Introduction
Homeostasis of the extracellular matrix within the
heart is dependent on cardiac fibroblasts, which account for 60-70%
of cells in the human heart. These cells are responsible for the
synthesis and breakdown of the extracellular matrix within the
heart (1,2). They are also able to detect physical
and biological stimuli; these stimuli may change the activity of
the cardiac fibroblasts and their potential for collagen synthesis
(2,3). It has been found that heart fibroblasts
secrete cytokines with autocrine or paracrine regulatory effects
(2,3): Fibroblasts isolated from the heart of a
patient with heart failure were found to release higher levels of
cytokines under inflammatory conditions, and this effect was
independent of hypoxia (3). The
bioactive molecules secreted by the fibroblasts may exert
regulatory effects on cardiomyocytes (2,4,5) or cardiac vessels (6,7).
Cardiac fibroblasts are involved in the regulation
of the heart extracellular matrix under both physiological and
pathological conditions (8).
Collagen, the fibrotic protein of the extracellular matrix, not
only provides mechanical support for cardiomyocytes (9), but is also responsible for the
distribution of mechanical force within the heart (2) and can influence the
electrophysiological processes within the myocardium (10). Collagen content determines compliance
of the heart. Cardiac fibroblasts can be transformed into a
profibrotic phenotype, known as myofibroblasts (11), which exhibit elevated migratory and
proliferative capacity, and secrete a range of bioactive molecules
(12).
The human heart is also home to mast cells (13). These can secrete proinflammatory,
angiogenic and lymphangiogenic factors, and may participate in the
pathogenesis of heart disease (13).
They are also an important source of histamine, and histidine
decarboxylase, the enzyme responsible for histamine synthesis, has
been found within the heart (14).
Histamine was found to exert positive chronotropic and inotropic
effects on the hearts of rats overexpressing the H2
receptors, and these effects were observed in vivo and in
vitro (15). After myocardial
infarction, the number of mast cells increases in the myocardium
(16), and histamine concentration
within the blood is elevated (17).
Moreover, histamine may promote fibrosis within the transgenic mice
via the H2 receptors (17). Although histamine regulates fibrosis
in the granulation tissue of the wound (18), it was not found to influence the
fibroblasts of the intact skin (18). In addition, rat myofibroblasts from
the myocardial scar were found to be subject to the regulatory
action of histamine (19).
The aim of the present study was to determine
whether histamine exerted a regulatory influence on collagen
accumulation in cells derived from intact heart tissues, and to
examine the participation of histamine in heart fibrosis
initiation. Additionally, the histamine receptor involved in heart
fibrosis regulation was identified, as well as the types of the
isolated cells.
Materials and methods
Cell culture and experimental
design
The cells (stored in liquid nitrogen) were obtained
from control rats of another project, which was approved by the
Local Commission of Ethics in Łódź (Łódź, Poland; approval no. 46ŁB
624/2012).
Cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with FBS (Biowest), gentamycin (25
µg/ml) amphotericin (2.5 µg/ml) at 37˚C in a humidified incubator,
supplied with 95% air and 5% CO2. Defrosted cells were
plated at an initial cell density of 8x104 cells per
well in a 6-well plate. The number of cells were counted using
trypan blue staining at room temperature (5 min) in a Bürker
chamber. After cells had reached confluence (70%), the cells were
trypsinized, passaged in new flasks and used for subsequent
experiments.
In vitro experiments
Myofibroblasts were grown in DMEM supplemented with
3% FBS and antibiotics as described above. The first part of the
study investigated the effects of histamine (Sigma-Aldrich; Merck
KGaA) on collagen content in the myofibroblast culture. Histamine
was used at concentrations ranging from
1x10-10-1x10-5 M. The results were compared
with control (untreated cells).
In the second portion of the study, receptor
agonists were used to identify receptors involved in the
histamine-dependent effects: 2-pyridylethylamine dihydrochloride
(H1 receptor agonist) (Tocris Bioscience), amthamine
dihydrobromide (H2 receptor agonist) (Tocris
Bioscience), imetit (H3 receptor agonist; Sigma-Aldrich;
Merck KGaA), 4-methylhistamine hydrochloride (H4
receptor agonist; Sigma-Aldrich; Merck KGaA). The agonists were
applied at concentrations of 1x10-8, 1x10-6 and
1x10-4 M, respectively.
The third portion of the study examined the effects
of the histamine receptor inhibitors ketotifen
(H1-receptor inhibitor; Sigma-Aldrich; Merck KGaA) and
ranitidine (H2-receptor inhibitor; Sigma-Aldrich; Merck
KGaA). The cells were divided into four groups: Untreated controls,
cells treated with histamine (1x10-6 M), a group treated
with both histamine (1x10-6 M) and histamine receptor
inhibitor (1x10-5 M), and a group treated only the with
histamine receptor inhibitor (1x10-5 M).
The inhibitors of the H3 (ciproxifan;
1x10-5 M) and H4 (JNJ7777120;
1x10-5 M; Sigma-Aldrich; Merck KGaA) receptors were
investigated. These experiment were comprised of the following
groups: Controls, cells treated with 0.001% DMSO (antagonist
solvent); cells treated with 1x10-6 M histamine; cells
treated with ciproxifan alone; cells treated with JNJ7777120 alone;
cells treated with 1x10-6 M histamine and ciproxifan;
and cells treated with 1x10-6 M histamine and
JNJ7777120). All groups in this portion of the study, except the
untreated control, were treated with 0.001% DMSO. Each group
consisted of 8 or 9 repeats.
Reverse transcription-quantitative
(RT-q)PCR
Gene expression was measured in four separate
cultures from each group, and each sample was measured twice. Total
RNA from cells was extracted using a Total RNA Mini kit (A&A
Biotechnology). Reverse transcription and cDNA synthesis was
performed using a PrimeScript RT-PCR kit according to the
manufacturer's protocol (Takara Bio, Inc.).
