARS-CoV
requires ACE-2(6), as the virus
appears to attack organs that express ACE2 (7-9).
The first stage of pathogenesis of the virus is the identification
ACE2 receptors by its spike protein. Thus, cells expressing ACE2
are likely the first cells to be infected (10). The ACE2 receptor is widely expressed
on the surface of numerous types of human cells, particularly the
alveolar type II cells of the lungs (11,12).
Other organs which express high quantities of ACE2 receptor are the
heart, liver, kidneys and digestive organs. In fact, a common cause
of spread of the virus within a host is that endothelial and smooth
muscle cells in almost all organs express ACE2 receptors, and thus,
the virus can enter the bloodstream with relative ease. Since any
tissue or organ expressing ACE2 may serve as the battlefield
between the novel coronavirus and immune cells, complications such
as acute respiratory distress syndrome, acute myocardial damage,
arrhythmia, acute kidney injury, shock and even death may be
observed (13,14). It has been reported that
human-to-human transmission of SARS-CoV occurs via the binding
between the receptor-binding domain of the virus spikes and
cellular ACE2 receptors (5,15).
The clinical spectrum of COVID-19 symptoms varies
from asymptomatic or pauci-symptomatic forms to clinical
conditions, characterized by respiratory failure requiring
mechanical ventilation and support in intensive care units, to
multiorgan and systemic manifestations such as sepsis, septic shock
and multiple organ dysfunction syndrome (16,17).
The aim of the present review is to discuss the role
of mesenchymal stem cells (MSCs), which are known to possess
regulatory functions on the immune system, as a means of
alleviating or eliminating the more severe consequences of the
cytokine storm along with pneumonia, two symptoms most commonly
associated with death in infected patients.
Pneumonia refers to the filling of air vesicles in
the lung with an inflammatory fluid. Viruses, bacteria and rarely
even fungal infections cause pneumonia as a complication of
infection. These pathogens begin to attack cells that form the
lining of the lungs and inflame small sacs where gaseous exchange
occurs. The breathing of a patient becomes shorter and harder, and
as the cells die, the lungs become filled with fluids and debris
further reducing breathing capacity, and secondary infections can
develop as a result. This condition is called pneumonia. In severe
cases, the patient requires a respirator to assist their breathing,
although the ventilator may not prove effective in some
individuals, and this is dependent on the specific reaction of a
patient's immune system. That is, the response mounted by the
immune system will dictate a patient's outcome. The immune system
of critically ill patients becomes overly activated, a condition
called cytokine storm, where a large number of white blood cells
are activated and release inflammatory cytokines that further
activate more white blood cells (18,19).
Pneumonia appears to be the most common severe
manifestation of COVID-19, distinguished primarily by fever, dry
cough, dyspnoea and bilateral infiltrates on chest imaging
(18). Models to predict outcomes of
patients infected with COVID-19 take into account three factors: i)
The severity of the infection, host response, physiological reserve
and comorbidities; ii) the ventilatory responsiveness of the
patient to hypoxemia and the time elapsed between the onset of the
disease; and iii) the unique observations/manifestations in
patients and the capacity of individual hospitals to manage
patients. The balance between these factors leads to the
development of a time-related disease spectrum with two primary
phenotypes. Type L is characterized by low elastance, low
ventilation-to-perfusion ratio, low lung weight and a low capacity
to recruit immune system actors. Type H is characterized by high
elastance, high right-to-left shunt, high lung weight and high a
high capacity to recruit immune system actors (19-22).
Cytokine storm syndrome refers to a range conditions
which ultimately manifests as systemic inflammation, multi-organ
failure, hyperferritinemia and, if untreated, often death (23). Numerous pathogenic viruses and
bacteria have been found to induce cytokine storms or
hypercytokinemia (24-26).
