Epimorphosis, the regeneration of a specific part of
an organism, such as a limb, does not occur in humans, and is
limited to regrowth of the tips of the digits (1). The underlying process, specifically
dedifferentiation (2), provides an
interesting prospect for generation of scaffolds (3), facilitation of wound healing (4) and guided tissue regeneration (5,6). Stem
cell therapy is now a well established field; however, treatments
based on stem cell therapies are limited, primarily due to the
ethical concerns regarding the sourcing of appropriate stem cells
(7). Pluripotent cells are difficult
to harvest without prior planning, and require the application of
differentiation factors, several of which have multiple, and
occasionally unpredictable effects on the cells (8). Multipotent cells are difficult to
harvest due to their scarcity within each individuals body and the
requirements for cell surface marker screening, prolonged
incubation to develop colonies and narrow therapeutic range limit
their use (9). These challenges can
also result in poor tissue integration and survival (10). By exploring the adipocytic tissue
niche through embryological, physiological and pathological
processes, the aim of the present review is to summarize the
potential of dedifferentiation of mature adipocytes for therapeutic
use. The physiological processes governing adipocyte development
and adipose tissue maintenance are summarized to provide an
understanding of some of the well established baseline
physiological processes that serve as checks for adipocytic lineage
commitment. These checks have been shown to possess a degree of
pliability, and this is subsequently explored. Fundamental
signalling elements are discussed, to provide an in depth look at
pathways involved in adipocyte regeneration. The similarities
between regeneration and growth of adipocyte tissue are compared to
the developmental potential of the adipose lineage. Furthermore,
the effects of molecules responsible for maintaining a homeostatic
balance of the adipose tissue through these developmental,
regenerative and pathological processes on the adjacent
vasculature, and the interactions with the immune system are
examined. To create a possible framework for clinical utilization
of these findings, as well as to stimulate further research in the
field, the potential for modulation of these pathways by
repurposing currently available techniques used in regenerative
medicine are highlighted.
MSCs have been shown to serve an adjuvant role with
beneficial effects as a clinical adjunct in non-healing ulcers
(21), tendon scarring (22) and osseous titanium implants (23), as well as in a variety of osseous,
titanium, polymer and cartilaginous scaffolds (24,25).
Injected aspirates rich in MSCs from bone marrow and adipose
harvests have been shown to increase the speed of repair and
longevity of torn tendons, non-union fractures, and osteoarthritis
(26,27). MSCs obtained from a variety of
sources, such as bone marrow and amniotic fluid, share a similar
proteome (28). Cell cultures from
the bone marrow, adipose tissue and umbilical cords also show
similar growth patterns and cellular architecture, differing by the
ease of harvest and size of the initial inoculum, which affects the
subsequent growth rate and viability of cultures and grafts
(29).
Adipose derived stem cells (ADSCs) are easy to
obtain due to the abundance of adipose tissue, as well as the fact
that the isolation time is reported to be as short as 30 min
(30). The allogenic nature of ADSCs
makes them more suitable for clinical use compared with the
considerably harder to harvest umbilical cord MSCs (31), whereas bone marrow MSC (BMSCs)
require a 24 h incubation to isolate suitable cultures on plastic
(32).
Mature adipocytes can also be utilised clinically,
for instance to form dedifferentiated fat (DFAT) cells, using a
ceiling culture method, which provides a physical dedifferentiation
stimulus on the cultured adipocytes. This physical stimulus
activates the Wnt pathway, resulting in MSC-like protein expression
and pluripotency (33). Activation
of the Wnt pathway causes downstream peroxisome
proliferator-activated receptor γ (PPARγ) inhibition (34). In addition to the ceiling culture
method, there are experimental pharmacological approaches such as
Chir98014 and Chir99021 that have been developed to achieve the
same results (35).
ADSCs have been shown to be more versatile in
adapting to surgical use than BMSCs (36). There is evidence to show that the
versatility of ADSCs may be due to an intrinsic dedifferentiation
potential, which is also partly involved in the wound healing
response of adipocytes (37). Deeper
insights into differentiation and dedifferentiation may shed light
on the adipose mechanisms that control the roles of adipocytes in
tissue repair.
PPARγ is considered the master regulator of
proadipogenic differentiation since all stimuli of adipogenesis
converge on it (38). Activation of
PPARγ is both necessary and sufficient to induce adipocyte
differentiation from MSCs (39).
PPARγ has also been shown to be responsible for vascularisation,
cardiac and placental development, and monocyte function (40,41).
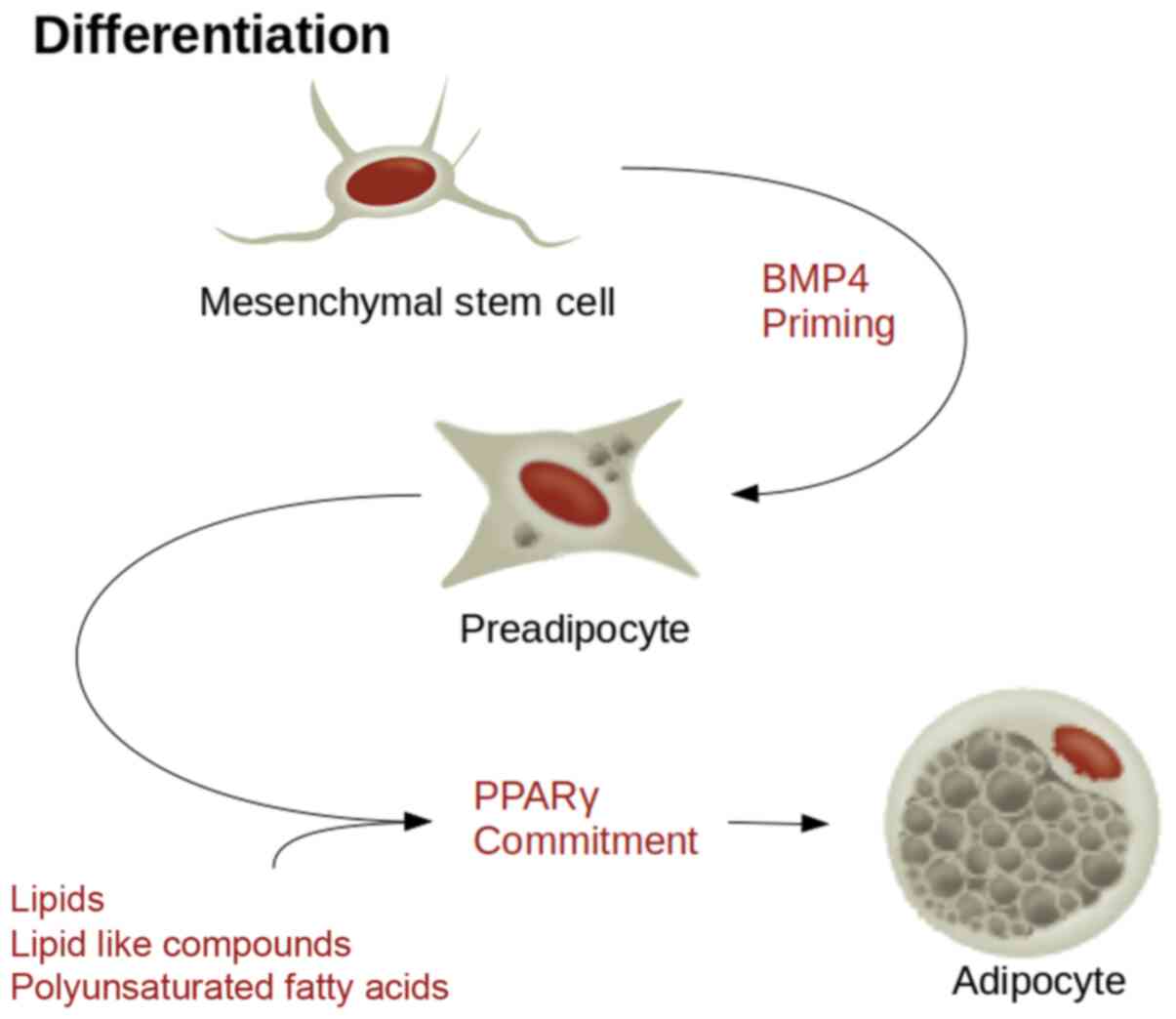
There are several molecules that can stimulate PPARγ mediated
adipogenic differentiation (39,42).
These include a variety of lipids and lipid-like compounds,
including naturally occurring polyunsaturated fatty acids (Fig. 1). Co-stimulators such as
CCAAT/enhancer binding protein β (CEBPβ) can significantly increase
the speed of this process.
MSC sensitivity to PPARγ is reliant on a cascade of
signalling factors. Bone morphogenic protein 4 signalling
determines the adipose lineage, whereas CEBPβ stimulation by
insulin like growth factor-1 or glucocorticoids stimulates the
preadipocytes from a growth arrested state to re-entry back into
the cell cycle, at which point PPARγ commits them to differentiate
into terminal adipocytes (43). It
is hypothesized that a potent factor in the induction of commitment
to an adipocyte lineage is the environment (44). Plating MSCs at a high density, used
to mimic heavy loading pressure in a bone environment or epiphyseal
plates, was shown to preferentially induce differentiation into
osteoblasts, as opposed to low density plating, which resulted in
adipocytes (45). Similarly, the
coculture of MSCs with mature adipocytes provides a positive
feedback mechanism as terminal adipocytes are a source of PPARγ,
stimulating further MSCs to differentiate into adipocytes (46). The molecules responsible for
adipocyte differentiation can also serve as targets for their
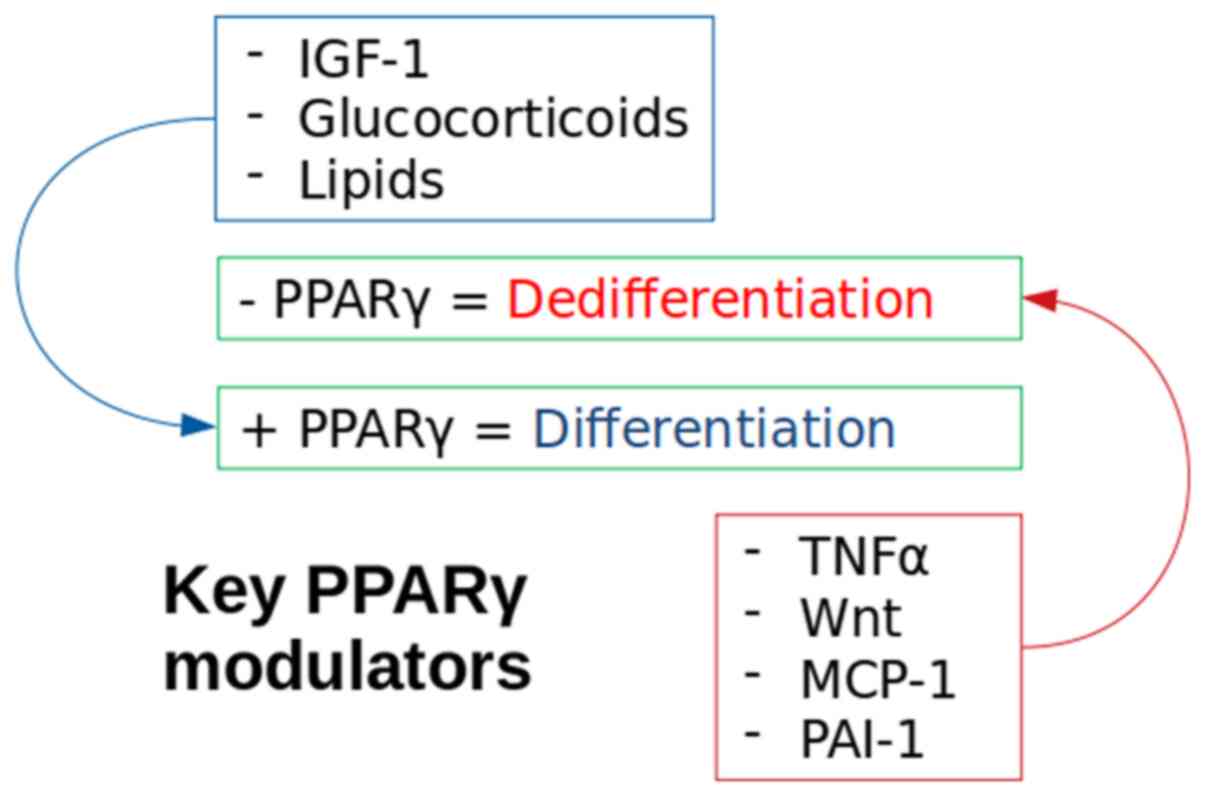
dedifferentiation (Fig. 2).