The expression of the collagen type I and III genes
was measured, and GAPDH, hypoxanthine-guanine
phosphoribosyltransferase (hprt1) and 60S ribosomal protein L13a
(rpl13a) were used as the reference genes. The genes coding for the
α1 chain of procollagen type I, procollagen type III and hprt1,
rpl13a were evaluated using an Universal Probe Library (UPL; Roche
Diagnostics GmbH). Real Time ready Custom Single assays (Roche
Diagnostics) were used to measure GAPDH expression. The sequences
of the primers used were: Collagen type 1 forward,
GGGATTCCCTGGACCTAAAG and reverse, GGAACACCTCGCTCTCCA, UPL probe
#67; collagen type III forward, TCCCCTGGAATCTGTGAATC and reverse
TGAGTCGAATTGGGGAGAAT, UPL probe #49; rpl13a forward,
CCCTCCACCCTATGACAAGA and reverse, GGTACTTCCACCCGACCTC, UPL probe
#74; and hprt1 forward, CTCCTCAGACCGCTTTTCC and reverse,
TCATAACCTGGTTCATCATCA, UPL probe #95.
The reactions were performed using the Fast Start
Essential Probe MasterMix (Roche Diagnostics GmbH) with the
following thermocycling conditions: Initial incubation at 95˚C for
10 min; followed by 55 cycles of 95˚C for 10 sec, 60˚C for 30 sec,
and incubation at 72˚C for 1 sec; with a final incubation at 40˚C
for 30 sec. A LightCycler® 96 software (Roche
Diagnostics) was used to calculate the relative gene expression
(20).
Flow cytometry
The expression of α-smooth muscle actin, vimentin
and desmin within cells was assessed using flow cytometry. In the
first step, the cells were fixed at 4˚C for 30 min (Fixation
Buffer; BD Biosciences) and then permeabilized with Perm Buffer (BD
Biosciences). In the next step, the cells were stained at a
temperature of 6˚C for 30 min in Stain Buffer (BD Biosciences).
Subsequently, the tested cells were treated with specific
antibodies conjugated to FITC at 4˚C for 30 min. For each
experiment, ~10,000 cells were assessed on the flow cytometer. The
following antibodies were used for the experiment: α-smooth muscle
actin Antibody (cat. no. NBP-2-34522F; 1:500; Novus Biologicals);
mouse IgG2a κ-Light Chain Isotype control (cat. no. NBP1-43955;
1:500; Novus Biologicals); Desmin antibody (cat. no. DES/1711;
1:500; Novus Biologicals); mouse IgG1 κ-Light Chain Isotype control
(1:500; Novus Biologicals); Vimentin Antibody (cat. no. LN-6;
1:250: Novus Biologicals); and mouse IgM Isotype control (1:250;
eBioscience, Inc; Thermo Fisher Scientific, Inc.). A FACS Canto II
Analytical Flow Cytometer (BD Biosciences) was used for analysis.
FACS data were analyzed using Kaluza Analysis version 1.5a (Beckman
Coulter, Inc.).
Determination of collagen levels
The total collagen levels in the cultured cells were
evaluated using the Woessner method (14). The samples were dried at 60˚C and
then hydrolyzed with 6 N HCl (3 ml/10 mg dry tissue) at 100˚C for
24 h in a water bath. The hydrolysates were evaporated and the
precipitates were dissolved in 3 ml deionized water. Subsequently,
the samples were neutralized with 1 N NaOH and diluted to 10 ml
with deionized water. For analysis, 0.2 ml sample was taken and
diluted with redistilled water to a final volume of 2 ml. The
sample was suspended in 1.25 ml Chloramine T in citrate buffer (pH
6.0), shaken for 5 min and incubated at room temperature for 20
min. During this time, the pyrrole was oxidized to hydroxyproline
by chloramine T. To remove the excess chloramine T, 1 ml perchloric
acid (3.15 M) was added. After 5 min, the samples were treated with
1 ml 20% p-dimethylaminobenzaldehyde and incubated in a water bath
at 60˚C for 20 min. The optical density of the samples was scanned
at 560 nm on a spectrophotometer.
Statistical analysis
Data were compared using a Kruskal-Wallis test.
Differences between all groups were compared using a multiple
comparisons of mean ranks test (Dunn's test). P<0.05 was
considered to indicate a statistically significant difference.
Statistica version 13 (StatSoft, Inc.) was used for statistical
analysis.