These pathogens disrupt the balance between a physiological and
pathophysiological inflammatory response, pushing from being
beneficial to destructive via positive feedback in immune cells and
upregulation of proinflammatory markers, in particular cytokines
such as TNF-α, IL-1β, IL-8 and IL-6. This results in symptoms such
as hypotension, fever and oedema, and may eventually result in
organ dysfunction and death (27).
Pathogen-induced lung injury can progress to acute
lung injury or its more severe form, acute respiratory distress
syndrome (ARDS), as observed with patients infected with SARS-CoV
or influenza viruses (28). A
hallmark of SARS-CoV-2 pathogenesis is the presence of a cytokine
storm in the lungs (29). One of the
primary mechanisms underlying development of ARDS is the cytokine
storm, a deadly uncontrolled systemic inflammatory response
resulting from the release of large amounts of pro-inflammatory
cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α
and TGF-β, amongst others) and chemokines (CCL2, CCL3, CCL5, CXCL8,
CXCL9 and CXCL10, amongst others) by immune effector cells in
response to SARS-CoV infection (14,30-32).
The cytokine storm initiates a violent attack by the immune system
on the host body, resulting in ARDS and multiple organ failure and
ultimately death in severe cases of SARS-CoV-2 infection, similar
to that observed in patients who were infected with SARS-CoV and
MERS-CoV (33). IL-1β is a key
cytokine driving proinflammatory activity in bronchoalveolar lavage
fluid of patients with lung injury (28). Pathophysiological levels of
inflammation in the lungs also can have other systemic effects on
other organs (34).
The self-renewal and differential capacity of stem
cells as potential tools for regeneration, restoration or
replacement therapies in a variety of disease conditions has been
described previously (35). MSCs are
a heterogeneous population of cells with the potential to
differentiate into a range of somatic lineages, and were originally
described as adherent cells with a fibroblast-like appearance
capable of differentiating into osteocytes, chondrocytes,
adipocytes, tenocytes and myocytes (36-38).
MSCs also support haematopoiesis, possess immunomodulatory
properties and specifically migrate to damaged sites. MSC migration
is mediated by growth factors, chemokines, adhesion molecules and
toll-like receptors (39). MSCs have
been successfully used to reverse graft-versus-host disease in
patients receiving bone marrow transplants (40,41),
particularly in patients diagnosed with severe steroid resistance
(42-44).
Similarly, in patients with systemic lupus erythematosus and
Crohn's disease, both autologous and allogeneic MSCs are able to
suppress inflammation and reduce damage to the kidneys and bowel,
possibly through the induction of regulatory T cells (45-48).
Following COVID-19 infection-mediated initiation of
immune overreaction in the body, the immune system produces large
quantities of inflammatory factors, causing a cytokine storm,
including an overproduction of immune cells and cytokines (49). At present, there are no specific
antiviral treatments recommended for treatment of COVID-19, and no
vaccines are currently widely available. Antibacterial agents are
ineffective due to the viral nature of the infection. Thus,
therapeutic strategies are limited to palliative care and assisted
ventilation for patients with severe pneumonia (50).
MSCs are considered a promising tool for cell
therapy, in particular for management of inflammatory diseases,
based on their immunomodulatory properties and paracrine effects
through trophic factors with anti-fibrotic, anti-apoptotic or
pro-angiogenic properties (51,52).
MSCs regulate the function of a broad range of immune cells
(52-59)
and are activated by inflammatory mediators released from activated
immune cells (such as IFN-γ, IL-1β and TNF-α) (60,61).
Studies have suggested that MSCs may exhibit
immunosuppressive or immunomodulatory properties (53,61,62-65).
MSCs are hypothesized to possess the ability to reduce inflammatory
effects and defend against a cytokine storm (66). MSCs home in on the injured site due
to the presence of local cytokine storm, produced by secretion of
activated immune cells. Activation and migration of MSCs results in
secretion of multiple immunomodulatory and growth factors.
Depending on the cytokine signal (acute vs. chronic inflammation),
MSCs initiate the immunoregulatory response and repair the injured
site, or are unable to inhibit the persisting chronic inflammatory
signals being generated as a result of cellular fibrosis (67).