Mature adipocytes have been shown to exhibit a
plastic phenotype in a variety of conditions (33,37).
Their dedifferentiation has been shown to function as a
physiological process in the mammary glands (47) and hair cycling (48). It also readily occurs in association
with pathological situations, notably inflammatory diseases
(49), dermal fibrosis (50), cancer (51) and wound healing (52,53).
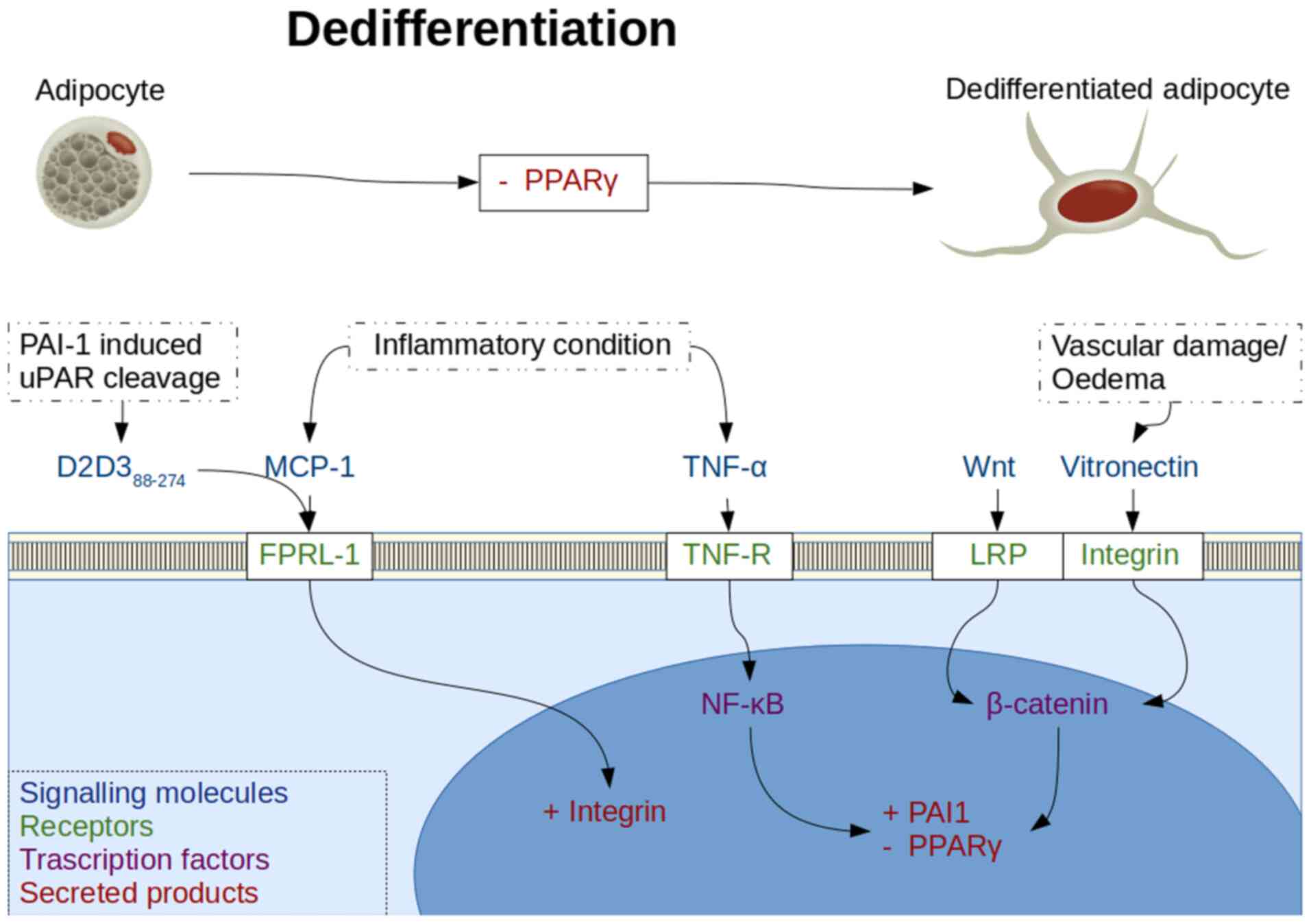
Inhibition of the PPARγ molecule is a method of
forced dedifferentiation of mature adipocytes and has been achieved
using tumour necrosis factor-α (TNF-α) (54) and wingless 3a (Wnt3a), a Wnt pathway
activator (35). Notably, a decrease
in PPARγ production in insulin resistant adipocytes has also been
reported following an increase in insulin stimulated release of
monocyte chemoattractant protein-1 (MCP-1) (55). TNF-α is present in the adipocyte
environment, where its systemic levels are increased under
inflammatory conditions such as obesity or insulin resistance
(56), and locally under the same
states following release of MCP-1 (57,58).
MCP-1 stimulation of integrin mediated cell adhesion
and migration has been shown to be abrogated by a naturally
occurring truncated soluble urokinase plasminogen activator
receptor (uPAR) lysis product termed D2D388-274, which
inhibits the human formyl peptide receptor like-1 (FPRL-1)
G-protein coupled receptor (59,60).
MCP-1 is also known to signal through a chemokine CC motif receptor
2 G-protein coupled receptor that is present in adipocytes
(61). This pattern of activation of
multiple G-protein activation coupled receptors is reminiscent of
that observed with alarmins, which are damage associated molecular
patterns (62). FPRL-1 activation
stimulates αvβ3 integrin production (63), whereas D2D388-274
formation occurs after urokinase plasminogen activator
(uPA)-plasminogen activator inhibitor-1 (PAI-1)-uPAR binding within
the plasminogen activation system (PAS), and is necessary for
fibroblast-to-myofibroblast differentiation (64). There are also reports of
D2D388-274 being chemotactic itself, by activating FPRL1
with LXA4R, thus highlighting the importance of cytokine receptors
and PAS in orchestrating inflammatory responses (65).
The αvβ3 vitronectin specific integrin has been
found to allow MSC to activate Wnt signalling and maintain
pluripotency (66), whereas Wnt
signalling in turn activates PAI-1 production and inhibits PPARγ
production (67). Since
MCP-1-mediated stimulation of monocyte chemotaxis is reliant on
FPRL-1(59), and this in turn
activates Wnt signalling through integrin production and
activation, it is hypothesized that this may be the system by which
MCP-1 exerts an inhibitory effect on PPARγ and subsequent adipose
dedifferentiation (Fig. 3).
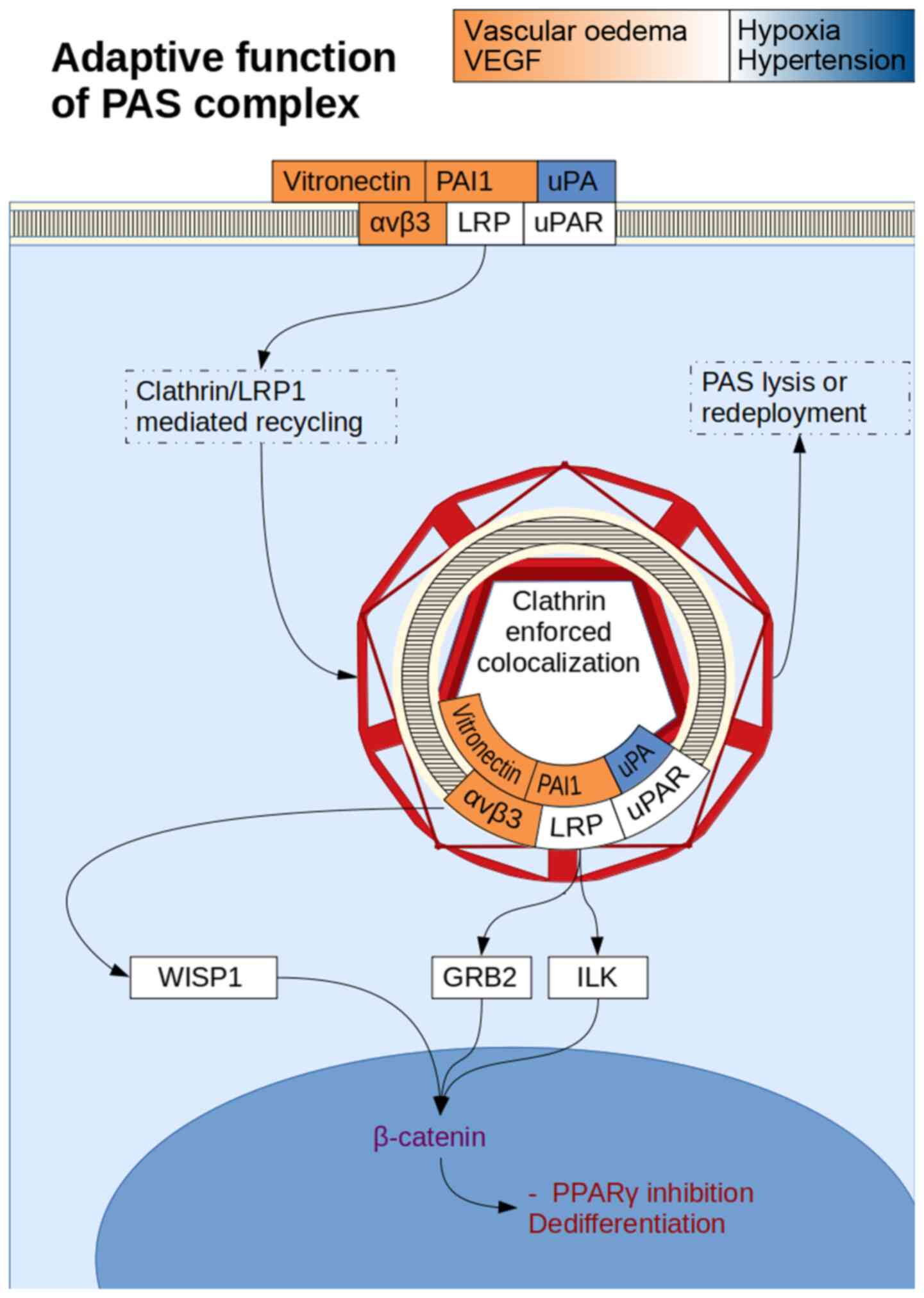
The activation of the Wnt signalling pathway has an
inhibitory effect on PPARγ production and therefore inhibited
adipocytic differentiation (68).