Results
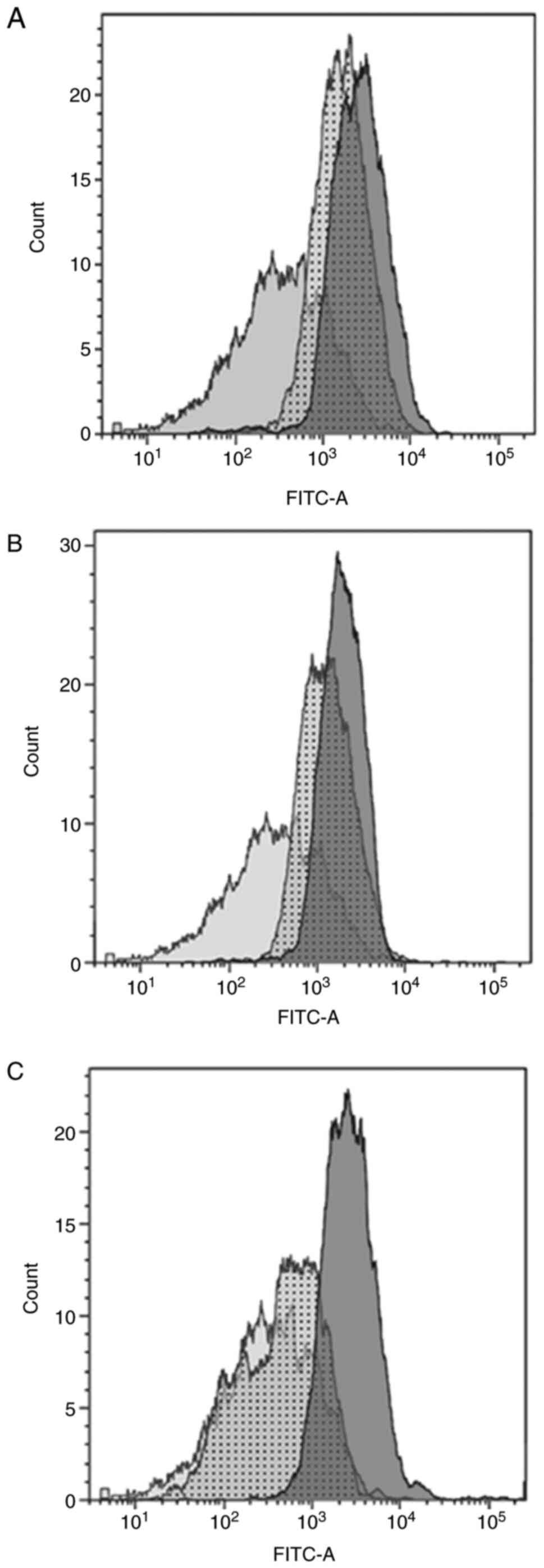
Flow cytometry
The isolated cells were found to have longitudinal,
fusiform, spindle and stellate morphology. The flow cytometry
experiments showed that the structure of isolated cells was typical
for myofibroblasts (20-22);
they were positive for all tested markers: α-smooth muscle actin
(Fig. 1A), desmin (Fig. 1B) and vimentin (Fig. 1C). The expression of these markers
indicates that this cell culture could be distinguished from smooth
muscle cells (vimentin negative, and positive for both α-smooth
muscle actin and desmin) and fibroblasts (negative for α-smooth
muscle actin, and positive for both desmin and vimentin) (23).
RT-qPCR
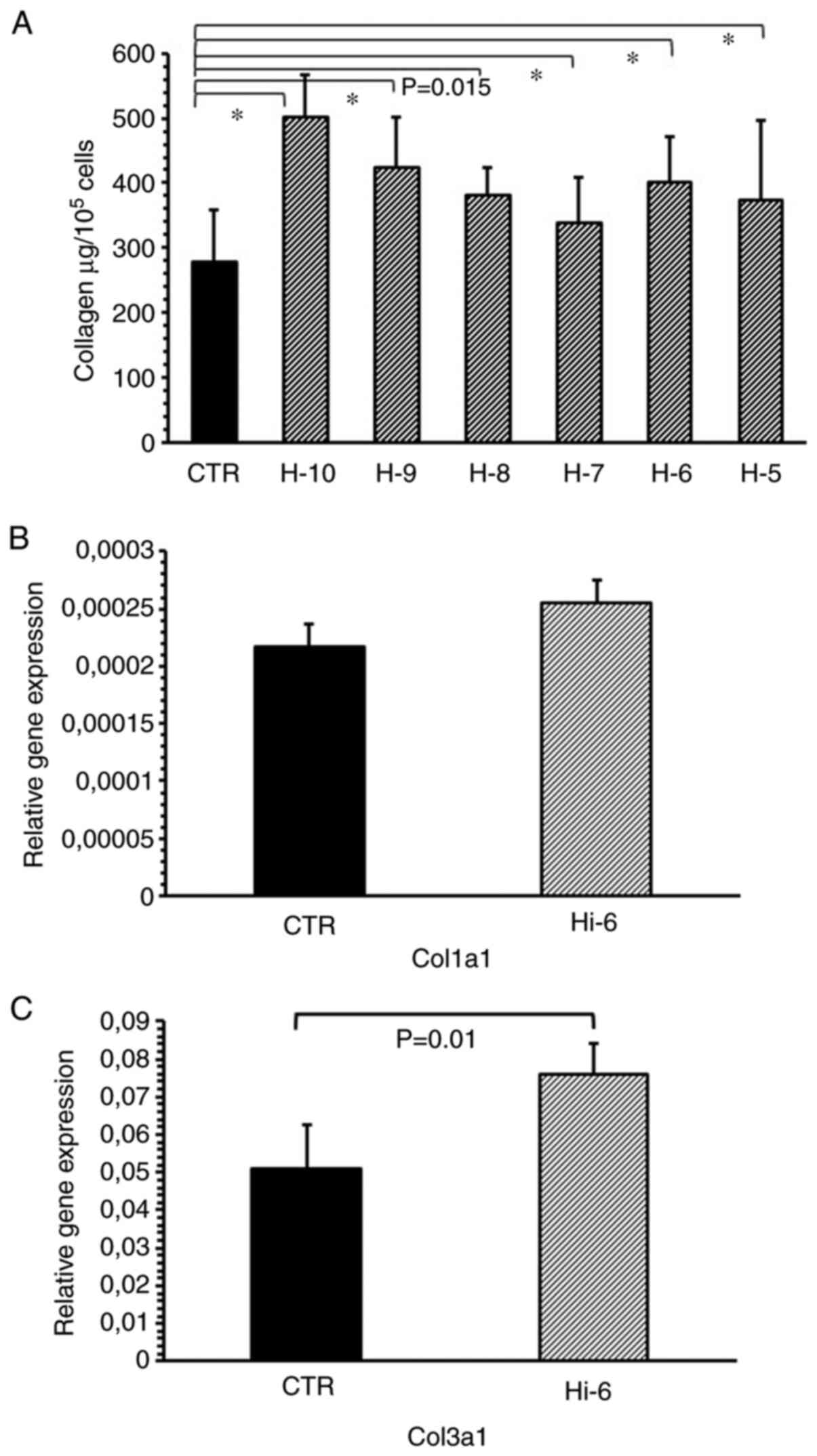
Histamine at concentrations ranging from
1x10-10-1x10-5 M significantly increased the
collagen levels in the cultured myofibroblasts compared with the
untreated control (Fig. 2A). The
maximal effect was observed for histamine administered at
1x10-10 M (P<0.001) and 1x10-9 M
(P<0.001). Histamine (1x10-6 M) increased the
expression of the collagen type III gene. (Fig. 2B and C).