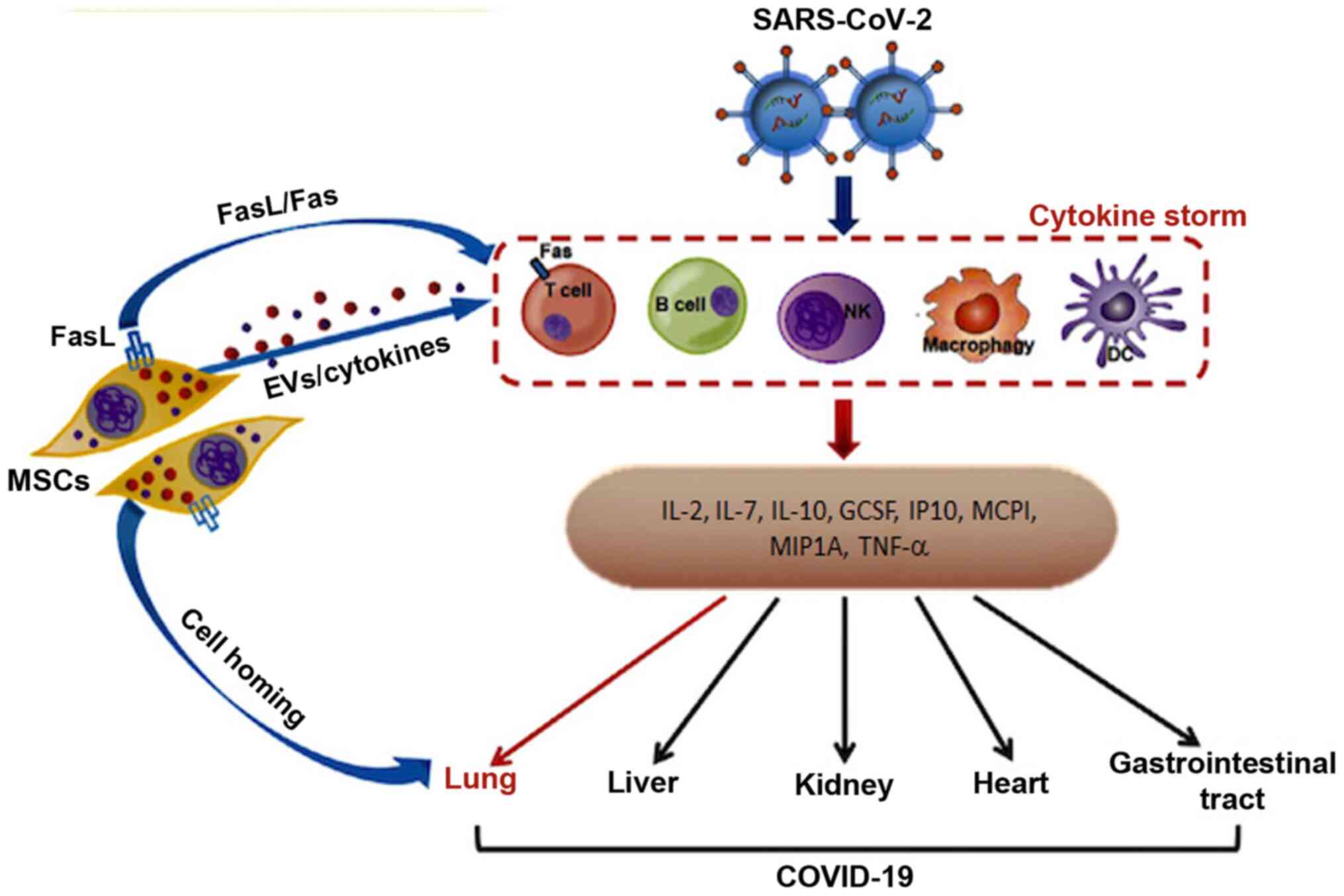
Alleviation of acute respiratory disease and
reversal of pulmonary fibrosis in SARS-CoV-2-infected patients is
mediated by three curative properties of MSCs: i) Directly inducing
the apoptosis of activated T cells to relieve the aberrant and
excessive immune responses; ii) homing toward specific sites of
injury in the lung to maintain homeostasis as well as promote
regeneration; and iii) releasing cytokines to diminish the
inflammatory response and release of extracellular vesicles to
stimulate tissue repair (Fig. 1)
(68). Notably, it has been shown
that cytokines released by MSCs may potently inhibit neutrophil
intravasation and enhance the differentiation of macrophages
(69,70).