5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, an
AMP-activated protein kinase activator, enhances lipoprotein
receptor-related protein (LRP)6 as well as β-catenin expression,
the latter of which activates the Wnt/β-catenin pathway causing
inhibition of expression of PPARγ, CEPBα, as well as their
downstream transcription targets, fatty acid binding protein 4 and
lipoprotein lipase (69). Wnt
expression was also found to directly stimulate PAI-1 expression
(67). Integrin secretion was found
to be mediated by the Wnt/β-catenin signalling pathway via LRP
(70).
TNF-α stimulated adipocyte dedifferentiation was
found to be mediated by PAI-1, since PAI-1 deficiency caused an
upregulation of PPARγ in TNF-α stimulated cells, resulting in
abrogation of the dedifferentiation caused by TNF-α (71). The mechanism by which PAI-1 is
upregulated is hypothesized to be mediated through TNF receptor
stimulated production of reactive oxygen species, which ultimately
propagates nuclear factor κ-light-chain-enhancer of activated B
cells (NF-κB) PAI-1 gene activation (72).
Due to the multiple sources indicating PAI-1 and Wnt
involvement in dedifferentiation of adipocytes, or maintenance of
MSC pluripotency, their interaction is discussed further below.
PAI-1 is a member of the PAS. The primary protease
of this system is plasmin, which is responsible for catalysing the
lysis of fibrin, glycoproteins and other components of the
extracellular matrix (ECM), but requires activation from a
precursor state (73).
The cleavage of the plasmin precursor, plasminogen,
is mediated by uPA and the tissue-type plasminogen activator (tPA),
activity of both of which is regulated by the PA-I family of
proteins, of which PAI-1 is the most rapidly acting and abundant
(74).
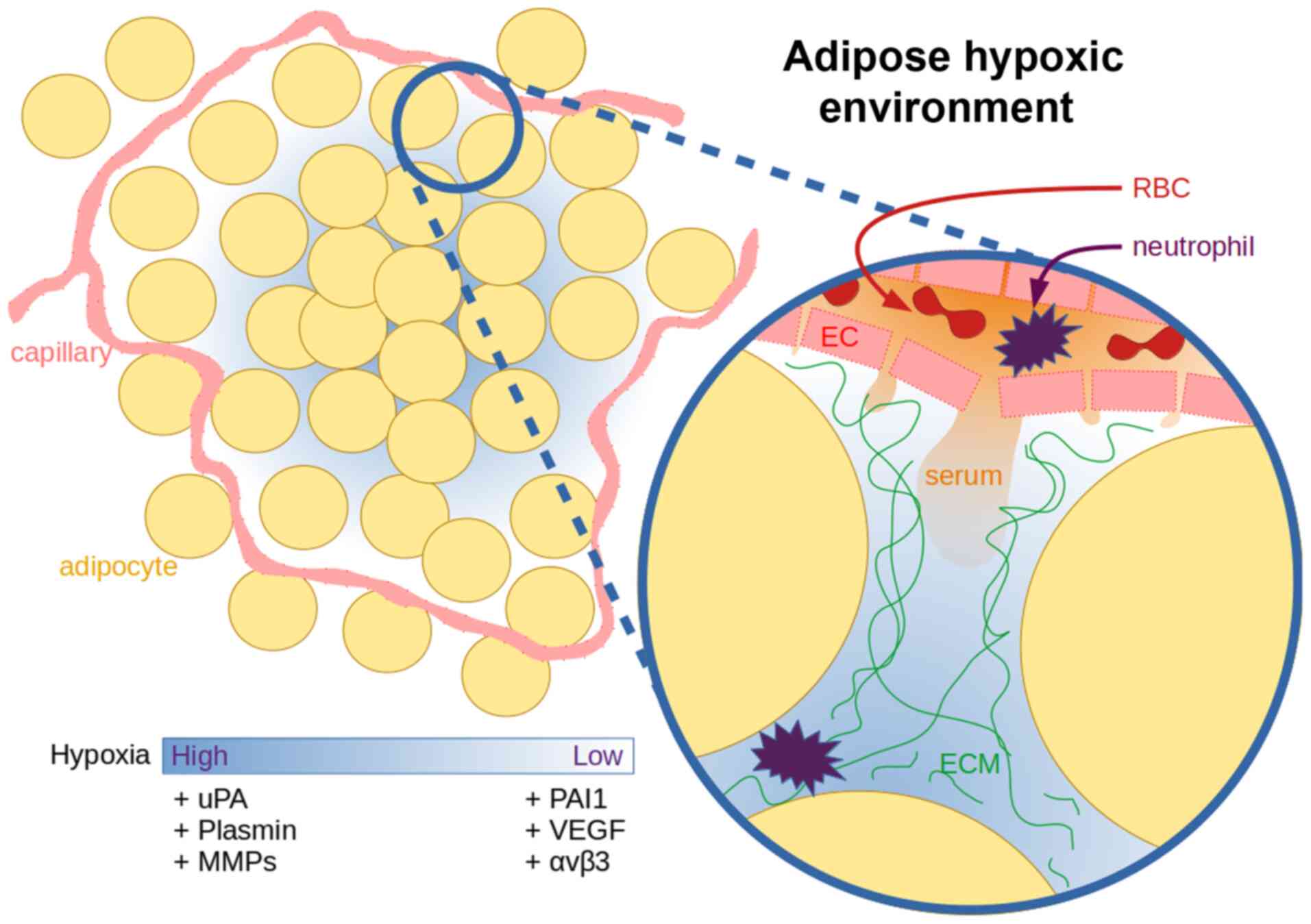
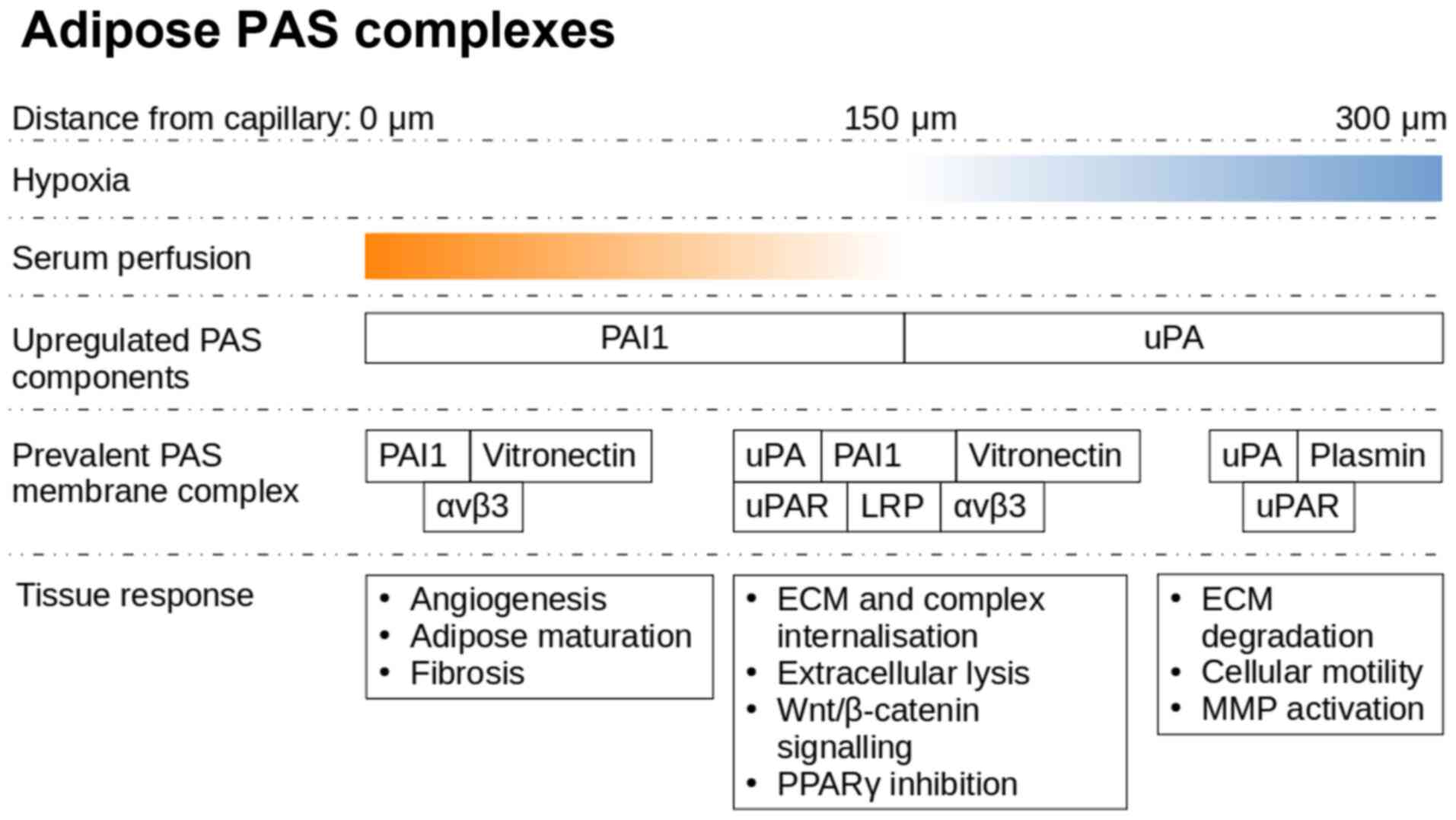
Under hypoxic conditions, the adipose tissue
actively utilises the PAS, both in physiological (75) and pathological (76) conditions, and this can be exploited
to improve our understanding of the complexity of this signalling
framework. Adipocytes secrete increased quantities of uPA under
hypoxic conditions in order to initiate the degradation of the
extracellular environment, in preparation for macrophages,
neutrophils and endothelial cells (ECs) necessary for the
resolution of the hypoxic state (77). In adipose tissue, a distance of 120
µm from the capillary is the limit of oxygen diffusion, while a
single adipose cell can reach sizes of up to 150 µm (78).
uPA activates plasminogen, and can itself initiate
the extracellular remodelling process (79). Once plasminogen is present, the ECM
degradation begins to cascade. uPA is truncated allowing it to form
dimers that have a lower affinity to cell surface uPAR, causing
increased plasminogen and matrix metalloproteinase (MMP) activation
due to lower PAI-1 affinity (80).