In vitro experiments
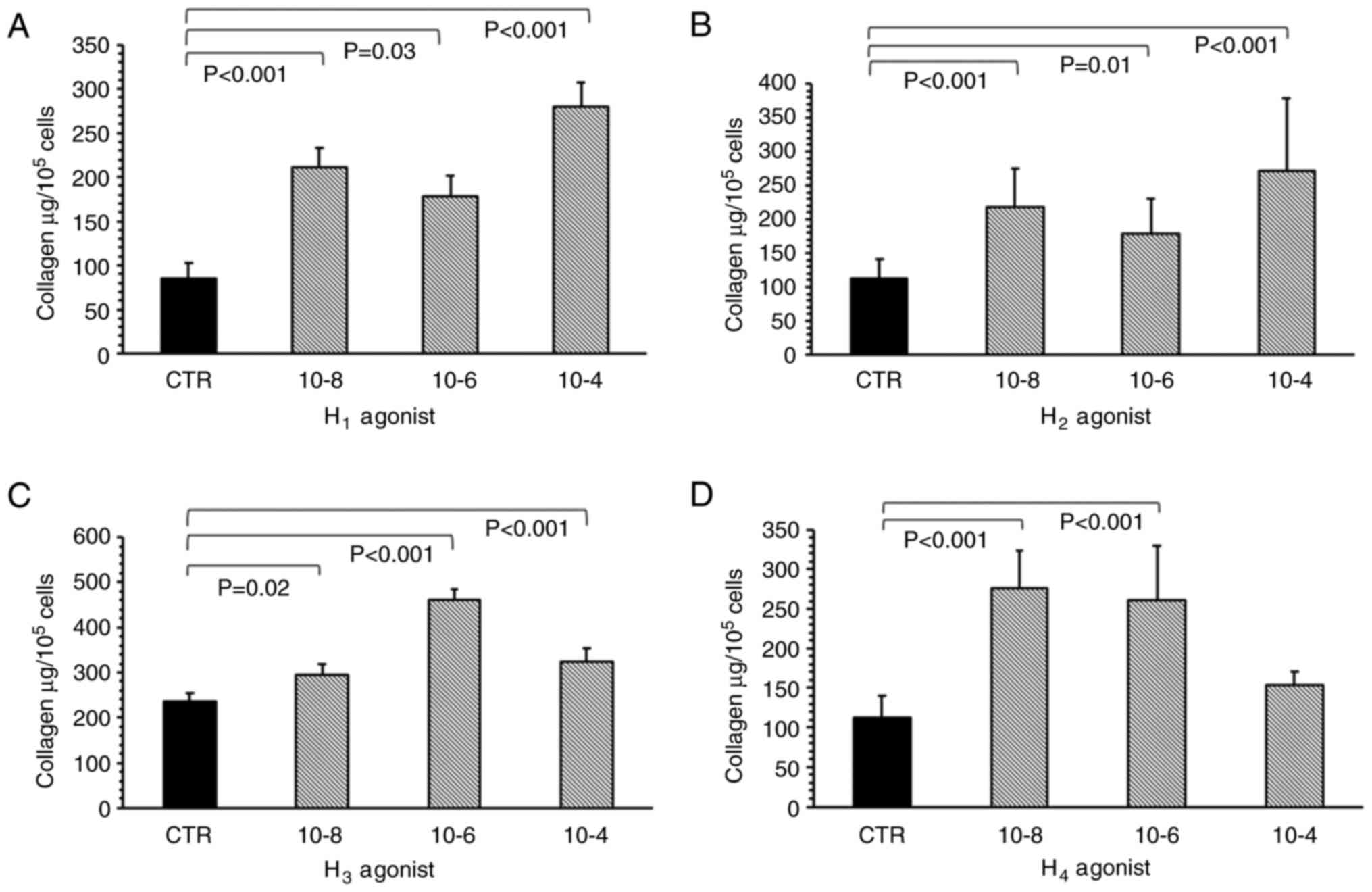
All histamine receptor agonists, used to mimic the
effects of histamine, significantly increased collagen levels in
the myofibroblast culture. The H1 receptor agonist
(2-pyridylethylamine dihydrochloride) significantly increased
collagen deposition compared with the control, at all tested
concentrations (1x10-8 M, P<0.001; 1x10-6
M, P=0.03; and 1x10-4 M, P<0.001; Fig. 3A). The most potent effect was
observed when cells were treated with 1x10-4 M of the
H1 receptor agonist. The H2 receptor agonist
(amthamine dihydrobromide) had a statistically significant effect
on collagen level in the myofibroblast cultures at all tested
concentrations (Fig. 3B).
Specifically, 1x10-8 (P<0.001), 1x10-6
(P<0.01) and 1x10-4 M (P<0.001) H2
receptor agonist significantly increased collagen content within
the cell cultures compared with the control.
Imetit, the H3 receptor agonist,
increased the collagen content compared with controls when
administered at 1x10-8 (P=0.02), 1x10-6
(P<0.001) and 1x10-4 M (P<0.001) (Fig. 3C), with the maximum effect observed
at 1x10-6 M. Elevated collagen levels were also observed
for 1x10-8 (P<0.001) and 1x10-6 M
(P<0.001) 4-methylhistamine hydrochloride, the H4
receptor agonist (Fig. 3D); however,
no such increase was observed at 1x10-4 M.