Due to a lack of expression of co-stimulatory
molecules and HLA-II, MSCs are regarded as non-immunogenic cells,
thus transplantation into an allogeneic host may not require the
use of immunosuppressive treatments (71,72).
Moreover, MSCs possess immunomodulatory properties and can suppress
and inhibit the activation, maturation and proliferation of innate
and adaptive immune cells (B cells, T cells, NK cells, dendritic
cells and macrophages) (73).
Following intravenous injection of MSCs (systemic
infusion), a proportion of the injected cells are trapped in the
lung, and this is normally considered a limitation of currently
used administration methods. However, with regard to treatment of
COVID-19 infection, this may prove beneficial, as these trapped
MSCs may promote repair of the pulmonary microenvironment, protect
alveolar epithelial cell regeneration, intercept pulmonary fibrosis
and reduce lung dysfunction resulting from the COVID-19 infection
and pneumonia (13).
Umbilical cord cells, umbilical cord blood,
Wharton's jelly, menstrual blood, dental pulp and commercially
produced-MSCs are important sources of MSCs that should be assessed
in clinical trials as potential treatment of patients infected with
COVID-19. However, the process of developing novel therapeutic
strategies and introducing them in a clinical setting may result in
identification of important practical implications/complications
which may not have taken into consideration beforehand (74).
Due to the novel coronavirus, >27 million
individuals have been infected and almost 900,000 deaths
COVID-19-realted deaths have been reported (correct as of 8th of
September, 2020). Whilst certain patients infected with COVID-19 do
not shown symptoms, predominantly younger healthy individuals, a
range of symptoms have been reported, which vary from those with
mild complaints (mild fever, cough and, transient loss of taste or
smell, amongst others) to more severe symptoms which require
admittance to intensive care and assisted mechanical ventilation.
The absence of a definitive treatment for management of the disease
and the absence of a vaccine imposes limitations on the management
of the spread of the disease, and thus has required governmental
bodies to rely on more rudimentary measures, such as social
distancing and lockdowns of certain regions to reduce the spread.
In addition, the unique immune systems of patients react
differently, and the extent of the cytokine storm produced by an
individuals may result in death if excessive. It is hypothesized
that the use of mesenchymal stem cells for their immunomodulatory
properties may result in improved patient outcomes. As mesenchymal
stem cells are pluripotent stromal stem cells, they may be
successful in treatment and management of COVID-19 infection due to
their immune regulatory properties, and thus may be useful for
treating patients who develop more severe symptoms. However,
additional studies, including clinical trials and meta-analyses are
required before widescale adoption in a clinical setting.
Not applicable.
This review was supported by funding from the
Scientific Research Project Coordination Unit of Istanbul
University (grant no. FBA-2017-24288).
Not applicable.
IC and MT both wrote and revised the article. Both
authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Uğraş Dikmen A, Kına HM, Özkan S and
İlhan MN: Epidemiology of COVID-19: What we learn from pandemic. J
Biotechnol and Strategic Health Res. 1:29–36. 2020.
|
|
2
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N Engl J Med. 382:727–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zumla A, Chan JFW, Azhar EI, Hui DS and
Yuen KY: Coronaviruses-drug discovery and therapeutic options. Nat
Rev Drug Discov. 15:327–347. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: Implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by novel coronavirus from Wuhan: An
analysis based on decade-long structural studies of SARS. J Virol.