MMPs are secreted either as membrane anchored or
free proteins, and in both instances as inactive zymogens,
requiring lysis for activation (81,82).
Plasmin, along with membrane bound and soluble uPA can activate MMP
extracellularly to allow for more specific targeting of the local
extracellular glycosaminoglycans and peptidoglycans, which is
necessary for correctly guiding the invasion of ECs, host immune
cells and stem cells (83). The
hypoxic stimulus is known to potentiate the dedifferentiation of
chondrocytes (84), and formation of
DFAT cells in ceiling culture (85).
In the context of adipose organs, continuous
remodelling is important physiologically to maintain stability
during adaptation to storage capacities of dietary nutrients, and
this puts pressure on the vasculature and connective tissues
(86). These pressures are
correlated between the adipocytes and ECs, which promote both
adipocyte-mediated stimulation of EC angiogenesis and EC
stimulation of preadipocyte formation in vitro (87). Hypoxic conditions arise spontaneously
as the adipose organ grows to meet the physiological demands
(88). PAS elements serve a key role
in mediating multiple aspects of these interactions, which are most
clearly observed in remodelling events following injury. Adipose
harvested MSC migration requires uPAR activation (89), whereas PAI-1 as well as αvβ3 are
strongly expressed by preadipocytes, resulting in a loss of a
migratory phenotype and maturation within the cell cluster
(90).
Hypoxia primed neutrophils start adhering and
migrating towards the affected ECs (91), whereas uPA activates proteolysis in
association with intracellular remodelling via vitronectin-integrin
binding (92). This allows new blood
vessels to extend towards the hypoxic locale. There is an increased
expression of vascular endothelial growth factor (VEGF) under
hypoxic conditions, either due to colder local temperatures
(93), trauma or oncogenesis
(94). VEGF is also known as a
vascular permeability factor, as beyond angiogenesis, VEGF often
causes a vascular leak, which can lead to oedema (95). The increased perfusion towards a
hypoxic locale by VEGF allows for influx of neutrophils and serum
contents, high molecular weight proteins (96), such as vitronectin (97) and fibronectin (98) (Fig.
4). Subsequently upon exposure to serum components, such as
vitronectin and fibronectin, integrin signalling along with
uPA/uPAR activation leads to Wnt activation and PAI-1 secretion
(99).
PAI-1 is found bound to vitronectin, in a latent
state which prevents its autolysis (100,101).
The affinity of PAI-1 is lower to vitronectin than it is to uPA.
This can result in the release of PAI-1 from vitronectin upon
secretion of uPA, making vitronectin available to bind to
integrins, which co-localise to uPAR on the lipid raft and mediate
uPA stimulated extracellular proteolysis and motility (102).
The uPA/uPAR/integrin complex allows for directional
endocytosis and proteolysis along the path of ECM degradation. The
presence of PAI-1 at the uPA/uPAR/integrin complex can cause
LRP-mediated intracellular recycling. This severs the extracellular
connection of uPA/uPAR/integrin and vitronectin to the degrading
ECM, and thus reduces the mobility caused by uPA/uPAR/integrin
association (103).
Vitronectin cellular attachment is mediated by
integrin αvβ3, which can also act in concert with αvβ5 to bind
fibronectin (104). Vitronectin
itself is secreted by the liver and the central nervous system
(105), and is primarily found in
the serum, although it is present in trace amounts in the ECM
(106), particularly in the lamina
elastica of vessels (107).
Fibronectin is more commonly found in areas of high cellular growth
or turnover (108), such as in a
wound (109). Collagen, the third
major ECM component, which binds to the cell surface via integrins,
is abundant throughout the ECM, its function is altered as a result
of changes in the function with its conformation and composition
(110).
Atherosclerotic vessels are a common complication of
hypoxia inducing conditions, such as obesity and diabetes (111). Studies have shown that cold induced
catabolism and hypoxia inducible factor (HIF) expression by
adipocytes inhibits the formation of atherosclerotic plaques
through lipid digestion (112).
Hypoxia inducible factor expression is known to stimulate PAI-1
expression (113). However,
increased PAI-1 expression results in a hypofibrinolytic state,
which leads to fibrosis and thrombosis of the fatty plaques
(114). The inhibition of
low-density lipoproteins by statins can reduce the adipocytic
commitment of fibroblast progenitors into adipose cells (115) resulting in reduced atherosclerotic
plaque formation (116). HIF
expression can also stimulate PAI-1 expression resulting in
angiogenesis (117). Increased
vitronectin presence at sites of vessel injury, such as
atherosclerotic plaques, are expected to be involved in the
mechanism underlying of increased platelet adhesion, and coupled
with the increased PAI-1 secretion, it may serve as an explanation
for the rapid thrombosis at these loci (118), much beyond the rate of PAI-1
induced angiogenesis.
Integrin binding to collagen and fibronectin can
increase the secretion of uPA, uPAR and PAI-1; however, αvβ3
binding to vitronectin was found to downregulate uPA and uPAR
antigen levels and upregulate PAI-1(99) (Fig.
5). The attachment of the uPA/uPAR complex to vitronectin and
other ECM proteins is performed via binding of the complex to
integrins, which is activated by the ECM proteins (119).
The integrin binding site on vitronectin is shared
by PAI-1, suggesting an interaction between signalling pathways
(124). It has been shown that the
extracellular signal-regulated kinase pathway is stimulated by αvβ5
integrin during chondrocyte dedifferentiation (125). Integrin αvβ1 has been identified in
the proteome of dedifferentiated chondrocytes (126), whereas αvβ3 has been reported to
mediate dedifferentiation of smooth muscle cells under
hypoglycaemic conditions (127).
Wnt-1 inducible signalling pathway protein 1 (WISP1)
is a downstream mediator of Wnt signalling (137) that has been found to be closely
associated with integrins. αvβ5 activation is now known as a
mediator of WISP1 in acute respiratory distress syndrome lung
injury (138). A separate study has
confirmed these findings across integrins αvβ5 and αvβ3(139). Upregulated WISP1 was found to
stimulate αvβ1 expression in transfected BMSCs (140). αvβ3 also allowed MSCs to activate
the Wnt/β-catenin pathway (66).
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (AKT) signalling pathway is necessary to stimulate
adipocyte differentiation from 3T3-L1 preadipocytes in the absence
of other inputs, as AKT1 can stimulate PPARγ production (141). uPA and uPAR downregulation inhibits
the PI3K/AKT pathway (142). The
downregulation of AKT by uPA/uPAR RNA inhibition results in
upregulation of PAI-1(143). In
fact, PAI-1 is a strong regulatory mechanism of adipocyte
differentiation, as microRNA (miRNA)-mediated inhibition of PAI-1
secretion in ADSCs was sufficient to stimulate differentiation into
adipocytes (144). miRNAs are mRNA
binding and modulating sequences, and have been strongly associated
with both stimulating adipocytic differentiation of 3T3-L1 cells
(144,145) and inhibiting it (146). The pre-miRNA requires endonuclease
activation. Once active, they can stimulate gene expression.
miR-130(147) and miR-27b (146) were found to stop adipocyte
differentiation by inhibiting PPARγ production via targeting coding
and untranslated mRNA regions. Stimulating the differentiation by
miRNA has been found via other pathways, namely miR-21 inhibition
of TGFBR2 secretion (148),
miR-17-92 reduction of tumour-suppressor Rb2/p130(145), and possibly miR-143 mediated
reduction of ERK5 production (149). Embryonic stem cell differentiation
into adipocytes is also inhibited by other uPA inhibitors, such as
amiloride (150).
The change in uPAR colocalization to integrins from
α3β1 to αvβ5 blocks the uPA signalling and activation of ERK or AKT
(151), which functionally
represents a shift from a laminin rich environment to a vitronectin
rich environment. Interestingly, Wnt signalling can be inhibited by
α3β1 complexes (152), while
activation of αvβ5 complexes stimulates Wnt signalling (153). uPAR complex internalisation by the
cell can result in Wnt mediated β-catenin gene transcription due to
nuclear colocalization, suggesting that uPAR in complex with
β-catenin can be a potent activator of stemness (154). PI3K/AKT signalling appears more
independent of uPAR internalisation, and tends towards maturation.
αvβ3/vitronectin complexes have been shown to activate a PI3K/AKT
induced stemness profile, whereas while α3β1/Laminin inhibits this
(155) (Fig. 6). PAI-1/LRP mediated clathrin
recycling of the uPA/uPAR complex and the vitronectin/αvβ3 complex
can be simultaneous due to different binding sites between
PAI-1/uPA and PAI-1/LRP, while uPA/uPAR itself also has a separate
integrin binding domain. The resultant vacuole is internalised with
the plasma membrane, and is isolated from the ECM, allowing only
for non-ECM dependent signalling.
Wound closure is initiated by thrombin, which
activates fibrinogen to create a fibrin clot (156). The activation of uPA, uPAR and
PAI-1 has been suggested as the mechanism underlying MSC
invasiveness into a fibrin clot of a wound (157). Fibronectin is an extracellular
glycoprotein, which creates a provisional matrix for cellular
adhesion to a wound environment via integrin binding (158). This in turn, has been shown to
stimulate uPA, uPAR and PAI-1 secretion (99). Adipocytes bind to fibronectin via
integrins (159). Preadipocyte
differentiation into adipocytes is marked by a loss of the
attachment to ECM components resulting in a rounded shape,
increased lipid content and reduced Wnt/βcatenin signalling
(160).
Fully differentiated adipocytes were reported to
lower PAI-1 concentration, and the subsequent addition of PAI-1 to
an osteoblast/fully differentiated adipocyte coculture did not
cause spontaneous transition of adipocytes to osteoblasts (161). Nonetheless, PAI-1 has been found to
push differentiated MSC lineages, of not only adipocytes but also
fibroblasts, back into the partially dedifferentiated growth arrest
state (162), as well as being
necessary for dedifferentiating osteosarcoma (163) into more malignant stem like cells,
suggesting the prevalence of this molecule in maintaining
pluripotency. It is also worth noting that the dedifferentiating
stimulus wrought by PAI-1 may partially explain why it is involved
in several types of tumours associated with a poor prognosis
(104).
Whilst detection of increased secretion of PAS
components within the tissue can be indicative of oncogenesis
(74,143) and diabetic dysregulation in obese
patients (61), localised activation
is both physiological and necessary for maintenance and healthy
tissue development. Currently, the fast-developing fields of
aesthetic and regenerative medicine, as well as dentistry, are
keenly focused on the bio-engineering potential of natural
products. Transplants of adipocytes, platelet rich fibrin or
platelet rich plasma are used for acceleration of healing, aiming
to stimulate fibroblasts or osteoblasts, and multiple approaches
are often combined to increase their effectiveness (164). It is hypothesized that adipocytes
primed with PAS system components may be used to improve outcomes
of bone regeneration, soft connective tissue regeneration and wound
healing.