Histamine significantly increased collagen levels in
the myofibroblast cultures compared with controls at a
concentration of 1x10-6 M (P=0.03; Fig. 4A). Treatment with 1x10-5 M
ketotifen, an H1 receptor inhibitor, did not modify the
effects of histamine when compared with histamine alone; the levels
of collagen in the ketotifen and histamine-treated groups were
still higher than that in the controls (P=0.04). Ketotifen (without
histamine), applied to the cultures of myofibroblasts at a
concentration of 1x10-5 M, did not alter the collagen
content in these cultures when compared with the control (Fig. 4A).
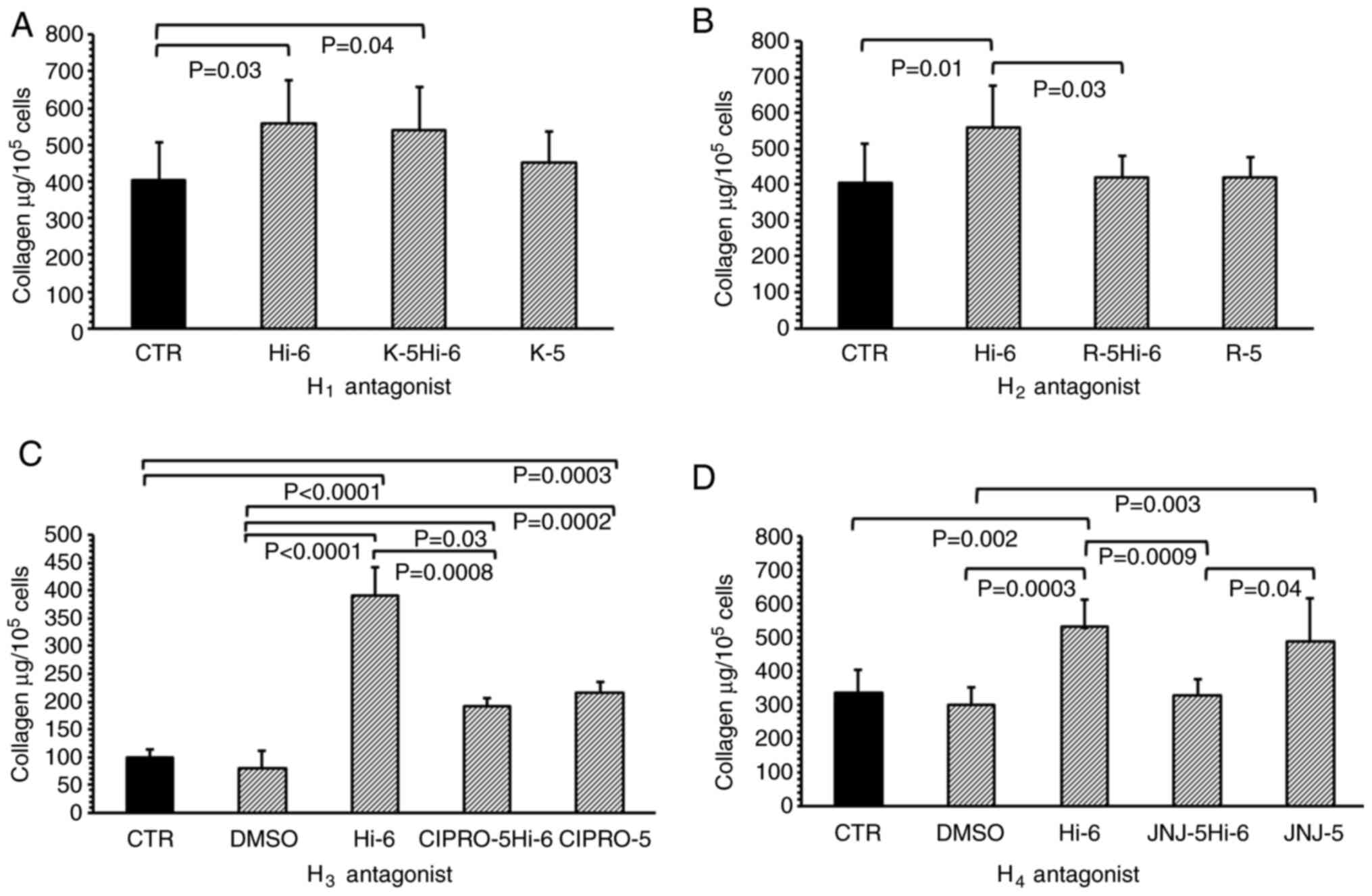 | Figure 4Total collagen content in isolated
heart cells treated with histamine receptor antagonists. (A)
Collagen content in myofibroblasts culture isolated from the heart
in the CTR or cells treated with Hi-6, K-5 (H1 receptor
antagonist) or both (K-5Hi-6); (B) treated with Hi-6, R-5
(H2 receptor antagonist) or both R-5Hi-6; (C) treated
with DMSO, Hi-6, CIPRO-5 (H3 receptor antagonist) or
both (CIPRO-5Hi-6); and (D) DMSO, Hi-6, J-5 (H4 receptor
antagonist) or both (J-5Hi-6). Data are presented as the mean ±
standard deviation. CTR, control; Hi-6, 1x10-6 M
histamine; K-5, 1x10-5 M ketotifen; R-5,
1x10-5 M ranitidine alone; DMSO, 0.001% DMSO; CIPRO-5,
1x10-5 M ciproxifan; J-5, 1x10-5 M
JNJ7777. |
The H2 receptor inhibitor Ranitidine
(1x10-5 M) significantly reduced the effects of
histamine (1x10-6 M) when compared with cells treated
with histamine alone (P=0.03; Fig.