94:e00127–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou P, Yang XL, Wang XG, Hu B, Zhang L,
Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al: A pneumonia outbreak
associated with a new coronavirus of probable bat origin. Nature.
579:270–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rose-John S: Interleukin-6 family
cytokines. Cold Spring Harb Perspect Biol.
10(a028415)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen J, Hu C, Che L, Tang L, Zhu Y, Xu X,
Chen L, Gao H, Lu X, Yu L, et al: Clinical study of mesenchymal
stem cell treatment for acute respiratory distress syndrome induced
by epidemic influenza A (H7N9) infection: A hint for COVID-19
treatment. Engineering (Beijing): Feb 28, 2020 (Epub ahead of
print).
|
|
9
|
Bennardo F, Buffone C and Giudice A: New
therapeutic opportunities for COVID-19 patients with Tocilizumab:
Possible correlation of interleukin-6 receptor inhibitors with
osteonecrosis of the jaws. Oral Oncol. 106(104659)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rothan HA and Byrareddy SN: The
epidemiology and pathogenesis of coronavirus disease (COVID-19)
outbreak. J Autoimmun. 109(102433)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hamming I, Timens W, Bulthuis ML, Lely AT,
Navis G and van Goor H: Tissue distribution of ACE2 protein, the
functional receptor for SARS coronavirus. A first step in
understanding SARS pathogenesis. J Pathol. 203:631–637.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vergano M, Bertolini G, Giannini A,
Giuseppe G, Livigni S, Mistraletti G and Petrini F: Raccomandazioni
di etica clinica per l'ammissione a trattamenti intensivi e per la
loro sospensione, in condizioni eccezionali di squilibrio tra
necessità e risorse disponibili. versione 01. SIAARTI, 2020.
urihttps://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/SIAARTI%20-%20Covid19%20-%20Raccomandazioni%20di%20etica%20clinica.pdfsimplehttps://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/SIAARTI%20-%20Covid19%20-%20Raccomandazioni%20di%20etica%20clinica.pdf.
Accessed March 6, 2020.
|
|
13
|
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han
Q, Shan G, Meng F, Du D, Wang S, et al: Transplantation of ACE2
mesenchymal stem cells improves the outcomes of patients with
COVID-19 pneumonia. Aging Dis. 11:216–228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jaimes JA, Millet JK, Stout AE, Andre NM
and Whittaker GR: A tale of two viruses: The distinct spike
glycoproteins of feline coronaviruses. Viruses.
12(83)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu Z and Mc Googan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: Summary of a report of 72 314 cases from the
Chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Del Rio C and Malani PN: 2019 novel
coronavirus-important information for clinicians. JAMA.
323:1039–1040. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
World Health Organization (WHO): WHO
Director-General's opening remarks at the media briefing on
COVID-19-24 February 2020. urihttps://www.who.int/dg/speeches/detail/who-director-general-s-op-ening-remarks-at-themedia-briefing-on-covid-19-24-february-2020simplehttps://www.who.int/dg/speeches/detail/who-director-general-s-op-ening-remarks-at-themedia-briefing-on-covid-19-24-february-2020.
Accessed February 26, 2020.
|
|
19
|
Gattinoni L, Pesenti A, Avalli L, Rossi F
and Bombino M: Pressure-volume curve of total respiratory system in
acute respiratory failure. Computed tomographic scan study. Am Rev
Respir Dis. 136:730–736. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gattinoni L, Caironi P, Cressoni M,
Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R
and Bugedo G: Lung recruitment in patients with the acute
respiratory distress syndrome. N Engl J Med. 354:1775–1786.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maiolo G, Collino F, Vasques F, Rapetti F,
Tonetti T, Romitti F, Cressoni M, Chiumello D, Moerer O, Herrmann
P, et al: Reclassifying acute respiratory distress syndrome. Am J
Respir Crit Care Med. 197:1586–1595. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gattinoni L, Chiumello D, Caironi P,
Busana M, Romitti F, Brazzi L and Camporota L: COVID-19 pneumonia:
Different respiratory treatments for different phenotypes?