Instances where establishing a sufficient blood
supply is of high concern, such as an osseoinductive transplant, a
collagenous cellular carrier implant, or for procedures such as
bone distraction, extensive surgical flaps, or any other surgical
intervention where scarring and grafting is an issue, may benefit
from the possible therapeutic applications of receptive stem cells.
When the protraction of healing is necessary to allow for complete
angiogenesis, and adequate deposition of ECM to support the
unformed tissue, uPA can ensure that the microenvironment is
maintained in a state of turnover (129). Degradation of the ECM increases
permeability to both cells and signalling molecules. However, local
inflammatory mediators tend to stimulate ECM degradation and uPA
release. However, at present, the clinical use for uPA alone is
limited to acellular pathologies, such as large thrombi (165) or thinning of tuberculous pleural
thickening (166).
The ability for adipocytes to secrete uPA and PAI-1
to modulate and maintain their pluripotent microenvironment has
been explored, and this may be conducive in wound healing or
bio-engineering stents (77). It is
hypothesized that balancing the vascularisation and remodelling
properties of uPA, with the dedifferentiating properties of PAI-1
production and recycling in association with integrins may be
achieved by cultivating an adipocyte harvest in the right ECM
protein environment (89).
The MSC like fraction can be boosted by
synthetically overloading harvested adipocytes with PAI-1 or any of
the other Wnt activating PPARγ inhibitors and re-introducing this
population into a wound or surgical microenvironment in order to
become more susceptible to local differentiation factors and
conduit healing or growth (157).
Alternatively, whole tissue adequately prepared to
stimulate activation of the PAS, such as in a wound healing or
hypoxic environment, may create a DFAT rich graft which would be
significantly more conducive to local cellular populations or ECM
architectures in mesenchymal lineage use cases (21).
For directed mobility via extracellular degradation,
there needs to be present a steady stream of uPA to counteract any
baseline PAI-1 secretion, to overcome the background PAI-1
recycling of the uPA/uPAR/integrin complex, which undergoes
intracellular recycling along with LRP and downstream targets. Such
interventions are difficult to perform in vivo due to the
delicate nature of surgical sites. Thus, it is more prudent to
identify self-regulating stents, which can stabilise harvested
adipocytes in a required state (3).
PAI-1 is found to be stable for 145 h when bound to
vitronectin, and for 2 h when expressed in isolation in
vitro (167). This suggests
that PAI-1 can cause a dormant inhibition of cellular motility
during a latent phase of the cell cycle when there are no uPA
stimulating environmental queues. However, when uPA is released,
the motility, sensing, and endocytic potential inhibited by the
latent PAI-1 secretion could explain a physiological receptiveness
to changes observed during pathological processes tending to
homeostasis (150).
Studies have shown that platelet rich plasma with
ADSCs can significantly improve tissue incorporation of synthetic
scaffolds and their neovascularisation (168). Additionally, ADSCs with platelet
rich fibrin (PRF) have exhibited a certain degree of improvement in
restoring salivary function following gland irradiation, which
neither ADSC nor PRF alone achieved (164). This effect could be due to the
presence of fibrin in the platelet rich plasma, which in turn
activates uPA and PAI-1 secretion through integrin activation of
Wnt. Fibrin has been shown to enhance Wnt signalling (169,170),
whereas PRF-stimulated BMSC healing of alveolar bone defects was
found to be mediated by expression of Wnt3a (171), further suggesting the promising
clinical applications of this approach. The high vitronectin
content of plasma, which has a selective PAI-1 up-regulatory
mechanism, could account for the multipotent stimuli that modifies
the local cell population for faster healing outcomes.
Adipocytes can be seen as a versatile cell lineage
which harbour mesenchymal stem potential in the form of MSCs, ADSCs
and the induced DFAT cells. The formation of the latter has been
found to rely on inhibition of PPARγ. Wnt signalling has been shown
to be stimulated by TNF-α and integrins, notably αvβ3 via fibrin.
The proteolytic cascade activated by PAS during inflammation is
mediated by multiple Wnt activators, acting on Wnt to stimulate uPA
release for ECM degradation, PAI-1 release for endocytosis, uPAR
for localisation to LRP, and integrins to provide motility and
directional specificity.
Since Wnt activation also inhibits PPARγ expression,
there is a high chance that adipose cells exposed to a fibrin or
vitronectin rich wound environment would undergo dedifferentiation.
PAI-1 expression has also been linked to dedifferentiation, which
could be explained by its ability to stimulate endocytotic clathrin
basket mediated recycling of uPA/uPAR/integrin complexes. After
wound resolution, in order to reach homeostasis and inhibit uPA
mediated extracellular ECM degradation, PAI-1 needs to be released
following its internalisation, suggesting that activation of Wnt
signalling is necessary in wound healing in order to produce PAI-1,
and consequently inhibit PPARγ. Coupled with Wnt mediated
stimulation of PAS expression to allow for remodelling of the ECM
and cellular motility, there is a strong suggestion that Wnt is
central to the success and high versatility of adipose tissue and
more importantly DFAT cells in pilot studies. An initial
dedifferentiating priming of adipose to DFAT cells from a
lipoaspirate harvest in a PRF could be followed by cytokine,
mineral or ECM exposure to initiate target cell differentiation
prior to clinical use, taking the DFAT cells one step closer to use
in a clinically applicable environment.
Not applicable.
The present review was supported by internal funding from Poznan
University of Medical Sciences.
Not applicable.
MWS, AE and conceived the topic of the review MN
wrote the manuscript. MN and AE reviewed and revised the
manuscript. All authors have read and approved the final
manuscript. Data sharing is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Muneoka K and Dawson LA: Evolution of
epimorphosis in mammals. J Exp Zool B Mol Dev Evol. 336:165–179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cai S, Fu X and Sheng Z:
Dedifferentiation: A new approach in stem cell research.
Bioscience. 57:655–662. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang C, Liu W, Shen Y, Chen J, Zhu H, Yang
X, Jiang X, Wang Y and Zhou J: Cardiomyocyte dedifferentiation and
remodeling in 3D scaffolds to generate the cellular diversity of
engineering cardiac tissues. Biomater Sci. 7:4636–4650.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Becker RO: Induced dedifferentiation: A
possible alternative to embryonic stem cell transplants.
NeuroRehabilitation. 17:23–31. 2002.PubMed/NCBI
|
|
5
|
Yao Y and Wang C: Dedifferentiation:
Inspiration for devising engineering strategies for regenerative
medicine. NPJ Regen Med. 5(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sugawara A and Sato S: Application of
dedifferentiated fat cells for periodontal tissue regeneration. Hum
Cell. 27:12–21. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Biehl JK and Russell B: Introduction to
stem cell therapy. J Cardiovasc Nurs. 24:98–105. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jiang M, He B, Zhang Q, Ge H, Zang MH, Han
ZH, Liu JP, Li JH, Zhang Q, Li HB, et al: Randomized controlled
trials on the therapeutic effects of adult progenitor cells for
myocardial infarction: Meta-analysis. Expert Opin Biol Ther.
10:667–680. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sobhani A, Khanlarkhani N, Baazm M,
Mohammadzadeh F, Najafi A, Mehdinejadiani S and Sargolzaei Aval F:
Multipotent stem cell and current application. Acta Med Iran.
55:6–23. 2017.PubMed/NCBI
|
|
10
|
Feyen DAM, Gaetani R, Doevendans PA and
Sluijter JPG: Stem cell-based therapy: Improving myocardial cell
delivery. Adv Drug Deliv Rev. 106:104–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sanchez-Gurmaches J and Guertin DA:
Adipocyte lineages: Tracing back the origins of fat. Biochim
Biophys Acta. 1842:340–351. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sebo ZL, Jeffery E, Holtrup B and
Rodeheffer MS: A mesodermal fate map for adipose tissue.
Development. 145(dev166801)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hepler C, Shan B, Zhang Q, Henry GH, Shao
M, Vishvanath L, Ghaben AL, Mobley AB, Strand D, Hon GC and Gupta
RK: Identification of functionally distinct fibro-inflammatory and
adipogenic stromal subpopulations in visceral adipose tissue of
adult mice. Elife. 7(e39636)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lynes MD and Tseng YH: Deciphering adipose
tissue heterogeneity. Ann N Y Acad Sci. 1411:5–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ronconi V, Turchi F, Bujalska IJ,
Giacchetti G and Boscaro M: Adipose cell-adrenal interactions:
Current knowledge and future perspectives. Trends Endocrinol Metab.
19:100–103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rippa AL, Kalabusheva EP and Vorotelyak
EA: Regeneration of dermis: Scarring and cells involved. Cells.
8(607)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Grant RW and Dixit VD: Adipose tissue as
an immunological organ. Obesity (Silver Spring). 23:512–518.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kassem M: Mesenchymal stem cells:
Biological characteristics and potential clinical applications.
Cloning Stem Cells. 6:369–374. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vojtaššák J, Danišovič Ľ, Kubeš M, Bakoš
D, Jarabek L, Uličná M and Blaško M: Autologous biograft and
mesenchymal stem cells in treatment of the diabetic foot.
Neuroendocrinol Lett. 27 (Suppl 2):S134–S137. 2006.PubMed/NCBI
|
|
22
|
Smith RKW: Mesenchymal stem cell therapy
for equine tendinopathy. Disabil Rehabil. 30:1752–1758.
2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou W, Han C, Song Y, Yan X, Li D, Chai
Z, Feng Z, Dong Y, Li L, Xie X, et al: The performance of bone
marrow mesenchymal stem cell-implant complexes prepared by cell
sheet engineering techniques. Biomaterials. 31:3212–3221.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pountos I, Jones E, Tzioupis C, McGonagle
D and Giannoudis PV: Growing bone and cartilage. The role of
mesenchymal stem cells. J Bone Joint Surg Br. 88:421–426.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Holmes B, Fang X, Zarate A, Keidar M and
Zhang LG: Enhanced human bone marrow mesenchymal stem cell
chondrogenic differentiation in electrospun constructs with carbon
nanomaterials. Carbon. 97:1–13. 2016.
|
|
26
|
Gianakos AL, Sun L, Patel JN, Adams DM and
Liporace FA: Clinical application of concentrated bone marrow
aspirate in orthopaedics: A systematic review. World J Orthop.
8:491–506. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sakamoto T, Miyazaki T, Watanabe S,
Takahashi A, Honjoh K, Nakajima H, Oki H, Kokubo Y and Matsumine A:
Intraarticular injection of processed lipoaspirate cells has
anti-inflammatory and analgesic effects but does not improve
degenerative changes in murine monoiodoacetate-induced
osteoarthritis. BMC Musculoskelet Disord. 20(335)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Roubelakis MG, Pappa KI, Bitsika V,
Zagoura D, Vlahou A, Papadaki HA, Antsaklis A and Anagnou NP:
Molecular and proteomic characterization of human mesenchymal stem
cells derived from amniotic fluid: Comparison to bone marrow
mesenchymal stem cells. Stem Cells Dev. 16:931–952. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bieback K, Kern S, Kocaömer A, Ferlik K
and Bugert P: Comparing mesenchymal stromal cells from different
human tissues: Bone marrow, adipose tissue and umbilical cord
blood. Biomed Mater Eng. 18 (1 Suppl):S71–S76. 2008.PubMed/NCBI
|
|
30
|
Francis MP, Sachs PC, Elmore LW and Holt
SE: Isolating adipose-derived mesenchymal stem cells from
lipoaspirate blood and saline fraction. Organogenesis. 6:11–14.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wouters G, Grossi S, Mesoraca A, Bizzoco
D, Mobili L, Cignini P and Giorlandino C: Isolation of amniotic
fluid-derived mesenchymal stem cells. J Prenat Med. 1:39–40.