4B). Ranitidine (without histamine), applied at a concentration
of 1x 10-5 M to the cell cultures, did not change the
collagen content when compared with the control group.
The control and DMSO-treated cultures were found to
have similar collagen levels. In addition, histamine increased the
levels of collagen within the culture when compared with the
untreated controls (P<0.001) and DMSO-treated (P<0.001)
cells. Ciproxifan, the H3 receptor inhibitor, applied at
concentrations of 1x 10-5 M, blocked the effects of
histamine, decreasing the levels of collagen when compared with
histamine alone (P=0.0008). Furthermore, the groups treated with
ciproxifan and histamine, or ciproxifan alone, demonstrated
augmentation of collagen content when compared with the untreated
controls (P=0.0003) or cells treated with DMSO (P=0.0002; Fig. 4C).
Comparable levels of collagen were found in the
untreated controls and DMSO-treated cultures; however, higher
collagen levels were observed in fibroblast cultures treated with
histamine when compared with the control (P=0.002) and DMSO
(P=0.0003) treated groups. JNJ (H4 receptor inhibitor)
blocked histamine-induced collagen elevation in the culture
(P=0.0009; Fig. 4D) when compared
with cells treated with histamine alone. Higher levels of collagen
were found in the JNJ-treated fibroblasts compared with the DMSO
treated group.
Discussion
In addition, flow cytometry analysis showed that the
cells were α-smooth muscle actin positive, vimentin positive and
desmin positive. These features differentiate them from smooth
muscle cells, which are desmin and α-smooth muscle actin positive,
but vimentin negative, and fibroblasts, which are negative for
α-smooth muscle actin, but positive for both desmin and vimentin
(23,24). The cells isolated in the present
study from intact hearts are hypothesized to transform into
myofibroblasts during isolation or culture. Recent data has
indicated that several factors may be responsible for this
transformation, and it has been suggested that it may be induced by
increased substrate stiffness amongst the cultured cells (25). An intrinsic mechanotransduction
mechanism may also be involved in the transformation of cells into
myofibroblasts (26).
Histamine appears to have a significant influence on
collagen accumulation in the heart myofibroblast cultures at all
applied concentrations. This observation clearly suggests that
histamine augments collagen content by acting directly on the
myofibroblasts, and that unstimulated myofibroblasts from the
intact heart may respond to histamine by increasing collagen
content within the cultures. These data correspond with our
previous studies, suggesting that histamine may increase the
collagen level within cultures of myofibroblasts derived from the
heart myocardial infarction scar (19), and that it may also accelerate the
metabolism of the myofibroblasts and increase collagen content
within the myofibroblasts derived from granulation tissue of skin
wound model of the rats (18). This
effect corresponds with increased secretion of TGF-β1 following
histamine treatment. Indeed, previous studies performed on
myofibroblasts derived from wounds or myocardial infarction
indicate that cells subjected previously to pro-inflammatory
factors may respond to histamine (19,18).
However, amongst cultures of fibroblasts derived from intact skin,
treatment with high (1x10-4-1x10-6 M) or low
(1x10-9 M) concentrations of histamine augment collagen
type I content (27). These effects
were confirmed by Takeda et al (28) on human foreskin fibroblasts.
Histamine exerts its effect on collagen content via stimulation of
procollagen type III expression; however, no such effect is
observed with regard to procollagen type I.
The tested inhibitors of the H2,
H3 and H4 histamine receptors were found to
block histamine-induced elevation of collagen content. These
findings are supported by the observation that the H2,
H3 and H4 agonists mimicked histamine action
and augmented collagen content within the cultures. Such expression
of H2, H3 and H4 histamine
receptors, and the confirmation of their activity within the heart
has been reported previously (29-31).
Interestingly, the H2-receptor agonist amthamine
dihydrobromide demonstrated its maximal effect at a high relatively
concentration (1x10-4 M), whereas imetit activity (an
H3 receptor agonist) peaked at 1x10-6 M, and
the H4-receptor agonist 4-methylhistamine hydrochloride
demonstrated maximal activity at both 1x10-6 and
1x10-8 M. Hence, stimulation of the H2,
H3 and H4 histamine receptors may be
responsible for the collagen augmentation observed within the
cardiac myofibroblast cultures.
Neither histamine itself nor any of the receptor
agonists tested in the present study were found to exert a
concentration-dependent effect, consistent with our previous study
(19). This effect has been
attributed to saturation of the receptors by lower concentrations
of the tested compounds. In contrast, the results of the present
study suggest that the three histamine receptors act in accord, and
the blockade of one receptor by a single antagonist negates the
full effect of histamine; however, this hypothesis required further
investigation. However, different effects were observed for
receptor H1. The H1-receptor agonist
2-pyridylethylamine dihydrochloride increased collagen content but
the H1 receptor antagonist ketotifen did not block
histamine action. Therefore, the present study failed to confirm
whether the H1 receptor regulates collagen content in
cardiac myofibroblasts. The effect of 2-pyridylethylamine
dihydrochloride is hypothesized to be unspecific; it is not
dependent on the H1 receptor.
Previously, it has been proposed that in the cardiac
myofibroblasts obtained from a myocardial infarction scar, the
histamine content is mediated by the H3 receptor
(19). However, the effect of
histamine was found to be influenced by the H1 receptor
in myofibroblasts from wound granulation tissue (18,32).
H1 receptor activation appears to be involved in the
regulation of collagen metabolism within dermal fibroblasts
(27), whereas blockade of the
H2 receptor decreased collagen type I gene expression in
human fibroblasts (28). These
results were also observed in vivo: H2 histamine
receptors appear to be involved in the regulation of collagen
levels in a model of cutaneous wounds in rats (33), and both H1 and
H2 receptors may regulate collagen levels in
fibroblast-like cells (34).