Intensive Care Med. 46:1099–1102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Behrens EM and Koretzky GA: Review:
Cytokine storm syndrome: Looking toward the precision medicine era.
Arthritis Rheumatol. 69:1135–1143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Us D: Cytokine storm in avian influenza.
Mikrobiyol Bul. 42:365–380. 2008.PubMed/NCBI(In Turkish).
|
|
25
|
Mares CA, Ojeda SS, Morris EG, Li Q and
Teale JM: Initial delay in the immune response to Francisella
tularensis is followed by hypercytokinemia characteristic of severe
sepsis and correlating with upregulation and release of
damage-associated molecular patterns. Infect Immun. 76:3001–3010.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
de Castro IF, Guzmán-Fulgencio M,
García-Alvarezand M and Resino S: First evidence of a
pro-inflammatory response to severe infection with influenza virus
H1N1. Crit Care. 14(115)2010.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Tisoncik JR, Korth MJ, Simmons CP, Farrar
J, Martin TR and Katze MG: Into the eye of the cytokine storm.
Microbiol Mol Biol Rev. 76:16–32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pugin J, Ricou B, Steinberg KP, Suter PM
and Martin TR: Proinflammatory activity in bronchoalveolar lavage
fluids from patients with ARDS, a prominent role for interleukin-1.
Am J Respir Crit Care Med. 153:1850–1856. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Metcalfe SM: Mesenchymal stem cells and
management of COVID-19 pneumonia. Med Drug Discov.
5(1000192)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Williams AE and Chambers RC: The mercurial
nature of neutrophils: Still an enigma in ARDS? Am J Physiol Lung
Cell Mol Physiol. 306:L217–L230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Channappanavar R and Perlman S: Pathogenic
human coronavirus infections: Causes and consequences of cytokine
storm and immunopathology. Semin Immunopathol. 39:529–539.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cameron MJ, Bermejo-Martin JF, Danesh A,
Muller MP and Kelvin DJ: Human immunopathogenesis of severe acute
respiratory syndrome (SARS). Virus Res. 133:13–19. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu Z, Shi L, Wang Y, Zhang J, Huang L,
Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al: Pathological findings
of COVID-19 associated with acute espiratory distress syndrome.
Lancet Resp Med. 8:420–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Imai Y, Parodo J, Kajikawa O, de Perrot M,
Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, et al:
Injurious mechanical ventilation and end-organ epithelial cell
apoptosis and organ dysfunction in an experimental model of acute
respiratory distress syndrome. JAMA. 289:2104–2112. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kögler G, Sensken S, Airey JA, Trapp T,
Müschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C,
et al: A new human somatic stem cell from placental cord blood with
intrinsic pluripotent differentiation potential. J Exp Med.
200:123–135. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Deng ZL, Sharff KA, Tang N, Song WX, Luo
J, Luo X, Chen J, Bennett E, Reid R, Manning D, et al: Regulation
of osteogenic differentiation during skeletal development. Front
Biosci. 13:2001–2021. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Friedenstein AJ, Petrakova KV, Kurolesova
AI and Frolova GP: Heterotopic of bone marrow. Analysis of
precursor cells for osteogenic and hematopoietic tissues.
Transplantation. 6:230–247. 1968.PubMed/NCBI
|
|
38
|
Luu HH, Song WX, Luo X, Manning D, Luo J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 25:665–677. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Entschladen F and Zänker KS (eds): Cell
migration: Signalling and mechanisms. Karger, Basel, pp1-6,
2010.