2007.PubMed/NCBI
|
|
32
|
Li C, Kilpatrick CD, Smith S, Glettig DL,
Glod DJ, Mallette J, Strunk MR, Chang J, Angle SR and Kaplan DL:
Assessment of multipotent mesenchymal stromal cells in bone marrow
aspirate from human calcaneus. J Foot Ankle Surg. 56:42–46.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Matsumoto T, Kano K, Kondo D, Fukuda N,
Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al:
Mature adipocyte-derived dedifferentiated fat cells exhibit
multilineage potential. J Cell Physiol. 215:210–222.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Turner NJ, Jones HS, Davies JE and
Canfield AE: Cyclic stretch-induced TGFbeta1/Smad signaling
inhibits adipogenesis in umbilical cord progenitor cells. Biochem
Biophys Res Commun. 377:1147–1151. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gustafson B and Smith U: Activation of
canonical wingless-type MMTV integration site family (Wnt)
signaling in mature adipocytes increases beta-catenin levels and
leads to cell dedifferentiation and insulin resistance. J Biol
Chem. 285:14031–14041. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Strioga M, Viswanathan S, Darinskas A,
Slaby O and Michalek J: Same or not the same? Comparison of adipose
tissue-derived versus bone marrow-derived mesenchymal stem and
stromal cells. Stem Cells Dev. 21:2724–2752. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liao Y, Zeng Z, Lu F, Dong Z, Chang Q and
Gao J: In vivo dedifferentiation of adult adipose cells. PLoS One.
10(e0125254)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tzameli I, Fang H, Ollero M, Shi H, Hamm
JK, Kievit P, Hollenberg AN and Flier JS: Regulated production of a
peroxisome proliferator-activated receptor-gamma ligand during an
early phase of adipocyte differentiation in 3T3-L1 adipocytes. J
Biol Chem. 279:36093–36102. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhuang H, Zhang X, Zhu C, Tang X, Yu F,
Shang GW and Cai X: Molecular mechanisms of PPAR-γ governing MSC
osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther.
11:255–264. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wieser F, Waite L, Depoix C and Taylor RN:
PPAR action in human placental development and pregnancy and its
complications. PPAR Res. 2008(527048)2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yessoufou A and Wahli W: Multifaceted
roles of peroxisome proliferator-activated receptors (PPARs) at the
cellular and whole organism levels. Swiss Med Wkly.
140(w13071)2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takada I, Kouzmenko AP and Kato S: Wnt and
PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev
Rheumatol. 5:442–447. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Otto TC and Lane MD: Adipose development:
From stem cell to adipocyte. Crit Rev Biochem Mol Biol. 40:229–242.
2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen L, Song J, Cui J, Hou J, Zheng X, Li
C and Liu L: microRNAs regulate adipocyte differentiation. Cell
Biol Int. 37:533–546. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
McBeath R, Pirone DM, Nelson CM,
Bhadriraju K and Chen CS: Cell shape, cytoskeletal tension, and
RhoA regulate stem cell lineage commitment. Dev Cell. 6:483–495.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Clabaut A, Delplace S, Chauveau C,
Hardouin P and Broux O: Human osteoblasts derived from mesenchymal
stem cells express adipogenic markers upon coculture with bone
marrow adipocytes. Differentiation. 80:40–45. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang QA, Song A, Chen W, Schwalie PC,
Zhang F, Vishvanath L, Jiang L, Ye R, Shao M, Tao C, et al:
Reversible De-differentiation of mature white adipocytes into
preadipocyte-like precursors during lactation. Cell Metab.
28:282–288.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kruglikov IL, Zhang Z and Scherer PE: The
role of immature and mature adipocytes in hair cycling. Trends
Endocrinol Metab. 30:93–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Maurizi G, Della Guardia L, Maurizi A and
Poloni A: Adipocytes properties and crosstalk with immune system in
obesity-related inflammation. J Cell Physiol. 233:88–97.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Marangoni RG, Korman BD, Wei J, Wood TA,
Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG and Varga J:
Myofibroblasts in murine cutaneous fibrosis originate from
adiponectin-positive intradermal progenitors. Arthritis Rheumatol.
67:1062–1073. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Motrescu ER and Rio MC: Cancer cells,
adipocytes and matrix metalloproteinase 11: A vicious tumor
progression cycle. Biol Chem. 389:1037–1041. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Plikus MV, Guerrero-Juarez CF, Ito M, Li
YR, Dedhia PH, Zheng Y, Shao M, Gay DL, Ramos R, His TC, et al:
Regeneration of fat cells from myofibroblasts during wound healing.
Science. 355:748–752. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Merrick D and Seale P: Skinny fat cells
stimulate wound healing. Cell Stem Cell. 26:801–803.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kurebayashi S, Sumitani S, Kasayama S,
Jetten AM and Hirose T: TNF-alpha inhibits 3T3-L1 adipocyte
differentiation without downregulating the expression of C/EBPbeta
and delta. Endocr J. 48:249–253. 2001.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sartipy P and Loskutoff DJ: Monocyte
chemoattractant protein 1 in obesity and insulin resistance. Proc
Natl Acad Sci USA. 100:7265–7270. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ruan H and Lodish HF: Insulin resistance
in adipose tissue: Direct and indirect effects of tumor necrosis
factor-alpha. Cytokine Growth Factor Rev. 14:447–455.
2003.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kanda H, Tateya S, Tamori Y, Kotani K,
Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K and
Kasuga M: MCP-1 contributes to macrophage infiltration into adipose
tissue, insulin resistance, and hepatic steatosis in obesity. J
Clin Invest. 116:1494–1505. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Panee J: Monocyte chemoattractant protein
1 (MCP-1) in obesity and diabetes. Cytokine. 60:1–12.
2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Furlan F, Orlando S, Laudanna C, Resnati
M, Basso V, Blasi F and Mondino A: The soluble D2D3(88-274)
fragment of the urokinase receptor inhibits monocyte chemotaxis and
integrin-dependent cell adhesion. J Cell Sci. 117:2909–2916.
2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Montuori N and Ragno P: Multiple
activities of a multifaceted receptor: Roles of cleaved and soluble
uPAR. Front Biosci (Landmark Ed). 14:2494–2503. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Catalán V, Frühbeck G and Gómez-Ambrosi J:
Chapter 8-Inflammatory and oxidative stress markers in skeletal
muscle of obese subjects. In: Obesity. del Moral AM and Aguilera
García CM (eds). Academic Press, pp163-189, 2018.
|
|
62
|
Yang D, Wei F, Tewary P, Howard OM and
Oppenheim JJ: Alarmin-induced cell migration. Eur J Immunol.
43:1412–1418. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ren P, Sun D, Xin D, Ma W, Chen P, Gao H,
Zhang S and Gong M: Serum amyloid A promotes osteosarcoma invasion
via upregulating αvβ3 integrin. Mol Med Rep. 10:3106–3112.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bernstein AM, Twining SS, Warejcka DJ,
Tall E and Masur SK: Urokinase receptor cleavage: A crucial step in
fibroblast-to-myofibroblast differentiation. Mol Biol Cell.
18:2716–2727. 2007.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Resnati M, Pallavicini I, Wang JM,
Oppenheim J, Serhan CN, Romano M and Blasi F: The fibrinolytic
receptor for urokinase activates the G protein-coupled chemotactic
receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 99:1359–1364.
2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W,
Yu T, Yang R, Wang R, Zhou Y and Shi S: Circulating apoptotic
bodies maintain mesenchymal stem cell homeostasis and ameliorate
osteopenia via transferring multiple cellular factors. Cell Res.
28:918–933. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
He W, Tan R, Dai C, Li Y, Wang D, Hao S,
Kahn M and Liu Y: Plasminogen activator inhibitor-1 is a
transcriptional target of the canonical pathway of Wnt/beta-catenin
signaling. J Biol Chem. 285:24665–24675. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Akimoto T, Ushida T, Miyaki S, Akaogi H,
Tsuchiya K, Yan Z, Williams RS and Tateishi T: Mechanical stretch
inhibits myoblast-to-adipocyte differentiation through Wnt
signaling. Biochem Biophys Res Commun. 329:381–385. 2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lee H, Kang R, Bae S and Yoon Y: AICAR, an
activator of AMPK, inhibits adipogenesis via the WNT/β-catenin
pathway in 3T3-L1 adipocytes. Int J Mol Med. 28:65–71.
2011.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Renner G, Noulet F, Mercier MC, Choulier
L, Etienne-Selloum N, Gies JP, Lehmann M, Lelong-Rebel I, Martin S
and Dontenwill M: Expression/activation of α5β1 integrin is linked
to the β-catenin signaling pathway to drive migration in glioma
cells. Oncotarget. 7:62194–62207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Liang X, Kanjanabuch T, Mao SL, Hao CM,
Tang YW, Declerck PJ, Hasty AH, Wasserman DH, Fogo AB and Ma LJ:
Plasminogen activator inhibitor-1 modulates adipocyte
differentiation. Am J Physiol Endocrinol Metab. 290:E103–E113.
2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Swiatkowska M, Szemraj J and Cierniewski
CS: Induction of PAI-1 expression by tumor necrosis factor alpha in
endothelial cells is mediated by its responsive element located in
the 4G/5G site. FEBS J. 272:5821–5831. 2005.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kruithof EK: Regulation of plasminogen
activator inhibitor type 1 gene expression by inflammatory
mediators and statins. Thromb Haemost. 100:969–975. 2008.PubMed/NCBI
|
|
74
|
Su SC, Lin CW, Yang WE, Fan WL and Yang
SF: The urokinase-type plasminogen activator (uPA) system as a
biomarker and therapeutic target in human malignancies. Expert Opin
Ther Targets. 20:551–566. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Prabhakar NR and Semenza GL: Oxygen
sensing and homeostasis. Physiology (Bethesda). 30:340–348.
2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Darby IA and Hewitson TD: Hypoxia in
tissue repair and fibrosis. Cell Tissue Res. 365:553–562.
2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Fierro FA, O'Neal AJ, Beegle JR, Chávez
MN, Peavy TR, Isseroff RR and Egaña JT: Hypoxic pre-conditioning
increases the infiltration of endothelial cells into scaffolds for
dermal regeneration pre-seeded with mesenchymal stem cells. Front
Cell Dev Biol. 3(68)2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ye J: Adipose tissue vascularization: Its
role in chronic inflammation. Curr Diab Rep. 11:203–210.