H3 histamine receptors may also regulate collagen
content within myofibroblasts derived from a myocardial infarction
scar (19). Hence, it appears that
different histamine receptors may be involved in the regulation of
collagen content, although the obtained results are dependent on
the experimental model, and the selected tissue or species.
Previous studies have suggested that different types of histamine
receptors participate in the regulation of collagen deposition in
various organs or tissues (19,32-34).
This phenomenon could be dependent on the varying influence of the
extracellular environment on myofibroblasts (2). The results of the present study
indicate that in myofibroblasts derived from intact rat heart, the
H2, H3 and H4 histamine receptors
participate in regulation of collagen accumulation within the
culture. In contrast, in myofibroblasts taken from a myocardial
infarction scar, only the H3 histamine receptor was
previously confirmed to influence collagen deposition (19). The myocardial scar myofibroblasts
were subjected to stimulation by mediators released by the
inflammatory environment (35). The
activity of some types of histamine receptors participating in
fibrosis regulation is hypothesized to be dependent on mediators
released during healing or inflammatory processes.
Histamine is hypothesized to act on collagen content
via a number of autocrine or paracrine effects (18,36).
Histamine stimulates the release of profibrotic TGF-β1 by
myofibroblasts from granulation tissue of a rat wound model in an
H1-dependent manner (18). Moreover, histamine is known to
increase secretion of ATP via activation of pannexin-1 hemichannels
and to increase collagen type I content within the subcutaneous
fibroblast cultures (36).
The results of the present study indicate that
histamine may directly increase collagen content on rat heart
myofibroblasts, possibly by augmentation of procollagen type III
expression. This effect is dependent on H2,
H3 and H4 histamine receptor stimulation.
These results highlight the role of histamine in heart fibrosis and
suggest that histamine receptors may be potential targets for
antifibrotic therapy.
Acknowledgements
We would like to thank Mrs Teresa Staszewska
(Department of Behavioral Pathophysiology, Medical University of
Łódź, Łódź, Poland) for her excellent technical assistance.
Funding
The present study was supported by a grant from the Medical
University of Łódź (grant no. 503/6-103-04/503-01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LP and MJ participated in the study design and
analysis of the data, performed all the experiments and
participated in the manuscript preparation. JS assisted with data
analysis and interpretation. JD supervised the laboratory analyses,
assisted with the design of the study and participated in
manuscript preparation. All authors have read and approved the
final manuscript. LP, JS, MJ and JD confirm the authenticity of all
the raw data.
Ethics approval and consent to
participate
The cells (stored in liquid nitrogen) were obtained
from control rats of another project, which was approved by the
Local Commission of Ethics in Łódź (approval no. 46ŁB
624/2012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Souders CA, Bowers SL and Baudino TA:
Cardiac fibroblast: The renaissance cell. Circ Res. 105:1164–1176.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gałdyszyńska M, Bobrowska E, Lekka M,
Radwańska P, Piera L, Szymański J and Drobnik J: . The
stiffness-controlled release of interleukin-6 by cardiac
fibroblasts is dependent on integrin α2β1. J Cell Mol Med.
24:13853–13862. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sandstedt J, Sandstedt M, Lundqvist A,
Jansson M, Sopasakis VR, Jeppsson A and Hultén LM: Human cardiac
fibroblasts isolated from patients with severe heart failure are
immune-competent cells mediating an inflammatory response.
Cytokine. 113:319–325. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandstedt M, Rotter Sopasakis V, Lundqvist
A, Vukusic K, Oldfors A, Dellgren G, Sandstedt J and Hultén LM:
Hypoxic cardiac fibroblasts from failing human hearts decrease
cardiomyocyte beating frequency in an ALOX15 dependent manner. PLoS
One. 13(e0202693)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bageghni SA, Hemmings KE, Zava N, Denton
CP, Porter KE, Ainscough JFX, Drinkhill MJ and Turner NA: Cardiac
fibroblast-specific p38α MAP kinase promotes cardiac hypertrophy
via a putative paracrine interleukin-6 signaling mechanism. FASEB
J. 32:4941–4954. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cao F, Liu X, Cao X, Wang S, Fu K, Zhao Y,
Shen F and Liu J: Fibroblast growth factor 21 plays an inhibitory
role in vascular calcification in vitro through OPG/RANKL system.
Biochem Biophys Res Commun. 491:578–586. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mcgettrick HM, Ward LS, Rainger GE and
Nash GB: Mesenchymal stromal cells as active regulators of
lymphocyte recruitment to blood vascular endothelial cells. Methods
Mol Biol. 1591:121–142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goh KY, He L, Song J, Jinno M, Rogers AJ,
Sethu P, Halade GV, Rajasekaran NS, Liu X, Prabhu SD, et al:
Mitoquinone ameliorates pressure overload-induced cardiac fibrosis
and left ventricular dysfunction in mice. Redox Biol.
21(101100)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Krenning G, Zeisberg EM and Kalluri R: The
origin of fibroblasts and mechanism of cardiac fibrosis. J Cell
Physiol. 225:631–637. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hiram R, Naud P, Xiong F, Al-U'datt D,
Algalarrondo V, Sirois MG, Tanguay JF, Tardif JC and Nattel S:
Right atrial mechanisms of atrial fibrillation in a rat model of
right heart disease. J Am Coll Cardiol. 74:1332–1347.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Philip JL, Xu X, Han M, Akhter SA and
Razzaque MA: Regulation of cardiac fibroblast-mediated maladaptive
ventricular remodeling by β-arrestins. PLoS One.