|
|
40
|
Müller I, Kordowich S, Holzwarth C,
Isensee G, Lang P, Neunhoeffer F, Dominici M, Greil J and
Handgretinger R: Application of multipotent mesenchymal stromal
cells in pediatric patients following allogeneic stem cell
transplantation. Blood Cells Mol Dis. 40:25–32. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Prasad VK, Lucas KG, Kleiner GI, Talano
JA, Jacobsohn D, Broadwater G, Monroy R and Kurtzberg J: Efficacy
and safety of ex vivo cultured adult human mesenchymal stem cells
(Prochymal™) in pediatric patients with severe refractory acute
graft-versus-host disease in a compassionate use study. Biol Blood
Marrow Transplant. 17:534–541. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kebriaei P, Isola L, Bahceci E, Holland K,
Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, et
al: Adult human mesenchymal stem cells added to corticosteroid
therapy for the treatment of acute graft-versus-host disease. Biol
Blood Marrow Transplant. 15:804–811. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wu KH, Chan CK, Tsai C, Chang YH, Sieber
M, Chiu TH, Ho M, Peng CT, Wu HP and Huang JL: Effective treatment
of severe steroid-resistant acute graft-versus- host disease with
umbilical cord-derived mesenchymal stem cells. Transplantation.
91:1412–1416. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun L, Wang D, Liang J, Zhang H, Feng X,
Wang H, Hua B, Liu B, Ye S, Hu X, et al: Umbilical cord mesenchymal
stem cell transplantation in severe and refractory systemic lupus
erythematosus. Arthritis Rheum. 62:2467–2475. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Carrion F, Nova E, Ruiz C, Diaz F,
Inostroza C, Rojo D, Mönckeberg G and Figueroa FE: Autologous
mesenchymal stem cell treatment increased T regulatory cells with
no effect on disease activity in two systemic lupus erythematosus
patients. Lupus. 19:317–322. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ciccocioppo R, Bernardo ME, Sgarella A,
Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A,
Calliada F, et al: Autologous bone marrow-derived mesenchymal
stromal cells in the treatment of fistulising Crohn's disease. Gut.
60:788–798. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Duijvestein M, Vos AC, Roelofs H,
Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning
F, Zwaginga JJ, Fidder HH, et al: Autologous bone marrow-derived
mesenchymal stromal cell treatment for refractory luminal Crohn's
disease: Results of a phase I study. Gut. 59:1662–1669.
2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mehta P, Mcauley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ: HLH Across Speciality Collaboration,
UK. Correspondence COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bari E, Ferrarotti I, Saracino L,
Perteghella S, Torre ML and Corsico AG: Mesenchymal stromal cell
secretome for severe COVID-19 infections: Premises for the
therapeutic use. Cells. 9(924)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Singer NG and Caplan AI: Mesenchymal stem
cells: Mechanisms of inflammation. Annu Rev Pathol. 6:457–478.
2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bernardo ME and Fibbe WE: Mesenchymal
stromal cells: Sensors and switchers of inflammation. Cell Stem
Cell. 13:392–402. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress t-lymphocyte proliferation
induced by cellular or non- specific mitogenic stimuli. Blood.
99:3838–3843. 2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E and Dazzi F: Bone marrow mesenchymal stem cells
inhibit the response of naive and memory antigen-specific T cells
to their cognate peptide. Blood. 101:3722–3729. 2003.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ghannam S, Pène J, Moquet-Torcy G,
Jorgensen C and Yssel H: Mesenchymal stem cells inhibit human Th17
cell differentiation and function and induce a T regulatory cell
phenotype. J Immunol. 185:302–312. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Prigione I, Benvenuto F, Bocca P,
Battistini L, Uccelli A and Pistoia V: Reciprocal interactions
between human mesenchymal stem cells and gammadelta T cells or
invariant natural killer T cells. Stem Cells. 27:693–702.
2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Corcione A, Benvenuto F, Ferretti E,
Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi
GL, Pistoia V and Uccelli A: Human mesenchymal stem cells modulate
B-cell functions. Blood. 107:367–372. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Raffaghello L, Bianchi G, Bertolotto M,
Montecucco F, Busca A, Dallegri F, Ottonello L and Pistoia V: Human
mesenchymal stem cells inhibit neutrophil apoptosis: A model for
neutrophil preservation in the bone marrow niche. Stem Cells.