2011.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lund IK, Nielsen BS, Almholt K, Rønø B,
Hald A, Illemann M, Green KA, Christensen IJ, Rømer J and Lund LR:
Concomitant lack of MMP9 and uPA disturbs physiological tissue
remodeling. Dev Biol. 358:56–67. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Carriero MV and Stoppelli MP: The
urokinase-type plasminogen activator and the generation of
inhibitors of urokinase activity and signaling. Curr Pharm Des.
17:1944–1961. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: Multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhao Y, Lyons CE Jr, Xiao A, Templeton DJ,
Sang QA, Brew K and Hussaini IM: Urokinase directly activates
matrix metalloproteinases-9: A potential role in glioblastoma
invasion. Biochem Biophys Res Commun. 369:1215–1220.
2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lafont JE: Lack of oxygen in articular
cartilage: Consequences for chondrocyte biology. Int J Exp Pathol.
91:99–106. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Shen J, Sugawara A, Yamashita J, Ogura H
and Sato S: Dedifferentiated fat cells: An alternative source of
adult multipotent cells from the adipose tissues. Int J Oral Sci.
3:117–124. 2011.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Hausman GJ and Richardson RL: Adipose
tissue angiogenesis. J Anim Sci. 82:925–934. 2004.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Lijnen HR: Angiogenesis and obesity.
Cardiovasc Res. 78:286–293. 2008.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Trayhurn P: Hypoxia and adipose tissue
function and dysfunction in obesity. Physiol Rev. 93:1–21.
2013.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Chabot V, Dromard C, Rico A, Langonné A,
Gaillard J, Guilloton F, Casteilla L and Sensebé L: Urokinase-type
plasminogen activator receptor interaction with β1 integrin is
required for platelet-derived growth factor-AB-induced human
mesenchymal stem/stromal cell migration. Stem Cell Res Ther.
6(188)2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Crandall DL, Busler DE, McHendry-Rinde B,
Groeling TM and Kral JG: Autocrine regulation of human preadipocyte
migration by plasminogen activator inhibitor-1. J Clin Endocrinol
Metab. 85:2609–2614. 2000.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Egners A, Erdem M and Cramer T: The
response of macrophages and neutrophils to hypoxia in the context
of cancer and other inflammatory diseases. Mediators Inflamm.
2016(2053646)2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Salasznyk RM, Zappala M, Zheng M, Yu L,
Wilkins-Port C and McKeown-Longo PJ: The uPA receptor and the
somatomedin B region of vitronectin direct the localization of uPA
to focal adhesions in microvessel endothelial cells. Matrix Biol.
26:359–370. 2007.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Xue Y, Petrovic N, Cao R, Larsson O, Lim
S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, et al:
Hypoxia-independent angiogenesis in adipose tissues during cold
acclimation. Cell Metab. 9:99–109. 2009.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jośko J and Mazurek M: Transcription
factors having impact on vascular endothelial growth factor (VEGF)
gene expression in angiogenesis. Med Sci Monit. 10:RA89–RA98.
2004.PubMed/NCBI
|
|
95
|
Weis SM and Cheresh DA: Pathophysiological
consequences of VEGF-induced vascular permeability. Nature.
437:497–504. 2005.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Vandoorne K, Addadi Y and Neeman M:
Visualizing vascular permeability and lymphatic drainage using
labeled serum albumin. Angiogenesis. 13:75–85. 2010.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kobayashi J, Yamada S and Kawasaki H:
Distribution of vitronectin in plasma and liver tissue:
Relationship to chronic liver disease. Hepatology. 20:1412–1417.
1994.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Goerges AL and Nugent MA: pH regulates
vascular endothelial growth factor binding to fibronectin: A
mechanism for control of extracellular matrix storage and release.
J Biol Chem. 279:2307–2315. 2004.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Hapke S, Kessler H, Arroyo de Prada N,
Benge A, Schmitt M, Lengyel E and Reuning U: Integrin
alpha(v)beta(3)/vitronectin interaction affects expression of the
urokinase system in human ovarian cancer cells. J Biol Chem.
276:26340–26348. 2001.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Chu Y, Bucci JC and Peterson CB:
Identification of a PAI-1-binding site within an intrinsically
disordered region of vitronectin. Protein Sci. 29:494–508.
2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Arroyo De Prada N, Schroeck F, Sinner EK,
Muehlenweg B, Twellmeyer J, Sperl S, Wilhelm OG, Schmitt M and
Magdolen V: Interaction of plasminogen activator inhibitor type-1
(PAI-1) with vitronectin. Eur J Biochem. 269:184–192.
2002.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang L, Ly CM, Ko CY, Meyers EE, Lawrence
DA and Bernstein AM: uPA binding to PAI-1 induces corneal
myofibroblast differentiation on vitronectin. Invest Ophthalmol Vis
Sci. 53:4765–4775. 2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Czekay RP, Aertgeerts K, Curriden SA and
Loskutoff DJ: Plasminogen activator inhibitor-1 detaches cells from
extracellular matrices by inactivating integrins. J Cell Biol.
160:781–791. 2003.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Mousa SA: Vitronectin receptors in
vascular disorders. Curr Opin Investig Drugs. 3:1191–1195.
2002.PubMed/NCBI
|
|
105
|
Seiffert D, Iruela-Arispe ML, Sage EH and
Loskutoff DJ: Distribution of vitronectin mRNA during murine
development. Dev Dyn. 203:71–79. 1995.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Aaboe M, Offersen BV, Christensen A and
Andreasen PA: Vitronectin in human breast carcinomas. Biochim
Biophys Acta. 1638:72–82. 2003.PubMed/NCBI View Article : Google Scholar
|
|
107
|
van Aken BE, Seiffert D, Thinnes T and
Loskutoff DJ: Localization of vitronectin in the normal and
atherosclerotic human vessel wall. Histochem Cell Biol.
107:313–320. 1997.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shi F and Sottile J: Caveolin-1-dependent
beta1 integrin endocytosis is a critical regulator of fibronectin
turnover. J Cell Sci. 121:2360–2371. 2008.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Lenselink EA: Role of fibronectin in
normal wound healing. Int Wound J. 12:313–316. 2015.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ricard-Blum S: The collagen family. Cold
Spring Harb Perspect Biol. 3(a004978)2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Scherer PE and Hill JA: Obesity, diabetes,
and cardiovascular diseases: A compendium. Circ Res. 118:1703–1705.
2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhang X, Zhang Y, Wang P, Zhang SY, Dong
Y, Zeng G, Yan Y, Sun L, Wu Q, Liu H, et al: Adipocyte
hypoxia-inducible factor 2α suppresses atherosclerosis by promoting
adipose ceramide catabolism. Cell Metab. 30:937–951.e5.
2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Kaneko M, Minematsu T, Yoshida M,
Nishijima Y, Noguchi H, Ohta Y, Nakagami G, Mori T and Sanada H:
Compression-induced HIF-1 enhances thrombosis and PAI-1 expression
in mouse skin. Wound Repair Regen. 23:657–663. 2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Samad F, Schneiderman J and Loskutoff D:
Expression of fibrinolytic genes in tissues from human
atherosclerotic aneurysms and from obese mice. Ann N Y Acad Sci.
811:350–360. 1997.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Qureshi R, Kindo M, Arora H, Boulberdaa M,
Steenman M and Nebigil CG: Prokineticin receptor-1-dependent
paracrine and autocrine pathways control cardiac tcf21+
fibroblast progenitor cell transformation into adipocytes and
vascular cells. Sci Rep. 7(12804)2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Li JQ, Zhao SP, Li QZ, Cai YC, Wu LR, Fang
Y and Li P: Atorvastatin reduces plasminogen activator inhibitor-1
expression in adipose tissue of atherosclerotic rabbits. Clin Chim
Acta. 370:57–62. 2006.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Geis T, Döring C, Popp R, Grossmann N,
Fleming I, Hansmann ML, Dehne N and Brüne B: HIF-2alpha-dependent
PAI-1 induction contributes to angiogenesis in hepatocellular
carcinoma. Exp Cell Res. 331:46–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Ekmekci H, Sonmez H, Ekmekci OB, Ozturk Z,
Domanic N and Kokoglu E: Plasma vitronectin levels in patients with
coronary atherosclerosis are increased and correlate with extent of
disease. J Thromb Thrombolysis. 14:221–225. 2002.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Reuning U, Magdolen V, Hapke S and Schmitt
M: Molecular and functional interdependence of the urokinase-type
plasminogen activator system with integrins. Biol Chem.
384:1119–1131. 2003.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Chiellini C, Cochet O, Negroni L, Samson
M, Poggi M, Ailhaud G, Alessi MC, Dani C and Amri EZ:
Characterization of human mesenchymal stem cell secretome at early
steps of adipocyte and osteoblast differentiation. BMC Mol Biol.
9(26)2008.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Ekström M, Liska J, Eriksson P,
Sverremark-Ekström E and Tornvall P: Stimulated in vivo synthesis
of plasminogen activator inhibitor-1 in human adipose tissue.
Thromb Haemost. 108:485–492. 2012.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Lijnen HR, Maquoi E, Demeulemeester D, Van
Hoef B and Collen D: Modulation of fibrinolytic and gelatinolytic
activity during adipose tissue development in a mouse model of
nutritionally induced obesity. Thromb Haemost. 88:345–353.
2002.PubMed/NCBI
|
|
123
|
Efimenko A, Starostina E, Kalinina N and
Stolzing A: Angiogenic properties of aged adipose derived
mesenchymal stem cells after hypoxic conditioning. J Transl Med.
9(10)2011.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Zhou A, Huntington JA, Pannu NS, Carrell
RW and Read RJ: How vitronectin binds PAI-1 to modulate
fibrinolysis and cell migration. Nat Struct Biol. 10:541–544.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
125
|
Fukui N, Ikeda Y, Tanaka N, Wake M,
Yamaguchi T, Mitomi H, Ishida S, Furukawa H, Hamada Y, Miyamoto Y,
et al: αvβ5 Integrin promotes dedifferentiation of
monolayer-cultured articular chondrocytes. Arthritis Rheum.
63:1938–1949. 2011.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Goessler UR, Bieback K, Bugert P, Heller
T, Sadick H, Hörmann K and Riedel F: In vitro analysis of
integrin expression during chondrogenic differentiation of
mesenchymal stem cells and chondrocytes upon dedifferentiation in
cell culture. Int J Mol Med. 17:301–307. 2006.PubMed/NCBI
|
|
127
|
Clemmons DR, Maile LA, Ling Y, Yarber J
and Busby WH: Role of the integrin alphaVbeta3 in mediating
increased smooth muscle cell responsiveness to IGF-I in response to
hyperglycemic stress. Growth Horm IGF Res. 17:265–270.
2007.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Czekay RP, Kuemmel TA, Orlando RA and
Farquhar MG: Direct binding of occupied urokinase receptor (uPAR)
to LDL receptor-related protein is required for endocytosis of uPAR
and regulation of cell surface urokinase activity. Mol Biol Cell.