14(e0219011)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Varricchi G, Loffredo S, Borriello F,
Pecoraro A, Rivellese F, Genovese A, Spadaro G and Marone G:
Superantigenic activation of human cardiac mast cells. Int J Mol
Sci. 20(E1828)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Drobnik J, Karbownik-Lewińska M,
Szczepanowska A, Słotwińska D, Olczak S, Jakubowski L and Dabrowski
R: Regulatory influence of melatonin on collagen accumulation in
the infarcted heart scar. J Pineal Res. 45:285–290. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gergs U, Bernhardt G, Buchwalow IB, Edler
H, Fröba J, Keller M, Kirchhefer U, Köhler F, Mißlinger N, Wache H,
et al: Initial characterization of transgenic mice overexpressing
human histamine H2 receptors. J Pharmacol Exp Ther. 369:129–141.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kwon JS, Kim YS, Cho AS, Cho HH, Kim JS,
Hong MH, Jeong HY, Kang WS, Hwang KK, Bae JW, et al: Regulation of
MMP/TIMP by HUVEC transplantation attenuates ventricular remodeling
in response to myocardial infarction. Life Sci. 101:15–26.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen J, Hong T, Ding S, Deng L,
Abudupataer M, Zhang W, Tong M, Jia J, Gong H, Zou Y, et al:
Aggravated myocardial infarction-induced cardiac remodeling and
heart failure in histamine-deficient mice. Sci Rep.
7(44007)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wolak M, Bojanowska E, Staszewska T,
Ciosek J, Juszczak M and Drobnik J: The role of histamine in the
regulation of the viability, proliferation and transforming growth
factor β1 secretion of rat wound fibroblasts. Pharmacol Rep.
69:314–321. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Piera L, Olczak S, Kun T, Ciosek J and
Drobnik J: Histamine augments collagen deposition in isolated from
the myocardial infarction scar myofibroblasts culture, via H3
receptor stimulation. J Physiol Pharmacol. 70:239–247. 2019.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Drobnik J, Słotwińska D, Olczak S, Tosik
D, Pieniążek A, Matczak K, Koceva-Chyła A and Szczepanowska A:
Pharmacological doses of melatonin reduce the glycosaminoglycan
level within the infarcted heart scar. J Physiol Pharmacol.
62:29–35. 2011.PubMed/NCBI
|
|
22
|
Drobnik J, Owczarek K, Piera L, Tosik D,
Olczak S, Ciosek J and Hrabec E: Melatonin-induced augmentation of
collagen deposition in cultures of fibroblasts and myofibroblasts
is blocked by luzindole - a melatonin membrane receptors inhibitor.
Pharmacol Rep. 65:642–649. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kawasaki H, Ohama T, Hori M and Sato K:
Establishment of mouse intestinal myofibroblast cell lines. World J
Gastroenterol. 19:2629–2637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
van den Borne SW, Isobe S, Verjans JW,
Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J,
et al: Molecular imaging of interstitial alterations in remodeling
myocardium after myocardial infarction. J Am Coll Cardiol.
52:2017–2028. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Olsen AL, Bloomer SA, Chan EP, Gaça MD,
Georges PC, Sackey B, Uemura M, Janmey PA and Wells RG: Hepatic
stellate cells require a stiff environment for myofibroblastic
differentiation. Am J Physiol Gastrointest Liver Physiol.
301:G110–G118. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nguyen DT, Nagarajan N and Zorlutuna P:
Effect of substrate stiffness on mechanical coupling and force
propagation at the infarct boundary. Biophys J. 115:1966–1980.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murota H, Bae S, Hamasaki Y, Maruyama R
and Katayama I: Emedastine difumarate inhibits histamine-induced
collagen synthesis in dermal fibroblasts. J Investig Allergol Clin
Immunol. 18:245–252. 2008.PubMed/NCBI
|
|
28
|
Takeda T, Goto H, Arisawa T, Hase S,
Hayakawa T and Asai J: Effect of histamine on human fibroblast in
vitro. Arzneimittelforschung. 47:1152–1155. 1997.PubMed/NCBI
|
|
29
|
McCaffrey SL, Lim G, Bullock M, Kasparian
AO, Clifton-Bligh R, Campbell WB, Widiapradja A and Levick SP: The
histamine 3 receptor is expressed in the heart and its activation
opposes adverse cardiac remodeling in the angiotensin II mouse
model. Int J Mol Sci. 21(9757)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gergs U, Kirchhefer U, Bergmann F,
Künstler B, Mißlinger N, Au B, Mahnkopf M, Wache H and Neumann J:
Characterization of stressed transgenic mice overexpressing
H2-histamine receptors in the heart. J Pharmacol Exp Ther.
374:479–488. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shahid M, Tripathi T, Sobia F, Moin S,
Siddiqui M and Khan RA: Histamine, histamine receptors and their
role in immunomodulation: An updated systematic review. Open
Immunol J. 2:9–41. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wolak M, Bojanowska E, Staszewska T, Piera
L, Szymański J and Drobnik J: Histamine augments collagen content
via H1 receptor stimulation in cultures of myofibroblasts taken
from wound granulation tissue. Mol Cell Biochem. 476:1083–1092.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dabrowski R and Maśliński C: The role of
histamine in wound healing. II. The effect of antagonists and
agonists of histamine receptors (H1 and H2) on collagen levels in
granulation tissue. Agents Actions. 11:122–124. 1981.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hatamochi A, Fujiwara K and Ueki H:
Effects of histamine on collagen synthesis by cultured fibroblasts
derived from guinea pig skin. Arch Dermatol Res. 277:60–64.
1985.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen B and Frangogiannis NG: Chemokines in
myocardial infarction. J Cardiovasc Transl Res. 14:35–52.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pinheiro AR, Paramos-de-Carvalho D, Certal
M, Costa MA, Costa C, Magalhães-Cardoso MT, Ferreirinha F, Sévigny
J and Correia-de-Sá P: Histamine induces ATP release from human
subcutaneous fibroblasts, via pannexin-1 hemichannels, leading to
Ca2+ mobilization and cell proliferation. J Biol Chem.
288:27571–27583. 2013.PubMed/NCBI View Article : Google Scholar
|