26:151–162. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
DelaRosa O, Sánchez-Correa B, Morgado S,
Ramírez C, del Río B, Menta R, Lombardo E, Tarazona R and Casado
JG: Human adipose-derived stem cells impair natural killer cell
function and exhibit low susceptibility to natural killer-mediated
lysis. Stem Cells Dev. 21:1333–1343. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Krampera M, Cosmi L, Angeli R, Pasini A,
Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G,
Vinante F, et al: Role for interferon-gamma in the immunomodulatory
activity of human bone marrow mesenchymal stem cells. Stem Cells.
24:386–398. 2006.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Prasanna SJ, Gopalakrishnan D, Shankar SR
and Vasandan AB: Proinflammatory cytokines, IFNgamma and TNFalpha,
influence immune properties of human bone marrow and Wharton jelly
mesenchymal stem cells differentially. PLoS One.
5(e9016)2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Frank MH and Sayegh MH: Immunomodulatory
functions of mesenchymal stem cells. Lancet. 363:1411–1412.
2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Horwitz EM, Gordon PL, Koo WK, Marx JC,
Neel MD, McNall RY, Muul L and Hofmann T: Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth in
children with osteogenesis imperfecta: Implications for cell
therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937.
2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Koç ON, Day J, Nieder M, Gerson SL,
Lazarus HM and Krivit W: Allogeneic mesenchymal stem cell infusion
for treatment of meta-chromatic leukodystrophy (MLD) and hurler
syndrome (MPS-IH). Bone Marrow Transplant. 30:215–222.
2002.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Le Blanc K: Immunomodulatory effects of
fetal and adult mesenchymal stem cells. Cytotherapy. 5:485–489.
2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Chen C, Zhang XR, Ju ZY and He WF:
Advances in the research of mechanism and related immunotherapy on
the cytokine storm induced by coronavirus disease 2019. Zhonghua
Shao Shang Za Zhi. 36:471–475. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
67
|
Rawat S, Gupta S and Mohanty S:
Mesenchymal stem cells modulate the immune system in developing
therapeutic interventions 2019.
|
|
68
|
Abraham A and Krasnodembskaya A:
Mesenchymal stem cell-derived extracellular vesicles for the
treatment of acute respiratory distress syndrome. Stem Cells Transl
Med. 9:28–38. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Xu AL, Rodriguez LA II, Walker KP III,
Mohammadipoor A, Kamucheka RM, Cancio LC, Batchinsky AI and Antebi
B: Mesenchymal stem cells reconditioned in their own serum exhibit
augmented therapeutic properties in the setting of acute
respiratory distress syndrome. Stem Cells Transl Med. 8:1092–1106.
2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Morrison TJ, Jackson MV, Cunningham EK,
Kissenpfennig A, McAuley DF, O'Kane CM and Krasnodembskaya AD:
Mesenchymal stromal cells modulate macrophages in clinically
relevant lung injury models by extracellular vesicle mitochondrial
transfer. Am J Respir Crit Care Med. 196:1275–1286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ji F, Li L, Li Z, Jin Y and Liu W:
Mesenchymal stem cells as a potential treatment for critically ill
patients with coronavirus disease 2019. Stem Cells Transl Med.
9:813–814. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Noël D, Djouad F, Bouffi C, Mrugala D and
Jorgensen C: Multipotent mesenchymal stromal cells and immune
tolerance. Leuk Lymphoma. 48:1283–1289. 2007.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chen X, Armstrong MA and Li G: Mesenchymal
stem cells in immunoregulation. Immunol Cell Biol. 84:413–421.
2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Golchin A, Seyedjafari E and
Ardeshirylajimi A: Mesenchymal stem cell therapy for COVID-19:
Present or future. Stem Cell Rev Rep. 16:427–433. 2020.PubMed/NCBI View Article : Google Scholar
|