12:1467–1479. 2001.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Binder BR, Mihaly J and Prager GW:
uPAR-uPA-PAI-1 interactions and signaling: A vascular biologist's
view. Thromb Haemost. 97:336–342. 2007.PubMed/NCBI
|
|
130
|
Cortese K, Sahores M, Madsen CD, Tacchetti
C and Blasi F: Clathrin and LRP-1-independent constitutive
endocytosis and recycling of uPAR. PLoS One.
3(e3730)2008.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Rabiej VK, Pflanzner T, Wagner T, Goetze
K, Storck SE, Eble JA, Weggen S, Mueller-Klieser W and Pietrzik CU:
Low density lipoprotein receptor-related protein 1 mediated
endocytosis of β1-integrin influences cell adhesion and cell
migration. Exp Cell Res. 340:102–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Crampton SP, Wu B, Park EJ, Kim JH,
Solomon C, Waterman ML and Hughes CC: Integration of the
beta-catenin-dependent Wnt pathway with integrin signaling through
the adaptor molecule Grb2. PLoS One. 4(e7841)2009.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Dejaeger M, Böhm AM, Dirckx N, Devriese J,
Nefyodova E, Cardoen R, St-Arnaud R, Tournoy J, Luyten FP and Maes
C: Integrin-linked kinase regulates bone formation by controlling
cytoskeletal organization and modulating BMP and Wnt signaling in
osteoprogenitors. J Bone Miner Res. 32:2087–2102. 2017.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Zucker MM, Wujak L, Gungl A, Didiasova M,
Kosanovic D, Petrovic A, Klepetko W, Schermuly RT, Kwapiszewska G,
Schaefer L and Wygrecka M: LRP1 promotes synthetic phenotype of
pulmonary artery smooth muscle cells in pulmonary hypertension.
Biochim Biophys Acta Mol Basis Dis. 1865:1604–1616. 2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Koraishy FM, Silva C, Mason S, Wu D and
Cantley LG: Hepatocyte growth factor (Hgf) stimulates low density
lipoprotein receptor-related protein (Lrp) 5/6 phosphorylation and
promotes canonical Wnt signaling. J Biol Chem. 289:14341–14350.
2014.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Masson O, Chavey C, Dray C, Meulle A,
Daviaud D, Quilliot D, Muller C, Valet P and Liaudet-Coopman E:
LRP1 receptor controls adipogenesis and is up-regulated in human
and mouse obese adipose tissue. PLoS One. 4(e7422)2009.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Jiang F, Parsons CJ and Stefanovic B: Gene
expression profile of quiescent and activated rat hepatic stellate
cells implicates Wnt signaling pathway in activation. J Hepatol.
45:401–409. 2006.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Ding X, Tong Y, Jin S, Chen Z, Li T,
Billiar TR, Pitt BR, Li Q and Zhang LM: Mechanical ventilation
enhances extrapulmonary sepsis-induced lung injury: Role of
WISP1-αvβ5 integrin pathway in TLR4-mediated inflammation and
injury. Crit Care. 22(302)2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Stephens S, Palmer J, Konstantinova I,
Pearce A, Jarai G and Day E: A functional analysis of Wnt inducible
signalling pathway protein-1 (WISP-1/CCN4). J Cell Commun Signal.
9:63–72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Ono M, Inkson CA, Kilts TM and Young MF:
WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J
Bone Miner Res. 26:193–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Xu J and Liao K: Protein kinase B/AKT 1
plays a pivotal role in insulin-like growth factor-1 receptor
signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem.
279:35914–35922. 2004.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Gondi CS, Kandhukuri N, Dinh DH, Gujrati M
and Rao JS: Down-regulation of uPAR and uPA activates
caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J
Oncol. 31:19–27. 2007.PubMed/NCBI
|
|
143
|
Whitley BR, Beaulieu LM, Carter JC and
Church FC: Phosphatidylinositol 3-kinase/Akt regulates the balance
between plasminogen activator inhibitor-1 and urokinase to promote
migration of SKOV-3 ovarian cancer cells. Gynecol Oncol.
104:470–479. 2007.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Karbiener M, Neuhold C, Opriessnig P,
Prokesch A, Bogner-Strauss JG and Scheideler M: MicroRNA-30c
promotes human adipocyte differentiation and co-represses PAI-1 and
ALK2. RNA Biol. 8:850–860. 2011.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg
RJ and Li X: miR-17-92 cluster accelerates adipocyte
differentiation by negatively regulating tumor-suppressor Rb2/p130.
Proc Natl Acad Sci USA. 105:2889–2894. 2008.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Karbiener M, Fischer C, Nowitsch S,
Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ and Scheideler M:
microRNA miR-27b impairs human adipocyte differentiation and
targets PPARgamma. Biochem Biophys Res Commun. 390:247–251.
2009.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim
MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, et
al: miR-130 suppresses adipogenesis by inhibiting peroxisome
proliferator-activated receptor gamma expression. Mol Cell Biol.
31:626–638. 2011.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Kim YJ, Hwang SJ, Bae YC and Jung JS:
MiR-21 regulates adipogenic differentiation through the modulation
of TGF-beta signaling in mesenchymal stem cells derived from human
adipose tissue. Stem Cells. 27:3093–3102. 2009.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Esau C, Kang X, Peralta E, Hanson E,
Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, et
al: MicroRNA-143 regulates adipocyte differentiation. J Biol Chem.
279:52361–52365. 2004.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Hadadeh O, Barruet E, Peiretti F, Verdier
M, Bernot D, Hadjal Y, Yazidi CE, Robaglia-Schlupp A, De Paula AM,
Nègre D, et al: The plasminogen activation system modulates
differently adipogenesis and myogenesis of embryonic stem cells.
PLoS One. 7(e49065)2012.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Tang CH, Hill ML, Brumwell AN, Chapman HA
and Wei Y: Signaling through urokinase and urokinase receptor in
lung cancer cells requires interactions with beta1 integrins. J
Cell Sci. 121:3747–3756. 2008.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Baldwin LA, Hoff JT, Lefringhouse J, Zhang
M, Jia C, Liu Z, Erfani S, Jin H, Xu M, She QB, et al: CD151-α3β1
integrin complexes suppress ovarian tumor growth by repressing
slug-mediated EMT and canonical Wnt signaling. Oncotarget.
5:12203–12217. 2014.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Zhao G, Kim EW, Jiang J, Bhoot C, Charles
KR, Baek J, Mohan S, Adams JS, Tetradis S and Lyons KM: CCN1/Cyr61
is required in osteoblasts for responsiveness to the anabolic
activity of PTH. J Bone Miner Res. 35:2289–2300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Asuthkar S, Gondi CS, Nalla AK, Velpula
KK, Gorantla B and Rao JS: Urokinase-type plasminogen activator
receptor (uPAR)-mediated regulation of WNT/β-catenin signaling is
enhanced in irradiated medulloblastoma cells. J Biol Chem.
287:20576–20589. 2012.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Gondi CS, Kandhukuri N, Kondraganti S,
Gujrati M, Olivero WC, Dinh DH and Rao JS: Down-regulation of uPAR
and cathepsin B retards cofilin dephosphorylation. Int J Oncol.
28:633–639. 2006.PubMed/NCBI
|
|
156
|
Weisel JW: Fibrinogen and fibrin. In:
Advances in Protein Chemistry. Vol 70. Academic Press, Cambridge,
MA, pp247-299, 2005.
|
|
157
|
Neuss S, Schneider RK, Tietze L, Knüchel R
and Jahnen-Dechent W: Secretion of fibrinolytic enzymes facilitates
human mesenchymal stem cell invasion into fibrin clots. Cells
Tissues Organs. 191:36–46. 2010.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Valenick LV, Hsia HC and Schwarzbauer JE:
Fibronectin fragmentation promotes alpha4beta1 integrin-mediated
contraction of a fibrin-fibronectin provisional matrix. Exp Cell
Res. 309:48–55. 2005.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Gavin KM, Majka SM, Kohrt WM, Miller HL,
Sullivan TM and Klemm DJ: Hematopoietic-to-mesenchymal transition
of adipose tissue macrophages is regulated by integrin β1 and
fabricated fibrin matrices. Adipocyte. 6:234–249. 2017.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Rodríguez Fernández JL and Ben-Ze'ev A:
Regulation of fibronectin, integrin and cytoskeleton expression in
differentiating adipocytes: Inhibition by extracellular matrix and
polylysine. Differentiation. 42:65–74. 1989.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Liu LF, Shen WJ, Zhang ZH, Wang LJ and
Kraemer FB: Adipocytes decrease Runx2 expression in osteoblastic
cells: Roles of PPARγ and adiponectin. J Cell Physiol. 225:837–845.
2010.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Kortlever RM and Bernards R: Senescence,
wound healing and cancer: The PAI-1 connection. Cell Cycle.
5:2697–2703. 2006.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Zhang Y, Pan Y, Xie C and Zhang Y: miR-34a
exerts as a key regulator in the dedifferentiation of osteosarcoma
via PAI-1-Sox2 axis. Cell Death Dis. 9(777)2018.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Wang Z, Xing H, Hu H, Dai T, Wang Y, Li Z,
An R, Xu H, Liu Y and Liu B: Intraglandular transplantation of
adipose-derived stem cells combined with platelet-rich fibrin
extract for the treatment of irradiation-induced salivary gland
damage. Exp Ther Med. 15:795–805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Gurewich V: Therapeutic fibrinolysis: How
efficacy and safety can be improved. J Am Coll Cardiol.
68:2099–2106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Cao GQ, Li L, Wang YB, Shi ZZ, Fan DY and
Chen HY: Treatment of free-flowing tuberculous pleurisy with
intrapleural urokinase. Int J Tuberc Lung Dis. 19:1395–1400.
2015.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Berkenpas MB, Lawrence DA and Ginsburg D:
Molecular evolution of plasminogen activator inhibitor-1 functional
stability. EMBO J. 14:2969–2977. 1995.PubMed/NCBI
|
|
168
|
Naderi N, Griffin MF, Mosahebi A, Butler
PE and Seifalian AM: Adipose derived stem cells and platelet rich
plasma improve the tissue integration and angiogenesis of
biodegradable scaffolds for soft tissue regeneration. Mol Biol Rep.
47:2005–2013. 2020.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Rahman SU, Park CH, Baek JH, Ryoo HM and
Woo KM: Fibrin-enhanced canonical Wnt signaling directs plasminogen
expression in cementoblasts. Int J Mol Sci. 18(2380)2017.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Tara S and Krishnan LK: Differentiation of
circulating neural progenitor cells in vitro on fibrin-based
composite-matrix involves Wnt-β-catenin-like signaling. J Cell
Commun Signal. 13:27–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Zhou C, Li S, Wenqiguli N, Yu L, Zhao L,
Wu P and Nijiati T: The expressions of the Notch and Wnt signaling
pathways and their significance in the repair process of alveolar
bone defects in rabbits with bone marrow stem cells compounded with
platelet-rich fibrin. Hua Xi Kou Qiang Yi Xue Za Zhi. 34:130–135.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|